Abstract
We evaluated the effects of feeding 60% dried corn distillers’ grains plus solubles (DDGS) or the equivalent sulfur as CaSO4 on sperm characteristics and transcript abundance. Thirty-six half-sibling Angus bulls (256 ± 8.5 d; initial BW = 320 ± 2.7 kg) were assigned to one of three treatments: (1) 60% concentrate as corn (CON); (2) 60% DDGS as corn replacement (60DDGS); and (3) CON diet + equivalent sulfur of 60DDGS added as CaSO4 (SULF). The acrosome/cell membrane integrity, mitochondrial energy potential, oxidation status, DNA integrity, and zinc signatures were analyzed via flow cytometry. Sperm-specific gene expression was assessed via RNA sequencing. The flow cytometry data were analyzed using PROC MIXED in SAS to determine the effects of treatment. Pairwise comparisons based on edgeR were used to identify differentially expressed genes. The percentage of polarized mitochondria tended to be greater (p = 0.08) for SULF compared with CON and 60DDGS. Protamine 1 was upregulated (p < 0.01; FDR = 0.10) in 60DDGS compared to CON. Zinc signature 1 in 60DDGS and SULF was reduced (p = 0.03) compared to CON. This study suggests that feeding bulls diets containing 60% DDGS had little effect on DNA integrity and gene expression.
1. Introduction
Early-life nutrition is vital for pubertal attainment and the reproductive success of bulls [1,2]. Feeding an increased plane of nutrition (high energy and/or high protein) may be beneficial for peri-pubertal bull development; however, caution must be taken because specific nutrients (i.e., sulfur) within certain high-energy/high-protein feeds may result in decreased reproductive performance [3,4,5]. An ingredient that has influenced yearling Angus bull reproductive performance is dried corn distillers’ grains plus solubles (DDGS) [6,7]. There is a possibility that the presence of elevated sulfur within DDGS may be detrimental to sperm motility and function [8,9,10].
Previous studies have reported that exposing mice or ram lambs to sulfur, as sulfur dioxide or DDGS, respectively, has resulted in decreased sperm concentration, decreased sperm motility, and increased abnormal sperm [10,11]. Previous studies by Kassetas et al. [6,7] have reported that feeding 60% DDGS to yearling Angus bulls altered sperm velocity parameters, glutathione peroxidase concentrations in seminal plasma, and trace mineral concentrations in serum and seminal plasma. These alterations to semen from increased percentages of dietary sulfur may negatively influence the ability of sperm to deliver quality intact DNA to the oocyte for proper fertilization and embryogenesis [12]. If damage occurs to DNA and/or RNA at a level where the cell cannot repair itself, reproductive processes (i.e., the ability for sperm to bind to the sperm oviductal reservoir, complete fertilization) will be negatively altered [13,14]. Flow cytometric assays can be used to evaluate novel measures of potential bull fertility including mitochondrial energy potential, oxidation potential, and sperm viability [15]. Furthermore, zinc signatures (a term describing the four zinc patterns [16]) can be used to evaluate the stages of sperm capacitation [17] and provide insight into sperm quality and fertilization capacity in bulls.
Evaluating the gene expression of sperm can provide insight into the indicators of potential sperm fertility. Certain genes, such as BMP2, NGF, TRADD, CCDC174, and others, have been identified as potential genetic markers for increased sperm quality [18,19]. Identifying genetic markers in sperm for the current study could explain whether sulfur within DDGS is influencing sperm quality and function at the genomic level. Therefore, it was hypothesized that DNA integrity and gene expression would be negatively altered for Angus bulls fed elevated concentrations of sulfur. The objectives of this study were to evaluate the effects of feeding 60% DDGS or the equivalent sulfur as CaSO4 on DNA structure and integrity in frozen/extended semen and gene expression in bull sperm.
2. Materials and Methods
All procedures were approved by the North Dakota State University Institution for Animal Care and Use Committee (#A19035).
2.1. Animals and Treatments
Thirty-six half-sibling Angus bulls (291 ± 8.5 d of age; mean initial body weight (BW) = 320 ± 2.7 kg) originating from a commercial cattle herd at the Central Grasslands Research Extension Center near Streeter, ND, were used in this experiment. Bulls were blocked by initial BW and sperm concentration, and then, randomly assigned to one of three treatments: (1) corn-based diet containing 60% concentrate as corn [CON; S = 0.18% dry matter (DM); n = 12]; (2) diet containing 60% DDGS as a replacement for corn (60DDGS; S = 0.55% DM; n = 12); (3) CON diet + equivalent sulfur of the 60DDGS diet added as CaSO4 (SULF; S = 0.54% DM; n = 12). Treatments were initiated on 20 December (d0) and continued for 112 days. All bulls were housed indoors in the Animal Nutrition and Physiology Center in Fargo, ND. Bulls were individually fed in a Calan gate system and body weights were recorded every 14 d during the 112-day study with the 2-day weight measured at the beginning and end of the study. Individual intakes were adjusted every 14 days to achieve a 1.6 kg/d average daily gain (ADG).
Diets were prepared as a total mixed ration by adding a vitamin premix, soybean meal, and CaSO4 (for the SULF diet only) to the mixer, and then, cracked corn or DDGS, hay, and corn silage were added after. After each diet was made and distributed to its respective bunk, the mixer and feed cart were emptied and cleaned. Diets were formulated to be equal in forage, concentrate, and total fat on a dry matter basis. Furthermore, the SULF diet was formulated to be equal in sulfur in the form of CaSO4 compared with total sulfur in the 60DDGS treatment. For more information regarding diet formulation and composition, refer to [6,7]. Data regarding pubertal status, scrotal circumference, semen characteristics, mineral concentrations, and metabolite concentrations for bulls were previously reported [6,7]. Samples for pubertal status, scrotal circumference, semen characteristics, mineral concentrations, and metabolite concentrations for bulls were collected every 28 d for 112 d before the morning feeding. However, the data presented in this paper are from semen collected on d 112. A schematic of semen processing for mRNA and flow cytometry evaluation is found in Figure 1.
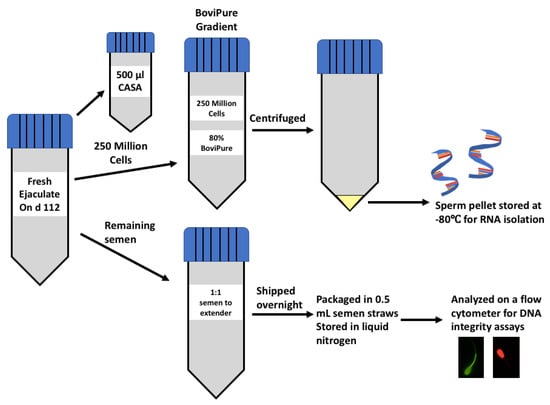
Figure 1.
Schematic showing the method for semen collection on d 112 across all dietary treatments ((1) corn-based diet containing 60% concentrate (CON; n = 12); (2) diet containing 60%; DDGS as a replacement for corn (60DDGS; n = 12); (3) equivalent sulfur of 60DDGS added to the CON diet as calcium sulfate (SULF; n = 12)).
2.2. Sample Preparation for RNA Isolation
Future efforts of our lab using this research model will involve evaluating the effects of our treatments on the paternal contribution to developmental programming. Therefore, before RNA isolation, we used density gradient centrifugation to target the population of cells most likely to fertilize oocytes. To remove contaminating somatic cells and those sperm cells of abnormal morphology via density gradient centrifugation, an 80% BoviPure solution (3.2 mL of 100% BoviPure + 0.8 mL of BoviDilute; Spectrum Technologies; Healdsburg, CA, USA) was prepared [20,21] in a sterile laminar flow hood and stored at 4 °C overnight. On the day of collection, BoviPure gradients were equilibrated to room temperature 30 min before use. Before the morning feeding on d 112, semen was collected one time per bull via electroejaculation (Pulsator IV; Lane Manufacturing Inc.; Denver, CO, USA) into disposable plastic semen collection bags. Semen evaluation and sperm concentration were determined as a component of semen evaluation via a Computer-Assisted Semen Analyzer (CASA; IVOS II; Hamilton Thorne; Beverly, MA, USA). Two hundred and fifty million cells were diluted in BoviWash (Spectrum Technologies; Healdsburg, CA, USA) to obtain 1 mL of the sample. For samples that were below that concentration, 250 million sperm were centrifuged at 1200× g for 5 min to concentrate the sample. Sperm were resuspended in 1 mL of BoviWash, and then, the sample was added to the BoviPure gradient (according to the manufacturer’s instructions). Semen containing 250 million sperm cells was pipetted onto the 80% BoviPure density gradient and centrifuged for 28 min at 300× g with no brake. Immediately after centrifugation, a glass Pasteur pipette was used to remove the supernatant, and the pellet was frozen and stored at −80 °C until RNA isolation (Figure 1).
2.3. Total RNA Isolation
BoviPure density gradient-separated sperm pellets were thawed at 20 °C, and then, kept on ice for 1 h during processing. Sperm cell concentration was determined using the CASA. Thirty million cells were pipetted into a fresh 2.0 mL RB sample tube (Qiagen; Germantown, MD, USA) and centrifuged at 1200× g for 10 min. The supernatant was removed, and the sample was vortexed to break up the pellet. A total of 500 μL RLT lysis buffer, 7.5 μL of β-mercaptoethanol, and 0.5 g of 0.2 mm stainless steel homogenization beads (Qiagen; Next Advance; Averill Park, NY, USA) were added and the samples homogenized using a TissueLyser LT (Qiagen; Germantown, MD, USA) for 5 min at 50 Hz. Next, 500 μL of Qiazol (Qiagen; Germantown, MD, USA) was added, and each tube was vortexed for two minutes. Samples were incubated for 5 min at 20 °C to dissolve nucleoprotein complexes, centrifuged for 5 min at 16,000× g, and the supernatant was transferred to a new tube.
Total RNA was purified using the Zymo Direct-zol RNA MiniPrep Kit (cat. # R2050; Zymo Research; Irvine, CA, USA) and an on-column DNase 1 treatment was performed following the manufacturers protocol. Total RNA (>18 nucleotide length) was eluted from the ZYMO column with 30 μl of heated DNase/RNase-free water after incubation at 20 °C for 5 min (17). The last elution step was repeated for a final total volume of 60 μL, and samples were stored at −80 °C until further analysis.
2.3.1. RNA Sample Quantification and Quality Evaluation
The isolated RNA was quantified via a Qubit 3.0 Fluorometer (Thermo Fisher Scientific; Waltham, MA, USA) using the Qubit microRNA assay kit (Thermo Fisher Scientific; Waltham, MA, USA) due to the low quantity of fragmented RNA typically obtained from sperm. RNA quality was assessed using the Agilent RNA 6000 Pico Kit (Agilent Technologies; Santa Clara, CA, USA; lot # 1923) via the 2100 Expert Software and a DV200 assay. An RNA Integrity Number (RIN) is generated by calculating the 18S-to-28S ribosomal band ratio. Due to the highly fragmented nature of sperm RNA, low RIN values of 2 to 4 are typical, and therefore, we also used the DV200 quality metric to represent the percentage of RNA fragments that were > 200 nucleotides in size (18). Six samples per treatment that had the highest DV200 values, and the best RINs were selected for RNA sequencing. The average amount of RNA isolated per sample was 46.0 ± 7.1 ng/μL, and samples ranged from 18.1 to 146.0 ng/μL. The average RIN for all samples was 2.3 ± 0.03. Two samples, one SULF and one CON, had DV200 values > 50%, eleven samples ranged from 30% to 50%, and five samples were <30%. Samples that had a DV200 < 50% were analyzed to achieve the goal of obtaining 6 samples per treatment for RNA sequencing.
2.3.2. RNA Library Preparation and Sequencing
Unstranded RNA libraries (n = 18; 6 per treatment) were prepared using the NEBNext Ultra II RNA Library Prep Kit (Illumina; Madison, WI, USA) following the manufacturer’s protocol. Sequencing was carried out using an Illumina NovaSeq SP 100 Cycle flow cell (Illumina; Madison, WI, USA). Library preparation and paired-end sequencing with 50 bp reads (target 10 million reads) were carried out at Wayne State University Genome Sciences Core (Detroit, MI, USA).
2.3.3. Data Quality Control and Differential Analysis
Sequencing adaptors and reads containing low-quality bases were removed in an initial data-filtering step. Cleaned reads were mapped to the Bos taurus reference genome (bosTau9) using the STAR aligner v. 2.7.5a build [22]. The number of mapped reads to each gene was obtained using the HTSeq software v. 0.13.5 [23]. Differential gene expression analysis was performed using edgeR v.3.24.3. Pairwise comparisons were evaluated, and differentially expressed genes were identified when the FDR < 0.1 and |log fold change| ≥ 2; p-value ≤ 0.05. The differentially expressed genes were classified as up- or downregulated based on the sign of the log2 fold change. The biological processes underlying the differentially expressed genes were retrieved using DAVID gene ontology analyses [24].
2.4. Sample Preparation for Flow Cytometry Assays
The remaining semen that was collected on d 112 was added 1:1 to a commercial semen extender (OptiXcell II; Ref. # 026218; IMV Technologies; Maple Grove, MN, USA), shipped overnight in a box containing ice packs to be processed in 0.5 mL straws each containing 40 million sperm, and stored in liquid nitrogen (Hawkeye Breeders Inc.; Adel, IA, USA; Figure 1). Semen straws from each bull (n = 36; 12 per treatment) were thawed for 40 s in a 37 °C water bath, and then, put in 0.5 mL screw-cap tubes. Concentrations were obtained using an Accureader (IMV Technologies; Maple Grove, MN, USA) and all samples were diluted in Sperm TL (Caisson Labs; Smithfield, UT) to achieve a cell count of 57 million cells per mL. The Guava easyCyte 5HT Flow Cytometer (Millipore Sigma; Burlington, MA, USA) was used to analyze acrosome/cell membrane integrity, mitochondrial energy potential, oxidation status, DNA integrity, and zinc signatures in frozen/extended bull semen. In general, sperm cells were diluted to 57 million/mL. The target flow rate for these assays was under 500 cells/μL and a 488 nm excitation laser was used in all assays. Side scatter and forward scatter gating were used to differentiate between sperm cells and debris so that further analyses would include evaluations of sperm cells only. The easyCyte 5HT Flow Cytometer has a bandpass filter with photomultiplier tubes, but does not contain sheath fluid. Guava EasyCheck calibration beads were used to calibrate the equipment. Logarithmic representation was used with one replicate. There was no data compensation for this experiment. GuavaSoft IMV 1.0 was used to analyze histograms and dot plots with a target of 5000 events per sample.
2.4.1. Mitochondrial Energy Potential
An EasyKit 2 (Ref. # 024864; IMV Technologies; Maple Grove, MN, USA) assay was used to evaluate mitochondrial activity. The 96-well plate included in the kit was protected from light, transferred to a working base, and then, wells required for the assay were removed from the plate. Ten microliters of 100% ethanol were added to each well to resuspend kit components at the bottom of each well; then, 190 μL of PBS (calcium- and magnesium-free) was added per well. Next, 43,000 cells were added to each well. The plate was covered and incubated in the dark for 30 min at 37 °C. After incubation, the cells were immediately analyzed using the flow cytometer. Cells that fluoresced orange (596 nm) were considered polarized (high mitochondrial energy potential), whereas cells that fluoresced green (525 nm) were depolarized.
2.4.2. Oxidation Potential
The EasyKit 3: Oxidation molecule D (Ref. # 025157; IMV Technologies; Maple Grove, MN, USA) was used to assess the oxidation status of sperm by measuring the ability of sperm to withstand exposure to reactive oxygen species (hydrogen peroxide) using flow cytometry. Each well contained 200 μL of filtered PBS and approximately 228,000 cells were added, covered, and incubated in the dark for 20 min at 37 °C. After 20 min, 2 μL of hydrogen peroxide (38 mM) was added to each well. The plate was covered and incubated in the dark for 40 min at 37 °C. From each well, 200 μL of PBS/cell solution was added to 600 μL of PBS and centrifuged for 5 min at 300× g. The supernatant was removed, and the pellet was resuspended in 300 μL PBS. This solution was analyzed using the flow cytometer. Cells that fluoresced red (620 nm) had a damaged plasma membrane and cells that emitted green fluorescence (530 nm) were oxidized. The cell population generated comprised Live ROS-, Live ROS+, Dead ROS-, and Dead ROS+. The population of interest is Live ROS+ as this is a challenge assay and Live ROS+ cells have the capability of responding to the stress of hydrogen peroxide.
2.4.3. DNA Integrity
A DNA compaction assay [25] (IMV Technologies; Maple Grove, MN, USA) was used to estimate the structural stability of sperm chromatin in the nucleus after acid digestion. Two microliters of semen (114,000 cells) were added to 98 μL of TNE buffer (0.15 M NaCl, 0.01 Tris, 1 mM EDTA; pH = 7.4) and digested for 30 s with 200 μL of acid detergent solution (0.15 M NaCl, 0.1% Triton-X-100, 0.08 N HCl; pH = 1.2). Acridine orange (600 µL) staining solution (6 μg/mL; 0.15 NaCl, 1 mM EDTA, 0.1 M citric acid, 0.2 M Na2HPO4; pH = 6) was added to each well and the reaction was incubated at 20 °C for 2.5 min. After incubation, samples were analyzed using the GuavaSoft/DNA compaction program via the flow cytometer. The Easysoft (IMV) software was used to identify fragmented DNA (orange; 620 nm) compared to intact DNA (green; 525 nm). Cells that fluoresced high green were characterized as cells with less chromatin (immature cells), and the DNA fragmentation index (DFI) was defined as the percentage of cells that had fragmented DNA. The DNA fragmentation index was calculated using Easysoft Software where the mean of the red population of cells was divided by the total red and green fluorescence intensity [13].
2.4.4. Viability Assay
The Sybr 14 (Ref. # L7011; Lifetech; Carlsbad, CA, USA) nucleic acid stain was diluted with dimethyl sulfoxide (DMSO; 900 nM). In each well, 266 µL PBS, 31.1 µL of 900 nM Sybr 14, 3 µL of 2.4 nM propidium iodide, and 57,000 sperm cells were added. The samples were analyzed via the flow cytometer using the assay protocol adapted from Garner and Johnson [26]. Live sperm was labeled with Sybr 14 fluoresced green (530 nm) and dead sperm was labeled with propidium iodide fluoresced red (620 nm).
2.4.5. Acrosome Integrity Assay
This assay was adapted from IMV Technologies [27]. A stain mix was prepared containing propidium iodide (48 µM), a peanut agglutinin probe with fluorescein isothiocyanate (PNA-FITC (0.01 mg/mL); Thermo Fisher Scientific; Waltham, MA, USA), and easy buffer B (IMV Technologies; Maple Grove, MN, USA). In each well, 57,000 cells were added with stain mix (50 µL) and easy buffer B (150 µL), and then, analyzed using the flow cytometer. This assay determined the cell viability (no propidium iodide binding) and the acrosomal integrity of each sample by assessing fluorescence and acrosomal damage (PNA-FITC bound). The cell populations generated include live acrosome intact, dead acrosome intact, live acrosome disrupted, and dead acrosome disrupted. The population of interest was the live intact cells as these cells are potentially capable of fertilization.
2.4.6. Zinc Signature Assay
The zinc signature assay was adapted from Kerns et al. [17]. Two working stock solutions were made prior to beginning the assay. A 1:50 FluoZin-3 working stock solution was made by diluting FluoZin-3, AM (Thermo Fisher Scientific; Waltham, MA, USA) in Sperm TL buffer. A 1:200 propidium iodide working stock solution was made by diluting propidium iodide in Sperm TL buffer. The concentration of each sample was obtained, and 1 million cells were diluted in 1 mL of warmed Sperm TL before centrifugation at 300× g for 5 min. The supernatant was removed, and the sperm cells were resuspended in 80 µL Sperm TL and 10 µL of FluoZin-3 stock solution at 20 °C for 30 min. The cells were centrifuged at 300× g for 5 min, the supernatant was removed, and 75 µL of Sperm TL and 25 µL propidium iodide were added. The samples were incubated under foil for 45 min to activate FluoZin-3. An aliquot (10 µL) of sperm (57,000 cells) was added to 200 µL of dPBS in each well and analyzed using the flow cytometer. FluoZin-3, AM is a cell-permeant zinc probe that has high binding affinity for Zn2+ and is excited at 488 nm. The assay distinguished the different signatures based on FluoZin-3, AM and propidium iodide intensity. Gating was used when analyzing the data to further distinguish between the zinc signature populations, as described previously [17,28].
2.4.7. Statistical Analysis for Flow Cytometry Analysis
For all DNA and zinc signature analyses, bull was the experimental unit. All data were analyzed using the MIXED procedures of SAS (SAS 9.4; Cary, NC, USA) with a model including treatment (CON, 60DDGS, and SULF). Normal distribution and homogeneity of variances were evaluated for every parameter before data were analyzed using PROC MIXED. Results were considered significant when p-values were ≤0.05, and tendencies were evaluated when 0.05 ≤ p ≤ 0.10.
3. Results
3.1. Sperm mRNA Quality and Transcript Abundance
On average, sperm RNA sequencing generated 16.57 M reads per sample with a PhredScore > 30, and 83.0% of the reads were uniquely mapped to genes in the bovine reference genome. Differential expression analysis was used to determine the effect of feeding DDGS or SULF diets on the expression profile of sperm RNA. The approach identified that the RNA Component of Signal Recognition Particle 7SL1 (RN7SL1), 18S Ribosomal RNA (RN18S1), and protamine 1 (PRM1) genes were upregulated and differentially expressed when comparing 60DDGS to CON (FDR < 0.10; Figure 2). However, no significant differences were identified when comparing SULF to CON or 60DDGS to SULF. Due to the relatively low number of differentially expressed genes, no significant changes in biological processes or pathways were identified.
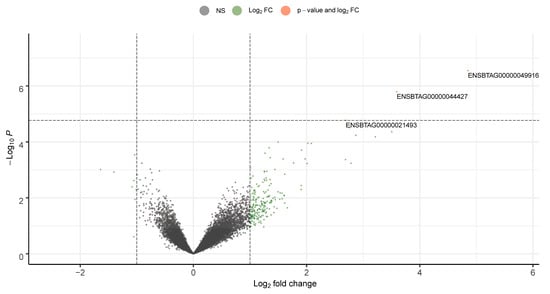
Figure 2.
Volcano plot showing the differentially expressed genes in sperm from 60DDGS (diet containing 60%; DDGS as a replacement for corn (n = 12)) treatment compared with CON (corn-based diet containing 60% concentrate (control; n = 12)). The log2 fold change indicates the mean expression level for each gene. Each dot represents one gene. Orange dots represent the genes that were differentially expressed (FDR < 0.10).
3.2. Flow Cytometry Analysis
No differences (p = 0.25) in post-thaw motility were observed among treatments (21.6, 19.5, and 24.7 ± 5.8% motile for CON, 60DDGS, and SULF, respectively). No differences (p ≥ 0.15) were observed among treatments for the viability, acrosome integrity, oxidation potential, and DNA integrity assays (Table 1). However, there was a tendency (p = 0.08) for an increased percentage of polarized mitochondria for SULF compared to CON and 60DDGS (Table 1).

Table 1.
Characteristics of frozen/thawed sperm from yearling bulls fed 60% DDGS or the equivalent sulfur as CaSO4, analyzed via flow cytometry.
No differences (p ≥ 0.11) were observed for zinc signatures 2, 3, or 4. However, there was a difference among treatments for zinc signature 1 where 60DDGS and SULF had reduced (p = 0.04) percentages of zinc signature 1 compared to CON (Table 2). Overall, the majority of the sperm within each treatment were identified as having zinc signature 3.

Table 2.
Least square means for the zinc signature 1 assay analyzed using the flow cytometer with frozen/extended semen from yearling Angus bulls at d 112.
4. Discussion
4.1. RNA Quality and Transcript Abundance
The current study hypothesized that elevated sulfur from DDGS would alter transcript abundance in the sperm of yearling Angus bulls. The bulls were on their respective diets for 112 days (approximately 1.8 spermatogenic cycles) to ensure that sperm from all stages were exposed to the metabolic environment imposed by the respective treatment diets. Additionally, most of the bulls utilized in this study were pre-pubertal at the beginning of the study and all became pubertal as the study progressed [6]. The peripubertal period has been identified as a time where sperm may be particularly susceptible to epigenetic changes from nutrition and/or environmental factors [29,30]. According to Jodar [31], the transcripts observed in sperm are mainly remnants of untranslated mRNAs transcribed before the DNA compaction process. Two differentially expressed genes (RN7SL1; RN18S1) were ribosomal RNAs (rRNA). rRNAs are the most abundant RNAs in sperm and have been suggested to be selectively cleaved [31]. There was a previous report of the RN7SL1 gene being highly abundant in Bos indicus spermatozoa [32]. This gene codes the signal recognition particle (SRP) required for co-translational regulation [33]. It has been suggested that rRNAs interact with argonaut (AGO) proteins and potentially silence target genes [34].
Protamine 1 was differentially expressed in this study and was upregulated in bulls fed the 60DDGS diet compared to bulls fed the CON diet. Previous research has observed that a deficiency in PRM1 may result in alterations to DNA integrity and DNA damage, leading to infertility in bulls [12]. Alternatively, greater sperm PRM1 abundance from cryopreserved semen was observed in high-fertility bulls compared with low-fertility bulls, indicating that PRM1 may be related to fertility [35]. In the current study, PRM1 was upregulated in 60 DDGS bulls but DNA integrity did not differ. Field fertility was not evaluated in the current study.
Based on the timeline and conditions tested in the current study, a large number of differentially expressed genes were not identified. Other researchers have utilized RNA sequencing methodologies and found no differences in gene expression when comparing bulls with low versus high sperm motility using the cold-TRIzol protocol [36]. An important factor to consider when interpreting the results from the current report is the experimental design, where no differences in body weight were observed because the bulls were weighed frequently and the diet deliveries were adjusted to achieve a weight gain of 1.6 kg/d (previously reported in [6]). Perhaps alterations in sperm RNA profiles are more sensitive to divergence in energy balance and other stressors that impact body weight and/or composition compared with changes in dietary ingredients with a similar body weight gain [37]. Additionally, as sperm acquire miRNA during their time in residence in the epididymis [38] and miRNAs are sensitive to environmental stressors [39], perhaps future work involving profiling miRNA in response to dietary ingredients is warranted.
Although 83% of the reads were uniquely mapped, it is worth mentioning that these reads were mainly mapped to the intronic (35%) or intergenic (63.6%) regions. This may be a result of the complex nature of the sperm RNA profile or a potential bias due to RNA isolation. However, Mao et al. [40] reported high levels of intronic (42.1%) and intergenic (53.8%) reads when evaluating the human sperm RNA profile. Additionally, the high percentage of intronic and intergenic reads identified in the current study is explained by the high percentage of functional non-coding RNAs present in the sperm [41,42].
4.2. Mitochondrial Energy Potential and DNA Integrity
Mitochondrial energy potential is crucial to the transport of electrons across the mitochondrial membrane to produce ATP. In the current study, there was a tendency for an increase in polarized mitochondria in the SULF treatment compared with CON and 60DDGS. Increased mitochondrial polarization indicates that sperm are in, or more capable of, an active state, thus indicating the greater long-term energy producing potential of sperm [43,44]. Increased mitochondrial potential has been correlated with increased sperm motility [45]. Alternatively, cells with a depolarized mitochondrion begin to die via apoptosis or necrosis. Therefore, it would be expected that as mitochondrial energy potential increases, an increase in motility or velocity parameters may be observed, but this was not the case for this study. The overall portion of motile and progressively motile sperm from fresh semen was not influenced by treatment, but the sperm velocity parameters in the motile and progressively motile populations of sperm were reduced for 60DDGS and SULF [6]. In addition, post-thaw motility was evaluated and no differences were observed among treatments. It is important to note that differences in sperm motion parameters were observed among treatments across the developmental period (d 0 to d 112). Other timepoints throughout the study may have driven the differences observed among treatments for sperm velocity parameters. To our knowledge, there is lack of studies in the literature evaluating the effects of feeding high-sulfur diets, specifically calcium sulfate or sulfuric acid, on mitochondrial energy potential in bulls.
Moreover, with the increase in mitochondrial energy potential in the SULF treatment, there was a possibility that more reactive oxygen species would be produced. An increase in reactive oxygen species combined with a lack of antioxidant enzymes in seminal plasma could negatively influence sperm DNA structure and function [8]. We previously reported that glutathione peroxidase concentrations in seminal plasma were greater for 60DDGS compared with SULF and CON at d 112 from the same study [6]. Glutathione peroxidase was the only antioxidant enzyme evaluated in the current study; therefore, the sperm in the SULF treatment may have utilized other enzymes (superoxide dismutase or catalase) to protect sperm from reactive oxygen species, especially since oxidation potential was not different between treatments.
Meng et al. [46] evaluated the effects of sulfur dioxide inhalation on DNA integrity and sperm characteristics in male mice. They observed that, within the testes, there was an increase in DNA damage as the inhalation of sulfur dioxide increased [46]. Although the form of sulfur in their study is different from that in the current study, it is possible that sulfur could cause an increase in sperm DNA damage. Other studies by this group have observed alterations in Sertoli cell and seminiferous tubule development in 8-week-old peripubertal male mice, further emphasizing the possible negative effects of sulfur dioxide inhalation [47]. The current study fed DDGS at a high concentration of the diet to evaluate the impacts of sulfur on sperm DNA in yearling beef bulls. We previously reported that the DDGS and SULF diets did result in increased concentrations of ruminal hydrogen sulfide compared with CON [6], which likely caused increased sulfur exposure to bulls via eructation and subsequent inhalation [48]. Although sperm DNA damage was observed in mice as sulfur inhalation increased [47], in the current study, there were no differences observed for DNA integrity. Feeding DDGS at 60% of the diet did not negatively influence sperm DNA integrity.
4.3. Zinc Signatures in Sperm
A study by Kerns et al. [17] evaluated zinc signatures in sperm within different fractions of boar ejaculates. The four zinc signatures corresponded with major events that occur in sperm during fertilization (i.e., zinc signature 1: non-capacitated; zinc signature 2: started the process of capacitation, may be hyperactivated; zinc signature 3: acrosomal remodeling; zinc signature 4: acrosome exocytosis) [16]. In the pre-sperm-rich fraction of boar semen (initial clear portion), the majority of sperm were identified as having zinc signature 1, but the remaining portion of the ejaculate contained primarily sperm with zinc signature 2 [16]. Furthermore, sperm with zinc signatures 1 and 2 bound to glycans that mimicked those in the sperm oviductal reservoir; however, sperm with signatures 3 and 4 did not bind to glycans, thus concluding that sperm with signatures 1 and 2 will be more likely to bind to the sperm oviductal reservoir compared to those with signatures 3 and 4 [17]. The binding of sperm to the oviductal reservoir is crucial to maintain fertilization and to regulate the capacitation and hyperactivation of sperm [49]. No differences were observed for zinc concentrations in the seminal plasma of ejaculates from the current study [7]. Decreased sperm with signature 1 from bulls fed the 60DDGS and SULF diets may indicate that sperm from bulls in the 60DDGS and SULF treatments may be in advanced stages of capacitation in comparison to CON, potentially limiting the sperms’ lifespan and ability to bind to the sperm oviductal reservoir and potentially negatively influencing fertilization [17]. More research is necessary to evaluate whether sperm from bulls fed diets containing 60% DDGS impacts sperm capacitation, fertilization, early embryogenesis, and ultimately, pregnancy rates.
5. Conclusions
In the current study, no differences were observed for post-thaw viability, acrosome membrane integrity, oxidation status, and DNA integrity in sperm from bulls receiving diets containing 60% DDGS (0.55% sulfur DM basis) or CaSO4 (0.54% sulfur DM basis) compared to bulls fed the CON diet. A tendency was observed for mitochondrial energy potential where more polarized sperm were observed in bulls fed the SULF diet compared with those fed CON and 60DDGS. In addition, differences were observed for zinc signature 1 where 60DDGS and SULF had reduced zinc signature 1 compared to CON, but most of the live sperm were identified as having zinc signature 3, indicating that sperm may be in advanced stages of sperm capacitation. Furthermore, PRM1 was the only gene identified to be upregulated in sperm by 60DDGS compared to CON. These findings suggest that the elevated percentage of sulfur from DDGS may not influence reproductive performance in yearling Angus bulls. However, more research is necessary to evaluate the effect of sperm in advanced stages of capacitation on field fertility.
Author Contributions
C.J.K.: investigation, formal analysis, data curation, visualization, writing—original draft, writing—review and editing. T.W.G.: investigation, writing—review and editing. A.L.Z.: investigation, data curation, writing—review and editing. J.S.C.: conceptualization, methodology, validation, writing—review and editing, funding acquisition. J.D.K.: investigation. S.T.D.: investigation, resources, data curation, writing—review and editing. W.J.S.D.: data curation, writing—review and editing. K.L.M.: investigation, writing—review and editing. M.S.C.: resources, validation, writing—review and editing. K.K.S.: resources, writing—review and editing. B.W.N.: investigation, resources, data curation, writing—review and editing. C.R.D.: conceptualization, methodology, investigation, validation, writing—review and editing, visualization, supervision, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the North Dakota Corn Council.
Institutional Review Board Statement
All procedures were approved by the North Dakota State University Institution for Animal Care and Use Committee (#A19035).
Data Availability Statement
Data are available upon request. All sequencing data are publicly available on the Gene Expression Omnibus (GSE242434).
Acknowledgments
The authors would like to thank the North Dakota Corn Council for their partial funding of this project and the NDSU Central Grasslands Research Extension Center for providing the bulls for this experiment. This work used resources of the Center for Computationally Assisted Science and Technology (CCAST) at North Dakota State University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bollwein, H.; Janett, F.; Kaske, M. Impact of nutritional programming on the growth, health, and sexual development of bull calves. Domest. Anim. Endocrinol. 2016, 56, S180–S190. [Google Scholar] [CrossRef]
- Kenny, D.A.; Byrne, C.J. Review: The effect of nutrition on timing of pubertal onset and subsequent fertility in the bull. Animal 2018, 12, s36–s44. [Google Scholar] [CrossRef] [PubMed]
- Barth, A.D.; Brito, L.F.; Kastelic, J.P. The effect of nutrition on sexual development of bulls. Theriogenology 2008, 70, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.F.; Barth, A.D.; Wilde, R.E.; Kastelic, J.P. Effect of growth rate from 6 to 16 months of age on sexual development and reproductive function in beef bulls. Theriogenology 2012, 77, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Dance, A.; Thundathil, J.; Wilde, R.; Blondin, P.; Kastelic, J. Enhanced early-life nutrition promotes hormone production and reproductive development in Holstein bulls. J. Dairy Sci. 2015, 98, 987–998. [Google Scholar] [CrossRef]
- Kassetas, C.J.; Caton, J.S.; Kirsch, J.D.; Dorsam, S.T.; McCarthy, K.L.; Crouse, M.S.; Sedivec, K.K.; Neville, B.W.; Dahlen, C.R. Effects of feeding 60% dried corn distillers grains plus solubles or the equivalent sulfur as CaSO4 on performance and reproductive traits of yearling Angus bulls. Theriogenology 2021, 162, 6–14. [Google Scholar] [CrossRef]
- Kassetas, C.J.; Caton, J.S.; Kirsch, J.D.; Dorsam, S.T.; McCarthy, K.L.; Crouse, M.S.; Sedivec, K.K.; Neville, B.W.; Dahlen, C.R. Effects of feeding bulls dried corn distiller’s grains plus solubles or CaSO4 on mineral and metabolite concentrations in serum and seminal plasma. Anim. Reprod. Sci. 2021, 226, 106703. [Google Scholar] [CrossRef]
- Bansal, A.K.; Bilaspuri, G.S. Impacts of oxidative stress and antioxidants on semen functions. Vet. Med. Int. 2010, 2010, 686137. [Google Scholar] [CrossRef]
- Crane, A.R.; Redden, R.R.; Crouse, M.S.; Kirsch, J.D.; Borowicz, P.P.; Held, J.E.; Swanson, K.C.; Schauer, C.S. Influence of distiller’s dried grains with solubles on ram lamb growth and reproductive traits. J. Anim. Sci. 2018, 96, 1484–1494. [Google Scholar] [CrossRef]
- Van Emon, M.L.; Vonnahme, K.A.; Berg, P.T.; Redden, R.R.; Thompson, M.M.; Kirsch, J.D.; Schauer, C.S. Influence of level of dried distillers grains with solubles on feedlot performance, carcass characteristics, serum testosterone concentrations, and spermatozoa motility and concentration of growing rams. J. Anim. Sci. 2013, 91, 5821–5828. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Qie, M.; Zheng, R.; Shetty, J.; Wang, J. Sodium fluoride and sulfur dioxide affected male reproduction by disturbing blood-testis barrier in mice. Food Chem. Toxicol. 2016, 94, 103–111. [Google Scholar] [CrossRef]
- Fortes, M.R.S.; Satake, N.; Corbet, D.H.; Corbet, N.J.; Burns, B.M.; Moore, S.S.; Boe-Hansen, G.B. Sperm protamine deficiency correlates with sperm DNA damage in Bos indicus bulls. Andrology 2014, 2, 370–378. [Google Scholar] [CrossRef]
- Evenson, D.P. The Sperm Chromatin Structure Assay (SCSA(®)) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim. Reprod. Sci. 2016, 169, 56–75. [Google Scholar] [CrossRef]
- Johnson, C.; Dance, A.; Kovalchuk, I.; Kastelic, J.; Thundathil, J. Enhanced early-life nutrition upregulates cholesterol biosynthetic gene expression and Sertoli cell maturation in testes of pre-pubertal Holstein bulls. Sci. Rep. 2019, 9, 6448. [Google Scholar] [CrossRef]
- Geary, T.W.; Waterman, R.C.; Van Emon, M.L.; Ratzburg, C.R.; Lake, S.; Eik, B.A.; Armstrong, D.R.; Zezeski, A.L.; Heldt, J.S. Effect of supplemental trace minerals on novel measures of bull fertility1. Transl. Anim. Sci. 2019, 3, 1813–1817. [Google Scholar] [CrossRef] [PubMed]
- Kerns, K.; Zigo, M.; Sutovsky, P. Zinc: A Necessary Ion for Mammalian Sperm Fertilization Competency. Int. J. Mol. Sci. 2018, 19, 4097. [Google Scholar] [CrossRef] [PubMed]
- Kerns, K.; Zigo, M.; Drobnis, E.Z.; Sutovsky, M.; Sutovsky, P. Zinc ion flux during mammalian sperm capacitation. Nat. Commun. 2018, 9, 2061. [Google Scholar] [CrossRef] [PubMed]
- Parthipan, S.; Selvaraju, S.; Somashekar, L.; Arangasamy, A.; Sivaram, M.; Ravindra, J.P. Spermatozoal transcripts expression levels are predictive of semen quality and conception rate in bulls (Bos taurus). Theriogenology 2017, 98, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, S.; Swathi, D.; Ramya, L.; Lavanya, M.; Archana, S.S.; Sivaram, M. Orchestrating the expression levels of sperm mRNAs reveals CCDC174 as an important determinant of semen quality and bull fertility. Syst. Biol. Reprod. Med. 2021, 67, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Samardzija, M.; Karadjole, M.; Matkovic, M.; Cergolj, M.; Getz, I.; Dobranic, T.; Tomaskovic, A.; Petric, J.; Surina, J.; Grizelj, J.; et al. A comparison of BoviPure and Percoll on bull sperm separation protocols for IVF. Anim. Reprod. Sci. 2006, 91, 237–247. [Google Scholar] [CrossRef]
- Arias, M.E.; Andara, K.; Briones, E.; Felmer, R. Bovine sperm separation by Swim-up and density gradients (Percoll and BoviPure): Effect on sperm quality, function and gene expression. Reprod. Biol. 2017, 17, 126–132. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Evenson, D.P.; Larson, K.L.; Jost, L.K. Sperm Chromatin Structure Assay: Its Clinical Use for Detecting Sperm DNA Fragmentation in Male Infertility and Comparisons With Other Techniques. J. Androl. 2002, 23, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.L.; Johnson, L.A. Viability assessment of mammalian sperm using SYBR-14 and propidium iodide. Biol. Reprod. 1995, 53, 276–284. [Google Scholar] [CrossRef]
- Tao, J.; Critser, E.S.; Critser, J.K. Evaluation of mouse sperm acrosomal status and viability by flow cytometry. Mol. Reprod. Dev. 1993, 36, 183–194. [Google Scholar] [CrossRef]
- Zoca, S.M.; Geary, T.W.; Zezeski, A.L.; Kerns, K.C.; Dalton, J.C.; Harstine, B.R.; Utt, M.D.; Cushman, R.A.; Walker, J.A.; Perry, G.A. Bull field fertility differences can be estimated with in vitro sperm capacitation and flow cytometry. Front. Anim. Sci. 2023, 4, 1180975. [Google Scholar] [CrossRef]
- Soubry, A.; Hoyo, C.; Jirtle, R.L.; Murphy, S.K. A paternal environmental legacy: Evidence for epigenetic inheritance through the male germ line. Bioessays 2014, 36, 359–371. [Google Scholar] [CrossRef]
- Stuppia, L.; Franzago, M.; Ballerini, P.; Gatta, V.; Antonucci, I. Epigenetics and male reproduction: The consequences of paternal lifestyle on fertility, embryo development, and children lifetime health. Clin. Epigenetics 2015, 7, 120. [Google Scholar] [CrossRef]
- Jodar, M. Sperm and seminal plasma RNAs: What roles do they play beyond fertilization? Reproduction 2019, 158, R113–R123. [Google Scholar] [CrossRef]
- Raval, N.P.; Shah, T.M.; George, L.B.; Joshi, C.G. Insight into bovine (Bos indicus) spermatozoal whole transcriptome profile. Theriogenology 2019, 129, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, N.; Walter, P. The Signal Recognition Particle (SRP) RNA Links Conformational Changes in the SRP to Protein Targeting. Mol. Biol. Cell 2007, 18, 2728–2734. [Google Scholar] [CrossRef]
- Sellem, E.; Jammes, H.; Schibler, L. Sperm-borne sncRNAs: Potential biomarkers for semen fertility? Reprod. Fertil. Dev. 2022, 34, 160–173. [Google Scholar] [CrossRef]
- Dogan, S.; Vargovic, P.; Oliveira, R.; Belser, L.E.; Kaya, A.; Moura, A.; Sutovsky, P.; Parrish, J.; Topper, E.; Memili, E. Sperm protamine-status correlates to the fertility of breeding bulls. Biol. Reprod. 2015, 92, 92. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, N.; Lévesque-Sergerie, J.P.; Thibault, C.; Boissonneault, G. Spermatozoal transcriptome profiling for bull sperm motility: A potential tool to evaluate semen quality. Reproduction 2009, 138, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Moura, F.H.; Macias-Franco, A.; Pena-Bello, C.A.; Archilia, E.C.; Batalha, I.M.; Silva, A.E.; Moreira, G.M.; Norris, A.B.; Schütz, L.F.; Fonseca, M.A. Sperm DNA 5-methyl cytosine and RNA N 6-methyladenosine methylation are differently affected during periods of body weight losses and body weight gain of young and mature breeding bulls. J. Anim. Sci. 2022, 100, skab362. [Google Scholar] [CrossRef]
- Sharma, U. Paternal Contributions to Offspring Health: Role of Sperm Small RNAs in Intergenerational Transmission of Epigenetic Information. Front. Cell Dev. Biol. 2019, 7, 215. [Google Scholar] [CrossRef]
- Alves, M.B.R.; Arruda, R.P.; Batissaco, L.; Garcia-Oliveros, L.N.; Gonzaga, V.H.G.; Nogueira, V.J.M.; Almeida, F.D.S.; Pinto, S.C.C.; Andrade, G.M.; Perecin, F.; et al. Changes in miRNA levels of sperm and small extracellular vesicles of seminal plasma are associated with transient scrotal heat stress in bulls. Theriogenology 2021, 161, 26–40. [Google Scholar] [CrossRef]
- Mao, S.; Sendler, E.; Goodrich, R.J.; Hauser, R.; Krawetz, S.A. A comparison of sperm RNA-seq methods. Syst. Biol. Reprod. Med. 2014, 60, 308–315. [Google Scholar] [CrossRef][Green Version]
- Sellem, E.; Marthey, S.; Rau, A.; Jouneau, L.; Bonnet, A.; Perrier, J.P.; Fritz, S.; Le Danvic, C.; Boussaha, M.; Kiefer, H.; et al. A comprehensive overview of bull sperm-borne small non-coding RNAs and their diversity across breeds. Epigenetics Chromatin 2020, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Sellem, E.; Marthey, S.; Rau, A.; Jouneau, L.; Bonnet, A.; Le Danvic, C.; Guyonnet, B.; Kiefer, H.; Jammes, H.; Schibler, L. Dynamics of cattle sperm sncRNAs during maturation, from testis to ejaculated sperm. Epigenetics Chromatin 2021, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.A.; Paasch, U.; Villegas, J.V. Mitochondrial membrane potential disruption pattern in human sperm. Hum. Reprod. 2009, 24, 2079–2085. [Google Scholar] [CrossRef]
- Starkov, A.A. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. N. Y. Acad. Sci. 2008, 1147, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.L.; Thomas, C.A.; Joerg, H.W.; DeJarnette, J.M.; Marshall, C.E. Fluorometric assessments of mitochondrial function and viability in cryopreserved bovine spermatozoa. Biol. Reprod. 1997, 57, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Qin, G.; Zhang, B. DNA damage in mice treated with sulfur dioxide by inhalation. Environ. Mol. Mutagen. 2005, 46, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Liu, Y. Cell Morphological Ultrastructural Changes in Various Organs from Mice Exposed by Inhalation to Sulfur Dioxide. Inhal. Toxicol. 2007, 19, 543–551. [Google Scholar] [CrossRef]
- Drewnoski, M.E.; Pogge, D.J.; Hansen, S.L. High-sulfur in beef cattle diets: A review. J. Anim. Sci. 2014, 92, 3763–3780. [Google Scholar] [CrossRef]
- Suarez, S.S. The oviductal sperm reservoir in mammals: Mechanisms of formation. Biol. Reprod. 1998, 58, 1105–1107. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).