Artificial Intelligence in Sports Medicine: Reshaping Electrocardiogram Analysis for Athlete Safety—A Narrative Review
Abstract
1. Introduction
2. Methods
3. Results
3.1. Cardiac Structural and Electrical Alterations
3.1.1. Atrial Fibrillation
3.1.2. Channelopathies
3.1.3. Hypertrophic Cardiomyopathy
3.1.4. Valvular Disease
3.2. Role of AI-ECG in Detecting Heart Failure and Arrhythmias
3.3. Internet of Things Wearables
3.4. eSports Athletes
4. Best Practice Example
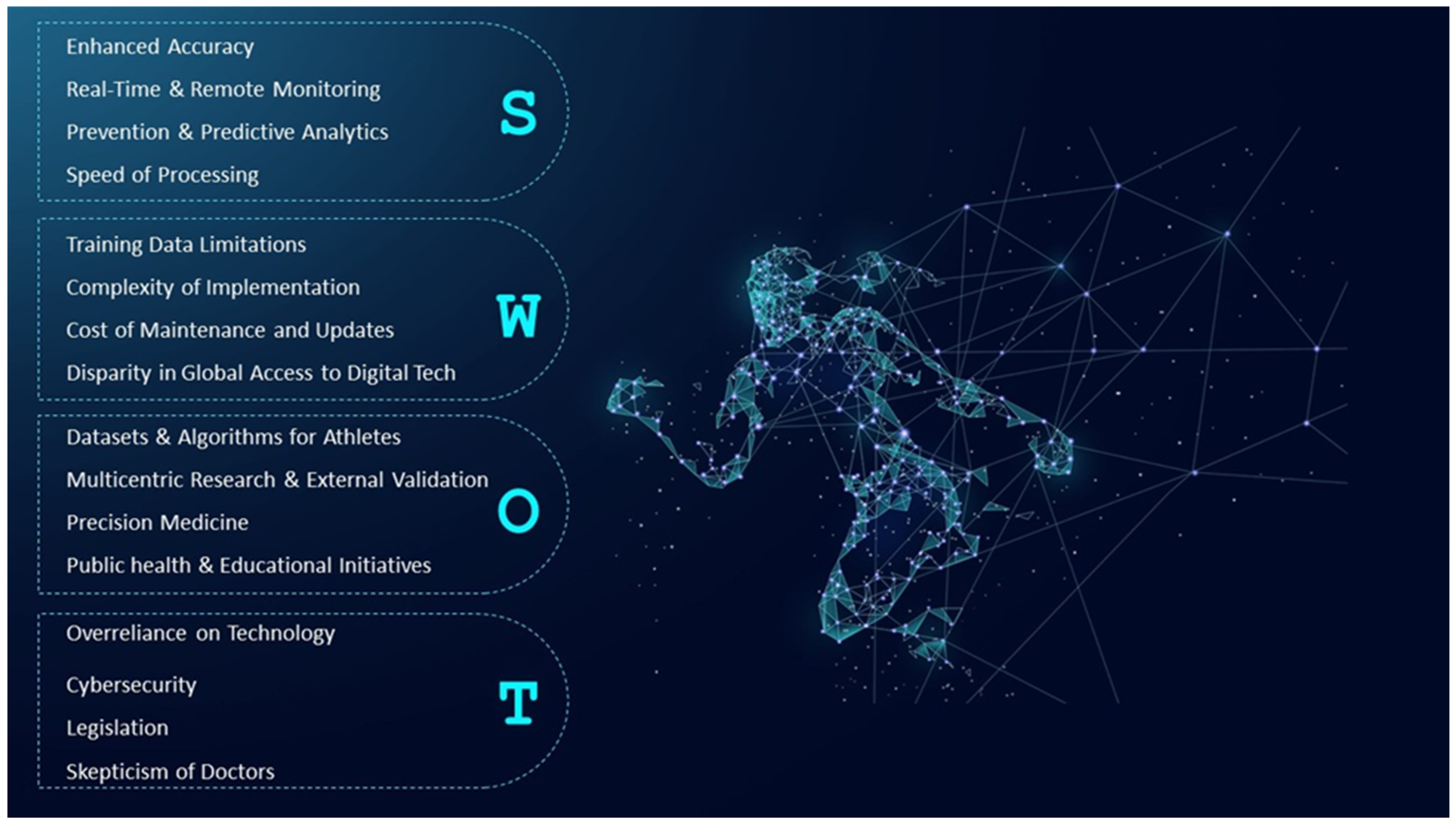
5. Limitations
6. Conclusions
7. Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Sharma, S.; Drezner, J.A.; Baggish, A.; Papadakis, M.; Wilson, M.G.; Prutkin, J.M.; La Gerche, A.; Ackerman, M.J.; Borjesson, M.; Salerno, J.C.; et al. International recommendations for electrocardiographic interpretation in athletes. Eur. Heart J. 2018, 39, 1466–1480. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.M.; Pitsiladis, Y.P.; Rozenstoka, S.; Bigard, X.; Löllgen, H.; Bachl, N.; Debruyne, A.; Pigozzi, F.; Casasco, M.; Jegier, A.; et al. Preparticipation medical evaluation for elite athletes: EFSMA recommendations on standardised preparticipation evaluation form in European countries. BMJ Open Sport Exerc. Med. 2021, 7, e001178. [Google Scholar] [CrossRef] [PubMed]
- Palermi, S.; Sirico, F.; Fernando, F.; Gregori, G.; Belviso, I.; Ricci, F.; D’Ascenzi, F.; Cavarretta, E.; De Luca, M.; Negro, F.; et al. Limited diagnostic value of questionnaire-based pre-participation screening algorithms: A “risk-exposed” approach to sports activity. J. Basic Clin. Physiol. Pharmacol. 2022, 33, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Caselli, S.; Sharma, S.; Basso, C.; Bax, J.J.; Corrado, D.; D’Andrea, A.; D’Ascenzi, F.; Di Paolo, F.D.; Edvardsen, T.; et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) joint position statement: Recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete’s heart. Eur. Heart J. 2018, 39, 1949–1969. [Google Scholar] [CrossRef] [PubMed]
- Palermi, S.; Cavarretta, E.; D’Ascenzi, F.; Castelletti, S.; Ricci, F.; Vecchiato, M.; Serio, A.; Cavigli, L.; Bossone, E.; Limongelli, G.; et al. Athlete’s Heart: A Cardiovascular Step-By-Step Multimodality Approach. Rev. Cardiovasc. Med. 2023, 24, 151. [Google Scholar] [CrossRef]
- Sezgin, E. Artificial intelligence in healthcare: Complementing, not replacing, doctors and healthcare providers. Digit. Health 2023, 9, 20552076231186520. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sellés, M.; Marina-Breysse, M. Current and Future Use of Artificial Intelligence in Electrocardiography. J. Cardiovasc. Dev. Dis. 2023, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Adasuriya, G.; Haldar, S. Next Generation ECG: The Impact of Artificial Intelligence and Machine Learning. Curr. Cardiovasc. Risk Rep. 2023, 17, 143–154. [Google Scholar]
- Harmon, K.G.; Asif, I.M.; Maleszewski, J.J.; Owens, D.S.; Prutkin, J.M.; Salerno, J.C.; Zigman, M.L.; Ellenbogen, R.; Rao, A.L.; Ackerman, M.J.; et al. Incidence, Cause, and Comparative Frequency of Sudden Cardiac Death in National Collegiate Athletic Association Athletes. Circulation 2015, 132, 10–19. [Google Scholar] [CrossRef]
- Harmon, K.G.; Asif, I.M.; Klossner, D.; Drezner, J.A. Incidence of Sudden Cardiac Death in National Collegiate Athletic Association Athletes. Circulation 2011, 123, 1594–1600. [Google Scholar] [CrossRef]
- Maron, B.J.; Doerer, J.J.; Haas, T.S.; Tierney, D.M.; Mueller, F.O. Sudden Deaths in Young Competitive Athletes. Circulation 2009, 119, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Danilov, A.; Aronow, W.S. Artificial Intelligence in Cardiology: Applications and Obstacles. Curr. Probl. Cardiol. 2023, 48, 101750. [Google Scholar] [CrossRef] [PubMed]
- Somani, S.; Russak, A.J.; Richter, F.; Zhao, S.; Vaid, A.; Chaudhry, F.; De Freitas, J.K.; Naik, N.; Miotto, R.; Nadkarni, G.N.; et al. Deep learning and the electrocardiogram: Review of the current state-of-the-art. EP Eur. 2021, 23, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Siontis, K.C.; Noseworthy, P.A.; Attia, Z.I.; Friedman, P.A. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nat. Rev. Cardiol. 2021, 18, 465–478. [Google Scholar] [CrossRef]
- Neri, L.; Oberdier, M.T.; van Abeelen, K.C.J.; Menghini, L.; Tumarkin, E.; Tripathi, H.; Jaipalli, S.; Orro, A.; Paolocci, N.; Gallelli, I.; et al. Electrocardiogram Monitoring Wearable Devices and Artificial-Intelligence-Enabled Diagnostic Capabilities: A Review. Sensors 2023, 23, 4805. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.W.; Torres Soto, J.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial Intelligence in Cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef] [PubMed]
- Bellfield, R.A.A.; Ortega-Martorell, S.; Lip, G.Y.H.; Oxborough, D.; Olier, I. The Athlete’s Heart and Machine Learning: A Review of Current Implementations and Gaps for Future Research. J. Cardiovasc. Dev. Dis. 2022, 9, 382. [Google Scholar] [CrossRef]
- Barbieri, D.; Chawla, N.; Zaccagni, L.; Grgurinović, T.; Šarac, J.; Čoklo, M.; Missoni, S. Predicting Cardiovascular Risk in Athletes: Resampling Improves Classification Performance. Int. J. Environ. Res. Public Health 2020, 17, 7923. [Google Scholar] [CrossRef] [PubMed]
- Caamal-Herrera, A.; Atoche-Enseñat, K.; Estrada-López, R.; Vázquez-Castillo, J.J.; Castillo-Atoche, J.; Palma-Marrufo, A.C.; Espinoza-Ruiz, O.; Efficient, A.E.; La Foresta, F.; Castillo-Atoche, A.; et al. Energy Efficient Framework for a AIoT Cardiac Arrhythmia Detection System Wearable during Sport. Appl. Sci. 2022, 12, 2716. [Google Scholar] [CrossRef]
- Noseworthy, P.A.; Attia, Z.I.; Behnken, E.M.; Giblon, R.E.; Bews, K.A.; Liu, S.; Gosse, T.A.; Linn, Z.D.; Deng, Y.; Yin, J.; et al. Artificial intelligence-guided screening for atrial fibrillation using electrocardiogram during sinus rhythm: A prospective non-randomised interventional trial. Lancet 2022, 400, 1206–1212. [Google Scholar] [CrossRef]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef]
- Harmon, D.M.; Sehrawat, O.; Maanja, M.; Wight, J.; Noseworthy, P.A. Artificial Intelligence for the Detection and Treatment of Atrial Fibrillation. Arrhythmia Electrophysiol. Rev. 2023, 12, e12. [Google Scholar] [CrossRef]
- Bos, J.M.; Attia, Z.I.; Albert, D.E.; Noseworthy, P.A.; Friedman, P.A.; Ackerman, M.J. Use of Artificial Intelligence and Deep Neural Networks in Evaluation of Patients With Electrocardiographically Concealed Long QT Syndrome From the Surface 12-Lead Electrocardiogram. JAMA Cardiol. 2021, 6, 532–538. [Google Scholar] [CrossRef]
- Raissi Dehkordi, N.; Raissi Dehkordi, N.; Karimi Toudeshki, K.; Farjoo, M.H. Artificial Intelligence in Diagnosis of Long QT Syndrome: A Review of Current State, Challenges, and Future Perspectives. Mayo Clin. Proc. Digit. Health 2024, 2, 21–31. [Google Scholar] [CrossRef]
- Vozzi, F.; Dimitri, G.; Piacenti, M.; Zucchelli, G.; Solarino, G.; Nesti, M.; Pieragnoli, P.; Gallicchio, C.; Persiani, E.; Morales, M.; et al. Artificial intelligence algorithms for the recognition of Brugada type 1 pattern on standard 12-leads ECG. EP Eur. 2022, 24, euac053-55. [Google Scholar] [CrossRef]
- Nakamura, T.; Aiba, T.; Shimizu, W.; Furukawa, T.; Sasano, T. Prediction of the Presence of Ventricular Fibrillation From a Brugada Electrocardiogram Using Artificial Intelligence. Circ. J. 2023, 87, 1007–1014. [Google Scholar] [CrossRef]
- Melo, L.; Ciconte, G.; Christy, A.; Vicedomini, G.; Anastasia, L.; Pappone, C.; Grant, E. Deep learning unmasks the ECG signature of Brugada syndrome. PNAS Nexus 2023, 2, pgad327. [Google Scholar] [CrossRef]
- Zanchi, B.; Faraci, F.D.; Gharaviri, A.; Bergonti, M.; Monga, T.; Auricchio, A.; Conte, G. Identification of Brugada syndrome based on P-wave features: An artificial intelligence-based approach. EP Eur. 2023, 25, euad334. [Google Scholar] [CrossRef]
- Kwon, J.M.; Jung, M.S.; Kim, K.H.; Jo, Y.Y.; Shin, J.H.; Cho, Y.H.; Lee, Y.J.; Ban, J.H.; Jeon, K.H.; Lee, S.Y.; et al. Artificial intelligence for detecting electrolyte imbalance using electrocardiography. Ann. Noninvasive Electrocardiol. 2021, 26, e12839. [Google Scholar] [CrossRef]
- Aro, A.L.; Jaakkola, I. Artificial intelligence in ECG screening: Ready for prime time? Int. J. Cardiol. 2021, 344, 111–112. [Google Scholar] [CrossRef]
- Siontis, K.C.; Suárez, A.B.; Sehrawat, O.; Ackerman, M.J.; Attia, Z.I.; Friedman, P.A.; Noseworthy, P.A.; Maanja, M. Saliency maps provide insights into artificial intelligence-based electrocardiography models for detecting hypertrophic cardiomyopathy. J. Electrocardiol. 2023, 81, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Adetiba, E.; Iweanya, V.C.; Popoola, S.I.; Adetiba, J.N.; Menon, C. Automated detection of heart defects in athletes based on electrocardiography and artificial neural network. Cogent Eng. 2017, 4, 1411220. [Google Scholar] [CrossRef]
- Lyon, A.; Ariga, R.; Mincholé, A.; Mahmod, M.; Ormondroyd, E.; Laguna, P.; de Freitas, N.; Neubauer, S.; Watkins, H.; Rodriguez, B. Distinct ECG phenotypes identified in hypertrophic cardiomyopathy using machine learning associate with arrhythmic risk markers. Front. Physiol. 2018, 9, 328675. [Google Scholar]
- Ko, W.Y.; Siontis, K.C.; Attia, Z.I.; Carter, R.E.; Kapa, S.; Ommen, S.R.; Demuth, S.J.; Ackerman, M.J.; Gersh, B.J.; Arruda-Olson, A.M.; et al. Detection of Hypertrophic Cardiomyopathy Using a Convolutional Neural Network-Enabled Electrocardiogram. J. Am. Coll. Cardiol. 2020, 75, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Orini, M.; Van Duijvenboden, S.; Young, W.J.; Ramírez, J.; Jones, A.R.; Tinker, A.; Munroe, P.B.; Lambiase, P.D. Premature atrial and ventricular contractions detected on wearable-format electrocardiograms and prediction of cardiovascular events. Eur. Heart J. Digit. Health 2023, 4, 112–118. [Google Scholar] [CrossRef]
- Helping Prevent Sudden Cardiac Arrest in Young Athletes with AI|AWS Public Sector Blog. Available online: https://aws.amazon.com/blogs/publicsector/helping-prevent-sudden-cardiac-arrest-young-athletes-ai/ (accessed on 29 January 2024).
- Ahmad, S.Z.R.S.; Yusoff, Y.; Zain, A.M.; Samsudin, R.; Ghazali, N.E. AI for Heart Rate Measurements for Sport Performance: A review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 551, 012041. [Google Scholar] [CrossRef]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Kashou, A.H.; Adedinsewo, D.A.; Siontis, K.C.; Noseworthy, P.A. Artificial Intelligence-Enabled ECG: Physiologic and Pathophysiologic Insights and Implications. Compr. Physiol. 2022, 12, 3417–3424. [Google Scholar] [CrossRef]
- Sehrawat, O.; Kashou, A.H.; Noseworthy, P.A. Artificial intelligence and atrial fibrillation. J. Cardiovasc. Electrophysiol. 2022, 33, 1932–1943. [Google Scholar] [CrossRef]
- Attia, Z.I.; Harmon, D.M.; Behr, E.R.; Friedman, P.A. Application of artificial intelligence to the electrocardiogram. Eur. Heart J. 2021, 42, 4717–4730. [Google Scholar] [CrossRef]
- Ragazzoni, G.L.; Cavigli, L.; Cavarretta, E.; Maffei, S.; Mandoli, G.E.; Pastore, M.C.; Valente, S.; Focardi, M.; Cameli, M.; Salvo, G.D.; et al. How to evaluate resting ECG and imaging in children practising sport: A critical review and proposal of an algorithm for ECG interpretation. Eur. J. Prev. Cardiol. 2023, 30, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.P. Artificial intelligence-augmented ECG assessment: The promise and the challenge. J. Cardiovasc. Electrophysiol. 2019, 30, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Biffi, A.; Pelliccia, A.; Verdile, L.; Fernando, F.; Spataro, A.; Caselli, S.; Santini, M.; Maron, B.J. Long-term clinical significance of frequent and complex ventricular tachyarrhythmias in trained athletes. J. Am. Coll. Cardiol. 2002, 40, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Culasso, F.; Di Paolo, F.M.; Accettura, D.; Cantore, R.; Castagna, W.; Ciacciarelli, A.; Costini, G.; Cuffari, B.; Drago, E.; et al. Prevalence of abnormal electrocardiograms in a large, unselected population undergoing pre-participation cardiovascular screening. Eur. Heart J. 2007, 28, 2006–2010. [Google Scholar] [CrossRef] [PubMed]
- Biffi, A.; Palermi, S.; D’Ascenzi, F.; Bonifazi, M.; Zorzi, A.; Corrado, D. Premature ventricular beats in athletes: To detrain or not to detrain? Br. J. Sports Med. 2024, 58, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Rossoni, A.; Vecchiato, M.; Brugin, E.; Tranchita, E.; Adami, P.E.; Bartesaghi, M.; Cavarretta, E.; Palermi, S. The eSports Medicine: Pre-Participation Screening and Injuries Management—An Update. Sports 2023, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Yamagata, L.M.; Abela, M. A review article of the cardiovascular sequalae in esport athletes: A cause for concern? Hell. J. Cardiol. 2022, 68, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Koshy, A.; Koshy, G.M. The potential of physiological monitoring technologies in esports. Int. J. Esports 2020, 1, 1–11. Available online: https://www.ijesports.org/article/22/html (accessed on 30 January 2024).
- Palermi, S.; Vecchiato, M.; Saglietto, A.; Niederseer, D.; Oxborough, D.; Ortega-Martorell, S.; Olier, I.; Castelletti, S.; Baggish, A.; Maffessanti, F.; et al. Unlocking the potential of artificial intelligence in sports cardiology: Does it have a role in evaluating athlete’s heart? Eur. J. Prev. Cardiol. 2024, 31, 470–482. [Google Scholar] [CrossRef]
| No | Authors | Study Design | Main Findings | AI Technology Used | Relevance to Sports Medicine | Limitations |
|---|---|---|---|---|---|---|
| 1 | Danilov A, Aronow WS (2023) [12] | Review | AI in cardiology is at an early stage but has potential benefits in diagnostics and treatments. Key applications include imaging, electrocardiography, wearable devices, risk prediction, and disease classification. Major hurdles remain in model understanding, bias, evaluation, legal and ethical dilemmas. | Machine Learning, Deep Learning, Neural Networks | The insights into AI-driven diagnostics and risk predictions could inform personalized training and rehabilitation programs for athletes, helping prevent cardiovascular issues. | The study notes the lack of extensive clinical trials and the challenges of model interpretability, which could impact the broader adoption and effectiveness of AI technologies in sports medicine. |
| 2 | Somani S, et al. (2021) [13] | Review | Reviews the integration of deep learning with electrocardiography, highlighting improved diagnosis and management of cardiac conditions such as arrhythmias, cardiomyopathy, and ischemia. Also notes the importance of large, diverse datasets and the potential of novel clinical applications. | Deep Learning | Can assist in enhancing monitoring and diagnostics in sports settings, particularly for conditions like arrhythmias which may be triggered by intense physical activity. | Challenges include the dependency on large, diverse datasets which may not always be available or of sufficient quality in sports settings, and the need for external validation in sports-specific populations. |
| 3 | Konstantinos C. Siontis KC, et al. (2021) [14] | Review | Discusses the transformative effect of AI on ECG interpretation, highlighting its potential to improve diagnostics in cardiovascular medicine. AI techniques can detect patterns in ECGs that are unrecognizable to humans, potentially identifying early signs of diseases like left ventricular dysfunction and hypertrophic cardiomyopathy. | Convolutional Neural Networks | The advanced detection capabilities of AI-enhanced ECGs can be crucial in sports medicine for early identification of athletes at risk of cardiovascular diseases. | As AI-ECG tools continue to evolve, their real-world implementation faces challenges like ensuring data quality, model validation across diverse populations, and integration into clinical workflows. |
| 4 | Neri L et al. (2023) [15] | Review | Examines advancements in AI for ECG monitoring via wearable devices, focusing on arrhythmias and coronary artery disease. Highlights the use of deep learning methods such as CNNs and RNNs for improved disease detection and prediction capabilities. | Convolutional Neural Networks (CNNs), Recurrent Neural Networks (RNNs) | Wearable ECG devices with AI can significantly enhance the monitoring and detection capabilities, important for athletes in managing arrhythmias and coronary health. | Limitations of wearable ECGs compared to standard multi-lead ECGs include less data richness and potential inaccuracies in data collection, especially during intense physical activity. |
| 5 | Johnson KW, et al. (2018) [16] | Review | Explores AI and ML in cardiology, highlighting how these technologies aid in the management of cardiovascular diseases through improved prediction and personalized treatment plans. Discusses the use of AI in feature selection for models, enhancing predictive accuracy beyond traditional statistical methods, and aiding in the interpretation of complex data from various sources. | Machine Learning, Deep Learning, Neural Networks, Convolutional Neural Networks, Recurrent Neural Networks, Support Vector Machines | Enhances data interpretation and management, crucial for monitoring athletes’ heart health and adapting training programs to individual cardiovascular profiles. | AI reliance on extensive, high-quality datasets, which may not always be available in sports settings, and the general complexity of AI models which require specialized expertise to manage and interpret. |
| 6 | Bellfield RAA, et al. (2022) [17] | Review | Reviews the use of ML techniques in researching the athlete’s heart, highlighting the integration of different ML approaches to better understand and manage physiological changes and disease risks due to intense physical training. Discusses current applications, identifies gaps, and suggests future directions for research including the need for larger, diverse datasets and the potential development of models for better disease prediction and management. | Machine Learning, Artificial Neural Networks | Directly addresses the integration of ML in sports cardiology to better understand the physiological impacts of intense training and to improve early diagnostic capabilities. | Highlights the challenges of limited dataset sizes and the need for more comprehensive studies to enhance the validity and applicability of ML models in sports cardiology. |
| 7 | Barbieri D, et al. (2020) [18] | Research | Evaluates the impact of resampling techniques on machine learning classification performance in predicting cardiovascular risk in athletes. Demonstrates that techniques like SMOTE can significantly improve sensitivity and predictive accuracy in imbalanced datasets, making it valuable for medical diagnostic systems, particularly in identifying at-risk athletes. | Decision Trees, Logistic Regression, SMOTE | Essential for assessing cardiovascular risk in athletes, enabling early intervention and tailored health management strategies. | While resampling improves classification accuracy, challenges persist in balancing sensitivity and specificity, especially in medical contexts where misclassification costs are high. |
| 8 | Caamal-Herrera A, et al. (2022) [19] | Observational Study | Demonstrated an energy-efficient framework for AIoT wearable cardiac arrhythmia detection system. Achieved a high arrhythmia detection precision of 98.6% using convolutional neural networks. | Convolutional Neural Networks | Focuses on real-time ECG monitoring and arrhythmia detection during sports activities, essential for monitoring athletes’ cardiac health. | Study limited to a small cohort of athletes, and did not compare with other diagnostic tools beyond ECG. Potential biases due to small sample size and limited environment test. |
| 10 | Noseworthy PA, et al. (2022) [20] | Prospective non-randomized interventional trial | Atrial fibrillation was detected in 1.6% of patients with low risk and 7.6% of patients with high risk. AI-guided screening increased atrial fibrillation detection compared to usual care (10.6% vs. 3.6% in high-risk group; 2.4% vs. 0.9% in low-risk group). | Artificial intelligence algorithm applied to ECG data | Although not directly related to sports medicine, the AI-guided screening approach could potentially be adapted for athletes to screen for cardiac arrhythmias or other heart conditions during routine check-ups or pre-participation evaluations. | Limitations include the potential for false positives or false negatives in AI algorithm predictions, as well as the need for further validation in diverse populations and settings. |
| 11 | Martínez-Sellés M, Manuel Marina-Breysse M (2023) [7] | Review | Reviewed the current and future applications of artificial intelligence in electrocardiography, including interpretation and detection of ECG abnormalities, risk prediction, monitoring ECG signals, signal processing, therapy guidance, and integration with other modalities. | Various AI algorithms | Provides insights into the potential use of AI in improving ECG diagnosis and management, which could have implications for sports medicine in detecting cardiovascular abnormalities in athletes. | Does not present original research findings; mainly a review of the existing literature and future perspectives. |
| 12 | Attia ZI, et al. (2019) [21] | Retrospective analysis | Developed an AI-enabled electrocardiograph using a convolutional neural network to detect the electrocardiographic signature of atrial fibrillation present during normal sinus rhythm using standard 10-s, 12-lead ECGs. | Convolutional neural network | Could potentially aid in the identification of individuals with atrial fibrillation during normal sinus rhythm, thus providing a rapid, inexpensive means of screening. | Limitations may include the retrospective nature of the analysis, potential biases in the dataset, and the need for further validation in prospective studies and clinical settings. |
| 13 | Harmon DM, et al. (2023) [22] | Review | Reviewed specific applications of AI for screening, diagnosis, and treatment of atrial fibrillation (AF), including prediction models, enhanced electrocardiographs (AI-ECG), photoplethysmography, risk stratification, and intracardiac signal analysis. Highlighted successes, limitations, and future directions. | Various AI algorithms, including machine learning and deep learning | Provides insights into how AI can enhance AF detection and management, potentially improving screening and treatment strategies for athletes with AF. | Limitations include lack of racial diversity in training/testing cohorts, concerns about data integrity, potential misdiagnosis, operator dependence, and the need for further validation and calibration. |
| 14 | Bos JM, et al. (2021) [23] | Diagnostic case-control study | The AI-ECG model successfully distinguished patients with long QT syndrome from those without and provided a simple and inexpensive method for early detection of congenital long QT syndrome. | Deep neural networks, convolutional neural network | Although not directly mentioned, the ability to diagnose and manage cardiac conditions like LQTS is crucial in sports medicine for athlete safety. | Limited to a single-centre study without external validation; focused on a highly specific patient group (genetic heart rhythm clinic patients), which may limit generalizability. |
| 15 | Raissi Dehkordi N, et al. (2023) [24] | Review Article | AI enhances the accuracy and efficiency of ECG interpretation for diagnosing Long QT Syndrome (LQTS). AI algorithms reduce interobserver variability and can identify risk individuals by analyzing subtle ECG features not apparent to the human eye. AI-driven devices like smartwatches may facilitate early detection of LQTS-related complications. | Deep learning, neural networks | Provides tools for early detection and continuous monitoring of LQTS in athletes, potentially improving safety and preventive care in sports medicine. | Challenges include potential bias in AI training data, patient privacy concerns, and the need for enhanced interpretability of AI decisions to gain trust among clinicians. |
| 16 | Vozzi F, et al. (2022) [25] | Diagnostic Study | Developed a novel system using Echo State Networks (ESNs) for diagnosing type 1 Brugada Syndrome (BrS) with good accuracy. The system uses ECG pattern recognition, showing good performance particularly when using lead V2 only. | Recurrent Neural Networks (Echo State Networks) | Can potentially improve early detection of BrS in athletes, aiding in safer participation in sports by identifying individuals at risk of arrhythmias. | Larger datasets needed to improve model performance and validate its effectiveness in clinical practice. |
| 17 | Nakamura T, et al. (2023) [26] | Diagnostic Study | An AI model using a convolutional neural network predicted the presence of ventricular fibrillation (VF) in patients with Brugada syndrome from ECGs with high precision and reliability. | Convolutional Neural Network | Useful for predicting VF in athletes with Brugada syndrome, potentially preventing sudden cardiac death during sports activities. | Requires further validation in broader clinical settings to enhance reliability and determine general applicability. |
| 18 | Melo L, et al. (2023) [27] | Diagnostic Study | Developed a deep learning model to diagnose Brugada Syndrome (BrS) from ECGs without the need for sodium channel blockers. The model achieved high accuracy and AUC, indicating potential for clinical application to detect BrS more safely and effectively. | Deep Neural Network | Enhances the detection of BrS in athletes, potentially allowing for earlier and safer identification of those at risk of sudden cardiac death. | Further validation required to confirm effectiveness and generalizability in diverse clinical settings. |
| 19 | Zanchi B, et al. (2023) [28] | Diagnostic Study | AI model can identify Brugada syndrome based only on P-wave characteristics from ECGs, showing an alternative diagnostic pathway beyond the typical ventricular phenotype analysis. This underscores an atrial aspect to BrS pathology with practical applications in diagnosis using AI. | Machine Learning (various classifiers, including AdaBoost) | Could aid in identifying at-risk athletes with atrial abnormalities potentially linked to BrS, enhancing preventive measures in sports contexts. | Larger and more diverse datasets are required to enhance the robustness and applicability of the model in clinical settings. |
| 20 | Kwon J myoung, et al. (2021) [29] | Diagnostic Study | Developed a deep learning model (DLM) to detect various electrolyte imbalances (e.g., hyperkalemia, hypokalemia, hypernatremia, hyponatremia, hypercalcemia, hypocalcemia) using ECG data. Achieved high AUCs in both internal and external validations, demonstrating effectiveness in non-invasively detecting these conditions. | Deep Learning Model (DLM) | Could significantly aid in monitoring electrolyte status in athletes, particularly for those undergoing intense training or endurance sports, where imbalance risks are elevated. | Further validation with prospective studies and in different clinical environments is needed to generalize the findings. |
| 21 | Aro AL, Jaakkola I (2021) [30] | Editorial | AI applications in cardiology, especially in ECG interpretation, show high efficacy in diagnosing conditions like left ventricular ejection fraction (LVEF) ≤ 35% and hypertrophic cardiomyopathy (HCM). Attia et al. reported AI-ECG algorithms from the Mayo Clinic demonstrating high discrimination abilities with impressive AUC, sensitivity, and specificity. | Deep Learning Algorithms | AI-ECG could significantly enhance screening for heart conditions in athletes, potentially identifying risks early. | Editorial does not discuss specific limitations, but general challenges include data diversity and algorithm transparency. |
| 22 | Siontis KC, et al. (2023) [31] | Research Article | A convolutional neural network (CNN) model using 12-lead ECG and one-lead median-beat saliency maps demonstrated effective identification of hypertrophic cardiomyopathy (HCM). The study highlighted specific ECG segments, such as the ST-T segment, atrial depolarization, and QRS complex, which were crucial for the CNN model’s performance in detecting HCM. The use of saliency maps helped reveal which segments of the ECG were most influential in diagnosing HCM. | Convolutional Neural Network (CNN) | AI-based ECG analysis could be crucial for early detection of HCM in athletes, allowing for timely intervention and management to prevent severe cardiac outcomes. | The study was limited by the subjective interpretation of saliency maps and only included patients with a high AI-ECG likelihood of HCM, potentially biasing the results towards already known cases of HCM. |
| 23 | Adetiba E, et al. (2017) [32] | Research Article | Developed an automated heart defect detection model using ECG and Artificial Neural Network (ANN) for athletes. The model achieved classification accuracy, sensitivity, and specificity of 90.00%, 91.96%, and 97.06%, respectively, and identified four classes of heart conditions. The model can help reduce sudden cardiac death among athletes by enabling early detection of heart defects. | Artificial Neural Network (ANN) | Implementing this model in sports could help monitor athletes’ heart health in real-time, potentially preventing sudden cardiac death. | Limited discussion on the real-world applicability and integration into daily athletic monitoring. Lack of validation in diverse athletic populations. |
| 24 | Lyon A, et al. (2018) [33] | Research Article | Used mathematical modeling and machine learning to analyze high-fidelity 12-lead Holter ECGs from HCM patients, identifying distinct phenotypic subgroups. The study linked these subgroups with variations in clinical risk factors and anatomical features, providing insights that might help refine risk stratification for sudden cardiac death in HCM patients. | Machine Learning, Mathematical Modeling | Advanced ECG phenotyping may refine risk stratification and aid in early diagnosis of conditions leading to sudden cardiac death in athletes, potentially improving management and preventive strategies. | The study’s scope and impact on practical clinical outcomes were limited by the size and specific characteristics of the patient population studied. |
| 25 | Ko WY, et al. (20220) [34] | Research Article | This study developed an AI approach using a convolutional neural network (CNN) to detect hypertrophic cardiomyopathy (HCM) based on 12-lead ECGs. The CNN was trained on a large dataset and achieved high diagnostic performance, particularly in younger patients, suggesting potential for broad HCM screening applications. | Convolutional Neural Network (CNN) | The AI-ECG model’s high accuracy in detecting HCM could revolutionize cardiac health monitoring in sports, offering a non-invasive and efficient screening tool for early detection. | Requires further refinement and external validation to ensure its effectiveness across diverse populations and clinical settings. |
| 26 | Orini M, et al. (2023) [35] | Research Article | Investigated the association between premature ventricular and atrial contractions (PVCs and PACs) detected on wearable-format ECGs and cardiovascular outcomes in individuals without cardiovascular disease (CVD). Found strong associations between PVCs and heart failure (HF) and between PACs and atrial fibrillation (AF), suggesting that wearable ECGs could be useful for early cardiovascular risk stratification. | None Specified | Wearable ECG monitoring of premature contractions could enhance early detection and management of cardiac risks in athletes, potentially improving preventative care and outcomes. | Study findings are based on associations and require further investigation to confirm causality and practical applications in routine clinical practice. |
| 27 | Inder M, Diwakar IB (2023) [36] | Initiative Description Blog | Developed an AI model to interpret pediatric ECGs for SCA risk, achieving high sensitivity and specificity. | Machine Learning, Deep Learning | Direct application in preventing sudden cardiac arrests in athletes through early detection. | Requires further validation in clinical settings; data on long-term outcomes are lacking. |
| 28 | Derman W, et al. (2019) [37] | Review Article | Outlines the potential of AI in improving sports performance through enhanced heart rate monitoring and analysis. | Artificial Neural Networks, Support Vector Regression | Can inform training regimes and monitor health in real-time, enhancing athlete performance and safety. | More empirical data needed to substantiate the theoretical claims; commercial accessibility and integration issues. |
| 30 | Perez MV, et al. (2019) [38] | Prospective Study | Smartwatch application effectively identified atrial fibrillation with high predictive value; a low notification rate may indicate specificity. | Smartwatch algorithm, Irregular pulse notification | Potential for early detection of atrial conditions in athletes, improving prevention strategies. | Limited follow-up response rate for ECG patch returns, generalizability concerns due to high attrition. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smaranda, A.M.; Drăgoiu, T.S.; Caramoci, A.; Afetelor, A.A.; Ionescu, A.M.; Bădărău, I.A. Artificial Intelligence in Sports Medicine: Reshaping Electrocardiogram Analysis for Athlete Safety—A Narrative Review. Sports 2024, 12, 144. https://doi.org/10.3390/sports12060144
Smaranda AM, Drăgoiu TS, Caramoci A, Afetelor AA, Ionescu AM, Bădărău IA. Artificial Intelligence in Sports Medicine: Reshaping Electrocardiogram Analysis for Athlete Safety—A Narrative Review. Sports. 2024; 12(6):144. https://doi.org/10.3390/sports12060144
Chicago/Turabian StyleSmaranda, Alina Maria, Teodora Simina Drăgoiu, Adela Caramoci, Adelina Ana Afetelor, Anca Mirela Ionescu, and Ioana Anca Bădărău. 2024. "Artificial Intelligence in Sports Medicine: Reshaping Electrocardiogram Analysis for Athlete Safety—A Narrative Review" Sports 12, no. 6: 144. https://doi.org/10.3390/sports12060144
APA StyleSmaranda, A. M., Drăgoiu, T. S., Caramoci, A., Afetelor, A. A., Ionescu, A. M., & Bădărău, I. A. (2024). Artificial Intelligence in Sports Medicine: Reshaping Electrocardiogram Analysis for Athlete Safety—A Narrative Review. Sports, 12(6), 144. https://doi.org/10.3390/sports12060144







