Thermal Properties of Expanded Amaranth Seed Oil †
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction Using the Soxhlet Apparatus
2.2. Determination of Oil’s Melting Characteristics
2.3. Determination of Oil Oxidation Time
2.4. Determination of Oil Crystallization Temperature
2.5. Determination of Fatty Acid Composition
3. Results and Discussion
3.1. Melting Profile
3.2. Oil Oxidation Time at Different Temperatures
3.3. Oil Crystallization Temperature
3.4. Fatty Acid Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ogrodowska, D.; Zadernowski, R.; Tańska, M.; Czaplicki, S. Physical properties of amaranth seeds (Amaranthus cruentus) from various regions of cultivation in Poland. Żyw. Nauk. Technol. Jak. 2011, 6, 91–104. (In Polish) [Google Scholar]
- Kulczyński, B.; Gramza-Michałowska, A.; Grdeń, M. Amaranth—Nutritional value and health-promoting properties. Brom. Chem. Toksykol. 2017, 1, 1–7. (In Polish) [Google Scholar]
- Szumera, M. Characteristics of selected thermal methods (part 1). LAB Lab. Apar. Bad. 2012, 17, 28–34. (In Polish) [Google Scholar]
- Kardas, M.; Grochowska-Niedworok, E. Differential scanning calorimetry as a thermoanalytical method used in pharmacy and food analysis. Brom Chem. Toksykol. 2009, 42, 224–230. (In Polish) [Google Scholar]
- Żontała, K.; Łopacka, J.; Lipińska, A.; Rafalska, U. Methods of thermal analysis of food with particular emphasis on differential scanning calorimetry. Post. Tech. Przet. Spoż. 2015, 2, 97–104. (In Polish) [Google Scholar]
- Ostrowska-Ligęza, E.; Mańko-Jurkowska, D.; Brozio, S.; Wirkowska-Wojdyła, M.; Bryś, J.; Głowacka, R.; Górska, A. The assesment of oxidative stability and melting characteristic of palm oil and cocoa butter. Zeszyt. Probl. Post. Nauk. Rol. 2019, 596, 45–54. [Google Scholar] [CrossRef]
- Zhou, J.; Xiong, Y.; Liu, X. Evaluation of the oxidation stability of biodiesel stabilized with antioxidants using the Rancimat and PDSC methods. Fuel 2017, 188, 61–68. [Google Scholar] [CrossRef]
- Luque de Castro, M.; Priego-Capote, F. Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska-Ligęza, E.; Szulc, K.; Wirkowska, M.; Górska, A.; Lenart, A. Influence of agglomeration and coating of infant formula powders on the stability of essential fatty acids. Acta Agrophys. 2012, 19, 77–88. (In Polish) [Google Scholar]
- Ratusz, K.; Kowalski, B.; Bekas, W.; Wirkowska, M. Monitoring of rapeseed and sunflower oil autooxidation. Oilseed Crops 2005, 26, 211–220. (In Polish) [Google Scholar]
- Tomaszewska-Gras, J. Influence of milk fat cooling speed on the crystallization process of milk fat triacylglycerols. Żyw. Nauk. Technol. Jak. 2014, 95, 97–107. (In Polish) [Google Scholar]
- Wirkowska, M.; Bryś, J.; Górska, A.; Ostrowska-Ligęza, E.; Tarnowska, K. An attempt to enrich milk fat with EPA and DHA acids. Żyw. Nauk. Technol. Jak. 2004, 2, 69–80. (In Polish) [Google Scholar]
- Pawłowicz, R. The influence of the temperature setting procedure and the method of measurement on the results of the evaluation of the solid phase content in selected fats. Żyw. Nauk. Technol. Jak. 2012, 3, 46–55. (In Polish) [Google Scholar]
- Anastasiu, A.; Chira, N.; Banu, I.; Ionescu, N.; Stan, R.; Rosca, S. Oil productivity of seven Romanian linseed varieties as affected by weather conditions. Ind. Crop Prod. 2016, 86, 219–230. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ratusz, K.; Ostrowska-Ligęza, E.; Krygier, K. Impact of Selected Chemical Characteristics of Cold-Pressed Oils on their Oxidative Stability Determined Using the Rancimat and Pressure Differential Scanning Calorimetry Method. Food Anal. Method. 2018, 11, 1095–1104. [Google Scholar] [CrossRef]
- Pietrzyk, S.; Fortuna, T.; Pabiś, E. Influence of modification temperature on starch oxidation and its physical and chemical properties. Nauka Przyr. Technol. 2012, 6, 1–9. (In Polish) [Google Scholar]
- Tomaszewska-Gras, J. Detection of butter adulteration with water using differential scanning calorimetry. J. Therm. Anal. Calorim. 2012, 108, 433–438. [Google Scholar] [CrossRef][Green Version]
- Ratusz, K.; Wirkowska, M. Characteristics of amaranth seeds and lipids. Oilseed Crops 2006, 27, 243–250. (In Polish) [Google Scholar]
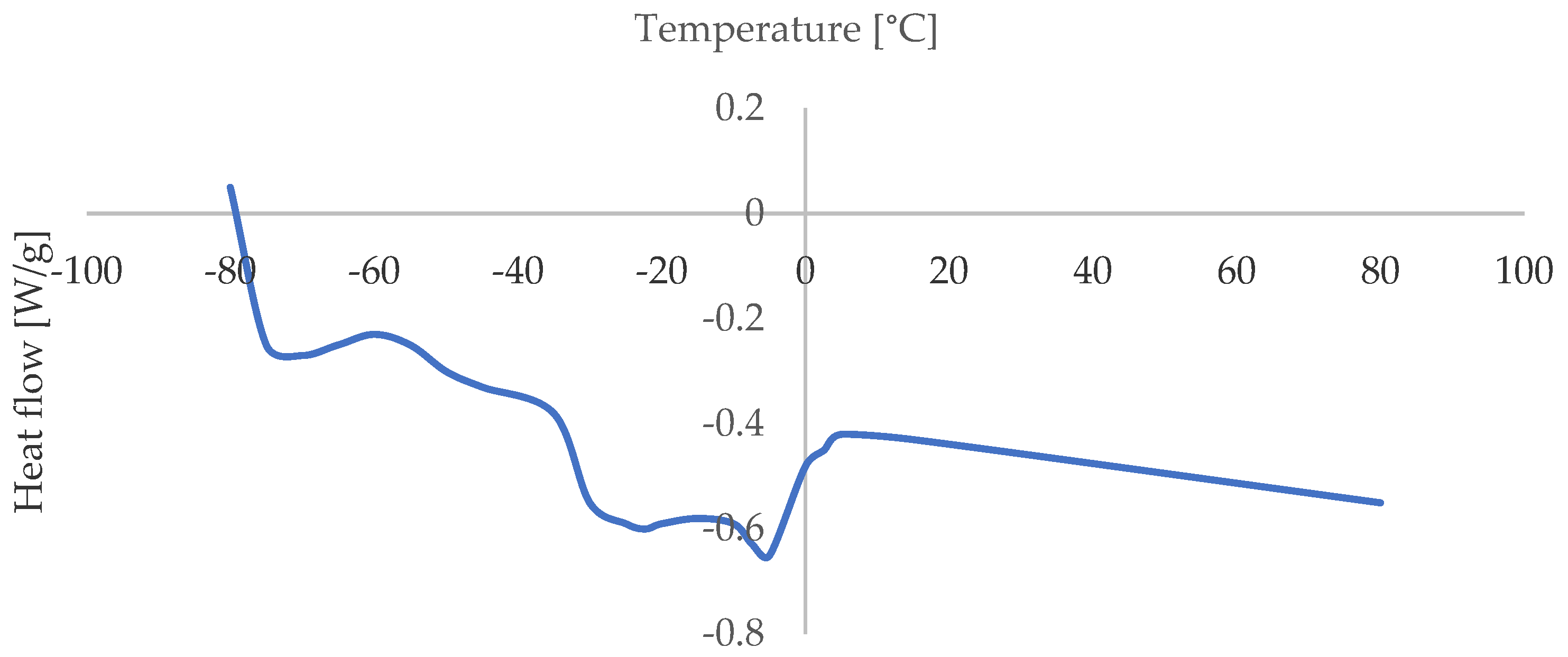

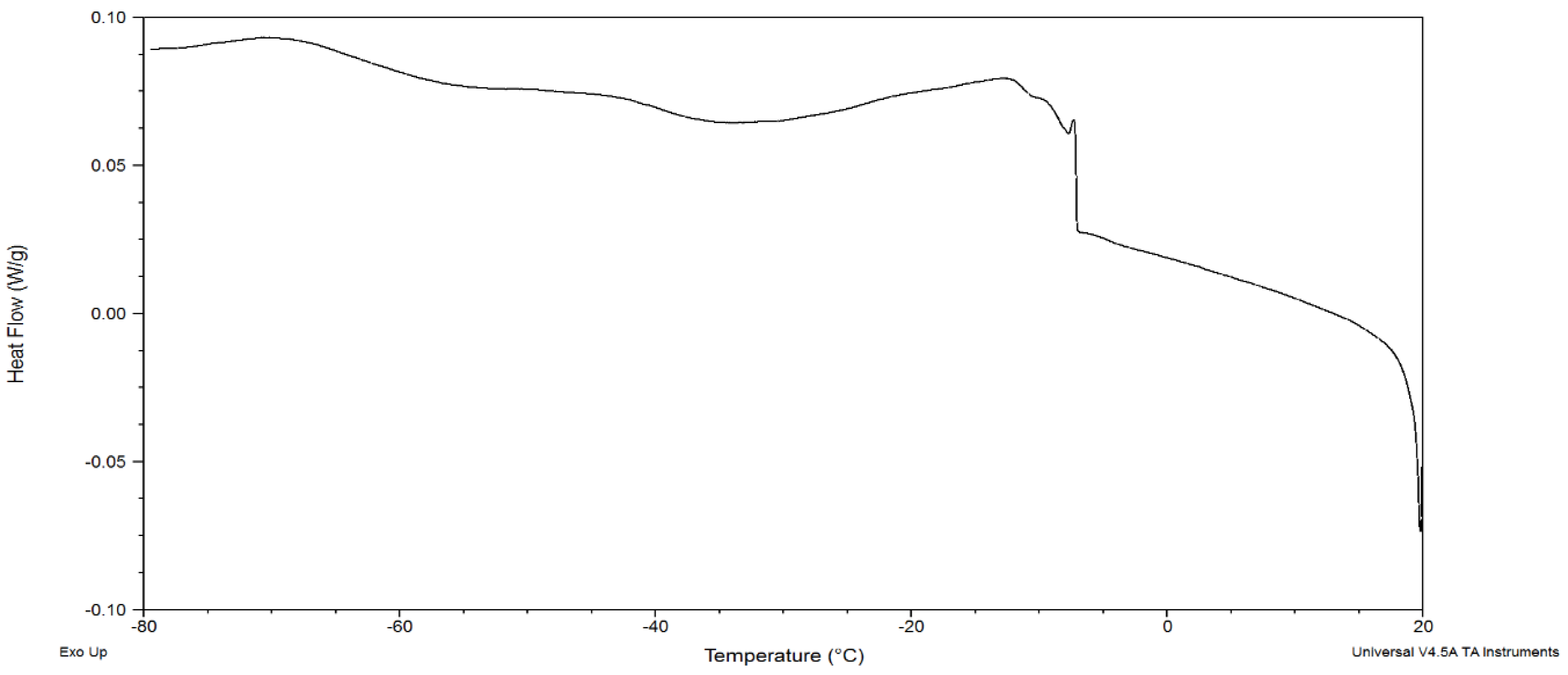
| Fatty Acid | Percentage (%) |
|---|---|
| Linoleic C18:2 n-6c | 34.65 ± 0.07 |
| Oleic C18:1 n-9c | 18.05 ± 0.07 |
| Palmitic C16:0 | 15.05 ± 0.21 |
| Docozadiene C22:2 n-6 | 8.85 ± 0.07 |
| Stearic C18:0 | 3.10 ± 0.0 |
| Others | 20.20 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostrowska-Ligęza, E.; Sidoruk, K.; Wirkowska-Wojdyła, M.; Brzezińska, R.; Piasecka, I.; Górska, A. Thermal Properties of Expanded Amaranth Seed Oil. Biol. Life Sci. Forum 2023, 26, 73. https://doi.org/10.3390/Foods2023-15101
Ostrowska-Ligęza E, Sidoruk K, Wirkowska-Wojdyła M, Brzezińska R, Piasecka I, Górska A. Thermal Properties of Expanded Amaranth Seed Oil. Biology and Life Sciences Forum. 2023; 26(1):73. https://doi.org/10.3390/Foods2023-15101
Chicago/Turabian StyleOstrowska-Ligęza, Ewa, Karolina Sidoruk, Magdalena Wirkowska-Wojdyła, Rita Brzezińska, Iga Piasecka, and Agata Górska. 2023. "Thermal Properties of Expanded Amaranth Seed Oil" Biology and Life Sciences Forum 26, no. 1: 73. https://doi.org/10.3390/Foods2023-15101
APA StyleOstrowska-Ligęza, E., Sidoruk, K., Wirkowska-Wojdyła, M., Brzezińska, R., Piasecka, I., & Górska, A. (2023). Thermal Properties of Expanded Amaranth Seed Oil. Biology and Life Sciences Forum, 26(1), 73. https://doi.org/10.3390/Foods2023-15101









