Microplastics in Atlantic Ribbed Mussels (Geukensia demissa) from the Delaware Inland Bays, USA
Abstract
1. Introduction
1.1. Microplastic Pollution
1.2. Atlantic Ribbed Mussels
1.3. Delaware Inland Bays
2. Methods and Materials
2.1. Study Area and Sampling Sites
2.2. Sample Collection and Preparation
2.3. Microplastic Identification
2.4. Quality Control and Quality Assurance
2.5. Data Analysis
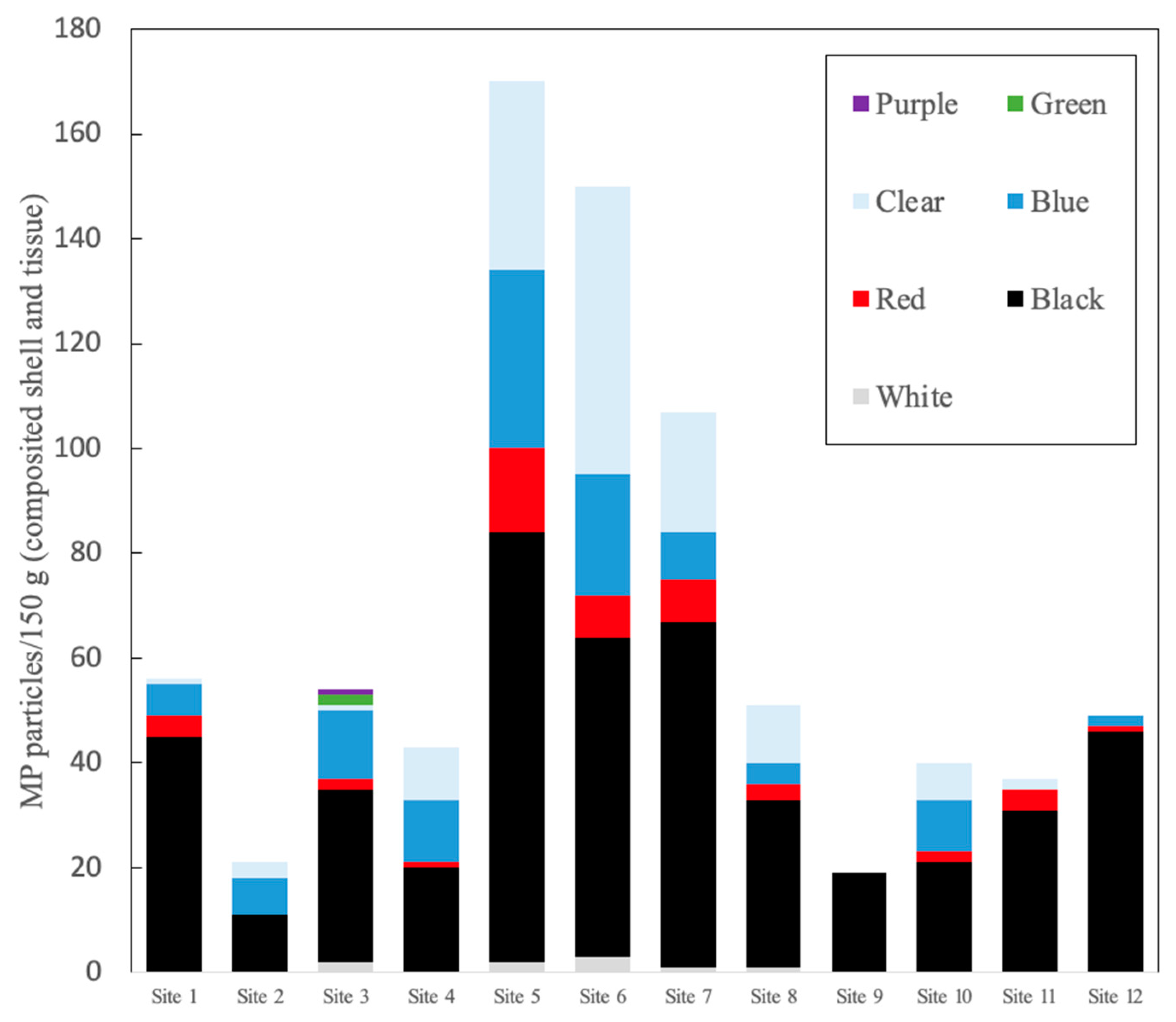
3. Results
4. Discussion
4.1. Issues When Identifying and Quantifying MPs
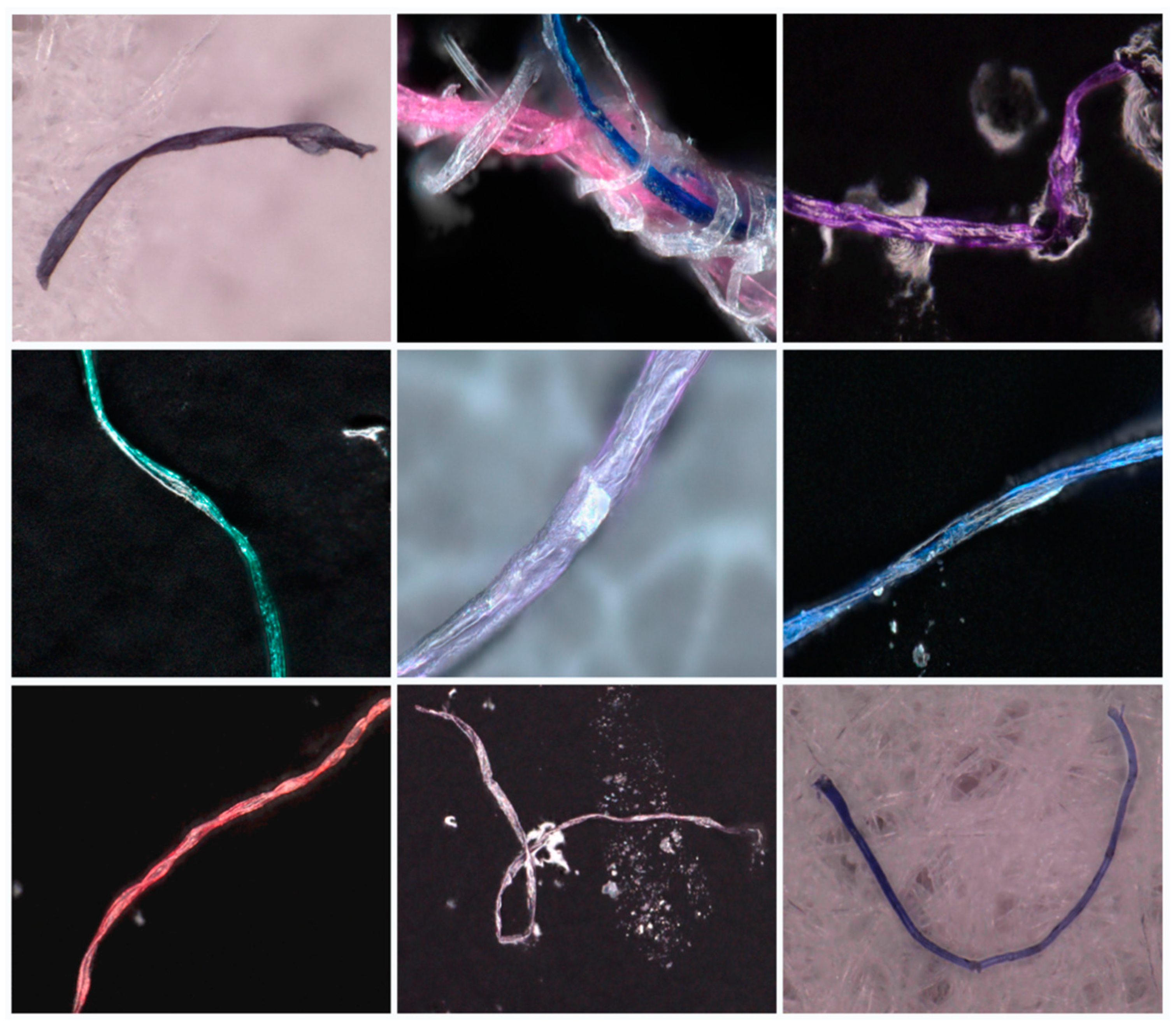
4.2. Dominant MP Types
4.3. Comparison to Other Data Sets
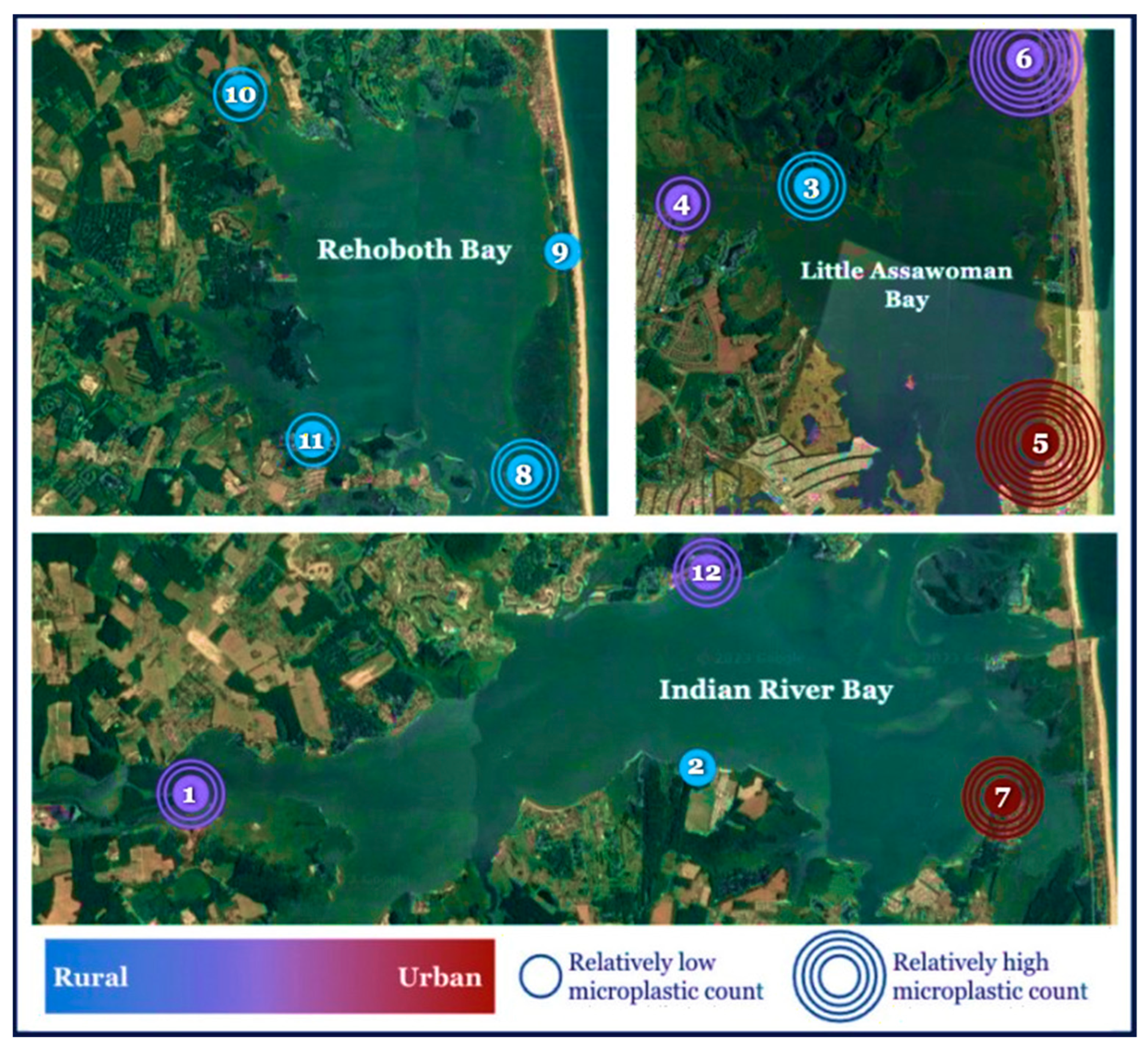
4.4. Regional Differences in MP Concentrations
4.5. Potential for Trophic Transfer of MPs
4.6. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arthur, C.; Baker, J.E.; Bamford, H.A. (Eds.) Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris, Tacoma, WA, USA, 9–11 September 2008; NOAA Technical Memorandum NOS-OR&R 30. Available online: https://repository.library.noaa.gov/view/noaa/2509 (accessed on 19 December 2023).
- Bergmann, M.; Wirzberger, V.; Krumpen, T.; Lorenz, C.; Primpke, S.; Tekman, M.B.; Gerdts, G. High quantities of microplastic in Arctic deep-sea sediments from the Hausgarten observatory. Environ. Sci. Technol. 2017, 51, 11000–11010. [Google Scholar] [CrossRef] [PubMed]
- Emberson-Marl, H.; Coppock, R.L.; Cole, M.; Godley, B.J.; Mimpriss, N.; Nelms, S.E.; Lindeque, P.K. Microplastics in the Arctic: A transect through the Barents Sea. Front. Mar. Sci. 2023, 10, 1241829. [Google Scholar] [CrossRef]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C. Plastic debris in marine environment: Consequences and solutions. In Marine Nature Conservation in Europe; Krause, J.C., von Nordheim, H., Brager, S., Eds.; Federal Agency for Nature Conservation: Stralsund, Germany, 2006; pp. 107–115. [Google Scholar]
- Gall, S.C.; Thompson, R.C. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Simon, N.; Schulte, M.L. Stopping Global Plastic Pollution: The Case for an International Convention; Ecology Publication Series; Heinrich-Böll-Foundation: Berlin, Germany, 2017; Volume 43, ISBN 978-3-86928-159-9. [Google Scholar]
- Yang, H.; Chen, G.; Wang, J. Microplastics in the marine environment: Sources, Fates, impacts and microbial degradation. Toxics 2021, 9, 14. [Google Scholar] [CrossRef]
- GESAMP (Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection). Sources, Fate and Effects of Microplastics in the Marine Environment: A Global Assessment; Kershaw, P.J., Ed.; International Maritime Organization: London, UK, 2015; 96p. [Google Scholar]
- Beaumont, N.J.; Aanesen, M.; Austen, M.C.; Börger, T.; Clark, J.R.; Cole, M.; Hooper, T.; Lindeque, P.K.; Pascoe, C.; Wyles, K.J. Global ecological, social and economic impacts of marine plastic. Mar. Pollut. Bull. 2019, 142, 189–195. [Google Scholar] [CrossRef]
- Li, N.; Wu, M.; Zhang, Y.; Yuan, W.; Jinlong Wu, J.; Shao, X. A review on microplastics pollution in coastal wetlands. Watershed Ecol. Environ. 2023, 5, 24–37. [Google Scholar] [CrossRef]
- Miller, M.E.; Hamann, M.; Kroon, F.J. Bioaccumulation and biomagnification of microplastics in marine organisms: A review and meta-analysis of current data. PLoS ONE 2020, 15, e0240792. [Google Scholar] [CrossRef]
- Schwarzer, M.; Brehm, J.; Vollmer, M.; Jasinski, J.; Xu, C.; Zainuddin, S.; Fröhlich, T.; Schott, M.; Greiner, A.; Scheibel, T.; et al. Shape, size, and polymer dependent effects of microplastics on Daphnia magna. J. Hazard. Mater. 2022, 426, 28136. [Google Scholar] [CrossRef]
- de Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef]
- Teuten, E.L.; Rowland, S.J.; Galloway, T.S.; Thompson, R.C. Potential for plastics to transport hydrophobic contaminants. Environ. Sci. Technol. 2007, 41, 7759–7764. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A detailed review study on potential effects of microplastics and additives of concern on human health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 12, 2588–2597. [Google Scholar] [CrossRef]
- Wang, F.; Wong, C.S.; Chen, D.; Lu, X.; Wang, F.; Zeng, E.Y. Interaction of toxic chemicals with microplastics: A critical review. Water Res. 2018, 139, 208–219. [Google Scholar] [CrossRef]
- Santos, L.H.M.L.M.; Rodríguez-Mozaz, S.; Barceló, D. Microplastics as vectors of pharmaceuticals in aquatic organisms—An overview of their environmental implications. Case Stud. Chem. Environ. Eng. 2021, 3, 100079. [Google Scholar] [CrossRef]
- Liu, S.; Shi, J.; Wang, J.; Dai, Y.; Li, H.; Li, J.; Liu, X.; Chen, X.; Wang, Z.; Zhang, P. Interactions between microplastics and heavy metals in aquatic environments: A review. Front. Microbiol. 2021, 12, 652520. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Hou, J.; Wang, X. A review of microplastic pollution in aquaculture: Sources, effects, removal strategies and prospects. Ecotoxicol. Environ. Saf. 2023, 252, 114567. [Google Scholar] [CrossRef] [PubMed]
- Kikaki, A.; Karantzalos, K.; Power, C.A.; Raitsos, D.E. Remotely Sensing the Source and Transport of Marine Plastic Debris in Bay Islands of Honduras (Caribbean Sea). Remote Sens. 2020, 12, 1727. [Google Scholar] [CrossRef]
- Themistocleous, K.; Papoutsa, C.; Michaelides, S.; Hadjimitsis, D. Investigating Detection of Floating Plastic Litter from Space Using Sentinel-2 Imagery. Remote Sens. 2020, 12, 2648. [Google Scholar] [CrossRef]
- Topouzelis, K.; Papakonstantinou, A.; Garaba, S.P. Detection of floating plastics from satellite and unmanned aerial systems (Plastic Litter Project 2018). Int. J. Appl. Earth Obs. Geoinf. 2019, 79, 175–183. [Google Scholar] [CrossRef]
- Biermann, L.; Clewley, D.; Martinez-Vicente, V.; Topouzelis, K. Finding plastic patches in coastal waters using optical satellite data. Sci. Rep. 2020, 10, 5364. [Google Scholar] [CrossRef]
- Li, J.; Lusher, A.L.; Rotchell, J.M.; Deudero, S.; Turra, A.; Bråte, I.L.N.; Sun, C.; Shahadat Hossain, M.; Li, Q.; Kolandhasamy, P.; et al. Using mussel as a global bioindicator of coastal microplastic pollution. Environ. Pollut. 2019, 244, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Nationwide monitoring of microplastics in bivalves from the coastal environment of Korea. Environ. Pollut. 2021, 270, 116175. [Google Scholar] [CrossRef]
- Ding, J.; Sun, C.; He, C.; Li, J.; Ju, P.; Li, F. Microplastics in four bivalve species and basis for using bivalves as bioindicators of microplastic pollution. Sci. Total Environ. 2021, 782, 146830. [Google Scholar] [CrossRef] [PubMed]
- Lent, C.M. Adaptations of the ribbed mussel, Modiolus demissus (Dillwyn), to the intertidal habitat. Am. Zool. 1969, 9, 283–294. [Google Scholar] [CrossRef]
- Kreeger, D.A.; Newell, R.I.E. Ingestion and assimilation of carbon from cellulolytic bacteria and heterotrophic flagellates by the mussels Geukensia demissa and Mytilus edulis (Bivalvia, Mollusca). Aquat. Microb. Ecol. 1996, 11, 205–214. [Google Scholar] [CrossRef]
- Riisgård, H.U. Efficiency of particle retention and filtration rate in 6 species of Northeast American bivalves. Mar. Ecol. Prog. Ser. 1988, 45, 217–223. [Google Scholar] [CrossRef]
- Delaware Center for the Inland Bays. Available online: https://www.inlandbays.org/about-the-bays/economic-value-of-the-inland-bays/ (accessed on 1 January 2024).
- Foekema, E.M.; De Gruijter, C.; Mergia, M.T.; van Franeker, J.A.; Murk, A.J.; Koelmans, A.A. Plastic in North Sea fish. Environ. Sci. Technol. 2013, 47, 8818–8824 . [Google Scholar] [CrossRef] [PubMed]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E. Evaluation of existing methods to extract microplastics from bivalve tissue: Adapted KOH digestion protocol improves filtration at single-digit pore size. Mar. Pollut. Bull. 2019, 142, 384–393. [Google Scholar] [CrossRef]
- Kotar, S.; McNeish, R.; Murphy-Hagan, C.; Renick, V.; Lee, C.T.; Steele, C.; Lusher, A.; Moore, C.; Minor, E.; Schroeder, J.; et al. Quantitative assessment of visual microscopy as a tool for microplastic research: Recommendations for improving methods and reporting. Chemosphere 2022, 308 Pt 3, 136449. [Google Scholar] [CrossRef] [PubMed]
- Noonan, M.J.; Grechi, N.; Mills, C.L.; Ferraz, M.M.M. Microplastics analytics: Why we should not underestimate the importance of blank controls. Microplastics Nanoplastics 2023, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Munno, K.; Lusher, A.L.; Minor, E.C.; Gray, A.; Ho, K.; Hankett, J.; Lee, C.T.; Primpke, S.; McNeish, R.E.; Wong, C.S.; et al. Patterns of microparticles in blank samples: A study to inform best practices for microplastic analysis. Chemosphere 2023, 333, 138883. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.L.; Santana, M.F.M.; Nelis, J.L.D.; Motti, C.A. Taking control of microplastics data: A comparison of control and blank data correction methods. J. Hazard. Mater. 2023, 443 Pt A, 130218. [Google Scholar] [CrossRef]
- Treilles, R.; Cayla, A.; Gaspéri, J.; Strich, B.; Ausset, P.; Tassin, B. Impacts of organic matter digestion protocols on synthetic, artificial and natural raw fibers. Sci. Total Environ. 2020, 748, 141230. [Google Scholar] [CrossRef] [PubMed]
- Huppertsberg, S.; Knepper, T.P. Instrumental analysis of microplastics—Benefits and challenges. Anal. Bioanal. Chem. 2018, 410, 6343–6352. [Google Scholar] [CrossRef] [PubMed]
- Gago, J.; Carretero, O.; Filgueiras, A.V.; Viñas, L. Synthetic microfibers in the marine environment: A review on their occurrence in seawater and sediments. Mar. Pollut. Bull. 2018, 127, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.L.; Gwinnett, C.; Robinson, L.F.; Woodall, L.C. Plastic microfibre ingestion by deep-sea organisms. Sci. Rep. 2016, 6, 33997. [Google Scholar] [CrossRef]
- Ross, P.S.; Chastain, S.; Vassilenko, E.; Etemadifar, A.; Zimmermann, S.; Quesnel, S.; Eert, J.; Solomon, E.; Patankar, S.; Posacka, A.M.; et al. Pervasive distribution of polyester fibres in the Arctic Ocean is driven by Atlantic inputs. Nat. Commun. 2021, 12, 106. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Vassilenko, E.; Watkins, M.; Chastain, S.; Mertens, J.; Posacka, A.M.; Patankar, S.; Ross, P.S. Domestic laundry and microfiber pollution: Exploring fiber shedding from consumer apparel textiles. PLoS ONE 2021, 16, 0250346. [Google Scholar] [CrossRef]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef]
- Mishra, S.; Rath, C.C.; Das, A.P. Marine microfiber pollution: A review on present status and future challenges. Mar. Pollut. Bull. 2019, 140, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Zubris, K.A.V.; Richards, B.K. Synthetic fibers as an indicator of land application of sludge. Environ. Pollut. 2005, 138, 201–211. [Google Scholar] [CrossRef]
- Habib, D.; Locke, D.C.; Cannone, L.J. Synthetic fibers as indicators of municipal sewage sludge, sludge products, and sewage treatment plant effluents. Water Air Soil Pollut. 1998, 103, 1–8. [Google Scholar] [CrossRef]
- Geyer, R.; Gavigan, J.; Jackson, A.M.; Saccomanno, V.R.; Suh, S.; Gleason, M.G. Quantity and fate of synthetic microfiber emissions from apparel washing in California and strategies for their reduction. Environ. Pollut. 2022, 298, 118835. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef]
- Lloret, J.; Pedrosa-Pamies, R.; Vandal, N.; Rorty, R.; Ritchie, M.; McGuire, C.; Chenoweth, K.; Valiela, I. Salt Marsh sediments act as sinks for microplastics and reveal effects of current and historical land use changes. Environ. Adv. 2021, 4, 100060. [Google Scholar] [CrossRef]
- Bashir, S.M.; Kimiko, S.; Mak, C.; Fang, J.K.; Gonçalves, D. Personal care and cosmetic products as a potential source of environmental contamination by microplastics in a densely populated Asian city. Front. Mar. Sci. 2021, 8, 683482. [Google Scholar] [CrossRef]
- Rochman, C.M.; Kross, S.M.; Armstrong, J.B.; Bogan, M.T.; Darling, E.S.; Green, S.J.; Smyth, A.R.; Veríssimo, D. Scientific evidence supports a ban on microbeads. Environ. Sci. Technol. 2015, 49, 10759–10761. [Google Scholar] [CrossRef]
- McDevitt, J.P.; Criddle, C.S.; Morse, M.; Hale, R.C.; Bott, C.B.; Rochman, C.M. Addressing the issue of microplastics in the wake of the microbead-free waters act—A new standard can facilitate improved policy. Environ. Sci. Technol. 2017, 51, 6611–6617. [Google Scholar] [CrossRef] [PubMed]
- Magni, S.; Gagné, F.; André, C.; Della Torre, C.; Auclair, J.; Hanana, H.; Parenti, C.C.; Bonasoro, F.; Binelli, A. Evaluation of uptake and chronic toxicity of virgin polystyrene microbeads in freshwater zebra mussel Dreissena polymorpha (Mollusca: Bivalvia). Sci. Total Environ. 2018, 631–632, 778–788. [Google Scholar] [CrossRef]
- Détrée, C.; Gallardo-Escárate, C. Polyethylene microbeads induce transcriptional responses with tissue-dependent patterns in the mussel Mytilus galloprovincialis. J. Molluscan Stud. 2017, 83, 220–225. [Google Scholar] [CrossRef]
- Kleinschmidt, J.M.; Janosik, A.M. Microplastics in Florida, United States: A case study of quantification and characterization with intertidal snails. Front. Ecol. Evol. 2021, 9, 645727. [Google Scholar] [CrossRef]
- Kazmiruk, T.N.; Kazmiruk, V.D.; Bendell, L.I. Abundance and distribution of microplastics within surface sediments of a key shellfish growing region of Canada. PLoS ONE 2018, 13, 0196005. [Google Scholar] [CrossRef] [PubMed]
- Bom, F.C.; Sá, F. Concentration of microplastics in bivalves of the environment: A systematic review. Environ. Monit. Assess. 2021, 193, 846. [Google Scholar] [CrossRef]
- Sparks, C.; Viljoen, N.; Hill, D.; Lassen, J.; Awe, A. Characteristics and risk assessment of microplastics in water and mussels sampled from Cape Town Harbour and Two Oceans Aquarium, South Africa. Bull. Environ. Contam. Toxicol. 2023, 110, 104. [Google Scholar] [CrossRef]
- Dambrosio, A.; Cometa, S.; Capuozzo, F.; Ceci, E.; Derosa, M.; Quaglia, N.C. Occurrence and Characterization of Microplastics in Commercial Mussels (Mytilus galloprovincialis) from Apulia Region (Italy). Foods 2023, 12, 1495. [Google Scholar] [CrossRef]
- Marques, F.; Vale, C.; Rudnitskaya, A.; Moreirinha, C.; Costa, S.T.; Botelho, M.J. Major characteristics of microplastics in mussels from the Portuguese coast. Environ. Res. 2021, 197, 110993. [Google Scholar] [CrossRef]
- Mercogliano, R.; Santonicola, S.; Raimo, G.; Gasperi, M.; Colavita, G. Extraction and identification of microplastics from mussels: Method development and preliminary results. Ital. J. Food. Saf. 2021, 10, 9264. [Google Scholar] [CrossRef]
- Truchet, D.A.; Forero López, A.D.; Ardusso, M.G.; Rimondino, G.N.; Buzzi, N.S.; Malanca, F.E.; Spetter, C.V.; Fernández Severini, M.D. Microplastics in bivalves, water and sediments from a touristic sandy beach of Argentina. Mar. Pollut. Bull. 2021, 173 Pt B, 113023. [Google Scholar] [CrossRef]
- Doucet, C.V.; Labaj, A.L.; Kurek, J. Microfiber content in freshwater mussels from rural tributaries of the Saint John River, Canada. Water Air Soil Pollut. 2021, 232, 32. [Google Scholar] [CrossRef]
- Khan, M.B.; Prezant, R.S. Microplastic abundances in a mussel bed and ingestion by the ribbed marsh mussel Geukensia demissa. Mar. Pollut. Bull. 2018, 130, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.; Branco, B. Variation in Microplastic Content of Marsh Sediment due to the Atlantic Ribbed Mussel (Geukensia demissa), Jamaica Bay, NY. In Final Reports of the Tibor T. Polgar Fellowship Program, 2017; Yozzo, D.J., Fernald, S.H., Andreyko, H., Eds.; Section IV; Hudson River Foundation: New York City, NY, USA, 2020; pp. 1–27. [Google Scholar]
- Seed, R. Predator-prey relationships between the mud crab Panopeus herbstii, the blue crab, Callinectes sapidus and the Atlantic ribbed mussel Geukensia (=Modiolus) demissa. Estuar. Coast. Mar. Sci. 1980, 11, 445–458. [Google Scholar] [CrossRef]
- Seed, R. Predation of the ribbed mussel Geukensia demissa by the blue crab callinectes sapidus. Neth. J. Sea Res. 1982, 16, 163–172. [Google Scholar] [CrossRef]
- Lin, J. Predator-prey interactions between blue crabs and ribbed mussels living in clumps. Estuar. Coast. Shelf Sci. 1991, 32, 61–69. [Google Scholar] [CrossRef]
- Lin, J. Mud crab predation on ribbed mussels in salt marshes. Mar. Biol. 1990, 107, 103–109. [Google Scholar] [CrossRef]
- Lin, J. Influence of location in a salt marsh on survivorship of ribbed mussels. Mar. Ecol. Prog. Ser. 1989, 56, 105–110. [Google Scholar] [CrossRef]
- Guillory, V.; Elliot, M. A review of blue crab predators. In Blue Crab Mortality Symposium, Proceedings of the Crustacean Society Annual Summer Meeting, Lafayette, LA, USA, 28–29 May 1999; Gulf States Marine Fisheries Commission: Ocean Springs, MS, USA, 2001; Volume 90, pp. 69–83. Available online: https://www.gsmfc.org/publications/GSMFC%20Number%20090.pdf (accessed on 18 December 2023).
- Fernández, B.; Albentosa, M. Insights into the uptake, elimination and accumulation of microplastics in mussel. Environ. Pollut. 2019, 249, 321–329. [Google Scholar] [CrossRef]
- Moreschi, A.C.; Callil, C.T.; Christo, S.W.; Ferreira Junior, A.L., Jr.; Nardes, C.; de Faria, E.; Girard, P. Filtration, assimilation and elimination of microplastics by freshwater bivalves. Case Stud. Chem. Environ. Eng. 2020, 2, 100053. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Envir. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Santonicola, S.; Volgare, M.; Di Pace, E.; Cocca, M.; Mercogliano, R.; Colavita, G. Occurrence of potential plastic microfibers in mussels and anchovies sold for human consumption: Preliminary results. Ital. J. Food Saf. 2021, 10, 9962. [Google Scholar] [CrossRef] [PubMed]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Goïc, N.L.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Walkinshaw, C.; Tolhurst, T.J.; Lindeque, P.K.; Thompson, R.C.; Cole, M. Impact of polyester and cotton microfibers on growth and sublethal biomarkers in juvenile mussels. Micropl. Nanopl. 2023, 3, 5. [Google Scholar] [CrossRef]
- Bringer, A.; Cachot, J.; Dubillot, E.; Lalot, B.; Thomas, H. Evidence of deleterious effects of microplastics from aquaculture materials on pediveliger larva settlement and oyster spat growth of Pacific oyster, Crassostrea gigas. Sci. Total Environ. 2021, 794, 148708. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Fan, C.; Liu, M.; Wolfe-Bryant, B. Effect of High-Density Polyethylene Microplastics on the Survival and Development of Eastern Oyster (Crassostrea virginica) Larvae. Int. J. Environ. Res. Public Health 2023, 20, 6142. [Google Scholar] [CrossRef]
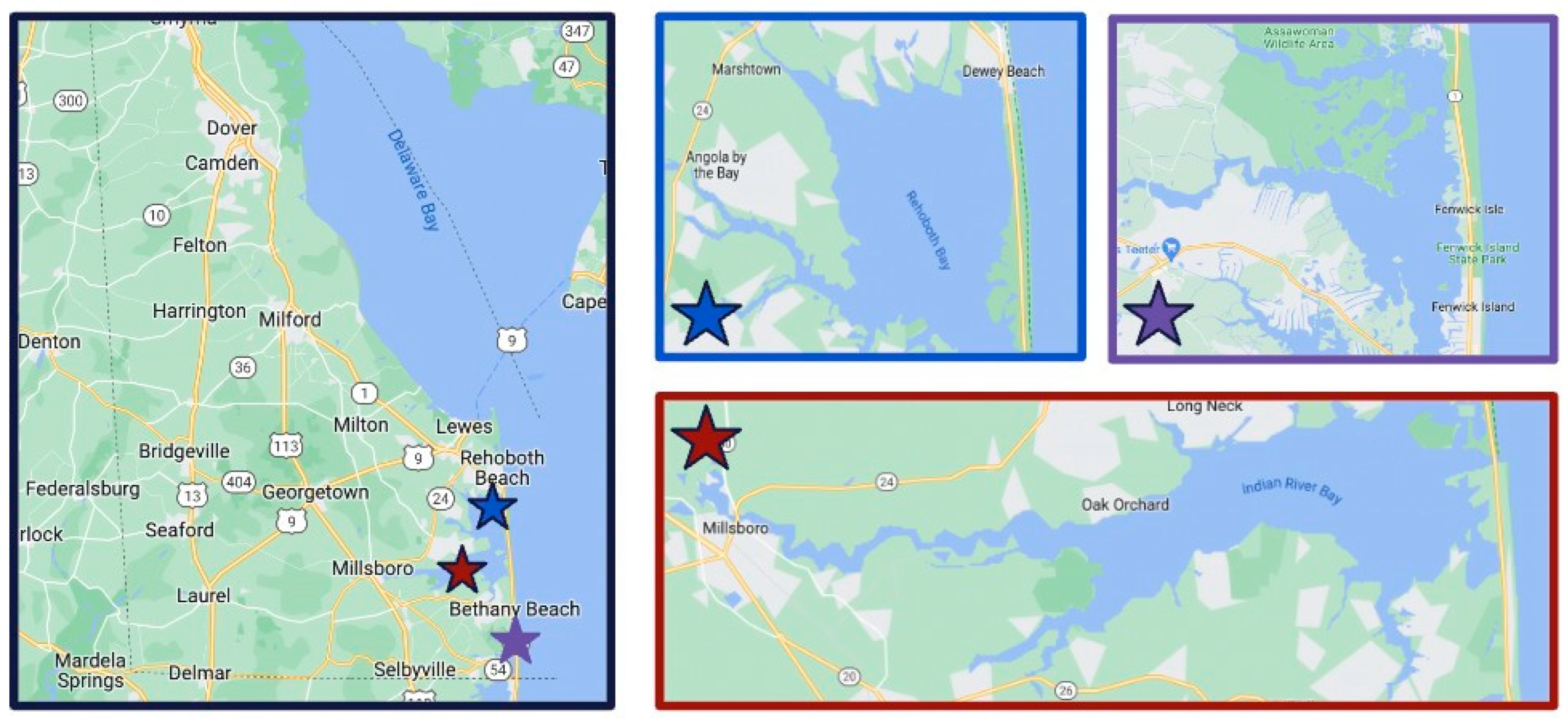

| Site Number | Site Location | Coordinates | Adjacent Land Use |
|---|---|---|---|
| 1 | NW Indian River Bay | 38.591868 and 75.215821 | Intermediate |
| 2 | SW Indian River Bay | 38.591367 and 75.126220 | Rural |
| 3 | NW Assawoman Bay | 38.485294 and 75.077185 | Rural |
| 4 | SW Assawoman Bay | 38.483374 and 75.089890 | Intermediate |
| 5 | SE Assawoman Bay | 38.483374 and 75.089890 | Urban |
| 6 | NE Assawoman Bay | 38.497810 and 75.056208 | Intermediate |
| 7 | SE Indian River Bay | 38.588284 and 75.076154 | Urban |
| 8 | SE Rehoboth Bay | 38.628009 and 75.070168 | Rural |
| 9 | NE Rehoboth Bay | 38.669167 and 75.071944 | Rural |
| 10 | NW Rehoboth Bay | 38.690833 and 75.136944 | Rural |
| 11 | SW Rehoboth Bay | 38.635278 and 75.125556 | Rural |
| 12 | NE Indian River Bay | 38.618056 and 75.124444 | Intermediate |
| Site | MPs/150 g Wet Tissue and Shells | MPs/g Wet Tissue |
|---|---|---|
| 1 | 56 | 0.67 |
| 2 | 21 | 0.25 |
| 3 | 55 | 0.66 |
| 4 | 43 | 0.52 |
| 5 | 170 | 2.06 |
| 6 | 150 | 1.81 |
| 7 | 107 | 1.29 |
| 8 | 51 | 0.62 |
| 9 | 19 | 0.23 |
| 10 | 40 | 0.48 |
| 11 | 37 | 0.44 |
| 12 | 49 | 0.51 |
| Range | 21–170 | 0.25–2.06 |
| Mean ± STD | 67 ± 49 | 0.80 ± 0.60 |
| Site | Replicate Number | Mass Whole Mussel (g) | Length of Mussel (cm) | MPs/Individual |
|---|---|---|---|---|
| 8 | 1 | 47.45 | 9.4 | 23 |
| 8 | 2 | 44.92 | 9.2 | 22 |
| 8 | 3 | 63.03 | 10 | 11 |
| 8 | 4 | 38.95 | 8.6 | 34 |
| 8 | 5 | 41.86 | 8.8 | 69 |
| 10 | 1 | 31.19 | 8.1 | 28 |
| 10 | 2 | 33.23 | 7.7 | 29 |
| 10 | 3 | 32.03 | 7.6 | 13 |
| 10 | 4 | 32.8 | 7.8 | 10 |
| 10 | 5 | 26.57 | 7 | 21 |
| Mean ± STD | 26 ± 17 |
| Organism/Species | Location | Reported Concentrations | Types of MPs Identified | Reference |
|---|---|---|---|---|
| Mussels | Cape Town Harbour and Two Oceans Aquarium, South Africa |
6.27 ± 0.59 MPs/individual 3.05 ± 1.09 MPs/g soft tissue wet weight | Black and grey filaments most prevalent | Sparks et al. [62] |
| Commercial Mussels (Mytilus galloprovincialis) | Apulia Region, Italy |
6.51 ± 4.32 MPs/individual 1.59 ± 0.95 MPs/g | Blue polyamide fragments most prevalent | Dambrosia et al. [63] |
| Mussels ( Mytilus spp) | Portuguese coast | 0.54 to 3.0 MPs/g | Microfibers the most abundant shape (50%) | Marques et al. [64] |
| Farmed Mussels | Tyrrhenian Sea and Adriatic Sea | 3.8 MPs/individual 0.5 MPs/g of tissue | Black MPs most prevalent | Mercogliano et al. [65] |
| fCommercial Mussels (Amarilladesma mactroides and Brachidontes rodriguezii) | Argentina | 0.15 to 0.5 MPs/g wet weight | Black and blue fibers of <0.5 and 0.5–1 mm the most abundant | Truchet et al. [66] |
| Oysters, Mussels | South Korean Coastline |
Oysters/Mussels 1.21 ± 0.68 MPs/individual 0.33 ± 0.23 MPs/g | Colorless fragments smaller than 300 μm most prevalent | Cho et al. [28] |
| Mussels ( Geukensia demissa ) | Delaware Inland Bays, United States | 0.80 ± 0.60 MPs/g wet weight 26 ± 16 MPs/individual | 96% microfibers; black MPs most prevalent | This Study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashley, J.; Pilat, A.; Ohlweiler, A.; Ogden, C.; Bradley, O.; Modi, P.; Talbot, S.; Smith, C.; O’Pella, J.; Ozbay, G. Microplastics in Atlantic Ribbed Mussels (Geukensia demissa) from the Delaware Inland Bays, USA. Microplastics 2024, 3, 147-164. https://doi.org/10.3390/microplastics3010009
Ashley J, Pilat A, Ohlweiler A, Ogden C, Bradley O, Modi P, Talbot S, Smith C, O’Pella J, Ozbay G. Microplastics in Atlantic Ribbed Mussels (Geukensia demissa) from the Delaware Inland Bays, USA. Microplastics. 2024; 3(1):147-164. https://doi.org/10.3390/microplastics3010009
Chicago/Turabian StyleAshley, Jeffrey, Amanda Pilat, Ariana Ohlweiler, Connor Ogden, Owen Bradley, Priya Modi, Spencer Talbot, Caya Smith, Justin O’Pella, and Gulnihal Ozbay. 2024. "Microplastics in Atlantic Ribbed Mussels (Geukensia demissa) from the Delaware Inland Bays, USA" Microplastics 3, no. 1: 147-164. https://doi.org/10.3390/microplastics3010009
APA StyleAshley, J., Pilat, A., Ohlweiler, A., Ogden, C., Bradley, O., Modi, P., Talbot, S., Smith, C., O’Pella, J., & Ozbay, G. (2024). Microplastics in Atlantic Ribbed Mussels (Geukensia demissa) from the Delaware Inland Bays, USA. Microplastics, 3(1), 147-164. https://doi.org/10.3390/microplastics3010009








