Abstract
Populus alba clone ‘Villafranca’ (white poplar), highly suitable for biomass production and ecosystem restoration, is a model system for molecular and physiological studies, but no reports are available concerning seed quality. Although clonal propagation is the preferred approach for commercial purposes, attention should be given to face genetic variability losses in the existing germplasm. To address this challenge, new populations should be developed starting from seeds, overcoming the issues of low germinability and viability during storage. This study proposes to develop tailored treatments to improve the germination of long-term stored white poplar seeds. Priming and soaking protocols, based on the use of water or spermidine (Spd, 50 and 100 μM), were tested. Treatment efficacy was assessed based on germination parameters, reactive oxygen species (ROS) profiles, and the expression patterns of genes with key roles in early seed germination. Soaking with 100 μM Spd for 4 h significantly enhanced germination percentage and speed. Low ROS levels were evidenced in the Spd-treated seeds, compared to water-soaked seeds. High expression of genes involved in desiccation tolerance acquisition, polyamine biosynthesis, and antioxidant defense was observed only in dry seeds. The results are discussed in view of the potential protective role of Spd.
1. Introduction
The genus Populus spp. consists of approximately 30 species including poplars, aspens and cottonwoods [1]. Among these, poplars have a significant impact on both the economy and the environment. Among wooden species, poplars are characterized by a fast growth which makes them promising candidates for sustainable biomass production. Additionally, their adaptability allows them to grow on poor soils, thus mitigating soil erosion due to the elaborate root networks [2,3]. Other characteristics are related to the elimination of airborne particulate matter and soil metal sequestration through phytoremediation [4]. In Europe, poplar plantations cover almost one million hectares, with France, Turkey, Italy, Spain, and Hungary being the largest producers. In Italy, hybrid poplar plantations are the primary source of industrial timber production, contributing more than 50% to the domestic supply of industrial hardwood [5].
Populus alba, commonly known as white poplar, is indigenous to central and southern Europe [6] and primarily utilized for soil erosion control, restoring rivers and floodplains and biomass production. The need to acclimate the species to harsh climatic conditions and meet the demands of the industry has resulted in the development of numerous clones. Among these, Populus alba clone ‘Villafranca’ was developed by the Forest and Wood Research Center of Casale Monferrato (Italy) in 1957. This clone is characterized by good quality wood with a basal density of 0.33 g/cm3, optimal vegetative propagation and the ability to quickly root in soil [6,7].
Clonal propagation is important for commercial wood production, leading to increased productivity and homogeneity in plantations [8]. Despite this benefit, clonal propagation has significantly reduced genetic diversity, which is detrimental for ensuring crop yields in the face of climate change and diseases. In this context, the breeding programs could benefit from a compromise strategy balancing the greater genetic gain from vegetative propagation (capturing non-additive genetic variance) and the maintenance of genetic diversity in the seed orchard. Seeds are of utmost importance as they serve as a primary source for generating plants for large-scale restoration efforts. Understanding the optimal conditions for seed germination and seedling establishment is crucial for ecological restoration and biodiversity preservation [9]. Given the preference for vegetative propagation in poplars, studies regarding seed germination are scanty. Furthermore, poplar seeds have a relatively short lifespan [10]. However, several studies have reported that fresh seeds have a germination rate of about 80% to 90% [11,12,13]. While extensive studies have been conducted on the physiological, molecular, and morphological aspects of germination and post-germination processes in model organisms and crops, a major gap of knowledge is underlined for woody species [14].
Long-term seed storage is the most effective approach for safeguarding the genetic diversity of plant materials. Storage parameters that influence seed quality include temperature, moisture content, time, and oxygen pressure; however seed desiccation tolerance (DT) is a crucial endogenous factor [15]. Based on DT, seeds are categorized into three storage categories: (1) orthodox seeds, able to endure low moisture levels, can be stored for extended periods of time; (2) recalcitrant seeds, highly susceptible to drying out and freezing, leading to rapid loss of viability; (3) intermediate seeds, falling between orthodox and recalcitrant in terms of viability [16]. In the past, poplar seeds were classified either as recalcitrant, since the seeds have a short lifespan [17], or as sub-orthodox given their capacity to maintain viability for extended periods at low temperatures [18]. However, according to a more recent classification, many poplar species are categorized as intermediate seeds [19]. Desiccation-sensitive seeds have a greater possibility of losing seed viability after water loss. This was evidenced in desiccation-sensitive Quercus acutissima seeds. The germination rate of Quercus acutissima seeds lowered as the water content decreased through the drying process [20]. A similar result has been observed in the seeds of Quercus coccifera and Quercus pubescens [21], which show recalcitrant storage behavior.
Seed priming, a pre-sowing method that involves imbibing seeds in water or other priming agents and subsequent drying to the original moisture content, represents a promising option to address seed quality issues in poplars. The advantages of seed priming, ranging from improved germination to enhanced resistance to biotic/abiotic stresses, are well documented in multiple species [22,23,24,25,26,27]. Generally, priming protocols are considered empiric and known to be species/genotype and even seed lot-dependent [28,29]. The seed response in terms of DT should also be considered when designing priming protocols [30,31,32]. Polyamines (PAs) are used as priming [33,34,35]. PAs are small nitrogenous bases with several amino groups, present in nearly all eukaryotic and prokaryotic species. Putrescine (Put), spermidine (Spd), and spermine (Spm) are the primary PAs found in plants. They play crucial roles in several biological processes such as flower and fruit development, embryogenesis, organogenesis, senescence, as well as abiotic and biotic stress responses [36,37,38,39,40,41,42]. Recently, numerous studies have demonstrated the positive effects of Spd on seed germination under stress in white clover [36], corn [37], rice [38], sorghum [41], and maple [42]. PAs, particularly Spd, contribute to the antioxidant response, essential for seed [40,43,44,45,46]. ROS (reactive oxygen species) accumulation causes seed deterioration during storage [47]; however, ROS also act as signaling molecules, promoting germination. The term ‘oxidative window’ is the critical limit of ROS accumulation that enables them to function as signaling molecules, without inducing oxidative stress [48].
Poplar seeds have a very short life span, and they have been previously classified both as recalcitrant or intermediate, due to their low dehydration tolerance, high water content at maturity, and high metabolic activity at dehiscence which makes their survival time in storage very short [13,14,16]. This reduced seed viability significantly impairs breeding and genetic conservation programs. The aim of the current study was to design tailored seed priming protocols to improve germination performance of long-term stored Populus alba clone ‘Villafranca’ seeds. Given that current literature promotes the use of spermidine as priming agent linked to the antioxidant response in different species [36,38,40,42], we wanted to test its efficiency also for poplar seeds. To do this, we focused on a representative case study using a poplar seed lot stored for 15 years. The questions that can be raised to address the aim are the following: (1) How viable are the representative of the seed lot under study; (2) Which is the dynamics of water uptake and loss of aged poplar seeds; (3) Why spermidine would improve the germinability of long-term stored poplar seeds? To reply to these questions, the seed viability and dynamics of rehydration–dehydration cycles were monitored using spermidine as a priming agent alongside water imbibition and untreated controls. To assess the efficiency of the treatments, phenotypical parameters (germination percentage and speed) and molecular analyses (ROS detection, gene expression profiles) were employed. This case study provides insights into developing tailored priming protocols for the post storage management of tree seeds.
2. Materials and Methods
2.1. Seed Material and Storage Conditions
Seeds of Populus alba clone ‘Villafranca’ were obtained from open pollination at the Forestry and Wood Department of CREA (Council for Agricultural Research and Analysis of Agricultural Economics), Casale Monferrato, Italy. The seeds were collected in April 2008 at the Institute’s facility. The seeds were dried in thermostatic chambers at a controlled temperature, and then vacuum-packed at −30 °C.
2.2. Experimental Flow to Design Tailored Priming Protocols
The experimental workflow designed in order to optimize the priming treatments protocol is depicted in Figure 1. The seed response along the rehydration–dehydration cycle was monitored by measuring the seed weight, thus producing imbibition and dehydration curves. Water uptake and loss (in presence/absence of Spd) were evaluated along multiple imbibition (1, 2, 3, 4, and 5 h) and dehydration (1, 2 and 3 h) timepoints by measuring the weight of 100 seeds at each timepoint. All measurements were performed in triplicates with two independent experiments (Figure 1A).
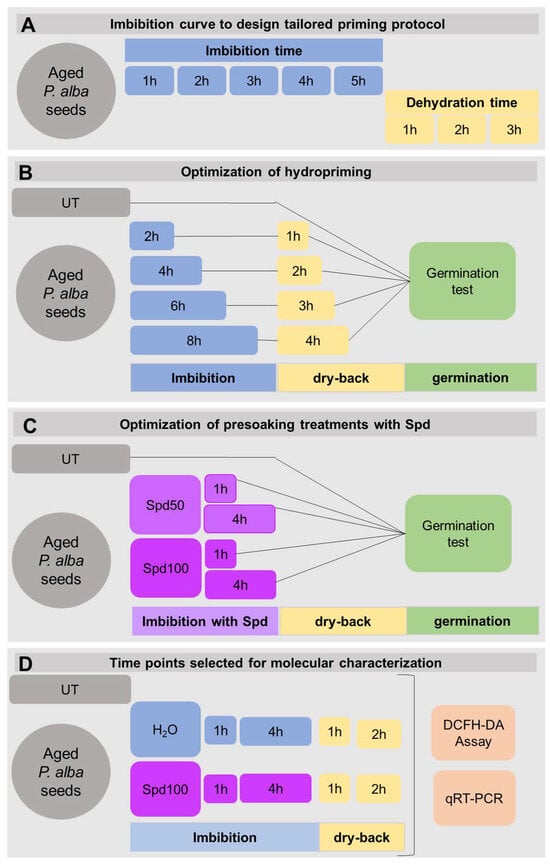
Figure 1.
Overview of the experimental flow. (A) Timepoints were selected to assess water loss and gain dynamics in long-term stored Populus alba clone ‘Villafranca’ seeds subjected to different intervals of rehydration (1, 2, 3, 4, 5 h) and dehydration (1, 2, 3 h). (B) Optimization of hydropriming in long-term stored Populus alba clone ‘Villafranca’ seeds using different imbibition timepoints (2, 4, 6, 8 h) followed by dry-back (DB) (1, 2, 3, 4 h). (C) Optimization of soaking with spermidine (Spd) in long-term stored Populus alba clone ‘Villafranca’ seeds different imbibition timepoints (1, 4 h). Two concentrations of Spd were tested: 50 µM Spd (Spd50) and 100 µM (Spd100). (D) Timepoints selected for molecular profiling of long-term stored Populus alba clone ‘Villafranca’ seeds along the rehydration–dehydration cycle. Untreated seeds (UT) were used as control in all experiments.
Seed treatments were devised as follows: seed priming in water (HP, hydropriming), seed presoaking in the presence/absence of Spd. For the HP treatments, seeds were imbibed in water for 2, 4, 6, and 8 h. For each imbibition treatment, seeds were then dried-back (DB) at 25 °C for 1, 2, 3 and 4 h (Figure 1B). For the Spd treatments, two Spd concentrations (50 µM, Spd50; 100 µM, Spd100) were applied for 1 h and 4 h. After each treatment, germination tests were conducted (Figure 1C). Additionally, untreated seeds (UT), were used as control. Samples for molecular analyses were collected at the indicated timepoints (Figure 1D), transferred to liquid N2 and stored at −80 °C.
2.3. Germination Tests
White poplar seeds were transferred to Petri dishes (diameter 90 mm) containing two filter papers moistened with 2.5 mL H2O, sealed with parafilm and kept in a growth chamber at 22 °C under light conditions with photon flux density of 150 µmol m−2 s−1, photoperiod of 16 h/8 h and 70–80% relative humidity (RH) inside the containers. Seeds with protrusion of the primary radicle were considered germinated. Germination tests were performed in triplicates of 30 seeds each. Germination was monitored for four days, and germination parameters were calculated [31]. For the evaluation of germination performance, the following parameters were used: germinability (G%) and time to reach 50% of final germinants (T50). G% is defined as the percentage of germinated seeds at the end of the germination test. T50 was calculated using the following formula: T50 = Ti + (N/2 − Ni) (Tj − Ti)/(Nj − Ni), where N is the final number of germinated seeds, N/2 is half of final number of germinated seed, Ni and Nj are the total number of seeds germinated in adjacent counts at time Ti and Tj [49].
2.4. Viability Assay
To measure seed viability, poplar seeds were first imbibed in H2O for 6 h to remove seed coat. The de-coated seeds were then incubated in a 1% (w/v) solution of 2,3,5-triphenyl tetrazolium chloride (TTC; Merck, Darmstadt, Germany), and maintained at 20 °C for 18 h in the dark [50]. The TTC test relies on the activity of dehydrogenase enzymes in mitochondria. TTC penetrates the seed tissues where it interferes with the reduction processes of the living cells by accepting a hydrogen ion. In the reduced form, the TTC-salt is a red-colored, stable, non-diffusible substance called triphenylformazan or formazan [51]. Stained tissues are considered viable, and unstained white tissues are considered dead. The TTC test was carried out using five replicates of 10 seeds per treatment.
2.5. ROS Quantification
ROS levels were quantified in dry and imbibed seeds collected at the indicated timepoints, using the fluorogenic dye 2′,7′-dichlorofluorescein diacetate (DCFH-DA, Sigma-Aldrich, Milan, Italy). The dye is converted to a non-fluorescent molecule following deacetylation mediated by cellular esterases, and it is subsequently oxidized by ROS into the fluorescent compound 2′,7′-dichlorofluorescein. DFC can be detected by fluorescence spectroscopy with maximum excitation and emission spectra of 495 nm and 529 nm, respectively [52]. The assay was carried out as described by Griffo et al., with the following modifications by [53]. Samples of 40 seeds per timepoint were incubated for 60 min with 50 μL of 10 μM DCFH-DA and subsequently fluorescence was determined at 517 nm using a Rotor-Gene 6000 PCR apparatus (Corbett Robotics, Brisbane, Australia), setting the program for one cycle of 30 s at 25 °C. As negative control, a sample containing only DCFH-DA was used to subtract the baseline fluorescence. The fluorescence was calculated by normalizing samples to controls and are expressed as Relative Fluorescence Units (R.F.U.).
2.6. RNA Extraction and cDNA Synthesis
Seeds imbibed with water and Spd were collected at different imbibition time (0, 1, 4 h) and DB timepoints (1, 2 h) for molecular analyses. Seed aliquots (80–90 mg) were grinded in liquid N2 and collected into 1.5 mL Eppendorf tubes. RNA was extracted as described by Oñate-Sánchez and Vicente-Carbajosa, with the following modifications [54]. A volume of 550 μL extraction buffer (0.4 M LiCl, 0.2 M Tris pH 8, 25 mM EDTA, 1% SDS) and 550 μL chloroform, were added to each Eppendorf tube containing approx. 50 mg of seed powder and vortexed for 10 s. The samples were centrifuged at 10,000 rpm for 3 min at 4 °C. The upper phase was collected and transferred to new 1.5 mL Eppendorf tubes, 500 µL of water-saturated acidic phenol was added and vortexed for 10 s. A volume of 200 μL chloroform was added to the sample, gently mixed, and centrifuged at 10,000 rpm for 3 min at 4 °C. The upper phase was collected and transferred to new 1.5 mL Eppendorf tubes, and a 1/3 volume of lithium chloride was added. The samples were incubated at 4 °C for 60 min then centrifuged at 10,000 rpm for 30 min at 4 °C. Supernatant was discarded, the RNA pellet was washed with 100 μL ice-cold 70% ethanol and centrifuged at 10,000 rpm for 1 min at 4 °C, followed by one additional wash with 100 μL ice-cold 100% ethanol. The RNA pellet was air dried and resuspended in nuclease-free H2O. The concentration of each RNA sample was measured using a Biowave spectrophotometer (Biochrom Ltd., Cambridge, UK), and RNA integrity was assessed using agarose gel electrophoresis. For cDNAs synthesis, the RevertAid First Strand cDNA Synthesis Kit (ThermoFisher Scientific, Monza, Italy) was used, according to the manufacturer’s recommendations.
2.7. Quantitative Real-Time PCR (qRT-PCR)
The qRT-PCR reactions were carried out using a CFX Duet Real-time PCR system machine (Bio-Rad Laboratories Inc., Milan, Italy) and the Maxima SYBR Green qPCR Master Mix (Thermo Fisher Scientific, Monza, Italy), as indicated by the manufacturer. The machine is operated using the Bio-ad CFX maestro software (Bio-Rad Laboratories Inc.) and the following amplification protocol was applied: denaturation at 95 °C, 10 min, and 40 cycles of 95 °C, 15 s and 60 °C, 30 s, final extension at 72 °C, 30 s.
Oligonucleotide primers were designed using the Real-Time PCR Primer Design program Primer3Plus (https://primer3plus.com) and further validated using Oligo Analyzer (https://eu.idtdna.com/calc/analyzer). Target specificity was assessed through Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). Relative quantification was carried out using Tub (tubulin beta-4 chain, LOC118055616) as reference gene. This gene was selected as a reference because it showed stable expression among the samples used (Figure S1). The following genes were tested: ABI3 (B3 domain-containing transcription factor ABI3, LOC118049976), FUS3 (B3 domain-containing transcription factor FUS3-LIKE, LOC118038131), SPDS (spermidine synthase, LOC118055381), SPMS (spermine syntase, LOC118033446), APX (ascorbate peroxidase, cytosolic-like, LOC118045884), CycB1 (cyclin B1-2, LOC118029686), CDKA1 (cyclin-dependent kinase A1, LOC118050756), and CycD2 (cyclin-D2-like, LOC118031573). Oligonucleotide sequences are provided in Table 1. The Thomsen method was employed for relative quantification of transcript accumulation using a standardized efficiency (E) value of 1.8 [55]. All reactions were carried out in triplicates. Z-score (mean-centered and divided by the standard deviation of each variable) was calculated on the linearized Ct values and used to generate heatmaps with the GraphPad Prism version 8.0.1 program.

Table 1.
List of oligonucleotide primers used for qRT-PCR analyses. Tub, Tubulin beta-4 chain; ABI3, B3 Domain-Containing Transcription Factor ABI3; FUS3, B3 Domain-Containing Transcription Factor FUS3-like; SPDS1, Spermidine synthase 1; SPMS, Spermine synthase; APX, Ascrobate peroxidase, cytosolic like; CycB1, Cyclin B1-2; CDKA1, Cyclin dependent kinase A; CycD2, Cyclin D2-1 like.
2.8. Statistical Analysis
For the germination tests and viability assay data, statistical analyses were performed using Student’s t-test. Asterisks indicate statistically significant differences determined using Student’s t-test (*, p ≤ 0.05). ROS quantification and gene expression analyses were analyzed through two-way analysis of variance (ANOVA) and the Duncan’s test, using the software Rapid Publication-Ready MS Word Tables Using Two-Way ANOVA 1.0 [56], available online (https://houssein-assaad.shinyapps.io/TwoWayANOVA/). Pearson’s correlation and Principal Component Analysis (PCA) were carried out using MetaboAnalyst 6.0 (https://www.metaboanalyst.ca/docs/Publications.xhtml), normalizing the values by Z-score and considering a p-value ≤ 0.05 as the threshold for [57].
3. Results
3.1. Reduced Seed Viability in Long-Term Stored P. alba Seeds
The estimated germination percentage (G%) of long-term stored ‘Villafranca’ seeds was 19.00 ± 4.47% (Figure 2A). TTC assay, performed to quantify the levels of seed viability showed that the percentage of non-viable seeds was significantly higher (68 ± 13.03%) compared to the viable ones (32 ± 13.03%) (Figure 2B). Taken together, these data indicate that the low germination observed in ‘Villafranca’ seeds was due to a substantial loss of viability during storage.
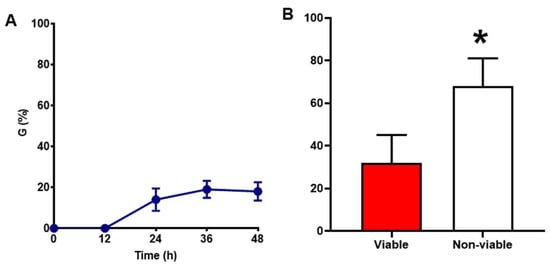
Figure 2.
Baseline characterization of long-term stored Populus alba clone ‘Villafranca’ seeds. (A) Germination percentage (G%). (B) Viability test performed through the TTC method. Statistically significant differences calculated using Student’s t-test are indicated with an asterisk (*, p ≤ 0.05).
3.2. Imbibition Curves Denote Rapid Water Loss During Dehydration
The dynamics of water uptake and loss (rehydration–dehydration cycle) were evaluated in naturally aged P. alba seeds at different timepoints during imbibition (1, 2, 3, 4, 5 h) with water (H2O), Spd50 (50 µM spermidine) and Spd100 (100 µM spermidine) as well as during dry-back (DB) (1, 2 h) (Figure 3). Across all treatments, a rapid water uptake was observed during the first two hours while at the subsequent timepoints the process was slower. No significant differences between the absorption of water or Spd solutions was evidenced. A very rapid water loss was evidenced already from the first hour of the dry-back process (Figure 3, 1 h DB). Additionally, the seed weight at 2 h DB (0.31 ± 0.03 mg) was lower than that of the initial dry state of the seeds (0.47 ± 0.01 mg). Also in this case, no significant differences were observed between the treatment with water or Spd (Figure 3).
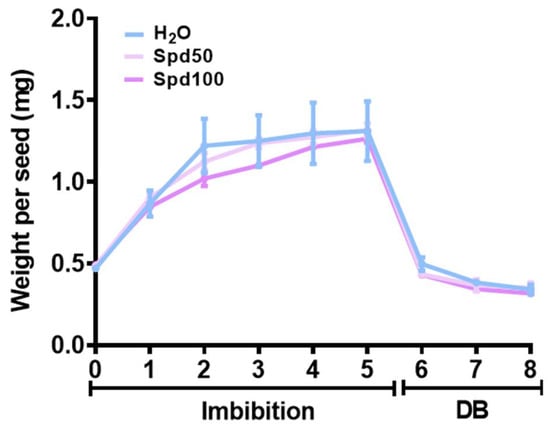
Figure 3.
Imbibition curves represented as seed weight gain and loss in long-term stored P. alba clone ‘Villafranca’ seeds subjected to different intervals of rehydration (1, 2, 3, 4, 5 h) and dehydration (1, 2, 3 h). DB, dry-back; Spd50, spermidine 50 µM; Spd100, spermidine 100 µM.
3.3. Hydropriming Does Not Improve Germination of Naturally Aged Seeds
To investigate if long-term stored seeds were responsive to priming treatments, different HP protocols were tested taking into consideration various imbibition and DB timepoints. The seeds were subjected to an increasing imbibition time of 2, 4, 6, and 8 h, associated with the corresponding DB time of 1, 2, 3, and 4 h. To test the efficiency of the treatments, germination tests were carried out alongside untreated (UT) seeds used as control. As shown in Figure 4A, none of the HP treatments applied was able to enhance G%. Moreover, a significant reduction in germinability was observed after the 8 h HP treatment (3.33 ± 5.77%) compared with the UT control (28 ± 2.88%). This indicates that HP was perceived as a stress treatment rather than a beneficial one. We hypothesize that this may be due to the rapid water loss evidenced previously during the DB process (Figure 3).
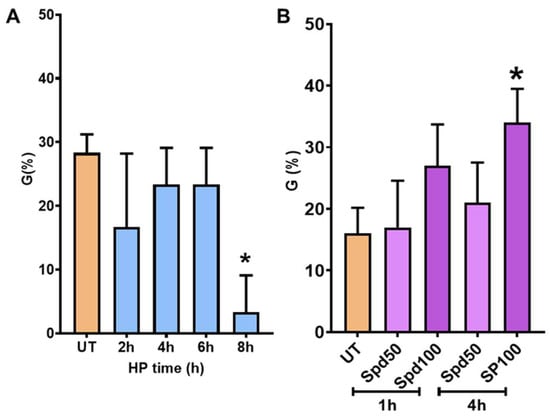
Figure 4.
(A) Germination percentage (G%) of long-term stored P. alba clone ‘Villafranca’ seeds treated/untreated with hydropriming (HP) at different imbibition timepoints (2, 4, 6, 8 h) followed by dry-back (DB). (B) Germination percentage (G%) of long-term stored P. alba clone ‘Villafranca’ seeds following soaking in spermidine (Spd) solutions (50 µM and 100 µM). Seeds were subjected to different soaking time intervals, namely 1 and 4 h. Statistically significant differences were calculated compared to untreated (UT) controls using Student’s t-test and are indicated with an asterisk (*, p ≤ 0.05).
Given the inefficiency of standard HP protocols, the next step consisted in applying only seed soaking treatments, thus avoiding the DB step. In this experiment, seeds were soaked in Spd solutions (50 µM, Spd50; 100 µM, Spd100) for different time intervals, namely 1 and 4 h. The timepoints were selected based on the imbibition curve (Figure 3). The germination tests conducted evidenced that soaking with 100 µM spermidine for 4 h was the most efficient treatment resulting in a significant rise of G% (36.66 ± 5.77%), compared to UT (15 ± 5%) (Figure 4B).
3.4. Seed Soaking with Spermidine Enhances Germination Performance
To confirm these results, another set of germination tests was conducted using the optimized timepoint (4 h) and soaking the seeds in water (H2O) or 100 Spd µM concentration (Spd100) (Figure 5). In this case, both G% and germination speed(T50) was assessed. Regarding G% (Figure 5A), only seeds treated with 100 µM Spd (Spd100) exhibited significantly enhanced germination (35.00 ± 5.00%) compared to UT controls (20.00 ± 5.00%). This finding was well corroborated by a significant reduction in the T50 values, observed only for the Spd100 treatment (Figure 5B). Overall, these results indicate that seed soaking with Spd represents an efficient treatment to improve the germination potential of long-time stored P. alba seeds.
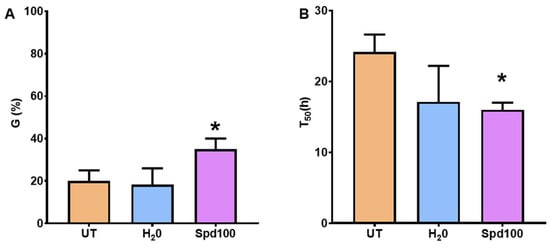
Figure 5.
Germination performance of long-term stored P. alba clone ‘Villafranca’ seeds soaked for 4 h in water or spermidine. (A) Germination percentage (G%). (B) Germination speed calculated in terms of T50. Statistically significant differences were calculated compared to untreated (UT) controls using Student’s t-test and are indicated with an asterisk (*, p ≤ 0.05). UT, untreated; H2O, water soaking; Spd100, 100 µM spermidine soaking; DB, dry-back.
3.5. Molecular Characterization of Long-Term Stored Populus Alba Clone ‘Villafranca’ Seeds Along the Hydration–Dehydration Cycle
3.5.1. SPD Enhances the Capacity of Aged Seeds to Effectively Scavenge ROS
To better understand the molecular mechanisms behind the efficacy of Spd on the germination performance of long-term stored ‘Villafranca’ seeds, a molecular characterization was carried out. The timepoints selected for the experimental system for molecular characterization were derived from those selected for the imbibition curve (Figure 3). The experimental system used was composed of dry seeds (DS, 0 h), seeds imbibed with H2O or 100 µM Spd for 1 h and 4 h, as well as following the dry-back process at 1 and 2 h DB (Figure 1D). With the chosen timepoints, which included both imbibition and dehydration timepoints. We selected imbibition timepoints to assess the beneficial effects of spermidine soaking on germination. We attempted to assess the adverse effects of dehydration on aged poplar seeds, which are desiccation sensitive by selecting the DB timepoints.
Molecular characterization included the evaluation of the seed oxidative status, by measuring the production of ROS through the DCFH-DA assay. ROS quantification was assessed given its significant role in relation to seed aging and stress response. In the case of the seeds treated with H2O, a significant increase in ROS levels was observed at 4 h imbibition and 1 and 2 h DB, with the highest accumulation registered for the 1DB samples (3.17 ± 0.62 R.F.U) (Figure 6). Interestingly, in the seeds treated with Spd100, ROS levels remained constant (with no significant differences compared to control) up to the 1DB step, and only a slightly significant increase was evidenced in the case of 2DB samples. When comparing the differences between H2O and Spd100 treatments during seed imbibition (4 h) and dry-back (1DB), it is possible to observe a significant decrease in ROS levels when the seeds are treated with spermidine (Figure 6). This suggests that Spd may act as an antioxidant mechanism to limit ROS production both during water uptake and loss.
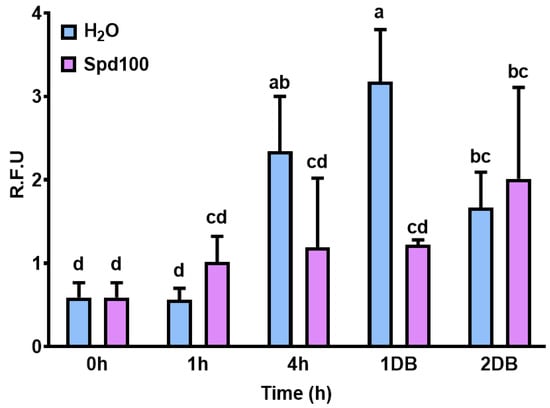
Figure 6.
Quantification of reactive oxygen species (ROS) through the DCFH-DA assay on the selected rehydration (1 and 4 h) and dehydration (1 and 2 h) timepoints. The imposed treatments included imbibition in H2O or a spermidine (Spd) solution at a concentration of 100 µM alongside dry seeds (0 h) used as untreated controls. Samples followed by different letters indicate statistically significant differences (p ≤ 0.05) as per Duncan test.
3.5.2. Effect of SPD on Antioxidant, DT, and Cell Cycle Related Gene Expression Profiles
To further characterize the experimental system, qRT-PCR analyses were performed to quantify the expression levels of selected genes with relevant roles during early seed germination. We examined the expression patterns of genes involved in polyamine biosynthesis (SPDS1, SPMS), antioxidant response (APX), DT acquisition (ABI3, FUS3), and cell cycle regulation (CycB1, CDKA1, CycD2). The mean ± standard deviation values are provided in Table S1, while Z score normalization was used to generate the heatmap, as described in Materials and Methods. Whitin the heatmap blue color indicates low expression and red color indicates high gene expression (Figure 7). Changes in gene expression profiles were observed during the rehydration and dehydration steps. An intriguing finding was that all the investigated genes were highly expressed in dry seeds (0 h). Compared to this, both H2O and Spd100 treatments resulted into a significant reduction in the expression of SPMS, ABI3, and CDKA1 genes at 1 h imbibition, and this low expression persisted at the subsequent timepoints, including DB. The Spd100 treatment seemed to induce the expression APX, FU3 and CycB1 genes in response to 2 h dehydration (2DB) and this upregulation was not observed in H2O-treated seeds. In this case, the only significantly upregulated genes compared to control (0 h) were SPDS1 at 1 h imbibition and CycD2 at 2 h DB.
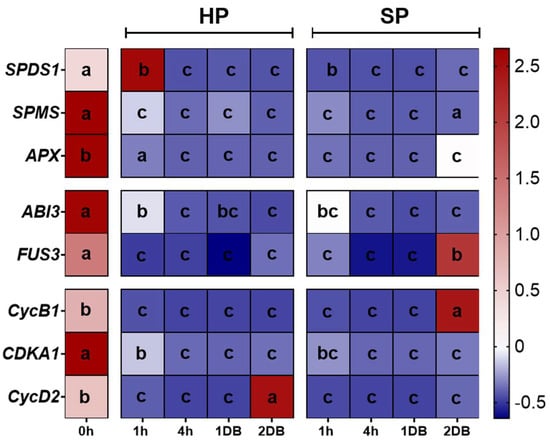
Figure 7.
Heatmaps representing the expression patterns of indicated genes obtained through qRT-PCR analyses on the selected rehydration (1 and 4 h) and dehydration (1 and 2 h) timepoints. The imposed treatments included imbibition in H2O or a spermidine (Spd) solution at a concentration of 100 µM alongside dry seeds (0 h) used as untreated controls. Blue color indicates low gene expression while red color indicates high expression. Samples followed by different letters indicate statistically significant differences (p ≤ 0.05) as per Duncan test. H2O, water soaking; Spd100, 100 µM spermidine soaking; DB, dry-back; SPDS1, Spermidine synthase 1; SPMS, Spermine synthase; APX, Ascrobate peroxidase; ABI3, B3 Domain-Containing Transcription Factor ABI3; FUS3, B3 Domain-Containing Transcription Factor FUS3-like; CycB1, Cyclin B1-2; CDKA1, Cyclin dependent kinase A; CycD2, Cyclin D2-1 like.
3.6. Clustering of Germination Parameters, ROS Accumulation, and Gene Expression Profiles
To integrate all the data obtained from the molecular and phenotyping analyses, a PCA (Principal Component Analysis) was carried out to illustrate the overall behavior of the applied treatments (Figure 8). The PCA clustering showed the formation of three distinct groups (UT, H2O, Spd100) corresponding to each of the imposed treatments (Figure 8A). Based on the biplot analysis, the separation of the UT samples was mainly due to gene expression data. The key variable contributing to the separation of H2O samples was the DCFH-DA data, while the germination parameters mostly contributed to the distinct separation of the Spd100 samples (Figure 8B). Globally, at the level of analyzed parameters, both PCA and biplot indicates that the improvement of germination in aged white poplar seeds can be associated with decrease in ROS accumulation at the early stages of germination.
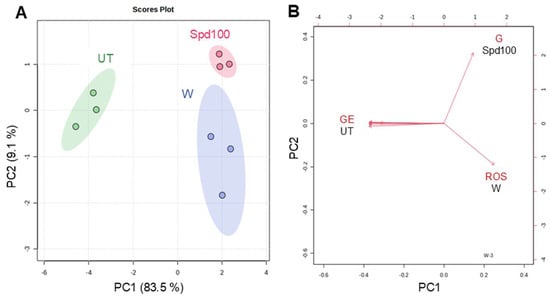
Figure 8.
Principal Component Analysis (PCA) plots using data gathered for the treatments (UT, H2O, Spd100) imposed on long-term stored Populus alba clone ‘Villafranca’ seeds. (A) Score plot clustering. (B) Biplot obtained with data from germination tests (G), ROS measurements (ROS) and gene expression (GE) data. UT, untreated; W, H2O water soaking; Spd100, 100 µM spermidine soaking.
4. Discussion
The current study aimed to develop seed treatments to enhance the germination performance of long-term stored P. alba clone ‘Villafranca’ seeds. This study focused on a batch of seeds stored under vacuum for 16 years at −30 °C. Given that poplar seeds have a short lifespan following harvest [17,58], they need to be prepared for storage immediately. Also, because they are defined as intermediate seeds [19], hence desiccation-sensitive, the storage conditions represent a limiting factor hampering seed viability.
The baseline characterization of the long-term stored ’Villafranca’ seeds evidenced low viability (approximately 20%). Loss of viability during storage has been reported in many trees, including Salix caprea and S. gracilistyla [59], as well as different poplar species like, Populus nigra [60] P. davidiana, P. koreana [10] and the P. alba × P. glandulosa hybrid [61]. When considering poplars, the storage time and temperature, along with the seed water content, are essential and species dependent. For instance, it was shown that P. nigra seeds were successfully stored for only two years at temperatures of −10 °C or −20 °C, while maintaining high levels of viability [60]. Significant differences in seed viability based on seed water content were reported for P. davidiana (orthodox seeds) and P. koreana (intermediate seeds) [10]. Cryopreservation was indicated as a more suitable storage method for short lifespan seeds [62]. P. alba × P. glandulosa hybrids exhibited an equivalent germination rate as the controls when cryopreserved at a water content of 0.07–0.10 g g−1 for 2 weeks [61]. Recently, seed priming has been promoted as part of the integrated resource management programs for fragile ecosystems [63]. HP, considered among the most simple, sustainable, and cost-effective seed priming methods [64,65], was first selected for ‘Villafranca’ seed treatments in this work.
In our study, multiple imbibition and DB timepoints were tested but none had positive effects on the germination of long-term stored ‘Villafranca’ seeds. Moreover, imbibition for 8 h followed by 4 h DB resulted in a significant reduction of G%. Such a deleterious effect might be due to the rapid water loss occurring during the dry-back phase of the treatment. This is also a typical feature of desiccation-sensitive seeds, both intermediate and recalcitrant [66,67]. HP proved to be ineffective also for other species with recalcitrant seeds like Cupania glabra and Cymbopetalum [68], or it had limited success after a mild dry-back treatment in the case of Quercus rugosa [69].
Given that HP was not successful, and the dry-back might be potentially harmful to seed viability, soaking treatments were then tested, thus eliminating the dehydration step. Soaking either in water or Spd50 and Spd100 solutions at 50 and 100 µM showed that the Spd100 applied for 4 h was the most effective treatment, doubling G% and increasing germination speed, compared to untreated seeds. These results are in agreement with previous reports showing that soaking treatments were efficient in improving germination of various species with intermediate and recalcitrant seeds, like Osyris lanceolata [70], Psidium guajava [71], Pinus roxburghii [72], Litchi chinensis [73], and Pouteria campachiana [74]. In our study, the germinability of long-term stored ‘Villafranca’ seeds was significantly enhanced only when Spd was added in the soaking solution. This essential polyamine has been extensively studied for its beneficial effect on seed germination under stress [36,37,38,39,40,41,42]. In a recent study, recalcitrant Acer saccharinum seeds treated with Spd showed improved germination after exposure to mild and severe desiccation [42].
The positive effect of Spd on the germination of aged seeds could be explained also through its antioxidant properties [42,75,76], thus contributing to maintaining the ROS balance during germination. ROS are important players given that they act as signaling molecules in many seed physiological processes [77] while high ROS accumulation leads to detrimental effects and loss of seed viability [78]. The DCFH-DA assay carried out in this study to monitor ROS production in ‘Villafranca’ imbibed seeds evidenced a significant decrease in ROS levels when seeds were treated with Spd. This is in agreement with results from other studies where Spd was applied to Oryza sativa [40], Solanum lycopersicum [43], Medicago sativa [44], Vigna radiata [45], and Cucumis sativus [46].
To investigate more in-depth the molecular events underlying the positive effect of Spd as well as the negative impact of the DB step on the germination of long-term stored ‘Villafranca’ seeds, gene expression analysis was conducted, focusing on genes related to DT acquisition, PA biosynthesis, antioxidant response, and cell cycle regulation. ABI3 and FUS3 genes play major roles in seed maturation and DT acquisition [79]. ABI3 functions as both an autoregulator and a regulator of FUS3 [80]. During germination, and therefore DT loss, the function of ABI3 and FUS3 genes are suppressed as they have a prominent role during embryo development and seed maturation [79]. This is in agreement with the qRT-PCR analyses carried out in this study, showing consistent downregulation of these two genes during imbibition with both water and Spd when compared to dry seeds. Considering PA biosynthesis, SPDS encodes for the enzyme that converts putrescine into Spd which is subsequently transformed into spermine by the SPMS enzyme [81]. In our experimental system, the SPDS1 gene was upregulated after 1 h of imbibition in H2O while SPMS was highly expressed only in dry seeds. Similar results were obtained in other studies where SPDS gene expression increased during imbibition in maize seeds [37] and the activity of the SPMS gene was mostly limited to dry seeds in Medicago truncatula [32]. These results indicate that the upregulation of these genes in mature seeds and during early imbibition could be associated with the activation of the antioxidant system in maintaining a proper ROS balance. In agreement with this statement is also the high expression of the APX gene, involved in H2O2 scavenging [82], in dry seed and subsequent downregulation afterwards. The APX gene was also upregulated at 2 h DB after SPD treatment. A similar upregulation was observed in aged oat seeds during, there was a significant amplification of the APX1 gene, indicating its involvement in mitigating the negative consequences of oxidative damage associated with drying [83]. Considering that germination involves the restart of cell cycle progression, the initiation of G1 phase and activation of the G1-to-S transition are crucial steps for a successful germination [84]. The expression profiles of cell cycle genes were evaluated during the early stages of ‘Villafranca’ seed germination to gain additional information. CDKA1 encodes for an A type cyclin-dependent kinase while CycD2 encodes a D-type cyclin, both playing roles in the transition from G1-to-S phase [85,86]. On the other hand, CycB1 encodes for a protein that promotes the progression of the cell cycle during M-phase [87], and it is also involved in the homologous recombination (HR) repair pathway [88]. In our experimental system, the CycB1 gene was highly expressed during DB after the Spd treatment while the CycD2 gene was most expressed at the same timepoint but when water was used. During the desiccation process, DNA damage can accumulate, and this damage must be effectively repaired before the start of germination [89]. The observed upregulation of the CycB1 and CycD2 genes may indicate that the cell cycle progression could be stalled to allow more time for an efficient repair. Other studies have also indicated that spermidine and spermine treatments promoted protection against DNA oxidative damage in wheat plants exposed to heavy metal stress [90].
5. Conclusions
To conclude, the current study reports the development of an optimized seed treatment able to improve germination of long-term stored P. alba clone ‘Villafranca’ seeds. The use of a spermidine solution at a concentration of 100 μM applied as seed soaking for 4 h, doubled G% and improved germination speed. HP treatments were ineffective in the case of poplar seeds because of the rapid water loss during the DB step. Molecular analyses revealed that the positive effect of the Spd treatment can be attributed to lower ROS production when the pre-germinative metabolism is resumed. Additionally, gene expression analyses indicate the upregulation of genes involved in DT acquisition, PA biosynthesis, and antioxidant defense in dry seeds. To our knowledge, this is the first report addressing the issues of seed quality in white poplars. The tailored protocol hereby developed will be validated in other poplar clones and species and further optimized to become a useful tool supporting seed bank operators, breeders, and seed technologists specialized in the agroforestry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/seeds4020025/s1, Figure S1: Ct values of Tub gene utilized as reference in the experimental system, including the selected rehydration (1 and 4 h) and dehydration (1 and 2 h) time points. Table S1: Relative expression of DDR, cyclin, and polyamines biosynthesis genes evaluated by qRT-PCR in P. alba clone Villafranca seeds. Data were subsequently normalized through Z score to generate the heatmap presented in the main text. UP, unprimed; HP, hydroprimed; SP, spermidine (10 mM)-treated seeds; UA, unaged dry seed; AA, artificially aged dry seed; Rh, aged imbibed seed; ABI3, B3 Domain-Containing Transcription Factor ABI3; FUS3, B3 Domain-Containing Transcription Factor FUS3-like; SPDS1, Spermidine synthase 1; SPMS, Spermine synthase; APX, Ascrobate peroxidase, cytosolic like; CycB1, Cyclin B1-2; CDKA1, Cyclin dependent kinase A; CycD2, Cyclin D2-1 like.
Author Contributions
Conceptualization, A.M. and A.B.; methodology, S.S.G., L.C., M.R. and A.P.; writing—original draft preparation S.S.G. and A.M.; writing—review and editing, A.G., P.M.C. and A.B.; funding acquisition, A.B. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4—Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union–NextGenerationEU; Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP B83C22002930006 (A.G.), CUP F13C22000720007 (A.B), and CUP F17G22000190007 (A.M.) Project title “National Biodiversity Future Center—NBFC”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used and/or analyzed during this study are available from the corresponding authors on reasonable request. The data hereby reported are part of a PhD thesis entitled “Exploring the molecular mechanisms of seed deterioration in the model species Medicago truncatula L. and Populus alba L.: designing anti-aging treatments to protect seeds in storage” deposited in the University of Pavia digital library, but it is not published in any open-access Journals.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Eckenwalder, J.E. Systematics and evolution of populus. In Biology of Populus; Stettler, R.F., Heilman, J.P.E., Hinckley, T.M., Eds.; National Research Council of Canada Research: Ottawa, ON, Canada, 1996; pp. 7–32. [Google Scholar]
- Pellegrino, E.; Di Bene, C.; Tozzini, C.; Bonari, E. Impact on soil quality of a 10-year-old short-rotation coppice poplar stand compared with intensive agricultural and uncultivated systems in a Mediterranean area. Agric. Ecosyst. Environ. 2011, 140, 245–254. [Google Scholar] [CrossRef]
- Cantamessa, S.; Rosso, L.; Giorcelli, A.; Chiarabaglio, P. The environmental impact of poplar stand Management: A life cycle assessment study of different scenarios. Forests 2022, 13, 464. [Google Scholar] [CrossRef]
- Nissim, W.G.; Castiglione, S.; Guarino, F.; Pastore, M.C.; Labra, M. Beyond cleansing: Ecosystem services related to phytoremediation. Plants 2023, 12, 1031. [Google Scholar] [CrossRef]
- Pra, A.; Pettenella, D. Investment returns from hybrid poplar plantations in northern Italy between 2001 and 2016: Are we losing a bio-based segment of the primary economy? Ital. Rev. Agric. Econ. 2019, 74, 49–71. [Google Scholar]
- Caudullo, G.; De Rigo, D. Populus alba in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; European Union: Luxemborg, 2016; pp. 134–135. [Google Scholar]
- Corona, P.; Bergante, S.; Castro, G.; Chiarabaglio, P.M.; Coaloa, D.; Facciotto, G.; Gennaro, M.; Giorcelli, A.; Rosso, L.; Vietto, L.; et al. Linee di Indirizzo per Una Pioppicoltura Sostenibile; Rete Rurale Nazionale, Consiglio per la Ricerca in Agricoltura e L’analisi Dell’economia Agraria: Rome, Italy, 2018; ISBN 978-88-99595-96-8.
- Mushtaq, T.; Banyal, R.; Mugloo, J.; Mushtaq, T.; Aziz, M.A. Clonal forestry: An effective technique for increasing the productivity of plantations. SKUAST J. Res. 2017, 19, 22–28. [Google Scholar]
- Dalziell, E.L.; Lewandrowski, W.; Commander, L.E.; Elliott, C.P.; Erickson, T.E.; Tudor, E.P.; Turner, S.R.; Merritt, D.J. Seed traits inform the germination niche for biodiverse ecological restoration. Seed Sci. Technol. 2022, 50, 103–124. [Google Scholar] [CrossRef]
- Kim, D.H. Extending Populus seed longevity by controlling seed moisture content and temperature. PLoS ONE 2018, 13, e0203080. [Google Scholar] [CrossRef]
- Karrenberg, S.; Suter, M. Phenotypic trade-offs in the sexual reproduction of Salicaceae from flood plains. Am. J. Bot. 2003, 90, 749–754. [Google Scholar] [CrossRef]
- González, E.; Bourgeois, B.; Masip, A.; Sher, A.A. Trade-offs in seed dispersal strategies across riparian trees: The how matters as much as the when. River Res. Appl. 2016, 32, 786–794. [Google Scholar] [CrossRef]
- Lefebvre, M.; Villar, M.; Boizot, N.; Delile, A.; Dimouro, B.; Lomenech, A.M.; Teyssier, C. Variability in seeds’physicochemical characteristics, germination and seedling growth within and between two French Populus nigra L. populations. Peer Community J. 2022, 2, e10. [Google Scholar] [CrossRef]
- Qu, C.; Zhang, S.; Zhao, H.; Chen, J.; Zuo, Z.; Sun, X.; Cheng, Y.; Xu, Z.; Liu, G. Analysis of the energy source at the early stage of poplar seed germination: Verification of Perl’s pathway. 3 Biotech 2020, 10, 418. [Google Scholar] [CrossRef] [PubMed]
- Kijak, H.; Ratajczak, E. What Do We Know About the Genetic Basis of Seed Desiccation Tolerance and Longevity? Int. J. Mol. Sci. 2020, 21, 3612. [Google Scholar] [CrossRef] [PubMed]
- Tweddle, J.C.; Dickie, J.B.; Baskin, C.C.; Baskin, J.M. Ecological aspects of seed desiccation sensitivity. J. Ecol. 2003, 91, 294–304. [Google Scholar] [CrossRef]
- Gosling, P. Raising Trees and Shrubs from Seeds; Practice Guide; Forestry Commission Practice Guide; Forestry Commission: Edinburgh, UK, 2007; pp. 1–28.
- Bonner, F.T. Storage of seed. In The Woody Plant Seed Manual, 1st ed.; Agriculture Handbook; United States Department of Agriculture, Forest Service: Washington, DC, USA, 2008; pp. 85–96. [Google Scholar]
- Michalak, M.; Plitta, B.P.; Tylkowski, T.; Chmielarz, P.; Suszka, J. Desiccation tolerance and cryopreservation of seeds of black poplar (Populus nigra L.), a disappearing tree species in Europe. Eur. J. For. Res. 2014, 134, 53–60. [Google Scholar] [CrossRef]
- Chen, H.; Shen, Y. Investigation of Water Distribution and Mobility Dynamics in Recalcitrant Quercus acutissima Seeds during Desiccation Using Magnetic Resonance Methods. Forests 2023, 14, 738. [Google Scholar] [CrossRef]
- Ganatsas, P.; Tsakaldimi, M. A comparative study of desiccation responses of seeds of three drought-resistant Mediterranean oaks. For. Ecol. Manag. 2013, 305, 189–194. [Google Scholar] [CrossRef]
- Bento, L.R.; Spaccini, R.; Cangemi, S.; Mazzei, P.; De Freitas, B.B.; De Souza, A.E.O.; Moreira, A.B.; Ferreira, O.P.; Piccolo, A.; Bisinoti, M.C. Hydrochar obtained with by-products from the sugarcane industry: Molecular features and effects of extracts on maize seed germination. J. Environ. Manag. 2021, 281, 11878. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Nafees, M.; Chen, J.; Darras, A.; Ferrante, A.; Hancock, J.T.; Ashraf, M.; Zaid, A.; Latif, N.; Corpas, F.J.; et al. Chemical priming enhances plant tolerance to salt stress. Front. Plant Sci. 2022, 13, 946922. [Google Scholar] [CrossRef]
- BiBi, R.; Elahi, N.N.; Danish, S.; Alahmadi, T.A.; Ansari, M.J. Enhancing germination and growth of canola (Brassica napus L.) through hydropriming and NaCl priming. Sci. Rep. 2024, 14, 14026. [Google Scholar] [CrossRef]
- Dueñas, C.; Pagano, A.; Calvio, C.; Srikanthan, D.S.; Slamet-Loedin, I.; Balestrazzi, A.; Macovei, A. Genotype-specific germination behavior induced by sustainable priming techniques in response to water deprivation stress in rice. Front. Plant Sci. 2024, 15, 1344383. [Google Scholar] [CrossRef]
- Gaonkar, S.S.; Sincinelli, F.; Balestrazzi, A.; Pagano, A. Quercetin and Rutin as Tools to Enhance Antioxidant Profiles and Post-Priming Seed Storability in Medicago truncatula. Agriculture 2024, 14, 738. [Google Scholar] [CrossRef]
- Gaonkar, S.S. Exploring the molecular mechanisms of seed deterioration in the model species Medicago truncatula L. and Populus alba L.: Designing Anti-Aging Treatments to Protect Seeds in Storage. Ph.D. Thesis, University of Pavia, Pavia, Italy, 2024. [Google Scholar]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Pagano, A.; Macovei, A.; Balestrazzi, A. Molecular dynamics of seed priming at the crossroads between basic and applied research. Plant Cell Rep. 2023, 42, 657–688. [Google Scholar] [CrossRef]
- Smolikova, G.; Leonova, T.; Vashurina, N.; Frolov, A.; Medvedev, S. Desiccation tolerance as the basis of Long-Term seed viability. Int. J. Mol. Sci. 2020, 22, 101. [Google Scholar] [CrossRef]
- Pagano, A.; Folini, G.; Pagano, P.; Sincinelli, F.; Rossetto, A.; Macovei, A.; Balestrazzi, A. ROS Accumulation as a Hallmark of Dehydration Stress in Primed and Overprimed Medicago truncatula Seeds. Agronomy 2022, 12, 268. [Google Scholar] [CrossRef]
- Pagano, A.; Zannino, L.; Pagano, P.; Doria, E.; Dondi, D.; Macovei, A.; Biggiogera, M.; De Sousa Araújo, S.; Balestrazzi, A. Changes in genotoxic stress response, ribogenesis and PAP (3′-phosphoadenosine 5′-phosphate) levels are associated with loss of desiccation tolerance in overprimed Medicago truncatula seeds. Plant Cell Environ. 2022, 45, 1457–1473. [Google Scholar] [CrossRef]
- Kusano, T.; Berberich, T.; Tateda, C.; Takahashi, Y. Polyamines: Essential factors for growth and survival. Planta 2008, 228, 367–381. [Google Scholar] [CrossRef]
- Lechowska, K.; Wojtyla, U.; Quinet, M.; Kubala, S.; Lutts, S.; Garnczarska, M. Endogenous Polyamines and Ethylene Biosynthesis in Relation to Germination of Osmoprimed Brassica napus Seeds under Salt Stress. Int. J. Mol. Sci. 2021, 23, 349. [Google Scholar] [CrossRef]
- Shao, J.; Huang, K.; Batool, M.; Idrees, F.; Afzal, R.; Haroon, M.; Noushahi, H.A.; Wu, W.; Hu, Q.; Lu, X.; et al. Versatile roles of polyamines in improving abiotic stress tolerance of plants. Front. Plant Sci. 2022, 1, 1003155. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y.; Zhang, X.Q.; Ma, X.; Huang, L.K.; Yan, Y.H. Exogenous Spermidine Improves Seed Germination of White Clover under Water Stress via Involvement in Starch Metabolism, Antioxidant Defenses and Relevant Gene Expression. Molecules 2014, 19, 18003–18024. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, C.; He, F.; Li, Z.; Guan, Y.; Hu, Q.; Hu, J. Exogenous spermidine improves seed germination of sweet corn via involvement in phytohormone interactions, H2O2 and relevant gene expression. BMC Plant Biol. 2017, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Roychoudhury, A. Seed priming with spermine and spermidine regulates the expression of diverse groups of abiotic stress-responsive genes during salinity stress in the seedlings of indica rice varieties. Plant Gene 2017, 11, 124–132. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine Function in Plants: Metabolism, Regulation on Development, and Roles in Abiotic Stress Responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Hu, Q.J.; Chen, M.X.; Song, T.; Cheng, C.L.; Tian, Y.; Hu, J.; Zhang, J.H. Spermidine enhanced the antioxidant capacity of rice seeds during seed aging. Plant Growth Regul. 2020, 91, 397–406. [Google Scholar] [CrossRef]
- Zhang, M.; Li, B.; Wan, Z.; Chen, X.; Liu, C.; Liu, C.; Zhou, Y. Exogenous Spermidine Promotes Germination of Aged Sorghum Seeds by Mediating Sugar Metabolism. Plants 2022, 11, 2853. [Google Scholar] [CrossRef]
- Fuchs, H.; Plitta-Michalak, B.P.; Małecka, A.; Ciszewska, L.; Sikorski, U.; Staszak, A.M.; Michalak, M.; Ratajczak, E. The chances in the redox priming of nondormant recalcitrant seeds by spermidine. Tree Physiol. 2023, 43, 1142–1158. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Hu, X.H. Exogenous spermidine-induced changes at physiological and biochemical parameters levels in tomato seedling grown in saline-alkaline condition. Bot. Stud. 2014, 55, 58. [Google Scholar] [CrossRef]
- Lou, Y.; Guan, R.; Sun, M.; Han, F.; He, W.; Wang, H.; Song, F.; Cui, X.; Zhuge, Y. Spermidine application alleviates salinity damage to antioxidant enzyme activity and gene expression in alfalfa. Ecotoxicology 2018, 27, 1323–1330. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, P.; Gu, Z.; Ma, M.; Yang, R. Spermidine improves antioxidant activity and energy metabolism in mung bean sprouts. Food Chem. 2020, 309, 125759. [Google Scholar] [CrossRef]
- Korbas, A.; Kubiś, J.; Rybus-Zając, M.; Chadzinikolau, T. Spermidine Modify Antioxidant Activity in Cucumber Exposed to Salinity Stress. Agronomy 2022, 12, 1554. [Google Scholar] [CrossRef]
- Li, W.; Niu, Y.; Zheng, Y.; Wang, Z. Advances in the understanding of reactive Oxygen Species-Dependent regulation on seed dormancy, germination, and deterioration in crops. Front. Plant Sci. 2022, 13, 826809. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. Comptes Rendus. Biol. 2008, 33, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Kim, J.H. Effect of storage temperature and cultivars on seed germination of Lilium × formolongi hort. J. Exp. Biol. Agric. 2020, 8, 621–627. [Google Scholar] [CrossRef]
- Faria, J.M.; Buitink, J.; van Lammeren, A.A.; Hilhorst, H.W. Changes in DNA and microtubules during loss and re-establishment of desiccation tolerance in germinating Medicago truncatula seeds. J. Exp. Bot. 2005, 56, 2119–2130. [Google Scholar] [CrossRef]
- De Barros França-Neto, J.; Krzyzanowski, F.C. Tetrazolium: An important test for physiological seed quality evaluation. J. Seed Sci. 2019, 41, 359–366. [Google Scholar] [CrossRef]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef]
- Griffo, A.; Bosco, N.; Pagano, A.; Balestrazzi, A.; Macovei, A. Noninvasive methods to detect reactive oxygen species as a proxy of seed quality. Antioxidants 2023, 12, 626. [Google Scholar] [CrossRef]
- Oñate-Sánchez, L.; Vicente-Carbajosa, J. DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Res. Notes 2008, 1, 93. [Google Scholar] [CrossRef]
- Thomsen, R.; Sølvsten, C.A.E.; Linnet, T.E.; Blechingberg, J.; Nielsen, A.L. Analysis of qPCR data by converting exponentially related Ct values into linearly related X0 values. J. Bioinform. Comput. Biol. 2010, 8, 885–900. [Google Scholar] [CrossRef]
- Assaad, H.I.; Hou, Y.; Zhou, L.; Carroll, R.J.; Wu, G. Rapid publication-ready MS-Word tables for two-way ANOVA. SpringerPlus 2015, 4, 33. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; De Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.T.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Gary, W.; Wyckoff, G.; Zasada, J.C.; Populus, L. Woody Plant Seed Manual. Agricultural Handbook 727; Bonner, F., Ed.; United States Department of Agriculture, Forest Service: Washington, DC, USA, 2008; pp. 856–871.
- Popova, E.V.; Kim, D.H.; Han, S.H.; Pritchard, H.W.; Lee, J.C. Narrowing of the critical hydration window for cryopreservation of Salix caprea seeds following ageing and a reduction in vigour. CryoLetters 2012, 33, 219–230. [Google Scholar]
- Suszka, J.; Plitta, B.P.; Michalak, M.; Bujarska-Borkowska, B.; Tylkowski, T.; Chmielarz, P. Optimal seed water content and storage temperature for preservation of Populus nigra L. germplasm. Ann. For. Sci. 2014, 71, 543–549. [Google Scholar] [CrossRef]
- Popova, E.V.; Kim, D.H.; Han, S.H.; Moltchanova, E.; Pritchard, H.W.; Hong, Y.P. Systematic overestimation of Salicaceae seed survival using radicle emergence in response to drying and storage: Implications for ex situ seed banking. Acta Physiol. Plant 2013, 35, 3015–3025. [Google Scholar] [CrossRef]
- Pritchard, H.W. Cryopreservation of desiccation tolerant seeds. Methods Mol. Biol. 2007, 368, 185–201. [Google Scholar]
- Devika, O.S.; Singh, S.; Sarkar, D.; Barnwal, P.; Suman, J.; Rakshit, A. Seed priming: A potential supplement in integrated resource management under fragile intensive ecosystems. Front. Sustain. Food Syst. 2021, 5, 65400. [Google Scholar] [CrossRef]
- Forti, C.; Shankar, A.; Singh, A.; Balestrazzi, A.; Prasad, V.; Macovei, A. Hydropriming and Biopriming Improve Medicago truncatula Seed Germination and Upregulate DNA Repair and Antioxidant Genes. Genes 2020, 11, 242. [Google Scholar] [CrossRef]
- Sushma, M.K.; Yadav, S.; Yadav, S.; Choudhary, R.; Anbalagan, A.; Navya, K.; Kaushal, K. Hydro-priming as a Sustainable Approach for Improving Germination and Seedling Growth in Tomato (Solanum lycopersicum L.). Seed Res. 2024, 51, 11–17. [Google Scholar]
- Lah, N.H.C.; Enshasy, H.A.E.; Mediani, A.; Azizan, K.A.; Aizat, W.M.; Tan, J.K.; Afzan, A.; Noor, N.M.; Rohani, E.R. An Insight into the behaviour of Recalcitrant Seeds by Understanding Their Molecular Changes upon Desiccation and Low Temperature. Agronomy 2023, 13, 2099. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Xian, Y.; Cui, C.; Xie, X.; Tong, B.; Han, B. Desiccation Sensitivity Characteristics and Low-Temperature Storage of Recalcitrant Quercus variabilis Seed. Forests 2023, 14, 1837. [Google Scholar] [CrossRef]
- Becerra-Vázquez, N.G.; Coates, R.; Sánchez-Nieto, S.; Reyes-Chilpa, R.; Orozco-Segovia, A. Effects of seed priming on germination and seedling growth of desiccation-sensitive seeds from Mexican tropical rainforest. J. Plant Res. 2020, 133, 855–872. [Google Scholar] [CrossRef] [PubMed]
- Castro-Colina, L.; Martínez-Ramos, M.; Sánchez-Coronado, M.E.; Huante, P.; Mendoza, A.; Orozco-Segovia, A. Effect of hydropriming and acclimation treatments on Quercus rugosa acorns and seedlings. Eur. J. For. Res. 2011, 131, 747–756. [Google Scholar] [CrossRef]
- Mwang’Ingo, P.; Teklehaimanot, Z.; Maliondo, S.; Msanga, H. Storage and pre-sowing treatment of recalcitrant seeds of Africa sandalwood (Osyris lanceolata). Seed Sci. Technol. 2004, 32, 547–560. [Google Scholar] [CrossRef]
- Bhanuprakash, K.; Yogeesha, H.; Vasugi, C.; Arun, M.; Naik, L. Effect of pre-soaking treatments and temperature on seed germination of guava (Psidium guajava L.). J. Drug Deliv. Sci. Technol. 2008, 36, 792–794. [Google Scholar] [CrossRef]
- Ghildiyal, S.K.; Sharma, C.M.; Khanduri, V.P. Effect of pre-soaking and pre-chilling treatments on seed germination of Pinus roxburghii provenances from western Himalaya. J. For. Res. 2009, 20, 323–330. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, J.; Fu, D.; Wang, L.; Chen, J.; Cai, C.; Ou, L. Soaking, Temperature, and Seed Placement Affect Seed Germination and Seedling Emergence of Litchi chinensis. HortScience 2015, 50, 628–632. [Google Scholar] [CrossRef]
- Amoakoh, O.A.; Nortey, D.D.N.; Sagoe, F.; Amoako, P.K.; Jallah, C.K. Effects of pre-sowing treatments on the germination and early growth performance of Pouteria campachiana. For. Sci. Technol. 2017, 13, 3–86. [Google Scholar] [CrossRef]
- Cao, D.; Huang, Y.; Mei, G.; Zhang, S.; Wu, H.; Zhao, T. Spermidine enhances chilling tolerance of kale seeds by modulating ROS and phytohormone metabolism. PLoS ONE 2023, 18, e0289563. [Google Scholar] [CrossRef]
- He, M.; Zhou, J.; Lyu, D.; Xu, G.; Qin, S. Exogenous spermidine alleviated Low-Temperature damage by affecting polyamine metabolism and antioxidant levels in apples. Plants 2024, 13, 1100. [Google Scholar] [CrossRef]
- Bailly, C. The signalling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive Oxygen Species as Potential Drivers of the Seed Aging Process. Plants 2019, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Lepiniec, L.; Devic, M.; Roscoe, T.; Bouyer, D.; Zhou, D.X.; Boulard, C.; Baud, S.; Dubreucq, B. Molecular and epigenetic regulations and functions of the LAFL transcriptional regulators that control seed development. Plant Reprod. 2018, 31, 291–307. [Google Scholar] [CrossRef] [PubMed]
- To, A.; Valon, C.; Savino, G.; Guilleminot, J.; Devic, M.; Giraudat, J.; Parcy, F. A network of local and redundant gene regulation governs Arabidopsis seed maturation. Plant Cell 2006, 18, 1642–1651. [Google Scholar] [CrossRef]
- Smirnova, O.A.; Bartosch, B.; Zakirova, N.F.; Kochetkov, S.N.; Ivanov, A.V. Polyamine Metabolism and Oxidative Protein Folding in the ER as ROS-Producing Systems Neglected in Virology. Int. J. Mol. Sci. 2018, 19, 1219. [Google Scholar] [CrossRef]
- Pandey, S.; Fartyal, D.; Agarwal, A.; Shukla, T.; James, D.; Kaul, T.; Negi, Y.K.; Arora, S.; Reddy, M.K. Abiotic Stress Tolerance in Plants: Myriad Roles of Ascorbate Peroxidase. Front. Plant Sci. 2017, 8, 581. [Google Scholar] [CrossRef]
- Kong, L.; Huo, H.; Mao, P. Antioxidant response and related gene expression in aged oat seed. Front. Plant Sci. 2015, 6, 158. [Google Scholar] [CrossRef]
- Barróco, R.M.; Van Poucke, K.; Bergervoet, J.H.; De Veylder, L.; Groot, S.P.; Inze, D.; Engler, G. The Role of the Cell Cycle Machinery in Resumption of Postembryonic Development. Plant Physiol. 2005, 13, 7127–7140. [Google Scholar] [CrossRef]
- Mironov, V.; De Veylder, L.; Van Montagu, M.; Inzé, D. Cyclin-Dependent Kinases and Cell Division in Plants—The Nexus. Plant Cell 1999, 11, 509–521. [Google Scholar]
- Sanz, L.; Dewitte, W.; Forzani, C.; Patell, F.; Nieuwland, J.; Wen, B.; Quelhas, P.; De Jager, S.; Titmus, C.; Campilho, A.; et al. The Arabidopsis D-Type Cyclin CYCD2;1 and the Inhibitor ICK2/KRP2 Modulate Auxin-Induced Lateral Root Formation. Plant Cell 2011, 23, 641–660. [Google Scholar] [CrossRef]
- Menges, M.; Hennig, L.; Gruissem, W.; Murray, J.A.H. Genome-wide gene expression in an Arabidopsis cell suspension. Plant Mol. Biol. 2003, 53, 423–442. [Google Scholar] [CrossRef]
- Weimer, A.K.; Biedermann, S.; Harashima, H.; Roodbarkelari, F.; Takahashi, N.; Foreman, J.; Guan, Y.; Pochon, G.; Heese, M.; Van Damme, D.; et al. The plant-specific CDKB 1-CYCB 1 complex mediates homologous recombination repair in Arabidopsis. EMBO J. 2016, 35, 2068–2086. [Google Scholar] [CrossRef] [PubMed]
- Waterworth, W.M.; Masnavi, G.; Bhardwaj, R.M.; Jiang, Q.; Bray, C.M.; West, C.E. A plant DNA ligase is an important determinant of seed longevity. Plant J. 2010, 63, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Taie, H.A.A.; El-Yazal, M.A.S.; Ahmed, S.M.A.; Rady, M.M. Polyamines modulate growth, antioxidant activity, and genomic DNA in heavy metal–stressed wheat plant. Environ. Sci. Pollut. Res. 2019, 26, 22338–22350. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).