Abstract
Australia is a minor producer of chilli, with the industry producing around 2500 tonnes of chillies per year. Due to the small size of the industry, there is currently limited research on the typical levels of capsaicinoids in the Australian crop and the relationship between these constituents and other agronomic and nutritional factors. This study applied a rapid, maceration-based extraction protocol with end-over-end shaking, coupled with a HPLC-DAD method for the analysis of capsaicin and dihydrocapsaicin in 20 Habanero chilli samples from Queensland, Australia. All samples were from the same growing season (2020) but were taken from different within-field locations to ensure that all of the variability within the growing site was sampled. In addition to the capsaicinoid measurements, the total phenolic content was measured using the Folin-Ciocalteu assay, while antioxidant activity was measured using the Ferric Reducing Antioxidant Power (FRAP) method. The capsaicin concentration of the samples ranged from 1474–3916 mg/kg, while the dihydrocapsaicin content ranged between 638–1757 mg/kg, giving total pungencies of approximately 32,000 to 83,000 Scoville Heat Units. Similarly, the total phenolic content varied from 1000–1608 mg gallic acid equivalents/100 g, while the antioxidant activity ranged from 301 to 455 mg Trolox equivalents/100 g. Pearson linear correlation analysis revealed that the capsaicin and dihydrocapsaicin contents were strongly positively correlated with one another (R2 = 0.73; p < 0.001), with a mean capsaicin: dihydrocapsaicin ratio of 2.4:1. Furthermore, there was a moderate positive correlation between the total capsaicinoid content and total phenolic content of the samples (R2 = 0.58; p < 0.01), as well as a strong correlation between the total capsaicinoid content and FRAP (R2 = 0.81; p < 0.001). However, dry matter content was not significantly correlated with capsaicinoid content, total phenolic content or antioxidant activity (p > 0.05 for all). These results may be used to inform future breeding programs for high-capsaicin chilli varieties and support further research into the agronomic and genetic factors driving capsaicin and dihydrocapsaicin content.
1. Introduction
Habanero chillies (Capsicum chinense cv Habanero) are among the hottest commonly consumed chillies in Australia, with a Scoville Heat rating of up to 200,000 Scoville Heat Units (SHU). The principal pungent constituents are the N-vanillylamides, namely capsaicin and dihydrocapsaicin (Figure 1) [1]. In addition, they contain a number of phenolic compounds [2,3] which may provide health benefits [4], including reducing the risk of cardiovascular disease [5].
Figure 1.
The chemical structures of capsaicin and dihydrocapsaicin. Reproduced from Guzmán and Bosland [6] under Creative Commons 3.0 License.
Both genotype [7] and environmental conditions [8] are known to influence the capsaicinoid and phenolic content of chilli; however, the correlations and interplay between these factors are understudied in the Australian setting, due to the small size of the industry. The total production of the Australian chilli sector has been estimated at 2500 t/year, with a market value of over $20 million (2013–2014 figures) [9]; however, the sector is currently undergoing rapid growth. There is also interest in breeding new varieties of Habanero chilli with higher capsaicinoid contents in order to meet the demands of niche high-value markets.
Consequently, the aim of this study was to investigate the relationships between capsaicin, dihydrocapsaicin and phenolic compounds in the Australian Habanero chilli crop. In addition to providing and insight into the inter-relationship and phytosynthetic pathways associated with these compounds, this could demonstrate if alternative parameters (such as total phenolics) could be used as a surrogate marker for detecting samples containing high levels of capsaicinoids.
2. Methods
2.1. Sample Procurement
Habanero chilli samples (n = 20 individual chillies) were sourced from a commercial chilli grower in Bundaberg, Queensland in January 2020. The samples were chosen from different within-field locations of a commercial chilli crop to incorporate a wide range of environmental variability. The mass (in g) and length (in mm) of each chilli was recorded, before being dried at 50 °C to a constant mass (Sunbeam Food Lab Dehydrator; Sunbeam, Botany, NSW) and ground to a fine powder (Retsch ZM1000 centrifugal grinding mill; Retsch, Haan, Germany). Dry matter content was determined gravimetrically from the loss in mass upon drying.
2.2. Extraction Protocol and Measurement of TP Content and FRAP
Polar phenolics and capsaicinoids were extracted from each powdered sample in duplicate, using 90% methanol and a previously described maceration-based extraction protocol with end-over-end shaking [10]. The total phenolic (TP) content was measured using the Folin-Ciocalteu assay [10], with results expressed in terms of gallic acid equivalents (GAE) per 100 g (dry weight basis). Antioxidant activity was measured using the Ferric Reducing Antioxidant Power (FRAP) method [11], with results expressed as Trolox equivalents (TE) per 100 g (DWB).
2.3. Measurement of Capsaicinoids by HPLC
A portion of each methanolic extract was syringe filtered (Livingstone 0.45 µm PTFE) prior to direct analysis by high performance liquid chromatography with diode array detection (HPLC-DAD). Capsaicinoids were separated on an Agilent 1100 HPLC system [12] with a C18 column (Agilent Eclipse XDB-C18; 150 × 4.6 mm; 5 µm pore size), with the instrument method adapted from Waite and Aubin [13]. The detection wavelength was 280 nm, injection volume 25 µL and flow rate 1 mL/min. The mobile phase gradient began at 40% methanol and 60% water, ramping to reach 85% methanol at 8 min and 99% methanol at 10 min, where it was held for 3 min, then returned to 40% methanol by 15 min, where it was held for a further minute. The post-run equilibration time was 2 min. Capsaicin and dihydrocapsaicin were quantified using external calibrations of authentic standards (Sigma Aldrich Australia) in the range of 1–200 mg L−1.
2.4. Data Analysis
Statistical analysis was conducted in R Studio running R 4.0.2 [14]. Where applicable, results are given as mean ± 1 SD.
3. Results and Discussion
3.1. Total Phenolic Content and Antioxidant Activity
The total phenolic content of the samples ranged from 1000–1608 mg GAE/100 g, with an average content of 1296 ± 185 mg GAE/100 g. This was higher than the TP content found by Shaha et al. [15] in three varieties of Capsicum annum (101–414 mg GAE/100 g); but comparable to the range of TP contents (639–1212 mg catechin equivalents/100 g) reported by Sricharoen et al. [16] in 14 chilli varieties from Thailand.
The FRAP of the samples showed somewhat less variability, with values ranging from 301–455 mg TE/100 g. The mean FRAP was 361 ± 45 mg TE/100 g, considerably higher than the 12–133 mg GAE/100 g reported by Dutta et al. [17] in Bird’s Eye chilli (Capsicum frutescens), but lower than the 502–1274 mg catechin equivalents/100 g found by Sricharoen et al. [16] in 14 different chilli varieties. Although all samples included in this study were of similar maturity, previous work has found that antioxidant activity generally increases during maturation of the fruit [18,19].
3.2. Capsaicinoids
The capsaicin content of the chillies ranged from 1474–3916 mg/kg, with a mean content of 2721 ± 662 mg/kg. The dihydrocapsaicin contents were approximately half that of the capsaicin contents, ranging between 638–1757 mg/kg (mean of 1169 ± 267 mg/kg). These values were quite comparable to those found for 10 varieties of hot chilli pepper from Thailand [20], and also comparable to results reported for Tabasco chilli from Spain [21].
The average capsaicin:dihydrocapsaicin ratio was 2.35 ± 0.43, with values ranging from 1.72–3.28. Most of these values were higher than the average ratio of 1.6:1 reported by Weaver et al. [22] and 1.78:1 found by Garcés-Claver et al. [21] in Orange Habanero chillies.
Using a conversion factor of 15 × total capsaicinoid concentration [23], the total pungencies of the samples were approximately 31,668–82,950 Scoville Heat Units (SHU) (mean of 58,361 ± 13,139 mg/kg). Again, these values were comparable to previous literature from international studies [24].
3.3. Correlation Analysis
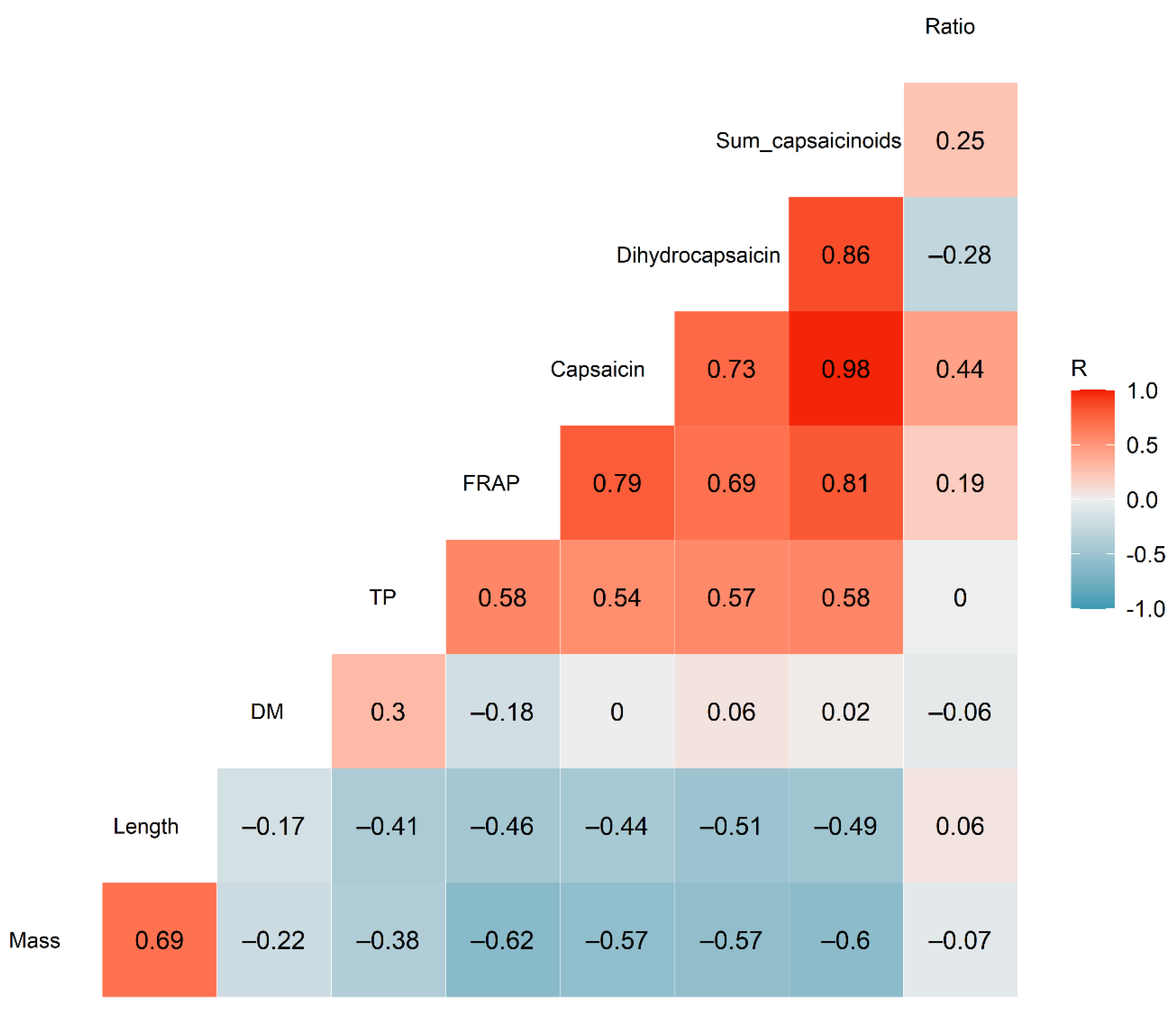
Pearson linear correlation analysis showed that the capsaicin and dihydrocapsaicin contents were strongly positively correlated with one another (R2 = 0.73; p < 0.001), as shown in Figure 2. This was expected, as both are produced by the same biosynthetic pathway [25,26], with the level of capsaicin produced being regulated by acyl-CoA synthetase expression [1].
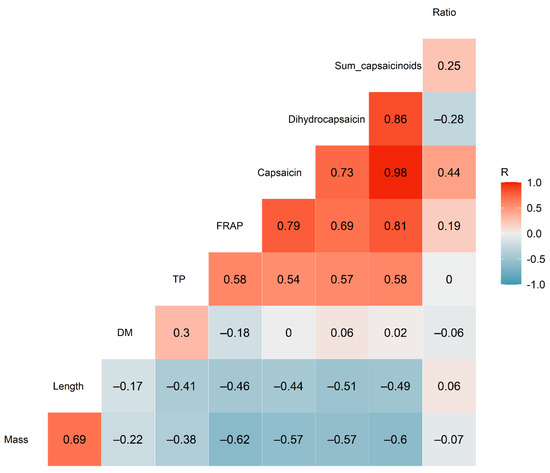
Figure 2.
Correlogram showing the correlations between the various parameters quantified in the chilli samples (n = 20). The numbers inside each square show the Pearson R correlation values.
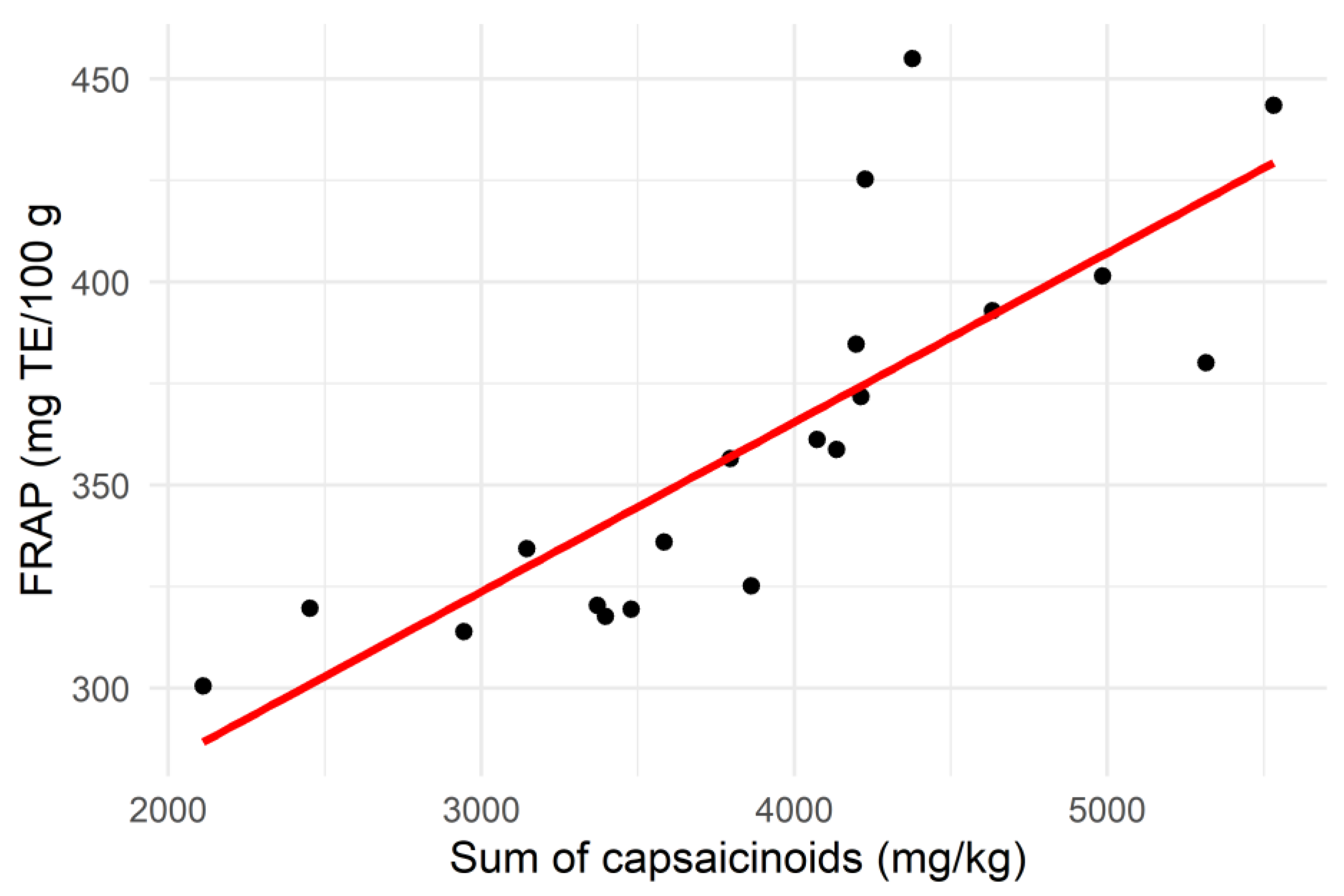
The total capsaicinoid content showed a moderate positive correlation with the total phenolic content of the samples (R2 = 0.58; p < 0.01), as well as a strong correlation with the FRAP (R2 = 0.81; p < 0.001), as illustrated in Figure 3. This contrasted with the findings of Sricharoen et al. [16], who found a weak negative correlation between these parameters and the total capsaicinoids in 14 different chilli varieties. Nevertheless, the strong positive relationship between total capsaicinoids and FRAP found in this study suggests that antioxidant activity could be used as a method for rapidly screening high-capsaicinoid samples of Habanero chilli.
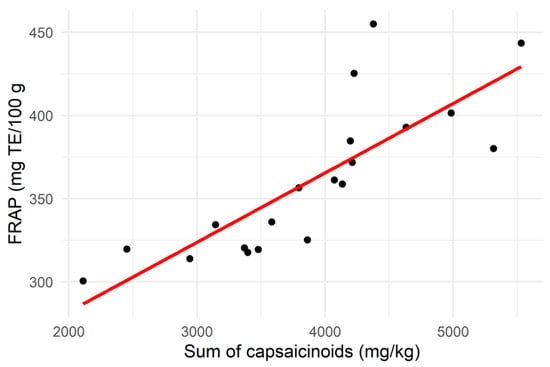
Figure 3.
Scatter plot showing the relationship between the sum of capsaicinoid contents and the Ferric Reducing Antioxidant Power (FRAP) of the chilli samples (n = 20).
The FRAP and TP content were only moderately correlated (R2 = 0.58; p < 0.01), in contrast to the strong correlations (R2 = 0.94–0.96) reported by others [16,27]. However, Dutta et al. [17] found a similarly moderate correlation between FRAP and TP in Bird’s Eye chilli (Capsicum frutescens) from India.
The chilli mass was negatively correlated with the capsaicin and dihydrocapsaicin concentration (R2 = –0.57; p < 0.01 for both), as well as being negatively correlated with the FRAP (R2 = –0.62; p < 0.01). In other words, larger chillies tended to contain lower concentrations of the pungent capsaicinoids. Although few previous studies have conducted chemical characterization on single chilli fruit, such as that performed in this study, Singh et al. [28] found a similarly negative correlation between capsaicin content and average dry fruit weight, fruit diameter and fruit length in their study across multiple chilli genotypes.
Dry matter content was not significantly correlated with any other parameter (p > 0.05 for all). Similarly, the capsaicin:dihydrocapsaicin ratio showed no significant correlations.
4. Conclusions
This study investigated the within-field variability in capsaicinoid content, phenolic content and antioxidant activity of Australian-grown samples of Habanero chilli. Overall, the results were indicative of a strong influence of environmental conditions on the capsaicinoid and total phenolic content. A strong positive correlation was observed between antioxidant activity and the total capsaicinoid content, suggesting the use of antioxidant activity as a rapid screening tool for capsaicinoid content. There was a moderate correlation between the total phenolic content and total capsaicinoids; while the capsaicinoid content and antioxidant activity were negatively associated with fruit size.
Author Contributions
Conceptualization, J.B.J. and M.N.; methodology, J.B.J.; software, J.B.J.; validation, J.B.J.; formal analysis, J.B.J.; investigation, J.B.J., J.S.M.; resources, J.B.J., M.N.; data curation, J.B.J.; writing—original draft preparation, J.B.J.; writing—review and editing, J.B.J., J.S.M., M.N.; visualization, J.B.J.; supervision, M.N.; project administration, M.N.; funding acquisition, M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a New Staff Grant from CQUniversity, grant number RSH/5343. One of the authors (J.B.J.) also acknowledges support from the Australian Government in the form of a Research Training Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank the assistance of Simon White and the chilli grower in procuring the samples used in this study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Aza-González, C.; Núñez-Palenius, H.G.; Ochoa-Alejo, N. Molecular biology of capsaicinoid biosynthesis in chili pepper (Capsicum spp.). Plant Cell Rep. 2011, 30, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, T.L.; Afolayan, A.J. The suitability of chili pepper (Capsicum annuum L.) for alleviating human micronutrient dietary deficiencies: A review. Food Sci. Nutr. 2018, 6, 2239–2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamed, M.; Kalita, D.; Bartolo, M.E.; Jayanty, S.S. Capsaicinoids, Polyphenols and Antioxidant Activities of Capsicum annuum: Comparative Study of the Effect of Ripening Stage and Cooking Methods. Antioxidants 2019, 8, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, M.; Higuera, I.; Goycoolea, F.M. Capsaicinoids: Occurrence, Chemistry, Biosynthesis, and Biological Effects. In Fruit and Vegetable Phytochemicals, 2nd ed.; Yahia, E.M., Ed.; Wiley: Hoboken, NJ, USA, 2017; pp. 499–514. [Google Scholar]
- Ofori-Asenso, R.; Mohsenpour, M.A.; Nouri, M.; Faghih, S.; Liew, D.; Mazidi, M. Association of Spicy Chilli Food Consumption With Cardiovascular and All-Cause Mortality: A Meta-Analysis of Prospective Cohort Studies. Angiology 2021, 72, 625–632. [Google Scholar] [CrossRef]
- Guzmán, I.; Bosland, P.W. A Matter of Taste: Capsaicinoid Diversity in Chile Peppers and the Importance to Human Food Preference. In Capsaicin and Its Human Therapeutic Development; Mozsik, G., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Canto-Flick, A.; Balam-Uc, E.; Bello-Bello, J.J.; Lecona-Guzmán, C.; Solís-Marroquín, D.; Avilés-Viñas, S.; Gómez-Uc, E.; López-Puc, G.; Santana-Buzzy, N.; Iglesias-Andreu, L.G. Capsaicinoids Content in Habanero Pepper (Capsicum chinense Jacq.): Hottest Known Cultivars. HortScience 2008, 43, 1344. [Google Scholar] [CrossRef] [Green Version]
- Naves, E.R.; de Ávila Silva, L.; Sulpice, R.; Araújo, W.L.; Nunes-Nesi, A.; Peres, L.E.P.; Zsögön, A. Capsaicinoids: Pungency beyond Capsicum. Trends Plant Sci. 2019, 24, 109–120. [Google Scholar] [CrossRef]
- AusVeg. Veggie Stats: Chillies; AusVeg: Melbourne, Australia, 2016; pp. 19–21. [Google Scholar]
- Johnson, J.; Collins, T.; Skylas, D.; Quail, K.; Blanchard, C.; Naiker, M. Profiling the varietal antioxidative content and macrochemical composition in Australian faba beans (Vicia faba L.). Legume Sci. 2020, 2, e28. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.B.; Walsh, K.B.; Bhattarai, S.P.; Naiker, M. Partitioning of nutritional and bioactive compounds between the kernel, hull and husk of five new chickpea genotypes grown in Australia. Future Foods 2021, 4, 100065. [Google Scholar] [CrossRef]
- Johnson, J.B.; Mani, J.S.; Skylas, D.; Walsh, K.B.; Bhattarai, S.P.; Naiker, M. Phenolic profiles and nutritional quality of four new mungbean lines grown in northern Australia. Legume Sci. 2020, 3, e70. [Google Scholar] [CrossRef]
- Waite, M.S.; Aubin, A.J. A Modular HPLC System for Routine Analysis of Capsaicin from Hot Sauces; Waters Corporation: Milford, MA, USA, 2008; p. 5. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 4.0.2; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Shaha, R.K.; Rahman, S.; Asrul, A. Bioactive compounds in chilli peppers (Capsicum annuum L.) at various ripening (green, yellow and red) stages. Ann. Biol. Res. 2013, 4, 27–34. [Google Scholar]
- Sricharoen, P.; Lamaiphan, N.; Patthawaro, P.; Limchoowong, N.; Techawongstien, S.; Chanthai, S. Phytochemicals in Capsicum oleoresin from different varieties of hot chilli peppers with their antidiabetic and antioxidant activities due to some phenolic compounds. Ultrason. Sonochem. 2017, 38, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.K.; Singh, S.B.; Saha, S.; Akoijam, R.S.; Boopathi, T.; Banerjee, A.; Lungmuana; Vanlalhmangaiha; Roy, S. Diversity in Bird’s Eye Chilli (Capsicum frutescens L.) Landraces of North-East India in Terms of Antioxidant Activities. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 1317–1326. [Google Scholar] [CrossRef]
- Conforti, F.; Statti, G.A.; Menichini, F. Chemical and biological variability of hot pepper fruits (Capsicum annuum var. acuminatum L.) in relation to maturity stage. Food Chem. 2007, 102, 1096–1104. [Google Scholar] [CrossRef]
- Jansasithorn, R.; East, A.R.; Hewett, E.W.; Molan, A.L.; Heyes, J.A.; Mawson, A.J. Harvest maturity influences the antioxidant activity in Jalapeño chili. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on Emerging Health Topics in Fruits and Vegetables, Lisbon, Portugal, 2012; pp. 379–384. [Google Scholar]
- Juangsamoot, J.; Ruangviriyachai, C.; Techawongstien, S.; Chanthai, S. Determination of capsaicin and dihydrocapsaicin in some hot chilli varieties by RP-HPLC-PDA after magnetic stirring extraction and clean up with C18 cartridge. Int. Food Res. J. 2012, 19, 1217–1226. [Google Scholar]
- Garcés-Claver, A.; Arnedo-Andrés, M.S.; Abadía, J.; Gil-Ortega, R.; Álvarez-Fernández, A. Determination of Capsaicin and Dihydrocapsaicin in Capsicum Fruits by Liquid Chromatography−Electrospray/Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 2006, 54, 9303–9311. [Google Scholar] [CrossRef] [Green Version]
- Weaver, K.M.; Luker, R.G.; Neale, M.E. Rapid quality control procedure for the determination of Scoville heat units and the detection of chillies in black pepper, via high-performance liquid chromatography. J. Chromatogr. A 1984, 301, 288–291. [Google Scholar] [CrossRef]
- Tainter, D.R.; Grenis, A.T. Spices and Seasonings: A Food Technology Handbook, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Othman, Z.A.A.; Ahmed, Y.B.H.; Habila, M.A.; Ghafar, A.A. Determination of Capsaicin and Dihydrocapsaicin in Capsicum Fruit Samples using High Performance Liquid Chromatography. Molecules 2011, 16, 8919–8929. [Google Scholar] [CrossRef] [Green Version]
- Prasad, B.C.N.; Gururaj, H.B.; Kumar, V.; Giridhar, P.; Ravishankar, G.A. Valine Pathway Is More Crucial than Phenyl Propanoid Pathway in Regulating Capsaicin Biosynthesis in Capsicum frutescens Mill. J. Agric. Food Chem. 2006, 54, 6660–6666. [Google Scholar] [CrossRef]
- Blum, E.; Mazourek, M.; O’Connell, M.; Curry, J.; Thorup, T.; Liu, K.; Jahn, M.; Paran, I. Molecular mapping of capsaicinoid biosynthesis genes and quantitative trait loci analysis for capsaicinoid content in Capsicum. Theor. Appl. Genet. 2003, 108, 79–86. [Google Scholar] [CrossRef]
- Sricharoen, P.; Techawongstein, S.; Chanthai, S. A high correlation indicating for an evaluation of antioxidant activity and total phenolics content of various chilli varieties. J. Food Sci. Technol. 2015, 52, 8077–8085. [Google Scholar] [CrossRef] [Green Version]
- Singh, Y.; Sharma, M.; Sharma, A. Genetic Variation, Association of Characters, and Their Direct and Indirect Contributions for Improvement in Chilli Peppers. Int. J. Veg. Sci. 2009, 15, 340–368. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).