Abstract
Grey oyster mushroom (Pleurotus sajor-caju (Fr.) Singer) is a popular edible mushroom in Thailand due to its high nutritional and medicinal benefits. This study aims to study the effects of temperature (100–140 °C), pressure (4–7 bar), and extraction time (20–60 min) on the extraction of crude polysaccharides with environmentally friendly pressurized hot water. The extraction conditions were optimized by the maximize yield using response surface method based on a central composite design (CCD). The temperature was the main factor affecting the increase in the extracted yield. The optimum extraction conditions were 140 °C, 10 bar, and 26.79 min, with a corresponding yield of 31.31 ± 2.55%. Under these conditions, the total phenolic content of crude polysaccharides was 401 ± 8.24 mg GAE/g dry mushroom. In addition, the total glucan content was indicated as 34.50 ± 1.79 g/100 g dry mushroom, which was separated as 32.47 ± 1.95 mg/100 g of β-glucans and 2.04 ± 0.98 mg/100 g of α-glucans.
1. Introduction
Grey oyster mushroom (Pleurotus sajor-caju (Fr.) Singer) is a popular edible mushroom worldwide due to its high nutritional value and medicinal benefits. It is a source of carbohydrates, protein, vitamins, and minerals [1]. The bioactive compounds in mushrooms, namely polysaccharides, especially of β-glucan, have been reported to possess immunomodulatory, antitumor, antiviral, wound healing, anti-obesity, and antidiabetics activities [2].
This study aims to investigate the effects of extraction temperature (100–140 °C), extraction pressure (4–7 bar), and extraction time (20–60 min) on the yield of extracting crude polysaccharides with environmentally friendly pressurized hot water [3]. This technique is regarded as a green and efficient technique to extract solid and semi-solid samples with water or other liquid solvents [3,4,5]. The extraction conditions were optimized to maximize the yield using the response surface method based on a central composite design (CCD). Under optimal conditions, the total phenolic and total glucan content of crude polysaccharides was indicated as 34.50 ± 1.79 g/100 g dry mushroom, separated as 32.47 ± 1.95 mg/100 g of β-glucans and 2.04 ± 0.98 mg/100 g of α-glucans.
2. Materials and Methods
2.1. Preparation of Sample
Grey oyster mushrooms (Pleurotus sajor-caju (Fr.) Singer) were harvested from the mushroom farm in The Institute of Biotechnology and Genetic Engineering, Chulalongkorn University. They were dried at 60 °C and stored in a desiccator at room temperature.
2.2. Extraction Method
The extraction of grey oyster mushrooms under pressurized hot water was performed in a 500 cm3 batch reactor (Parr Instrument Company, Moline, IL, USA). Dried mushrooms of approximately 10 ± 0.1000 g in weight and 300 mL distilled water were loaded into the reactor. Then, the reactor was heated according to the desired conditions, with a temperature of 100–140 °C, a pressure of 4–7 bar and an extraction time of 20–60 min. To stop the reaction, the reactor was cooled to room temperature by tap water. The obtained extract was first filtered through a filter cloth and second filtered through Whatman paper filter No. 1 and stored at 4 °C for the recovery of polysaccharides.
2.3. Recovery of Crude Polysaccharides
The recovery of crude polysaccharides was performed by means of precipitation with absolute ethanol. The extract solution was mixed with a 2-fold volume of absolute ethanol and kept overnight at 4 °C. The mixture was then centrifuged at 1000 rpm for 10 min. The precipitate was dried in an oven at 40 °C for 24 h until constant weight was observed. The recovery yield of polysaccharides was calculated by Equation (1).
% yield = (sediment weight after extract (g)/dry mushroom weight (g)) × 100
2.4. Experimental Design and Data Analysis
The influence of temperature (A), pressure (B) and time (C) on crude polysaccharides yield was investigated by the central composite design (CCD) method. The optimal conditions were obtained using the response surface method (RSM). Design-Expert® software (Stat-Ease, Inc., Minneapolis, MN, USA) was employed to design the experiment and to carry out the analysis of variance (ANOVA), regression model, and statistical analysis.
3. Results and Discussion
3.1. Effect of Factors on the Extraction Yield
Table 1 shows the analysis of variance (ANOVA); temperature and time wereobserved as the significant factors in the extraction yield due to a p-value less than 0.0001. In addition, the interactions of temperature and pressure (AB) and pressure and time (BC) were also presented regarding their effect on the yield. As shown in Figure 1, the % yield increased with the increase in temperature and pressure. However, it dramatically decreased with a long extraction time. This is due to the degradation of polysaccharides [6,7].

Table 1.
The analysis of variance (ANOVA) of the effect of reaction conditions on the extraction yield.
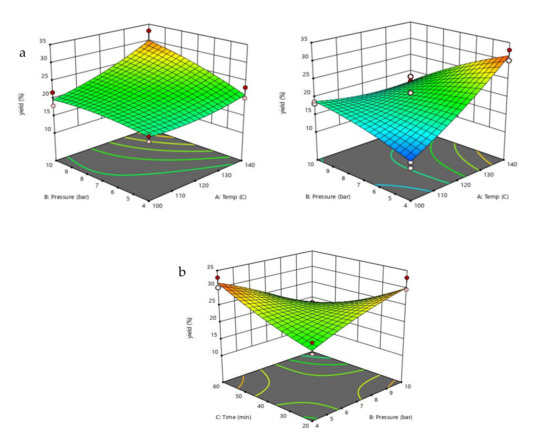
Figure 1.
(a) Effect of the interaction of temperature and pressure and (b) effect of the interaction of pressure and time on extraction yield. A is temperature in term of coded unit, B is pressure in term of coded unit, and C is time in term of coded unit.
3.2. Optimal Conditions for the Highest Yield
The optimum conditions obtained were implemented with the assistance of the optimization function embedded in the Design-Expert® software as shown in Table 2. Under these optimal conditions, a predicted % yield of 27.3307 was obtained. The experimental runs under the suggested optimal conditions in triplicate provided the actual value of 31.31 ± 2.55. As β-glucan is an important bioactive compound in mushrooms, the total glucan content was also analyzed using the mushroom and yeast beta glucan assay kit (Megazyme International, Wicklow, Ireland). The total glucan content from the obtained polysaccharide was indicated as 34.50 ± 1.79 g/100 g dry mushroom, which was separated as 32.47 ± 1.95 mg/100 g of β-glucans and 2.04 ± 0.98 mg/100 g of α-glucans.

Table 2.
Optimal conditions for SCGs extraction with pressurized hot water and the predicted and the actual values of the response.
4. Conclusions
Crude polysaccharides of grey oyster mushrooms were successfully extracted by pressurized hot water. The temperature was the main factor affecting the increase in the extracted yield. The optimum extraction conditions were 140 °C, 10 bar, and 26.79 min with a corresponding yield of 31.31 ± 2.55%. Under these conditions, the total phenolic content of crude polysaccharides was 401 ± 8.24 mg GAE/g dry mushroom. In addition, the total glucan content was indicated as 34.50 ± 1.79 g/100 g dried mushroom, which was separated as 32.47 ± 1.95 mg/100 g of β-glucans and 2.04 ± 0.98 mg/100 g of α-glucans.
Author Contributions
Conceptualization, W.S. and R.S.; methodology, W.S., P.A. and S.P.; validation, W.S. and R.S.; formal analysis, W.S., P.A. and S.P.; investigation, W.S., P.A. and S.P.; resources, W.S.; data curation, P.A. and S.P.; writing—original draft preparation, W.S. writing—review and editing, W.S. and R.S.; visualization, W.S. and R.S.; supervision, W.S. and R.S.; project administration, W.S.; funding acquisition, W.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the School of Food Industry, King Mongkut’s Institute of Technology Ladkrabang [Grant number 2564-02-07-004].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The lab equipment was facilitated by the Future Food Lab of Food Innopolis at King Mongkut’s Institute of Technology Ladkrabang.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eswaran, A.; Ramabadran, R. Studies on some physiological, cultural and postharvest aspects of oyster mushroom, Pleurotus eous (Berk.) sacc. Trop. Agric. Res. 2000, 12, 360–374. [Google Scholar]
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Dilshad, S.M.R. Beta Glucan: A Valuable Functional Ingredient in Foods. Crit. Rev. Food Sci. Nutr. 2012, 52, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Pressurized hot water extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.F.; Kim, S.M.; Lee, W.J.; Um, B.-H. Pressurized liquid method for fucoxanthin extraction from Eisenia bicyclis (Kjellman) Setchell. J. Biosci. Bioeng. 2011, 111, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Tena, M.T. Extraction|Pressurized Liquid Extraction. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2019; pp. 78–83. [Google Scholar] [CrossRef]
- Zou, Y.; Jiang, A.; Tian, M. Extraction optimization of antioxidant polysaccharides from Auricularia auricula fruiting bodies. Food Sci. Technol. 2015, 35, 428–433. [Google Scholar] [CrossRef][Green Version]
- Zou, Y.; Xie, C.; Fan, G.; Gu, Z.; Han, Y. Optimization of ultrasound-assisted extraction of melanin from Auricularia auricula fruit bodies. Innov. Food Sci. Emerg. Technol. 2010, 11, 611–615. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).