Optimization of Polysaccharides Extraction from Spent Coffee Grounds (SCGs) by Pressurized Hot Water Extraction †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of SCGs Sample
2.2. Pressurized Hot Water Extraction
2.3. Recovery of Polysaccharides
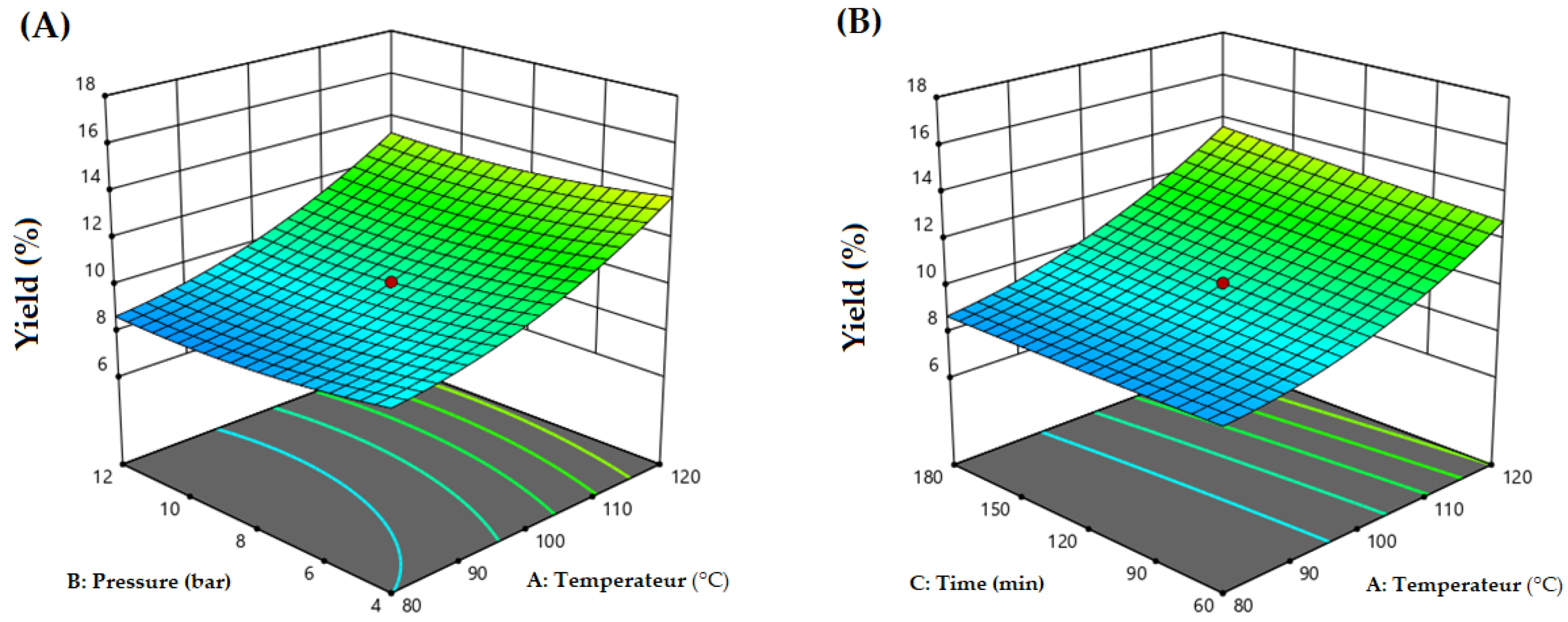
2.4. Experimental Design and Data Analysis
2.5. Determination of Phenolic Compounds
3. Results and Discussion
3.1. Data Analysis and Response Surface Model Building
3.2. Process Optimization by RSM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burniol-Figols, A.; Cenian, K.; Skiadas, I.V.; Gavala, H.N. Integration of chlorogenic acid recovery and bioethanol production from spent coffee grounds. Biochem. Eng. J. 2016, 116, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Shang, Y.F.; Kim, S.M.; Lee, W.J.; Um, B.-H. Pressurized liquid method for fucoxanthin extraction from Eisenia bicyclis (Kjellman) Setchell. J. Biosci. Bioeng. 2011, 111, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Tena, M.T. Extraction | Pressurized Liquid Extraction. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2019; pp. 78–83. [Google Scholar]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Santos, D.T.; Veggi, P.C.; Meireles, M.A.A. Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins. J. Food Eng. 2012, 108, 444–452. [Google Scholar] [CrossRef] [Green Version]
- Shang, Y.-F.; Xu, J.-L.; Lee, W.-J.; Um, B.-H. Antioxidative polyphenolics obtained from spent coffee grounds by pressurized liquid extraction. S. Afr. J. Bot. 2017, 109, 75–80. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
| Independent Variables | Code Units | Coded Levels | ||||
|---|---|---|---|---|---|---|
| −α | −1 | 0 | 1 | +α | ||
| Temperature (°C) | A | 70 | 80 | 100 | 120 | 130 |
| Pressure (bar) | B | 2 | 4 | 8 | 12 | 14 |
| Extraction time (min) | C | 30 | 60 | 120 | 180 | 210 |
| Run | Actual Process Variables | %Yield | ||
|---|---|---|---|---|
| Temperature (°C) | Pressure (bar) | Extraction Time (min) | ||
| 1 | 100 | 2 | 120 | 11.5100 |
| 2 | 100 | 2 | 120 | 11.5550 |
| 3 | 80 | 4 | 60 | 10.1562 |
| 4 | 80 | 4 | 60 | 9.1000 |
| 5 | 80 | 4 | 180 | 9.4061 |
| 6 | 80 | 4 | 180 | 9.4061 |
| 7 | 120 | 4 | 60 | 12.2650 |
| 8 | 120 | 4 | 60 | 15.2737 |
| 9 | 120 | 4 | 180 | 14.1212 |
| 10 | 120 | 4 | 180 | 13.2937 |
| 11 | 100 | 8 | 30 | 10.6625 |
| 12 | 100 | 8 | 30 | 9.8187 |
| 13 | 70 | 8 | 120 | 8.6712 |
| 14 | 70 | 8 | 120 | 8.2537 |
| 15 | 100 | 8 | 120 | 9.9675 |
| 16 | 100 | 8 | 120 | 10.1650 |
| 17 | 130 | 8 | 120 | 14.9100 |
| 18 | 130 | 8 | 120 | 16.1700 |
| 19 | 100 | 8 | 210 | 10.7050 |
| 20 | 100 | 8 | 210 | 9.9962 |
| 21 | 80 | 12 | 60 | 7.3437 |
| 22 | 80 | 12 | 60 | 9.6975 |
| 23 | 120 | 12 | 60 | 10.6075 |
| 24 | 120 | 12 | 60 | 13.7875 |
| 25 | 80 | 12 | 180 | 9.3637 |
| 26 | 80 | 12 | 180 | 8.5587 |
| 27 | 120 | 12 | 180 | 13.6300 |
| 28 | 120 | 12 | 180 | 14.8700 |
| 29 | 100 | 14 | 120 | 11.4375 |
| 30 | 100 | 14 | 120 | 9.5612 |
| Source | Sum of Squares | df | Mean Square | p-Value |
|---|---|---|---|---|
| Model | 140.62 | 9 | 15.62 | <0.0001 |
| A (Temperature) | 125.66 | 1 | 125.66 | <0.0001 |
| B (Pressure) | 2.73 | 1 | 2.73 | 0.1033 |
| C (Extraction Time) | 0.9019 | 1 | 0.9019 | 0.3384 |
| AB | 0.0684 | 1 | 0.0684 | 0.7898 |
| AC | 0.7850 | 1 | 0.7850 | 0.3710 |
| BC | 1.93 | 1 | 1.93 | 0.1670 |
| A2 | 6.91 | 1 | 6.91 | 0.0133 |
| B2 | 1.38 | 1 | 1.38 | 0.2384 |
| C2 | 0.0132 | 1 | 0.0132 | 0.9069 |
| Operating Parameter | Optimal Condition | %Yield | |
|---|---|---|---|
| Predicted Value | Actual Value | ||
| Temperature (°C) | 120 | 13.782 | 13.71 ± 53 |
| Pressure (bar) | 4 | ||
| Extraction time (min) | 60 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakdasri, W.; Khamseng, K.; Wattanavaree, M.; Chandeang, Y.; Sawangkeaw, R. Optimization of Polysaccharides Extraction from Spent Coffee Grounds (SCGs) by Pressurized Hot Water Extraction. Biol. Life Sci. Forum 2021, 6, 33. https://doi.org/10.3390/Foods2021-11053
Sakdasri W, Khamseng K, Wattanavaree M, Chandeang Y, Sawangkeaw R. Optimization of Polysaccharides Extraction from Spent Coffee Grounds (SCGs) by Pressurized Hot Water Extraction. Biology and Life Sciences Forum. 2021; 6(1):33. https://doi.org/10.3390/Foods2021-11053
Chicago/Turabian StyleSakdasri, Winatta, Kanyagorn Khamseng, Mookanda Wattanavaree, Yaowapa Chandeang, and Ruengwit Sawangkeaw. 2021. "Optimization of Polysaccharides Extraction from Spent Coffee Grounds (SCGs) by Pressurized Hot Water Extraction" Biology and Life Sciences Forum 6, no. 1: 33. https://doi.org/10.3390/Foods2021-11053
APA StyleSakdasri, W., Khamseng, K., Wattanavaree, M., Chandeang, Y., & Sawangkeaw, R. (2021). Optimization of Polysaccharides Extraction from Spent Coffee Grounds (SCGs) by Pressurized Hot Water Extraction. Biology and Life Sciences Forum, 6(1), 33. https://doi.org/10.3390/Foods2021-11053







