Decoding Plant-Based Beverages: An Integrated Study Combining ATR-FTIR Spectroscopy and Microscopic Image Analysis with Chemometrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Lyophilization of the Plant-Based Beverages
2.2. Color Measurement, Microscopy and Image Analysis Evaluation
2.3. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR) of the Lyophilized Plant-Based Beverages
2.4. Univariate Statistical Analysis
2.5. Chemometrics and Multivariate Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition and Color Parameters of Plant-Based Beverages
3.2. Evaluation of Plant-Based Beverages’ Samples Based on Image Texture Analysis of the Microscopy Pictures
3.3. ATR-FTIR Spectra Interpretation of Plant-Based Beverages
| Frequency (cm–1) | Bond and Vibration Type | Expected Compounds | References |
|---|---|---|---|
| 3300–3270 | N-H stretching vibration (Amide I) | Amides and proteins | [38] |
| 3010 | cis- or trans-C(sp2)-H stretching vibrations in vinyl groups | Unsaturated compounds (lipids, fatty acids) | |
| 2922 | Asymmetric stretching vibrations of C(sp3)-H in methylene groups | Triglycerides, carboxylic and fatty acids, carbohydrates, amino acids, waxes | [23,39] |
| 2854 | Symmetric stretching vibrations of C(sp3)-H in methylene groups | ||
| 1743 | Stretching vibrations of carbonyl group (C=O) | Triglycerides, cholesterol esters, polysaccharides | [40,41,42] |
| 1649–1631 | Stretching vibrations of carbonyl group (C=O): Amide I absorption band | Amides and proteins | [24] |
| 1545–1535 | combined C-N stretching and N-H bending vibrations: Amide II absorption band | Amides and proteins | |
| 1460–1440 and 1421–1396 | C(sp3)-H bending vibration in -CH3 and -CH2 | Lipids, amides, proteins, polysaccharides | [43] |
| 1314 | Combined C-N stretching and O=C-N and N-H bending vibrations: Amide III absorption band | Amides and proteins | [44,45] |
| 1240 and 1157 | Asymmetric and symmetric C-O stretching vibrations | Polysaccharides, amides and proteins | [44,46,47] |
| 1107–1100 | C-O stretching vibrations | Secondary alcohols | [38] |
| 1075–1045 | Polysaccharides | [48] | |
| 1020–1010 | Lipids, primary alcohols, and pyranose structure of the carbohydrates | [44,49] | |
| 999–979 | Sucrose | [50] | |
| 932–920 | β-configuration of carbohydrate anomers | carbohydrates | [46,47] |
| 870–835 and 819–817 | α-configuration, of carbohydrate anomers | ||
| 770–750 | pyran ring | ||
| 721–718 | cis-C(sp2)-H out-of-plane bending vibrations or O–H out of plane bending vibrations | Carotenoids, unsaturated fatty acids Amylose and amylopectin | [22,38,51,52] |
| 667–630 | C-C-O deformation within the glycosidic linkage | Carbohydrates | [51] |
| 575–532 and 526–516 | C–O–C in plane bending vibrations of the glycosidic linkage | Amylose and amylopectin | [22,51] |
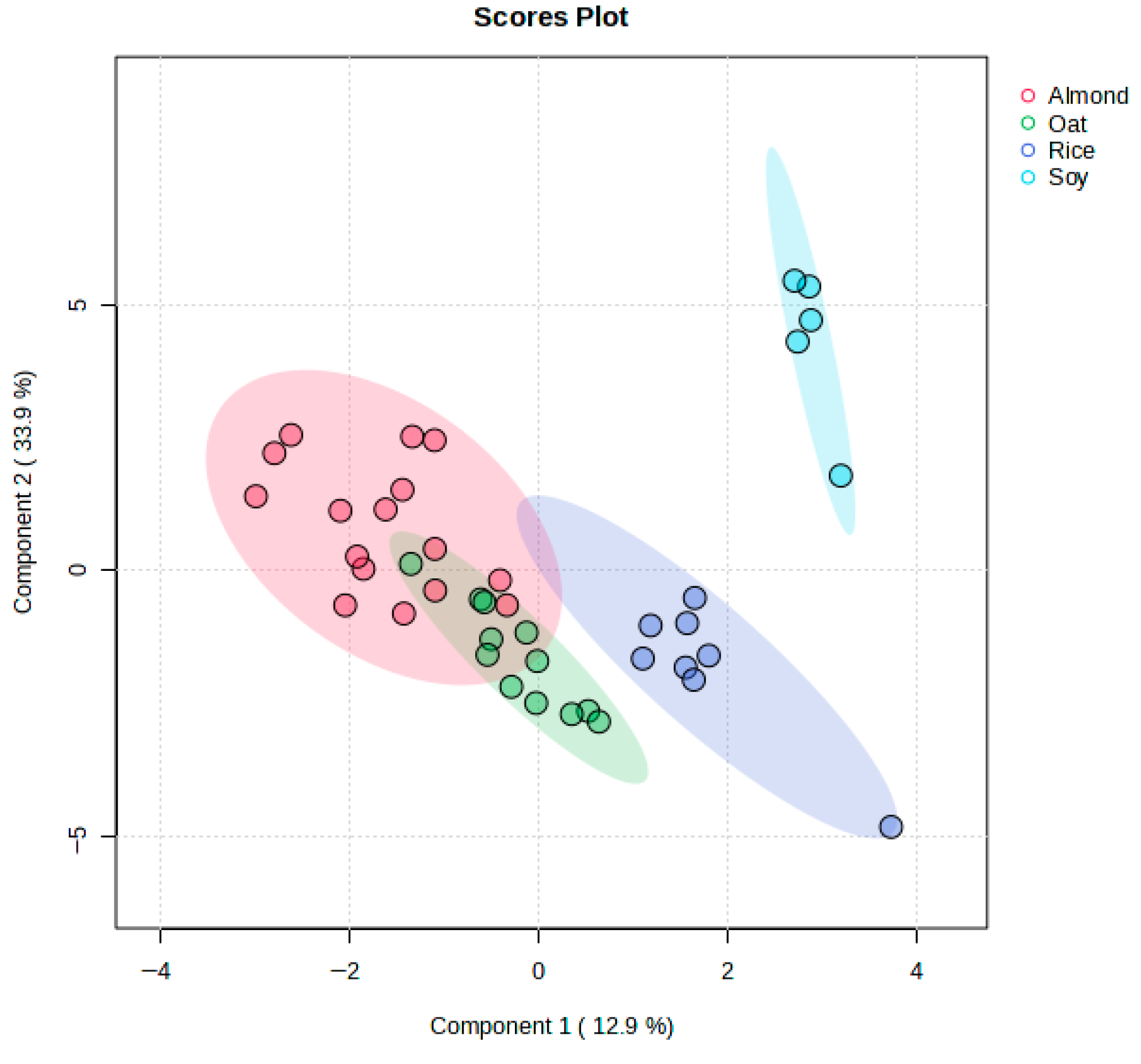
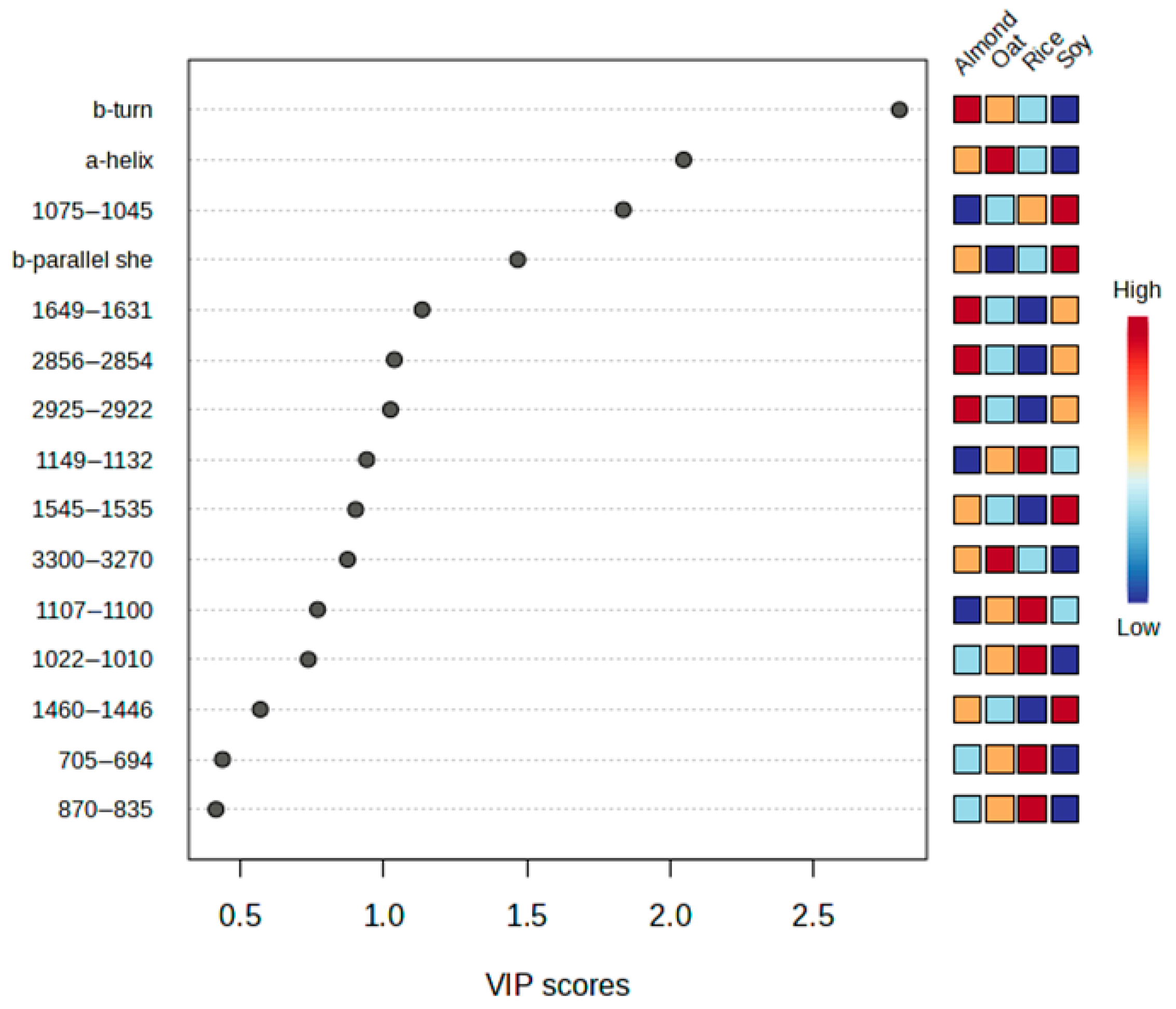
3.4. Discrimination of Plant-Based Beverages Samples Using PLS-DA
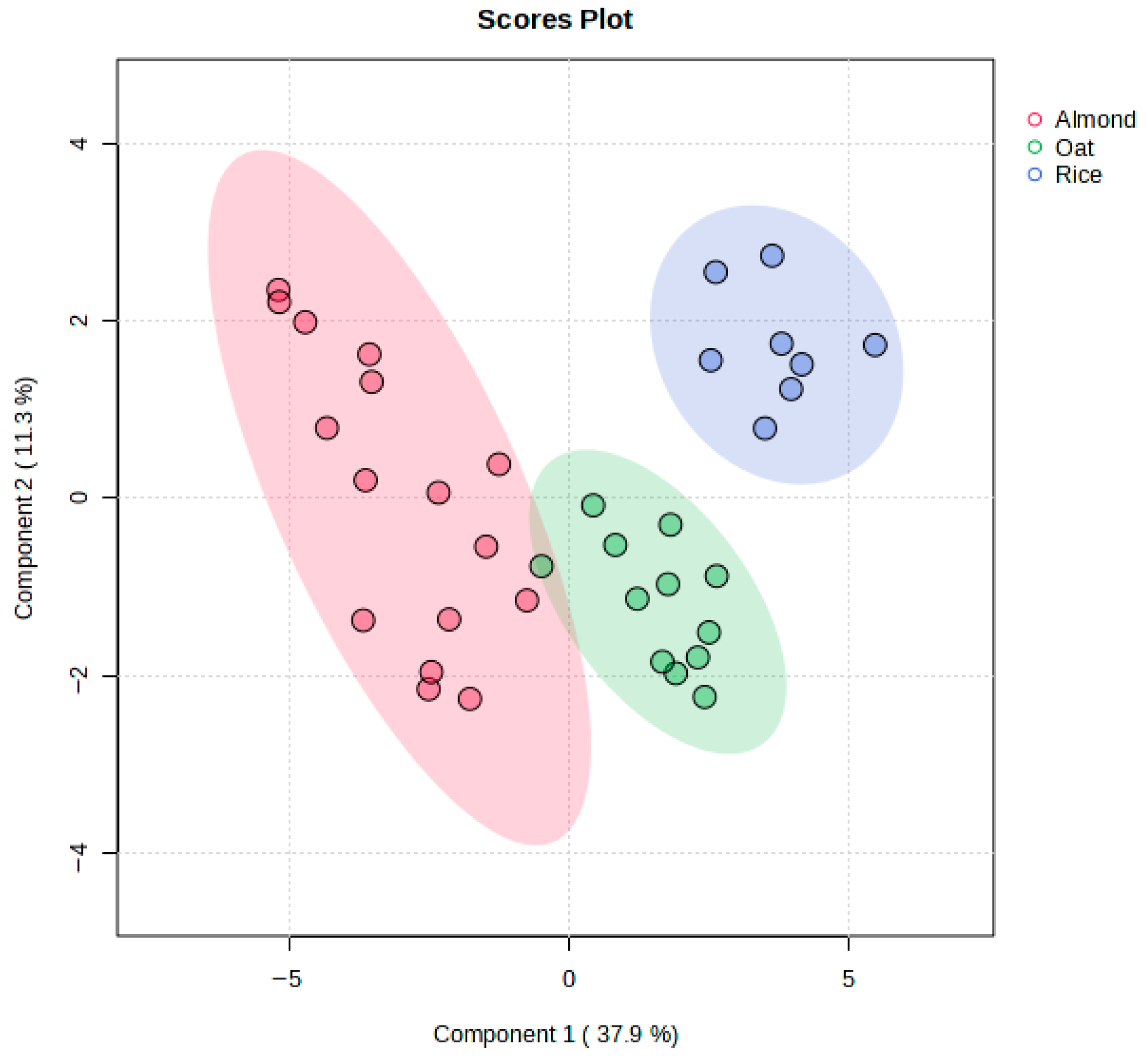
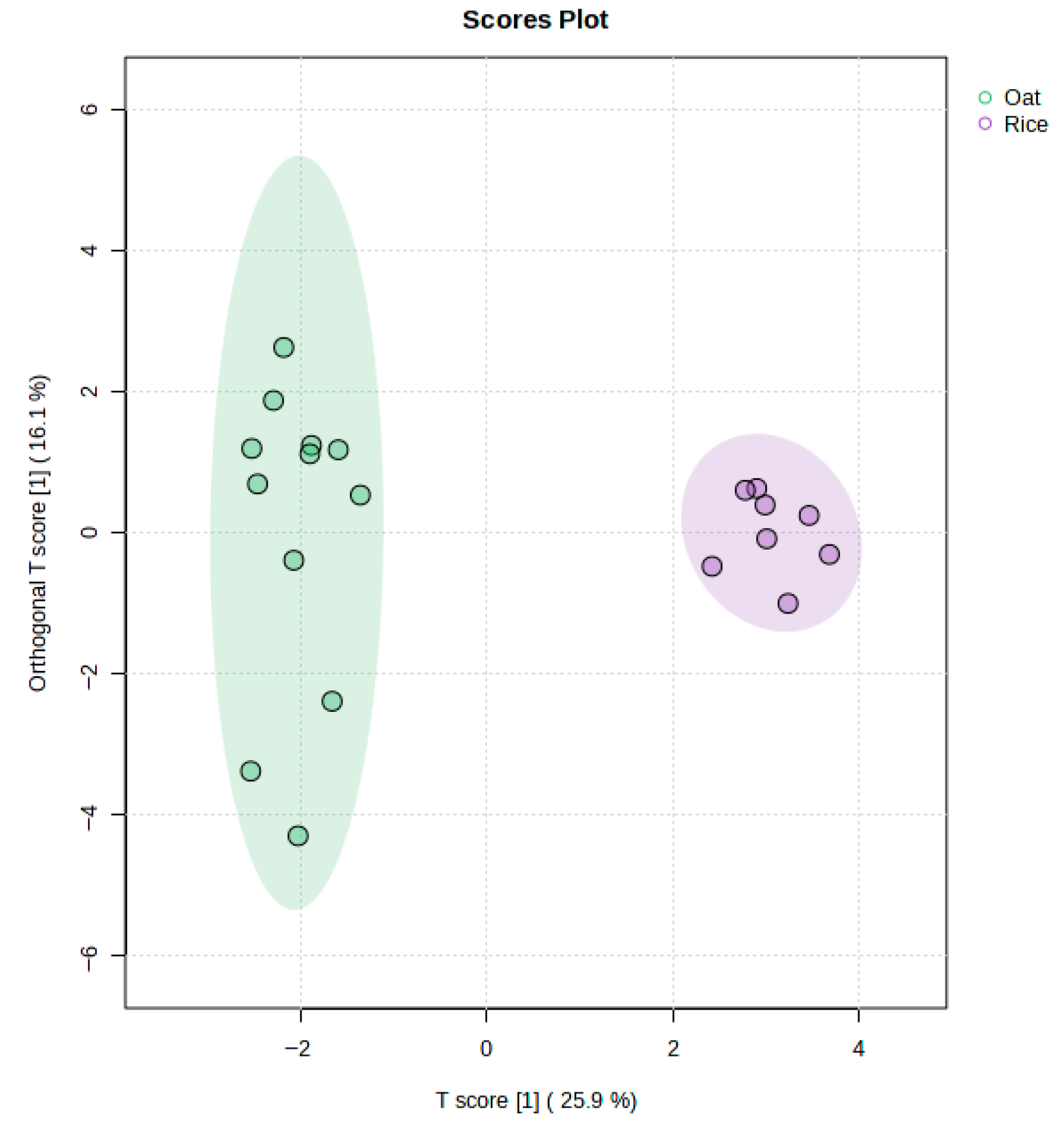
3.5. Discrimination Between Almond, Rice, and Oat Beverage Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milani, F.X.; Nutter, D.; Thoma, G. Invited Review: Environmental Impacts of Dairy Processing and Products: A Review. J. Dairy Sci. 2011, 94, 4243–4254. [Google Scholar] [CrossRef] [PubMed]
- Warren, V.; Bell, R.; Bruning-Mescher, S. Plant-Based Milk Alternatives: Consumer Needs and Marketing Strategies. In Plant-Based Food Consumption; Elsevier: Amsterdam, The Netherlands, 2024; pp. 153–177. ISBN 978-0-323-98828-5. [Google Scholar]
- Velangi, M.; Savla, M. Role of Plant Based Milk Alternatives as a Functional Beverage: A Review. Int. J. Health Sci. Res. 2022, 12, 273–281. [Google Scholar] [CrossRef]
- Ismail, M.M. Chemical Composition, Sensory Evaluation and Starter Activity in Cow, Soy, Peanut and Rice Milk. J. Nutr. Health Food Eng. 2016, 5, 00175. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Peñas, E. Health Benefits of Oat: Current Evidence and Molecular Mechanisms. Curr. Opin. Food Sci. 2017, 14, 26–31. [Google Scholar] [CrossRef]
- Singh, R.; De, S.; Belkheir, A. Avena sativa (Oat), A Potential Neutraceutical and Therapeutic Agent: An Overview. Crit. Rev. Food Sci. Nutr. 2013, 53, 126–144. [Google Scholar] [CrossRef] [PubMed]
- Bocker, R.; Silva, E.K. Innovative Technologies for Manufacturing Plant-Based Non-Dairy Alternative Milk and Their Impact on Nutritional, Sensory and Safety Aspects. Future Foods 2022, 5, 100098. [Google Scholar] [CrossRef]
- Romulo, A. Food Processing Technologies Aspects on Plant-Based Milk Manufacturing: Review. IOP Conf. Ser. Earth Environ. Sci. 2022, 1059, 012064. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, R.; Upadhyay, A.; Mishra, S.; Shukla, S. Emerging Trends in Processing for Cereal and Legume-Based Beverages: A Review. Future Foods 2023, 8, 100257. [Google Scholar] [CrossRef]
- Curl, S.; Rivero-Mendoza, D.; Dahl, W.J. Plant-Based Milks: Hemp. EDIS 2020, 2020, 5. [Google Scholar] [CrossRef]
- Boukid, F. Oat Proteins as Emerging Ingredients for Food Formulation: Where We Stand? Eur. Food Res. Technol. 2021, 247, 535–544. [Google Scholar] [CrossRef]
- Chalupa-Krebzdak, S.; Long, C.J.; Bohrer, B.M. Nutrient Density and Nutritional Value of Milk and Plant-Based Milk Alternatives. Int. Dairy J. 2018, 87, 84–92. [Google Scholar] [CrossRef]
- Medic, J.; Atkinson, C.; Hurburgh, C.R. Current Knowledge in Soybean Composition. J. Am. Oil Chem. Soc. 2014, 91, 363–384. [Google Scholar] [CrossRef]
- Al Tamimi, J.Z. Effects of Almond Milk on Body Measurements and Blood Pressure. Food Nutr. Sci. 2016, 7, 466–471. [Google Scholar] [CrossRef]
- Tomishima, H.; Luo, K.; Mitchell, A.E. The Almond (Prunus dulcis): Chemical Properties, Utilization, and Valorization of Coproducts. Annu. Rev. Food Sci. Technol. 2022, 13, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Gul, O.; Saricaoglu, F.T.; Mortas, M.; Atalar, I.; Yazici, F. Effect of High Pressure Homogenization (HPH) on Microstructure and Rheological Properties of Hazelnut Milk. Innov. Food Sci. Emerg. Technol. 2017, 41, 411–420. [Google Scholar] [CrossRef]
- McClements, D.J.; Newman, E.; McClements, I.F. Plant-based Milks: A Review of the Science Underpinning Their Design, Fabrication, and Performance. Comp. Rev. Food Sci. Food Safe 2019, 18, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.D.C.G.; Mendonça, A.C.; Viana, K.W.C.; Maia, M.D.P.; Carvalho, A.F.D.; Minim, V.P.R.; Stringheta, P.C. Beverages Formulated with Whey Protein and Added Lutein. Cienc. Rural 2017, 47, e20151606. [Google Scholar] [CrossRef]
- Abe-Inge, V.; Aidoo, R.; Moncada De La Fuente, M.; Kwofie, E.M. Plant-Based Dietary Shift: Current Trends, Barriers, and Carriers. Trends Food Sci. Technol. 2024, 143, 104292. [Google Scholar] [CrossRef]
- Coşkun, N.; Sarıtaş, S.; Jaouhari, Y.; Bordiga, M.; Karav, S. The Impact of Freeze Drying on Bioactivity and Physical Properties of Food Products. Appl. Sci. 2024, 14, 9183. [Google Scholar] [CrossRef]
- Christodoulou, P.; Ladika, G.; Tsiantas, K.; Kritsi, E.; Tsiaka, T.; Cavouras, D.; Zoumpoulakis, P.; Sinanoglou, V.J. Quality Assessment of Greenhouse-Cultivated Cucumbers (Cucumis sativus) during Storage Using Instrumental and Image Analyses. Appl. Sci. 2024, 14, 8676. [Google Scholar] [CrossRef]
- Sinanoglou, V.J.; Tsiaka, T.; Aouant, K.; Mouka, E.; Ladika, G.; Kritsi, E.; Konteles, S.J.; Ioannou, A.-G.; Zoumpoulakis, P.; Strati, I.F.; et al. Quality Assessment of Banana Ripening Stages by Combining Analytical Methods and Image Analysis. Appl. Sci. 2023, 13, 3533. [Google Scholar] [CrossRef]
- Ladika, G.; Strati, I.F.; Tsiaka, T.; Cavouras, D.; Sinanoglou, V.J. On the Assessment of Strawberries’ Shelf-Life and Quality, Based on Image Analysis, Physicochemical Methods, and Chemometrics. Foods 2024, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Kritsi, E.; Ladika, G.; Stavropoulou, N.A.; Oikonomakou, M.; Ioannou, A.-G.; Christodoulou, P.; Konteles, S.J.; Cavouras, D.; Sinanoglou, V.J. Evaluation of the Quality Changes in Three Commercial Pastourma Samples during Refrigerated Storage Using Physicochemical, Microbiological, and Image Analyses Combined with Chemometrics. Foods 2024, 13, 1017. [Google Scholar] [CrossRef] [PubMed]
- Cichońska, P.; Ziarno, M. Legumes and Legume-Based Beverages Fermented with Lactic Acid Bacteria as a Potential Carrier of Probiotics and Prebiotics. Microorganisms 2021, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.S.M.; Wang, P.; Wang, N.; Yang, L.; Xiao, Z. Brown Rice Versus White Rice: Nutritional Quality, Potential Health Benefits, Development of Food Products, and Preservation Technologies. Comp. Rev. Food Sci. Food Safe 2019, 18, 1070–1096. [Google Scholar] [CrossRef] [PubMed]
- Ravichanthiran, K.; Ma, Z.F.; Zhang, H.; Cao, Y.; Wang, C.W.; Muhammad, S.; Aglago, E.K.; Zhang, Y.; Jin, Y.; Pan, B. Phytochemical Profile of Brown Rice and Its Nutrigenomic Implications. Antioxidants 2018, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Latifah, R.N.; Setiawan, C. Increase of Shelf Life Stability and Kinetics Study of Type 1 Brown Rice Milk through Addition of Alginate Extract from Sargassum Binderi. Food Sci. J. 2019, 1, 83. [Google Scholar] [CrossRef]
- Hidalgo-Fuentes, B.; de Jesús-José, E.; Cabrera-Hidalgo, A.d.J.; Sandoval-Castilla, O.; Espinosa-Solares, T.; González-Reza, R.M.; Zambrano-Zaragoza, M.L.; Liceaga, A.M.; Aguilar-Toalá, J.E. Plant-Based Fermented Beverages: Nutritional Composition, Sensory Properties, and Health Benefits. Foods 2024, 13, 844. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Domínguez, R.; Budaraju, S.; Roselló-Soto, E.; Barba, F.J.; Mallikarjunan, K.; Roohinejad, S.; Lorenzo, J.M. Effect of Innovative Food Processing Technologies on the Physicochemical and Nutritional Properties and Quality of Non-Dairy Plant-Based Beverages. Foods 2020, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.A.; Roriz, C.; Calhelha, R.C.; Rodrigues, P.; Pires, T.C.S.P.; Prieto, M.A.; Ferreira, I.C.F.R.; Barros, L.; Heleno, S.A. Valorization of Natural Resources—Development of a Functional Plant-Based Beverage. Food Chem. 2025, 472, 142813. [Google Scholar] [CrossRef] [PubMed]
- Tobolková, B.; Durec, J. Colour Descriptors for Plant-Based Milk Alternatives Discrimination. J. Food Sci. Technol. 2023, 60, 2497–2501. [Google Scholar] [CrossRef] [PubMed]
- Patra, T.; Rinnan, Å.; Olsen, K. The Physical Stability of Plant-Based Drinks and the Analysis Methods Thereof. Food Hydrocoll. 2021, 118, 106770. [Google Scholar] [CrossRef]
- Kayeye, T.N. Particle Size and Stability of Plant-Based Milks—Insights from Various Analytical Techniques. Master’s Thesis, Macquarie University, Sydney, NSW, Australia, 2023. [Google Scholar]
- Maskan, M.; Göǧüş, F. Effect of Sugar on the Rheological Properties of Sunflower Oil–Water Emulsions. J. Food Eng. 2000, 43, 173–177. [Google Scholar] [CrossRef]
- Pirsa, S.; Hafezi, K. Hydrocolloids: Structure, Preparation Method, and Application in Food Industry. Food Chem. 2023, 399, 133967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Q.; Liu, Z.; Zhi, L.; Jiao, B.; Hu, H.; Ma, X.; Agyei, D.; Shi, A. Plant Protein-Based Emulsifiers: Mechanisms, Techniques for Emulsification Enhancement and Applications. Food Hydrocoll. 2023, 144, 109008. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97. [Google Scholar] [CrossRef]
- Kozłowicz, K.; Różyło, R.; Gładyszewska, B.; Matwijczuk, A.; Gładyszewski, G.; Chocyk, D.; Samborska, K.; Piekut, J.; Smolewska, M. Identification of Sugars and Phenolic Compounds in Honey Powders with the Use of GC–MS, FTIR Spectroscopy, and X-Ray Diffraction. Sci. Rep. 2020, 10, 16269. [Google Scholar] [CrossRef] [PubMed]
- Vladimír, M.; Matwijczuk, A.P.; Niemczynowicz, A.; Kycia, R.A.; Karcz, D.; Gładyszewska, B.; Ślusarczyk, L.; Burg, P. Chemometric Approach to Characterization of the Selected Grape Seed Oils Based on Their Fatty Acids Composition and FTIR Spectroscopy. Sci. Rep. 2021, 11, 19256. [Google Scholar] [CrossRef] [PubMed]
- De Souza, T.R.P.; Olenka, L.; Peternella, W.S. A Study of Degradation in Vegetable Oils by Exposure to Sunlight Using Fourier Transform Infrared Spectroscopy. Mater. Sci. Appl. 2020, 11, 678–691. [Google Scholar] [CrossRef]
- Strati, I.F.; Tsiantas, K.; Psouni, A.; Ladika, G.; Cavouras, D.; Sinanoglou, V.J. Quality Assessment of Edible Plant-Based Fixed Oils Using Different Analytical Techniques and Machine Learning Approaches. Appl. Sci. 2024, 14, 10305. [Google Scholar] [CrossRef]
- Giang, L.T.; Thien, T.L.T.; Yen, D.H. Rapid Classification of Rice in Northern Vietnam by Using FTIR Spectroscopy Combined with Chemometrics Methods. Vietnam J. Chem. 2020, 58, 372–379. [Google Scholar] [CrossRef]
- Patra, T.; Olsen, K.; Rinnan, Å. A Multivariate Perspective on the Stability of Oat-Based Drinks Assessed by Spectroscopy. Food Hydrocoll. 2022, 131, 107831. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Ur Rehman, I. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Ioannou, A.G.; Kritsi, E.; Sinanoglou, V.J.; Cavouras, D.; Tsiaka, T.; Houhoula, D.; Zoumpoulakis, P.; Strati, I.F. Highlighting the Potential of Attenuated Total Reflectance—Fourier Transform Infrared (ATR-FTIR) Spectroscopy to Characterize Honey Samples with Principal Component Analysis (PCA). Anal. Lett. 2023, 56, 789–806. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Applications of Infrared Spectroscopy in Polysaccharide Structural Analysis: Progress, Challenge and Perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-J.; Zhou, Y.; Lee, J.S.; Shin, B.K.; Seo, J.-A.; Lee, D.; Kim, Y.-S.; Choi, H.-K. Discrimination and Prediction of the Origin of Chinese and Korean Soybeans Using Fourier Transform Infrared Spectrometry (FT-IR) with Multivariate Statistical Analysis. PLoS ONE 2018, 13, e0196315. [Google Scholar] [CrossRef] [PubMed]
- Ying, D.; Hlaing, M.M.; Lerisson, J.; Pitts, K.; Cheng, L.; Sanguansri, L.; Augustin, M.A. Physical Properties and FTIR Analysis of Rice-Oat Flour and Maize-Oat Flour Based Extruded Food Products Containing Olive Pomace. Food Res. Int. 2017, 100, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Greulich, O.; Duedahl-Olesen, L.; Mikkelsen, M.S.; Smedsgaard, J.; Bang-Berthelsen, C.H. Fourier Transform Infrared Spectroscopy Tracking of Fermentation of Oat and Pea Bases for Yoghurt-Type Products. Fermentation 2024, 10, 189. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and Infrared Spectroscopy of Carbohydrates: A Review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Mukhametov, A.; Mamayeva, L.; Kazhymurat, A.; Akhlan, T.; Yerbulekova, M. Study of Vegetable Oils and Their Blends Using Infrared Reflectance Spectroscopy and Refractometry. Food Chem. X 2023, 17, 100386. [Google Scholar] [CrossRef] [PubMed]
- Bureau, S.; Cozzolino, D.; Clark, C.J. Contributions of Fourier-Transform Mid Infrared (FT-MIR) Spectroscopy to the Study of Fruit and Vegetables: A Review. Postharvest Biol. Technol. 2019, 148, 1–14. [Google Scholar] [CrossRef]
- Yang, H.; Li, Y.; Zhao, J.; Chen, Z.; Huang, X.; Fan, G. Regulating the Composition and Secondary Structure of Wheat Protein through Canopy Shading to Improve Dough Performance and Nutritional Index. Food Res. Int. 2023, 173, 113399. [Google Scholar] [CrossRef] [PubMed]
- Roland, I.S.; Aguilera-Toro, M.; Nielsen, S.D.-H.; Poulsen, N.A.; Larsen, L.B. Processing-Induced Markers in Proteins of Commercial Plant-Based Drinks in Relation to Compositional Aspects. Foods 2023, 12, 3282. [Google Scholar] [CrossRef] [PubMed]
- Sotelo-Lara, D.M.; Amador-Espejo, G.G.; Álvarez-Araiza, D.F.; Cordero-Rivera, A.K.; Millán-Quintero, K.G.; Campos-Vega, R.; Velázquez-Estrada, R.M. Ultrasound and Thermosonication as Promising Technologies for Processing Plant-Based Beverages: A Review. Food Technol. Biotechnol. 2024, 62, 538–552. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; You, X.; Zhang, S.; Zhu, Z.; Yi, J.; Jin, G. Enhancing Functional Properties and Protein Structure of Almond Protein Isolate Using High-Power Ultrasound Treatment. Molecules 2024, 29, 3590. [Google Scholar] [CrossRef] [PubMed]
- Vanga, S.K.; Wang, J.; Orsat, V.; Raghavan, V. Effect of Pulsed Ultrasound, a Green Food Processing Technique, on the Secondary Structure and in-Vitro Digestibility of Almond Milk Protein. Food Res. Int. 2020, 137, 109523. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wu, J.; Li-Chan, E.C.Y.; Zhu, L.; Zhang, F.; Xu, X.; Fan, G.; Wang, L.; Huang, X.; Pan, S. Effects of Ultrasound on Structural and Physical Properties of Soy Protein Isolate (SPI) Dispersions. Food Hydrocoll. 2013, 30, 647–655. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.; Xu, B.; Wang, Y.; Zhang, C.; Cao, Y.; Xing, X. Research Progress on Classification, Sources and Functions of Dietary Polyphenols for Prevention and Treatment of Chronic Diseases. J. Future Foods 2023, 3, 289–305. [Google Scholar] [CrossRef]
- Bai, M.; Qin, G.; Sun, Z.; Long, G. Relationship between Molecular Structure Characteristics of Feed Proteins and Protein In Vitro Digestibility and Solubility. Asian Australas. J. Anim. Sci. 2015, 29, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Espinosa, M.E.; Guevara-Oquendo, V.H.; He, J.; Zhang, W.; Yu, P. Research Updates and Progress on Nutritional Significance of the Amides I and II, Alpha-Helix and Beta-Sheet Ratios, Microbial Protein Synthesis, and Steam Pressure Toasting Condition with Globar and Synchrotron Molecular Microspectroscopic Techniques with Chemometrics. Crit. Rev. Food Sci. Nutr. 2025, 65, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Grimsley, G.R.; Razvi, A.; Scholtz, J.M.; Pace, C.N. Increasing Protein Stability by Improving Beta-turns. Proteins 2009, 77, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.L.B.; Duarte, C.; Fausto, R. Analysis of Plant-Based Commercial Milk Substitutes Using ATR-FTIR Spectroscopy. J. Food Compos. Anal. 2025, 145, 107786. [Google Scholar] [CrossRef]
- Tsapou, E.A.; Sinanoglou, V.J.; Ntourtoglou, G.; Koussissi, E. Emphasizing the Potential of Attenuated Total Reflectance–Fourier Transform Infrared (ATR-FTIR) Spectroscopy Combined with Chemometrics, for Classification of Greek Grape Marc Spirits. Beverages 2024, 10, 42. [Google Scholar] [CrossRef]
- Kuhnen, S.; Ogliari, J.B.; Dias, P.F.; Boffo, E.F.; Correia, I.; Ferreira, A.G.; Delgadillo, I.; Maraschin, M. ATR-FTIR Spectroscopy and Chemometric Analysis Applied to Discrimination of Landrace Maize Flours Produced in Southern Brazil. Int. J. Food Sci. Technol. 2010, 45, 1673–1681. [Google Scholar] [CrossRef]
- Balan, B.; Dhaulaniya, A.S.; Jamwal, R.; Yadav, A.; Kelly, S.; Cannavan, A.; Singh, D.K. Rapid Detection and Quantification of Sucrose Adulteration in Cow Milk Using Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy Coupled with Multivariate Analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118628. [Google Scholar] [CrossRef] [PubMed]
- Balan, B.; Dhaulaniya, A.S.; Jamwal, R.; Amit; Sodhi, K.K.; Kelly, S.; Cannavan, A.; Singh, D.K. Application of Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Spectroscopy Coupled with Chemometrics for Detection and Quantification of Formalin in Cow Milk. Vib. Spectrosc. 2020, 107, 103033. [Google Scholar] [CrossRef]


| Composition (g/100 g) | Soy-Based Beverages | Rice-Based Beverages | Oat-Based Beverages | Brown Rice-Based Beverages | Almond-Based Beverages |
|---|---|---|---|---|---|
| Total fat | 2.1 ± 0.4 a | 1.2 ± 0.2 b | 1.4 ± 0.3 ab | 1.1 ± 0.2 b | 1.7 ± 0.7 ab |
| Saturated fat | 0.3 ± 0.1 a | 0.2 ± 0.1 a | 0.2 ± 0.1 a | 0.2 ± 0.1 a | 0.2 ± 0.2 a |
| Carbohydrates | 2.2 ± 0.8 a | 11.6 ± 0.9 b | 7.2 ± 1.9 c | 11.7 ± 1.5 b | 2.3 ± 2.0 a |
| Sugars | 1.8 ± 1.0 a | 6.2 ± 1.4 b | 4.2 ± 1.1 b | 5.1 ± 0.4 b | 1.8 ± 1.1 a |
| Protein | 3.7 ± 0.8 a | 0.3 ± 0.2 b | 0.9 ± 0.8 b | 0.5 ± 0.0 b | 1.1 ± 0.9 b |
| Salt | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a |
| Fibers | 0.3 ± 0.4 a | - | 0.6 ± 0.7 a | 0.5 ± 0.1 a | 0.5 ± 0.6 a |
| Color Parameters | Soy-Based Beverages | Rice-Based Beverages | Oats-Based Beverages | Brown Rice-Based Beverages | Almond-Based Beverages |
|---|---|---|---|---|---|
| L* | 62.30 ± 4.54 a | 59.65 ± 5.42 ab | 56.89 ± 3.04 ab | 54.15 ± 1.11 b | 60.01 ± 5.50 ab |
| a* | −1.09 ± 0.32 a | −0.42 ± 0.15 bd | −0.65 ± 0.21 b | −0.08 ± 0.06 c | −0.26 ± 0.08 d |
| b* | 8.77 ± 2.22 a | 3.42 ± 1.43 b | 6.16 ± 1.68 ab | 4.29 ± 1.11 b | 5.94 ± 1.59 ab |
| h | 96.67 ± 3.86 a | 100.02 ± 6.68 a | 96.56 ± 4.37 a | 95.37 ± 3.24 a | 92.89 ± 2.98 a |
| Relative Intensities % | Soy-Based Beverages | Rice-Based Beverages | Oat-Based Beverages | Brown Rice-Based Beverages | Almond-Based Beverages |
|---|---|---|---|---|---|
| 3300–3270 | 0.518 ± 0.026 a | 0.649 ± 0.044 b | 0.781 ± 0.052 c | 0.808 ± 0.021 c | 0.732 ± 0.065 cb |
| 3010 | 0.028 ± 0.002 a | 0.003 ± 0.001 b | 0.007 ± 0.001 c | 0.001 ± 0.000 d | 0.013 ± 0.002 e |
| 2922 | 0.409 ± 0.021 a | 0.204 ± 0.016 b | 0.265 ± 0.055 b | 0.240 ± 0.027 b | 0.449 ± 0.043 a |
| 2854 | 0.128 ± 0.007 a | 0.023 ± 0.007 b | 0.041 ± 0.005 c | 0.023 ± 0.009 b | 0.136 ± 0.023 a |
| 1743 | 0.192 ± 0.010 a | 0.129 ± 0.021 b | 0.121 ± 0.024 b | 0.175 ± 0.021 c | 0.170 ± 0.018 c |
| 1649–1631 | 0.407 ± 0.021 a | 0.049 ± 0.005 b | 0.120 ± 0.013 c | 0.045 ± 0.003 b | 0.373 ± 0.018 a |
| 1545–1535 | 0.224 ± 0.013 a | 0.001 ± 0.000 b | 0.025 ± 0.005 c | 0.005 ± 0.001 d | 0.157 ± 0.012 e |
| 1460–1440 | 0.053 ± 0.003 a | - | 0.007 ± 0.003 b | - | 0.049 ± 0.006 a |
| 1421–1396 | 0.066 ± 0.004 a | 0.038 ± 0.001 b | 0.032 ± 0.004 c | 0.039 ± 0.003 b | 0.054 ± 0.006 d |
| 1314 | 0.071 ± 0.007 a | 0.003 ± 0.000 b | 0.046 ± 0.004 c | 0.013 ± 0.002 d | 0.091 ± 0.006 e |
| 1240 | 0.060 ± 0.003 a | 0.033 ± 0.004 b | 0.034 ± 0.003 b | 0.050 ± 0.011 ac | 0.043 ± 0.003 c |
| 1157 | 0.043 ± 0.003 a | 0.117 ± 0.008 b | 0.088 ± 0.011 c | 0.113 ± 0.005 b | 0.045 ± 0.011 a |
| 1107–1100 | 0.006 ± 0.001 a | 0.022 ± 0.002 b | 0.010 ± 0.001 c | 0.021 ± 0.002 b | 0.010 ± 0.001 c |
| 1075–1045 | 0.021 ± 0.002 a | 0.051 ± 0.003 b | 0.036 ± 0.005 c | 0.055 ± 0.002 b | 0.047 ± 0.008 bc |
| 1022–1010 | 0.161 ± 0.020 a | 0.615 ± 0.021 b | 0.568 ± 0.050 b | 0.635 ± 0.038 b | 0.314 ± 0.043 c |
| 999–979 | 0.110 ± 0.013 a | - | - | - | 0.092 ± 0.014 a |
| 932–920 | 0.032 ± 0.004 a | 0.052 ± 0.004 b | 0.042 ± 0.005 c | 0.052 ± 0.002 b | 0.047 ± 0.003 c |
| 870–835 | 0.018 ± 0.002 a | 0.039 ± 0.003 b | 0.024 ± 0.004 a | 0.038 ± 0.003 b | 0.020 ± 0.002 a |
| 819–817 | - | - | - | - | 0.009 ± 0.002 |
| 770–750 | - | 0.035 ± 0.004 ab | 0.028 ± 0.005 a | 0.037 ± 0.002 b | 0.034 ± 0.003 ab |
| 721–718 | 0.007 ± 0.001 a | 0.023 ± 0.002 b | 0.020 ± 0.003 b | 0.023 ± 0.002 b | 0.015 ± 0.002 c |
| 667–630 | 0.004 ± 0.001 a | - | 0.007 ± 0.001 b | - | 0.001 ± 0.000 c |
| 575–532 | 0.015 ± 0.002 a | 0.029 ± 0.004 b | 0.026 ± 0.008 bc | 0.020 ± 0.001 c | 0.031 ± 0.002 b |
| 526–516 | - | 0.016 ± 0.004 a | 0.016 ± 0.004 a | 0.017 ± 0.002 a | 0.008 ± 0.001 b |
| Secondary Structure of Proteins (%) | Soy-Based Beverages | Rice-Based Beverages | Oats-Based Beverages | Brown Rice-Based Beverages | Almond-Based Beverages |
|---|---|---|---|---|---|
| β-parallel sheet (1610–1642 cm−1) | 68.07 ± 3.37 aA | 38.77 ± 1.11 bA | 34.75 ± 2.47 cA | 42.05 ± 1.05 dA | 42.70 ± 2.34 dA |
| random coil (1642–1650 cm−1) | 12.18 ± 1.58 aB | 32.68 ± 1.40 bB | 25.47 ± 1.50 cB | 26.16 ± 1.57 cB | 19.28 ± 1.80 dB |
| α-helix (1650–1660 cm−1) | 12.20 ± 1.71 aB | 17.01 ± 0.26 bC | 24.58 ± 1.51 cB | 16.81 ± 0.61 bC | 20.26 ± 1.42 dB |
| β-turn (1660–1680 cm−1) | 7.55 ± 0.87 aC | 11.54 ± 0.52 bD | 15.20 ± 1.08 cC | 14.98 ± 1.37 cC | 17.75 ± 0.82 dC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christodoulou, P.; Athanasopoulou, S.; Ladika, G.; Konteles, S.J.; Cavouras, D.; Sinanoglou, V.J.; Kritsi, E. Decoding Plant-Based Beverages: An Integrated Study Combining ATR-FTIR Spectroscopy and Microscopic Image Analysis with Chemometrics. AppliedChem 2025, 5, 16. https://doi.org/10.3390/appliedchem5030016
Christodoulou P, Athanasopoulou S, Ladika G, Konteles SJ, Cavouras D, Sinanoglou VJ, Kritsi E. Decoding Plant-Based Beverages: An Integrated Study Combining ATR-FTIR Spectroscopy and Microscopic Image Analysis with Chemometrics. AppliedChem. 2025; 5(3):16. https://doi.org/10.3390/appliedchem5030016
Chicago/Turabian StyleChristodoulou, Paris, Stratoniki Athanasopoulou, Georgia Ladika, Spyros J. Konteles, Dionisis Cavouras, Vassilia J. Sinanoglou, and Eftichia Kritsi. 2025. "Decoding Plant-Based Beverages: An Integrated Study Combining ATR-FTIR Spectroscopy and Microscopic Image Analysis with Chemometrics" AppliedChem 5, no. 3: 16. https://doi.org/10.3390/appliedchem5030016
APA StyleChristodoulou, P., Athanasopoulou, S., Ladika, G., Konteles, S. J., Cavouras, D., Sinanoglou, V. J., & Kritsi, E. (2025). Decoding Plant-Based Beverages: An Integrated Study Combining ATR-FTIR Spectroscopy and Microscopic Image Analysis with Chemometrics. AppliedChem, 5(3), 16. https://doi.org/10.3390/appliedchem5030016







