Abstract
Plastic pollution is a major environmental concern. In humans, ingestion through contaminated seafood is a recognized exposure route to microplastics, which may impact gut health. However, the extent to which microplastics interfere with digestion and nutrient absorption remains unclear. To this end, the present work aimed to assess, for the first time, the influence of microplastic particles (polyethylene terephthalate, PET, and polylactic acid, PLA) on the digestibility of three selected seafood species (gilthead seabream, Sparus aurata; Atlantic salmon, Salmo salar; and hard clam, Mercenaria mercenaria) using an in vitro human digestion model. Furthermore, this study evaluated the potential degradability of microplastics along the gastrointestinal tract and examined how particle type and exposure level (10 or 20 particles) may influence seafood digestibility. Protein digestibility in S. aurata and S. salar filets was ~86%, while in M. mercenaria it was ~73%, regardless of microplastic presence or quantity. PET and PLA integrity was affected differently by digestion, with PLA showing greater surface degradation. These findings provide preliminary insight into the mutual interactions between microplastics and the human digestive process, highlighting the importance for further research into how the leaching of plastics additives may or may not influence the bioaccessibility of essential nutrients.
1. Introduction
Currently, the world produces approximately more than 450 million tons of plastic waste annually, of which 0.5% ends up in the oceans [1]. Recent projections indicate that, without efficient management and regulatory strategies, the amount of plastic waste could triple by 2060, raising significant ecological and public health concerns [2]. Although plastics are highly resistant to degradation in the marine environment, they can undergo significant structural and chemical changes, often breaking down into smaller particles of different sizes (i.e., microplastics, ≥1 μm–5 mm) and shapes [3,4]. These degradation processes are promoted by a combination of various factors, such as photodegradation (due to the ultraviolet (UV) radiation) and oxygen (O2) exposure [3,5]. This environmental problem, raised by the intensive production, combined with inadequate waste management, weak circular economy, a limited recycling system and improper disposal of commercial plastics, has directed the attention of the scientific and industrial communities towards the development of new and more sustainable alternative materials made from natural ingredients, i.e., the so-called biopolymers or bioplastics [6]. Biopolymers are becoming a suitable and explored alternative to commonly used polymers because they minimize the carbon dioxide (CO2) footprint, municipal solid waste and reliance on petroleum-based resources [7]. The empirical-based knowledge is still very limited regarding the toxicological attributes/effects of bioplastics on humans who unintentionally come into contact with them [8,9]. Recent studies have raised concerns suggesting that bioplastics (e.g., polylactic acid, PLA) may not be as harmless as previously thought, because their enhanced biodegradability can facilitate fragmentation into very small particles and/or leaching out polymer additives. This subsequently increases the chances of tissue damage and systemic absorption of compounds [10]. This general idea that “bioderived and more biodegradable is not necessarily less hazardous” highlights the need for more comprehensive studies and risk assessments on the toxicity of bioplastics to both humans and biota.
Seafood consumption (including fish, crustaceans and mollusks) has increased worldwide over the last 50 years [11], and it plays an essential role in human diet, as it is the primary source of animal protein in many regions of the world [12]. In general, seafood species are low in cholesterol and high in essential minerals, including proteins of high biological value, vitamins, and health-promoting oils (e.g., omega-3 fatty acids), whose benefits have been shown for consumer’ health [13]. Despite all these nutritional benefits, seafood consumption can also pose risks, as it constitutes a route of exposure to environmental contaminants, namely microplastics [3,14,15], for which the toxicological outcomes are not yet fully understood. Chronic discomfort, swelling, inflammation, alterations in digestive and antioxidant enzymes, and tissue blockage are examples of the potential effects that microplastics can trigger in both humans and animals [15]. The lack of regulation regarding the presence of microplastics in food commodities, as well as the absence of tolerable intake recommendations has led to a growing number of recent studies devoted to estimating human dietary exposure to these emerging contaminants and the inherent toxicological responses that it can prompt [15,16,17,18]. Yet, the influence that microplastics’ unintentional ingestion can have on food digestibility and nutrient uptake is a research topic that has been strikingly underexplored.
When an organism ingests food, it is assumed that all of it will be assimilated and absorbed. However, this assumption is not entirely accurate, since only a fraction of the ingested material becomes bioaccessible upon digestion [19,20]. Bioaccessibility, i.e., the amount of a given nutrient/contaminant that becomes available for systemic absorption and used by the organisms, after gastrointestinal digestion, can be seen as an indicator of the maximum oral bioavailability of any food constituent [19]. Hence, in vitro bioaccessibility assays are crucial to accurately determine the nutritional value of foods, estimate nutrient intake, and, on that basis, design appropriate nutrition strategies and public health recommendations. Such assays are equally important from a toxicological standpoint, as the data they generate enable realistic estimations of contaminant exposure and risk assessment, being fundamental in modern environmental and food toxicological studies.
To this end, this study aimed to evaluate, for the first time, the influence of one petroleum-based (polyethylene terephthalate, PET) and one bio-derived (polylactic acid, PLA) microplastic on the protein bioaccessibility of selected seafood species (gilthead seabream Sparus aurata, Atlantic salmon Salmo salar, and hard clam Mercenaria mercenaria) using an in vitro human digestion model. Furthermore, the study assessed the potential degradability of microplastics along the gastrointestinal tract and examined how particle type and exposure level (10 or 20 particles of PET and PLA) may affect the digestibility of the seafood matrix.
2. Materials and Methods
2.1. Preparation of Microplastics Particles
Commercially available polyethylene terephthalate (PET) and polylactic acid (PLA) pellets (~5 mm) were provided by LOGOPLASTE company (Portugal). Pellets were broken down into smaller-sized particles, to obtain sizes ranging between 700 μm and 1000 μm (PET—874.4 ± 101.3 μm; PLA—896.6 ± 75.2 μm), using a cryogenic mill (Spex Sampleprep, 6770 Freezer/Mill, New Jersey, NJ, USA) with nitrogen.
Briefly, after the particles were broken down, sieves with various mesh sizes (1000 μm, 500 μm, 250 μm and 125 μm) were assembled. Then, the particles were placed on the top sieve (1000 μm) and sieved while washed with Milli-Q water so that they could move to the next sieve, if they did not match the size range of that specific sieve. After sieving, the particles were washed into a beaker for subsequent vacuum filtration through a 20 μm filter, to remove as much water as possible. Finally, the samples were freeze-dried (−81 °C, 3 × 10−3; LABCONCO, Kansas City, KS, USA) to remove all remaining water from samples, and then transferred to small Petri dishes.
2.2. In Vitro Human Digestion Model
2.2.1. Sample Preparation
Fish (S. aurata and S. salar) and clam (M. mercenaria) specimens were acquired at a local market in Setúbal, Portugal. Samples were trimmed to remove the edible fraction to be homogenized using a sample homogenizer (Restch, Knife Mill Grindomix GM200, Haan-Gruiten, Germany). Afterwards, for each seafood sample, 1.5 g sample aliquots (3 replicate samples, per species, polymer and number of particles) were weighed in NalgeneTM high-speed PPCO centrifuge tubes (Thermo ScientificTM, 3119-0050, New York, NY, USA) and minced with 10 or 20 PET or PLA particles. This number of microplastic particles added was selected based on previous studies reporting human exposure through seafood consumption. Estimates indicate that European consumers may ingest roughly 30 particles per day from shellfish [21], while edible fish tissues may vary from an average of 9 to 70 particles per fish [22,23]. Considering these values, the use of 10 and 20 particles was chosen to reproduce low-exposure (6.67 particles g−1 of sample) and high-exposure (13.3 particles g−1 of sample) scenarios under controlled in vitro digestion conditions.
2.2.2. In Vitro Digestion Protocol
The standardized static in vitro human gastrointestinal model described by Minekus et al. [24] was employed to simulate the digestion of fish filets and clam’s edible part. This well-established method involves the digestion of ~1.5 g of food sample in steps representing the oral, gastric and small intestine. During these steps, different digestive juices (with distinct pH, chemical and enzyme compositions; see Supplementary Table S1) are sequentially added. Briefly, the digestion process involved three stages: (a) the oral phase, where 4 mL of saliva fluid was added to the fish and clam samples, which were then incubated at 37 °C for 5 min at pH 7.0 ± 0.2; (b) gastric phase, where 8 mL of gastric fluid was added and incubated at 37 °C for 2 h at pH 2.0 ± 0.2; and (c) intestinal phase, where 8 mL of duodenal fluid and 4 mL of bile fluid were introduced and incubated at 37 °C for 2 h at pH 7.0 ± 0.2 (all digestive fluids were prepared before the digestion protocol to prevent enzyme inhibition, and the pH was adjusted immediately before each digestion step using HCl (1M) or NaOH (1M)). During the incubation period, samples were placed in the oven (Thermo ScientificTM, Thermo Heratherm IMH60, Waltham, MA, USA) in a rotation disk (RS Lab, RS Lab-9, Madrid, Spain) set at 100 rpm to simulate peristaltic movements during digestion. The following reagents and commercial enzymes were used to prepare the digestive fluids mentioned above: KCl (Merck Millipore, Burlington, MA, USA), KSCN (Sigma-Aldrich, St. Louis, MO, USA), NaH2PO4 (Panreac AppliChem, Darmstadt, Germany), Na2SO4 (Merck Millipore, Burlington, MA, USA), NaCl (Biochem Chemopharma, Cosne-Cours-sur-Loire, France), NaHCO3 (Merck Millipore, Burlington, MA, USA), CaCl2.2H2O (Merck Millipore, Burlington, MA, USA), NH4Cl (CHEM-LAB, Zedelgem, Belgium), KH2PO4 (Aros Organics, Darmstadt, Germany), MgCl2 (Acros Organics, Geel, Belgium), Urea (Sigma-Aldrich, St. Louis, MO, USA), Glucuronic acid (Sigma-Aldrich, St. Louis, MO, USA), Glucose (Sigma-Aldrich, St. Louis, MO, USA), Glucoseamine hydrochloride (Sigma-Aldrich, St. Louis, MO, USA), α-amylase (Sigma-Aldrich, St. Louis, MO, USA), Uric acid (Sigma-Aldrich, St. Louis, MO, USA), Mucin (Carl ROTH, Karlsruhe, Germany), Bovine serum albumin (Sigma-Aldrich St. Louis, MO, USA), Pepsin (Sigma-Aldrich, St. Louis, MO, USA), Pancreatin (Sigma-Aldrich, St. Louis, MO, USA), Lipase (Sigma-Aldrich, St. Louis, MO, USA), Trypsin (Sigma-Aldrich, St. Louis, MO, USA), α-chymotrypsin (Sigma-Aldrich, St. Louis, MO, USA), and Bile bovine (Sigma-Aldrich, St. Louis, MO, USA).
Once the digestion process was concluded, sample tubes were placed on ice to stop digestion reactions and centrifuged at 2750× g (Kubota 6800, Osaka, Japan) at 10 °C for 10 min, to allow separation of the bioaccessible (i.e., supernatant, BIO) and the non-bioaccessible fractions (i.e., pellet; NBIO). Control reaction tubes, containing only digestive fluids, were also included in the assays. The microplastics were recovered from the NBIO fraction by washing with Milli-Q water and filtration through a 125 µm sieve.
2.3. Protein Bioaccessibility
To assess potential effects of microplastics digestion on seafood digestibility, protein contents were determined in all samples before (fish filets and clam’s edible part) and after (bioaccessible fraction) digestion through the Dumas method in a Dumatherm N Pro automatic nitrogen analyzer (Gerhardt, 14-0400, Königswinter, Germany). In brief, 100 mg from the bioaccessible fraction (liquid) and 50 mg of super-absorber (DumaSorb, Gerhardt GHR14-0022, Königswinter, Germany) were weighed into tin foils and placed on the equipment rack for protein measurements. Sample analysis was conducted under the following conditions: combustion furnace at 1100 °C, reduction furnace at 450 °C, and degassing furnace at 300 °C. The equipment was calibrated using 25 calibration points of EDTA (Duma-EDTA, Gerhardt, GHR14-0032, Königswinter, Germany). Before each analysis, six bypasses (blanks) and six standards (EDTA) were measured to ensure the quality of the equipment’s work.
2.4. Microplastic Particle Characterization
PET and PLA particles were characterized (before and after the in vitro human digestion) through three distinct techniques: (1) binocular stereomicroscopy; (2) Fourier transform infrared spectroscopy (FTIR); and (3) Scanning Electron Microscopy (SEM). Particles were first analyzed using a binocular stereomicroscope (Leica S9i, Wetzlar, Germany) at 10× magnification to determine their general color, size, and shape. Then, they were examined using Fourier transform infrared spectroscopy (FTIR Spectrum TwoTM ATR Universal spectrometer, Perkin Elmer, Shelton, CT, USA), in attenuated total reflectance (ATR), to collect spectra in the transmission mode in the region of 500 cm−1 and 4000 cm−1, so that it would be possible to characterize their infrared spectrum. The resolution was fixed at 4 cm−1 with a minimum of 40 scans. Plastic samples were pressed against the diamond ATR crystal with a force of 90–120 N. Spectra were analyzed using a PerkinElmer Spectrum IR software (version 10.7.2) and compared with reference spectra from different databases from PerkinElmer. A level of acceptance of the polymer spectra was established using a threshold for identifying spectra of 90% similarity when comparing the particle spectrum with the reference spectra from the database. Finally, PET and PLA particles were analyzed through benchtop SEM (Hitachi, TM4000 PLUS, Tokyo, Japan) to identify structural changes (e.g., pore formation) promoted by the digestive process. The images were taken at 10 kV beam voltage using a backscattered electron detector (BSE) at a magnification of 400×.
2.5. Statistical Analysis
Significant differences between treatments and matrices in total bioaccessible protein were assessed using a one-way ANOVA analysis. Square root and Log were performed, whenever necessary, to comply with the assumption of homogeneity of variances (Levene’s test) and normality (Kolmogorov–Smirnov’s test) required for the analysis. Afterwards, a post hoc Tukey HSD test was carried out to identify significant differences between treatments. All statistical analyses considered a significance level of 0.05 using STATISTICATM software (version 7.0, StatSoft Inc., Tulsa, OK, USA).
3. Results
3.1. Characterization of Pristine PET and PLA Particles
The appearance, morphology (Figure 1), chemical composition (Figure 2), and surface structure (Figure 3) of PET and PLA microplastics were characterized (three particles per replicate, three replicates per treatment) before the in vitro simulation of the digestive process. Overall, observations through the binocular stereomicroscope showed that PET and PLA particles had different shapes and edges, although both had a whitish color (Figure 1). Unlike PET, PLA particles presented a slight transparency (Figure 1). The FTIR spectra of the PET and PLA particles show the typical vibration bands of these polymers. Both the PLA and PET carbonyl stretching bands (C=O) can be observed at ca. 1750 cm−1 and 1714 cm−1, respectively, while the CH3, CH2, and CH stretching vibrations are confirmed between 3000 and 2850 cm−1. For PET, the C=O bending can be identified at 1245 cm−1, the O–C stretching at 1118 and 1098 cm–1 and the vibration modes associated with the aromatic ring at 1578, 1505, 1014, 874 and723 cm–1 [25,26]. For the PLA, the O–C=O stretching can be detected at ca. 190–1080 cm−1, the CH3 asymmetric stretching at 1355–1380 cm−1, the CH3 bending at 1453 cm−1 and the O–C=O stretching at ca. 1190–1080 cm−1 [25,26]. As for SEM observation (Figure 3), pristine particles’ surface evidenced fractures with multiple irregularities, river marks, folds and tears. These fractures were generated during pellet cryomilling with liquid nitrogen, necessary to obtain particles with the desired size.
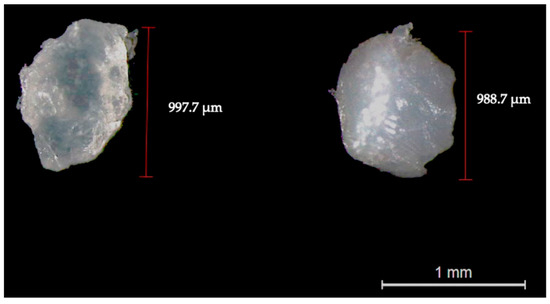
Figure 1.
Representative image of pristine PLA (left side) and PET (right side) particles observed through binocular stereomicroscopy (magnification: 10×).
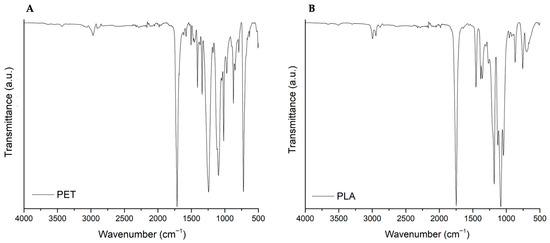
Figure 2.
Representative spectra of pristine PET (A) and PLA (B) particles. The y-axis corresponds to the percentage transmittance (a.u.) and the x-axis to the wave numbers (cm−1).
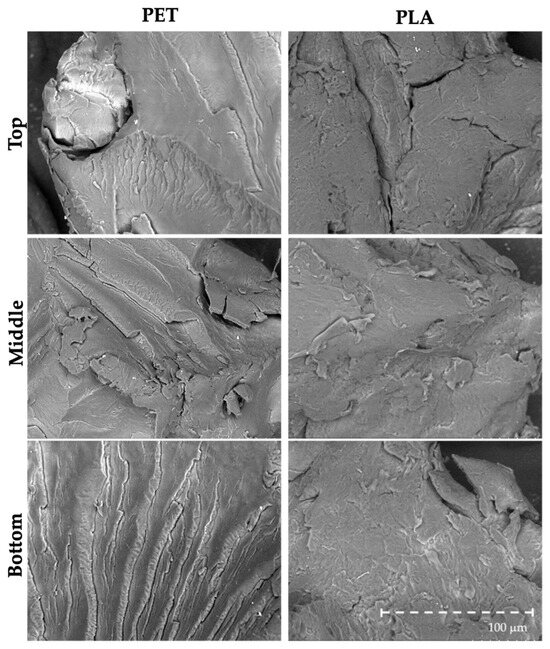
Figure 3.
Representative images of pristine PET and PLA particles observed through SEM (magnification: 400×).
3.2. Effects of the Human Digestive Process on the Morphology and Integrity of PET and PLA Particles
Observation through the binocular stereomicroscope revealed the formation of a yellowish biofilm, consisting of organic layer/matter adhered to the microplastic surface of both PET and PLA particles after the digestive process (Figure 4). Despite the substantial differences between the three seafood species in terms of the proximate chemical composition and pigments that constitute them, the color and extent to which the biofilm was formed on the particles’ surface did not seem to be influenced by the type of digested seafood species.
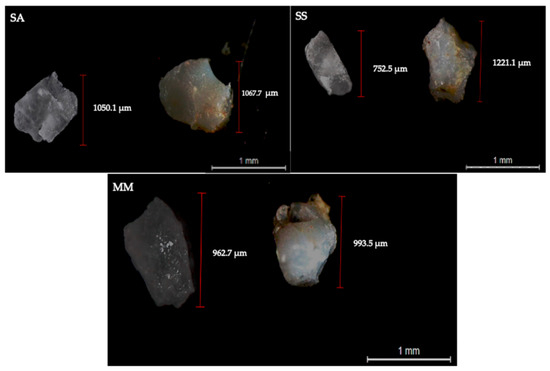
Figure 4.
Representative images of PLA (left side) and PET (right side) particles recovered from the non-bioaccessible fraction upon seafood digestion (SA—S. aurata; SS—S. salar; MM—M. mercenaria), acquired through binocular stereomicroscope (magnification: 10×).
Representative images of the spectra of PET and PLA particles upon the digestion of S. aurata samples are presented in Figure 5A and Figure 5B, respectively. Spectra of PLA and PET particles upon digestion of S. salar and M. mercenaria samples can be found in the Appendix A, Figure A1 and Figure A2. Spectra observations of PET revealed the appearance of a peak at 1640 cm−1, which, in combination with a more intense band between 3700 and 3020 cm−1, confirms the presence of water in particles after digestion. The appearance of two peaks at 2846 and 2925 cm−1, related to C-H stretching, can also be associated with the presence of amino acids. When observing the peak of the C=O stretching vibration, at 1750 cm−1 for PLA and at 1714 cm−1 for PET, a decrease in its intensity is detected for all the samples submitted to digestion, more typically for the particles of the study involving 10 particles.
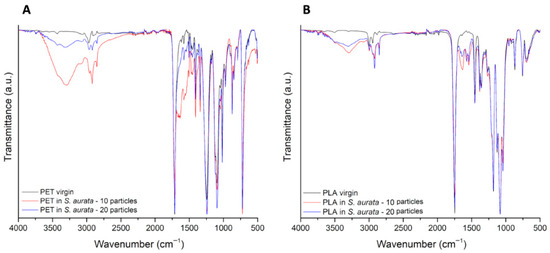
Figure 5.
FTIR-spectra comparing representative spectra from pristine (black line) and digested PET (A) and PLA (B) particles (10 particles: red line; 20 particles: blue line), with S. aurata filet samples. The y-axis corresponds to the percentage transmittance (a.u.) and the x-axis to the wave numbers (cm−1).
Overall, the number and type/extent of morphological irregularities (e.g., river marks, folds and tears) in PET particles were similar in pristine particles and those that suffered digestion with seafood (Figure 6), meaning that the human digestive process did not have a preponderant impact on particles’ surface and integrity. Regarding PLA, the in vitro digestive process induced evident changes in particle morphology, by forming and/or enhancing and accentuating existing pits and pores (Figure 6). Moreover, PET and PLA particles showed the formation of small biofilms on their surfaces (Figure S1).
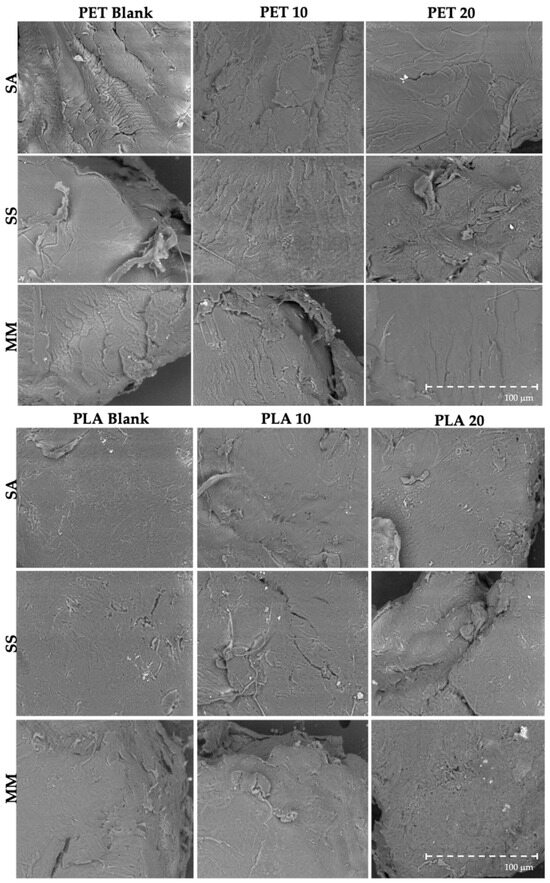
Figure 6.
Representative SEM images (magnification: 400×) of PET and PLA particles exposed to the in vitro human digestive process. PET Blank are 10 particles only with digestive juices; PET 10 and PET 20 are samples with 6.67 particles of PET g−1 of sample and 13.3 particles of PET g−1 of sample, respectively. PLA Blank are 10 particles only with digestive juices; PLA 10 and PLA 20 are samples with 6.67 particles of PLA g−1 of sample and 13.3 particles of PLA g−1 of sample, respectively. (SA—S. aurata; SS—S. salar; MM—M. mercenaria).
3.3. Effects of PET and PLA Particles on Seafood Protein Digestibility
Protein digestibility (i.e., the percentage of bioaccessible protein upon seafood in vitro digestion) for S. aurata, S. salar and M. mercenaria filets is shown in Figure 7A–C. S. aurata protein bioaccessibility varied between 80% (PET 20) and 95% (PLA 20), while S. salar varied between 81% (PLA 10) and 93% (PLA 10). As for M. mercenaria, protein bioaccessibility varied between 69% (CTR) and 79% (PLA 10). Significant differences in protein digestibility were not observed between non-contaminated seafood samples and those contaminated with PET and PLA (p > 0.05; Figure 7A–C), indicating that microplastics do not influence seafood protein digestibility, regardless of polymer type. In addition, the number of particles did not have a significant effect either, in all three studied matrices (p > 0.05; Figure 7A–C).
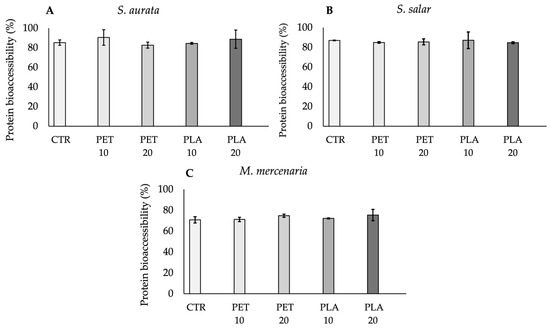
Figure 7.
Protein bioaccessibility percentage (%) (mean ± standard deviation), after the in vitro digestion, for S. aurata (A), S. salar (B) and M. mercenaria (C). Abbreviations: CTR—samples with seafood and digestive juices and no plastic particles; PET 10—seafood samples spiked with 6.67 particles of PET g−1; PET 20—seafood samples piked with 13.3 particles of PET g−1; PLA 10—seafood samples spiked with 6.67 particles of PLA g−1; PLA 20—seafood samples piked with 13.3 particles of PLA g−1.
4. Discussion
The widespread presence of microplastics in seafood has been raising growing concerns about human health. Both conventional plastics and emerging bioplastics can enter the food chain, potentially carrying chemical additives or undergoing degradation during digestion, thus releasing health warning monomers that constitute them. Understanding how these interact with food and the digestive process is crucial to assess their potential risks for human consumption. This study provides not only novel data on how conventional plastics (i.e., PET) and bioplastics (i.e., PLA) influence protein bioaccessibility during simulated digestion but also important insights from a food safety perspective.
4.1. Impact of Simulated Human Digestive Process on the Structural Integrity of PET and PLA Particles
The presence of a more notable biofilm in PET particles than in PLA particles is likely related to the fact that PET’s hydrophobicity is higher, which contributes to its increased ability to adsorb organic matter, facilitating a more extensive biofilm formation [27]. This biofilm can have a significant and potentially hazardous role on the transition of these particles across the intestinal lumen [28,29]. Indeed, recent evidences link the presence of biofilm to an increased intestinal permeability [28]. In turn, this facilitates the uptake of small particles by the intestinal epithelium, their consequent transfer to the bloodstream and, subsequently, to different tissues, where they possibly bioaccumulate and cause a myriad of hazardous effects. Moreover, the biofilm formation in the particles surfaces is evidenced by the presence of two new peaks, at 1574 cm−1 and 1540 cm−1, in PET spectra. These peaks correspond to vibrations typically found in proteins and peptides (N-H bending and C-N stretching of the peptide bond), therefore confirming that organic matter from the digestive process adhered to the particles’ surface [30,31].
Following the in vitro digestion process, PET particles did not show noticeable changes in their surface morphology, whereas PLA particles exhibited clear morphological changes. The lack of changes on the PET particle surface was somewhat expected, given the high level of resistance to degradation exhibited by petroleum-based polymers [32]. For this reason, PET’s mechanical and chemical properties are believed to hinder the action of enzymes and/or strong pH fluctuations that occur during human digestion [33]. On the other hand, the changes seen in PLA are likely related to the fact that it is a biopolymer produced from lactic acid fermentation of natural ingredients (e.g., corn, sugar beets and potato starch), which, in turn, is more easily degraded [34]. Interestingly, while SEM analysis did not reveal obvious surface changes in PET, FTIR analysis indicated a decrease in the intensity of the C=O stretching vibration at 1714 cm−1 in digested PET particles. This suggests that, although PET maintained its surface morphology, subtle chemical modification may have occurred at the molecular level. Such changes in PET and PLA, likely related to hydrolytic processes, were also supported by the appearance of a more intense OH stretching band between 3700 cm−1 and 3020 cm−1 [35,36,37]. Our findings are consistent with those reported by Stock et al. [38] and Tamargo et al. [28] (though different particle sizes were tested in those studies). Both studies suggested that particle size and biofilm formation play a critical role in bioavailability, significantly modulating the intestinal uptake rate of the particles and their subsequent toxicological effects and health risks. PLA results also align with those reported in other studies, despite the particle size difference [39,40]. As acidic pH conditions are known to elicit drastic changes to the surface of polymers [39], one hypothesis would be that the gastric step is the most critically destructive to PLA particles. Yet, a recent study conducted by Peng et al. [40] reported that most changes observed in digested PLA particles only become evident after the small intestine step, i.e., at neutral (7.0) pH conditions. In other words, PLA’s surface degradation, potentially resulting in loss of integrity and leaching of monomers and/or additives to the bioaccessible fraction [41], might have been promoted not only by the action of enzymes, but also by the pH condition to which particles were subjected during both the gastric and small intestine steps. This degradation and potential contaminant leaching can pose serious risks to human health [42]. Monomers and/or plastic chemical additives are responsible for providing specific characteristics improving their properties (e.g., plasticity, malleability, color, processability, among others) [42]. Some studies stated that many of these additives are classified as hazardous according to EU regulations [43], and a myriad of them have been linked to deleterious health effects [42]. Carcinogenic and mutagenic effects, reproductive toxicity, as well as metabolic and neurological disorders, are examples of the consequences arising from plastics additives [43]. In light of this, the surface changes seen in PLA surface may pose risks to the human health, due to the potential toxicity of these monomers and/or additives. Additionally, recent studies have shown that aquatic and terrestrial organisms, including humans, presented more severe toxicological responses when exposed to PLA microplastics, compared to petroleum-based microplastics [44,45,46]. Moreover, it is important to note that the particle size range used in this study (700–1000 μm) is considerably larger than the sizes typically ingested by humans. According to the literature, ingested microplastics are generally reported to be below 100 μm, and in many cases even smaller than 10 μm [47]. Nevertheless, the use of larger sizes in the present study reflects both methodological and technical constraints. Handling, isolating, and characterizing particles <100 μm is highly challenging, due to possible losses during digestion assays, and analytical resolution limits. Considering all the above, it is clear that PLA particles are susceptible to changes during digestion. These results, together with the controversial outcomes of other comparative studies on PET and PLA toxicity [45,48,49], calls for further research to reinforce the current knowledge on bioplastics biodegradability within human and animal gastrointestinal systems, as to better estimate the environmental and human health hazards posed by these “presumably” eco-friendly polymer. Alongside this, the potential of microplastics to adsorb nutrients and their influence on the human gut microbiome represent an emerging area of concern. A recent study by Huang et al. [50] demonstrated that polyethylene microplastics, in combination with chemical additives like tetrabromobisphenol A (TBBPA), can disrupt gut microbial communities, suggesting potential health risks. Moreover, microplastics have the potential to adsorb essential nutrients onto their surfaces, posing a potential risk to human health through dietary exposure [15]. These findings also highlight the need for further research to not only understand the long-term effects of different microplastics and their additives on nutrient digestion and absorption, but also to explore their potential impacts on gut microbiome composition and function.
4.2. Influence of PET and PLA Particles on the Digestibility of Seafood Proteins
In both fish species, the percentage of digested (i.e., bioaccessible) protein was above 80%, which are values in accordance with those reported in previous bioaccessibility studies with fish samples [51,52]. As for M. mercenaria samples, protein digestibility was approximately 70%, regardless of the presence, type, and number of particles, being in accordance with values reported in the literature [52]. This slightly lower protein bioaccessibility was most likely related to the fact that bivalves’ edible part presents a distinct chemical composition (high glycogen and collagen fiber contents) that renders into a thicker muscle tissue with lower digestibility, as well as a typical chewy texture [53]. On the other hand, fish tissues contain fewer complex proteins, denser muscles, and a softer texture than clams, leading to easier digestion [54,55,56]. Despite the lack of significance regarding protein bioaccessibility, it should be noted that the present results may be limited to the experimental condition used, namely the number, size, shape, and type of tested particles. Given the panoply of plastic particles that can be found in the marine environment and in seafood species, additional studies should be carried out with different experimental conditions to those followed in this study (e.g., different particle number and size range, as well as a wider range of polymers), in order to gain a broader coverage of microplastics influence on the human digestive process.
5. Conclusions
The present study provides novel and relevant data that can contribute to a better understanding of the toxicological outcomes of petroleum-based (PET) and bio-based (PLA) plastics from a seafood safety perspective. Even though the simulated co-ingestion of PET and PLA had noeffects on the digestibility of animal proteins, irrespective of the seafood matrix (S. aurata, S. salar, or M. mercenaria) and the number of particles ingested, it should be noted that these findings may be limited to the tested experimental conditions. Hence, further studies in this area are encouraged in the future, accounting for the potential effects of different ecologically relevant particle types, shapes, and sizes.
This work also evidenced that the human digestive process has distinct effects on microplastic particles, depending on the type of polymer. Overall, PLA particles were impacted by digestion (increased number and enlargement of pores), but the same was not evident for PET particles. This is most likely due to the fact that PLA is a less resistant polymer designed to be easily biodegradable, and is consistent with its increased toxicity reported in recent studies. Taken together, these findings highlight the importance of assessing the bioaccessibility of potential monomers, polymers, and additives that can be released from microplastics, to validate the eco-friendliness of alternative biopolymers released on the market.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microplastics4040083/s1, Table S1: Composition of digestive juices; Figure S1: Representative SEM images (magnification: 400×) of PET and PLA particles with organic matter remains in the surfaces. Red circles show remains of organic matter after the in vitro digestive process. PET 10 and PET 20 are samples with 6.67 particles of PET g−1 of sample and 13.3 particles of PET g−1 of sample, respectively. PLA 10 and PLA 20 are samples with 6.67 particles of PLA g−1 of sample and 13.3 particles of PLA g−1 of sample, respectively (SA—S. aurata; SS—S. salar; MM—M. mercenaria).
Author Contributions
Conceptualization, D.B. and A.L.M.; Methodology, D.B., A.C.A., C.L., J.R., M.V.L., A.S.P. and A.L.M.; Formal analysis, D.B.; Investigation, D.B., R.V.C.G., A.C.A., M.V.L. and A.L.M.; Resources, A.L.M.; Data curation, D.B.; Writing—original draft preparation, D.B.; Writing—review and editing, D.B., R.V.C.G., A.C.A., C.L., J.R., M.V.L., A.S.P., P.S., A.M., T.R. and A.L.M.; Visualization, D.B.; Supervision, T.R., A.M. and A.L.M.; Project administration, A.L.M.; Funding acquisition, A.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the NextGenerationEU programme under the Recovery and Resilience Facility Plan for Portugal—Agenda VIIAFOOD—Platform for Valorization, Industrialization, and Commercial Innovation for the Agri-Food Sector (No. C644929456-00000040).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Acknowledgments
The data present in this study was supported by the EMSO-GOLD laboratory of the European Research Infrastructure EMSO-PT (European Multidisciplinary Seafloor and Water Column Observatory, Portugal) under Grant Agreement No. PINFRA/22157/2016.
Conflicts of Interest
Authors R.V.C.G, M.V.L. and A.S.P. were employed by the company SOVENA Portugal Consumer Goods. Author P.S. was employed by LOGOPLASTE Innovation Lab (ILAB). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| UV | Ultraviolet |
| O2 | Oxygen |
| CO2 | Carbon dioxide |
| FTIR | Fourier Infrared Spectroscopy |
| SEM | Scanning Electron Microscopy |
| PLA | Polylactic acid |
| PET | Polyethylene terephthalate |
| ANOVA | Analysis of variance |
| SA | Sparus aurata |
| SS | Salmo salar |
| MM | Mercenaria mercenaria |
Appendix A. Representative Spectra from Pristine and Digested PET and PLA Particles
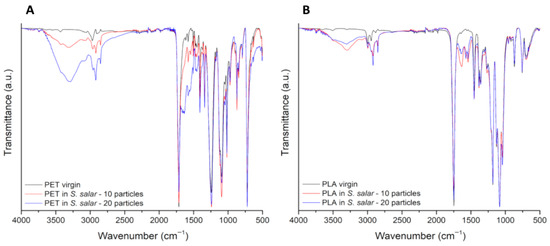
Figure A1.
FITR-spectra comparing representative spectra from pristine (black line) and digested PET (A) and PLA (B) particles (10 particles: red line; 20 particles: blue line), with S. salar samples. The y-axis corresponds to the percentage transmittance (a.u.) and the x-axis to the wave numbers (cm−1).
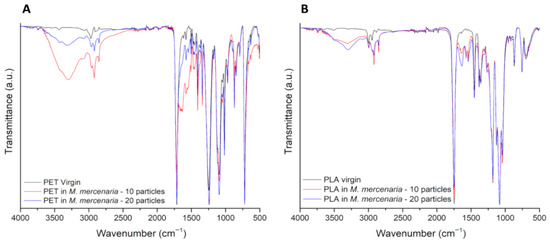
Figure A2.
FTIR-spectra comparing representative spectra from pristine (black line) and digested PET (A) and PLA (B) particles (10 particles: red line; 20 particles: blue line), with M. mercenaria samples. The y-axis corresponds to the percentage transmittance (a.u.) and the x-axis to the wave numbers (cm−1).
References
- Ritchie, H.; Samborska, V.; Roser, M. Plastic Pollution. Our World Data. 2023. Available online: https://ourworldindata.org/plastic-pollution (accessed on 5 July 2025).
- OECD. Global Material Resources Outlook to 2060: Economic Drivers and Environmental Consequences; OECD: Paris, France, 2019; ISBN 978-92-64-30744-5. [Google Scholar]
- Bajt, O. From Plastics to Microplastics and Organisms. FEBS Open Bio 2021, 11, 954–966. [Google Scholar] [CrossRef]
- Lim, X. Microplastics are everywhere—But are they harmful? Nature 2021, 593, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Dimassi, S.N.; Hahladakis, J.N.; Yahia, M.N.D.; Ahmad, M.I.; Sayadi, S.; Al-Ghouti, M.A. Degradation-Fragmentation of Marine Plastic Waste and Their Environmental Implications: A Critical Review. Arab. J. Chem. 2022, 15, 104262. [Google Scholar] [CrossRef]
- Jayakumar, A.; Radoor, S.; Siengchin, S.; Shin, G.H.; Kim, J.T. Recent Progress of Bioplastics in Their Properties, Standards, Certifications and Regulations: A Review. Sci. Total Environ. 2023, 878, 163156. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a Circular Economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Venâncio, C.; Lopes, I.; Oliveira, M. Bioplastics: Known Effects and Potential Consequences to Marine and Estuarine Ecosystem Services. Chemosphere 2022, 309, 136810. [Google Scholar] [CrossRef]
- Liang, B.; Deng, Y.; Zhong, Y.; Chen, X.; Huang, Y.; Li, Z.; Huang, X.; Yang, X.; Du, J.; Ye, R.; et al. Gastrointestinal Incomplete Degradation Exacerbates Neurotoxic Effects of PLA Microplastics via Oligomer Nanoplastics Formation. Adv. Sci. 2024, 11, 2401009. [Google Scholar] [CrossRef] [PubMed]
- Supartini, A.; Oishi, T.; Yagi, N. Changes in Fish Consumption Desire and Its Factors: A Comparison between the United Kingdom and Singapore. Foods 2018, 7, 97. [Google Scholar] [CrossRef]
- Tidwell, J.H.; Allan, G.L. Fish as Food: Aquaculture’s Contribution: Ecological and Economic Impacts and Contributions of Fish Farming and Capture Fisheries. EMBO Rep. 2001, 2, 958–963. [Google Scholar] [CrossRef]
- Mohanty, B.P.; Mahanty, A.; Ganguly, S.; Mitra, T.; Karunakaran, D.; Anandan, R. Nutritional Composition of Food Fishes and Their Importance in Providing Food and Nutritional Security. Food Chem. 2019, 293, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, E.; Jenner, L.C.; Twiddy, M.; Rotchell, J.M. Microplastic Contamination of Seafood Intended for Human Consumption: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2020, 128, 126002. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, S. Effects of Microplastics on Fish and in Human Health. Front. Environ. Sci. 2022, 10, 827289. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J. Investigation of Microplastics in Aquatic Environments: An Overview of the Methods Used, from Field Sampling to Laboratory Analysis. Trends Anal. Chem. 2018, 108, 195–202. [Google Scholar] [CrossRef]
- De-la-Torre, G.E. Microplastics: An Emerging Threat to Food Security and Human Health. J. Food Sci. Technol. 2020, 57, 1601–1608. [Google Scholar] [CrossRef]
- Versantvoort, C.H.M.; Oomen, A.G.; Van De Kamp, E.; Rompelberg, C.J.M.; Sips, A.J.A.M. Applicability of an in Vitro Digestion Model in Assessing the Bioaccessibility of Mycotoxins from Food. Food Chem. Toxicol. 2005, 43, 31–40. [Google Scholar] [CrossRef]
- Marmelo, I.; Barbosa, V.; Maulvault, A.L.; Duarte, M.P.; Marques, A. Does the Addition of Ingredients Affect Mercury and Cadmium Bioaccessibility in Seafood-Based Meals? Food Chem. Toxicol. 2020, 136, 110978. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in Bivalves Cultured for Human Consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef]
- Hossain, M.B.; Pingki, F.H.; Azad, A.S.; Nur, A.-A.U.; Banik, P.; Paray, B.A.; Arai, T.; Yu, J. Microplastics in Different Tissues of a Commonly Consumed Fish, Scomberomorus Guttatus, from a Large Subtropical Estuary: Accumulation, Characterization, and Contamination Assessment. Biology 2023, 12, 1422. [Google Scholar] [CrossRef]
- Ibrahim, Y.S.; Abd Razak, N.I.; Roslan, N.S.; Yusof, K.M.K.K.; Mohd Ali, A.A.; Omar, N.F.; Chinglenthoiba, C.; Mohamad, N.N.; Anuar, S.T. Morphochemical Information on Microplastic Fibers Found in Edible Tissue of Local Commercial Fishes from the South China Sea and the Straits of Malacca for Potential Human Consumption. Environ. Sci. Adv. 2025, 4, 964–979. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Torres-Huerta, A.M.; Del Angel-López, D.; Domínguez-Crespo, M.A.; Palma-Ramírez, D.; Perales-Castro, M.E.; Flores-Vela, A. Morphological and Mechanical Properties Dependence of PLA Amount in PET Matrix Processed by Single-Screw Extrusion. Polym.-Plast. Technol. Eng. 2016, 55, 672–683. [Google Scholar] [CrossRef]
- Rocca-Smith, J.R.; Lagorce-Tachon, A.; Iaconelli, C.; Bellat, J.P.; Marcuzzo, E.; Sensidoni, A.; Piasente, F.; Debeaufort, F.; Karbowiak, T. How High Pressure CO2 Impacts PLA Film Properties. Express Polym. Lett. 2017, 11, 320–333. [Google Scholar] [CrossRef]
- Ertli, T.; Marton, A.; Földényi, R. Effect of pH and the Role of Organic Matter in the Adsorption of Isoproturon on Soils. Chemosphere 2004, 57, 771–779. [Google Scholar] [CrossRef]
- Tamargo, A.; Molinero, N.; Reinosa, J.J.; Alcolea-Rodriguez, V.; Portela, R.; Bañares, M.A.; Fernández, J.F.; Moreno-Arribas, M.V. PET Microplastics Affect Human Gut Microbiota Communities during Simulated Gastrointestinal Digestion, First Evidence of Plausible Polymer Biodegradation during Human Digestion. Sci. Rep. 2022, 12, 528. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C. Microplastics and Human Health: Integrating Pharmacokinetics. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1489–1511. [Google Scholar] [CrossRef]
- Sizeland, K.H.; Hofman, K.A.; Hallett, I.C.; Martin, D.E.; Potgieter, J.; Kirby, N.M.; Hawley, A.; Mudie, S.T.; Ryan, T.M.; Haverkamp, R.G.; et al. Nanostructure of Electrospun Collagen: Do Electrospun Collagen Fibers Form Native Structures? Materialia 2018, 3, 90–96. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-Calculated IR Spectrum Amide I, II, and III Band Contributions of N-Methylacetamide Fine Components. ACS Omega 2020, 5, 8572–8578. [Google Scholar] [CrossRef]
- Venkatachalam, S.; Nayak, S.G.; Labde, J.V.; Gharal, P.R.; Rao, K.; Kelkar, A.K. Degradation and Recyclability of Poly (Ethylene Terephthalate). In Polyester; Saleh, H.E.-D.M., Ed.; IntechOpen: London, UK, 2012; ISBN 978-953-51-0770-5. [Google Scholar]
- Laria, J.G.; Gaggino, R.; Kreiker, J.; Peisino, L.E.; Positieri, M.; Cappelletti, A. Mechanical and Processing Properties of Recycled PET and LDPE-HDPE Composite Materials for Building Components. J. Thermoplast. Compos. Mater. 2023, 36, 418–431. [Google Scholar] [CrossRef]
- Swetha, T.A.; Ananthi, V.; Bora, A.; Sengottuvelan, N.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A Review on Biodegradable Polylactic Acid (PLA) Production from Fermentative Food Waste—Its Applications and Degradation. Int. J. Biol. Macromol. 2023, 234, 123703. [Google Scholar] [CrossRef]
- Rosli, N.A.; Karamanlioglu, M.; Kargarzadeh, H.; Ahmad, I. Comprehensive Exploration of Natural Degradation of Poly(Lactic Acid) Blends in Various Degradation Media: A Review. Int. J. Biol. Macromol. 2021, 187, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Koterwa, A.; Kaczmarzyk, I.; Mania, S.; Cieslik, M.; Tylingo, R.; Ossowski, T.; Bogdanowicz, R.; Niedziałkowski, P.; Ryl, J. The Role of Electrolysis and Enzymatic Hydrolysis Treatment in the Enhancement of the Electrochemical Properties of 3D-Printed Carbon Black/Poly(Lactic Acid) Structures. Appl. Surf. Sci. 2022, 574, 151587. [Google Scholar] [CrossRef]
- Lv, S.; Liu, C.; Li, H.; Zhang, Y. Assessment of Structural Modification and Time-Dependent Behavior of Poly (Lactic Acid) Based Composites upon Hydrolytic Degradation. Eur. Polym. J. 2022, 166, 111058. [Google Scholar] [CrossRef]
- Stock, V.; Fahrenson, C.; Thuenemann, A.; Dönmez, M.; Voss, L.; Böhmert, L.; Braeuning, A.; Lampen, A.; Sieg, H. Impact of Artificial Digestion on the Sizes and Shapes of Microplastics Particles. Food Chem. Toxicol. 2020, 135, 111010. [Google Scholar] [CrossRef]
- Jiménez-Arroyo, C.; Tamargo, A.; Molinero, N.; Reinosa, J.J.; Alcolea-Rodriguez, V.; Portela, R.; Bañares, M.A.; Fernández, J.F.; Moreno-Arribas, M.V. Simulated Gastrointestinal Digestion of Polylactic Acid (PLA) Biodegradable Microplastics and Their Interaction with the Gut Microbiota. Sci. Total Environ. 2023, 902, 166003. [Google Scholar] [CrossRef]
- Peng, Y.; Lu, J.; Fan, L.; Dong, W.; Jiang, M. Simulated Gastrointestinal Digestion of Two Different Sources of Biodegradable Microplastics and the Influence on Gut Microbiota. Food Chem. Toxicol. 2024, 185, 114474. [Google Scholar] [CrossRef]
- Prabhu, K.; Ghosh, S.; Sethulekshmi, S.; Shriwastav, A. In Vitro Digestion of Microplastics in Human Digestive System: Insights into Particle Morphological Changes and Chemical Leaching. Sci. Total Environ. 2024, 934, 173173. [Google Scholar] [CrossRef]
- Kumar, P. Role of Plastics on Human Health. Indian J. Pediatr. 2018, 85, 384–389. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [PubMed]
- Givissis, P.K.; Stavridis, S.I.; Papagelopoulos, P.J.; Christodoulou, A.G. Delayed Foreign-Body Reaction to Absorbable Implants in Metacarpal Fracture Treatment. Clin. Res. 2010, 468, 3377–3383. [Google Scholar] [CrossRef]
- Parolini, M.; Stucchi, M.; Ambrosini, R.; Romano, A. A Global Perspective on Microplastic Bioaccumulation in Marine Organisms. Ecol. Indic. 2023, 149, 110179. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, X.; Lu, Y.; Guo, L. Behavioral Toxicity and Neurotoxic Mechanisms of PLA-PBAT Biodegradable Microplastics in Zebrafish. Sci. Total Environ. 2024, 928, 172354. [Google Scholar] [CrossRef] [PubMed]
- De Ruijter, V.N.; Redondo-Hasselerharm, P.E.; Gouin, T.; Koelmans, A.A. Quality Criteria for Microplastic Effect Studies in the Context of Risk Assessment: A Critical Review. Environ. Sci. Technol. 2020, 54, 11692–11705. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Dombrowski, A.; Völker, C.; Wagner, M. Are Bioplastics and Plant-Based Materials Safer than Conventional Plastics? T In Vitro Toxicity and Chemical Composition. Environ. Int. 2020, 145, 106066. [Google Scholar] [CrossRef]
- Zhang, C.; Li, F.; Liu, X.; Xie, L.; Zhang, Y.T.; Mu, J. Polylactic Acid (PLA), Polyethylene Terephthalate (PET), and Polystyrene (PS) Microplastics Differently Affect the Gut Microbiota of Marine Medaka (Oryzias melastigma) after Individual and Combined Exposure with Sulfamethazine. Aquat. Toxicol. 2023, 259, 106522. [Google Scholar] [CrossRef]
- Huang, W.; Yin, H.; Yang, Y.; Jin, L.; Lu, G.; Dang, Z. Influence of the Co-Exposure of Microplastics and Tetrabromobisphenol A on Human Gut: Simulation In Vitro with Human Cell Caco-2 and Gut Microbiota. Sci. Total Environ. 2021, 778, 146264. [Google Scholar] [CrossRef]
- Costa, S.; Afonso, C.; Cardoso, C.; Batista, I.; Chaveiro, N.; Nunes, M.L.; Bandarra, N.M. Fatty Acids, Mercury, and Methylmercury Bioaccessibility in Salmon (Salmo salar) Using an in Vitro Model: Effect of Culinary Treatment. Food Chem. 2015, 185, 268–276. [Google Scholar] [CrossRef]
- Alves, R.N.; Maulvault, A.L.; Barbosa, V.L.; Cunha, S.; Kwadijk, C.J.A.F.; Álvarez-Muñoz, D.; Rodríguez-Mozaz, S.; Aznar-Alemany, Ò.; Eljarrat, E.; Barceló, D.; et al. Preliminary Assessment on the Bioaccessibility of Contaminants of Emerging Concern in Raw and Cooked Seafood. Food Chem. Toxicol. 2017, 104, 69–78. [Google Scholar] [CrossRef]
- Otwell, S.; Garrido, L.; Sturmer, L. Sensory Characterization Program for Cultured Hard Clams Mercenaria Species. 2012. Available online: https://repository.library.noaa.gov/view/noaa/45864/noaa_45864_DS1.pdf (accessed on 20 July 2025).
- Hyldig, G.; Nielsen, D. Texture of Fish, Fish Products, and Shellfish. In Handbook of Meat, Poultry and Seafood Quality; Nollet, L.M.L., Ed.; Wiley: Hoboken, NJ, USA, 2007; pp. 549–562. ISBN 978-0-8138-2446-8. [Google Scholar]
- Ariño, A.; Beltrán, J.A.; Herrera, A.; Roncalés, P. Fish and Seafood: Nutritional Value. In Encyclopedia of Human Nutrition; Elsevier: Amsterdam, The Netherlands, 2013; pp. 254–261. ISBN 978-0-12-384885-7. [Google Scholar]
- Ryu, B.; Shin, K.-H.; Kim, S.-K. Muscle Protein Hydrolysates and Amino Acid Composition in Fish. Mar. Drugs 2021, 19, 377. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).