Abstract
The presence of 2-methylisoborneol (2-MIB) in water is a critical global concern due to its low threshold and resistance to conventional processes. In the present study, activated carbon/alginate (AC/alginate) composite beads were synthesized via ionic gelation method for the removal of 2-MIB from aqueous solution. The physicochemical characteristics of the adsorbent were determined using scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR) techniques. The effects of contact time, solution pH, initial 2-MIB concentration and adsorbent dose on adsorption were examined. Over 95% of 2-MIB removal was obtained under optimum conditions within 360 min. The adsorption equilibrium was well described by Langmuir (R2 = 0.97) and Freundlich (R2 = 0.96) models suggesting that 2-MIB adsorption involves both monolayer and multilayer adsorption. Kinetic modeling revealed that the pseudo-first order model showed strong fits to the experimental data, indicating the role of surface adsorption in controlling the rate of adsorption. The adsorbent demonstrated reasonable stability, retaining 59% removal efficiency after four adsorption–desorption cycles, highlighting its potential for repeated application in water treatment. Overall, the AC/alginate composite beads were found to be promising for the effective elimination of 2-MIB from water.
1. Introduction
The occurrence of taste and odor in drinking water has become a growing global concern [1,2,3,4]. 2-methylisoborneol (2-MIB) is one of the primary taste and odor compounds in water, typically originating from cyanobacteria and actinomycetes [5,6,7]. 2-MIB can impart an unpleasant earthy or musty odor to water, which can be detected by humans at concentrations as low as 2.5 ng/L, leading to consumer dissatisfaction and concerns over drinking water quality [8,9]. According to Japan’s drinking water quality standards the concentration of 2-MIB in tap water must not exceed 10 ng/L [10,11]. It has been reported that conventional treatment methods, including coagulation, sedimentation, and filtration are inefficient for the removal of 2-MIB [12,13]. Compared to conventional techniques, adsorption is regarded as a more effective approach for 2-MIB removal owing to its low cost, high removal efficiency and simplicity in operation and handling [14,15]. Different adsorbents have already been studied for 2-MIB removal including activated carbon (AC), graphene oxide, porous coordination polymers, zeolites, metal doped TiO2, ceramic adsorbents and bentonite-based composites [16,17,18,19,20,21]. Activated carbon-based adsorbents have been the primary focus of most previous studies. Different types of AC materials such as powdered activated carbon (PAC), superfine PAC, granular activated carbon (GAC), and activated carbon nanotubes have been reported to remove 2-MIB in aqueous solutions [22,23,24,25,26,27,28,29,30,31]. PAC has been shown to be more effective than GAC for the removal of 2-MIB due to its high adsorption kinetics and greater surface area [32,33,34]. However, due to their fine size, PAC particles are difficult to separate from treated water, requiring additional treatment steps that increase sludge generation and operational complexity [35,36]. Moreover, PAC regeneration is less efficient due to its small size, making thermal or chemical regeneration energy intensive. As a result, spent PAC is often discarded, raising economic and environmental concerns. These limitations reduce the practical applicability of powdered activated carbon in industrial wastewater treatment. Encapsulating powdered activated carbon within polymer composite beads has emerged as a promising strategy to overcome the challenges of regeneration and recovery [37,38].
Sodium alginate, a naturally occurring polysaccharide extracted from brown seaweed, has gained increasing attention as a versatile biopolymer in environmental applications. Its unique gel forming ability in the presence of divalent cations such as Ca2+ makes it particularly suitable for fabricating hydrogel beads and composite materials with tailored porosity and stability [39,40]. Sodium alginate serves as an effective immobilization matrix for immobilizing powder-type adsorbents, facilitating easier handling, separation and reusability [41,42]. Previous studies have shown that alginate-based polymeric composite beads exhibit advantageous properties, including high porosity, large surface area, stability, biodegradability, environmental safety, and abundant availability [43,44].
In this study, spherical composite beads were developed by incorporating powdered activated carbon into alginate for efficient 2-MIB removal. The morphology and functional groups of the adsorbent were examined using scanning electron microscopy (SEM) and Fourier transformed infrared spectroscopy (FTIR). The effects of various experimental parameters on 2-MIB adsorption were investigated along with adsorption isotherms and kinetics. Additionally, desorption and reusability of the adsorbent were examined.
2. Materials and Methods
2.1. Materials
Activated carbon Norit SX plus-powder, sodium alginate powder (300–400 cP), calcium chloride (CaCl2), hydrochloric acid (HCl), sodium hydroxide (NaOH), sodium chloride (NaCl), and 2-MIB standard material were purchased from FUJIFILM Wako Pure Chemical Corporation, Japan. All reagents were of analytical grade and used without further purification. Deionized (DI) water was used for the preparation of all solutions.
2.2. Methods
2.2.1. Preparation of the Adsorbent
The ionic gelation method was employed to fabricate calcium AC/alginate composite beads [45,46,47,48,49]. About 3 g of sodium alginate was dissolved in 200 mL of deionized water at 60 °C under vigorous stirring. The solution was stirred vigorously for 1 h to obtain a uniform viscous mixture. Subsequently, 1.2 g of PAC was dispersed in 100 mL of DI water and slowly incorporated into the sodium alginate solution under vigorous stirring. The mixture was further homogenized using a sonicator. The resulting suspension was then introduced dropwise to a 0.5 mol/L calcium chloride solution using a peristaltic pump to form spherical beads. The beads were immersed in calcium chloride solution for 24 h to ensure complete crosslinking. The obtained AC/alginate composite beads were gently collected from the crosslinking medium and subsequently washed with distilled water and used for adsorption experiments.
2.2.2. Characterization of the Adsorbent
The surface morphology of the synthesized materials was examined using SEM (SU1510, Hitachi, Tokyo, Japan). Fourier transform infrared (FTIR) spectra were obtained using a Tensor II FT-IR spectrometer (Bruker Optics, Tokyo, Japan) within the spectral range of 4000–500 cm−1. The point of zero charge (pHPZC) of the adsorbent was determined using the ∆pH drift method. About 0.45 g of the adsorbent was added to 50 mL of 0.01 M NaCl solutions with initial pH values ranging from 1 to 12. The solutions were agitated for 24 h under room temperature after which the final pH was recorded to determine pHPZC [50].
2.2.3. Adsorption Experiments
Adsorption experiments were conducted to determine the effects of contact time, dosage, initial concentration, and solution pH.
Isotherm experiments were performed at room temperature (25 ± 1 °C) by mixing 0.45 g of the synthesized AC/alginate composite with 50 mL of 2-MIB solutions with different initial concentrations (250–1250 ng/L). The mixtures were shaken at 100 rpm for 360 min until equilibrium was achieved. Upon separation of the beads, the residual 2-MIB concentrations were quantified using gas chromatography–mass spectrometry.
For kinetic studies, 0.45 g of the AC/alginate composite was shaken with 50 mL of 500 ng/L 2-MIB solution at 100 rpm for different periods of time, varying from 60 to 360 min, using separate tubes for each contact time. The 2-MIB concentration in each solution was determined following isolation of the adsorbent.
The removal efficiency (R%), the removal capacity at time t (qt, ng/g), and the removal capacity at equilibrium (qe, ng/g), were calculated from the following equations:
where C0 and Ce are initial and equilibrium concentrations (ng/L), respectively, Ct (ng/L) is the concentrations of adsorbate at time t. V is the solution volume (L) and m is the adsorbent dosage (g).
2.2.4. Adsorption Kinetic Models
The kinetics of 2-MIB adsorption on AC/alginate composite were investigated using pseudo-first order, and pseudo-second order models, which are described below.
- Pseudo-first order kinetic model
The pseudo-first order kinetic model assumes that the rate of adsorption is proportional to the number of unoccupied sites on the adsorbent surface.
This model can be represented by the following equation:
where qt and qe represent the adsorption capacity at time t (min) and equilibrium, respectively, and k1 (min−1) is the pseudo-first order rate constant.
- Pseudo-second order kinetic model
This model assumes that the rate limiting step is controlled by electron sharing or exchange between the adsorbent and the adsorbate.
The pseudo-second order equation is shown below:
where k2 is the pseudo-second order rate constant and qe is the adsorption capacity at equilibrium.
2.2.5. Adsorption Isotherm Models
The adsorption isotherms of 2-MIB onto the AC/alginate composite were examined using Langmuir, Freundlich and Temkin isotherm models.
- Langmuir isotherm model
The Langmuir isotherm model assumes that maximum adsorption is achieved when a complete monolayer of solute molecules uniformly covers the adsorbent surface.
The linearized form of Langmuir model is given below:
where k is the Langmuir constant and qmax is the maximum monolayer adsorption capacity.
The key features of the Langmuir isotherm can also be represented using a dimensionless separation factor, or equilibrium parameter, RL, defined as follows:
where C0 (ng L−1) represents the highest initial concentration of the adsorbate, and K (L ng−1) is the Langmuir constant. The dimensionless separation factor, RL, reflects the favorability of the adsorption process. Specifically, RL > 1 indicates unfavorable adsorption, RL = 1 denotes linear adsorption, 0 < RL <1 corresponds to favorable adsorption, and RL = 0 suggests irreversible adsorption.
- Freundlich isotherm model
The Freundlich isotherm is an empirical model that assumes the surface of the adsorbent material is heterogenous. This heterogeneity results from the coexistence of different functional groups on the adsorbent surface and the diverse interactions occurring between the adsorbent and adsorbate.
The Freundlich isotherm model, in its linearized form, is presented below:
where Ce is the equilibrium concentration of the adsorbate, Kf is the Freundlich adsorption constant, and n is the adsorption intensity or heterogeneity factor.
- Temkin model
The Temkin isotherm is a two-parameter model based on the assumption that the heat of adsorption decreases linearly with the amount of adsorbate adsorbed, owing to interactions between the adsorbate and the adsorbent.
The linearized equation can be expressed as follows:
where, R is the universal gas constant (8.314 J/mol K), T is the absolute temperature (K), KT is the Temkin isotherm equilibrium binding constant (L/g), and n is the Temkin isotherm constant (J/mol) related to the heat of adsorption.
2.2.6. Regeneration Experiments
Reusability and regeneration capacity of an adsorbent are important characteristics that determine its feasibility. Accordingly, regeneration experiments were conducted to examine the reusability of the prepared adsorbent. Approximately 0.45 g of the adsorbent was mixed with 50 mL of 500 ng/L 2-MIB solution and stirred at 25 °C and 100 rpm for 360 min. After adsorption, the saturated adsorbent was separated and treated with a 0.5 M NaCl in a 30% v/v ethanol solution for desorption of 2-MIB at 25 °C and 100 rpm for 30 min. The beads were then thoroughly washed with deionized water and reused for adsorption under identical conditions. This regeneration–adsorption process was repeated for four successive cycles.
2.2.7. Analysis of 2-MIB
2-MIB was analyzed by the headspace-gas chromatography/mass spectrometry (HS-GC/MS) method using a Shimadzu GCMS-QP2010 instrument (Shimadzu, Kyoto, Japan) equipped with a Restek Rtx-5ms capillary GC column (30 m × 0.25 mm× 0.25 µm; Restek, Bellefonte, PA, USA). Headspace 2-MIB extraction was performed using a solid phase microextraction (SPME) fiber (Supelco 57293-U, Sigma Aldrich, Japan) at 70 °C for 30 min with agitation at 700 rpm. The fiber was subsequently introduced into the GC injection port, where the analyte was thermally desorbed at 230 °C for 3 min under splitless conditions. Carrier gas was helium at a constant flow rate of 5 mL/min. The GC oven temperature was kept constant at 40 °C for 3 min, followed by a temperature increase to 250 °C at 15 °C/min and stabilized for 5 min. The GC–MS interface temperature was maintained at 250 °C. Total analysis time was 20 min. The monitoring ions m/z 95 and 107 were used to detect 2-MIB [51]. The concentrations of 2-MIB was determined using calibration curves prepared from standard solutions. Stock solutions of 2-MIB (CRM47523, Sigma, Japan) were used to achieve target concentrations of 5, 10, 25, 50, 100, 250, 500, 1000, and 1250 ng/L. To reduce measurement errors, 50 mL of each concentration was prepared, and there 2 mL aliquots from each were used to construct the calibration curves, ensuring reproducibility and accuracy in quantification.
3. Results and Discussion
3.1. Characterization of the Adsorbent
The surface morphology of the dried AC/alginate composite beads was examined using SEM at different magnifications (Figure 1). At low magnification (Figure 1a) the beads exhibited a nearly spherical shape with an average diameter of about 1.14 mm. The external surface appears rough and irregular, which reflects the successful incorporation of ACinto the alginate matrix. At higher magnification (Figure 1b) the bead surface reveals a heterogenous morphology with pores indicating the formation of a porous structure during the ionic gelation process. Further magnification (Figure 1c,d) highlights the presence of pores ranging from 679 nm to 4.8 µm, which represent macropores within the bead structure.
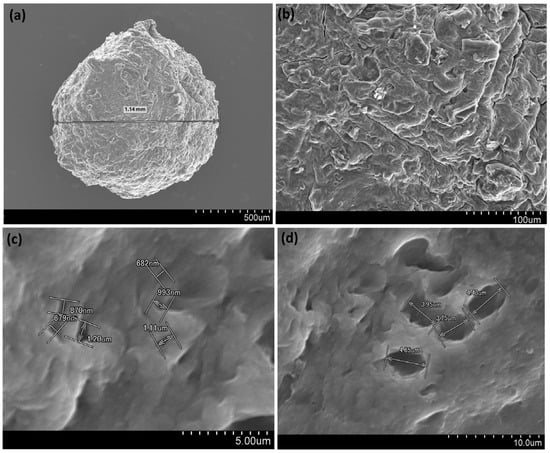
Figure 1.
SEM images of AC/alginate composite beads (dry) under different magnification, scale bar = 500 µm (a) scale bar = 100 µm (b) scale bar = 5 µm (c) scale bar = 10 µm (d).
FTIR spectra of the AC/alginate composite is shown in Figure 2a. The distinct peaks associated with alginate structure were observed at 3275, 1591, 1409, and 1022 cm−1. The broad adsorption band in the range of 3600–3000 cm−1 is attributed to the O−H stretching vibrations of the hydroxyl groups present in the polymer structure [52]. The peak at 1591 cm−1 is due to the asymmetric stretching vibration of the C=O bond in the carboxylate group and the peak at 1409 cm−1 is assigned to symmetric C=O vibration (from COO−) [53]. The C−O−C stretching vibrations, which are characteristic to the glycosidic bonds in alginate polysaccharide structure, appeared at 1022 cm−1 [54].
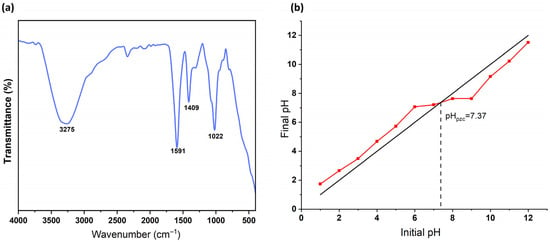
Figure 2.
FTIR spectra (a) and pHpzc determination (b) of the studied AC/alginate beads.
The point of zero charge (pHpzc) of the AC/alginate composite was evaluated using the pH drift method. As shown in Figure 2b, the ∆pH values approached zero at a pH of 7.4, which was identified as the pHpzc of the adsorbent. This indicates that the composite surface carries a net positive charge at solution pH below 7.4 and a net negative charge at values above 7.4. The result provides important insight into the surface chemistry of the adsorbent, as it governs the nature of electrostatic interactions with 2-MIB under different pH conditions.
3.2. Effect of Contact Time
The removal efficiency of AC/Alginate composite was determined by mixing 0.45 g of the adsorbent with 50 mL of 500 ng/L 2-MIB solutions varying the contact time in the range of 60–480 min at 100 rpm. The results in Figure 3 revealed the adsorption efficiency increased rapidly during the initial stages, with about 50% removal within the first 120 min and reached approximately 95% after 360 min. Beyond 360 min only a slight increase was observed, with a maximum adsorption efficiency of 99% at 480 min. The initial fast adsorption may be governed by surface interactions, primarily hydrophobic interactions with the activated carbon domains of the composite.
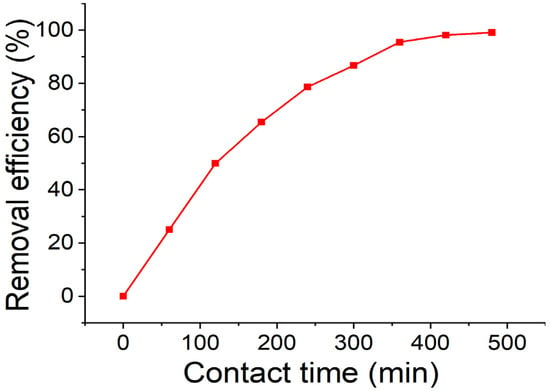
Figure 3.
Effect of contact time on the 2-MIB removal efficiency of AC/alginate composite.
3.3. Effect of Adsorbent Dosage
To investigate the effect of adsorbent dosage on 2-MIB removal, different amounts of the adsorbent (0.05–0.50 g) were mixed with 50 mL of 2-MIB at an initial concentration of 500 ng/L for 360 min at 100 rpm. The effect of adsorbent dose on removal efficiency is depicted in Figure 4. Increasing the adsorbent dosage from 0.05 to 0.45 g enhanced removal efficiency from 25.95% to 95.48%, respectively. The increased removal efficiency is likely due to the increased surface area of the adsorbent and the greater availability of binding sites for 2-MIB removal. However, a further increase in dosage up to 0.50 g showed no significant improvement in 2-MIB removal efficiency. Therefore, 0.45 g was determined to be the optimum adsorbent dose for the removal 500 ng/L of 2-MIB from aqueous solution.
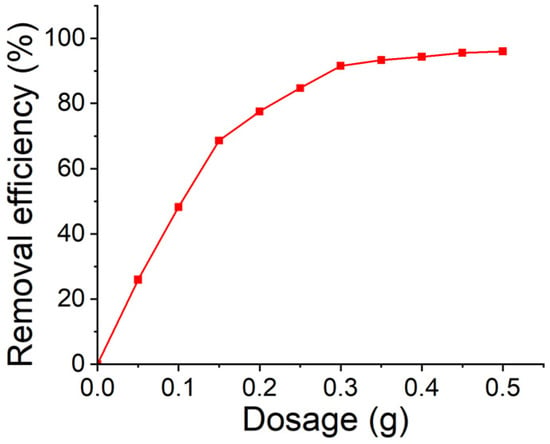
Figure 4.
Effect of adsorbent dose on the 2-MIB removal efficiency of the AC/alginate composite.
3.4. Effect of Initial Concentration of 2-MIB
The effect of initial concentration on the adsorption of 2-MIB by AC/alginate composite was examined using initial concentrations ranging from 250 to 1250 ng/L and a constant adsorbent dose of 0.45 g for 360 min at 100 rpm (see Figure 5). This concentration range (250–1250 ng L−1) was selected to reflect environmentally relevant 2-MIB levels in surface water. The results indicated that adsorption capacity increased with increasing initial 2-MIB concentration, whereas the removal efficiency decreased. The enhanced adsorption capacity observed at elevated initial concentrations can be attributed to the greater availability of 2-MIB molecules, which generates a stronger driving force for mass transfer. Conversely, the decrease in removal efficiency at higher initial concentrations can be explained by the relatively high surface area and adsorption site availability at low initial concentrations, where 2-MIB molecules are more easily absorbed and removed. At elevated concentrations, saturation of accessible adsorption sites occurs leading to a reduction in removal efficiency.
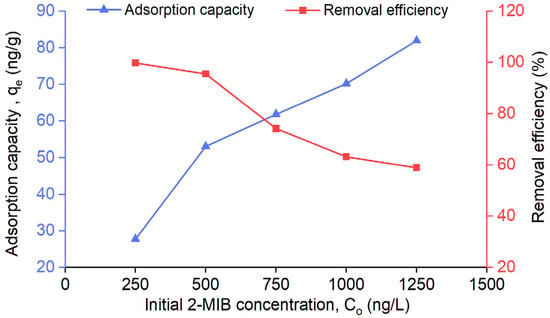
Figure 5.
Effect of initial concentration on the adsorption capacity and removal efficiency of AC/alginate composite.
3.5. Effect of Initial pH
The influence of pH on the sorption process of 2-MIB on AC/Alginate beads was studied in the pH range from 3 to 11 (see Figure 6). About 0.45 g of the adsorbent was added to 50 mL of 500 ng/L 2-MIB solutions adjusted to the pH values of 3, 5, 7, 9, and 11 followed by agitation for 360 min at 100 rpm. The maximum removal of 2-MIB was achieved at pH 7, with a removal efficiency of 95%. The results indicate that the removal efficiency increased with increasing initial pH from 3 to 7. Increasing the pH above 7 resulted in a slight decline in percent removal. This behavior can be explained in terms of the pHPZC of the adsorbent. The pHPZC for AC/alginate composite was determined to be 7.4, indicating that the adsorbent surface possesses no net charge at this pH. At values below 7.4, the surface tends to be positively charged, while at higher pH levels, it carries a negative charge. Although 2-MIB is a neutral compound, the surface charge of the adsorbent can still influence adsorption indirectly by affecting how accessible the hydrophobic regions are and how water molecules are arranged near the surface. The adsorbate 2-MIB has an extremely low pKa value of −0.42 [55], indicating that it remains essentially neutral under typical aqueous conditions, including the pH range studied (3–11). Since 2-MIB is largely uncharged at environmental pH values, electrostatic interactions between 2-MIB and the AC/alginate composite are minimal. Therefore, the adsorption mechanism is dominated by hydrophobic interactions and van der Waals forces. The observation that maximum adsorption occurred close to the pHpzc (7.4) supports this interpretation, as electrostatic effects are minimized under near-neutral conditions, allowing hydrophobic interactions between 2-MIB and the activated carbon domains of the adsorbent to dominate. At more alkaline pH values, the slightly negatively charged adsorbent surface likely becomes more hydrated, which may reduce the availability of hydrophobic adsorption sites.
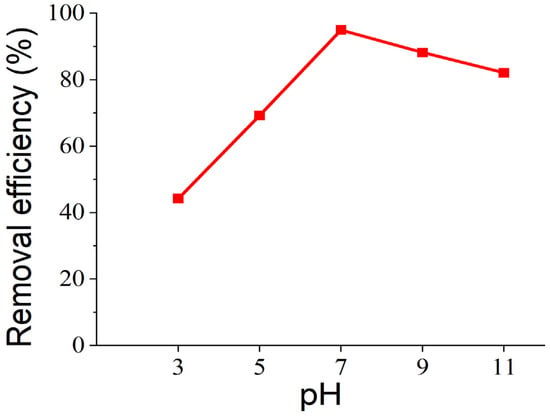
Figure 6.
Effect of pH on the removal efficiency of 2-MIB onto AC/alginate composite.
These findings align with previous studies demonstrating that adsorption of neutral organic compounds is favored when the adsorbent is at a pH near its pHpzc, which enhances hydrophobic interactions [56]. Overall, the AC/alginate composite shows optimal 2-MIB removal under near-neutral conditions, reducing the need for extensive pH adjustment in practical water treatment scenarios.
3.6. Adsorption Kinetic Study
The adsorption kinetics of 2-MIB onto AC/alginate composite were evaluated using the pseudo-first order (PFO), and pseudo-second order (PSO) models. Figure 7 shows the nonlinear plots of PFO and PSO kinetic models. The kinetic parameters for 2-MIB adsorption onto AC/alginate adsorbent are summarized in Table 1.
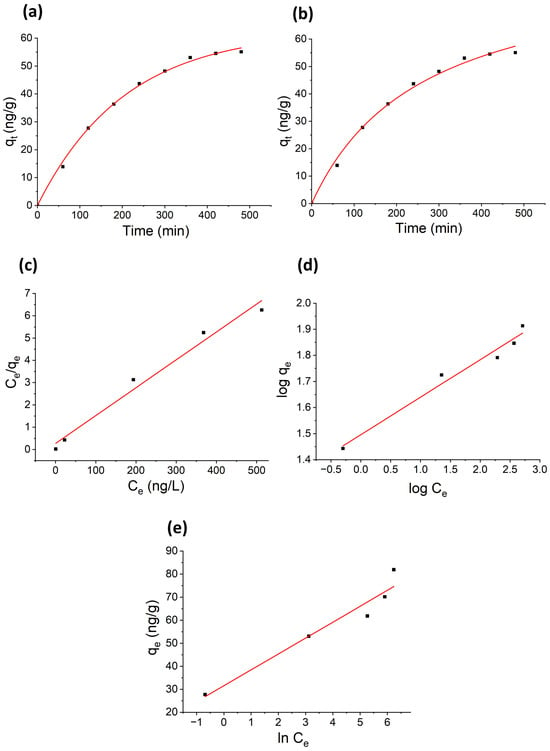
Figure 7.
Nonlinear kinetic adsorption models: (a) pseudo-first order kinetic model, (b) pseudo-second order kinetic model, and linear isotherm models: (c) Langmuir isotherm model, (d) Freundlich isotherm model, and (e) Temkin isotherm model.

Table 1.
Kinetic parameters for the adsorption of 2-MIB onto AC/alginate composite.
Both the PFO and PSO models exhibited high correlation coefficients (R2 = 0.99), indicating that both could describe the experimental data well. To determine the most applicable model, we compared their Akaike information criterion (AIC) and Bayesian information criterion (BIC) values. The PFO model exhibited lower AIC (9.73) and BIC (5.52) compared to the PSO model (AIC = 16.81, BIC = 12.6), indicating that it provides a better statistical fit. This suggests that the adsorption rate is primarily governed by the availability of unoccupied surface sites, with physical adsorption dominating the process. Mechanistically, the 2-MIB molecules are likely adsorbed onto the AC/alginate composite through van der Waals forces and hydrophobic interactions between the nonpolar regions of 2-MIB and the carbon surfaces, while the alginate matrix helps stabilize the beads and may slightly influence surface accessibility. The adsorption is therefore surface site-controlled, with rapid initial uptake as the most accessible sites are occupied first, followed by slower approach to equilibrium as remaining sites are gradually filled. Overall, these results highlight that the PFO model accurately captures the dominant surface adsorption mechanism for 2-MIB on AC/alginate beads.
3.7. Adsorption Isotherm Study
The equilibrium adsorption of 2-MIB on the AC/alginate composite was analyzed using Langmuir, Freundlich, and Temkin isotherm models to explain the adsorption mechanism. The adsorption isotherms are presented in Figure 7, and corresponding isotherm parameters are summarized in Table 2. The adsorption data were well described by both isotherms, with the linearized Langmuir isotherm model (R2 = 0.97) showing a slightly better fit than Freundlich model (R2 = 0.96). The Langmuir model predicted a maximum adsorption capacity of 79.94 ngg−1. The calculated RL values were in the range from 0.07 to 0.01, indicating a favorable adsorption process. Moreover, the decline in RL values at higher 2-MIB concentrations suggests that adsorption becomes more effective with increasing concentration. The Freundlich constant, kf = 31.31, reflects the high adsorption capacity of the AC/alginate composite. The adsorption intensity (1/n = 0.144), being less than one, confirms that the sorption process was favorable and that the sorbent surface possesses heterogenous binding sites, which facilitate additional adsorption at high concentrations. The Temkin model (R2 = 0.92), which considers adsorbate–adsorbent interactions and a linear decrease in adsorption heat with coverage, indicates that such interactions may also contribute to the overall adsorption process. These findings suggest that 2-MIB adsorption on the AC/alginate composite involves both monolayer adsorption at high affinity sites and multilayer adsorption on heterogeneous sites, with surface interactions contributing at moderate coverage.

Table 2.
Parameters and correlation coefficients of the equilibrium isotherm models for the adsorption of 2-MIB onto AC/alginate composite.
3.8. Regeneration Studies
The reusability of the AC/alginate composite for 2-MIB removal was evaluated over multiple adsorption–desorption cycles, and the results are depicted in Figure 8. The removal efficiency gradually declined from 81.5% in the first cycle to 59.0% by the fourth cycle, indicating that the adsorbent retains a substantial portion of its adsorption capacity even after repeated use. This decrease in performance is likely due to partial saturation of adsorption sites or incomplete desorption of 2-MIB during regeneration, which reduces the availability of hydrophobic sites in subsequent cycles. Nevertheless, the composite maintained more than half of its initial removal efficiency after four cycles, demonstrating reasonable stability and effective regeneration capability.
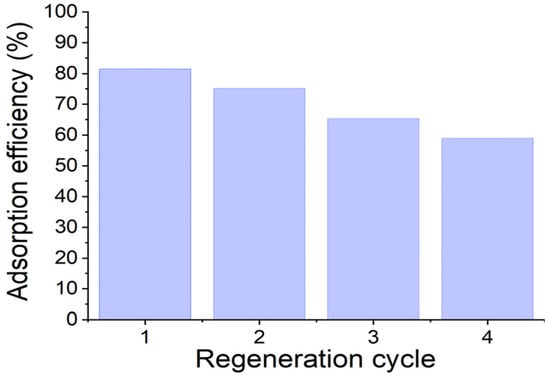
Figure 8.
Adsorption efficiency of the adsorbent over multiple regeneration cycles.
A simple mass-balance estimate further indicates that, for an influent 2-MIB concentration of 250 ng/L and an odor threshold of 10 ng/L, 1 g of AC could treat approximately 1.16 L of water before reaching the regulatory limit. This estimate provides practical context for the material’s performance and underscores the potential of the AC/alginate composite for real-world water treatment applications, particularly considering its combination of adsorption efficiency, durability, and environmental compatibility.
Compared to conventional PAC and previously reported composites (see Table 3), the AC/alginate composites developed in this study exhibit comparable or superior 2-MIB removal performance while offering distinct advantages in terms of ease of recovery, structural integrity in water, and reusability over multiple cycles. While polymer–carbon composites have been explored previously, their application specifically for 2-MIB remains limited. Importantly, our results show that the alginate matrix does not hinder the intrinsic adsorption capacity of AC; rather it provides stable, environmentally benign, and regenerable support that enhances the practical applicability of AC for 2-MIB control. These findings underscore the novelty of the present composite, distinguishing it from prior adsorbents and highlighting its potential as a cost-effective and sustainable alternative to conventional AC-based water treatment methods.

Table 3.
Comparison of the adsorbents reported for 2-MIB removal from aqueous solution.
For practical application, several operational factors should be considered. The separation and regeneration process involves desorption using 0.5 M NaCl in 30% ethanol, followed by thorough washing, which may contribute to operational costs and solvent handling requirements at larger scales. Additionally, when implementing packed columns or high throughput systems, the composite beads could introduce pressure drop concerns due to their size and packing characteristics, potentially affecting flow rates and energy consumption. Optimization of bead size, packing density, and regeneration protocols will be critical to minimize these limitations and enhance the feasibility of industrial-scale applications.
4. Limitations and Future Work
This study demonstrates the promise of AC/alginate composite beads for 2-MIB removal at the laboratory scale, but several aspects warrant further investigation. Pilot or full-scale column studies are needed to capture adsorption kinetics, mass transfer limitations, and flow distribution under continuous operation. Experiments were conducted in model solutions and future work should evaluate performance in natural waters where natural organic matter (NOM) and co-existing ions may compete for adsorption sites. Additional materials characterization, such as nitrogen sorption, thermogravimetric analysis, swelling behavior, bead size distribution, zeta potential across pH, ionic strength effects, as well as mechanical strength, would provide deeper insight into pore structure stability and physicochemical properties. Post-regeneration structural analyses are also essential to clarify mechanisms of sorbent deactivation and to assess long-term durability under realistic water treatment conditions. Finally, the development of greener regeneration strategies should be explored to enhance the sustainability of the composite system.
5. Conclusions
This study examined the effectiveness of the AC/alginate composite beads for the removal of 2-MIB from aqueous solutions. The beads exhibited rapid initial uptake, achieving nearly complete removal at equilibrium, with adsorption maximized at neutral pH (7), close to the pHpzc of the adsorbent. This optimal performance under near-neutral conditions is particularly significant, as it aligns with the natural pH range of most drinking water sources, thereby enhancing the practical applicability of the composite in real-world treatment processes. Isotherm analyses indicated that the process involves both monolayer adsorption at high-affinity sites and multilayer adsorption across heterogeneous surfaces, while kinetic modeling emphasized the influence of surface adsorption in governing uptake. Collectively, these findings highlight AC/alginate composites as a stable, effective, and reusable adsorbent, demonstrating their potential utility in water treatment processes.
Author Contributions
Conceptualization, I.L.B. and M.D.H.J.S.; Investigation, I.L.B.; Methodology, M.D.H.J.S. and W.W.; Formal Analysis, I.L.B.; Resources, M.D.H.J.S. and W.W.; Supervision, M.D.H.J.S.; Visualization, I.L.B.; Writing—original draft preparation, I.L.B.; Writing—review and editing, M.D.H.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used in the study are available from the corresponding author on reasonable request.
Acknowledgments
We are grateful to the Comprehensive Analysis Center for Science, Saitama University, for FTIR analysis with the Tensor II spectrometer and their expert guidance.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 2-MIB | 2-methylisoborneol |
| AC/alginate | Activated carbon/alginate |
| SEM | Scanning electron microscopy |
| FTIR | Fourier transform infrared spectroscopy |
| PAC | Powdered activated carbon |
| GAC | Granular activated carbon |
| GCMS | Gas chromatography/mass spectrometry |
| SPME | Solid phase microextraction |
| PFO | Pseudo first order |
| PSO | Pseudo second order |
References
- Chapra, S.C.; Boehlert, B.; Fant, C.; Bierman, V.J.; Henderson, J.; Mills, D.; Mas, D.M.L.; Rennels, L.; Jantarasami, L.; Martinich, J.; et al. Climate Change Impacts on Harmful Algal Blooms in U.S. Freshwaters: A Screening-Level Assessment. Environ. Sci. Technol. 2017, 51, 8933–8943. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate Change: Links to Global Expansion of Harmful Cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-S.; Yuan, D.-X.; Weng, T.-P. Pilot Study of Drinking Water Treatment with GAC, O3/BAC and Membrane Processes in Kinmen Island, Taiwan. Desalination 2010, 263, 271–278. [Google Scholar] [CrossRef]
- Sugiura, N.; Iwami, N.; Inamori, Y.; Nishimura, O.; Sudo, R. Significance of Attached Cyanobacteria Relevant to the Occurrence of Musty Odor in Lake Kasumigaura. Water Res. 1998, 32, 3549–3554. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Elbestawy, A.R.; Ellakany, H.F.; Abaza, S.S.; Geneedy, A.M.; Salem, H.M.; Taha, A.E.; Swelum, A.A.; Omer, F.A. Undesirable Odour Substances (Geosmin and 2-Methylisoborneol) in Water Environment: Sources, Impacts and Removal Strategies. Mar. Pollut. Bull. 2022, 178, 113579. [Google Scholar] [CrossRef]
- Gerber, N.N. Volatile Substances from Actinomycetes: Their Role in the Odor Pollution of Water. CRC Crit. Rev. Microbiol. 1979, 7, 191–214. [Google Scholar] [CrossRef]
- Persson, P.-E. Odorous Algal Cultures in Culture Collections. Water Sci. Technol. 1988, 20, 211–213. [Google Scholar] [CrossRef]
- Young, W.; Horth, H.; Crane, R.; Ogden, T.; Arnott, M. Taste and Odour Threshold Concentrations of Potential Potable Water Contaminants. Water Res. 1996, 30, 331–340. [Google Scholar] [CrossRef]
- Bruce, D.; Westerhoff, P.; Brawley-Chesworth, A. Removal of 2-Methylisoborneol and Geosmin in Surface Water Treatment Plants in Arizona. J. Water Supply Res. Technol.—AQUA 2002, 51, 183–198. [Google Scholar] [CrossRef]
- Hafuka, A.; Nagasato, T.; Yamamura, H. Application of Graphene Oxide for Adsorption Removal of Geosmin and 2-Methylisoborneol in the Presence of Natural Organic Matter. Int. J. Environ. Res. Public Health 2019, 16, 1907. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, L.; Gao, J.; Zhu, J.; Zhou, J. Process Optimization and Mechanism Revealing of KMnO4 Pre-Oxidation Coupled Powdered Activated Carbon Adsorption for 2-MIB Removal. J. Water Process Eng. 2023, 53, 103705. [Google Scholar] [CrossRef]
- Zamyadi, A.; Henderson, R.; Stuetz, R.; Hofmann, R.; Ho, L.; Newcombe, G. Fate of Geosmin and 2-Methylisoborneol in Full-Scale Water Treatment Plants. Water Res. 2015, 83, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Hoefel, D.; Bock, F.; Saint, C.P.; Newcombe, G. Biodegradation Rates of 2-Methylisoborneol (MIB) and Geosmin through Sand Filters and in Bioreactors. Chemosphere 2007, 66, 2210–2218. [Google Scholar] [CrossRef]
- Cook, D.; Newcombe, G.; Sztajnbok, P. The Application of Powdered Activated Carbon for MIB and Geosmin Removal: Predicting PAC Doses in Four Raw Waters. Water Res. 2001, 35, 1325–1333. [Google Scholar] [CrossRef]
- Graham, M.; Summers, R.; Simpson, M.; MacLeod, B. Modeling Equilibrium Adsorption of 2-Methylisoborneol and Geosmin in Natural Waters. Water Res. 2000, 34, 2291–2300. [Google Scholar] [CrossRef]
- Yuan, J.; Mortazavian, S.; Crowe, G.; Flick, R.; Passeport, E.; Hofmann, R. Evaluating the Relative Adsorption and Biodegradation of 2-Methylisoborneol and Geosmin across Granular Activated Carbon Filter-Adsorbers. Water Res. 2022, 215, 118239. [Google Scholar] [CrossRef]
- Tosa, K.; Nakamura, G.; Miyabayashi, K.; Ishisaki, H.; Takahashi, Y. Adsorption of Geosmin and 2-MIB to Porous Coordination Polymer MIL-53 (Al). J. Water Environ. Technol. 2022, 20, 212–218. [Google Scholar] [CrossRef]
- Ellis, J.; Korth, W. Removal of Geosmin and Methylisoborneol from Drinking Water by Adsorption on Ultrastable Zeolite-Y. Water Res. 1993, 27, 535–539. [Google Scholar] [CrossRef]
- Asghar, A.; Khan, Z.; Maqbool, N.; Qazi, I.A.; Awan, M.A. Comparison of Adsorption Capability of Activated Carbon and Metal Doped TiO2 for Geosmin and 2-MIB Removal from Water. J. Nanomater. 2015, 2015, 479103. [Google Scholar] [CrossRef]
- Xue, Q.; Chen, R.; Sakharkar, M.K.; Utsumi, M.; Li, M.; Shimizu, K.; Zhang, Z.; Sugiura, N. Development of a Ceramic Adsorbent for the Removal of 2-Methylisoborneol from Aqueous Solution. Desalination 2011, 281, 293–297. [Google Scholar] [CrossRef]
- Liu, L.; Li, L.; Zuo, Y.; Huang, Y.; Song, L. Adsorption of 2-Methylisoborneol and Geosmin by a Low-Cost Hybrid Adsorbent Synthesized from Fly Ash and Bentonite. J. Water Supply Res. Technol.—AQUA 2011, 60, 478–485. [Google Scholar] [CrossRef]
- Matsui, Y.; Nakao, S.; Sakamoto, A.; Taniguchi, T.; Pan, L.; Matsushita, T.; Shirasaki, N. Adsorption Capacities of Activated Carbons for Geosmin and 2-Methylisoborneol Vary with Activated Carbon Particle Size: Effects of Adsorbent and Adsorbate Characteristics. Water Res. 2015, 85, 95–102. [Google Scholar] [CrossRef]
- Zoschke, K.; Engel, C.; Börnick, H.; Worch, E. Adsorption of Geosmin and 2-Methylisoborneol onto Powdered Activated Carbon at Non-Equilibrium Conditions: Influence of NOM and Process Modelling. Water Res. 2011, 45, 4544–4550. [Google Scholar] [CrossRef] [PubMed]
- Chae, A.N.; Shin, J.W.; Cho, K.W.; Lee, B.C.; Song, K.G. Removal of Geosmin and 2-Methylisoborneol in Drinking Water by Powdered Activated Carbon. KSCE J. Civ. Environ. Eng. Res. 2017, 37, 475–483. [Google Scholar] [CrossRef][Green Version]
- Park, S.-M.; Heo, T.-Y.; Park, N.-B.; Na, K.-J.; Jun, H.-B.; Jung, J.-Y. Application of Air Stripping to Powdered Activated Carbon Adsorption of Geosmin and 2-Methylisoborneol. J. Water Supply Res. Technol.—AQUA 2010, 59, 492–500. [Google Scholar] [CrossRef]
- Bong, T.; Kang, J.-K.; Yargeau, V.; Nam, H.-L.; Lee, S.-H.; Choi, J.-W.; Kim, S.-B.; Park, J.-A. Geosmin and 2-Methylisoborneol Adsorption Using Different Carbon Materials: Isotherm, Kinetic, Multiple Linear Regression, and Deep Neural Network Modeling Using a Real Drinking Water Source. J. Clean. Prod. 2021, 314, 127967. [Google Scholar] [CrossRef]
- Watanabe, T.; Amano, Y.; Machida, M. The Adsorption Mechanism and Rapid Screening of Activated Carbon for 2-Methylisoborneol Adsorption. TANSO 2013, 2013, 124–134. [Google Scholar] [CrossRef][Green Version]
- Yuan, J.; Huang, Y.; Nie, Z.; Hofmann, R. The Effect of Water Temperature on the Removal of 2-Methylisoborneol and Geosmin by Preloaded Granular Activated Carbon. Water Res. 2020, 183, 116065. [Google Scholar] [CrossRef]
- Pan, L.; Matsui, Y.; Matsushita, T.; Shirasaki, N. Superiority of Wet-Milled over Dry-Milled Superfine Powdered Activated Carbon for Adsorptive 2-Methylisoborneol Removal. Water Res. 2016, 102, 516–523. [Google Scholar] [CrossRef]
- Xin, X.; Wang, M.; Ge, X.; Zhao, Q.; Sun, S.; Jia, R. Highly Efficient Removal of Geosmin and 2-Methylisoborneol by Carboxylated Multi-Walled Carbon Nanotubes. Monatshefte Chem.-Chem. Mon. 2014, 145, 747–754. [Google Scholar] [CrossRef]
- Yuan, J.; Hofmann, R. Adsorption and Biodegradation of 2-Methylisoborneol and Geosmin in Drinking Water Granular Activated Carbon Filters: A Review and Meta-Analysis. J. Hazard. Mater. 2022, 440, 129838. [Google Scholar] [CrossRef]
- Newcombe, G.; Morrison, J.; Hepplewhite, C.; Knappe, D. Simultaneous Adsorption of MIB and NOM onto Activated Carbon: II. Competitive Effects. Carbon 2002, 40, 2147–2156. [Google Scholar] [CrossRef]
- Matsui, Y.; Nakao, S.; Taniguchi, T.; Matsushita, T. Geosmin and 2-Methylisoborneol Removal Using Superfine Powdered Activated Carbon: Shell Adsorption and Branched-Pore Kinetic Model Analysis and Optimal Particle Size. Water Res. 2013, 47, 2873–2880. [Google Scholar] [CrossRef]
- Wang, F.; Li, X.; Liu, T.; Li, X.; Cui, Y.; Xu, L.; Huo, S.; Zou, B.; Qian, J.; Ma, A. Removal of Taste and Odor Compounds from Water: Methods, Mechanism and Prospects. Catalysts 2023, 13, 1356. [Google Scholar] [CrossRef]
- Kim, K.T.; Park, Y.-G. Geosmin and 2-MIB Removal by Full-Scale Drinking Water Treatment Processes in the Republic of Korea. Water 2021, 13, 628. [Google Scholar] [CrossRef]
- Andreadakis, A.D.; Mamais, D.; Gavalakis, E.A.; Noutsopoulos, C.; Kouris, N.; Nikitopoulos, G. Removal of Taste and Odour from Potable Water by Ozone and Powdered Activated Carbon (PAC). Int. J. Environ. Waste Manag. 2010, 5, 392–409. [Google Scholar] [CrossRef]
- Pawar, R.R.; Ingole, P.G.; Lee, S.-M. Use of Activated Bentonite-Alginate Composite Beads for Efficient Removal of Toxic Cu2+ and Pb2+ Ions from Aquatic Environment. Int. J. Biol. Macromol. 2020, 164, 3145–3154. [Google Scholar] [CrossRef] [PubMed]
- Benhouria, A.; Islam, M.A.; Zaghouane-Boudiaf, H.; Boutahala, M.; Hameed, B. Calcium Alginate–Bentonite–Activated Carbon Composite Beads as Highly Effective Adsorbent for Methylene Blue. Chem. Eng. J. 2015, 270, 621–630. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Rhee, S.K. (Eds.) Polysaccharides and Polyamides in the Food Industry: Properties, Production, and Patents; WILEY-VCH: Weinheim, Germany, 2005; pp. 1–30. ISBN 978-3-527-31345-7. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Chitosan-Based Biosorbents: Modification and Application for Biosorption of Heavy Metals and Radionuclides. Bioresour. Technol. 2014, 160, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Sutirman, Z.A.; Sanagi, M.M.; Aini, W.I.W. Alginate-Based Adsorbents for Removal of Metal Ions and Radionuclides from Aqueous Solutions: A Review. Int. J. Biol. Macromol. 2021, 174, 216–228. [Google Scholar] [CrossRef]
- Torres, M.R.; Sousa, A.P.; Silva Filho, E.A.; Melo, D.F.; Feitosa, J.P.; de Paula, R.C.; Lima, M.G. Extraction and Physicochemical Characterization of Sargassum Vulgare Alginate from Brazil. Carbohydr. Res. 2007, 342, 2067–2074. [Google Scholar] [CrossRef]
- Faidi, A.; Lassoued, M.A.; Becheikh, M.E.H.; Touati, M.; Stumbé, J.-F.; Farhat, F. Application of Sodium Alginate Extracted from a Tunisian Brown Algae Padina Pavonica for Essential Oil Encapsulation: Microspheres Preparation, Characterization and in Vitro Release Study. Int. J. Biol. Macromol. 2019, 136, 386–394. [Google Scholar] [CrossRef]
- Hassan, A.; Abdel-Mohsen, A.; Fouda, M.M. Comparative Study of Calcium Alginate, Activated Carbon, and Their Composite Beads on Methylene Blue Adsorption. Carbohydr. Polym. 2014, 102, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Bée, A.; Talbot, D.; Abramson, S.; Dupuis, V. Magnetic Alginate Beads for Pb (II) Ions Removal from Wastewater. J. Colloid Interface Sci. 2011, 362, 486–492. [Google Scholar] [CrossRef]
- Fiol, N.; Poch, J.; Villaescusa, I. Chromium (VI) Uptake by Grape Stalks Wastes Encapsulated in Calcium Alginate Beads: Equilibrium and Kinetics Studies. Chem. Speciat. Bioavailab. 2004, 16, 25–33. [Google Scholar] [CrossRef]
- Vincent, T.; Parodi, A.; Guibal, E. Pt Recovery Using Cyphos IL-101 Immobilized in Biopolymer Capsules. Sep. Purif. Technol. 2008, 62, 470–479. [Google Scholar] [CrossRef]
- Hassan, A.; Abdel-Mohsen, A.; Elhadidy, H. Adsorption of Arsenic by Activated Carbon, Calcium Alginate and Their Composite Beads. Int. J. Biol. Macromol. 2014, 68, 125–130. [Google Scholar] [CrossRef]
- Davranche, M.; Lacour, S.; Bordas, F.; Bollinger, J.-C. An Easy Determination of the Surface Chemical Properties of Simple and Natural Solids. J. Chem. Educ. 2003, 80, 76. [Google Scholar] [CrossRef]
- Senavirathna, M.D.H.J.; Jayasekara, M.A.D.D. Temporal Variation of 2-MIB and Geosmin Production by Pseudanabaena Galeata and Phormidium Ambiguum Exposed to High-intensity Light. Water Environ. Res. 2023, 95, e10834. [Google Scholar] [CrossRef]
- Ahmad, A.; Loh, M.; Aziz, J. Preparation and Characterization of Activated Carbon from Oil Palm Wood and Its Evaluation on Methylene Blue Adsorption. Dye. Pigment. 2007, 75, 263–272. [Google Scholar] [CrossRef]
- Saarai, A.; Kasparkova, V.; Sedlacek, T.; Sáha, P. On the Development and Characterisation of Crosslinked Sodium Alginate/Gelatine Hydrogels. J. Mech. Behav. Biomed. Mater. 2013, 18, 152–166. [Google Scholar] [CrossRef]
- Larosa, C.; Salerno, M.; de Lima, J.S.; Meri, R.M.; da Silva, M.F.; de Carvalho, L.B.; Converti, A. Characterisation of Bare and Tannase-Loaded Calcium Alginate Beads by Microscopic, Thermogravimetric, FTIR and XRD Analyses. Int. J. Biol. Macromol. 2018, 115, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, S.; Tijani, J.O.; Ndamitso, M.; Abdulkareem, A.S.; Shuaib, D.T.; Mohammed, A.K. A Critical Review on Geosmin and 2-Methylisoborneol in Water: Sources, Effects, Detection, and Removal Techniques. Env. Monit Assess 2021, 193, 204. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yu, J.; Wang, Q.; Wang, C.; Du, Y.; Liu, Z.; Zhang, L.; Liu, Z.; Jing, C.; Tang, J. Enhancing the Adsorption Performance of 2-Methylisoborneol by Activated Carbon by Promoting Hydrophobic Effects. ACS EST Water 2022, 2, 1789–1798. [Google Scholar] [CrossRef]
- Thiel, P.; Cullum, P. Evaluating the Performance of Different Powdered Activated Carbons (PAC) for Taste and Odour Reduction. In Proceeding of the 32nd Annual Water Industry Operations Workshop, Rockhampton, QLD, Australia, 17–19 April 2007. [Google Scholar]
- Newcombe, G.; Drikas, M.; Hayes, R. Influence of Characterised Natural Organic Material on Activated Carbon Adsorption: II. Effect on Pore Volume Distribution and Adsorption of 2-Methylisoborneol. Water Res. 1997, 31, 1065–1073. [Google Scholar] [CrossRef]
- Yu, J.; Yang, M.; Lin, T.-F.; Guo, Z.; Zhang, Y.; Gu, J.; Zhang, S. Effects of Surface Characteristics of Activated Carbon on the Adsorption of 2-Methylisobornel (MIB) and Geosmin from Natural Water. Sep. Purif. Technol. 2007, 56, 363–370. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).