Natural Product-Based Drug Discovery for Monkeypox Virus: Integrating In Silico Approaches and Therapeutic Development Strategies
Abstract
1. Introduction
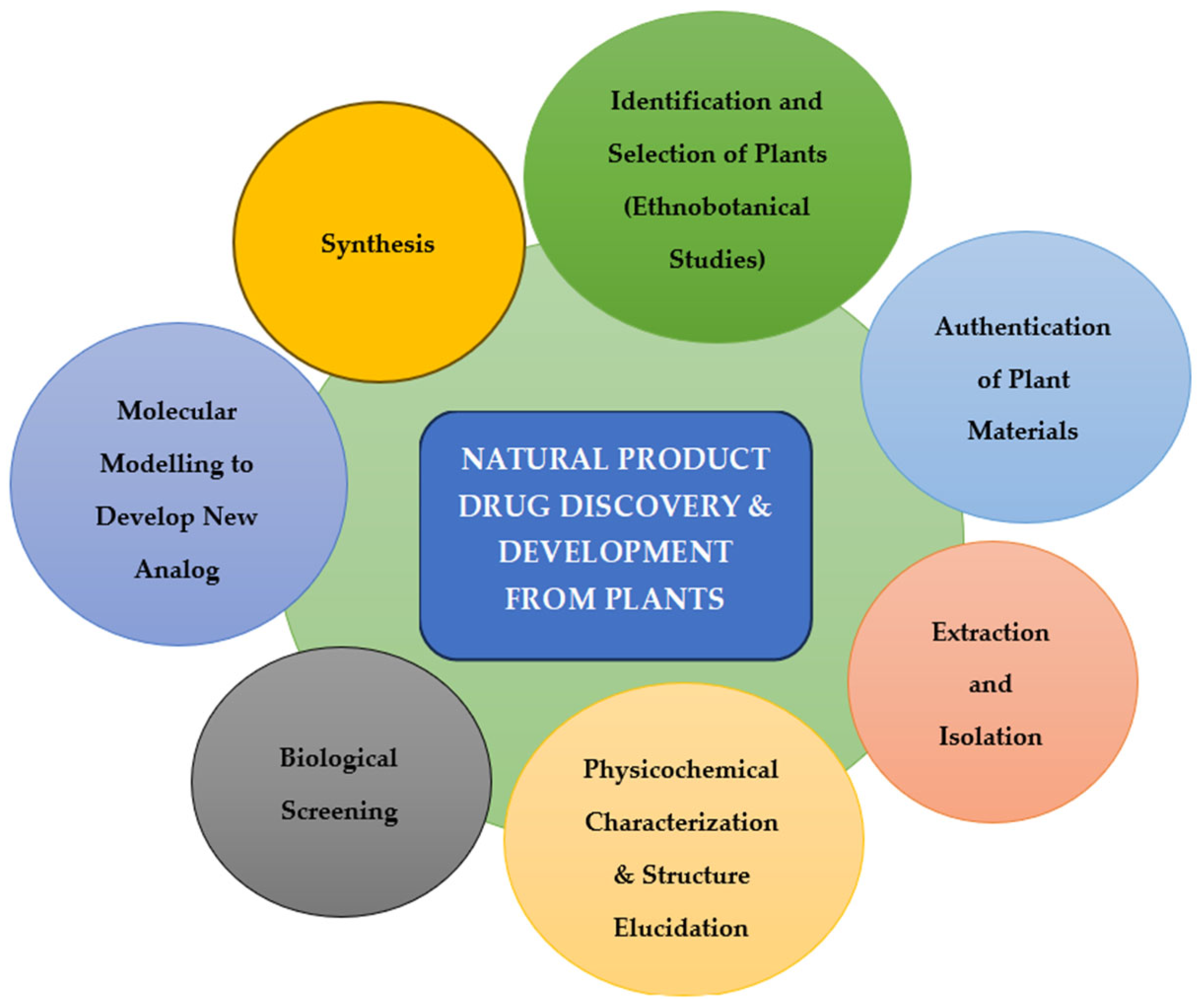
2. Natural Compounds in Antiviral Drug Discovery
2.1. Potential of Bioactive Natural Compounds as Antiviral Agents
| Natural Compound and Chemical Structure | Plant Source | Molecular Target | Antiviral Activity | Other reported Therapeutic Indications/Benefits | Probable Mechanism of Action Against Poxviruses | Refs |
|---|---|---|---|---|---|---|
Curcumin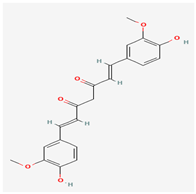 | Curcuma longa (Turmeric) | DNA Polymerase, Methyltransferase VP39, A42R Profilin-like Protein, Envelope Protein E8 | Inhibits viral replication | Anti-inflammatory, Antioxidant, Anticancer, Neurological Health, Cardiovascular Support, Metabolic Disorders | Inhibit viral replication by binding to viral proteins and exhibit antioxidant and anti-inflammatory properties. | [20,30] |
Demethoxycurcumin (DMC)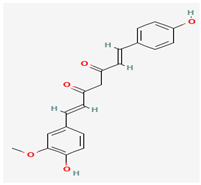 | Curcuma longa (Turmeric) | DNA Polymerase, Thymidylate Kinase, Profilin-like Protein | Potential inhibition of viral activity | Anti-inflammatory, Antioxidant, Apoptosis Induction, Inhibition of Cancer Cell Invasion, Neuroprotective Effects, Inhibition of Cancer Cell Invasion | Interacts with viral proteins, potentially inhibiting their function | [31] |
Bisdemethoxycurcumin (BDMC)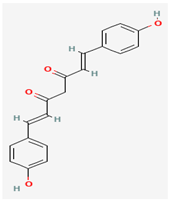 | Curcuma longa (Turmeric) | Thymidylate Kinase | Potential disruption of viral replication | Anti-inflammatory, Antioxidant, Apoptosis Induction, Antimicrobial Activity | Contributes to therapeutic efficacy through complementary biological effects. | [30] |
Epigallocatechin Gallate (EGCG)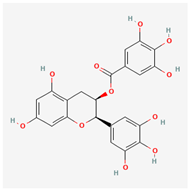 | Camellia sinensis (Green Tea) | Under investigation (Interact with viral proteins and host cell receptors) | Inhibits viral infections by directly binding to viral particles, preventing attachment to host cells. | modulates various signaling pathways, including the MAPK, PI3K/Akt, and NF-κB pathways, Cancer Suppression, Neuroprotection, Diabetes Management, Cardiovascular Health | Exhibits virucidal effects by binding to viral particles and inhibiting host cell attachment. | [32,33] |
Folic Acid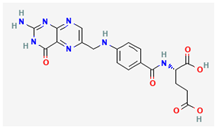 | Various plant sources | Methyltransferase (MTase) | Inhibits MTase activity, reducing viral replication | Essential vitamin for DNA synthesis and repair. Possesses therapeutic potential in neurological disorders, cancer, cardiovascular diseases, and metabolic syndromes. | Occupies active site of MTase, hindering its interaction with mRNA substrate | [34] |
1,2,4,6-Tetragalloylglucose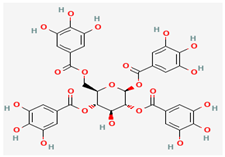 | Inhibits MTase activity, reducing viral replication | Antioxidant, Antimicrobial, Anticomplement Activity, UDP Glucuronosyltransferase Inhibition | Binds to MTase, inhibiting its function | [34] | ||
Gedunin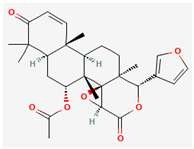 | Azadirachta indica (Neem) | Profilin-like Protein | Potential inhibition of viral replication | Antimalarial, Anticancer (Inhibition of Hsp90, modulates the Shh/Gli signaling pathway), Neuroprotection | Binds to profilin-like protein, disrupting its function | [28] |
Piperine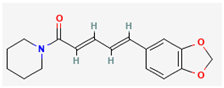 | Piper nigrum (Black Pepper) | Potential inhibition of viral replication | Bioavailability enhancer, Anti-Inflammatory and Antioxidant Effects, Blood Sugar Regulation, Cholesterol Management, Cognitive Function | Interacts with profilin-like protein, inhibiting its activity | [28,35] | |
Coumadin (Warfarin)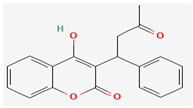 | Synthetic derivative of natural compounds | Potential inhibition of viral replication | Anticoagulant | Binds to profilin-like protein, disrupting its function | [22,28] | |
Rosmarinic Acid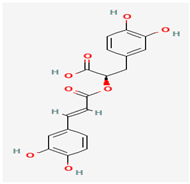 | Rosmarinus officinalis (Rosemary)/Salvia rosmarinus (Rosemary) | Various viral proteins | Antiviral activity against poxviruses | Anti-inflammatory, Antioxidant, Neuroprotective Properties, Anticancer Potential, Cardioprotective Effects, Metabolic Benefits | Inhibits viral replication through multiple mechanisms | [36,37,38] |
Caffeic Acid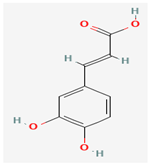 | Various plant sources | Various viral proteins | Antiviral activity against poxviruses | Anti-inflammatory, Antioxidant, Cancer Treatment Adjuvant, | Inhibits viral replication through multiple mechanisms | [37] |
Resveratrol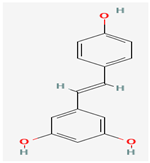 | Vitis vinifera (Grapes) | Various viral proteins | Antiviral activity against poxviruses | Cardioprotective (Cardiovascular Health), Antioxidant, Metabolic Effects, Anti-Aging and Longevity, Cancer Prevention, Neuroprotection | Inhibits viral replication through multiple mechanisms | [37,39] |
Myricitrin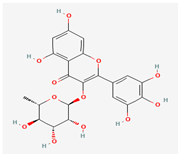 | Various plant sources | Various viral proteins | Antiviral activity against poxviruses | Anti-inflammatory, Antioxidant, Neuropharmacological Effects, Inhibition of Nitric Oxide and Protein Kinase C | Inhibits viral replication through multiple mechanisms | [38,40] |
Gingerol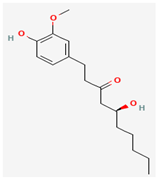 | Zingiber officinale (Ginger) | Various viral proteins | Antiviral activity against poxviruses | Anti-inflammatory and Immunomodulatory Effects, Antioxidant, Anticancer Properties, Neuroprotective Effects, Antimicrobial Activity, Inhibition of Nitric Oxide and Protein Kinase C/ | Inhibits viral replication through multiple mechanisms | [19,41,42] |
Gallotannins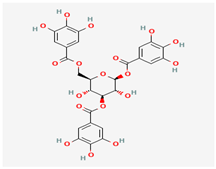 | Various plant sources | Various viral proteins | Antiviral activity against poxviruses | Antioxidant, Antimicrobial, Anticancer Properties, Anti-inflammatory Action | Inhibits viral replication through multiple mechanisms | [19,22] |
Propolis-benzofuran A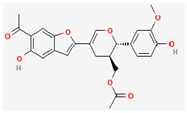 | Apis mellifera (Honeybee Propolis) | Various viral proteins | Antiviral activity against poxviruses | Antimicrobial Photodynamic Therapy, Anti-inflammatory, Cytotoxic Activity, | Inhibits viral replication through multiple mechanisms | [43,44] |
Galanthamine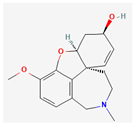 | Galanthus species (Snowdrop) | Various viral proteins | Antiviral activity against poxviruses | Treatment of Alzheimer disease, Vascular Dementia, Cognitive Impairment in Various Conditions, Autism Spectrum Disorders, Organophosphate Poisoning | Inhibits viral replication through multiple mechanisms | [22,45] |
Thalimonine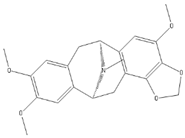 | Various plant sources (Thalictrum simplex,…) | Various viral proteins | Antiviral activity against poxviruses | Antimicrobial. | Inhibits viral replication through multiple mechanisms | [22,46] |
Luteolin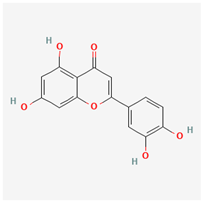 | Potential inhibition of viral entry | Anti-inflammatory, Antioxidant, Antiviral Activity, Anticancer Potential, Neuroprotective Properties | Modulates various cellular pathways, including inhibition of pro-inflammatory cytokines and enzymes, suppression of cancer cell proliferation, and enhancement of antioxidant defenses | [19,47] | ||
Quercitrin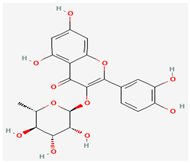 | Antiviral activity against poxviruses | Anti-inflammatory, Antioxidant, Cardiovascular protection, antimicrobial properties | Inhibits quinone reductase 2 (QR2) | [48,49,50] |
2.2. Historical Success of Natural Products in Drug Development
2.3. Existing Evidence of Natural Compounds with Antiviral Activity Against Poxviruses
3. In Silico Strategies for Drug Repurposing in Mpox
4. Molecular Docking and Binding Affinity
4.1. Identifying Key Monkeypox Viral Proteins as Therapeutic Targets
| Compound Class | Representative Natural Compounds | Putative MPXV Viral/Host Targets | Proposed Mechanism of Interaction | Evidence Level (Typical) | Translational Notes/Limitations | Refs |
|---|---|---|---|---|---|---|
| Flavonoids | Amentoflavone, Quercetin derivatives, Rutin | Viral proteases (I7L), VP39 methyltransferase, envelope/attachment factors (E8) | Direct binding to catalytic or substrate pockets → inhibition of proteolysis or methylation; also host-kinase modulation (MAPK/NF-κB) | Mostly in silico docking + MD; occasional orthogonal in vitro antiviral assays in related poxviruses | Exhibit high polarity, poor oral bioavailability, and rapid metabolism. Require formulation (nanocarriers) or semi-synthetic optimization. | [38,77] |
| Alkaloids | Catharanthine, Harmine-type analogs | I7L protease, structural proteins (D13L), host kinases | Occupy protease active sites or allosteric pockets; some show predicted multi-target binding to host factors that virus co-opts | In silico docking/MD with some cross-study consensus; limited phenotypic follow-up | Exhibit potential cytotoxicity at high doses, interact with P-glycoprotein, and cause drug–drug interactions; require cytotoxicity and pharmacokinetic profiling. | [22,38] |
| Polyphenols/Tannins | Punicalagin, EGCG (epigallocatechin gallate) | Envelope/attachment proteins (E8), entry mediators, viral surface proteins | High-affinity surface binding that can block attachment/entry; may also chelate metal cofactors | In silico + some in vitro viral inhibition (plaque/foci assays) in related viruses | Very polar, large MW → poor systemic exposure; topical or localized delivery may be more practical. | [32,46] |
| Phenolic acids | Rosmarinic acid, Caffeic acid derivatives | Host signaling hubs (STAT3, NF-κB), viral envelope proteins | Modulate host inflammatory pathways and may indirectly reduce viral replication; predicted binding to envelope proteins | In silico + supporting literature on host modulation (limited direct MPXV phenotypic data) | Anti-inflammatory benefit may reduce immunopathology but not a direct antiviral; ADME optimization needed. | [22,78] |
| Naphthodianthrones/Photoactive polycycles | Pseudohypericin, Hypericin-like | Multi-target binding (VP39, F13L/VP37, polymerase interfaces) | Predicted stable binding to several viral proteins; photodynamic activity reported for related viruses | In silico docking/MD; known antiviral activity in other viruses (photoactivated) but MPXV data limited | Phototoxicity and formulation challenges; safety concerns require careful evaluation. | [62,79] |
| Terpenoids/Triterpenes | Betulinic acid derivatives, Ursolic acid analogs | Viral envelope maturation factors, host membrane processes | Disrupt membrane fusion/egress by interacting with viral envelope proteins or host lipid pathways | In silico + some cell-based assays against enveloped viruses | Low water solubility: chemical modification or nanoparticle delivery often required. | [22,46] |
| Stilbenoids & Resveratrol-type | Resveratrol, Pterostilbene | Host factors (SIRT, NF-κB) and putative weak binding to viral enzymes | Host-directed immunomodulation and modest direct inhibition predicted against replication/minor viral enzymes | In silico + broad antiviral literature; limited direct MPXV phenotypic validation | Rapid metabolism and low plasma exposure; prodrugs or sustained-release formulations may help. | [39,80] |
| Coumarins & other phenolics | Esculetin, Scopoletin | Viral decapping enzymes (D9), replication-associated proteins | Predicted competitive binding to enzyme active sites, possible synergy with nucleoside analogs | In silico with occasional MD refinement | Moderate drug-likeness; further target deconvolution and ADME profiling needed. | [22,30] |
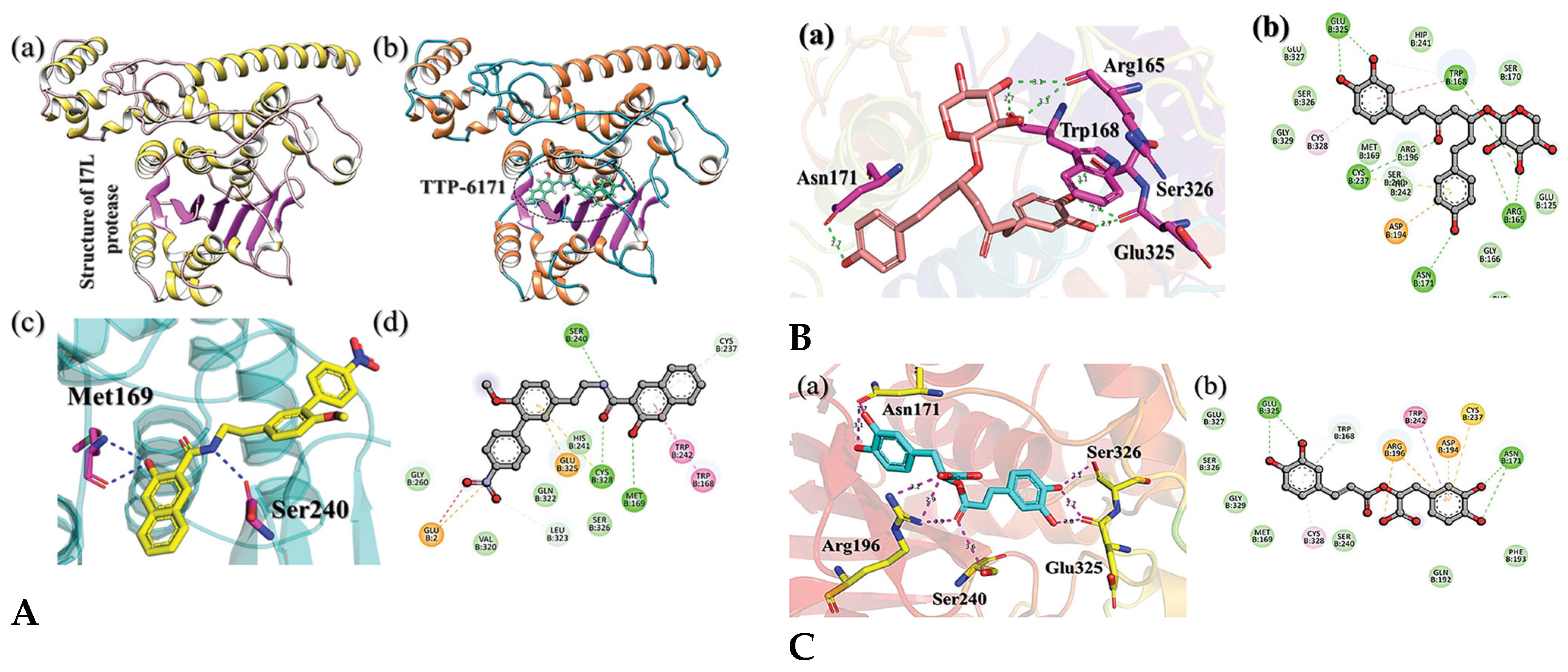
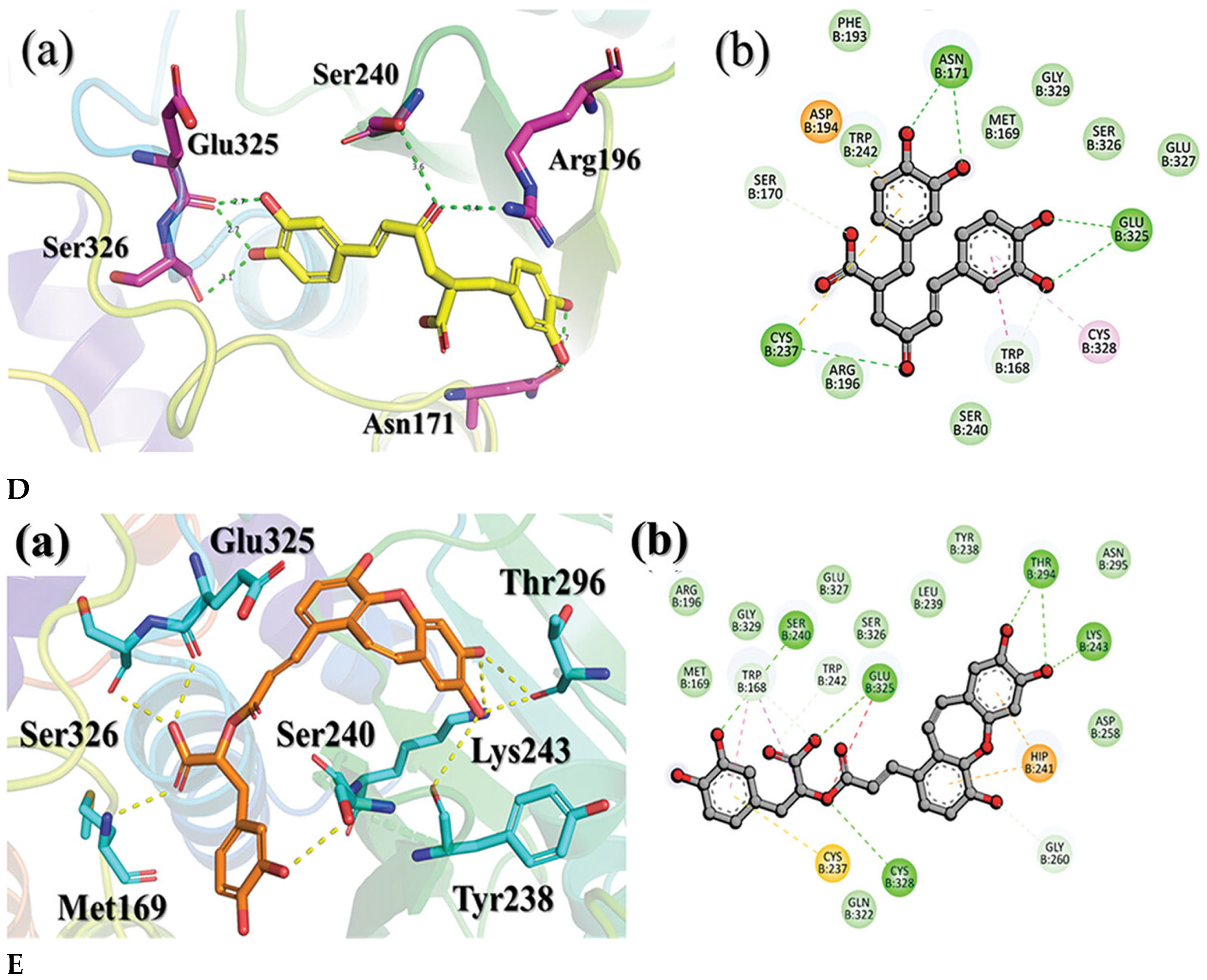
4.2. Docking Natural Compounds Against Viral Proteins
4.3. Evaluating Binding Affinity and Interaction Mechanisms
5. Pharmacokinetics and ADMET Predictions in MPXV Drug Discovery
5.1. Importance of Drug-Likeness, and ADMET Profiling
5.2. Computational Tools for Assessing Pharmacokinetics Properties of Repurposed Compounds
5.3. Case Studies of Natural Compounds with Favorable ADMET Properties
6. Future Perspectives and Challenges
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karagoz, A.; Tombuloglu, H.; Alsaeed, M.; Tombuloglu, G.; AlRubaish, A.A.; Mahmoud, A.; Smajlović, S.; Ćordić, S.; Rabaan, A.A.; Alsuhaimi, E. Monkeypox (mpox) virus: Classification, origin, transmission, genome organization, antiviral drugs, and molecular diagnosis. J. Infect. Public Health 2023, 16, 531–541. [Google Scholar] [CrossRef]
- Begum, J.S.; Ngangom, L.; Semwal, P.; Painuli, S.; Sharma, R.; Gupta, A. Emergence of monkeypox: A worldwide public health crisis. Hum. Cell 2023, 36, 877–893. [Google Scholar] [CrossRef]
- Ebede, S.O.; Orabueze, I.N.; Maduakor, U.C.; Nwafia, I.N.; Ohanu, M.E. Recurrent Mpox: Divergent virulent clades and the urgent need for strategic measures including novel vaccine development to sustain global health security. BMC Infect. Dis. 2025, 25, 536. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Singh, P.K.; Branda, F.; Mishra, S.; Kutikuppala, L.S.; Suvvari, T.K.; Kandi, V.; Ansari, A.; Desai, D.N.; Alfaresi, M. Transmission dynamics, complications and mitigation strategies of the current mpox outbreak: A comprehensive review with bibliometric study. Rev. Med. Virol. 2024, 34, e2541. [Google Scholar] [CrossRef] [PubMed]
- Mushebenge, A.G.-A.; Mphuthi, D.D. Emerging Insights into Monkeypox: Clinical Features, Epidemiology, Molecular Insights, and Advancements in Management. BioMed 2025, 5, 21. [Google Scholar] [CrossRef]
- Mushebenge, A.G.-A.; Mphuthi, D.D. Deciphering Drug Repurposing Strategies: Antiviral Properties of Candidate Agents Against the Mpox Virus. Sci. Pharm. 2025, 93, 51. [Google Scholar] [CrossRef]
- Lu, J.; Xing, H.; Wang, C.; Tang, M.; Wu, C.; Ye, F.; Yin, L.; Yang, Y.; Tan, W.; Shen, L. Mpox (formerly monkeypox): Pathogenesis, prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Cambaza, E.M. A Review of the Molecular Understanding of the Mpox Virus (MPXV): Genomics, Immune Evasion, and Therapeutic Targets. Zoonotic Dis. 2025, 5, 3. [Google Scholar] [CrossRef]
- Ezat, A.A.; Abduljalil, J.M.; Elghareib, A.M.; Samir, A.; Elfiky, A.A. The discovery of novel antivirals for the treatment of mpox: Is drug repurposing the answer? Expert Opin. Drug Discov. 2023, 18, 551–561. [Google Scholar] [CrossRef]
- Srivastava, V.; Naik, B.; Godara, P.; Das, D.; Mattaparthi, V.S.K.; Prusty, D. Identification of FDA-approved drugs with triple targeting mode of action for the treatment of monkeypox: A high throughput virtual screening study. Mol. Divers. 2024, 28, 1093–1107. [Google Scholar] [CrossRef]
- Mohanto, S.; Faiyazuddin, M.; Gholap, A.D.; Jc, D.; Bhunia, A.; Subbaram, K.; Ahmed, M.G.; Nag, S.; Akhtar, M.S.; Bonilla-Aldana, D.K. Addressing the resurgence of global monkeypox (Mpox) through advanced drug delivery platforms. Travel Med. Infect. Dis. 2023, 56, 102636. [Google Scholar] [CrossRef]
- Nandu, T.G.; Jithesh, K. Natural Product-Based Anti-Viral Agents Against RNA Viruses: An Important Strategy for Pandemic Preparedness. In Drugs from Nature: Targets, Assay Systems and Leads; Springer: Singapore, 2024; pp. 411–440. [Google Scholar]
- Gulati, P.; Chadha, J.; Harjai, K.; Singh, S. Targeting envelope proteins of poxviruses to repurpose phytochemicals against monkeypox: An in silico investigation. Front. Microbiol. 2023, 13, 1073419. [Google Scholar] [CrossRef]
- Vardhan, S.; Sahoo, S.K. Computational studies on searching potential phytochemicals against DNA polymerase activity of the monkeypox virus. J. Tradit. Complement. Med. 2023, 13, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Mushebenge, A.G.-A.; Ugbaja, S.C.; Mbatha, N.A.; Khan, B.R.; Kumalo, H.M. Assessing the potential contribution of in silico studies in discovering drug candidates that interact with various SARS-CoV-2 receptors. Int. J. Mol. Sci. 2023, 24, 15518. [Google Scholar] [CrossRef]
- Mushebenge, A.G.; Ugbaja, S.C.; Mtambo, S.E.; Ntombela, T.; Metu, J.I.; Babayemi, O.; Chima, J.I.; Appiah-Kubi, P.; Odugbemi, A.I.; Ntuli, M.L. Unveiling the Inhibitory Potentials of Peptidomimetic Azanitriles and Pyridyl Esters towards SARS-CoV-2 Main Protease: A Molecular Modelling Investigation. Molecules 2023, 28, 2641. [Google Scholar] [CrossRef]
- Morais, G.C.d.F.; de Souza Sena, C.P.; Silva, G.V.R.; Alves, G.B.; Vieira, D.S.; Akash, S.; Aktaruzzaman, M.; Al-Dies, A.-A.M.; Fulco, U.L.; Oliveira, J.I.N. Comprehensive evaluation of potential EBOV inhibitors using advanced molecular modeling techniques: Implications for anti-Ebola therapeutics and rational drug design. Future J. Pharm. Sci. 2025, 11, 145. [Google Scholar] [CrossRef]
- Siddika, A. Computational Investigation of nsP2 Inhibitors for Chikungunya Virus Treatment: Advancing Therapeutic Strategies Against Viral Infections; BRAC University: Dhaka, Bangladesh, 2025. [Google Scholar]
- Ndayambaje, M.; Munyeshyaka, E.; Dieumerci, O.; Habyarimana, T.; Ndishimye, P.; Naya, A.; Oudghiri, M. Plant-derived molecules in monkeypox management: Insight and alternative therapeutic strategies. Beni-Suef Univ. J. Basic Appl. Sci. 2025, 14, 17. [Google Scholar] [CrossRef]
- Maurya, V.K.; Kumar, S.; Maurya, S.; Ansari, S.; Paweska, J.T.; Abdel-Moneim, A.S.; Saxena, S.K. Structure-based drug designing for potential antiviral activity of selected natural product against Monkeypox (Mpox) virus and its host targets. VirusDisease 2024, 35, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Ridho, F.M.; Nur’aini, A.D.; Al Atsariyah, H.; Syachputra, A.J.; Wardana, W.E.; Nurhuda, A. Curcumin and its derivatives as potential antiviral candidates against monkeypox (mpox): A review of computational studies. Ars Pharm. 2025, 66, 225–232. [Google Scholar] [CrossRef]
- Ali, S.I.; Salama, A. Natural immunomodulatory agents as a complementary therapy for poxviruses. In Poxviruses; Springer: Cham, Switzerland, 2024; pp. 337–354. [Google Scholar] [CrossRef]
- Priya, S.P.; Amalraj, S.; Padmanabhan, V.; Rahman, M.M.; Chaitanya, N.C.; Hashim, N.T.; Prabhu, S.; Ayyanar, M.; Gurav, S.; Ceasar, S.A.; et al. Evaluating the Antiviral Potential of Polyherbal Formulation (Kabasura kudineer) Against Monkeypox Virus: Targeting E5, Poxin, and DNA Polymerase Through Multifaceted Drug Discovery Approaches. Life 2025, 15, 771. [Google Scholar] [CrossRef]
- Ashley, C.N.; Broni, E.; Wood, C.M.; Okuneye, T.; Ojukwu, M.T.; Dong, Q.; Gallagher, C.; Miller, W.A., 3rd. Identifying potential monkeypox virus inhibitors: An in silico study targeting the A42R protein. Front. Cell. Infect. Microbiol. 2024, 14, 1351737. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Adil, S.; Qudsia, H.A.; Waheed, Y.; Alshabrmi, F.M.; Wei, D.-Q. Structure-based design of promising natural products to inhibit thymidylate kinase from Monkeypox virus and validation using free energy calculations. Comput. Biol. Med. 2023, 158, 106797. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Dwivedi, S.; Agrawal, R.; Sadashiv; Fatima, G.; Abidi, A. The human Monkeypox virus and host immunity: Emerging diagnostic and therapeutic challenges. Infect. Disord.-Drug Targets 2025, 25, E18715265309361. [Google Scholar] [CrossRef]
- Dubey, A.; Alawi, M.M.; Alandijany, T.A.; Alsaady, I.M.; Altwaim, S.A.; Sahoo, A.K.; Dwivedi, V.D.; Azhar, E.I. Exploration of microbially derived natural compounds against monkeypox virus as viral core cysteine proteinase inhibitors. Viruses 2023, 15, 251. [Google Scholar] [CrossRef]
- Banik, A.; Ahmed, S.R.; Shahid, S.B.; Ahmed, T.; Tamanna, H.K.; Marma, H. Therapeutic Promises of Plant Metabolites against Monkeypox Virus: An In Silico Study. Adv. Virol. 2023, 2023, 9919776. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Mahal, A.; Mohapatra, P.K.; Sarangi, A.K.; Mishra, S.; Alsuwat, M.A.; Alshehri, N.N.; Abdelkhalig, S.M.; Garout, M.; Aljeldah, M.; et al. Structure-based discovery of F. religiosa phytochemicals as potential inhibitors against Monkeypox (mpox) viral protein. J. Biosaf. Biosecurity 2024, 6, 157–169. [Google Scholar] [CrossRef]
- Akash, S.; Hossain, A.; Hossain, M.S.; Rahman, M.M.; Ahmed, M.Z.; Ali, N.; Valis, M.; Kuca, K.; Sharma, R. Anti-viral drug discovery against monkeypox and smallpox infection by natural curcumin derivatives: A Computational drug design approach. Front. Cell. Infect. Microbiol. 2023, 13, 1157627. [Google Scholar]
- Boudou, F.; Belakredar, A.; Keziz, A.; Aissani, L.; Alsaeedi, H.; Cronu, D.; Bechelany, M.; Barhoum, A. Therapeutic potential of Curcuma longa against monkeypox: Antioxidant, anti-inflammatory, and computational insights. Front. Chem. 2025, 12, 1509913. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Szopa, D.; Witek-Krowiak, A. Antiviral properties of polyphenols from plants. Foods 2021, 10, 2277. [Google Scholar] [CrossRef]
- Capasso, L.; De Masi, L.; Sirignano, C.; Maresca, V.; Basile, A.; Nebbioso, A.; Rigano, D.; Bontempo, P. Epigallocatechin Gallate (EGCG): Pharmacological Properties, Biological Activities and Therapeutic Potential. Molecules 2025, 30, 654. [Google Scholar] [CrossRef]
- Hashim, H.O.; Al-Shuhaib, J.M.B.; Mohammed, M.K.; Al-Shuhaib, M.B.S. Targeting Monkeypox Virus Methyltransferase: Virtual Screening of Natural Compounds from Middle-Eastern Medicinal Plants. Mol. Biotechnol. 2024, 67, 3194–3216. [Google Scholar] [CrossRef]
- Tiwari, A.; Mahadik, K.R.; Gabhe, S.Y. Piperine: A comprehensive review of methods of isolation, purification, and biological properties. Med. Drug Discov. 2020, 7, 100027. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Aslam Gondal, T.; Imran, A.; Shahbaz, M.; Muhammad Amir, R.; Wasim Sajid, M.; Batool Qaisrani, T.; Atif, M.; Hussain, G.; et al. Therapeutic Potential of Rosmarinic Acid: A Comprehensive Review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef]
- Langland, J.; Jacobs, B.; Wagner, C.E.; Ruiz, G.; Cahill, T.M. Antiviral activity of metal chelates of caffeic acid and similar compounds towards herpes simplex, VSV-Ebola pseudotyped and vaccinia viruses. Antiviral. Res. 2018, 160, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; An, S.; Chu, J.; Liang, B.; Liao, Y.; Jiang, J.; Lin, Y.; Ye, L.; Liang, H. Potential inhibitors of monkeypox virus revealed by molecular modeling approach to viral DNA topoisomerase I. Molecules 2023, 28, 1444. [Google Scholar] [CrossRef]
- Adepoju, O.A.; Danazumi, A.U.; Dibba, L.B.S.; Ibrahim, B.; Gital, S.I.; Ibrahim, J.G.; Jibrailu, M.L.; Balogun, E.O. Computational interrogation of natural compounds identified resveratrol-3-O-D-glucopyranoside as a potential inhibitor of essential monkeypox virus proteins. Intell. Med. 2025, 5, 5–13. [Google Scholar] [CrossRef]
- Rout, M.; Mishra, S.; Dey, S.; Singh, M.K.; Dehury, B.; Pati, S. Exploiting the potential of natural polyphenols as antivirals against monkeypox envelope protein F13 using machine learning and all-atoms MD simulations. Comput. Biol. Med. 2023, 162, 107116. [Google Scholar] [CrossRef]
- Shaukat, M.N.; Nazir, A.; Fallico, B. Ginger Bioactives: A Comprehensive Review of Health Benefits and Potential Food Applications. Antioxidants 2023, 12, 2015. [Google Scholar] [CrossRef]
- Sharma, S.; Shukla, M.K.; Sharma, K.C.; Tirath; Kumar, L.; Anal, J.M.H.; Upadhyay, S.K.; Bhattacharyya, S.; Kumar, D. Revisiting the therapeutic potential of gingerols against different pharmacological activities. Naunyn Schmiedeberg’s Arch. Pharmacol. 2023, 396, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Prichard, M.N.; Kern, E.R. Orthopoxvirus targets for the development of new antiviral agents. Antivir. Res. 2012, 94, 111–125. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Bahador, A. Virtual screening and computational simulation analysis of antimicrobial photodynamic therapy using propolis-benzofuran A to control of Monkeypox. Photodiagnosis Photodyn. Ther. 2023, 41, 103208. [Google Scholar] [CrossRef]
- Ghate, S.D.; Pinto, L.; Alva, S.; Srinivasa, M.G.; Vangala, R.K.; Naik, P.; Revanasiddappa, B.; Rao, R.S.P. In silico identification of potential phytochemical inhibitors for mpox virus: Molecular docking, MD simulation, and ADMET studies. Mol. Divers. 2024, 28, 4067–4086. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, K.V.; Prabha, T.; Karthikeyan, J.; Rani, C.I.; Vethamoni, P.I.; Bharathi, A. Harnessing nature’s arsenal: A comprehensive review of phytomedicine approaches in Monkeypox treatment. Ann. Phytomedicine 2024, 13, 23–32. [Google Scholar] [CrossRef]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytother. Res. 2022, 36, 266–278. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.; Fakola, E.G.; Ghosh, S.; Ghosh, S.; Das, S. From phytomedicine to conventional drug research, to look for new drug molecule against monkey pox virus; a molecular docking, virtual screening and ADME analysis. Silico Pharmacol. 2025, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Israr, J.; Pandey, J.; Misra, S. Current Approaches and Implications in Discovery of Novel Bioactive Products from Microbial Sources. Curr. Microbiol. 2025, 82, 258. [Google Scholar] [CrossRef]
- Cech, N.B.; Oberlies, N.H. From plant to cancer drug: Lessons learned from the discovery of taxol. Nat. Prod. Rep. 2023, 40, 1153–1157. [Google Scholar] [CrossRef]
- Dsouza, L.; Pant, A.; Offei, S.; Priyamvada, L.; Pope, B.; Satheshkumar, P.S.; Wang, Z.; Yang, Z. Antiviral activities of two nucleos(t)ide analogs against vaccinia, mpox, and cowpox viruses in primary human fibroblasts. Antivir. Res. 2023, 216, 105651. [Google Scholar] [CrossRef]
- Ahmad, F.; Sachdeva, A.; Sah, B.K.; Kumar, A.; Kumar, R.; Seksaria, B. A review of herbal therapeutics for the prevention and management of poxvirus infections. Arch. Microbiol. 2025, 207, 186. [Google Scholar] [CrossRef]
- Sharifan, A. Natural Compounds against Mpox: Mapping Evidence and Identifying Gaps. Planta Med. 2025, 91, 271–273. [Google Scholar] [CrossRef]
- Grajales, D.B.; Kar, S. Exploring Monkeypox: Prospects for therapeutics through computational-aided drug discovery. Mol. Divers. 2023, 28, 3497–3521. [Google Scholar] [CrossRef] [PubMed]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef] [PubMed]
- Shaker, B.; Ahmad, S.; Lee, J.; Jung, C.; Na, D. In silico methods and tools for drug discovery. Comput. Biol. Med. 2021, 137, 104851. [Google Scholar] [CrossRef]
- Shrivastava, R.; Modi, G.; Satpathy, P.S.; Bandyopadhyay, S.; Kumar, Y.; Yadav, I. Natural Products Revolutionizing and Innovative Drug Discovery and Development Strategies: Healthcare Challenges and Future Perspectives. J. Appl. Bioanal. 2024, 10, 20–38. [Google Scholar]
- Moore, M.J.; Rathish, B.; Zahra, F. Mpox (Monkeypox); StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Polyphenols and their potential role to fight viral diseases: An overview. Sci. Total Environ. 2021, 801, 149719. [Google Scholar] [CrossRef] [PubMed]
- Abduljalil, J.M.; Elfiky, A.A.; Elgohary, A.M. Exploration of natural compounds against the human mpox virus DNA-dependent RNA polymerase in silico. J. Infect. Public Health 2023, 16, 996–1003. [Google Scholar] [CrossRef]
- Aydemir, D.; Ulusu, N.N. The possible importance of the antioxidants and oxidative stress metabolism in the emerging monkeypox disease: An opinion paper. Front. Public Health 2022, 10, 1001666. [Google Scholar] [CrossRef]
- Rajak, P.; Ganguly, A.; Dey, S.; Sen, K. In silico targeting of pox virus proteins to repurpose triterpenes against monkeypox infection. Comput. Struct. Biotechnol. Rep. 2025, 2, 100027. [Google Scholar] [CrossRef]
- Winkler, D.A. Computational repurposing of drugs for viral diseases and current and future pandemics. J. Math. Chem. 2024, 62, 2844–2879. [Google Scholar] [CrossRef]
- Khan, S.; Ray, I. Bioactive Phytochemicals for Human Monkeypox Outbreak. Asian J. Res. Infect. Dis. 2025, 16, 24–43. [Google Scholar] [CrossRef]
- Trepte, P.; Secker, C.; Olivet, J.; Blavier, J.; Kostova, S.; Maseko, S.B.; Minia, I.; Silva Ramos, E.; Cassonnet, P.; Golusik, S.; et al. AI-guided pipeline for protein-protein interaction drug discovery identifies a SARS-CoV-2 inhibitor. Mol. Syst. Biol. 2024, 20, 428–457. [Google Scholar] [CrossRef]
- Pathan, I.; Raza, A.; Sahu, A.; Joshi, M.; Sahu, Y.; Patil, Y.; Raza, M.A.; Ajazuddin. Revolutionizing pharmacology: AI-powered approaches in molecular modeling and ADMET prediction. Med. Drug Discov. 2025, 28, 100223. [Google Scholar] [CrossRef]
- Kushwaha, J.M.; Rani, M.S.S.; Singh, S. Targeting monkeypox virus (MPXV): Strategies for molecular docking studies on protein inhibition. Virus Genes 2025, 61, 523–534. [Google Scholar] [CrossRef]
- Mishra, S.K.; Chandra, A.; Mitra, N.; Krishna, N.; Singh, N.; Akash, S.; Jardan, Y.A.B.; Bourhia, M.; Georrge, J.J. Exploring the Dynamics of Asparagus racemosus Phytochemicals as Dual Target Inhibitors of Monkeypox Virus. Curr. Med. Chem. 2025, 32, 8678–8700. [Google Scholar] [CrossRef] [PubMed]
- Al Mashud, M.A.; Kumer, A.; Mukerjee, N.; Chandro, A.; Maitra, S.; Chakma, U.; Dey, A.; Akash, S.; Alexiou, A.; Khan, A.A.; et al. Mechanistic inhibition of Monkeypox and Marburg virus infection by O-rhamnosides and Kaempferol-o-rhamnosides derivatives: A new-fangled computational approach. Front. Cell. Infect. Microbiol. 2023, 13, 1188763. [Google Scholar] [CrossRef]
- Athar, M.; Sona, A.N.; Bekono, B.D.; Ntie-Kang, F. Fundamental physical and chemical concepts behind “drug-likeness” and “natural product-likeness”. Phys. Sci. Rev. 2019, 4, 20180101. [Google Scholar] [CrossRef]
- Chen, A.; Fang, N.; Zhang, Z.; Wen, Y.; Shen, Y.; Zhang, Y.; Zhang, L.; Zhao, G.; Ding, J.; Li, J. Structural basis of the monkeypox virus mRNA cap N7 methyltransferase complex. Emerg. Microbes Infect. 2024, 13, 2369193. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Xie, Y.; Kuai, L.; Wang, H.; Qi, J.; Gao, G.F.; Shi, Y. Structure of monkeypox virus DNA polymerase holoenzyme. Science 2023, 379, 100–105. [Google Scholar] [CrossRef]
- Grifoni, L.; Vanti, G.; Donato, R.; Sacco, C.; Bilia, A.R. Promising Nanocarriers to Enhance Solubility and Bioavailability of Cannabidiol for a Plethora of Therapeutic Opportunities. Molecules 2022, 27, 6070. [Google Scholar] [CrossRef]
- Adeosun, W.B.; Loots, D.T. Medicinal Plants against Viral Infections: A Review of Metabolomics Evidence for the Antiviral Properties and Potentials in Plant Sources. Viruses 2024, 16, 218. [Google Scholar] [CrossRef]
- Balasundar, A.; Parsanathan, R. In Silico Screening of Phenylpropanoid and Polyketide Phytochemicals as Potential Inhibitors of Mpox Viral Proteins. Exon 2025, 2, 132–151. [Google Scholar] [CrossRef]
- Astakala, R.V.; Preet, G.; Haj Hasan, A.; Desai, R.; Alfurayh, M.; Ebel, R.; Jaspars, M. Computational repurposing of polyphenols for anti-Mpoxviral activity. Silico Pharmacol. 2025, 13, 65. [Google Scholar] [CrossRef]
- Alandijany, T.A.; El-Daly, M.M.; Tolah, A.M.; Bajrai, L.H.; Khateb, A.M.; Kumar, G.S.; Dubey, A.; Dwivedi, V.D.; Azhar, E.I. A multi-targeted computational drug discovery approach for repurposing tetracyclines against monkeypox virus. Sci. Rep. 2023, 13, 14570. [Google Scholar] [CrossRef]
- Duta-Bratu, C.G.; Nitulescu, G.M.; Mihai, D.P.; Olaru, O.T. Resveratrol and Other Natural Oligomeric Stilbenoid Compounds and Their Therapeutic Applications. Plants 2023, 12, 2935. [Google Scholar] [CrossRef]
- Khan, A.; Shahab, M.; Nasir, F.; Waheed, Y.; Alshammari, A.; Mohammad, A.; Zichen, G.; Li, R.; Wei, D. Exploring the Traditional Chinese Medicine (TCM) database chemical space to target I7L protease from monkeypox virus using molecular screening and simulation approaches. SAR QSAR Environ. Res. 2023, 34, 689–708. [Google Scholar] [CrossRef]
- Shah, B.M.; Modi, P. Breaking Barriers: Current Advances and Future Directions in Mpox Therapy. Curr. Drug Targets 2024, 25, 62–76. [Google Scholar] [CrossRef]
- Miah, M.M.; Tabassum, N.; Afroj Zinnia, M.; Islam, A.B.M.M.K. Drug and anti-viral peptide design to inhibit the monkeypox virus by restricting A36R protein. Bioinform. Biol. Insights 2022, 16, 11779322221141164. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, Y.; Ye, Z.; Ali, A.; Yao, S. Bioactive Deep Eutectic Solvent-Involved Sprayable Versatile Hydrogel for Monkeypox Virus Lesions Treatment. ACS Appl. Mater. Interfaces 2024, 17, 2148–2168. [Google Scholar] [CrossRef] [PubMed]
- Dodaro, A.; Pavan, M.; Moro, S. Targeting the I7L protease: A rational design for anti-monkeypox drugs? Int. J. Mol. Sci. 2023, 24, 7119. [Google Scholar] [CrossRef]
- Yousaf, M.A.; Basheera, S.; Sivanandan, S. Inhibition of Monkeypox Virus DNA polymerase using Moringa oleifera Phytochemicals: Computational studies of drug-Likeness, molecular docking, molecular dynamics simulation and density functional theory. Indian J. Microbiol. 2024, 64, 1057–1074. [Google Scholar] [CrossRef] [PubMed]
- Raen, R.; Islam, M.M.; Islam, R.; Islam, M.R.; Jarin, T. Functional characterization and structural prediction of hypothetical proteins in monkeypox virus and identification of potential inhibitors. Mol. Divers. 2025, 29, 1589–1617. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Mahal, A.; Ansari, A.; Kumar, M.; Guru, J.P.; Sarangi, A.K.; Abdou, A.; Mishra, S.; Aljeldah, M.; AlShehail, B.M. Comparison of the binding energies of approved mpox drugs and phytochemicals through molecular docking, molecular dynamics simulation, and ADMET studies: An in silico approach. J. Biosaf. Biosecurity 2023, 5, 118–132. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Halwani, M.A.; Alshehri, A.A.; Al-Subaie, M.F.; Almansour, Z.H.; AlShehail, B.M.; Alotaibi, N.; Khamis, F.; Al Kaabi, N.A.; Alsomali, G. Bioprospecting of Meliaceae family phytomolecules for the treatment of monkeypox virus infection: A QSAR modeling and MD simulation approach. J. Biomol. Struct. Dyn. 2025, 43, 2277–2299. [Google Scholar] [CrossRef]
- Imran, M.; Abida; Alotaibi, N.M.; Thabet, H.K.; Alruwaili, J.A.; Eltaib, L.; Alshehri, A.; Alsaiari, A.A.; Kamal, M.; Alshammari, A.M.A. Repurposing anti-dengue compounds against monkeypox virus targeting core cysteine protease. Biomedicines 2023, 11, 2025. [Google Scholar] [CrossRef]
- Dutt, M.; Kumar, A.; Rout, M.; Dehury, B.; Martinez, G.; Ndishimye, P.; Kelvin, A.A.; Kelvin, D.J. Drug repurposing for Mpox: Discovery of small molecules as potential inhibitors against DNA-dependent RNA polymerase using molecular modeling approach. J. Cell. Biochem. 2023, 124, 701–715. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Z.; Huang, Y.; Liu, L.; He, D.; Wang, W.; Fang, X.; Zhang, X.; Wang, F.; Wu, H.; et al. HelixADMET: A robust and endpoint extensible ADMET system incorporating self-supervised knowledge transfer. Bioinformatics 2022, 38, 3444–3453. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; Lin, Y.; Zhang, M.; Liu, O.; Shuai, J.; Zhao, Q. A Multi-Task Self-Supervised Strategy for Predicting Molecular Properties and FGFR1 Inhibitors. Adv. Sci. 2025, 12, e2412987. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Saha, S.; Basu, S.; Chakraborti, T. Computational analysis of pathogen-host interactome for fast and low-risk in-silico drug repurposing in emerging viral threats like Mpox. Sci. Rep. 2024, 14, 18736. [Google Scholar] [CrossRef] [PubMed]
- Asif, S.; Zhao, M.; Li, Y.; Tang, F.; Ur Rehman Khan, S.; Zhu, Y. AI-based approaches for the diagnosis of Mpox: Challenges and future prospects. Arch. Comput. Methods Eng. 2024, 31, 3585–3617. [Google Scholar] [CrossRef]
- Abdizadeh, T. Identification of novel potential inhibitors of monkeypox virus thymidine kinase using molecular docking, molecular dynamics simulation and MM/PBSA methods. Mol. Divers. 2024, 28, 2513–2546. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-P.; Zhao, R.; Wang, H.-F.; Wang, M.-Y.; Hu, W.-S.; Lin, M.-M.; Shu, W.; Sun, Y.-J.; Cao, J.-M.; Cui, W. Crystal structure of mRNA cap (guanine-N7) methyltransferase E12 subunit from monkeypox virus and discovery of its inhibitors. Int. J. Biol. Macromol. 2023, 253, 127565. [Google Scholar] [CrossRef]
- Arasu, M.V.; Vijayaragavan, P.; Purushothaman, S.; Rathi, M.A.; Al-Dhabi, N.A.; Gopalakrishnan, V.K.; Choi, K.C.; Ilavenil, S. Molecular docking of monkeypox (mpox) virus proteinase with FDA approved lead molecules. J. Infect. Public Health 2023, 16, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.; Khan, A.A.; Alanazi, A.M.; Abdikakharovich, S.A.; Shah, J.A.; Ren, Z.-G.; Khattak, S. Identification of the myxobacterial secondary metabolites Aurachin A and Soraphinol A as promising inhibitors of thymidylate kinase of the Monkeypox virus. Mol. Divers. 2024, 28, 3349–3362. [Google Scholar] [CrossRef]
- Tupally, K.R.; Seal, P.; Pandey, P.; Lohman, R.J.; Smith, S.; Ouyang, D.; Parekh, H. Integration of Dendrimer-Based Delivery Technologies with Computational Pharmaceutics and Their Potential in the Era of Nanomedicine. Explor. Comput. Pharm. AI Model. Pharma 4.0 2024, 12, 328–378. [Google Scholar]
- Al-Sulaiti, H.; Almaliti, J.; Naman, C.B.; Al Thani, A.A.; Yassine, H.M. Metabolomics approaches for the diagnosis, treatment, and better disease management of viral infections. Metabolites 2023, 13, 948. [Google Scholar] [CrossRef]
- El-kenawy, E.-S.M. Leveraging Advanced Machine Learning for Pioneering Monkeypox Diagnosis: A New Paradigm in Infectious Disease Detection. Metaheuristic Optim. Rev. 2024, 1, 1–16. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mushebenge, A.G.-A.; Mphuthi, D.D. Natural Product-Based Drug Discovery for Monkeypox Virus: Integrating In Silico Approaches and Therapeutic Development Strategies. Future Pharmacol. 2025, 5, 69. https://doi.org/10.3390/futurepharmacol5040069
Mushebenge AG-A, Mphuthi DD. Natural Product-Based Drug Discovery for Monkeypox Virus: Integrating In Silico Approaches and Therapeutic Development Strategies. Future Pharmacology. 2025; 5(4):69. https://doi.org/10.3390/futurepharmacol5040069
Chicago/Turabian StyleMushebenge, Aganze Gloire-Aimé, and David Ditaba Mphuthi. 2025. "Natural Product-Based Drug Discovery for Monkeypox Virus: Integrating In Silico Approaches and Therapeutic Development Strategies" Future Pharmacology 5, no. 4: 69. https://doi.org/10.3390/futurepharmacol5040069
APA StyleMushebenge, A. G.-A., & Mphuthi, D. D. (2025). Natural Product-Based Drug Discovery for Monkeypox Virus: Integrating In Silico Approaches and Therapeutic Development Strategies. Future Pharmacology, 5(4), 69. https://doi.org/10.3390/futurepharmacol5040069





