Abstract
Rambutan seeds (RS) are industrial waste often generated in the canned fruit industry. The aim of this study was to extract polysaccharides from defatted rambutan seeds or crude polysaccharides (POLS-DRSs) with subcritical water. Defatted seed powder (DRS) was extracted by hot-water extraction (HWE) at 100 °C as a reference condition. Subcritical water extraction (SWE) was performed at 120–140 °C and an initial pressure of 2 MPa. A sample-to-water ratio of 1:10 (w/w) and an extraction time of 15–60 min were used for both methods. The results show that gravimetric extraction yields of 53.01 g/100 g DRS and 7.71–41.70 g/100 g DRS were obtained from HWE and SWE, respectively. Additionally, HWE provided a total sugar content of 30.75 g/100 g POLS-DRSs, while SWE generated a total sugar content in the range of 27.00–49.76 g/100 POLS-DRSs. The antioxidant activities of POLS-DRSs were measured with a 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay. An amount of 40 mg of POLS-DRSs obtained at 120 °C after 60 min provided the highest DPPH activity of 82.93% inhibition. The POLS-DRSs were suitable for growing microorganisms because they had a high sugar content and a low total phenolic content. A prebiotic activity assay will be carried out in future studies.
1. Introduction
Rambutan (Nephelium lappaceum Linn.) is a native seasonal fruit in Southeast Asia. It can be consumed as fresh fruit or processed into various food products. The rambutan production capacity of Thailand is approximately 0.3–0.4 million tons per year. It is reported that annually, an average of 1900 tons of rambutan seed is wasted [1,2].
The extraction and purification of seed polysaccharides by hot-water extraction (HWE) and subcritical water extraction (SWE) have been recently summarized elsewhere [3]. HWE is performed below 100 °C, whereas SWE is performed between 100 °C and 374 °C under a pressure of 0.1–22.1 MPa. Increasing the temperature enhances the heat and mass transfer during the extraction process, reduces the dielectric constant (polarity) and viscosity of water, and induces the dissociation of hydronium ions [4]. However, SWE carries the risk of the thermal degradation of active compounds when performed at higher temperatures. Although the protein and carbohydrate compounds in rambutan seeds are valuable, there are relatively few studies on the obtainment of these compounds by HWE and SWE because of the high lipid content of the seeds. Defatting the seeds with n-hexane prior to aqueous and/or ethanolic extraction of carbohydrates is necessary [2]. For example, a study on polysaccharide extraction from selected plants from Southern Thailand reported problems with the extraction from rambutan seeds. Aqueous and/or ethanolic polysaccharide extraction from RSs was impaired by the formation of a fat layer on the surface [5]. The aim of this work was to produce a crude polysaccharide extract containing the highest total sugar content with low phenolic compound content by subcritical water extraction.
2. Materials and Methods
2.1. Rambutan Seed Preparation
Rambutan seed waste was supplied by Pissanumhon Food Products Company Limited, Thayang, Muang, Chumphon Province, Thailand. Feedstock was cleaned under running tap water prior to drying under hot-air circulation at 60 °C for 8 h. The fat from dried rambutan seeds was pre-extracted with a screw press machine at a feed rate of 7 kg/h. A kilogram of screw-pressed cake was subsequentially extracted by supercritical CO2–ethanol extraction at 30 MPa and a temperature of 50 ± 5 °C for a static extraction time of 90 min. Proximate analysis of DRS was conducted using AOAC standard methods.
2.2. Subcritical Water Extraction and Biological Activity Assay
The SWE was performed at a constant ratio of 20 g of sample to 200 mL of DI water (1:10 w/v). Extraction conditions were achieved by using the central composite design, as shown in Table 1. In this work, SWE was compared to HWE at 100 °C for 60 min. The extractor (Parr company, Series 4625, 500 mL working volume) was changed for every DRS sample and pressurized with nitrogen to 2 MPa. After extraction time was achieved, the extractor was quenched in an ice-water bath to room temperature. An amount of 100 mL of extract was filtered through a paper filter, centrifuged, and mixed with 95% ethanol at a sample-to-ethanol ratio of 1:4 (v/v). The mixture was incubated overnight at 4 °C before being subjected to centrifugation at 8000 rpm for 20 min at 4 °C to separate the precipitated POLS-DRSs. The POLS-DRSs were dried in a hot-air oven at 40 °C for 24 h. POLS-DRS yield was calculated from the weight of dried precipitate divided by the weight of DRS. The POLS-DRSs were ground for further bioactive analysis. The total sugar content was determined by the phenol–sulfuric acid method using D-glucose as a standard. Antioxidant activities and total phenolic content (TPC) of POLS-DRSs were measured with a DPPH radical scavenging assay at substrate concentration of 40 mg/mL [6].

Table 1.
Experimental conditions of POLS-DRS.
2.3. Statistical Analysis
The statistical analysis results of all data are reported in terms of average ± S.D. The total variation in data was estimated by one-way analysis of variance (ANOVA). Duncan’s multiple range test (DMRT) was used for determining significance (p ≤ 0.05) with SPSS program version 22.
3. Results and Discussion
3.1. Proximate Analysis of DRS
The proximate analysis of DRS showed a moisture content of 2.57 ± 0.07% (w/w), carbohydrate content of 72.31 ± 0.08% (w/w), fat content of 8.07 ± 0.32% (w/w), protein content of 8.95 ± 0.21% (w/w), crude fiber content of 6.19 ± 0.11% (w/w), and ash content of 1.91 ± 0.01 %(w/w). In our previous work, RS had contents of 29.00 ± 0.18% (w/w) of fat and 42.96 ± 0.15% (w/w) of carbohydrates [7]. It was clear that screw-press and supercritical CO2–ethanol extractions effectively removed fat from RSs and enhanced carbohydrates in DRS.
3.2. Effects of Temperature and Time on POLS-DRS Yield
POLS-DRS yields obtained under various conditions are illustrated in Figure 1. The HWE generated the highest POLS-DRS yield of 51.01 g/100 g DRS because the extracted polysaccharides were water-soluble starch [8]. At a temperature of 120 °C, the maximum POLS-DRS yield of 41.71 g/100 g DRS was produced at 30 min, whereas the SWE conducted at 140 °C gave a maximum POLS-DRS yield of 36.72 g/100 g DRS at 15 min. At an escalated temperature, subcritical water partially hydrolyzed polysaccharides to oligosaccharides [9]. Hence, temperature and time are crucial parameters for SWE.
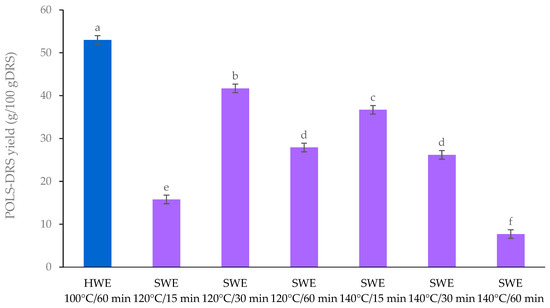
Figure 1.
POLS-DRS yields obtained from HWE and SWE. Mean values with different superscript letters in each column are significantly different (p ≤ 0.05, DMRT).
3.3. Total Sugar, DPPH Activity, and pH of POLS-DRS Solution
According to Table 2, for SWE at a temperature of 120 °C and extraction time of 60 min, a highest total sugar content of 49.76 g/100 g POLS-DRSs and DPPH scavenging activity of 82.93% inhibition at 40 mg of POLS-DRS/mL were observed, while a lower TPC of 28.78 mg of gallic acid equivalent (GAE)/100 g POLS-DRSs was seen. The total sugar content and DPPH activity of POLS-DRSs obtained from SWE were higher than those obtained from HWE. TPC was found to be low for all samples, except the sample produced at 140 °C for 60 min. The pH values of POLS-DRS solutions were within the range of 6.55–7.15, which is suitable for microbiological growth. The monosaccharide profile of POLS-DRSs will be analyzed in a further study.

Table 2.
Total sugar, TPC (mg/100 g POLS-DRS), DPPH (% inhibition), and pH of POLS-DRSs obtained under conditions.
4. Conclusions
POLS-DRSs were extracted from DRS using HWE and SWE. Under the optimum extraction conditions, a temperature of 120 °C and an extraction time of 60 min, SWE resulted in a yield of 27.91 g/100 g DRS, with higher total sugar and DPPH contents than those obtained with HWE. Considering the use of POLS-DRSs as a prebiotic, a higher total sugar content, lower TPC, and neutral pH were expected. Except for the sample obtained at 140 °C for 60 min, all samples were suitable for testing prebiotic activity. The exponentially increasing TPC after an extraction time of 30 min at 140 °C indicates that the degradation of lignin and tannin took place under this condition.
Author Contributions
Conceptualization, R.S. and S.N. (Somkiat Ngamprasertsith); methodology, W.S. and P.J.; formal analysis and investigation, K.N.; resources, K.N. and S.N. (Sajee Noitang); data curation, K.N. and R.S.; writing—original draft preparation, K.N.; writing—review and editing, S.N. (Somkiat Ngamprasertsith), A.K. and R.S.; supervision, A.K. and R.S.; funding acquisition, R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by Thailand Science Research and Innovation Fund Chulalongkorn University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors are grateful to Pissanumhon Food Products Company Limited, Chumphon Province, Thailand, for suppling the rambutan seeds. The authors also express their appreciation to Sarintip Sooksai and Acting Capt. Weradaj Sukaead for their helpful guidance and sample handling.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Sirisompong, W.; Jirapakkul, W.; Klinkesorn, U. Response surface optimization and characteristics of rambutan (Nephelium lappaceum L.) kernel fat by hexane extraction. LWT-Food Sci. Technol. 2011, 44, 1946. [Google Scholar] [CrossRef]
- Jirarattanarangsri, W.; Muangrat, R. Comparison of supercritical CO2 and screw press extraction methods for producing oil from Camellia sinensis var. assamica seeds: Physicochemical properties and antioxidant activity. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100413. [Google Scholar] [CrossRef]
- Muthusamy, S.; Udayakumar, G.P.; Narala, V.R. Recent advances in the extraction and characterization of seed polysaccharides, and their bioactivities: A review. Bioact. Carbohydr. Diet. Fibre 2021, 26, 100276. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183. [Google Scholar] [CrossRef]
- Witayaudom, P.; Klinkesorn, U. Effect of surfactant concentration and solidification temperature on the characteristics and stability of nanostructured lipid carrier (NLC) prepared from rambutan (Nephelium lappaceum L.) kernel fat. J. Colloid Interface Sci. 2017, 505, 1082. [Google Scholar] [CrossRef] [PubMed]
- Sakdasri, W.; Sila-ngam, P.; Chummengyen, S.; Sukruay, A.; Ngamprasertsith, S.; Supang, W.; Sawangkeaw, R. Optimization of yield and thymoquinone content of screw press-extracted black cumin seed oil using response surface methodology. Ind. Crops Prod. 2023, 191, 113895. [Google Scholar] [CrossRef]
- Nilmat, K.; Sakdasri, W.; Karnchanatat, A.; Sawangkeaw, R. Biocomposite film from Rambutan seed oil extracted by green extraction technologies. In Proceedings of the International Symposium on Green Chemistry (ISGC2022), La Rochelle, France, 16–20 May 2022; National Center for Scientific Research (CNRS): Paris, France, 2022. [Google Scholar]
- Gu, J.; Zhang, H.; Yao, H.; Zhou, J.; Duan, Y.; Ma, H. Comparison of characterization, antioxidant and immunological activities of three polysaccharides from Sagittaria sagittifolia L. Carbohydr. Polym. 2020, 235, 115939. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Liu, H.M.; Yan, Y.Y.; Fan, L.Y.; Yang, J.N.; Wang, X.D.; Qin, G.Y. Structural characterization and antioxidant activity of polysaccharides extracted from jujube using subcritical water. LWT-Food Sci. Technol. 2020, 117, 108645. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).