Zygosity Genotyping by Pyrosequencing of SNPs rs601338 and rs1047781 of the FUT2 Gene in Children Living in the Amazon Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Synthetic Controls of FUT2 Gene Fragments
2.3. Histo Blood Group Antigen Phenotyping
2.4. PCR Amplification of FUT2 Gene
2.5. Validation of the Pyrosequencing Method for SNPs rs601338 and rs1047781
2.6. Pyrosequencing for SNPs rs601338 and rs1047781 of Sixty-Eight Saliva Samples from Children of Different Indigenous Ethnicities
2.7. Statistical Analysis
2.8. Sanger Nucleotide Sequencing of the FUT2 Gene
3. Results
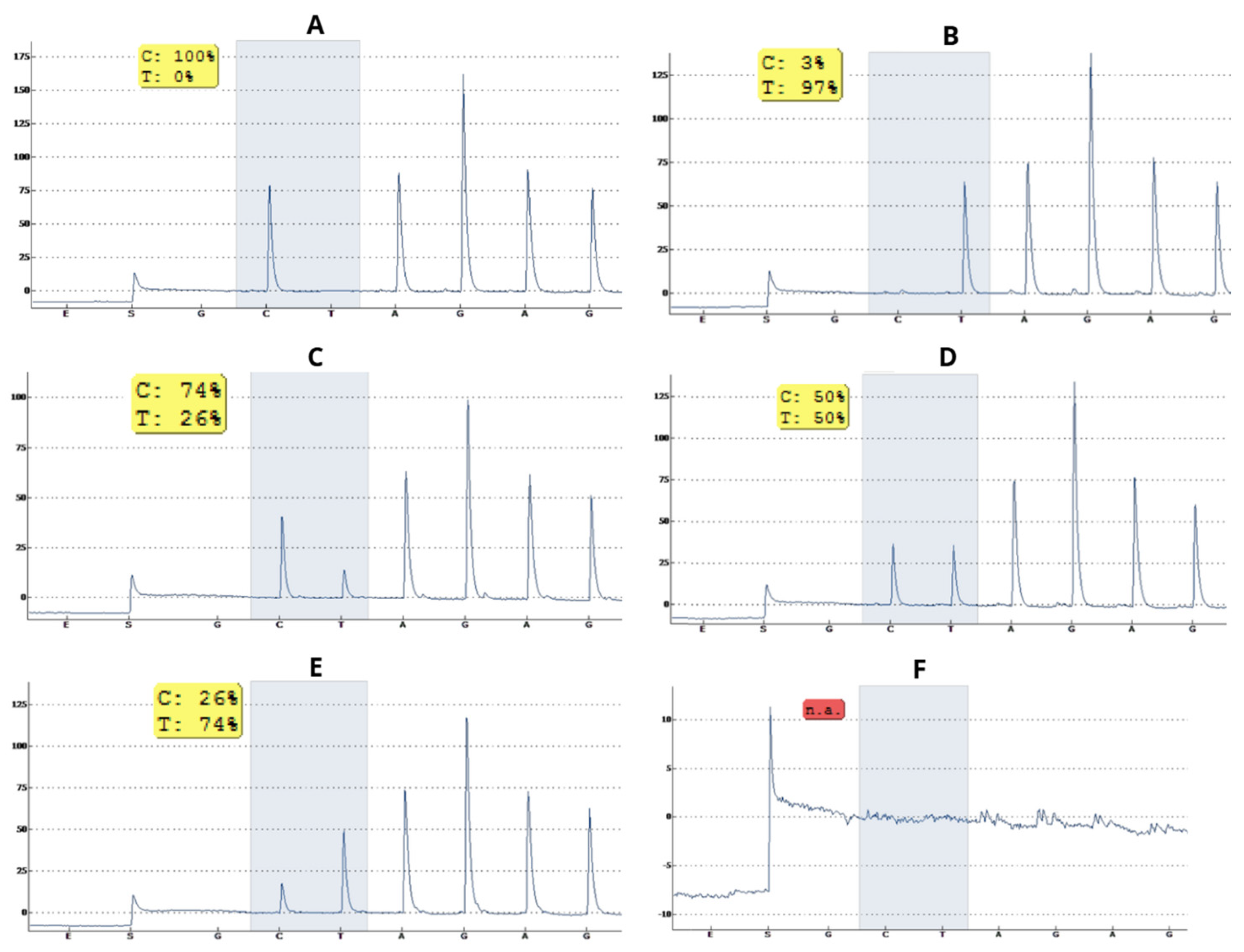
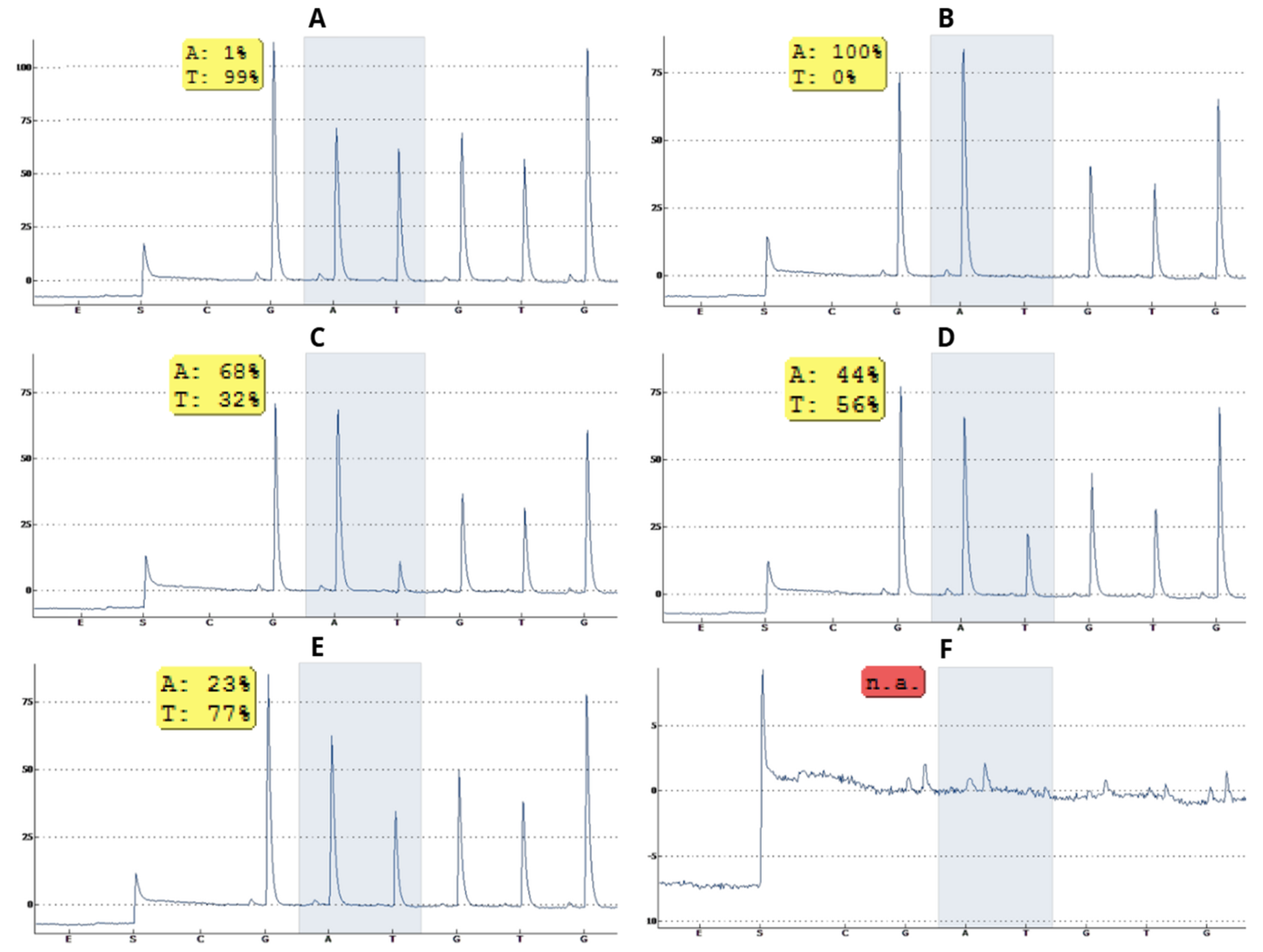
3.1. Specificity of Pyrosequencing for Detection of SNPs rs601338 and rs1047781
3.2. Zygosity Profile of SNPs rs601338 and rs1047781 of the FUT2 Gene of Children Living in the Amazon Region Is Predominantly Homozygous Secretor
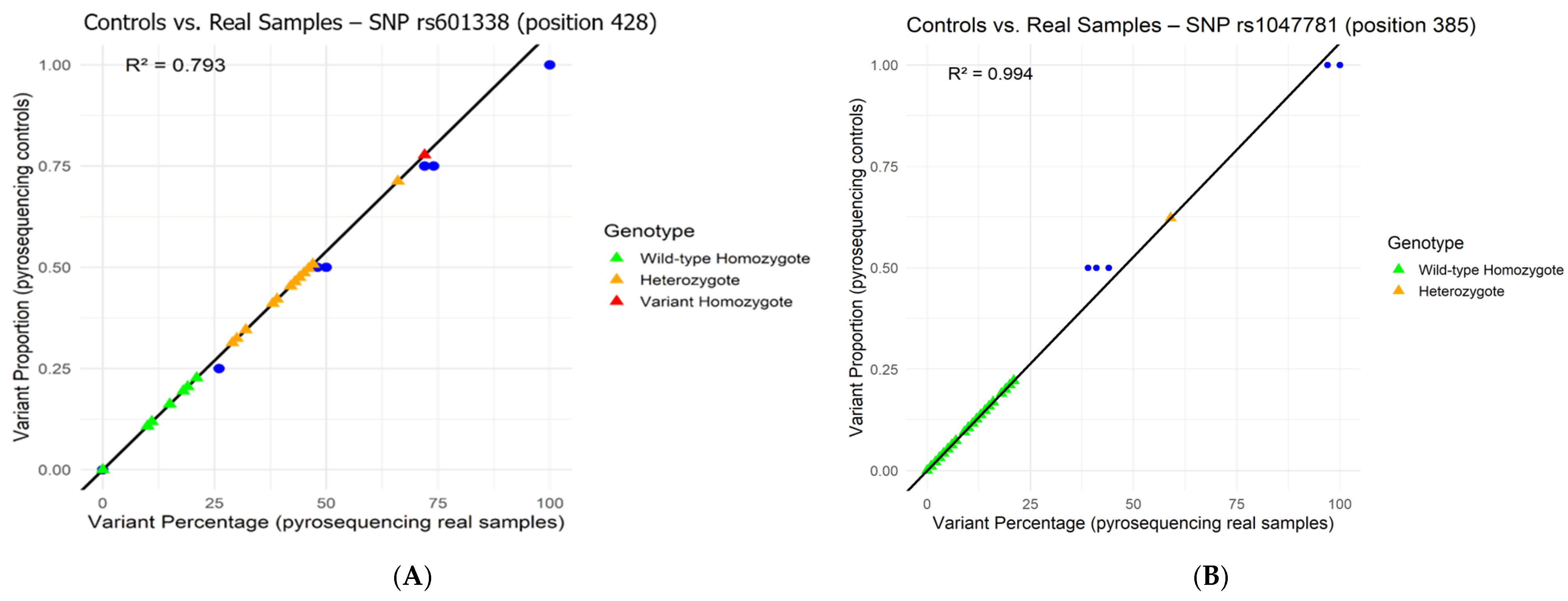
3.3. Correlation Between the Results Obtained by HBGA Phenotyping, Pyrosequencing, and Sanger Nucleotide Sequencing and Methods Applied to Samples in This Study
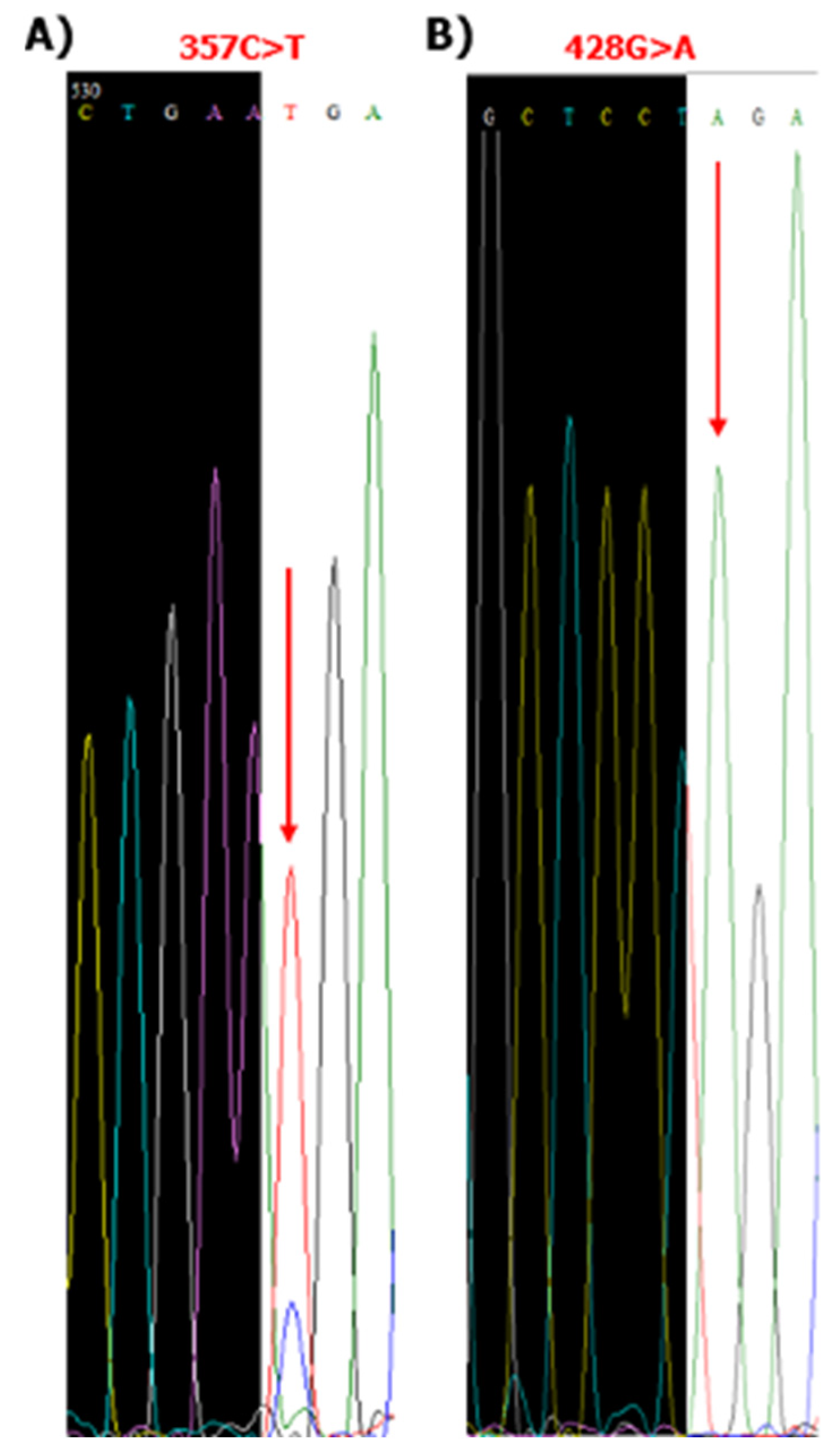
3.4. Sese428 Tightly Linked SNPs (171A>G, 216C>T, 357T>C, 428G>A>A, 739G, 960A>G) Were Detected in the Children Living in the Northwest of the Brazilian Amazon Region by Sanger Nucleotide Sequencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, R. Cancer cells and viruses share common glycoepitopes: Exciting opportunities toward combined treatments. Front. Immunol. 2024, 15, 1292588. [Google Scholar] [CrossRef]
- Cooling, L. Blood Groups in Infection and Host Susceptibility. Clin. Microbiol. Rev. 2015, 28, 801–870. [Google Scholar] [CrossRef]
- Nordgren, J.; Svensson, L. Genetic Susceptibility to Human Norovirus Infection: An Update. Viruses 2019, 11, 226. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Hagbom, M.; Svensson, L.; Nordgren, J. The Impact of Human Genetic Polymorphisms on Rotavirus Susceptibility, Epidemiology, and Vaccine Take. Viruses 2020, 12, 324. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Herrera, H.; Block, T. Glycosylation and liver cancer. Adv. Cancer Res. 2015, 126, 257–279. [Google Scholar] [PubMed]
- Maroni, L.; Hohenester, S.D.; van de Graaf, S.F.J.; Tolenaars, D.; van Lienden, K.; Verheij, J.; Marzioni, M.; Karlsen, T.H.; Oude Elferink, R.P.J.; Beuers, U. Knockout of the primary sclerosing cholangitis-risk gene Fut2 causes liver disease in mice. Hepatology 2017, 66, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Ernst, L.K.; Larsen, R.D.; Bryant, J.G.; Robinson, J.S.; Lowe, J.B. Molecular basis for H blood group deficiency in Bombay (Oh) and para-Bombay individuals. Proc. Natl. Acad. Sci. USA 1994, 91, 5843–5847. [Google Scholar] [CrossRef]
- Daniels, G. ABO, H, and Lewis systems. In Human Blood Groups; Wiley-Blackwell: West Sussex, UK, 2013; pp. 11–95. [Google Scholar]
- Ferrer-Admetlla, A.; Sikora, M.; Laayouni, H.; Esteve, A.; Roubinet, F.; Blancher, A.; Calafell, F.; Bertranpetit, J.; Casals, F. A natural history of FUT2 polymorphism in humans. Mol. Biol. Evol. 2009, 26, 1993–2003. [Google Scholar] [CrossRef]
- Kelly, R.J.; Rouquier, S.; Giorgi, D.; Lennon, G.G.; Lowe, J.B. Sequence and expression of a candidate for the human Secretor blood group alpha (1,2) fucosyltransferase gene (FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J. Biol. Chem. 1995, 270, 4640–4649. [Google Scholar] [CrossRef]
- Soejima, M.; Koda, Y. Molecular mechanisms of Lewis antigen expression. Leg. Med. 2005, 7, 266–269. [Google Scholar] [CrossRef]
- Soejima, M.; Fujimoto, R.; Agusa, T.; Iwata, H.; Fujihara, J.; Takeshita, H.; Minh, T.B.; Trang, P.T.; Viet, P.H.; Nakajima, T.; et al. Genetic variation of FUT2 in a Vietnamese population: Identification of two novel Se enzyme-inactivating mutations. Transfusion 2012, 52, 1268–1275. [Google Scholar] [CrossRef]
- Koda, Y.; Tachida, H.; Pang, H.; Liu, Y.; Soejima, M.; Ghaderi, A.A.; Takenaka, O.; Kimura, H. Contrasting patterns of polymorphisms at the ABO-secretor gene (FUT2) and plasma alpha (1,3) fucosyltransferase gene (FUT6) in human populations. Genetics 2001, 158, 747–756. [Google Scholar] [CrossRef]
- Henry, S.; Mollicone, R.; Fernandez, P.; Samuelsson, B.; Oriol, R.; Larson, G. Molecular basis for erythrocyte Le (a+ b+) and salivary ABH partial-secretor phenotypes: Expression of a FUT2 secretor allele with an A-->T mutation at nucleotide 385 correlates with reduced alpha (1,2) fucosyltransferase activity. Glycoconj. J. 1996, 13, 985–993. [Google Scholar] [CrossRef]
- Ruiz-Linares, A. How genes have illuminated the history of early Americans and Latino Americans. Cold Spring Harb. Perspect. Biol. 2014, 7, a008557. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cavalli-Sforza, L.L.; Menozzi, P.; Piazza, A. The History and Geography of Human Genes, Abridged Paperback ed.; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Chang, J.G.; Ko, Y.C.; Lee, J.C.; Chang, S.J.; Liu, T.C.; Shih, M.C.; Peng, C.T. Molecular analysis of mutations and polymorphisms of the Lewis secretor type alpha (1,2)-fucosyltransferase gene reveals that Taiwan aborigines are of Austronesian derivation. J. Hum. Genet. 2002, 47, 60–65. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moraes, M.T.B.; Olivares, A.I.O.; Fialho, A.M.; Malta, F.C.; da Silva, S.M.J.; Bispo, R.S.; Velloso, A.J.; Leitão, G.A.A.; Cantelli, C.P.; Nordgren, J.; et al. Phenotyping of Lewis and secretor HBGA from saliva and detection of new FUT2 gene SNPs from young children from the Amazon presenting acute gastroenteritis and respiratory infection. Infect. Genet. Evol. 2019, 70, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, C.J.; Huang, Y.H.; Pan, M.H.; Lee, M.H.; Yu, K.J.; Pfeiffer, R.M.; Viard, M.; Yuki, Y.; Gao, X.; et al. HLA Zygosity Increases Risk of Hepatitis B Virus-Associated Hepatocellular Carcinoma. J. Infect. Dis. 2021, 224, 1796–1805. [Google Scholar] [CrossRef]
- Soejima, M.; Koda, Y. FUT2 polymorphism in Latin American populations. Clin. Chim. Acta 2020, 505, 1–5. [Google Scholar] [CrossRef]
- Yang, B.; Wagner, J.; Yao, T.; Damaschke, N.; Jarrard, D.F. Pyrosequencing for the rapid and efficient quantification of allele-specific expression. Epigenetics 2013, 8, 1039–1042. [Google Scholar] [CrossRef][Green Version]
- Yang, B.; Damaschke, N.; Yao, T.; McCormick, J.; Wagner, J.; Jarrard, D. Pyrosequencing for accurate imprinted allele expression analysis. J. Cell Biochem. 2015, 116, 1165–1170. [Google Scholar] [CrossRef]
- Chen, C.T.; Liao, W.Y.; Hsu, C.C.; Hsueh, K.C.; Yang, S.F.; Teng, Y.H.; Yu, Y.L. FUT2 genetic variants as predictors of tumor development with hepatocellular carcinoma. Int. J. Med. Sci. 2017, 14, 885–890. [Google Scholar] [CrossRef]
- Olivares, A.I.O.; Leitão, G.A.A.; Pimenta, Y.C.; Cantelli, C.P.; Fumian, T.M.; Fialho, A.M.; da Silva, S.M.J.; Delgado, I.F.; Nordgren, J.; Svensson, L.; et al. Epidemiology of enteric virus infections in children living in the Amazon region. Int. J. Infect. Dis. 2021, 108, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.R.; Sampaio, R.M.A.; Nunes, P.F.; Guimarães, V.S.; Costa, C.C.D.S.; Coelho, E.D.C.; Souza, M.V.; Almeida, L.W.C.; Fuzii, H.T.; Filho, A.B.O.; et al. Immunological and Virological Responses in Patients with Monoinfection and Coinfection with Hepatitis B and C Viruses in the Brazilian Amazon. Trop. Med. Infect. Dis. 2025, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Moraes, M.T.B.; Leitão, G.A.A.; Olivares, A.I.O.; Xavier, M.D.P.T.P.; Bispo, R.S.; Sharma, S.; Leite, J.P.G.; Svensson, L.; Nordgren, J. Molecular Epidemiology of Sapovirus in Children Living in the Northwest Amazon Region. Pathogens 2021, 10, 965. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, Y.C.; Moreira, G.M.S.; da Silva Souza, W.; Olivares, A.I.O.; Svensson, L.; Leite, J.P.G.; Nordgren, J.; de Moraes, M.T.B. Epidemiological profiles of norovirus genotypes in children with and without acute gastroenteritis from the northwestern Amazon region. Infect. Genet. Evol. 2025, 134, 105804. [Google Scholar] [CrossRef]
- Barros, J.J.; Peres, L.R.; Sousa, P.S.; Mello, F.C.A.; Araujo, N.M.; Gomes, S.A.; Niel, C.; Lewis-Ximenez, L.L. Occult infection with HBV intergenotypic A2/G recombinant following acute hepatitis B caused by an HBV/A2 isolate. J. Clin. Virol. 2015, 67, 31–35. [Google Scholar] [CrossRef]
- RCore Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.r-project.org/ (accessed on 6 August 2025).
- RStudio Team. RStudio: Integrated Development Environment for R; Posit Software, PBC: Boston, MA, USA, 2024; Available online: https://posit.co/ (accessed on 6 August 2025).
- ISBT 018 H (FUT1FUT2) Blood Group Alleles v6.1 31-MAR-2023. Available online: https://www.isbtweb.org/asset/376793E9-EBB1-47D1-9A00E079476910FD/ (accessed on 6 August 2025).
- Fernandez-Mateos, P.; Cailleau, A.; Henry, S.; Costache, M.; Elmgren, A.; Svensson, L.; Larson, G.; Samuelsson, B.E.; Oriol, R.; Mollicone, R. Point mutations and deletion responsible for the Bombay H null and the Reunion H weak blood groups. Vox Sang. 1998, 75, 37–46. [Google Scholar] [CrossRef]
- Matzhold, E.M.; Helmberg, W.; Wagner, T.; Drexler, C.; Ulrich, S.; Winkler, A.; Lanzer, G. Identification of 14 new alleles at the fucosyltransferase 1, 2, and 3 loci in Styrian blood donors, Austria. Transfusion 2009, 49, 2097–2108. [Google Scholar] [CrossRef]
- Soejima, M.; Nakajima, T.; Fujihara, J.; Takeshita, H.; Koda, Y. Genetic variation of FUT2 in Ovambos, Turks, and Mongolians. Transfusion 2008, 48, 1423–1431. [Google Scholar] [CrossRef]
- Song, M.; Zhao, S.; Jiang, T.; Lu, H. A Very Rare Case with Particular H-deficient Phenotypes. Indian J. Hematol. Blood Transfus. 2018, 34, 788–791. [Google Scholar] [CrossRef]
- Scharberg, E.A.; Olsen, C.; Bugert, P. An update on the H blood group system. Immunohematology 2019, 35, 67–68. [Google Scholar] [CrossRef]
- Scharberg, E.A.; Olsen, C.; Bugert, P. The H blood group system. Immunohematology 2016, 32, 112–118. [Google Scholar] [CrossRef]
- Soejima, M.; Koda, Y. Genetic variation of FUT2 in a Peruvian population: Identification of a novel LTR-mediated deletion and characterization of 4 nonsynonymous single-nucleotide polymorphisms. Transfusion 2019, 59, 2415–2421. [Google Scholar] [CrossRef]
- Cantelli, C.P.; Velloso, A.J.; Assis, R.M.S.; Barros, J.J.; Mello, F.C.D.A.; Cunha, D.C.D.; Brasil, P.; Nordgren, J.; Svensson, L.; Miagostovich, M.P.; et al. Rotavirus A shedding and HBGA host genetic susceptibility in a birth community-cohort, Rio de Janeiro, Brazil, 2014–2018. Sci. Rep. 2020, 10, 6965. [Google Scholar] [CrossRef]
- Soejima, M.; Koda, Y. Survey and characterization of nonfunctional alleles of FUT2 in a database. Sci. Rep. 2021, 11, 3186. [Google Scholar] [CrossRef]
- Soejima, M.; Koda, Y. Distinct single nucleotide polymorphism pattern at the FUT2 promoter among human populations. Ann. Hematol. 2008, 87, 19–25. [Google Scholar] [CrossRef]

| Sample ID | Phenotyping | Pyrosequencing | Sanger Sequencing 1 |
|---|---|---|---|
| 26255 | Inconclusive | rs602662 Heterozygous | 171A>G, 216C>T, 357T>C, 428G>A, 739G>A, 960A>G |
| 27682 | Inconclusive | rs602662 Heterozygous | 171A>G, 216C>T, 357T>C, 428G>A, 739G>A, 960A>G |
| 27752 | Inconclusive | rs602662 Heterozygous | 171A>G, 216C>T, 357T>C, 428G>A, 739G>A, 960A>G |
| 27824 | Inconclusive | rs602662 Heterozygous | 171A>G, 216C>T, 428G>A, 739G>A, 960A>G |
| 27852 | Inconclusive | rs602662 Heterozygous | 171A>G, 216C>T, 428G>A, 739G>A, 960A>G |
| 28265 | Inconclusive | rs602662 Heterozygous | 171A>G, 216C>T, 428G>A, 739G>A, 960A>G |
| 32213 | Inconclusive | rs602662 Heterozygous | 171A>G, 216C>T, 357T>C, 428G>A, 739G>A, 960A>G |
| 32343 | Weak secretor; Lea+Leb+ | rs602662/rs1047781 Heterozygous | 171A>G, 216C>T, 357T>C, 385A>T, 428G>A, 739G>A, 960A>G |
| 32371 | Weak secretor; Lea+Leb+ | rs602662 Heterozygous | 171A>G, 216C>T, 357T>C, 428G>A, 739G>A, 863C>T, 960A>G |
| 32525 | Non-secretor; Lea+Leb- | rs602662 Homozygous | 171A>G, 216C>T, 357T>C, 428G>A, 739G>A, 960A>G |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.F.; Rodrigues, D.A.O.; Campos, L.B.; Pimenta, Y.C.; Oliveira, S.d.S.; Pedroso, B.L.d.A.; Ramalho, E.; Olivares, A.I.O.; Leite, J.P.G.; de Barros, J.J.F.; et al. Zygosity Genotyping by Pyrosequencing of SNPs rs601338 and rs1047781 of the FUT2 Gene in Children Living in the Amazon Region. DNA 2025, 5, 54. https://doi.org/10.3390/dna5040054
Silva MF, Rodrigues DAO, Campos LB, Pimenta YC, Oliveira SdS, Pedroso BLdA, Ramalho E, Olivares AIO, Leite JPG, de Barros JJF, et al. Zygosity Genotyping by Pyrosequencing of SNPs rs601338 and rs1047781 of the FUT2 Gene in Children Living in the Amazon Region. DNA. 2025; 5(4):54. https://doi.org/10.3390/dna5040054
Chicago/Turabian StyleSilva, Mauro França, Diego Archanjo Oliveira Rodrigues, Letícia Bomfim Campos, Yan Cardoso Pimenta, Silas de Souza Oliveira, Bruno Loreto de Aragão Pedroso, Emanuelle Ramalho, Alberto Ignacio Olivares Olivares, José Paulo Gagliardi Leite, José Júnior França de Barros, and et al. 2025. "Zygosity Genotyping by Pyrosequencing of SNPs rs601338 and rs1047781 of the FUT2 Gene in Children Living in the Amazon Region" DNA 5, no. 4: 54. https://doi.org/10.3390/dna5040054
APA StyleSilva, M. F., Rodrigues, D. A. O., Campos, L. B., Pimenta, Y. C., Oliveira, S. d. S., Pedroso, B. L. d. A., Ramalho, E., Olivares, A. I. O., Leite, J. P. G., de Barros, J. J. F., & de Moraes, M. T. B. (2025). Zygosity Genotyping by Pyrosequencing of SNPs rs601338 and rs1047781 of the FUT2 Gene in Children Living in the Amazon Region. DNA, 5(4), 54. https://doi.org/10.3390/dna5040054






