Abstract
Seed germination is a crucial phase where a plant embryo transitions from dormancy to active growth, emerging as a seedling. This intricate process is highly susceptible to environmental cues, particularly abiotic stress factors including drought, salinity, and temperature extremes, which can profoundly influence both germination success and subsequent plant development. Among the various cellular components that modulate plant responses to these stresses, Rho of Plants (ROP) emerges as a pivotal regulator. Under abiotic stress, ROP signaling components integrate with the core abscisic acid (ABA) signaling pathway by regulating gene transcription and protein stability, modulating subcellular localization, converting protein activity, and engaging in competitive interactions. This review summarizes recent findings on roles of ROP signaling in regulating plant adaptive responses to abiotic stress, whilst explores potential involvement of ROPs in seed germination. This review summarizes the effects of ROP proteins and their effectors, such as GEF, on the seed germination process. It preliminarily elucidates the crosstalk mechanisms between these proteins and the ABA signaling pathway, thereby gaining a deeper understanding of the role of ROP signaling in regulating plant adaptive responses to abiotic stresses.
1. Introduction
Seed germination is a critical phase in the plant life cycle, marking the transition from a dormant embryo to an actively growing seedling. This process is highly susceptible to abiotic stress, which refers to any environmental condition that adversely affects plant growth and development [1]. Drought, salinity, and extreme temperatures are major abiotic stresses that critically impair seed germination [2,3]. These stresses can further reduce seedling vigor and ultimately impact plant distribution and agricultural yield [4,5]. Understanding the impact of these stressors on germination is crucial for enhancing plant survival and adaptation, thereby improving crop productivity and ensuring food security in a changing environment [6,7].
Drought primarily induces hyperosmotic stress in plant cells, impeding seed imbibition and radicle protrusion through the seed coat [8]. Salinity stress similarly triggers hyperosmotic conditions, while high concentrations of Na+ and Cl− ions exert cytotoxic effects by disrupting ion homeostasis [2,9,10]. Extreme temperatures mainly exert their effects by disrupting cellular homeostasis, membrane integrity, and enzyme activity. Generally, high temperatures denature proteins, disrupt protein folding, and increase membrane fluidity, leading to electrolyte leakage, while low temperatures reduce membrane fluidity, hinder nutrient transport and signal transduction, and slow down metabolic processes [11,12,13]. Moreover, drought, salinity, and high temperatures all lead to the overproduction of reactive oxygen species (ROS), which causes oxidative damage to cellular components such as lipids, proteins, and nucleic acids [14,15,16]. These abiotic stresses also trigger the accumulation of abscisic acid (ABA), which subsequently activates adaptive responses in plants and inhibits seed germination [17].
Abscisic acid (ABA) is a key phytohormone that plays a central role in modulating seed germination, particularly under abiotic stress conditions such as drought, high salinity, and extreme temperatures (reviewed in [18,19]). ABA is synthesized in response to stress signals and accumulates in seeds where it acts as a crucial regulator of dormancy and germination. During seed maturation, ABA levels peak, contributing to the establishment of dormancy, which is essential for preventing premature germination in unfavorable conditions. In the imbibed state, ABA continues to play a pivotal role through its signaling pathway with several key components. The PYR/PYL/RCAR family of receptors perceives ABA, leading to the activation of downstream signaling cascades [20]. These receptors bind to PP2Cs (protein phosphatase 2C, such as ABI1 and ABI2), thereby inhibiting the phosphatase activity [21]. The inhibition allows activation of downstream SNF1-related protein kinases (SnRK2s), which in turn phosphorylate and activate transcription factors such as ABI5, ultimately controlling expression of the activation of ABA-responsive genes [22]. These genes, including those encoding Late Embryogenesis Abundant (LEA) proteins and heat shock proteins, enhance stress tolerance and delay the onset of germination until conditions become more favorable [23].
The Rho-related GTPases of Plants (ROPs), also known as RACs, represent a unique family of small GTP-binding proteins that are exclusively found in plants. These proteins play crucial roles in various plant processes, including cell growth, polarity establishment, and responses to biotic and abiotic stresses [24]. ROPs cycle between an active GTP-bound state and an inactive GDP-bound state to act as multifunctional molecular switches in cellular processes, including organization and dynamics of the actin and microtubule cytoskeletons, endocytosis, exocytosis, and the activation of NADPH oxidase, which produces reactive oxygen species (ROS) [25]. Although recent studies have shown that ROP plays roles in regulation of seed germination under abiotic stress, the current knowledge mainly depends on the crosstalk between ROP and ABA signaling pathways. Moreover, the explicit mechanisms have not been well elaborated yet. This review commences with a brief introduction of seed germination, ABA signaling under abiotic stress, and ROP signaling. Then, the role of ROPs in seed germination is introduced and followed by the detailed exploration of crosstalk between ROP and ABA signaling pathways during seed germination under abiotic stress, which is the core of this review. Finally, we highlight some current knowledge gaps and future direction related to ROP signaling in seed germination under abiotic stress.
2. Germination
Seed germination is a pivotal developmental transition in the plant life cycle, signifying the transformation of a metabolically dormant embryo into an actively growing seedling that initiates autotrophic growth. This intricate process is tightly regulated by a combination of internal and external factors to ensure plant survival and optimal growth under favorable environmental conditions. Germination sensu stricto begins with water uptake (imbibition) and is completed when the radicle (or other embryonic tissue) emerges from the enclosing seed tissues. However, in most studies, it is essential to consider several critical events during germination, including dormancy release, imbibition, and embryo growth, each characterized by distinct physiological and biochemical changes [5,26].
Dormancy is an adaptive mechanism that delays germination until specific environmental or physiological cues are met, thereby optimizing seedling survival and dispersal across variable conditions [6,27]. ABA maintains dormancy, while gibberellins (GA) promote dormancy release [28,29,30]. These hormones dominate the regulation of dormancy and germination, overshadowing other factors such as temperature, water, and oxygen [27,31,32,33]. Imbibition is the initial water uptake by dry seeds, triggering cellular repair, metabolic reactivation, and preparation for radicle emergence [34]. Imbibed seeds generate limited amounts of reactive oxygen species (ROS) to regulate germination by activating GA signaling and inactivating ABA, facilitating the transition from dormancy to growth [35]. ROS also promote cell wall loosening and α-amylase induction, providing energy for germination [35,36]. In the late stage of imbibition, the antioxidant system is activated to control ROS homeostasis, thereby avoiding the adverse effects of excessive ROS on seed germination [35,37,38]. Seed germination culminates with radicle emergence, and the subsequent embryo growth forms the seedling, endowing it with the capability for photoautotrophy. Embryo growth involves metabolic reprogramming, hormonal regulation, and cell wall remodeling through enzymes like EXPANSINs and xyloglucan endotransglucosylases (XETs) [39,40]. The apical hook is a simple curved structure formed in the upper part of the hypocotyl when dicotyledonous plant seeds germinate in the dark. The formation of the apical hook, a protective structure in dicot seedlings, ensures successful soil emergence by minimizing mechanical damage [41]. Ethylene and auxin gradients further regulate this process by promoting differential cell elongation [42,43,44].
3. ABA Signaling in Response to Abiotic Stress
Studies have revealed that ABA plays a central role in regulating seed germination under various abiotic stresses. The significant increase in ABA content in seeds under drought and water deficiency conditions [45,46] can induce the expression of ABI3 and trigger the accumulation of ABI5. ABI3 can directly bind to the ABI5 promoter, promoting its expression and thereby initiating the late embryonic development program, inhibiting embryo growth, and reducing seed germination rates [47]. Additionally, genes involved in ABA synthesis and signal transduction can respond to salt stress by upregulating ABA expression [45,46]. For instance, overexpression of ABA2 (ABA DEFICIENT2) in Arabidopsis leads to increased ABA levels, delayed seed germination, and salt sensitivity, while aba2 mutant seeds are insensitive to salt stress [48]. Similarly, seeds overexpressing the ABA receptor PYL8/RCAR3 exhibit salt sensitivity, with significantly lower germination rates than controls [49]. Transcription factors in the ABA signaling pathway, such as abi3, abi4, and abi5 mutants, show insensitivity to both salt and drought stresses [50,51]. High temperatures can induce secondary dormancy or thermal inhibition of seed germination by accelerating ABA synthesis and inhibiting GA signaling [52]. Seeds of ABA-deficient mutants (e.g., aba1, aba2) and ABA-insensitive mutants (e.g., abi1, abi3) are insensitive to high-temperature stress [52,53]. Under high-temperature conditions, the interaction between ABI3, ABI5, and DELLA proteins on the SOMNUS (SOM) promoter activates SOM and other high-temperature-induced genes, altering GA/ABA metabolism to inhibit seed germination [54]. While seeds primarily adapt to low-temperature stress by modulating levels of reactive oxygen species (ROS), it has been established that ROS can enhance the transcription of abscisic acid 8-hydroxylase (CYP707As). This enzyme degrades ABA, thereby altering the ratio of ABA to GA. This shift in hormone balance subsequently breaks seed dormancy and activates the potential for germination [55]. These findings collectively demonstrate that ABA signaling during seed germination is crucial for responding to environmental stresses such as drought, high salinity, and extreme temperatures. Water deficiency prompts a dramatic surge in ABA levels, which in turn sets off stomatal closure and activates stress-responsive genes [51,56]. It is a widely accepted fact that when confronted with drought stress, plants display the most pronounced and immediate response to ABA. Under high salinity conditions, ABA accumulates and assumes a crucial role in regulating ion homeostasis and osmotic adjustment [2,10]. However, the plant response to ABA in this scenario is somewhat less immediate than that under drought conditions. In contrast, high and low temperature stresses influence ABA signaling through more indirect pathways [12,52,53,55], which results in a relatively weaker response to ABA.
4. Structures and Signaling Pathway of ROPs
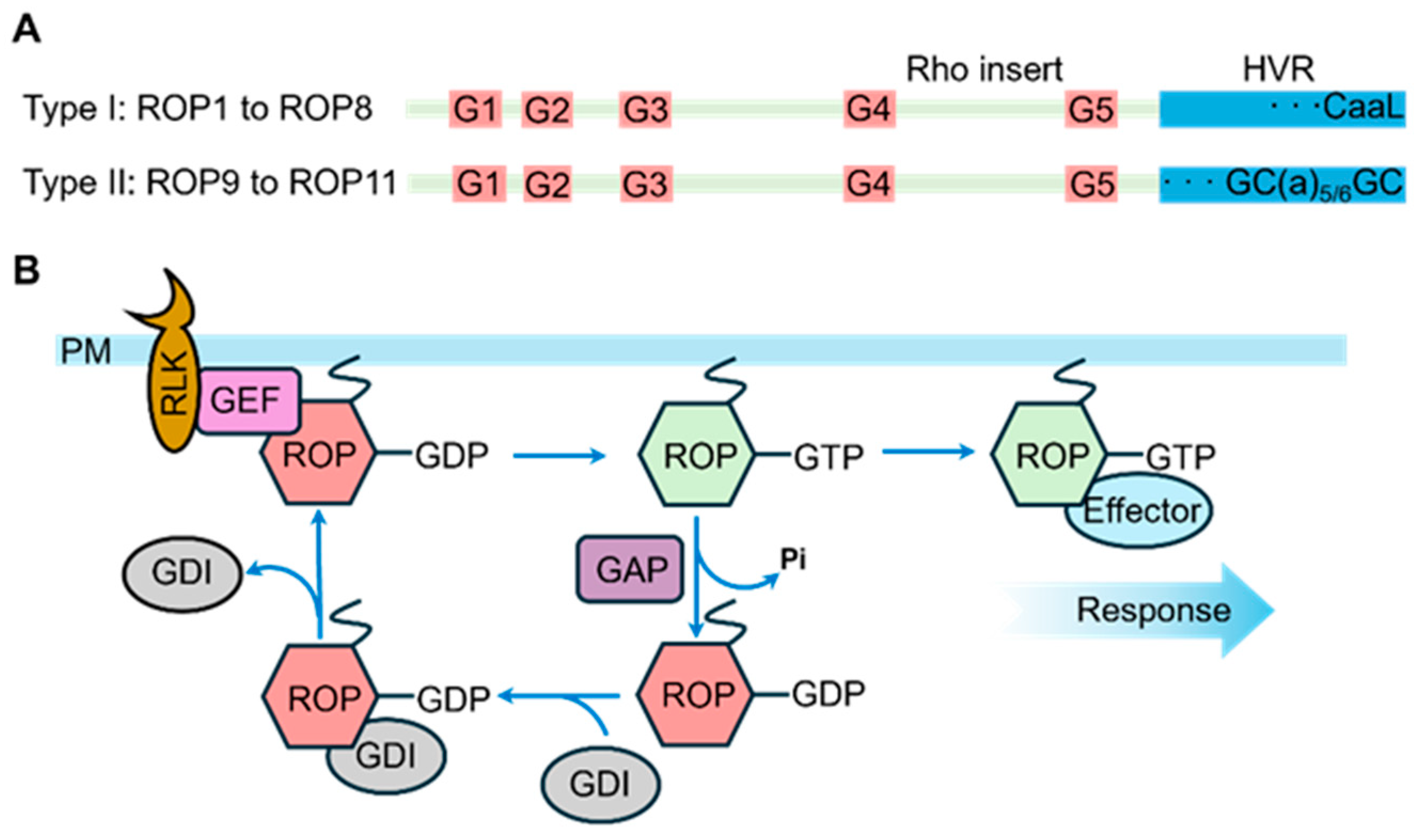
ROP genes are widely conserved across plant species, and their encoded proteins exhibit high structural conservation (Figure 1A). A typical ROP protein consists of five highly conserved G-box motifs (G1 to G5 loops) for binding of nucleotides and effectors, a Rho insert region, and a C-terminal hyper-variable region (HVR) for subcellular localization [57,58,59]. Among these motifs, G1 to G5 loops are crucial for GTP/GDP binding and GTP hydrolysis, while G2 and G3 loops, also known as Switch I and Switch II, are responsible for Mg2+ binding [58], interaction with GTPase-activating proteins (GAPs), and engagement with downstream effector proteins. The Rho insert region undergoes conformational changes upon binding to GTP and GDP, and these changes are crucial for the function of ROP proteins [60]. However, due to limited research in this area, the precise roles that the insert region plays in ROP proteins remain to be fully elucidated. Based on their C-terminal HVRs, ROP proteins are classified into two groups. Type I ROPs (ROP1 to ROP8) terminate with a CaaL box motif, where the cysteine residue is modified by geranylgeranyl [59,61]. Type II ROPs contain GC-CG boxes with two cysteine residues separated by five or six aliphatic residues, undergoing S-acylation by palmitate or stearate [59,61,62].
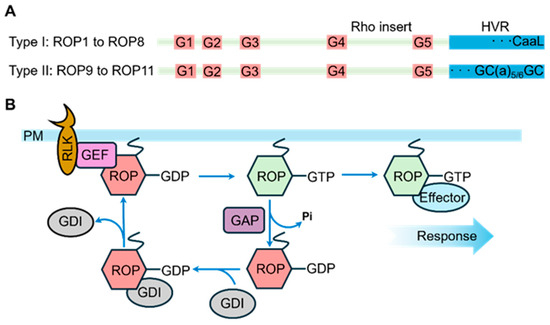
Figure 1.
Structural and Regulatory Mode Diagram of ROP Proteins. (A) ROP structures. ROP comprises five G loops, in addition to the Rho insert and the C-terminal HVR. ROPs are classified into two types based on the C-terminal tail in the HVR: type I ROPs end with the CaaL motif, while type II ROPs ends with the GC-GC box. (B) Signaling pathway of ROPs. Upon perceiving extracellular stimuli, some RLKs activate GEFs to convert ROPs from their GDP-bound inactive state to the GTP-bound active status. The active GTP-ROP subsequently interacts with specific effectors to trigger downstream signaling pathways. Meanwhile, GAPs promote the conversion of GTP-ROPs back to GDP-ROPs through GTP hydrolysis. Additionally, GDIs sequester ROPs and inhibit the activation of GDP-ROPs by GEFs.
The ROP signaling transduction can be systematically delineated through three sequential steps (Figure 1B): (1) the perception of extracellular stimuli by plasma membrane-localized receptor-like kinases (RLKs) initiates the signaling cascade [63]; (2) the activated RLKs subsequently phosphorylate ROP guanine nucleotide exchange factors (ROPGEFs), which catalyze the conversion of ROP-GDP (inactive form) to ROP-GTP (active form) [64]; (3) the activated ROP-GTP interacts with downstream effector proteins, particularly ROP-interactive CRIB motif-containing proteins (RICs), to modulate diverse signaling pathways that orchestrate plant growth and developmental processes [65]. In addition to ROP-GEFs, ROP GTPase-activating proteins (ROP-GAPs) and ROP guanine nucleotide dissociation inhibitors (ROP-GDIs) are also key regulators of ROP activity. ROP-GAPs accelerate the hydrolysis of ROP-bound GTP to GDP, inactivating ROP, while ROP-GDIs sequester ROP in the cytoplasm, inhibiting its activity and preventing its participation in signaling [59,66].
5. Regulation of ROP in Seed Germination Under Abiotic Stress
5.1. ROPs in Seed Germination and ABA Signaling
In Arabidopsis, there are 11 ROP proteins, and current research has identified the involvement of six ROPs (ROP1, ROP2, ROP6, ROP9, ROP10, and ROP11) in seed germination (Table 1), suggesting functional redundancy among these proteins. However, their roles in this process are not entirely consistent. For instance, single mutants of rop1 and rop6 exhibited better seed germination than the wild type, and this effect was even more pronounced in the rop1 rop6 double mutant. These results indicate that ROP1 and ROP6 negatively regulate seed germination [67]. Similarly, ROP9 also negatively impacts germination, as transgenic seeds with silenced ROP9 expression germinated faster than the wild type [68]. In contrast, ROP2 acts as a positive regulator of seed germination. Seeds overexpressing a constitutively active ROP2 mutant (CA-rop2) germinated faster than wild-type seeds, while those overexpressing a dominant-negative ROP2 mutant (DN-rop2) showed delayed germination [69]. A similar phenotype has been observed in Arabidopsis seeds overexpressing MaROP5g from Musa acuminata [70]. The inconsistent effects of ROPs on seed germination may be attributed to their influence on seed dormancy. For example, the effects of CA-rop2, DN-rop2, and ROP9-silenced transgenic seeds were eliminated after cold treatment [68,69]. Notably, the influence on seed germination by some ROPs merely accelerates the germination, without compromising seed viability. This is evidenced by the consistent germination observed when the germination period is extended [67,68,69,71]. Additionally, recent studies have shown no discernible differences in seed germination for rop10, rop11, DN-rop10, DN-rop11, and seeds overexpressing CsRAC1 (a ROP homolog in Camellia sinensis) compared to their respective wild types [71,72,73].

Table 1.
Roles of ROPs and physically interactive proteins in seed germination.
During seed germination, ABA signaling plays a crucial role in regulating seed germination and stress tolerance [79]. Most ROPs (ROP2, ROP10, and ROP11) consistently exhibit negative roles in ABA responsiveness during seed germination. Under ABA treatment, seeds of rop mutants or those overexpressing inactive ROPs display enhanced inhibition of germination [69,71,72,74]. Additionally, ROP10 has been shown to negatively regulate responsiveness to salt and drought (mannitol) during seed germination [74]. Consistent with this, reduced sensitivity to salt was found in Arabidopsis seeds overexpressing MaROP5g from Musa acuminata [70], highlighting a similar role for this ROP homolog. However, ROP9 from Arabidopsis and CsRAC1 from Camellia sinensis exhibit the opposite effect: silencing ROP9 reduces sensitivity, while overexpression of CsRAC1 enhances ABA, salt (NaCl), and drought sensitivity in Arabidopsis seeds [68,73]. Notably, CsRAC1 is the ortholog of Arabidopsis ROP3, whose role in seed germination remains uncharacterized. Whether this discrepancy is due to functional or evolutionary divergence of ROP proteins awaits further investigation.
5.2. ROP Signaling Regulates ABA Responsiveness
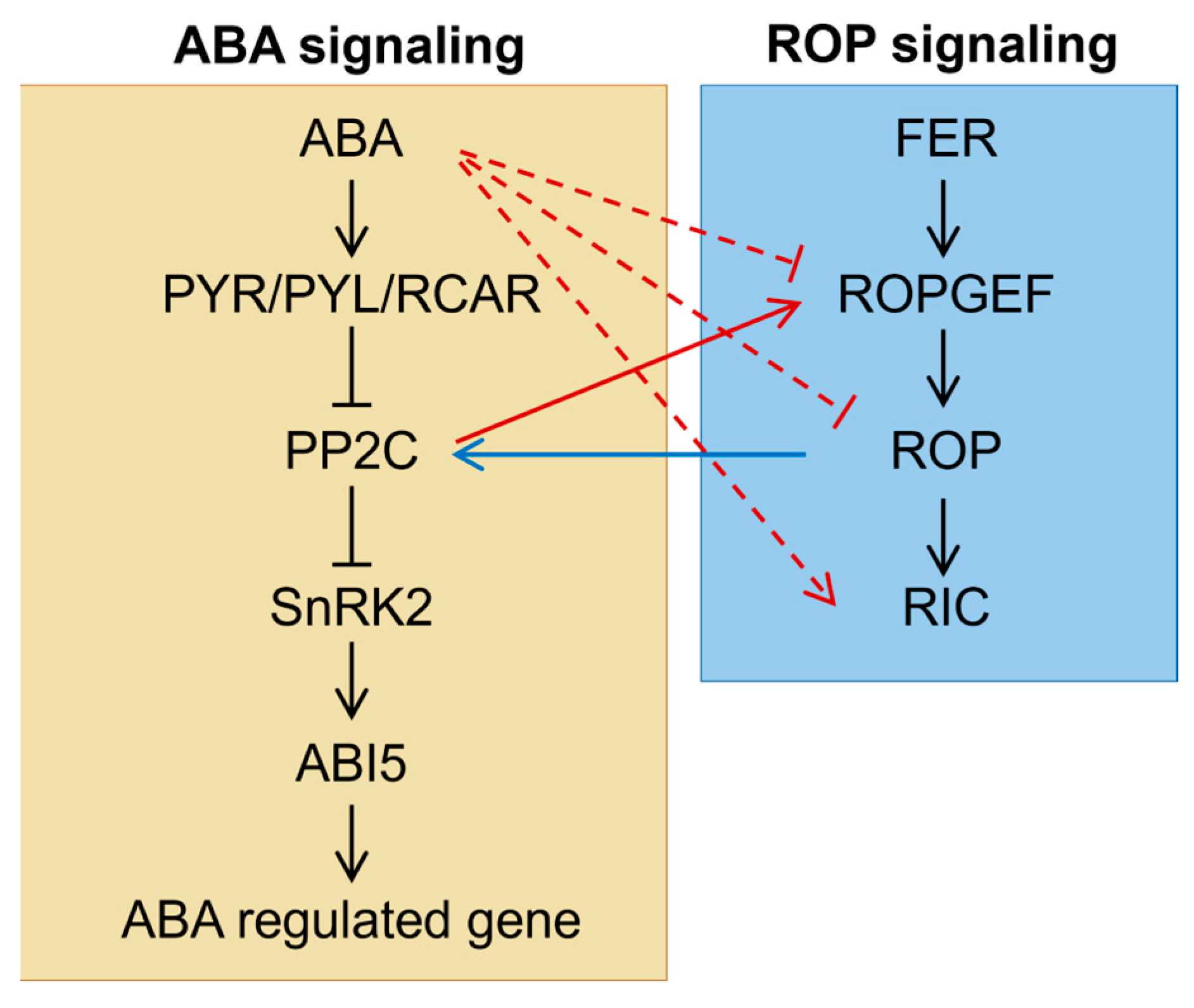
Numerous studies have demonstrated that ROP signaling negatively regulates ABA responsiveness during seed germination. Conversely, ABA also negatively regulates ROP signaling, thereby forming a complex feedback loop (Figure 2). The interaction between ROPs and PP2Cs (protein phosphatase 2Cs) is a well-established mechanism by which ROP signaling modulates ABA responsiveness. ABI1 and ABI2 are two major PP2Cs that negatively regulate downstream ABA signaling by dephosphorylating SnRK2 kinases. A recent study has revealed that ROP11 interacts with ABI1, competing with the binding of the ABA receptor RCAR1/PYL9 to ABI1 and thereby negatively regulating downstream ABA signaling [80]. Similarly, ROP11 was also shown to interact with ABI2 [75], further highlighting the central role of these interactions in modulating ABA responsiveness.
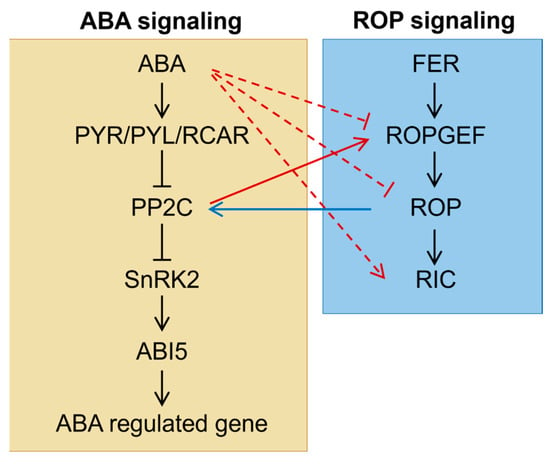
Figure 2.
Crosstalk between ABA and ROP signaling pathways during seed germination under abiotic stress. The solid lines represent direct effects, while the dashed lines indicate indirect effects, the underlying mechanisms of which are still unclear. The blue lines illustrate the regulation of the ABA pathway by ROP signaling, and the red lines depict the influence of ABA signaling on the ROP pathway. The interactions depicted are also described in the main text.
Since DN-rop11 exhibited impaired interaction with ABI1 while CA-rop11 maintained comparable interaction [80], it suggests that upstream regulators of ROP signaling may play a role in negatively regulating ABA responsiveness. Seeds from ROP-GEF2 knockout and silencing mutants displayed ABA hypersensitivity during germination, indicating that ROP-GEF2 is involved in modulating ABA responsiveness [77]. Moreover, ROP-GEF2 can interact with ROP2, ROP6, and ROP10 [77], with ROP10 functionally redundant with ROP11 in ABA response [71]. In addition, ROP-GEF1, ROP-GEF4, ROP-GEF10, and ROP-GEF14 likely act at this regulatory level, as mutants of gef1gef4, gef1gef4gef10, and gef1gef4gef10gef14 exhibited enhanced ABA inhibition during seed germination [75,76]. Furthermore, FERONIA (FER), a receptor-like kinase that directly regulates ROPGEFs [81], may act upstream of ROPGEFs. Consistent with this, fer mutants also displayed enhanced ABA sensitivity [75].
5.3. ABA Regulates ROP Signaling
On the other hand, ABA regulates ROP signaling through multiple pathways (Figure 2). One pathway involves downregulating the expression of ROPs. For example, ROP10 is preferentially expressed in the radicle, and a GUS reporter system driven by the ROP10 promoter showed reduced activity in the presence of ABA, indicating that ABA transcriptionally represses ROP10 [72]. A similar inhibitory effect was also observed for the ROP9 promoter [68]. Interestingly, the ABA inhibition of ROP10 transcription was not detected in tissues other than radicles [72]. Moreover, other plant hormones do not affect ROP10 transcription [68,72]. In contrast, ABA has no effect on the transcription or protein abundance of ROP11 [71]. These findings suggest that the effect of ABA on ROP transcription may be specific in hormones, tissues, and ROP proteins. In contrast, expressions of most ROP effector RICs were induced by ABA and auxin [78], implying that ABA exerts differential hierarchical regulation on ROP signaling pathway.
The solid lines represent direct effects, while the dashed lines indicate indirect effects, the underlying mechanisms of which are still unclear. The blue lines illustrate the regulation of the ABA pathway by ROP signaling, and the red lines depict the influence of ABA signaling on the ROP pathway. The interactions depicted are also described in the main text.
Apart from the regulation of ROP transcription, ABA also modulates the activity of ROP proteins. Recent studies indicated that ABA promotes ROP-GEF2 degradation via the ubiquitin-26S proteosome system [77], thereby inhibiting the conversion of ROP proteins from the GDP-bound to the GTP-bound status. In addition, ABA also promotes ROP-GEF1 proteolysis but through the vacuolar degradation system [76]. These findings align with the observation that ABA reduces the activity of ROP6/AtRAC1 [82]. However, the finding that the binding of ROP-GEF2 to ROP proteins (ROP2, ROP6, ROP10) alleviated ABA-induced ROP-GEF2 degradation [77] suggests a competitive relationship between ROP expression and ABA signaling. Similar protection of ROP-GEF1 from degradation were also detected due to the interaction with PP2Cs [76].
ABA may also modulate the subcellular localization of ROP signaling components. Direct evidence comes from the observation that ABA promoted the accumulation of GFP-CA-rop11 in the nucleus, whereas GFP-CA-rop11 normally localized at the plasma membrane in the absence of ABA [71]. Given the dual localization of ROP11 in the nucleus and plasma membrane, whether this localization regulation of ABA is related to its effect on ROP activation through ROP-GEF degradation remains to be further explored. However, for the degradation of ROP-GEF1, 4, 10, 12, and 14, ABA indeed promotes their degradation by targeting them from the plasma membrane to prevacuolar compartments [76].
5.4. Other Regulation of ROPs in Response to Abiotic Stress
In addition to modulating ABA signaling, the ROP2-RIC1 module may regulate plant responses to abiotic stresses during seed germination by influencing microtubule reorganization and reactive oxygen species (ROS) levels [83]. Specifically, activated ROP2 promotes the dissociation of RIC1 from microtubules, thereby facilitating microtubule reassembly. The movement and positioning of CSCs along microtubules are thus altered, further influencing oriented deposition of cellulose microfibrils in the cell wall [84,85]. By regulating cell wall rigidity, this process may control intracellular water loss [86,87] and consequently enhance plant survival under high salinity conditions. For example, overexpression of NtRop1 from Nicotiana tabacum has been shown to increase H2O2 production, leading to enhanced salt sensitivity in transgenic plants [88]. This suggests that ROPs may regulate ROS levels through downstream effectors like respiratory burst oxidase homologs (Rbohs), thereby influencing plant stress tolerance [89].
6. Perspectives
As a class of plant-specific proteins, ROP signaling plays an important and differentiated regulatory role in seed germination. This is not only reflected in its influence on seed dormancy and germination but also in its response to abiotic stress. Although crosstalk between ROP signaling and ABA signaling during seed germination have been partly understood, research in many other aspects remains limited. Future work is still needed to elucidate how environmental stimuli activate ROP signaling during seed germination in response to abiotic stresses, the dependence of ROP signaling on ABA signaling pathway, the regulatory mechanisms of crosstalk between the two signaling pathways at various levels, and how downstream signaling of ROP regulates plant adaptive responses.
Current results have already hinted that FER may act as the most upstream receptor of ROP signaling, sensing environmental stimuli. However, which abiotic stresses can activate the downstream ROP signaling remains to be further explored. Moreover, ROP also plays a role in seed dormancy [69]. How ROP signaling is weakened after cold treatment has not been studied. Further examination of expression and active states of ROPs would benefit the understanding. Downstream of FER receptor, whether ROP-GEF activation by FER and ROP activation by ROP-GEF are selective and how these interactions are determined also need further genetic evidence to support. Although the active state of ROP affects downstream regulation of seed germination [80], no mutants related to ROP-GAPs or ROP-GDIs have been identified yet in this process. How ROP-GAP and ROP-GDI are specifically activated to balance the active state of ROP remains to be explored.
ABA signaling plays an important role in both seed germination and abiotic stress responses. ROP signaling interplays with ABA signaling at multiple levels. However, the limited research cannot distinguish well whether ROP signaling can regulate seed germination independently of ABA signaling. Although the effects of ROP signaling on germination have been explored under some ABA signaling pathway mutations (e.g., PP2C mutants) [72,76], it is still necessary to investigate the dependence of ROP signaling on ABA signaling in response to abiotic stress using higher-order mutants that completely block ABA signaling.
Regarding the crosstalk between ROP and ABA signaling pathways, it is questionable whether the binding of ROP11 to PP2Cs is universal. Detailed investigation into the interactions between other ROPs and PP2Cs, as well as the genetic proof supporting their involvement in this process, are still insufficient. Moreover, ROP9, which positively regulates ABA responses [68], likely employs different regulatory mechanisms and affects ABA signaling. Conversely, the influence of ABA signaling on ROP signaling is even more uncertain. Although some ROP promoters contain cis-acting elements responsive to ABA [68], it is still unclear whether ABA directly downregulates ROP transcription. Additionally, ABA induced the opposite change in RIC transcription [78], the biological significance of which remains in mystery. Even the relatively clear effect of ABA on ROP-GEF degradation [76,77] has not yet identified the specific regulatory components. The genetic evidence supporting the impact of ROP-GEF degradation on downstream ROP activity in response to ABA induction also awaits further investigation.
Activated ROP signaling exerts various biological functions by binding to a series of downstream effectors. Currently, RIC1 and RIC4 are the only effectors reported to be involved in abiotic stress responses during seed germination [67,78]. RIC1 may regulate plant salt tolerance through the rearrangement of microtubules [83]. Moreover, ROP may regulate the level of reactive oxygen species (ROS) within cells through the effector Rboh [89], thereby affecting seed germination and stress responses, but this still needs to be verified. Whether other ROP effectors are involved awaits further investigation.
Manipulating seed germination can considerably enhance plant adaptability to environmental changes and promote growth. For example, transgenic Arabidopsis overexpressing CsRAC1 exhibits remarkable salt tolerance and drought resistance [73]. Based on current research, several targets within ROP signaling pathways have been identified and evaluated for their potential to improve stress resistance through breeding. However, the growth of plants following germination must be carefully assessed. For instance, while CA-rop11 is transgenic and Arabidopsis shows enhanced seed germination under ABA treatment, its mature plants exhibit poorer growth performance compared to the wild type [71]. Despite these challenges, further elucidation of the regulatory roles and molecular mechanisms of ROP signaling in seed germination will provide robust theoretical foundations and strategic recommendations for addressing environmental changes and improving crop stress resistance.
7. Conclusions
An increasing number of studies have uncovered the involvement of ROP signaling in the response to abiotic stress during seed germination. However, research in this area remains very limited, with the current knowledge still focusing on the crosstalk between ROP and ABA signaling pathways. The relatively clear regulations are the protection of ROPGEFs in the ROP signaling from degradation by PP2Cs in the ABA signaling, and the maintenance of PP2C activity through competitive binding of ROPs to PP2Cs. The establishment of the detailed regulatory model of seed germination by ROP signaling under abiotic stress awaits in-depth exploration in the future.
Author Contributions
The draft was written by M.Z. and L.Z. The manuscript was reviewed and edited by M.X., Q.L. and L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Southwest University and Fuling Academy Joint Construction Project (FLYJY202401), and the Fundamental Research Funds for the Central Universities (Grant No. XDJK2020B040).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- He, M.; He, C.-Q.; Ding, N.-Z. Abiotic Stresses: General Defenses of Land Plants and Chances for Engineering Multistress Tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, K.K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Ma, L.; Li, J.; Hou, D.; Zeng, B.; Zhang, L.; Liu, C.; Bi, Q.; Tan, J.; Yu, X.; et al. Factors influencing seed dormancy and germination and advances in seed priming technology. Plants 2024, 13, 1319. [Google Scholar] [CrossRef]
- Klupczyńska, E.A.; Pawłowski, T.A. Regulation of seed dormancy and germination mechanisms in a changing environment. Int. J. Mol. Sci. 2021, 22, 1357. [Google Scholar] [CrossRef]
- Nanda, A.K.; El Habti, A.; Hocart, C.H.; Masle, J.; Leubner, G. ERECTA receptor-kinases play a key role in the appropriate timing of seed germination under changing salinity. J. Exp. Bot. 2019, 70, 6417–6435. [Google Scholar] [CrossRef]
- Footitt, S.; Clewes, R.; Feeney, M.; Finch-Savage, W.E.; Frigerio, L. Aquaporins influence seed dormancy and germination in response to stress. Plant Cell Environ. 2019, 42, 2325–2339. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Yadav, S.K. Cold stress tolerance mechanisms in plants. A review. Agron. Sustain. Dev. 2010, 30, 515–527. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Olvera-Carrillo, Y.; Garciarrubio, A.; Campos, F.; Covarrubias, A.A. The enigmatic LEA proteins and other hydrophilins. Plant Physiol. 2008, 148, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Shu, K.; Zhou, W.; Chen, F.; Luo, X.; Yang, W. Abscisic Acid and Gibberellins Antagonistically Mediate Plant Development and Abiotic Stress Responses. Front. Plant Sci. 2018, 9, 416. [Google Scholar] [CrossRef]
- Sajeev, N.; Koornneef, M.; Bentsink, L. A commitment for life: Decades of unraveling the molecular mechanisms behind seed dormancy and germination. Plant Cell 2024, 36, 1358–1376. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic Stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef]
- Wang, Z.-Z.; Cao, M.-J.; Yan, J.; Dong, J.; Chen, M.-X.; Yang, J.-F.; Li, J.-H.; Ying, R.-N.; Gao, Y.-Y.; Li, L.; et al. Stabilization of dimeric PYR/PYL/RCAR family members relieves abscisic acid-induced inhibition of seed germination. Nat. Commun. 2024, 15, 8077. [Google Scholar] [CrossRef]
- Née, G.; Kramer, K.; Nakabayashi, K.; Yuan, B.; Xiang, Y.; Miatton, E.; Finkemeier, I.; Soppe, W.J.J. DELAY OF GERMINATION1 requires PP2C phosphatases of the ABA signalling pathway to control seed dormancy. Nat. Commun. 2017, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Skubacz, A.; Daszkowska-Golec, A.; Szarejko, I. The role and regulation of ABI5 (ABA-Insensitive 5) in plant development, abiotic stress responses and phytohormone crosstalk. Front. Plant Sci. 2016, 7, 1884. [Google Scholar] [CrossRef] [PubMed]
- Galland, M.; Huguet, R.; Arc, E.; Cueff, G.; Job, D.; Rajjou, L. Dynamic proteomics emphasizes the importance of selective mRNA translation and protein turnover during Arabidopsis seed germination. Mol. Cell. Proteom. 2014, 13, 252–268. [Google Scholar] [CrossRef]
- Shi, B.; Wang, J.; Gao, H.; Yang, Q.; Wang, Y.; Day, B.; Ma, Q. The small GTP-binding protein TaRop10 interacts with TaTrxh9 and functions as a negative regulator of wheat resistance against the stripe rust. Plant Sci. 2021, 309, 110937. [Google Scholar] [CrossRef]
- Ganotra, J.; Sharma, B.; Biswal, B.; Bhardwaj, D.; Tuteja, N. Emerging role of small GTPases and their interactome in plants to combat abiotic and biotic stress. Protoplasma 2022, 260, 1007–1029. [Google Scholar] [CrossRef]
- Shu, K.; Meng, Y.J.; Shuai, H.W.; Liu, W.G.; Du, J.B.; Liu, J.; Yang, W.Y.; Weber, A. Dormancy and germination: How does the crop seed decide? Plant Biol. 2015, 17, 1104–1112. [Google Scholar] [CrossRef]
- Tuan, P.A.; Kumar, R.; Rehal, P.K.; Toora, P.K.; Ayele, B.T. Molecular mechanisms underlying abscisic acid/gibberellin balance in the control of seed dormancy and germination in cereals. Front. Plant Sci. 2018, 9, 668. [Google Scholar] [CrossRef]
- Toora, P.K.; Tuan, P.A.; Nguyen, T.-N.; Badea, A.; Ayele, B.T. Modulation in the ratio of abscisic acid to gibberellin level determines genetic variation of seed dormancy in barley (Hordeum vulgare L.). J. Plant Physiol. 2024, 301, 154301. [Google Scholar] [CrossRef] [PubMed]
- Laspina, N.V.; Batlla, D.; Benech-Arnold, R.L.; Penfield, S. Dormancy cycling is accompanied by changes in ABA sensitivity in polygonum aviculare seeds. J. Exp. Bot. 2020, 71, 5924–5934. [Google Scholar] [CrossRef]
- Nonogaki, H. Seed dormancy and germination emerging mechanisms and new hypotheses. Front. Plant Sci. 2014, 5, 233. [Google Scholar] [CrossRef]
- Jhanji, S.; Goyal, E.; Chumber, M.; Kaur, G. Exploring fine tuning between phytohormones and ROS signaling cascade in regulation of seed dormancy, germination and seedling development. Plant Physiol. Biochem. 2024, 207, 108352. [Google Scholar] [CrossRef] [PubMed]
- Chiu, R.S.; Saleh, Y.; Gazzarrini, S. Inhibition of FUSCA3 degradation at high temperature is dependent on ABA signaling and is regulated by the ABA/GA ratio. Plant Signal. Behav. 2016, 11, e1247137–e1247141. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Steinbrecher, T.; Leubner-Metzger, G. Tissue and cellular mechanics of seeds. Curr. Opin. Genet. Dev. 2018, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ishibashi, Y.; Kasa, S.; Sakamoto, M.; Aoki, N.; Kai, K.; Yuasa, T.; Hanada, A.; Yamaguchi, S.; Iwaya-Inoue, M. A role for reactive oxygen species produced by NADPH oxidases in the embryo and aleurone cells in barley seed germination. PLoS ONE 2015, 10, e0143173. [Google Scholar] [CrossRef]
- Ischebeck, T.; Werner, S.; Krishnamoorthy, P.; Lerche, J.; Meijón, M.; Stenzel, I.; Löfke, C.; Wiessner, T.; Im, Y.J.; Perera, I.Y.; et al. Phosphatidylinositol 4,5-bisphosphate influences PIN polarization by controlling clathrin-mediated membrane trafficking in Arabidopsis. Plant Cell 2013, 25, 4894–4911. [Google Scholar] [CrossRef]
- Zhong, C.; Xu, H.; Ye, S.; Wang, S.; Li, L.; Zhang, S.; Wang, X. AtGASA6 serves as an integrator of gibberellin-, abscisic acid- and glucose-signaling during seed germination in Arabidopsis. Plant Physiol. 2015, 169, 15–00858. [Google Scholar] [CrossRef]
- Hu, C.-C.; Wu, C.-Y.; Yang, M.-Y.; Huang, J.-Z.; Wu, C.-W.; Hong, C.-Y. Catalase associated with antagonistic changes of abscisic acid and gibberellin response, biosynthesis and catabolism is involved in eugenol-inhibited seed germination in rice. Plant Cell Rep. 2023, 43, 10. [Google Scholar] [CrossRef]
- Steinbrecher, T.; Leubner-Metzger, G. The biomechanics of seed germination. J. Exp. Bot. 2016, 68, 765–783. [Google Scholar] [CrossRef]
- Sechet, J.; Frey, A.; Effroy-Cuzzi, D.; Berger, A.; Perreau, F.; Cueff, G.; Charif, D.; Rajjou, L.; Mouille, G.; North, H.M.; et al. Xyloglucan metabolism differentially impacts the cell wall characteristics of the endosperm and embryo during Arabidopsis seed germination. Plant Physiol. 2016, 170, 1367–1380. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, Y.; Guo, H. To curve for survival: Apical hook development. J. Integr. Plant Biol. 2023, 65, 324–342. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Vandenbussche, F.; Van Der Straeten, D. Regulation of seedling growth by ethylene and the ethylene-auxin crosstalk. Planta 2017, 245, 467–489. [Google Scholar] [CrossRef] [PubMed]
- Lorrai, R.; Erguvan, Ö.; Raggi, S.; Jonsson, K.; Široká, J.; Tarkowská, D.; Novák, O.; Griffiths, J.; Jones, A.M.; Verger, S.; et al. Cell wall integrity modulates HOOKLESS1 and PHYTOCHROME INTERACTING FACTOR4 expression controlling apical hook formation. Plant Physiol. 2024, 196, 1562–1578. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, H. On hormonal regulation of the dynamic apical hook development. New Phytol. 2019, 222, 1230–1234. [Google Scholar] [CrossRef]
- Shu, K.; Qi, Y.; Chen, F.; Meng, Y.; Luo, X.; Shuai, H.; Zhou, W.; Ding, J.; Du, J.; Liu, J.; et al. Salt stress represses soybean seed germination by negatively regulating GA biosynthesis while positively mediating ABA biosynthesis. Front. Plant Sci. 2017, 8, 1372. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Kai, W.; Zhao, B.; Chen, P.; Sun, L.; Ji, K.; Li, Q.; Dai, S.; Sun, Y.; et al. Transcriptional regulation of abscisic acid signal core components during cucumber seed germination and under Cu2+, Zn2+, NaCl and simulated acid rain stresses. Plant Physiol. Biochem. 2014, 76, 67–76. [Google Scholar] [CrossRef]
- Lopez-Molina, L.; Mongrand, S.; McLachlin, D.T.; Chait, B.T.; Chua, N.H. ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. Plant J. 2002, 32, 317–328. [Google Scholar] [CrossRef]
- Lin, P.-C.; Hwang, S.-G.; Endo, A.; Okamoto, M.; Koshiba, T.; Cheng, W.-H. Ectopic expression of ABSCISIC ACID 2/GLUCOSE INSENSITIVE 1 in Arabidopsis promotes seed dormancy and stress tolerance. Plant Physiol. 2007, 143, 745–758. [Google Scholar] [CrossRef]
- Saavedra, X.; Modrego, A.; Rodriguez, D.; Gonzalez-Garcia, M.P.; Sanz, L.; Nicolas, G.; Lorenzo, O. The nuclear interactor PYL8/RCAR3 of Fagus sylvatica FsPP2C1 is a positive regulator of abscisic acid signaling in seeds and stress. Plant Physiol. 2010, 152, 133–150. [Google Scholar] [CrossRef]
- Yuan, K.; Rashotte, A.M.; Wysocka-Diller, J.W. ABA and GA signaling pathways interact and regulate seed germination and seedling development under salt stress. Acta Physiol. Plant. 2010, 33, 261–271. [Google Scholar] [CrossRef]
- Verslues, P.E.; Bray, E.A. Role of abscisic acid (ABA) and Arabidopsis thaliana ABA-insensitive loci in low water potential-induced ABA and proline accumulation. J. Exp. Bot. 2006, 57, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Toh, S.; Imamura, A.; Watanabe, A.; Nakabayashi, K.; Okamoto, M.; Jikumaru, Y.; Hanada, A.; Aso, Y.; Ishiyama, K.; Tamura, N.; et al. High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiol. 2008, 146, 1368–1385. [Google Scholar] [CrossRef] [PubMed]
- Tamura, N.; Yoshida, T.; Tanaka, A.; Sasaki, R.; Bando, A.; Toh, S.; Lepiniec, L.; Kawakami, N. Isolation and characterization of high temperature-resistant germination mutants of Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 1081–1094. [Google Scholar] [CrossRef]
- Lim, S.; Park, J.; Lee, N.; Jeong, J.; Toh, S.; Watanabe, A.; Kim, J.; Kang, H.; Kim, D.H.; Kawakami, N.; et al. ABA-insensitive3, ABA-insensitive5, and DELLAs Interact to activate the expression of SOMNUS and other high-temperature-inducible genes in imbibed seeds in Arabidopsis. Plant Cell 2013, 25, 4863–4878. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, N.; Liu, R.; Chen, M.; Zhang, J. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J. Exp. Bot. 2010, 61, 2979–2990. [Google Scholar] [CrossRef]
- Zhu, J.-K. Salt and Drought Stress Signal Transduction in Plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Boureux, A.; Vignal, E.; Faure, S.; Fort, P. Evolution of the Rho family of Ras-Like GTPases in eukaryotes. Mol. Biol. Evol. 2007, 24, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Yalovsky, S.; Bloch, D.; Sorek, N.; Kost, B. Regulation of membrane trafficking, cytoskeleton dynamics, and cell polarity by ROP/RAC GTPases. Plant Physiol. 2008, 147, 1527–1543. [Google Scholar] [CrossRef]
- Feiguelman, G.; Fu, Y.; Yalovsky, S. ROP GTPases structure-function and signaling pathways. Plant Physiol. 2018, 176, 57–79. [Google Scholar] [CrossRef]
- Kosami, K.-i.; Ohki, I.; Nagano, M.; Furuita, K.; Sugiki, T.; Kawano, Y.; Kawasaki, T.; Fujiwara, T.; Nakagawa, A.; Shimamoto, K.; et al. The Crystal Structure of the Plant Small GTPase OsRac1 Reveals Its Mode of Binding to NADPH Oxidase. J. Biol. Chem. 2014, 289, 28569–28578. [Google Scholar] [CrossRef]
- Yalovsky, S. Protein lipid modifications and the regulation of ROP GTPase function. J. Exp. Bot. 2015, 66, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Sorek, N.; Gutman, O.; Bar, E.; Abu-Abied, M.; Feng, X.; Running, M.P.; Lewinsohn, E.; Ori, N.; Sadot, E.; Henis, Y.I.; et al. Differential effects of prenylation and s-acylation on type I and II ROPS membrane interaction and function. Plant Physiol. 2011, 155, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Araya, T.; von Wirén, N.; Takahashi, H. CLE peptide signaling and nitrogen interactions in plant root development. Plant Mol. Biol. 2016, 91, 607–615. [Google Scholar] [CrossRef]
- Fehér, A.; Lajkó, D.B. Signals fly when kinases meet Rho-of-plants (ROP) small G-proteins. Plant Sci. 2015, 237, 93–107. [Google Scholar] [CrossRef]
- Wu, G.; Gu, Y.; Li, S.; Yang, Z. A genome-wide analysis of Arabidopsis Rop-interactive CRIB motif–containing proteins that act as Rop GTPase targets. Plant Cell 2001, 13, 2841–2856. [Google Scholar] [CrossRef]
- Engelhardt, S.; Trutzenberg, A.; Hückelhoven, R. Regulation and Functions of ROP GTPases in Plant–Microbe Interactions. Cells 2020, 9, 2016. [Google Scholar] [CrossRef]
- Venus, Y.; Oelmüller, R. Arabidopsis ROP1 and ROP6 influence germination time, root morphology, the formation of F-actin bundles, and symbiotic fungal interactions. Mol. Plant 2013, 6, 872–886. [Google Scholar] [CrossRef] [PubMed]
- Nibau, C.; Tao, L.; Levasseur, K.; Wu, H.-M.; Cheung, A.Y. The Arabidopsis small GTPase AtRAC7/ROP9 is a modulator of auxin and abscisic acid signalling. J. Exp. Bot. 2013, 64, 3425–3437. [Google Scholar] [CrossRef]
- Li, H.; Shen, J.-J.; Zheng, Z.-L.; Lin, Y.; Yang, Z. The Rop GTPase switch controls multiple developmental processes in Arabidopsis. Plant Physiol. 2001, 126, 670–684. [Google Scholar] [CrossRef]
- Miao, H.; Sun, P.; Liu, J.; Wang, J.; Xu, B.; Jin, Z. Overexpression of a novel ROP gene from the banana (MaROP5g) confers increased salt stress tolerance. Int. J. Mol. Sci. 2018, 19, 3108. [Google Scholar] [CrossRef]
- Li, Z.; Kang, J.; Sui, N.; Liu, D. ROP11 GTPase is a negative regulator of multiple ABA responses in Arabidopsis. J. Integr. Plant Biol. 2012, 54, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-L.; Nafisi, M.; Tam, A.; Li, H.; Crowell, D.N.; Chary, S.N.; Schroeder, J.I.; Shen, J.; Yang, Z. Plasma membrane-associated ROP10 small GTPase is a specific negative regulator of abscisic acid responses in Arabidopsis. Plant Cell 2002, 14, 2787–2797. [Google Scholar] [CrossRef]
- Xu, X.; Ye, X.; Xing, A.; Wu, Z.; Li, X.; Shu, Z.; Wang, Y. Camellia sinensis small GTPase gene (CsRAC1) involves in response to salt stress, drought stress and ABA signaling pathway. Gene 2022, 821, 146318. [Google Scholar] [CrossRef]
- Xin, Z.; Zhao, Y.; Zheng, Z.-L. Transcriptome analysis reveals specific modulation of abscisic acid signaling by ROP10 small GTPase in Arabidopsis. Plant Physiol. 2005, 139, 1350–1365. [Google Scholar] [CrossRef]
- Yu, F.; Qian, L.; Nibau, C.; Duan, Q.; Kita, D.; Levasseur, K.; Li, X.; Lu, C.; Li, H.; Hou, C.; et al. FERONIA receptor kinase pathway suppresses abscisic acid signaling in Arabidopsis by activating ABI2 phosphatase. Proc. Natl. Acad. Sci. USA 2012, 109, 14693–14698. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Waadt, R.; Schroeder, J.I. Release of GTP exchange factor mediated down-regulation of abscisic acid signal transduction through ABA-induced rapid degradation of RopGEFs. PLoS Biol. 2016, 14, e1002461. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, Y.; He, Y.; Wang, Y.; Xiao, J.; Li, L.; Wang, Y.; Chen, X.; Xiong, W.; Wu, Y. RopGEF2 is involved in ABA-suppression of seed germination and post-germination growth of Arabidopsis. Plant J. 2015, 84, 886–899. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, Y.; Kim, S.Y.; Lee, Y.; Hwang, J.U. Arabidopsis ROP-interactive CRIB motif-containing protein 1 (RIC1) positively regulates auxin signalling and negatively regulates abscisic acid (ABA) signalling during root development. Plant Cell Environ. 2013, 36, 945–955. [Google Scholar] [CrossRef]
- Huang, G.-T.; Ma, S.-L.; Bai, L.-P.; Zhang, L.; Ma, H.; Jia, P.; Liu, J.; Zhong, M.; Guo, Z.-F. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 2011, 39, 969–987. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Gao, X.; Chinnusamy, V.; Bressan, R.; Wang, Z.X.; Zhu, J.K.; Wu, J.W.; Liu, D. ROP11 GTPase negatively regulates ABA signaling by protecting ABI1 phosphatase activity from inhibition by the ABA receptor RCAR1/PYL9 in Arabidopsis. J. Integr. Plant Biol. 2012, 54, 180–188. [Google Scholar] [CrossRef]
- Tang, W.; Lin, W.; Zhou, X.; Guo, J.; Dang, X.; Li, B.; Lin, D.; Yang, Z. Mechano-transduction via the pectin-FERONIA complex activates ROP6 GTPase signaling in Arabidopsis pavement cell morphogenesis. Curr. Biol. 2022, 32, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Lemichez, E.; Wu, Y.; Sanchez, J.-P.; Mettouchi, A.; Mathur, J.; Chua, N.-H. Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev. 2001, 15, 1808–1816. [Google Scholar] [CrossRef]
- Li, C.; Lu, H.; Li, W.; Yuan, M.; Fu, Y. A ROP2-RIC1 pathway fine-tunes microtubule reorganization for salt tolerance in Arabidopsis. Plant Cell Environ. 2017, 40, 1127–1142. [Google Scholar] [CrossRef]
- Schneider, R.; Ehrhardt, D.W.; Meyerowitz, E.M.; Sampathkumar, A. Tethering of cellulose synthase to microtubules dampens mechano-induced cytoskeletal organization in Arabidopsis pavement cells. Nat. Plants 2022, 8, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lei, L.; Yingling, Y.G.; Gu, Y. Microtubules and cellulose biosynthesis: The emergence of new players. Curr. Opin. Plant Biol. 2015, 28, 76–82. [Google Scholar] [CrossRef]
- Colin, L.; Ruhnow, F.; Zhu, J.-K.; Zhao, C.; Zhao, Y.; Persson, S. The cell biology of primary cell walls during salt stress. Plant Cell 2023, 35, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, W.; Long, S.; Zhao, C. Maintenance of Cell Wall Integrity under High Salinity. Int. J. Mol. Sci. 2021, 22, 3260. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Z.; Chen, T.; Zhang, Z.; Zhang, J.; Chen, S. Overexpression of a tobacco small G protein gene NtRop1 causes salt sensitivity and hydrogen peroxide production in transgenic plants. Sci. China Ser. C Life Sci. 2008, 51, 383–390. [Google Scholar] [CrossRef]
- Wong, H.L.; Pinontoan, R.; Hayashi, K.; Tabata, R.; Yaeno, T.; Hasegawa, K.; Kojima, C.; Yoshioka, H.; Iba, K.; Kawasaki, T.; et al. Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell 2007, 19, 4022–4034. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).