Abstract
Commiphora wightii is an important medicinal tree of arid and semi-arid regions of India. It is commonly known as Guggal and belongs to the family Burseraceae. It is a slow growing poor viable seed producing plant; moreover, excessive destructive tapping for oleogum resin (known as guggul) has put this plant under critically endangered categories according to the IUCN. This plant produces two types of seeds, viz., black highly viable seed and white non-viable seeds. Therefore, the present study was carried out with the aim to estimate seed production in summer and winter and their viability ratios (black:white), which will be helpful in raising nurseries and large-scale plantations of Guggul. Mature seeds were collected from 647 guggal plants (10 years old) from Deesa, Gujarat, India in summer (March–July 2017) and winter (November–December 2017). There is no significant difference in percentage of plants bearing mature seeds, but the number of mature seeds per seed producing plant is significantly higher in winter (72.51 seeds) as compared with summer (10.19 seeds). The proportion of black seed in summer and winter was almost opposite, i.e., in winter 70.9% of seeds were black, whereas in summer only 30% black seeds were found. Seed germination data revealed that black seeds collected in winter showed higher seed germination (13.6%) than in summer (2.1%). No germination was recorded in summer collected white seed, whereas very low seed germination was observed in winter (1.2%) white seeds.
1. Introduction
Commiphora wightii is a slow growing, endangered, medicinally important plant and commonly known as Guggal. It is a perennial, dioecious, small to medium size thorny plant belonging to the Burseraceae family. The population is dominated by females, with male and hermaphrodite plants being extremely rare. With the scarcity of male plants, it produces seeds through apomixis [1,2]. The plant remains leafless most of time. Leaves are present only in the rainy season and are small and sessile with irregularly toothed edges, and they are aromatic in nature [3]. The flowers are small and red in colour. The fruits are oval in shape, pulpy in nature, green when immature, pinkish when partially mature, and become red at maturity [4]. It is mainly distributed in arid and semi-arid areas of Rajasthan and Gujarat, and also occurs in some areas of Pakistan and Bangladesh [5]. The plant is valued for oleogum resin exudate produced from its bark, known as guggul. It has been used in Ayurvedic medicine to treat arthritis inflammation, hypercholesterolemia, cardiovascular diseases, and cancerous diseases, and to improve hepatic antioxidant defence systems [6,7].
This plant is categorized as critically endangered by the IUCN [8] due to excessive destructive harvesting to obtain oleogum resin, and poor regeneration and seed germination. Previous literature on guggal seeds has reported that these seeds are apomictic [2], black and white seeds with significantly different seed viability [9]. Because of the abovementioned reason, raising nurseries and plantations and developing agrotechniques for domestication of this species is a serious problem for forestry departments. In this plant, the flowering and fruiting are highly asynchronous [10]. Previously, Prakash et al. [11] collected C. wightii seeds in February–March and calculated seed weight, seed viability, seed germination percentage, and percentage of polyembryony. In 2014, [10] reported two peaks for both flowering and fruiting intensity in C. wightii, but they included both mature and immature seeds in their study and the results were based on relative score. Present studies were carried out on actual seed yield (mature seeds only) with an aim to compare seed production in summer and winter, along with black and white seed ratio and their germination. This study is essential for the development of agro and nursery techniques.
2. Materials and Methods
Surveys were carried out in different guggal growing areas. A ten-year-old, four field plantation, having 647 plants from Ranpur Forest nursery, Datiwada (Deesa, Gujarat), India was selected for seed collection, being well protected and maintained. It is situated between 24°17′ N (Latitude) and 72°12′ E (Longitude). The spacing between plants was 3 m and between rows was also 3 m in all four fields.
Mature red fruits and seeds without epicarp and mesocarp from individual plant were collected from four fields at Deesa, Gujarat. Seeds from individual plant were kept in separate brown paper bags and their identity was maintained by marking the plant number. These fruits were de-pulped by manually rubbing in cloth and washed with fresh water then dried in shade.
Guggal seeds were collected from Deesa, Gujarat in in summer (March–July 2017) and winter (November–December 2017). The pooled seed data from March, May, and July (summer) and November and December (winter) were used to estimate the seed yield: the average number of mature seeds per plant.
Seed ratio and seed weight in summer (July 2017) and winter (November–December 2017) were calculated by counting the black and white seeds of each plant and then weighing them. Seed weight (1000 seeds) is calculated by the following method:
(Total seed weight of all the seeds of a plant/number of seed in that plant) × 1000
A total of 3000 seeds (1500 black and 1500 white) collected in summer and 25,738 seeds (19,816 black and 5922 white) collected in winter were sown in a root trainer filled with fine sand, coarse sand, and compost in a 3:2:1 ratio in March and were kept in polyhouse conditions (35.9 ± 0.12 °C temperature and 53.8 ± 0.24% humidity) of AFRI. The germination medium was kept moist with regular watering or misting. Criteria for germination was the emergence of plumule above the germination medium. Seed germination was recorded after four weeks.
3. Results
3.1. Total Mature Seed Production
The percentage of plants bearing mature seeds is not much varied in summer (50.6%) and winter (53.0%). However, the intensity of seed production per seed producing plant was greatly influenced by the season (Figure 1). Th enumber of seeds per seed producing plant was higher in winter (72.5 seeds) as compared with summer (10.2 seeds). In winter, a higher average number of seeds per seed producing plant was observed in field C (105.3 seeds) and lowest in field D (13.9 seeds).
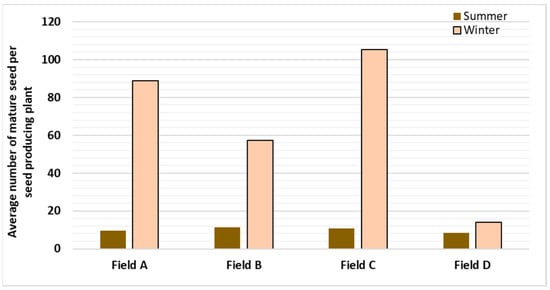
Figure 1.
Effect of season on mature seed yield in C. wightii.
3.2. Black and White Seed Ratio and Seed Weight
The ratio of black and white seeds changed with the season. In summer, the guggal plant produced more white seeds (70%) than black seeds (30%), whereas in winter black seed (70.9%) was higher than white seed (29.1%). The black and white seed ratio in summer and winter was almost opposite in all four fields (Figure 2). It appears that higher temperature is not favourable for seed maturity and viability.
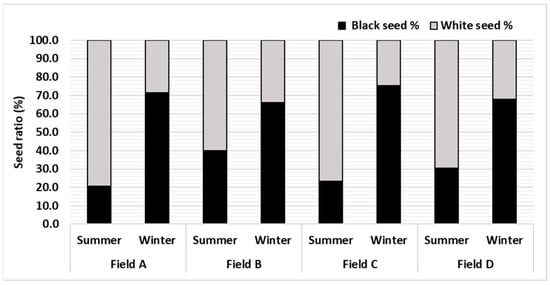
Figure 2.
Effect of season on seed ratio in four fields of C. wightii.
Comparison of black and white seed weight in summer and winter showed that black seed weight was almost similar both in summer and winter (39.6 and 40.0, respectively) but higher than white seed weight (summer, 25.8 g and winter, 26.1 g). Black seed weight was highest in field C (43.5 gm), whereas in winter higher black seed weight was found in field D (42.87 gm; Figure 3).
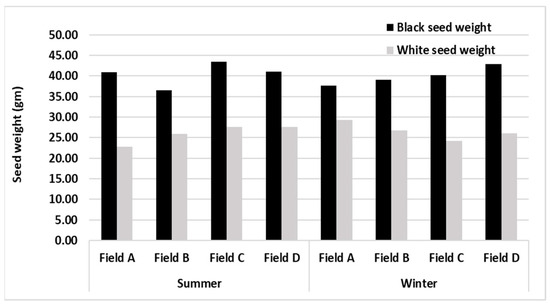
Figure 3.
Effect of season on seed weight in four fields of C. wightii.
3.3. Seed Germination
Overall germination (about 1%) was very poor in the summer collected seeds. In summer, black seed germination percentage was 2.1% and white seeds failed to germinate (zero percent), whereas, winter collected black seeds germination was relatively higher (13.6%), and few white seeds germinated (1.2%; Figure 4). The amount of total mature seeds collected in winter was almost seven times higher as compared with summer.
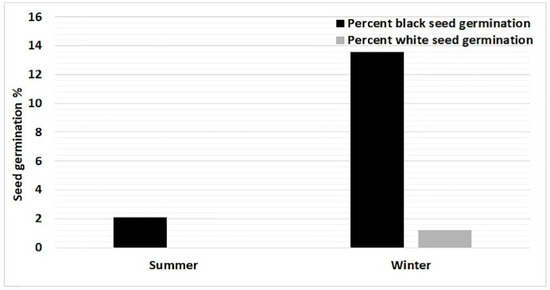
Figure 4.
Effect of season on seed germination of C. wightii.
4. Discussion
Seed maturation and viability is influence by several environmental factors as well as by species genetic structure. In 2005, [12] reported the effect of higher temperature and lower relative humidity and reported that it caused lower seed yield in Helianthus annuus. Prasad et al. [13] reported that higher temperature in Sorghum bicolor resulted in low seed set and seed yield. Similarly, we found that higher temperatures in summer greatly influence total mature seed yield in C. wightii. In summer, a lower availability of water and nutrients caused lower conversion of flowers to mature fruits, whereas the temperature during the period October to December is ideal for the ripening of fruits in C. wightii. Previously, Singhal et al. [10] reported that maximum flowering and fruiting intensity in C. wightii was in April and May, respectively. In their study, they considered all fruits (mature red and immature green). They also reported a smaller peak of fruit intensity in winter. They did not study the fully mature fruit yield. It may be possible that immature seeds remain attached to plants and require a longer period (four to six months) for seed maturation, which results in a higher mature seed yield in winter.
Time of seed maturation affect the germination efficiency in Portulaca oleracea [14] and Prosopis juliflora [15]. Similarly, germination of C. wightii seeds was also affected by the time of seed maturation. The high temperature during seed maturation causes seed dormancy in Soya bean [16] and Thlaspi arvense [17]. In Portulaca oleracea [14] and Arabidopsis thaliana [18], exposure of the mother plant to short days during seed development and maturation was reported to increase seed germination. According to Evenari et al. [19], the higher germination in seeds matured under shorter days compared to those matured under longer days was due to their greater ability to imbibe water during germination. Similarly, in our case, both low temperature and short days are characteristic of the winter season, which may be the main reason behind high seed germination in winter matured seeds.
In the present work, it was found that the ratio of black and white seeds was greatly influenced by the season. The winter season supported more viable black seed production. Miralles et al. [20] reported that high temperature and low relative humidity had adverse effect on pollen viability and pollen quality, which resulted in empty and sterile achenes (seeds). This could also be the case in C. wightii. Seed weight denotes the presence of a mature embryo inside the seed coat. A high seed weight indicates a fully developed embryo, and a light seed weight indicates a deteriorated embryo or empty seed. In the present study, black seeds had a higher seed weight, both in summer and in winter as compared with white seeds.
5. Conclusions
Winter is the right harvesting time for guggal seeds. Seed screening is necessary to remove white seed and seeds having low seed weight. This study is useful to stakeholders who are interested in raising large scale plants of Guggul through seeds.
Supplementary Materials
The poster presentation is available online at https://www.mdpi.com/article/10.3390/IECPS2020-08889/s1. The video presentation is available online at: https://sciforum.net/event/IECPS2020/keynote/2c55d838f4d9a0444749d8357c21c25e/presentation_video/guggal%20ppt%20presentation%20for%20plant%20science%20conference%20-%20Copy%203.mp4.
Author Contributions
U.K.T. conceived and designed the experiments; M.C. and S.B. performed the experiments and wrote the paper; M.C. and U.K.T. analysed the data; U.K.T. edited and finalized the manuscript for submission. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available with Meena Choudhary.
Acknowledgments
We acknowledge the financial support received from the National Medicinal Plant Board (NMPB), New Delhi, India.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IUCN | International Union for Conservation of Nature |
| AFRI | Arid Forest Research Institute |
References
- Gupta, P.; Shivanna, K.R.; Ram, H.Y.M. Apomixis and Polyembryony in the Guggul Plant, Commiphora wightii. Ann. Bot. 1996, 78, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Geetha, K.A.; Kawane, A.; Bishoyi, A.K. Characterization of mode of reproduction in Commiphora wightii [(Arnot) Bhandari] reveals novel pollen–pistil interaction and occurrence of obligate sexual female plants. Trees 2013, 27, 567–581. [Google Scholar] [CrossRef]
- Mohan, C.; Naresh, B.; Kumar, K.B.; Reddy, V.; Manjula; Keerthi, B.; Sreekanth, D.; Manzelat, S.F.; Cherku, P.D. Micropropagation studies and phytochemical analysis of the endangered tree Commiphora wightii. J. Appl. Res. Med. Aromat. Plants 2017, 6, 70–79. [Google Scholar] [CrossRef]
- Kulhari, A.; Sheorayan, A.; Singh, R.; Dhawan, A.K.; Kalia, R.K. Survey, collection and conservation of Commiphora wightii (Arn.) Bhandari—An important medicinal plant heading towards extinction. Indian For. 2014, 140, 1171–1183. [Google Scholar]
- Sarup, P.; Bala, S.; Kamboj, S. Pharmacology and Phytochemistry of Oleo-Gum Resin of Commiphora wightii (Guggulu). Scientifica 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, R. Therapeutic Effects of Guggul and Its Constituent Guggulsterone: Cardiovascular Benefits. Cardiovasc. Drug Rev. 2007, 25, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Al-Rejaie, S.S. Effect of oleo-gum-resin on ethanol-induced hepatotoxicity in rats. J. Med. Sci. 2012, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ved, D.; Saha, D.; Ravikumar, K.; Haridasan, K. Commiphora wightii. The IUCN Red List of Threatened Species 2015: E.T31231A50131117. Available online: http://dx.doi.org/10.2305/IUCN.UK.20152.RLTS.T31231A50131117 (accessed on 22 August 2019).
- Lal, H.; Kasera, K.P. Status and distribution range of Guggul: A critically endangered medicinal plant from the Indian Thar Desert. Sci. Cult. 2010, 76, 531–533. [Google Scholar]
- Singhal, H.; Gaur, A.; Tomar, U.K. Observation on flowering and fruiting in Commiphora wightii (Arnott) Bhandari. Eur. J. Med. Plants 2014, 4, 1087–1097. [Google Scholar] [CrossRef]
- Prakash, J.; Kasera, P.K.; Chawan, D.D. A report on polyembryony in Commiphora wightii from thar desert, India. Curr. Sci. 2000, 78, 1185–1187. [Google Scholar]
- Baydar, H.; Erbas, S. Influence of Seed Development and Seed Position on Oil, Fatty Acids and Total Tocopherol Contents in Sunflower (Helianthus annuus L.). Turk. J. Agric. For. 2005, 29, 179–186. [Google Scholar]
- Prasad, P.V.V.; Boote, K.J.; Allen, L.H. Adverse high temperature effects on pollen viability, seed-set, seed yield and harvest index of grain-sorghum [Sorghum bicolor (L.) Moench] are more severe at elevated carbon dioxide due to higher tissue temperatures. Agric. For. Meteorol. 2006, 139, 237–251. [Google Scholar] [CrossRef]
- Keblawy, A.E.; Ansari, F.A. Effects of site of origin, time of seed maturation, and seed age on germination behavior of Portulaca oleracea from the Old and New Worlds. Can. J. Bot. 2000, 78, 279–287. [Google Scholar]
- Keblawy, A.E.; Rawai, A.A. Effects of seed maturation time and dry storage on light and temperature requirements during germination in invasive Prosopis juliflora. Flora Morphol. Distrib. Funct. Ecol. Plants 2006, 201, 135–143. [Google Scholar]
- Keigley, P.J.; Mullen, E.E. Changing in soybean seed quality from high temperature during seed fill and maturation. Crop Sci. 1986, 26, 1212–1216. [Google Scholar] [CrossRef]
- Hume, L. Maternal environment effects on plant growth and germination of two strains of Thlaspi arvense L. Int. J. Plant Sci. 1994, 155, 180–186. [Google Scholar] [CrossRef]
- Munir, J.; Dorn, L.A.; Donohue, K.; Schmitt, J. The effect of maternal photoperiod on seasonal dormancy in Arabidopsis thaliana (Brassicaceae). Am. J. Bot. 2001, 88, 1240–1249. [Google Scholar] [CrossRef] [Green Version]
- Evenari, M.; Koller, D.; Gutterman, Y. Effects of the environment of the mother plant on germination by control of seed-coat permeability to water in Ononis sicula Guss. Aust. J. Biol. Sci. 1966, 19, 1007–1016. [Google Scholar] [CrossRef] [Green Version]
- Miralles, O.M.; Valero, J.A.; Olalla, F.M. Growth, development and yield of five sunflower hybrids. Eur. J. Agron. 1997, 6, 47–59. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).