Abstract
Best known as an organizer of the mitotic spindle, the protein product of the human assembly factor for spindle microtubules (ASPM) gene has recently been shown to function in the interphase nucleus during multiple DNA-associated processes, including BRCA1-mediated DNA DSB repair, ATR-CHK1 activation during replication stress, and transcription regulation alongside the transcription factor FOXM1. In this review, we provide an overview of these DNA-related roles of ASPM. Additionally, we suggest the facilitation of liquid–liquid phase separation (LLPS) as a potential unifying mechanism underlying ASPM function. We also consider the implications of LLPS and ASPM dysfunction in disease, and highlight the impact of cellular context including cell cycle phase-dependent post-translational protein modifications and ion concentrations. An increased understanding of LLPS in ASPM function relevant to genome stability may enable future drug discovery for diseases such as cancer.
1. Introduction
The human Assembly factor for Spindle Microtubules (ASPM) gene and its orthologues have been studied since 1985, when mutants of the Drosophila melanogaster orthologue abnormal spindle (asp) showed the morphologically abnormal spindles for which it was named []. Since then, human ASPM has been regarded primarily as a mitotic spindle gene, since both Asp and ASPM protein products localize and contribute to spindle organization [,,,,,,,]. However, the roles of ASPM protein products have recently been expanded to include influences on multiple developmentally important signaling pathways (for review, see []) and most recently, to include multiple roles within the nucleus, contributing to overall genomic stability [,,,], with recent evidence also suggesting transcription-related roles for the Drosophila homolog Asp []. Human ASPM mutations are the most common cause of primary microcephaly, a disorder resulting in small brains [,,], while ASPM overexpression is associated with many cancers [,,,].
In this review, we overview the multiple nuclear roles of ASPM including in DNA replication stress, homologous recombination, and transcription initiation. Next, we highlight the liquid–liquid phase separation (LLPS)-initiating potential for the protein product of ASPM and propose LLPS as a unifying hypothesis underlying ASPM protein functions, including DNA-associated functions. Improved understanding of ASPM function is of great interest as overexpression has been robustly associated with cancer progression, making it a promising drug target for novel therapies [].
2. The Multiple DNA-Associated Roles of ASPM
ASPM has been implicated in multiple nuclear roles, including homologous recombination after DNA double strand break, coping with DNA replication stress, and the regulation of translation initiation (Figure 1). Early work in 2011 showed that ASPM knockdown impaired DNA double-strand break (DSB) repair, sensitizing cells to ionizing radiation []. In a 2021 study, it was found that ASPM is recruited to DNA DSB lesions, where it interacts with Breast Cancer Type 1 Susceptibility Protein (BRCA1) and its E3 Ubiquitin ligase HERC2 []. In this interaction, ASPM prevents HERC2 from accessing BRCA1, safeguarding BRCA1 stability. BRCA1 is an essential mediator of homologous recombination (HR), one of two main mechanisms of DNA DSB repair. Therefore, this study showed that ASPM promotes DNA DSB repair via HR, which likely contributes to its role as an oncogene [].
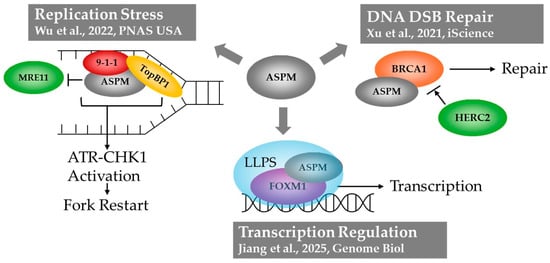
Figure 1.
Graphic representing three DNA-associated roles of ASPM: (1) coping with replication stress through loading of 9-1-1 complex components and TopBP1 on stalled replication forks, enabling ATR-CHK1 activation []. (2) Protection of BRCA1 enabling DNA DSB repair via homologous recombination []. (3) Transcription regulation alongside the transcription factor FOXM1 through condensate formation via liquid–liquid phase separation [].
In 2022, ASPM was also shown to mediate the response to DNA replication stress []. When replication forks become stalled due to stressors, such as the presence of DNA secondary structures or lesions, single-stranded DNA (ssDNA) becomes exposed []. Replication protein A (RPA) coats this ssDNA and recruits RAD17, the RAD9, RAD1, HUS1 (9-1-1) complex, and TopBP1 to the stalled replication fork. Their recruitment leads to the activation of Ataxia-telangiectasia and Rad3-related (ATR) kinase, which subsequently activates Checkpoint Kinase 1 (CHK1): this ATR-CHK1-signaling axis ultimately leads to replication fork restart []. While ASPM is dispensable for DNA replication in the absence of stress, when replication forks are stalled, ASPM is recruited in a RAD17-dependent manner to promote the loading of RAD9 and TopBP1 onto chromatin, and facilitate ATR-CHK1 pathway activation []. ASPM was also found to protect nascent DNA strands at stalled replication forks from degradation by the nuclease MRE11 [].
Most recently, a role for ASPM in transcription has been identified. ASPM interacts with transcription factor Forkhead box protein 1 (FOXM1; []), a transcription factor important for regulating the transcription of genes involved in mitosis, DNA damage repair, and tissue regeneration []. FOXM1 has been recognized as an oncofetal protein, as it is important during development but also plays a role in tumor initiation and progression []. The physical interaction between ASPM and FOXM1 was found to trap FOXM1 in the nucleus, preventing its proteasome-dependent degradation and promoting its transcriptional activity. The mechanism of nuclear entrapment involved the formation of condensates via Liquid–Liquid Phase separation (LLPS; []), a phenomenon in which biomolecules form droplets separate from the bulk fluid (further discussed in Section 2.1). This finding suggests the possibility that other roles of ASPM may similarly involve LLPS. This is an attractive hypothesis as it may provide a unified underlying mechanism for the many, seemingly disparate functions of ASPM. In the below sections, we explore two lines of reasoning which suggest LLPS as a unifying mechanism of ASPM function: the protein structure itself, and the subcellular locations in which it functions.
2.1. Liquid–Liquid Phase Separation as an Organizer of Cellular Functions
The transcription, replication, and DNA damage repair events described above all need to occur within the interphase nucleus at precise regions and precise times, but the molecular mechanisms allowing for timely recruitment and localized function of the required molecules remain incompletely understood. Because both recruitment and retention of the required multiprotein assemblies are unlikely to be driven in an effective timescale by diffusion alone, the phenomenon of LLPS and protein condensate formation have recently become research focal points as expediting mechanisms regulating transcription [] and DNA damage-repair [,]. LLPS can result in the formation of biomolecular condensates with liquid-like properties, which concentrate molecules into distinct regions and have been implicated in a wide variety of cellular processes (reviewed in []). Biomolecular condensates have alternatively been described as “membraneless organelles”, due to their influence on local reaction kinetics of cellular processes by concentrating or sequestering the relevant components []. The formation of a phase separated condensate is driven by multiple interactions between multivalent biomolecules: for example, interactions between short linear motifs (SLiMs), interactions between folded and disordered domains, and electrostatic interactions can all drive phase separation. The threshold for this occurring can be modified by post-translational modifications, biomolecule availability or oligomerization creating de novo macromolecules able to drive condensate formation although the individual components could not (for review see [,,]). A model describing these multivalent interactions is “stickers and spacers” [], where multiple smaller regions allowing various types of inter-molecular interactions (stickers) are interspersed with non-contributing regions (spacers). Intrinsic stickers may be determined by amino acid sequence, whereas hierarchical macromolecular assembly can create new, context-dependent “emergent stickers” []. The molecular crowding within the condensate region can create a porous structure that allows only certain molecules to move freely within, producing a restricted environment that can concentrate permitted molecules while still allowing local exchange with the surroundings ([]; Figure 2).
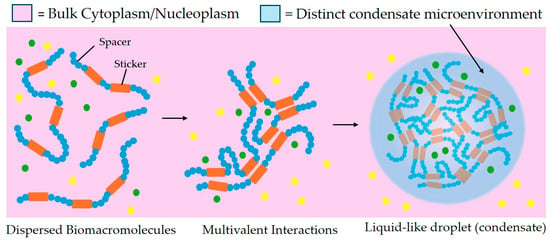
Figure 2.
Schematic of liquid–liquid phase separation under the spacers and stickers model. Stickers (shown in orange) are defined as regions of the macromolecule that participate in attractive interactions. Stickers may be folded domains such as binding sites, or they may be single amino acids or short linear motifs found in disordered protein regions. Spacers (shown in blue) are regions interspersed between stickers that do not significantly drive attractive interactions. Multivalent interactions between stickers drive a phase transition forming a biomolecule dense, liquid-like, phase-separated biomolecular condensate distinct from the surrounding dilute phase. Green and yellow dots indicate factors (such as other proteins, DNA or RNA molecules, other small molecules, or ions) which may be included or excluded from the condensate, creating a distinct microenvironment.
2.2. ASPM Has Characteristics of a Phase-Separating Protein
The ASPM gene codes for two predominant protein isoforms: ASPM-i1 is the full-length protein, with 3477 amino acids comprising 28 exons, and the shorter ASPM-i2 which is 1892 amino acids in length. The larger ASPM-i1 is most abundant, although the two isoforms have not been reported to show tissue specificity, with both being expressed in a variety of proliferating tissues []. However, intracellularly, the larger ASPM-i1 has been reported to localize predominantly to the cytoplasm, while the smaller ASPM-i2 localizes to the nucleus []. Both ASPM proteins contain multiple functional domains (Figure 3). The N-terminal region contains a Hydin domain, also called an ASH (ASPM, SPD-2, Hydin) domain, which is present in ciliary, centrosomal, and Golgi-associated proteins and is predicted to have a microtubule-binding function []. Both isoforms also contain two calponin-homology (CH) domains, which are involved in the binding of actin and tubulin in some proteins []. The CH domains of ASPM have been shown to bind microtubules, but not actin []. Much of the middle of the sequence consists of 81 repeated isoleucine-glutamine (IQ) motifs, which have been shown to bind the calcium-sensing protein calmodulin (CaM; [,]). The shorter ASPM-i2 excludes exon 18, which encodes much of the IQ array []. The C-terminal region of both isoforms contains a predicted HEAT motif, which consists of a repetitive array of alpha-helices [], which may contribute to protein flexibility and function in crowded environments [].
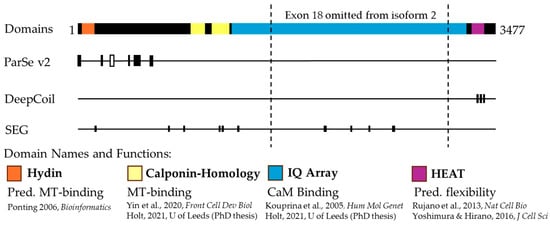
Figure 3.
Schematic representation of ASPM protein features. ASPM isoform 1 is represented, highlighting the Exon 18 region omitted from isoform 2. From left to right, the annotated protein domains are as follows: hydin (orange), calponin-homology (CH, yellow), isoleucine-glutamine (IQ) array (blue), and HEAT (purple). Functions and predicted functions (Pred.) are listed with the supporting literature [,,,,,]. ParSe v2, DeepCoil, and SEG predict phase separation-prone characteristics in the ASPM protein. ParSe V2 predicts multiple IDRs (black) and one phase separation-prone IDR (white) in the N-terminal region of ASPM. DeepCoil predicts three coiled-coil regions (black) in the C-terminal region, aligning with the annotated HEAT motif. SEG predicts multiple short low-complexity regions throughout the ASPM protein.
The ASPM protein contains characteristics suggesting phase separation. As described above, LLPS is driven by multivalency, which is the ability to form multiple interactions with other proteins or biomolecules [,]. Protein characteristics that enable multivalent interactions include intrinsically disordered regions (IDRs), low-complexity regions (LCRs), and the possession of certain secondary structures. IDRs are regions of proteins that lack a stable three-dimensional structure. IDRs are highly varied, and may be enriched with basic, acidic, or polar uncharged amino acids []. IDRs facilitate multivalent interactions between amino acids such as cation–anion, cation–π, π–stacking, hydrophobic contacts, H–bond, and dipole–dipole interactions [,] and are considered primary drivers of LLPS []. Much of the ASPM N-terminal region is predicted to be intrinsically disordered by ParSe v2 [], a predictive software which identifies protein regions predicted to be either folded, IDRs, or phase separation-prone IDRs. Of particular interest, the region from residue 278 to 301 was predicted as a phase separation-prone IDR, which aligns with experimental data showing that the N-terminal region of ASPM (residues 1-443) was necessary for LLPS upon interaction with FOXM1 []. These N-term IDRs vary in composition, with some areas enriched with acidic and some with basic amino acids. This N-term IDR is also enriched with phosphorylation sites [], which is of note given the role phosphorylation and other post-translational modifications play in the regulation of LLPS ([,,], discussed further in Section 3).
Analysis of IDRs often identifies low-complexity regions (LCRs) with short sequence repeats, which may also contribute to LLPS []. The SEG algorithm, which predicts low-complexity protein regions [], detected short LCRs throughout the ASPM protein sequence. Additionally, folded protein regions may contribute to multivalent interactions and therefore LLPS. Of interest here are coiled-coil (CC) domains, which consist of multiple alpha-helices organized into a super-helical structure. Experimental evidence and simulations have indicated that CC domains are sufficient to drive LLPS through multivalent interactions between CCs [,]. DeepCoil, a neural network-based tool for predicting coiled-coil domains [], predicts three regions of CCs within the HEAT domain of ASPM.
PhaSePred, a machine-learning model that integrates multiple protein features, predicts whether a given protein falls into two categories: proteins which can self-assemble into condensates spontaneously (termed PS-Self proteins), and those which interact with partners to undergo phase separation (PS-Part proteins; []). The model outputs PS-Self and PS-Part scores between 0 and 1, with values close to 1 indicating a high predicted likelihood that a protein is a PS-self or PS-part protein. For ASPM-i1, the PhaSePred PS-Part score was higher than the PS-Self score (0.955 and 0.887, respectively). This aligns with the findings of Jiang et al., 2025, who concluded that neither FOXM1 nor ASPM could undergo LLPS independently, but rather that the interaction between the two drives condensate formation []. It is possible that the interaction of ASPM with other IDR-containing proteins may enable LLPS in other contexts.
2.3. ASPM Functions in Areas of Condensate Formation
Many processes in which ASPM functions have also been reported as sites of condensate formation (Figure 4). As discussed above, ASPM has recently been implicated in both DNA double strand-break (DSB) repair and the stabilization of stalled replication forks. Multiple DNA repair factors have been demonstrated to form phase-separated condensates at DNA repair centers, including the p53 binding protein 53BP1 [] and the DNA/RNA-binding protein Fused in Sarcoma (FUS; []). Some of these proteins, including FUS, are recruited to sites of DNA damage by PARylation: the building of Poly(ADP-ribose) or PAR chains in response to single or double-strand DNA breaks [,]. This recruitment has recently been demonstrated to involve LLPS, with PAR “seeding” the phase-separation of IDR-containing proteins at DNA damage sites []. Intriguingly, ASPM has been shown to be recruited to DSBs in a Poly(ADP-ribose) polymerase 2 (PARP2)-dependent manner, although the molecular mechanism of this recruitment remains unknown []. PARP2 enables the construction of branched PAR chains []. This finding, considered together with the fact that both the IDR-containing N-term of ASPM, as well as the CC-containing C-term of ASPM were sufficient for recruitment to DSB, while the structured middle of the protein was not [], suggests that, like other IDR-containing proteins, ASPM recruitment to DSBs may be mediated by LLPS induced by PAR. As discussed above, ASPM has furthermore been shown to localize to stalled replication forks and promote their stabilization in cooperation with TOPBP1 [], which is also a condensate-forming protein []. It will be of interest to determine whether the IDR-containing N- or CC-containing C-term similarly impacts ASPM influence on this.
Outside the nucleus, ASPM is most well known as a mitotic spindle protein, localizing to the minus ends of microtubules at the spindle poles, as well as the central spindle during cytokinesis []. The organization of mitotic spindle poles has previously been proposed to involve phase separation [,]. Indeed, multiple microtubule-binding spindle proteins have been shown to undergo phase separation (reviewed in []). Intriguingly, many of these proteins contain coiled-coil domains, and it has been suggested that CC-mediated phase separation may be particularly important for the formation of mitotic spindle condensates [,]. Considering both the localization of ASPM to the spindle poles and its CC-containing HEAT domain, it would be interesting to determine whether ASPM undergoes LLPS in its function as a spindle-pole organizing protein. If so, the ability to promote LLPS could be a unifying mechanism of ASPM function, with its various roles dictated by interactor availability (e.g., access to microtubules vs. DNA as dictated by nuclear envelope breakdown) or cell cycle-dependent regulation [].
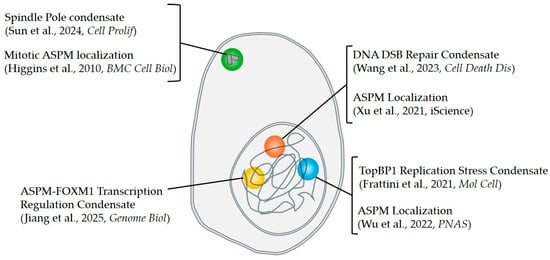
Figure 4.
ASPM localizes to areas known to harbor biomolecular condensates. A schematic depicting an overlap between known nuclear and centrosomal condensates with functions of ASPM from the literature. ASPM localizes to sites of DNA double-strand break repair [], where other repair factors have been shown to form liquid-like condensates (orange) []. ASPM localizes to stalled replication forks and facilitates the loading of TopBP1 [], which has been shown to form replication stress condensates on chromatin (blue) []. ASPM and FOXM1 interact to form liquid-like condensates which occupy promotor regions, regulating gene transcription (yellow) []. Mitotic localization of ASPM [] coincides with liquid-like condensates at the spindle poles (green) [].
3. Future Directions: Understanding ASPM Dysfunction in Disease by Considering LLPS
As the contribution of LLPS to the molecular organization required for many cellular events during development and throughout adulthood is increasingly recognized, it follows that abnormal phase separation (impaired or aberrant condensate formation, location, timing, size or dissolution) can contribute to developmental disorder due to the disruption of any these processes (for review, see []). Dysregulated LLPS has also been implicated in driving cancer (for review, see [,]). As described above, ASPM mis-expression is the leading genetic cause of the developmental disorder microcephaly [,,], while ASPM overexpression is associated with cancer in many tissue types [,,,]. The DNA-associated roles of ASPM reviewed above must be considered alongside mitotic roles to gain a comprehensive understanding of the molecular events underlying disease pathology. Future work will likely benefit from experimental approaches informed by a greater understanding of the biophysical properties of LLPS, which will also become important when considering therapeutic drug delivery as condensates can influence drug metabolism or localization, and therefore efficacy [].
The consideration of LLPS as a contributing factor to molecular events including DNA damage repair, transcription and replication will be challenging because the local environment within the cell can change the interaction properties of the contributing biomolecules. In the stickers-and-spaces LLPS model ([], described above), “emergent stickers” are created by specific molecular contexts such as interactor concentration, macromolecule formation, or post-translational/post-transcriptional modifications that alter protein/RNA binding affinity or solubility. For example, we previously described ASPM as a prime candidate for multi-site cyclin-dependent kinase (CDK1)-mediated phosphorylation [], which itself is a characteristic of highly disordered proteins that switch between favorable vs. inhibitory modes for protein condensate formation relevant to cell cycle progression []. Rather than considering single amino acid phosphorylation or motifs, cell cycle-specific LLPS can be influenced by “charge blockiness” of a large disordered region [], increasing the variety of potential events that could lead to the same outcome. Similarly, the local environment status of free ions such as calcium can influence LLPS in a variety of contexts (for review, see []). ASPM isoforms contain multiple repeat IQ motifs and may accumulate up to several hundred calcium ions through interactions with calmodulin (CaM; [,,,]). Such modifications warrant consideration as calcium and CaM are abundant in the interphase nucleus [] and have long been implicated in cell cycle progression events such as DNA replication [].
The above are only two examples amongst many that could influence LLPS and experimental or therapeutic strategy outcome. Predictive software has made rapid advances as a powerful tool in developing hypotheses relevant to molecular interactions (for example, []), with new developments aimed at predicting the molecules driving LLPS as dictated by the molecular sequence-predicted structure (for example, []). Continued work will improve current limitations due to the inherent disorder of contributing IDRs, which typically render a mix of high- and low-confidence areas modeled with overall low certainty. Continued work is further required to account for the nuances of the cytoplasmic or nuclear local environment context, which in turn will require experimentally derived information specific to IDRs and relevant to LLPS. Due to its multiple mitotic, signaling, and DNA-associated roles, oncogenic ASPM represents an attractive target for the development of novel cancer therapies. Therefore, the improved understanding of the molecular mechanisms of ASPM function, which may include phase separation, is of interest in future research.
Author Contributions
L.B. and G.F.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, writing (original draft) and writing (review and editing). L.B.: project administration, resources and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by lab funding from the Canada Foundation for Innovation (CFI JELF #40709 to L.B.), Research Nova Scotia (2021-1871 to L.B. and 2024-2984 to G.F.), the Natural Sciences and Engineering Research Council of Canada (RGPIN-2025-05831 and DGECR-2025-00086 to L.B.), the Royal Society (AL\231042 and NF151014 to L.B.) and MSVU (Internal Project Grant to L.B. and Jeanne Sauvé Endowed Award for Women in Science Policy and Procedures to G.F.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This manuscript does not report data.
Acknowledgments
The authors thank members of the MSVU Cell Proliferation lab for constructive discussion, as well as colleagues at the Nova Scotia Insect Research Group and the University of Exeter.
Conflicts of Interest
The authors declare there are no competing interests.
References
- Ripoll, P.; Pimpinelli, S.; Valdivia, M.M.; Avila, J. A Cell Division Mutant of Drosophila with a Functionally Abnormal Spindle. Cell 1985, 41, 907–912. [Google Scholar] [CrossRef]
- Gai, M.; Bianchi, F.T.; Vagnoni, C.; Vernì, F.; Bonaccorsi, S.; Pasquero, S.; Berto, G.E.; Sgrò, F.; Chiotto, A.M.; Annaratone, L.; et al. ASPM and CITK Regulate Spindle Orientation by Affecting the Dynamics of Astral Microtubules. EMBO Rep. 2016, 17, 1396–1409. [Google Scholar] [CrossRef]
- Higgins, J.; Midgley, C.; Bergh, A.-M.; Bell, S.M.; Askham, J.M.; Roberts, E.; Binns, R.K.; Sharif, S.M.; Bennett, C.; Glover, D.M.; et al. Human ASPM Participates in Spindle Organisation, Spindle Orientation and Cytokinesis. BMC Cell Biol. 2010, 11, 85. [Google Scholar] [CrossRef]
- Jiang, K.; Rezabkova, L.; Hua, S.; Liu, Q.; Capitani, G.; Altelaar, A.F.M.; Heck, A.J.R.; Kammerer, R.A.; Steinmetz, M.O.; Akhmanova, A. Microtubule Minus-End Regulation at Spindle Poles by an ASPM-Katanin Complex. Nat. Cell Biol. 2017, 19, 480–492. [Google Scholar] [CrossRef]
- Wakefield, J.G.; Bonaccorsi, S.; Gatti, M. The Drosophila Protein Asp Is Involved in Microtubule Organization during Spindle Formation and Cytokinesis. J. Cell Biol. 2001, 153, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Schoborg, T.; Zajac, A.L.; Fagerstrom, C.J.; Guillen, R.X.; Rusan, N.M. An Asp-CaM Complex Is Required for Centrosome-Pole Cohesion and Centrosome Inheritance in Neural Stem Cells. J. Cell Biol. 2015, 211, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Saunders, R.D.C.; Avides, M.d.C.; Howard, T.; Gonzalez, C.; Glover, D.M. The Drosophila Gene Abnormal Spindle Encodes a Novel Microtubule-Associated Protein That Associates with the Polar Regions of the Mitotic Spindle. J. Cell Biol. 1997, 137, 881–890. [Google Scholar] [CrossRef]
- Ito, A.; Goshima, G. Microcephaly Protein Asp Focuses the Minus Ends of Spindle Microtubules at the Pole and within the Spindle. J. Cell Biol. 2015, 211, 999–1009. [Google Scholar] [CrossRef]
- Razuvaeva, A.V.; Graziadio, L.; Palumbo, V.; Pavlova, G.A.; Popova, J.V.; Pindyurin, A.V.; Bonaccorsi, S.; Somma, M.P.; Gatti, M. The Multiple Mitotic Roles of the ASPM Orthologous Proteins: Insight into the Etiology of ASPM-Dependent Microcephaly. Cells 2023, 12, 922. [Google Scholar] [CrossRef]
- Tsai, K.K.; Bae, B.-I.; Hsu, C.-C.; Cheng, L.-H.; Shaked, Y. Oncogenic ASPM Is a Regulatory Hub of Developmental and Stemness Signaling in Cancers. Cancer Res. 2023, 83, 2993–3000. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.A.; Okayasu, R.; Jeggo, P.A.; Fujimori, A. ASPM Influences DNA Double-Strand Break Repair and Represents a Potential Target for Radiotherapy. Int. J. Radiat. Biol. 2011, 87, 1189–1195. [Google Scholar] [CrossRef]
- Xu, S.; Wu, X.; Wang, P.; Cao, S.-L.; Peng, B.; Xu, X. ASPM Promotes Homologous Recombination-Mediated DNA Repair by Safeguarding BRCA1 Stability. iScience 2021, 24, 102534. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, S.; Wang, P.; Wang, Z.-Q.; Chen, H.; Xu, X.; Peng, B. ASPM Promotes ATR-CHK1 Activation and Stabilizes Stalled Replication Forks in Response to Replication Stress. Proc. Natl. Acad. Sci. USA 2022, 119, e2203783119. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, J.; Wang, K.; Sun, J.; Yin, H.; Jiang, Y.; Liu, Y.; Wang, N.; Ding, X.; Gao, P.; et al. ASPM Mediates Nuclear Entrapment of FOXM1 via Liquid-Liquid Phase Separation to Promote Progression of Hepatocarcinoma. Genome Biol. 2025, 26, 68. [Google Scholar] [CrossRef]
- Mannino, M.C.; Cassidy, M.B.; Florez, S.; Rusan, Z.; Chakraborty, S.; Schoborg, T. Mutations in Abnormal Spindle Disrupt Temporal Transcription Factor Expression and Trigger Immune Responses in the Drosophila Brain. Genetics 2023, 225, iyad188. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Z.; Wang, Z.-Q.; Xu, X. The Neurological and Non-Neurological Roles of the Primary Microcephaly-Associated Protein ASPM. Front. Neurosci. 2023, 17, 1242448. [Google Scholar] [CrossRef] [PubMed]
- Létard, P.; Drunat, S.; Vial, Y.; Duerinckx, S.; Ernault, A.; Amram, D.; Arpin, S.; Bertoli, M.; Busa, T.; Ceulemans, B.; et al. Autosomal Recessive Primary Microcephaly Due to ASPM Mutations: An Update. Hum. Mutat. 2018, 39, 319–332. [Google Scholar] [CrossRef]
- Muhammad, F.; Mahmood Baig, S.; Hansen, L.; Sajid Hussain, M.; Anjum Inayat, I.; Aslam, M.; Anver Qureshi, J.; Toilat, M.; Kirst, E.; Wajid, M.; et al. Compound Heterozygous ASPM Mutations in Pakistani MCPH Families. Am. J. Med. Genet. Part A 2009, 149A, 926–930. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Q.; Luh, F.; Jin, B.; Liu, X. Overexpression of the ASPM Gene Is Associated with Aggressiveness and Poor Outcome in Bladder Cancer. Oncol. Lett. 2019, 17, 1865–1876. [Google Scholar] [CrossRef]
- Lin, P.; Liang, L.; Dong, Y.; Ren, Z.; Zhao, H.; Li, G. Identification of Abnormal Spindle Microtubule Assembly as a Promising Therapeutic Target for Osteosarcoma. Orthop. Surg. 2020, 12, 1963–1970. [Google Scholar] [CrossRef]
- Ibrahim, A.; Atallah, N.M.; Makhlouf, S.; Toss, M.S.; Green, A.; Rakha, E. Deciphering the Role of ASPM in Breast Cancer: A Comprehensive Multicohort Study. Cancers 2024, 16, 3814. [Google Scholar] [CrossRef]
- Cabral de Carvalho Corrêa, D.; Dias Oliveira, I.; Mascaro Cordeiro, B.; Silva, F.A.; de Seixas Alves, M.T.; Saba-Silva, N.; Capellano, A.M.; Dastoli, P.; Cavalheiro, S.; Caminada de Toledo, S.R. Abnormal Spindle-like Microcephaly-Associated (ASPM) Gene Expression in Posterior Fossa Brain Tumors of Childhood and Adolescence. Childs Nerv. Syst. 2021, 37, 137–145. [Google Scholar] [CrossRef]
- Zeman, M.K.; Cimprich, K.A. Causes and Consequences of Replication Stress. Nat. Cell Biol. 2014, 16, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Rundle, S.; Bradbury, A.; Drew, Y.; Curtin, N.J. Targeting the ATR-CHK1 Axis in Cancer Therapy. Cancers 2017, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Bella, L.; Zona, S.; Nestal de Moraes, G.; Lam, E.W.-F. FOXM1: A Key Oncofoetal Transcription Factor in Health and Disease. Semin. Cancer Biol. 2014, 29, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.; Park, G.; Cho, W.-K. Emerging Insights into Transcriptional Condensates. Exp. Mol. Med. 2024, 56, 820–826. [Google Scholar] [CrossRef]
- Altmeyer, M.; Neelsen, K.J.; Teloni, F.; Pozdnyakova, I.; Pellegrino, S.; Grøfte, M.; Rask, M.-B.D.; Streicher, W.; Jungmichel, S.; Nielsen, M.L.; et al. Liquid Demixing of Intrinsically Disordered Proteins Is Seeded by Poly(ADP-Ribose). Nat. Commun. 2015, 6, 8088. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Zhao, W.-W.; Shi, J.; Wan, X.-B.; Zheng, J.; Fan, X.-J. Liquid-Liquid Phase Separation in DNA Double-Strand Breaks Repair. Cell Death Dis. 2023, 14, 746. [Google Scholar] [CrossRef]
- Choi, J.-M.; Holehouse, A.S.; Pappu, R.V. Physical Principles Underlying the Complex Biology of Intracellular Phase Transitions. Annu. Rev. Biophys. 2020, 49, 107–133. [Google Scholar] [CrossRef]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular Condensates: Organizers of Cellular Biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef]
- Sipko, E.L.; Chappell, G.F.; Berlow, R.B. Multivalency Emerges as a Common Feature of Intrinsically Disordered Protein Interactions. Curr. Opin. Struct. Biol. 2024, 84, 102742. [Google Scholar] [CrossRef]
- Kouprina, N.; Pavlicek, A.; Collins, N.K.; Nakano, M.; Noskov, V.N.; Ohzeki, J.-I.; Mochida, G.H.; Risinger, J.I.; Goldsmith, P.; Gunsior, M.; et al. The Microcephaly ASPM Gene Is Expressed in Proliferating Tissues and Encodes for a Mitotic Spindle Protein. Hum. Mol. Genet. 2005, 14, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Liao, W.; Chan, T.; Chen, W.; Lee, C.; Shan, Y.; Huang, P.; Hou, Y.; Li, C.; Tsai, K.K. The Differential Distributions of ASPM Isoforms and Their Roles in Wnt Signaling, Cell Cycle Progression, and Pancreatic Cancer Prognosis. J. Pathol. 2019, 249, 498–508. [Google Scholar] [CrossRef]
- Ponting, C.P. A Novel Domain Suggests a Ciliary Function for ASPM, a Brain Size Determining Gene. Bioinformatics 2006, 22, 1031–1035. [Google Scholar] [CrossRef]
- Yin, L.-M.; Schnoor, M.; Jun, C.-D. Structural Characteristics, Binding Partners and Related Diseases of the Calponin Homology (CH) Domain. Front. Cell Dev. Biol. 2020, 8, 342. [Google Scholar] [CrossRef]
- Holt, M.J.E. The Role of the Autosomal Recessive Primary Microcephaly Protein ASPM: A Protein Involved in Mitosis. Ph.D. Thesis, University of Leeds, Leeds, UK, 2021. [Google Scholar]
- Rujano, M.A.; Sanchez-Pulido, L.; Pennetier, C.; le Dez, G.; Basto, R. The Microcephaly Protein Asp Regulates Neuroepithelium Morphogenesis by Controlling the Spatial Distribution of Myosin II. Nat. Cell Biol. 2013, 15, 1294–1306. [Google Scholar] [CrossRef]
- Yoshimura, S.H.; Hirano, T. HEAT Repeats—Versatile Arrays of Amphiphilic Helices Working in Crowded Environments? J. Cell Sci. 2016, 129, 3963–3970. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhang, Z.; Zong, Z.; Zhang, L.; Zhou, F. Emerging Implications of Phase Separation in Cancer. Adv. Sci. 2022, 9, 2202855. [Google Scholar] [CrossRef]
- Dignon, G.L.; Best, R.B.; Mittal, J. Biomolecular Phase Separation: From Molecular Driving Forces to Macroscopic Properties. Annu. Rev. Phys. Chem. 2020, 71, 53–75. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.Y.; Khaodeuanepheng, N.P.; Amarasekara, D.L.; Correia, J.J.; Lewis, K.A.; Fitzkee, N.C.; Hough, L.E.; Whitten, S.T. Intrinsically Disordered Regions That Drive Phase Separation Form a Robustly Distinct Protein Class. J. Biol. Chem. 2023, 299, 102801. [Google Scholar] [CrossRef]
- Burns, M.C.; Borgal, L. Asp/ASPM Phospho-Regulation throughout the Cell Cycle. Genome 2024, 68, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, M.; Ma, W.; Yang, B.; Lu, H.; Zhou, F.; Zhang, L. Post-Translational Modifications in Liquid-Liquid Phase Separation: A Comprehensive Review. Mol. Biomed. 2022, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Valverde, J.M.; Dubra, G.; Phillips, M.; Haider, A.; Elena-Real, C.; Fournet, A.; Alghoul, E.; Chahar, D.; Andrés-Sanchez, N.; Paloni, M.; et al. A Cyclin-Dependent Kinase-Mediated Phosphorylation Switch of Disordered Protein Condensation. Nat. Commun. 2023, 14, 6316. [Google Scholar] [CrossRef]
- Yamazaki, H.; Takagi, M.; Kosako, H.; Hirano, T.; Yoshimura, S.H. Cell Cycle-Specific Phase Separation Regulated by Protein Charge Blockiness. Nat. Cell Biol. 2022, 24, 625–632. [Google Scholar] [CrossRef]
- Martin, E.W.; Mittag, T. Relationship of Sequence and Phase Separation in Protein Low-Complexity Regions. Biochemistry 2018, 57, 2478–2487. [Google Scholar] [CrossRef]
- Wootton, J.C. Non-Globular Domains in Protein Sequences: Automated Segmentation Using Complexity Measures. Comput. Chem. 1994, 18, 269–285. [Google Scholar] [CrossRef]
- Ramirez, D.A.; Hough, L.E.; Shirts, M.R. Coiled-Coil Domains Are Sufficient to Drive Liquid-Liquid Phase Separation in Protein Models. Biophys. J. 2024, 123, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Ludwiczak, J.; Winski, A.; Szczepaniak, K.; Alva, V.; Dunin-Horkawicz, S. DeepCoil—A Fast and Accurate Prediction of Coiled-Coil Domains in Protein Sequences. Bioinformatics 2019, 35, 2790–2795. [Google Scholar] [CrossRef]
- Chen, Z.; Hou, C.; Wang, L.; Yu, C.; Chen, T.; Shen, B.; Hou, Y.; Li, P.; Li, T. Screening Membraneless Organelle Participants with Machine-Learning Models That Integrate Multimodal Features. Proc. Natl. Acad. Sci. USA 2022, 119, e2115369119. [Google Scholar] [CrossRef]
- Kilic, S.; Lezaja, A.; Gatti, M.; Bianco, E.; Michelena, J.; Imhof, R.; Altmeyer, M. Phase Separation of 53BP1 Determines Liquid-like Behavior of DNA Repair Compartments. EMBO J. 2019, 38, e101379. [Google Scholar] [CrossRef]
- Levone, B.R.; Lenzken, S.C.; Antonaci, M.; Maiser, A.; Rapp, A.; Conte, F.; Reber, S.; Mechtersheimer, J.; Ronchi, A.E.; Mühlemann, O.; et al. FUS-Dependent Liquid-Liquid Phase Separation Is Important for DNA Repair Initiation. J. Cell Biol. 2021, 220, e202008030. [Google Scholar] [CrossRef]
- Chen, Q.; Kassab, M.A.; Dantzer, F.; Yu, X. PARP2 Mediates Branched Poly ADP-Ribosylation in Response to DNA Damage. Nat. Commun. 2018, 9, 3233. [Google Scholar] [CrossRef]
- Singatulina, A.S.; Hamon, L.; Sukhanova, M.V.; Desforges, B.; Joshi, V.; Bouhss, A.; Lavrik, O.I.; Pastré, D. PARP-1 Activation Directs FUS to DNA Damage Sites to Form PARG-Reversible Compartments Enriched in Damaged DNA. Cell Rep. 2019, 27, 1809–1821.e5. [Google Scholar] [CrossRef]
- Frattini, C.; Promonet, A.; Alghoul, E.; Vidal-Eychenie, S.; Lamarque, M.; Blanchard, M.-P.; Urbach, S.; Basbous, J.; Constantinou, A. TopBP1 Assembles Nuclear Condensates to Switch on ATR Signaling. Mol. Cell 2021, 81, 1231–1245.e8. [Google Scholar] [CrossRef] [PubMed]
- Akhmanova, A.; Steinmetz, M.O. Microtubule Minus-End Regulation at a Glance. J. Cell Sci. 2019, 132, jcs227850. [Google Scholar] [CrossRef]
- Borgal, L.; Wakefield, J.G. Context-Dependent Spindle Pole Focusing. Essays Biochem. 2018, 62, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yang, Y.; Zhou, J.; Liu, P. Liquid–Liquid Phase Separation of Microtubule-Binding Proteins in the Regulation of Spindle Assembly. Cell Prolif. 2024, 57, e13649. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ho, D.B.T.; Mahe, K.; Mia, J.; Sepulveda, G.; Antkowiak, M.; Jiang, L.; Yamada, S.; Jao, L.-E. Condensation of Pericentrin Proteins in Human Cells Illuminates Phase Separation in Centrosome Assembly. J. Cell Sci. 2021, 134, jcs258897. [Google Scholar] [CrossRef]
- Parra, A.S.; Johnston, C.A. Phase Separation as a Driver of Stem Cell Organization and Function during Development. J. Dev. Biol. 2023, 11, 45. [Google Scholar] [CrossRef]
- Mehta, S.; Zhang, J. Liquid–Liquid Phase Separation Drives Cellular Function and Dysfunction in Cancer. Nat. Rev. Cancer 2022, 22, 239–252. [Google Scholar] [CrossRef]
- Boija, A.; Klein, I.A.; Young, R.A. Biomolecular Condensates and Cancer. Cancer Cell 2021, 39, 174–192. [Google Scholar] [CrossRef]
- Sołtys, K.; Tarczewska, A.; Bystranowska, D. Modulation of Biomolecular Phase Behavior by Metal Ions. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2023, 1870, 119567. [Google Scholar] [CrossRef] [PubMed]
- Bähler, M.; Rhoads, A. Calmodulin Signaling via the IQ Motif. FEBS Lett. 2002, 513, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.G.; Guimarães, E.S.; Andrade, L.M.; Menezes, G.B.; Fatima Leite, M. Decoding Calcium Signaling across the Nucleus. Physiology 2014, 29, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Takuwa, N.; Zhou, W.; Takuwa, Y. Calcium, Calmodulin and Cell Cycle Progression. Cell. Signal. 1995, 7, 93–104. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Monti, M.; Fiorentino, J.; Miltiadis-Vrachnos, D.; Bini, G.; Cotrufo, T.; Sanchez de Groot, N.; Armaos, A.; Tartaglia, G.G. catGRANULE 2.0: Accurate Predictions of Liquid-Liquid Phase Separating Proteins at Single Amino Acid Resolution. Genome Biol. 2025, 26, 33. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).