Abstract
Microplastic particles with a size of less than 5 mm make up a significant component of the plastic pollution in freshwater and the ocean. This study was designed to investigate the effects of eight-week exposure to high-molecular-weight polyvinyl chloride (HMW-PVC) on young rats. A total of 40 rats were divided into two assay groups of 15 rats (Group 1, Group 2, a total of 30 rats) and a control group of 10 rats. The rats in the first and second assay groups were fed with food containing HMW-PVC at rates of 1 and 2% of their weight, respectively. The control group was fed food without HMW-PVC. The rats’ weights were recorded every 15 days. After eight weeks of feeding, the rats’ intestines, kidneys, and livers were removed and underwent histopathological examinations. Additionally, mRNA expression levels of Cyp3A2, Pepck, and Fasn genes in the liver, UT-A1, UT-A2, renin, and Cyp27B1 genes in the kidney, and Muc2, Fabp2, and PepT1 genes in the intestine were determined by using the RT-PCR technique. Our study revealed that rats exposed to microplastic particles exhibited non-significant weight loss and obvious organ degeneration. Furthermore, mRNA expression levels of the examined genes were either elevated or suppressed by regular exposure to high-molecular-weight PVC.
1. Introduction
Since the 2000s, scientists have started focusing on plastic pollution as they gain a deeper understanding of its harmful effects. Plastic particles fall into the following categories according to their size: macroplastics (>50 cm), megaplastics (5–50 cm), mesoplastics (0.5–5 cm), microplastics (<5 mm), and nanoplastics (≤0.1 µm) []. A single piece of plastic that is thrown away in nature as a waste material can be degraded into millions of micro- and nanoplastics (MNPs), which constitute the main form of plastic particles ingested by living creatures. Numerous studies have investigated the effects of MNP accumulation on various aquatic and terrestrial organisms. Those studies showed that following ingestion or uptake, MNPs can easily build up and cause damage in living tissues, and their tissue accumulation may result in a number of negative effects, including intestinal defects, decreased motility, increased inflammation, genotoxicity, physical injury, decreased feeding activity, growth and development inhibition, energy deficiency, immune responses, oxidative stress, neurotoxic response, metabolic disorder, and changes in the microbiome [,,,,,,,,,,,]. Organisms are exposed to environmental MNPs via respiratory, digestive, and cutaneous routes [,,]. MNPs cannot penetrate skin layers or living epidermis cells, but can be size-dependently deposited within the epidermal layer. Furthermore, numerous studies conducted on human AC and lung epithelial cells showed that polystyrene MNPs of various sizes can cause inflammation, the production of reactive oxygen species, decreased transepithelial electrical resistance, changes in cell viability, apoptosis, alteration of gene expression levels, and disturbed barrier function of alveolar epithelium [,,]. Furthermore, MNP particles can carry toxic materials, including contaminants, pesticides, toxic chemicals, and bioactive compounds, such as chemicals that interfere with hormones []. It has also been reported that polystyrene MNPs can induce lipid accumulation in the body []. MNPs can also impede the digestive tract of organisms and decrease nutrient absorption [,]. Furthermore, many studies showed that exposure to toxic material, including MNPs, cause alterations in the expression levels of Cyp3A2, Pepck, and Fasn genes in the liver; UT-A1, UT-A2, renin, and Cyp27B1 genes in the kidneys; and Muc2, Fabp2, and PepT1 genes in the gut [,,,,,,,,,,,,,].
Because the majority of research on the harmful effects of MNPs has been conducted on aquatic animals rather than mammals, and studies regarding the possible health risks of tissue accumulation of MNPs in mammals are limited [,], we designed this study to examine the consequences of MNPs exposure on the total weight, organs’ histopathology, and the mRNA expression profiles of specific genes of young rats that are exposed to MNP particles over an extended period of time.
2. Materials and Methods
Approval of the experimental protocols was obtained from the Local Ethics Committee of the Cukurova University Medical Sciences Experimental Search and Application Center. This study was carried out in accordance with the NIH Guide for Animal Care and Use.
2.1. Test Substances
Microplastic (HMW-PVC) particles in powder form with no additives were purchased from Sigma-Aldrich (catalog number 81387; 2, Burlington, MA, USA). The particles, which have a particle size of 75.6 ± 15.3 μm and a density of 1.4 g/mL at 25 °C, were considered as a perfect sphere (Jovanović et al. 2018) []. HMW-PVC particles have the chemical formula of (CH2CHCl)n and the international chemical identifier (InChl) of 1S/C2H3Cl/c1-2-3/h2H,1H2. The food for rats was obtained from a facility that produces pellets specifically for research purposes through the Cukurova University Experimental Application and Research Center (CU-EARC), Adana/TURKEY. This special solid feeding pellet mostly contained starch, amino acids, vitamins, and some trace elements. The supplier confirmed that the pellet was additive-free and pure. For rats in the first and second experimental groups, foods containing 1% and 2% HMW-PVC, respectively, were prepared. Likewise, food that did not contain HMW-PVC was provided to control rats.
2.2. Animals
This investigation was conducted on 40 young male Wistar rats (weighing 200 ± 20 g) that were raised at the Cukurova University Center for Experimental Application and Research. Rats were given free access to water and food at all times. The animals were housed in a chamber that had a 12 h light–dark cycle and a temperature of 21 ± 2 °C.
2.3. Assay Procedure
This 8-week study was conducted on 40 Wistar rats, which were divided into three groups. Two experimental groups, each containing fifteen rats, and one control group containing ten Wistar rats. Rats in study Groups 1 and 2 were fed with solid pellets containing 1% and 2% HMW-PVC, respectively. Having an average weight of 220 g, rats consumed food equal to 10% of their weight per day, which means that they ate 22 g of food and ingested 220 mg and 440 mg of microplastics per day, respectively. Rats in the control group were fed food that did not contain HMW-PVC. All rats received unlimited food. Rat weights were recorded at 15-day intervals. At the end of the eighth week, the rats’ livers, kidneys, and intestines were removed for histopathological and gene expression analyses.
2.4. Histopathological Study
The removed rats’ liver, kidney, and intestine tissues showed no disease symptoms in macroscopic examination. For microscopic examination, tissues were embedded within paraffin blocks and sectioned into thin slices, and then paraffin slices were dyed with hematoxylin and eosin (H&E) and finally examined under a light microscope.
2.5. Real-Time PCR Analysis Study
The mRNA expression levels of the CYP3A2, Pepck, and Fasn genes in the liver, the UT-A1, UT-A2, renin, and CYP27B1 genes in the kidneys, and the Muc2, Fabp2, and Pept1 genes in the gut were determined by the RT-PCR technique, and the results of the study groups were compared with the results of the control group.
2.6. RNA Extraction, cDNA Synthesis, and RT-PCR
Following the manufacturer’s instructions, TRIpure total RNA extraction reagent (ELK Biotechnology, Denver, CO, USA) was used to extract total RNA from the liver, kidney, and intestines of rats in the control and study groups. Then, the quality of total RNA was assessed by spectrophotometry using the absorbance ratio of 260 nm and 280 nm (Implen NanoPhotometer, Bavyera, Germany). cDNA synthesis was performed by employing the EntiLinkTM 1st Strand cDNA Synthesis Kit (ELK Biotechnology). The qPCR reactions were carried out utilizing hot Firepol Evagreen qPCR mix plus (Solis Biodyne, Tartu, Estonia). All quantitative PCR assays were conducted using the BioRad DX Real-Time Systems device (Hercules, CA, USA). The 40 cycles of qPCR reactions at the cycle conditions of 95 °C for 15 s (denaturation), 60 °C for 30 s (annealing), and 72 °C for 30 s (extension) were performed. The Gapdh and Actb genes were used as internal references. Table 1 contains all primer sequences used in this study. The variations in the expression levels of the CYP3A2, Pepck, Fasn, UT-A1, UT-A2, renin, CYP27B1, Muc2, Fabp2, and Pept1 genes were calculated by using the 2Ct formula and by utilizing threshold cycle (Ct) values that were obtained from replicates.

Table 1.
Primer sequences used for real-time quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR).
2.7. Statistical Analyses
Prior to this study, power analysis was conducted using the criteria of a type 1 error rate of (α) 0.05, power of 0.90, and effect size of 1 in order to determine the minimum required sample size for the study and control groups. The statistical power analysis results showed that n = 5 for each group was the minimum required sample size. Despite the minimum needed sample size of five rats, each study group consisted of 15 rats, and the control group consisted of 10 rats because it was thought some animals could die during the experimental processes.
2.7.1. Statistical Analysis of the Results of RT-PCR
IBM SPSS 20.0 software was used for statistical analysis. Intergroup differences were tested for each gene. When data met parametric assumptions and variances were homogeneous (Levene’s test p > 0.05), one-way ANOVA was used, followed by Tukey’s HSD for pairwise comparisons; when variances were unequal, Tamhane’s T2 was applied. For non-normal data, Kruskal–Wallis tests were used, followed by pairwise Mann–Whitney U tests with Bonferroni adjustment. To account for multiple testing across the panel of [n] genes, p-values from the primary group comparisons were adjusted using the Benjamini–Hochberg false discovery rate (FDR) procedure (q = 0.05). Both unadjusted p-values and FDR-adjusted q-values are reported; statistical significance was defined as q ≤ 0.05. Gene expression data are presented as mean ± standard error.
2.7.2. Statistical Analysis of Weight
The weight differences were compared using IBM SPSS 20. The GLM Repeated Measures was applied to test changes and differences in weight gain over time among the study groups.
3. Results
3.1. Results of Weight Comparison
The weight values of the rats were taken at the beginning of the feeding period (at day 1), every two weeks during the feeding period, and at the conclusion of the feeding period. A total of five weight measurements were taken, and at the end of this study, the weights of the rats in the study and control groups were compared both within and across groups. According to the results of intergroup comparisons, there was no significant difference between the mean value of rats’ weights between the study groups and the control group (p = 0.585). Nevertheless, it should be noted that the weights of rats in the first and second study groups tend to be lower than the control group, but the differences were not statistically significant. In contrast, the results of within-group comparisons showed that the weight averages of the rats in Group 1, Group 2, and the control group were significantly higher at all measurements as compared with earlier measurements (p < 0.001). (Figure 1 and Table 2).
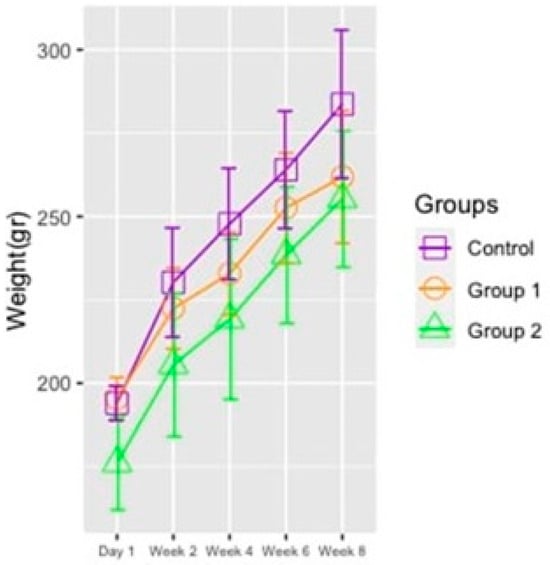
Figure 1.
Changes in the rat groups’ average body weight values across five measurements.

Table 2.
Average weight values of rat groups across five measurements during the eight-week feeding period.
3.2. The Outcomes of Histopathological Analyses
The dissected gut, liver, and kidney of Wistar rats that are exposed to HMW-PVC microplastics for 8 weeks are histopathologically examined. The results are shown in Table 3 and Figure 2, Figure 3 and Figure 4. Table 3 and Figure 2 demonstrate that all rats exposed to HMW-PVC developed active, chronic inflammation of the intestinal mucous membrane and lamina propria. The intensity of inflammation in the surface epithelium of the intestines was scored as +: slight, ++: moderate, and +++: severe. The degree of inflammation (+; ++; +++) was evaluated according to the intensity of inflammation infiltrating the surface epithelium.

Table 3.
Histopathological analysis results of liver, kidney, and intestine tissues in rats exposed to HMW-PVC.
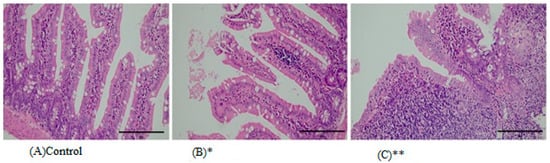
Figure 2.
Representative images from histopathological examination of intestinal paraffin slices of rats that were exposed to HMW-PVC for 8 weeks. Slices were dyed with H&E and magnified ×200. (A) An intestinal slice from control rats. (B) An intestinal slice of a rat from Group 1 shows loss of mucin and inflammation. (C) An intestinal slice of a rat from Group 2 demonstrates loss of mucin and inflammation. * indicates Group 1, and ** indicates Group 2.
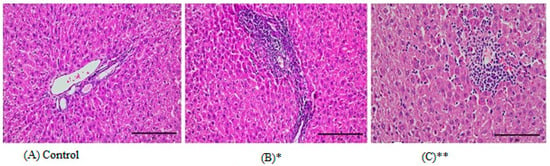
Figure 3.
Representative images from histopathological examination of liver paraffin slices of rats that were exposed to HMW-PVC for 8 weeks. Slices were dyed with H&E and magnified ×200. (A) A liver slice from a control rat. (B) A liver slice from a rat in Group 1 demonstrates moderately severe chronic inflammation and piecemeal necrosis in the liver tissue portal area. (C) A liver slice from a rat in Group 2 demonstrates spotty necrosis of liver tissue. * indicates Group 1, and ** indicates Group 2.
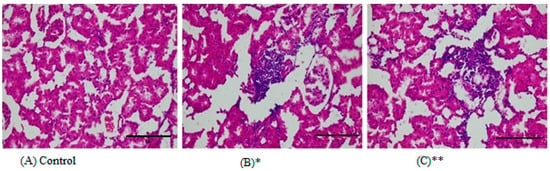
Figure 4.
Representative images from the histopathological examination of kidney paraffin slices from rats subjected to HMW-PVC for eight weeks. Slices were dyed with H&E and magnified ×400. (A) An image from the kidney of a control rat. (B) A kidney slice from a rat in Group 1 demonstrates interstitial nephritis. (C) A kidney slice from a rat in Group 2 demonstrates interstitial nephritis. * indicates Group 1, and ** indicates Group 2.
Edema, mucus loss, and surface epithelium loss were occasionally observed. Fifty percent (50%) of the liver tissues of the first and second study groups showed portal inflammation, 33% of tissues had piecemeal necrosis, and again 50% had patchy necrosis (Table 3 and Figure 3). Eighty-three percent (83%) of the kidney tissues of rats in the first and second study groups showed interstitial nephritis with chronic inflammation in the interstitium (Table 3 and Figure 4).
3.3. mRNA Gene Expression Data from RT-PCR
In this study, the mRNA expression profiles of the Muc2, Fabp2, and Pept1 genes in the gut tissue, the Cyp3A2, Pepck, and Fasn genes in the liver tissue, and the UT-A1, UTA2, renin, and Cyp27B1 genes in the kidney tissue were analyzed. Our findings showed that the Fabp2 gene expression in the rat gut significantly decreased in the 2% HMW-PVC treatment group, whereas the expression levels of the Pept1 and Muc2 genes in the study groups were non-significantly changed compared with the control group (Figure 5).
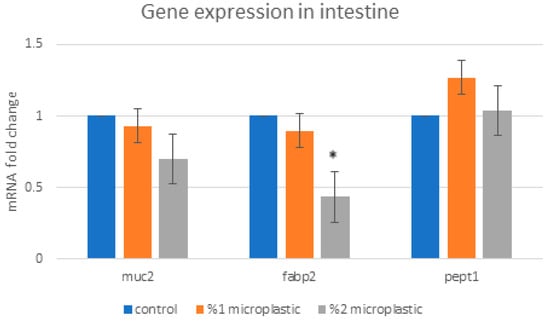
Figure 5.
Changes in expression levels of the Muc2, Fabp2, and Pept1 genes in the rat intestine. Asterisks on the column displaying mRNA expression level in the study group indicate a significant difference from the mRNA expression level in the control group (* p < 0.05).
As for liver genes, we found that the expression levels of Cyp3a were significantly increased in the first study group compared with the control group. The expression level of the Cyp3a gene in the second study group was not significantly decreased. The expression level of the Pepck and Fasn genes non-significantly increased in the group of rats exposed to 1% HMW-PVC (first study group) compared with the control rats, while the expression levels of the same genes reduced in the group of rats exposed to 2% HMW-PVC (second study group) (Figure 6). Likewise, when the mRNA expression levels of kidney genes in the rats of the treatment groups were compared with control rats, it was detected that UT-A1, UT-A2, and Cyp27B1 gene expressions significantly increased, but renin gene expression non-significantly decreased in the rats of the second study group compared with the controls (Figure 7). The expression levels of the UT-A1 and renin genes in rats of the first study group were significantly increased and reduced, respectively. The expression levels of the UT-A2 and Cyp27B1 genes in this group of rats were increased, but these increases were not statistically significant.
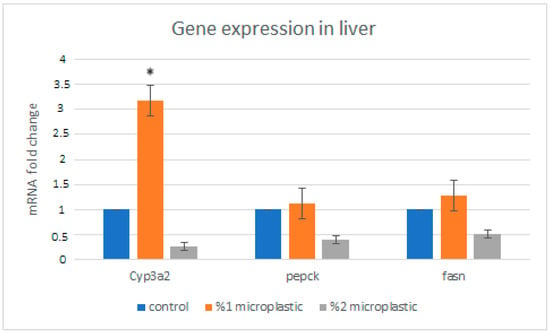
Figure 6.
Changes in expression levels of Cyp3a, Pepck, and Fasn genes in the rat liver. Asterisks on the column displaying mRNA expression level in the study group indicate a significant difference from the mRNA expression level in the control group (* p < 0.05).
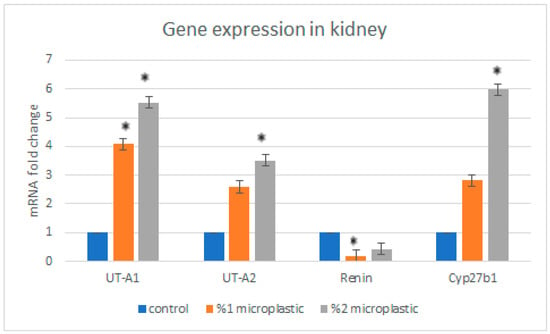
Figure 7.
The changes in expression levels of UT-A1, UT-A2, renin, and Cyp27B1 genes in rat kidney. Asterisks on the columns displaying mRNA expression levels in the study groups indicate a significant difference from the mRNA expression level in the control group (* p < 0.05).
4. Discussion
This study examined a total of 40 young rats, which were divided into two assay groups of 15 rats, which were provided food containing 1% and 2% of HMW-PVC, respectively, and a control group of 10 rats, which were provided food without HMW-PVC. Having an average weight of 220 g, rats consumed food equal to 10% of their weight per day, which means that they ate 22 g of food and ingested 220 mg (1% of 22 g) and 440 mg (2% of 22) of microplastics per day, respectively. This indicates that rats fed 1% and 2% HMW-PVC were exposed to HMW-PVC at daily doses of 1 to 2 g per kg body weight per day. Similar studies mixed different amounts of microplastic particles with feeding pellets. Papp et al. [] investigated the effects of polyvinyl chloride (PVC) microplastics on organ and total body weights of rabbits. They fed rabbit groups with food containing 0.5% and 0.05% PVC. In their investigation, exposure to those levels of PVC resulted in no significant change in total body and organ weights of rabbits but produced some minor macroscopic changes in organs. Because they did not provide the precise quantity of PVC rabbits exposed to per kg body weight per day, we were unable to compare the PVC doses used in their investigation with our own. However, in gross comparison, the percentages of PVC they used were far lower than those we used in our research. Yang et al. [] conducted a study comparable to ours, giving mice 210 mg of PVC microplastics in their chow on a daily basis. They concluded that ingestion of that dose of PVC microplastics caused impairment of the intestinal barrier, disruption of the intestinal microflora, and damage to the reproductive system. Their results are in agreement with our results in causing damage to the intestines. Therefore, different studies tested the effects of varying concentrations of microplastic particles on diverse animal species, making precise comparison difficult.
The reasons for providing very high doses of HMW-PVC to rats in our study were as follows: (a) The study design required the rats to consume HMW-PVC-mixed food based on their eating needs instead of direct treatment of microplastic particles by oral gavage, meaning that the amount of food and so the amount of HMW-PVC that would be ingested by rats was not definitely determined. (b) Any organism, including human beings, might be exposed to microplastic particles directly or indirectly via any item that can be consumed by any animal. (c) The last but not least reason is that it is aimed at testing the outcomes of in vivo exposure to very high doses of HMW-PVC.
Our study results showed that there was no significant difference between the mean value of rats’ weights between the study groups and the control group (p = 0.585). In a very similar study, researchers tried to test the effects of PVC on the reproductive capacity of adult male Wistar rats. Archana et al. [] provided two groups of rats, the PVC doses of 100 and 500 mg per kg body weight per day within the rodent feed for 60 days. Although they applied very high doses compared with our doses of 22 and 44 mg, they reported that there is no significant difference between body weight gain in the rats of the study and control groups. However, they still found that the group of rats that were provided 500 mg PVC per day showed reduced relative weights for the testes and different reproductive organs (epididymis, seminal vesicles, prostate gland, and vas deferens) [].
In another study conducted by Huang et al. [], mice were treated with five different concentrations of microplastic particles for 10 weeks. Of the four groups (n = 10–12 for each), MPs were given in drinking water with concentrations of 0.001, 0.1, 1, and 10 μg/mL (about 8.12 × 107, 8.12 × 109, 8.12 × 1010, and 8.12 × 1011 particles/mL, respectively). In the fifth group of mice (n = 6), microplastic particles were provided at 100 µg/day by gavage of 200 µL water containing microplastic particles. They showed that high concentrations of microplastic particles (more than 1 μg/mL) caused significant weight losses, the lowest concentration caused little change, and relatively low concentrations caused weight gain in mice. They concluded that the different doses of microplastic particles caused bidirectional modulatory effects on mice []. In a different investigation, Qiao et al. [] exposed zebrafish to polystyrene microplastics having shapes of beads, fragments, and fibers at a dose of 20 mg/L for two weeks. They reported that microplastic fibers significantly reduced the body weight of zebrafish compared with the controls []. Contrary to the results of those studies, Papp et al. [] investigated the effects of polyvinyl chloride (PVC) microplastics on organ and total body weights of rabbits. For this purpose, they provided high (500 g of 60–250 µm PVC microplastics per 100 kg of food, equivalent to 0.5% of the feeding mixture) and low (50 g of 60–250 µm PVC microplastics per 100 kg of food, equivalent to 0.05% of the feeding mixture) doses of PVC to rabbits within the feeding mixtures for 35 days. They measured the weight of the rabbits every week during and at the end of the feeding period. They reported that mean body weight values were not significantly changed across experimental groups and the control group during and after the feeding period. Since it is not specified how much PVC microplastic rabbits are exposed to during the course of the Papp et al. study, and we were also unable to determine how much HMW-PVC microplastic was consumed by rats during the feeding period of our study, we were unable to precisely compare the findings of the two studies. However, overall, it seems the results of Papp et al.’s study are in agreement with our results. So, there are contradictory findings about how exposure to PVC microplastics affects changes in total body weight. For precise results, we require more carefully planned studies.
MNPs can pass the gut barrier, enter into lymphatic and systemic circulations, and accumulate in tissues such as the liver, kidney, lungs, and brain. Some studies reported that accumulation of various-sized plastic particles in the tissues of animals caused oxidative stress, inflammation, and diminished cell viability [,]. Accordingly, Gaspar et al. [] reported that female mice exposed to various doses of polystyrene microplastic particles (0.0025 mg/mL, 0.025 mg/mL, and 0.125 mg/mL) for three weeks showed the presence of plastic particles in all tissues of the liver, kidney, gastrointestinal tract, lung, spleen, and heart, and they detected increased mRNA expression of the inflammatory cytokine TNF-α as a sign of liver inflammation. Similarly, Qiao et al. [] showed that zebrafish exposed to polystyrene microplastics at a dose of 20 mg/L for two weeks accumulated microplastics in their intestines and that the accumulation of MP particles in the fish intestine caused multiple toxic effects, such as increased permeability, mucosal damage, and inflammation []. In this study, we also examined the histopathological consequences of exposure to HMW-PVC in rats and found that the high doses of HMW-PVC caused common inflammation in the gut, liver, and kidney tissues. Additionally, tissue necrosis in the liver and surface epithelial loss and edema in the gut were observed (Table 2). Our results are in good agreement with previous study results [,,].
Numerous studies conducted in different settings showed that the expression levels of some genes, particularly the genes playing roles in oxidative stress and inflammation, were affected by microplastics’ exposure [,,,,,]. Accordingly, in our study, we analyzed the expression levels of the Cyp3A2, Pepck, and Fasn genes in the liver; the UT-A1, UT-A2, renin, and Cyp27B1 genes in the kidneys; and the Muc2, Fabp2, and PepT1 genes in the gut, which are prominent genes that have roles in intoxication-caused oxidative stress and inflammation of those organs.
As to the genes whose expressions were analyzed in the liver, the cytochrome P450, family 3, subfamily a, polypeptide 2 (Cyp3A2) gene is a member of the cytochrome P450 family, which is mainly expressed in the liver and plays an important role in xenobiotic metabolism. The other two genes, phosphoenolpyruvate carboxykinase (Pepck) and fatty acid synthase (Fasn), encode proteins playing roles in gluconeogenesis and hepatic de novo lipogenesis pathways, respectively [,,,]. Our results showed that the expression levels of Cyp3a, Pepck, and Fasn genes increased in the liver of rats exposed to 1% HMW-PVC but reduced in the liver of rats exposed to 2% HMW-PVC. One possible reason why exposure to 2% HMW-PVC caused downregulated expression of the Cyp3a, Pepck, and Fasn genes, while exposure to 1% HMW-PVC caused upregulated expression of the same genes in the rat liver, may be the hormesis phenomenon.
Hormesis is a toxicological concept that explains the beneficial effects of mild stress. According to this concept, a low-dose stressor can trigger an adaptive response and strengthen biological performance, while a high-dose stressor can cause a negative response and weaken biological resilience. We need more studies, possibly molecular-based studies, for a thorough interpretation of this dose-dependent dual effect of HMW-PVC on the expression levels of liver genes []. Some previous studies reported that microplastic exposure increased the expression levels of these genes in the liver, digestive glands, and adipose tissues of mice, oysters, and marine medaka species. These studies used small MNPs doses of 0.5 and 10 mg/L and 1 × 104 particles/L compared with the doses we exposed the Wistar rats to in our study [,,].
UT-A1 and UT-A2 are urea transporter proteins encoded by the UT-A (SLC14A2, Solute Carrier Family 14 Member 2) gene. In the rat kidney, UT-A1 localizes to the apical membrane and cytoplasm of the middle and terminal inner medullary collecting duct. In contrast, UT-A2 is located in type 1 (short) thin descending limbs and type 3 (long) thin descending limbs of the loop of Henle. These urea transporters mediate the transepithelial movement of urea across the membranes of the inner medullary collecting duct and descending limbs of the loop of Henle. The CYP27B1 (cytochrome P450, family 27, subfamily b, polypeptide 1) gene encodes an enzyme that is a member of the cytochrome P450 superfamily of enzymes that regulates the level of biologically active vitamin D and plays an important role in calcium homeostasis. It is known that some intoxications, such as lithium intoxication and nephrotoxic drugs, show effects on protein levels of urea transporters [,]. In an earlier study, Limonta et al. exposed adult zebrafish to a mix of polystyrene (PS) and high-density polyethylene (PE) microplastics at concentrations of 100 μg and 1000 μg per liter []. They reported that RT-qPCR results showed that exposure to a microplastic dose of 100 μg/L caused upregulated mRNA expression of the CYP gene family member CYP2P8 gene in fish head kidney. Likewise, in agreement with the literature results, our study results showed that exposure to HMW-PVC significantly upregulated UT-A1, UT-A2, and Cyp27B1 gene expressions in the kidneys of rats in the high-dose (second study) group.
The Ren (renin) gene encodes a protease enzyme that is secreted by the kidney and plays an important role in the renin–angiotensin–aldosterone system, which ultimately regulates blood pressure []. It has been reported that MNPs exposure has the potential to induce inflammation and cell apoptosis in the kidney, which ultimately induces kidney fibrosis [], and the renin–angiotensin–aldosterone system and, intimately, the renin gene have a particular role in kidney fibrosis []. In a study conducted by Itani et al. [], it has been reported that As4.1 cells treated with H2O2, as a common agent of triggering cellular oxidative stress, showed a dose-dependent and time-dependent decrease in levels of endogenous renin mRNA. In agreement with the study results of Itani et al. [], our results also showed that the mRNA expression level of the renin gene decreased in rat kidneys as a result of exposure to high doses of HMW-PVC. This suggests that HMW-PVC showed an impact akin to that of toxic H2O2.
The mRNA expression levels of Muc2, Fabp2, and PEPT1 genes were analyzed in the rat intestine. Muc2 is one of the mucin genes that encodes a protein forming an insoluble mucous barrier that protects the gut epithelia from the damaging environment of the lumen []. The Fabp2 gene is one of the intracellular fatty acid-binding proteins that is present throughout small intestine tissue, and its ablation causes a variety of metabolic defects, including defects in hepatic lipid metabolism [].
As a member of the solute carrier family 15 genes, the PEPT1 (solute carrier family 15 members, SLC15A1) gene encodes a protein that is placed within the apical membrane of the intestinal epithelium and mediates the transport of di- and tripeptides from the lumen to the intestinal cells across the brush border membrane (in humans) [].
Some previous studies investigated the effects of microplastic particles on expression levels of Muc2, Fabp2, and Pept1 genes in various organs [,]. A study conducted by Cui et al. [] reported that exposure to polystyrene nanoplastics at doses of 10, 50, and 250 mg/kg body weight for 28 days increased Muc2 expression in mice intestines. In another study, it has been reported that exposure to 200 nm-sized polystyrene microspheres at doses of 0.5 and 5 mg/L for 21 days reduced the expression level of the FABP gene (fatty acid binding protein) in the hepatopancreatic tissue of redclaw crayfish, Cherax quadricarinatus []. In agreement with the results of the Chen et al. study [], we found that the rat gut’s exposure to HMW-PVC reduced the mRNA expression level of the Fabp2 gene in a dose-dependent manner. We need more studies to draw an exact conclusion about the effects of MNPs on the expression levels of those genes.
5. Conclusions
Our findings demonstrated that long-term exposure to microplastics has the potential to upregulate or downregulate the mRNA expression of all genes related to the metabolism of toxicity or toxicity-caused oxidative stress and inflammation. Our results also showed that exposure to high doses of HMW-PVC caused inflammation in the liver, kidney, and gut, and it is predicted that inflammation in those organs will persist for a very long time. The increased use of MN particles with each passing day will have long-term effects on organisms ingesting those particles and also on organisms that consume those MNP-ingested organisms. Possibly, we do not know many harmful effects of microplastics on living things, and so more research is required for a thorough understanding of the hazards that these synthetic particles pose for our world. Therefore, raising awareness about the potential consequences of excessive plastic use on all living creatures is thought to be important.
Author Contributions
Conceptualization, A.B.P. and M.E.; Methodology, A.B.P. and M.E.; Software, A.B.P.; Validation, A.B.P., M.E. and E.T.; Formal Analysis, T.E. and Y.S.; Investigation, A.B.P., M.E., B.K., S.I., H.O., S.E., T.T. and N.E.; Resources, A.B.P.; Data Curation, A.B.P.; Writing—Original Draft Preparation, A.B.P.; Writing—Review and Editing, A.B.P. and E.T.; Visualization, A.B.P. and E.T.; Supervision, M.E.; Project Administration, A.B.P.; Funding Acquisition(only for purchase of equipment and chemical materials), A.B.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported (for purchase of equipment and chemical materials) by the Çukurova University Research Fund (I.U.BAP) (Project no.: TSA-2021-13781).
Institutional Review Board Statement
The animal study protocol was approved by the Çukurova University Animal Experiments Local Ethics Committee (No. 3; 18 March 2021).
Data Availability Statement
Data supporting this study’s findings are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| HMW-PVC | High-Molecular-Weight Polyvinyl Chloride |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| MNPs | Micro-and nanoplastics |
References
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, M.; Wang, L.; Gu, W.; Li, X.; Han, Z.; Fu, X.; Wang, X.; Li, X.; Su, Z. Continuous oral exposure to micro- and nanoplastics induced gut microbiota dysbiosis, intestinal barrier and immune dysfunction in adult mice. Environ. Int. 2023, 182, 108353. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.K.; Ge, S.J.; Su, Q.L.; Chen, J.J.; Wu, J.K. Effects of polyvinyl chloride microplastics on the reproductive system, intestinal structure, and microflora in male and female mice. Vet. Sci. 2024, 11, 488. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Zhang, S.; Xu, Y.; Wen, L.; Feng, X. Effects of microplastic exposure on the early developmental period and circadian rhythm of zebrafish (Danio rerio): A comparative study of polylactic acid and polyglycolic acid. Ecotoxicol. Environ. Saf. 2023, 258, 114994. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhuang, J.; Chen, Q.; Xu, L.; Yue, X.; Qiao, D. Polyvinyl chloride microplastics induced gut barrier dysfunction, microbiota dysbiosis and metabolism disorder in adult mice. Ecotoxicol. Environ. Saf. 2022, 241, 113809. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, P.; Tan, H.; Shen, R.; Chen, D. Polylactic Acid Microplastics Do Not Exhibit Lower Biological Toxicity in Growing Mice Compared to Polyvinyl Chloride Microplastics. J. Agric. Food Chem. 2023, 71, 19772–19782. [Google Scholar] [CrossRef]
- Iheanacho, S.C.; Odo, G.E. Neurotoxicity, oxidative stress biomarkers and haematological responses in African catfish (Clarias gariepinus) exposed to polyvinyl chloride microparticles. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 232, 108741. [Google Scholar] [CrossRef]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef]
- Papp, P.P.; Hoffmann, O.I.; Libisch, B. Effects of polyvinyl chloride (pvc) microplastic particles on gut microbiota composition and health status in rabbit livestock. Int. J. Mol. Sci. 2024, 25, 12646. [Google Scholar] [CrossRef]
- Jewett, E.; Arnott, G.; Connolly, L. Microplastics and their impact on reproduction-can we learn from the c. elegans model? Front. Toxicol. 2022, 4, 748912. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef]
- Murphy, F.; Quinn, B. The effects of microplastic on freshwater Hydra attenuata feeding, morphology & reproduction. Environ. Pollut. 2018, 234, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Akçay, S.; Törnük, F.; Yetim, H. Mikroplastikler: Gıdalarda bulunuşu ve sağlık üzerine etkileri. Eur. J. Sci. Technol. 2020, 20, 530–538. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, B.; Yao, Q.; Feng, X.; Shen, T.; Guo, P.; Wang, P.; Bai, Y.; Li, B.; Wang, P.; et al. Toxicological effects of micro/nano-plastics on mouse/rat models: A systematic review and meta-analysis. Front. Public Health 2023, 11, 1103289. [Google Scholar] [CrossRef]
- Brown, D.M.; Wilson, M.R.; MacNee, W. Size-dependent proinflammatory effects of ultrafine polystyrene particles: A role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol. Appl. Pharmacol. 2001, 175, 191–199. [Google Scholar] [CrossRef]
- Dong, C.D.; Chen, C.W.; Chen, Y.C.; Chen, H.H.; Lee, J.S.; Lin, C.H. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J. Hazard. Mater. 2020, 385, 121575. [Google Scholar]
- Yang, S.; Cheng, Y.; Chen, Z.; Liu, T.; Yin, L.; Pu, Y.; Liang, G. In vitro evaluation of nanoplastics using human lung epithelial cells, microarray analysis and co-culture model. Ecotoxicol. Environ. Saf. 2021, 226, 112837. [Google Scholar] [CrossRef]
- Shen, Q.; Liu, Y.J.; Qiu, T.T.; Loon K, S.; Zhou, D. Microplastic-induced NAFLD: Hepatoprotective effects of nanosized selenium. Ecotoxicol. Environ. Saf. 2024, 272, 115850. [Google Scholar] [CrossRef]
- Kershaw, P.J.; Rochman, C.M. Sources, Fate and Effects of Microplastics in the Marine Environment: Part 2 of a Global Assessment. In Reports and Studies-IMO/FAO/Unesco-IOC/WMO/IAEA/UN/UNEP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection (GESAMP) Eng; International Maritime Organization: London, UK, 2015. [Google Scholar]
- Rist, S.; Almroth, B.C.; Hartmann, N.B.; Karlsson, T.M. A critical perspective on early communications concerning human health aspects of microplastics. Sci. Total Environ. 2018, 626, 720–726. [Google Scholar] [PubMed]
- Allen, A.; Hutton, D.A.; Jeffrey, P.; Pearson, J.P. The MUC2 gene product: A human intestinal mucin. Int. J. Biochem. Cell Biol. 1998, 30, 797–801. [Google Scholar] [CrossRef] [PubMed]
- AlQudah, M.; Hale, T.M.; Czubryt, M.P. Targeting the renin-angiotensin-aldosterone system in fibrosis. Matrix Biol. 2020, 91–92, 92–108. [Google Scholar] [CrossRef]
- Blount, M.A.; Sim, J.H.; Zhou, R.; Martin, C.F.; Lu, W.; Sands, J.M.; Klein, J.D. Expression of transporters involved in urine concentration recovers differently after cessation of lithium treatment. Am. J. Physiol. Ren. Physiol. 2010, 298, F6018. [Google Scholar] [CrossRef]
- Cassuto, H.; Kochan, K.; Chakravarty, K.; Cohen, H.; Blum, B.; Olswang, Y.; Hakimi, P.; Xu, C.; Massillon, D.; Hanson, R.W.; et al. Glucocorticoids Regulate Transcription of the Gene for Phosphoenolpyruvate Carboxykinase in the Liver via an Extended Glucocorticoid Regulatory Unit. J. Biol. Chem. 2005, 280, 33873–33884. [Google Scholar] [CrossRef]
- Chen, Y.; Agellon, L.B. Distinct Alteration of Gene Expression Programs in the Small Intestine of Male and Female Mice in Response to Ablation of Intestinal Fabp Genes. Genes 2020, 11, 943. [Google Scholar] [CrossRef]
- Cui, M.; He, Q.; Wang, Z.; Yu, Y.; Gao, H.; Liu, Z.; Peng, H.; Wang, H.; Zhang, X.; Li, D.; et al. Mucin 2 regulated by Ho1/p38/IL-10 axis plays a protective role in polystyrene nanoplastics-mediated intestinal toxicity. Environ. Pollut. 2023, 330, 121808. [Google Scholar] [CrossRef]
- Cyp3a2 Cytochrome. P450, Family 3, Subfamily a, Polypeptide 2 [Rattus Norvegicus (Norwayrat)]. Available online: https://www.ncbi.nlm.nih.gov/gene/266682/ (accessed on 30 September 2024).
- CYP27B1 Cytochrome. P450, Family 27, Subfamily B Member 1 [(Homo Sapiens (Human)]. Available online: https://www.ncbi.nlm.nih.gov/gene/1594 (accessed on 3 December 2024).
- Fenton, R.A.; Stewart, G.S.; Carpenter, B.; Howorth, A.; Potter, E.A.; Cooper, G.J.; Smith, C.P. Characterization of mouse urea transporters UT-A1 and UT-A2. Am. J. Physiol. Ren. Physiol. 2002, 283, F817–F825. [Google Scholar] [CrossRef]
- Gene Cards: The Human Gene Database. Available online: https://www.genecards.org/ (accessed on 30 September 2024).
- Klein, J.D.; Blount, M.A.; Sands, J.M. Urea transport in the kidney. Compr. Physiol. 2011, 1, 699–729. [Google Scholar] [CrossRef]
- Patra, I.; Huy, D.T.N.; Alsaikhan, F.; Opulencia, M.J.C.; Tuan, P.V.; Nurmatova, K.C.; Majdi, A.; Shoukat, S.; Yasin, G.; Margiana, R.; et al. Toxic effects on enzymatic activity, gene expression and histopathological biomarkers in organisms exposed to microplastics and nanoplastics: A review. Environ. Sci. Eur. 2022, 34, 80. [Google Scholar] [CrossRef]
- Xiong, X.; Gao, L.; Chen, C.; Zhu, K.; Luo, P.; Li, L. The microplastics exposure induce the kidney injury in mice revealed by RNA-seq. Ecotoxicol. Environ. Saf. 2023, 56, 114821. [Google Scholar] [CrossRef]
- Pekmezekmek, A.B.; Emre, M.; Erdogan, S.; Yilmaz, B.; Tunc, E.; Sertdemir, Y.; Emre, Y. Effects of high-molecular-weight polyvinyl chloride on Xenopus laevis adults and embryos: The mRNA expression profiles of Myf5, Esr1, Bmp4, Pax6, and Hsp70 genes during early embryonic development. Environ. Sci. Pollut. Res. 2022, 29, 14767–14779. [Google Scholar] [CrossRef]
- Jovanović, B.; Gökdag, K.; Güven, O.; Emre, Y.; Whitley, E.M.; Kideys, A.E. Virgin microplastics are not causing imminent harm to fish after dietary exposure. Mar. Pollut. Bull. 2018, 130, 123–131. [Google Scholar] [CrossRef]
- Archana, D.; Supriya, C.; Girish, B.P.; Kishori, B.; Reddy, P.S. Alleviative effect of resveratrol on polyvinyl chloride-induced reproductive toxicity in male Wistar rats. Food Chem. Toxicol. 2018, 116, 173–181. [Google Scholar] [CrossRef]
- Huang, H.; Wei, F.; Qiu, S.; Xing, B.; Hou, J. Polystyrene microplastics trigger adiposity in mice by remodeling gut microbiota and boosting fatty acid synthesis. Sci. Total Environ. 2023, 890, 164297. [Google Scholar] [CrossRef]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.M.; Romero, A.S.; Merkley, A.S.; Meyer-Hagen, J.L.; Forbes, C.; Hayek, E.E.; Sciezka, D.P.; Templeton, R.; Gonzalez-Estrella, J.; Jin, Y. In Vivo Tissue Distribution of Microplastics and Systemic Metabolomic Alterations After Gastrointestinal Exposure. bioRxiv 2023, 3, 542598. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.; Bartman, S.; Coppotelli, G.; Ross, J.M. Acute Exposure to Microplastics Induced Changes in Behavior and Inflammation in Young and Old Mice. Int. J. Mol. Sci. 2023, 24, 12308. [Google Scholar] [CrossRef]
- Tu, P.; Chi, L.; Bodnar, W.; Zhang, Z.; Gao, B.; Bian, X.; Stewart, J.; Fry, R.; Lu, K. Gut Microbiome Toxicity: Connecting the Environment and Gut Microbiome-Associated Diseases. Toxics 2020, 8, 19. [Google Scholar] [CrossRef]
- Cornick, S.; Tawiah, A.; Chadee, K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers 2015, 3, e982426. [Google Scholar] [CrossRef]
- Du, Y.; Teng, J.; Zhao, J.; Ren, J.; Ma, H.; Zhang, T.; Xia, B.; Sun, S.; Wang, Q. Effects of ocean acidification and polystyrene microplastics on the oysters Crassostrea gigas: An integrated biomarker and metabolomic approach. Mar. Environ. Res. 2024, 196, 106434. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, Y.; Xu, R.; Li, D.; Waiho, K.; Wang, Y.; Hu, M. Combined toxic effects of nanoplastics and norfloxacin on antioxidant and immune genes in mussels. Mar. Environ. Res. 2024, 193, 106277. [Google Scholar] [CrossRef]
- Schmidt, C.; Höcherl, K.; Bucher, M. Cytokine-mediated regulation of urea transporters during experimental endotoxemia. Am. J. Physiol. Ren. Physiol. 2007, 292, F1479–F1489. [Google Scholar] [CrossRef]
- Matsukawa, T.; Yagi, T.; Uchida, T.; Sakai, M.; Mitsushima, M.; Naganuma, T.; Yano, H.; Inaba, Y.; Inoue, H.; Yanagida, K.; et al. Hepatic FASN deficiency differentially affects nonalcoholic fatty liver disease and diabetes in mouse obesity models. J. Clin. Investig. 2023, 8, e161282. [Google Scholar] [CrossRef]
- Sun, T.; Zhan, J.F.; Li, F.; Ji, C.; Wu, H. Effect of microplastics on aquatic biota: A hormetic perspective. Environ. Pollut. 2021, 15, 117206. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, K.; Ding, D.; Liu, J.; Lei, Z.; Chen, X.; Ye, G.; Zhang, J.; Shen, H.; Yan, C. Size-dependent adverse effects of microplastics on intestinal microbiota and metabolic homeostasis in the marine medaka (Oryzias melastigma). Environ. Int. 2021, 151, 106452. [Google Scholar] [CrossRef]
- Limonta, G.; Mancia, A.; Abelli, L.; Fossi, M.C.; Caliani, I.; Panti, C. Effects of microplastics on head kidney gene expression and enzymatic biomarkers in adult zebrafish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 24, 109037. [Google Scholar] [CrossRef]
- REN Renin [Homo Sapiens (Human) Gene ID: 5972]. National Institutes of Health (NIH). Available online: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=5972 (accessed on 21 September 2025).
- Itani, H.; Liu, X.; Sarsour, E.H.; Goswami, P.C.; Born, E.; Keen, H.L.; Sigmund, C.D. Regulation of renin gene expression by oxidative stress. Hypertension 2009, 53, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- SLC15A1 (Pept1) Solute Carrier Family 15 Member 1 [Homo Sapiens (Human)]. National Institutes of Health (NIH). Available online: https://www.ncbi.nlm.nih.gov/gene/6564 (accessed on 30 September 2024).
- Chen, Q.; Lv, W.; Jiao, Y.; Liu, Z.; Li, Y.; Cai, M.; Wu, D.; Zhou, W.; Zhao, Y. Effects of exposure to waterborne polystyrene microspheres on lipid metabolism in the hepatopancreas of juvenile redclaw crayfish, Cherax quadricarinatus. Aquat. Toxicol. 2020, 224, 105497. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).