Antiproliferative Potential of Eugenia uniflora L. Leaf Essential Oil in Normal and Tumoral Human Colon Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Herbal Material
2.2. Essential Oil Extraction and Solutions
2.3. Essential Oil Analysis: Gas Chromatography Coupled with Mass Spectrometry (GC-MS)
2.4. Cell Culture
2.5. MTT Assay
2.6. Trypan Blue Viability Assay
2.7. Assessment of Cell Proliferation Kinetics
2.8. DNA Damage Assay
2.9. Reactive Oxygen Species Detection Assay
2.10. Statistical Analysis
3. Results
3.1. Chemical Characterization of E. uniflora EO
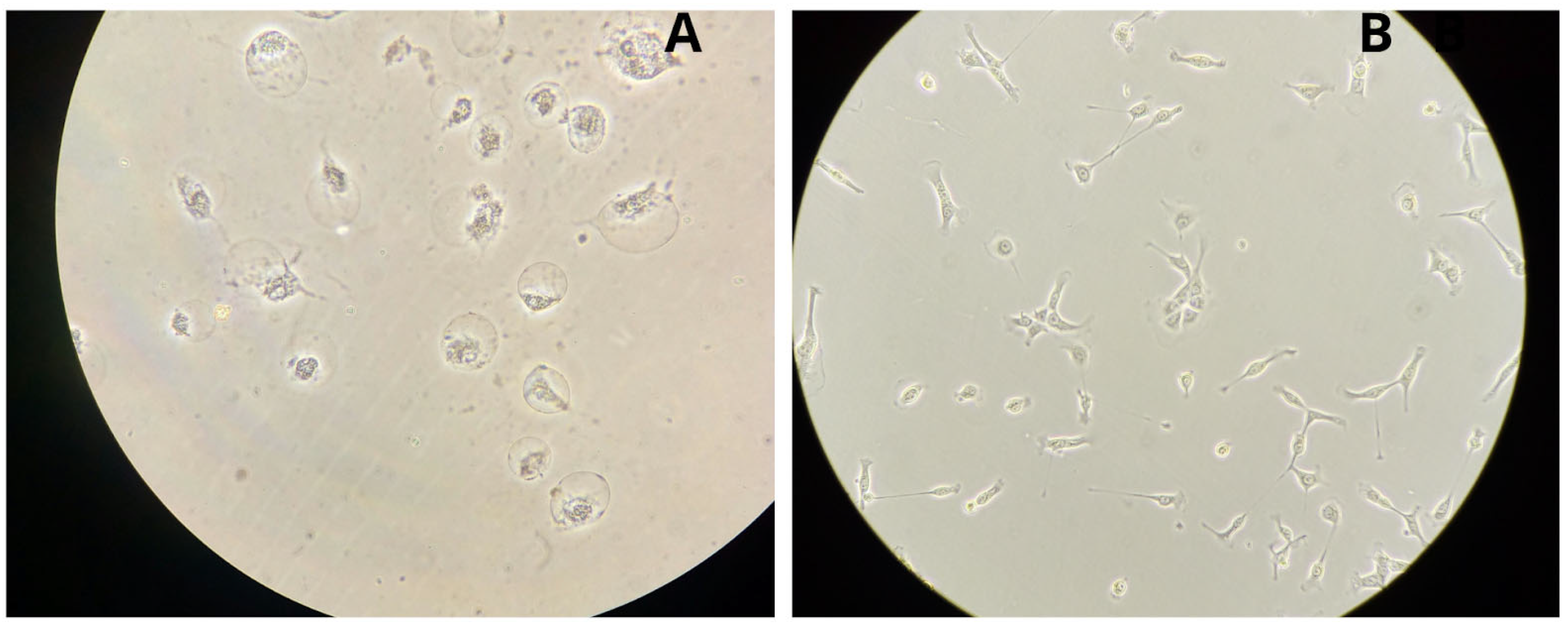
3.2. Microscopic Morphology of Colonic Cell Lines
3.3. Evaluation of the Cytotoxic Effect of E. uniflora EO on Cell Lines
3.4. Cell Proliferation Kinetics of CCD 841 CoN and Caco-2 Cell Lines
3.5. DNA Damage Produced by the E. uniflora EO
3.6. Oxidative Stress Produced by E. uniflora EO
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EO | Essential Oil |
| TB | Trypan Blue |
| DT | Cell Doubling Time |
| FBS | Fetal Bovine Serum |
| PBS | Phosphate Buffer saline |
| CRC | Colorectal Cancer |
References
- Dzobo, K. 2.20—The Role of Natural Products as Sources of Therapeutic Agents for Innovative Drug Discovery. In Comprehensive Pharmacology; Kenakin, T., Ed.; Elsevier: Oxford, UK, 2022; pp. 408–422. [Google Scholar]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 2013. [Google Scholar] [CrossRef] [PubMed]
- Keszenman, D.J.; German, P. Traditional medicine usage of pitanga’s leaves for medicinally valuable infusions is directing the search for potential chemotherapeutic agents against colon cancer. In ABS Is Genetic Resources for Sustainable Development; UNDP and Global Environment Facility: Montreal, QC, Canada, 2018; pp. 212–219. [Google Scholar]
- WHO. WHO Global Report on Traditional and Complementary Medicine 2019; World Health Organization: Geneva, Switzerland, 2019.
- Deligiannidou, G.-E.; Kyrgou, C.; Nena, E.; Manolopoulos, V.G.; Bezirtzoglou, E.; Kontogiorgis, C.A.; Constantinidis, T.C. Use of Edible, Medicinal, and Aromatic Plants in Various Health Disorders: A Cross-Sectional Evaluation among Inhabitants in the Area of Thrace, North-Eastern Greece. Int. J. Environ. Res. Public Health 2022, 19, 12576. [Google Scholar] [CrossRef] [PubMed]
- Allegra, S.; De Francia, S.; Turco, F.; Bertaggia, I.; Chiara, F.; Armando, T.; Storto, S.; Mussa, M.V. Phytotherapy and Drugs: Can Their Interactions Increase Side Effects in Cancer Patients? J. Xenobiotics 2023, 13, 75–89. [Google Scholar] [CrossRef]
- Asiimwe, J.B.; Nagendrappa, P.B.; Atukunda, E.C.; Kamatenesi, M.M.; Nambozi, G.; Tolo, C.U.; Ogwang, P.E.; Sarki, A.M. Prevalence of the Use of Herbal Medicines among Patients with Cancer: A Systematic Review and Meta-Analysis. Evid. Based Complement. Altern. Med. 2021, 2021, 9963038. [Google Scholar] [CrossRef] [PubMed]
- Tayeb, B.A.; Kusuma, I.Y.; Osman, A.A.M.; Minorics, R. Herbal compounds as promising therapeutic agents in precision medicine strategies for cancer: A systematic review. J. Integr. Med. 2024, 22, 137–162. [Google Scholar] [CrossRef]
- Youn, B.-Y.; Kim, J.-H.; Jo, Y.-K.; Yoon, S.; Im, J.-Y.; Kim, H.-J.; Lee, J.-D.; Ko, S.-G. Current Characteristics of Herbal Medicine Interventions for Cancer on Clinical Databases: A Cross-Sectional Study. Integr. Cancer Ther. 2023, 22, 15347354231218255. [Google Scholar] [CrossRef]
- Wu, Z.; Xia, F.; Lin, R. Global burden of cancer and associated risk factors in 204 countries and territories, 1980–2021: A systematic analysis for the GBD 2021. J. Hematol. Oncol. 2024, 17, 119. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Chambers, A.C.; Dixon, S.W.; White, P.; Williams, A.C.; Thomas, M.G.; Messenger, D.E. Demographic trends in the incidence of young-onset colorectal cancer: A population-based study. Br. J. Surg. 2020, 107, 595–605. [Google Scholar] [CrossRef]
- Howren, A.; Sayre, E.C.; Loree, J.M.; Gill, S.; Brown, C.J.; Raval, M.J.; Farooq, A.; De Vera, M.A. Trends in the Incidence of Young-Onset Colorectal Cancer with a Focus on Years Approaching Screening Age: A Population-Based Longitudinal Study. JNCI J. Natl. Cancer Inst. 2021, 113, 863–868. [Google Scholar] [CrossRef]
- Musetti, C.; Garau, M.; Alonso, R.; Piñeros, M.; Soerjomataram, I.; Barrios, E. Colorectal Cancer in Young and Older Adults in Uruguay: Changes in Recent Incidence and Mortality Trends. Int. J. Environ. Res. Public Health 2021, 18, 8232. [Google Scholar] [CrossRef]
- Piñeros, M.; Laversanne, M.; Barrios, E.; Cancela, M.d.C.; de Vries, E.; Pardo, C.; Bray, F. An updated profile of the cancer burden, patterns and trends in Latin America and the Caribbean. Lancet Reg. Health Am. 2022, 13, 100294. [Google Scholar] [CrossRef]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Arrillaga, B. Plantas Usadas en Medicina Natural; Editorial Everest: La Paz, Bolivia, 1997. [Google Scholar]
- Davies, P. Fichas Técnicas de Cultivo. In Estudios de Domesticación y Cultivo de Especies Medicinales y Aromáticas Nativas; INIA: Montevideo, Uruguay, 2004; pp. 37–120. [Google Scholar]
- Paz, E.A.; Bassagoda, M.J.; Ferreira, F. Yuyos: Uso Racional de las Plantas Medicinales; Editorial Fin de Siglo: Montevideo, Uruguay, 1992. [Google Scholar]
- Tabakian, G. Estudio comparativo de plantas medicinales vinculadas a tradiciones indígenas y europeas en Uruguay. Bonplandia 2019, 28, 135–158. [Google Scholar] [CrossRef]
- Stefanello, M.É.A.; Pascoal, A.C.R.F.; Salvador, M.J. Essential Oils from Neotropical Myrtaceae: Chemical Diversity and Biological Properties. Chem. Biodivers. 2011, 8, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Brussa, C.A.; Grela González, A. Flora Arbórea del Uruguay: Con Énfasis en las Especies de Rivera y Tacuarembó; Editorial Fin de Siglo: Montevideo, Uruguay, 2007; p. 544. [Google Scholar]
- Jolochín Manorani, G. Estudios Biogeográficos en Poblaciones Uruguayas de Eugenia uniflora L. Master’s Thesis, Facultad de Ciencias, Universidad de la República, Montevideo, Uruguay, 2016. [Google Scholar]
- Bagetti, M.; Facco, E.M.P.; Piccolo, J.; Hirsch, G.E.; Rodriguez-Amaya, D.; Kobori, C.N.; Vizzotto, M.; Emanuelli, T. Physicochemical characterization and antioxidant capacity of pitanga fruits (Eugenia uniflora L.). Food Sci. Technol. 2011, 31, 147–154. [Google Scholar] [CrossRef]
- Vizzotto, M. Fitoquímicos em pitanga (Eugenia uniflora L.): Seu potencial na prevencao e comabte a doencas. In Proceedings of the III Simpósio Nacional do Morango II Encontro Sobre Pequenas Frutas e Frutas Nativas do Mercosul; Embrapa Clima Temperado: Pelotas, Brazil, 2006; pp. 29–34. [Google Scholar]
- Ferragut, G.; Lombardo, P.; Severi, M.A.; Vignale, B.; Cedano, J.; Dellacassa, E.; Pérez, E. Estudios de bioactividad de extractos de plantas nativas uruguayas y su papel inmunológico. In Proceedings of the XV Jornadas de la Sociedad Uruguaya de Biociencias, Piriápolis, Uruguay, 5–7 September 2014. [Google Scholar]
- Fidelis, E.M.; Savall, A.S.P.; de Oliveira Pereira, F.; Quines, C.B.; Ávila, D.S.; Pinton, S. Pitanga (Eugenia uniflora L.) as a source of bioactive compounds for health benefits: A review. Arab. J. Chem. 2022, 15, 103691. [Google Scholar] [CrossRef]
- Lombardo, P. Caracterización Química y Bioactividad de Aceites Esenciales Contra Patógenos de los Cítricos. Master’s Thesis, Universidad de la República, Montevideo, Uruguay, 2015. [Google Scholar]
- Lombardo, P.; Guimaraens, A.; Franco, J.; Dellacassa, E.; Pérez Faggiani, E. Effectiveness of essential oils for postharvest control of Phyllosticta citricarpa (citrus black spot) on citrus fruit. Postharvest Biol. Technol. 2016, 121, 1–8. [Google Scholar] [CrossRef]
- Rodrigues, K.A.d.F.; Amorim, L.V.; Oliveira, J.M.G.d.; Dias, C.N.; Moraes, D.F.C.; Andrade, E.H.d.A.; Maia, J.G.S.; Carneiro, S.M.P.; Carvalho, F.A.d.A. Eugenia uniflora L. Essential Oil as a Potential Anti-Leishmania Agent: Effects on Leishmania amazonensis and Possible Mechanisms of Action. Evid. Based Complement. Altern. Med. 2013, 2013, 279726. [Google Scholar] [CrossRef]
- Sánchez, A.G.; Ferragut, G.; Lombardo, P.; Severi, M.A.; Cedano, J.; Vignale, B.; Vázquez, A.; Dellacassa, E.; Keszenman, D.J. Efectos celulares de extractos de Pitanga. In Proceedings of the 8vo Encuentro Nacional de Frutos Nativos, Rocha, Uruguay, 30–31 March 2017. [Google Scholar]
- Victoria, F.N.; Lenardão, E.J.; Savegnago, L.; Perin, G.; Jacob, R.G.; Alves, D.; Silva, W.P.d.; Motta, A.d.S.d.; Nascente, P.d.S. Essential oil of the leaves of Eugenia uniflora L.: Antioxidant and antimicrobial properties. Food Chem. Toxicol. 2012, 50, 2668–2674. [Google Scholar] [CrossRef]
- Vignale, B.; Cabrera, D.; Rodríguez, P.; Machado, G. Selección de frutales nativos en Uruguay. Hortic. Argent. 2016, 35, 19–29. [Google Scholar]
- Weyerstahl, P.; Marschall-Weyerstahl, H.; Christiansen, C.; Oguntimein, B.O.; Adeoye, A.O. Volatile Constituents of Eugenia uniflora Leaf Oil. Planta Med. 1988, 54, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Benfatti, C.S.; Cordova, S.M.d.; Guedes, A.; Magina, M.D.A.; Cordova, C.M.M.d. Atividade antibacteriana in vitro de extratos brutos de espécies de Eugenia sp. frente a cepas de molicutes. Rev. Pan. Amaz. Saúde 2010, 1, 33–39. [Google Scholar] [CrossRef]
- de Souza Prestes, L.; Damé Schuch, L.F.; Hörnke Alves, G.; Ziemann dos Santos, M.A.; Alves Rodrigues, M.R.; Araújo Meireles, M.C. Evaluación de la actividad bactericida de aceites esenciales de hojas de guayabo, pitango y arazá. Rev. Cuba. Plantas Med. 2011, 16, 324–330. [Google Scholar]
- Borsoi, F.T.; Rosa, B.B.d.S.; Filomena, M.; Oliveira, F.D.L.d.; Dulce, B.M.; Kempka, A.P. Eugenia uniflora L. seed and pulp extracts: Phytochemical profile, cytotoxic potential, antitumoral activity, and α-amylase and α-glucosidase inhibition capacity. Nat. Prod. Res. 2023, 37, 3862–3867. [Google Scholar] [CrossRef]
- Falcão, T.R.; de Araújo, A.A.; Soares, L.A.L.; de Moraes Ramos, R.T.; Bezerra, I.C.F.; Ferreira, M.R.A.; de Souza Neto, M.A.; Melo, M.C.N.; de Araújo, R.F.; de Aguiar Guerra, A.C.V.; et al. Crude extract and fractions from Eugenia uniflora Linn leaves showed anti-inflammatory, antioxidant, and antibacterial activities. BMC Complement. Altern. Med. 2018, 18, 84. [Google Scholar] [CrossRef]
- Núñez, J.G.; Pinheiro, J.d.S.; Silveira, G.F.; Beckenkamp, A.; Buffon, A.; Bruno, A.N. Antineoplastic potential of the aqueous crude extract of Eugenia uniflora L. in human cervical cancer. Braz. J. Pharm. Sci. 2018, 54, e17267. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Carol. Stream 2005, 16, 65–120. [Google Scholar]
- McLafferty, F.W.; Stauffer, D.B.; Loh, S.Y. Comparative evaluations of mass spectral data bases. J. Am. Soc. Mass. Spectrom. 1991, 2, 438–440. [Google Scholar] [CrossRef]
- Mondello, L. Mass Spectra of Flavors and Fragrances of Natural and Synthetic Compounds; Wiley Blackwell: Hoboken, NJ, USA, 2015; Volume 10. [Google Scholar]
- Dellacassa, E.; Lorenzo, D.; Paz, D. Procesos de extracción aplicados a la obtención de productos aromáticos de origen vegetal. In Estudios en Domesticación y Cultivos de Especies Medicinales y Aromáticas Nativas; Davies, P., Ed.; INIA: Montevideo, Uruguay, 2004; pp. 155–160. [Google Scholar]
- Dellacassa, E.; Lorenzo, D.; Paz, D. Caracterización fisicoquímica de los aceites esenciales. In Estudios en Domesticación y Cultivos de Especies Medicinales y Aromáticas Nativas; Davies, P., Ed.; INIA: Montevideo, Uruguay, 2004; pp. 161–169. [Google Scholar]
- de Abreu Costa, L.; Henrique Fernandes Ottoni, M.; Dos Santos, M.G.; Meireles, A.B.; Gomes de Almeida, V.; de Fátima Pereira, W.; Alves de Avelar-Freitas, B.; Eustáquio Alvim Brito-Melo, G. Dimethyl Sulfoxide (DMSO) Decreases Cell Proliferation and TNF-α, IFN-γ, and IL-2 Cytokines Production in Cultures of Peripheral Blood Lymphocytes. Molecules 2017, 22, 1789. [Google Scholar] [CrossRef]
- Chen, X.; Thibeault, S. Effect of DMSO concentration, cell density and needle gauge on the viability of cryopreserved cells in three dimensional hyaluronan hydrogel. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2013, 2013, 6228–6231. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Avelar-Freitas, B.A.; Almeida, V.G.; Pinto, M.C.; Mourao, F.A.; Massensini, A.R.; Martins-Filho, O.A.; Rocha-Vieira, E.; Brito-Melo, G.E. Trypan blue exclusion assay by flow cytometry. Braz. J. Med. Biol. Res. 2014, 47, 307–315. [Google Scholar] [CrossRef]
- Keszenman, D.J.; Salvo, V.A.; Nunes, E. Effects of bleomycin on growth kinetics and survival of Saccharomyces cerevisiae: A model of repair pathways. J. Bacteriol. 1992, 174, 3125–3132. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Bribiesca, L.; Sanchez-Suarez, P. Oxidative damage, bleomycin, and gamma radiation induce different types of DNA strand breaks in normal lymphocytes and thymocytes. A comet assay study. Ann. N. Y. Acad. Sci. 1999, 887, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Azqueta, A.; Sanz-Serrano, J.; Bakuradze, T.; Richling, E.; Eyluel Bankoglu, E.; Stopper, H.; Claudino Bastos, V.; Langie, S.A.S.; Jensen, A.; et al. Visual comet scoring revisited: A guide to scoring comet assay slides and obtaining reliable results. Mutagenesis 2023, 38, 253–263. [Google Scholar] [CrossRef]
- Keszenman, D.J.; Carmen Candreva, E.; Nunes, E. Cellular and molecular effects of bleomycin are modulated by heat shock in Saccharomyces cerevisiae. Mutat. Res. 2000, 459, 29–41. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Costa-Font, J.; Sato, A.; Saenz-de-Miera, B. Cultural persistence of self-assessed health: A study of first- and second-generation migrants. J. Migr. Health 2025, 11, 100280. [Google Scholar] [CrossRef]
- Da Silva, J.K.R.; Andrade, E.H.A.; Barreto, L.H.; Da Silva, N.C.F.; Ribeiro, A.F.; Montenegro, R.C.; Maia, J.G.S. Chemical Composition of Four Essential Oils of Eugenia from the Brazilian Amazon and Their Cytotoxic and Antioxidant Activity. Medicines 2017, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Ascari, J.; Felipe Maciel Pereira, M.; Schaffka, V.M.; Nunes, D.S.; Magalhães, C.G.; Santos, J.S.; Granato, D.; Carmo, M.; Azevedo, L.; Archilha, M.; et al. Selina-1,3,7(11)-trien-8-one and Oxidoselina-1,3,7(11)-trien-8-one from Eugenia uniflora Leaf Essential Oil and Their Cytotoxic Effects on Human Cell Lines. Molecules 2021, 26, 740. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.L.B.; Pinto, L.C.; da Costa, J.S.; da Silva, A.R.C.; Mourão, R.H.V.; Montenegro, R.C.; da Silva, J.K.R.; Maia, J.G.S. Composition, antioxidant capacity and cytotoxic activity of Eugenia uniflora L. chemotype-oils from the Amazon. J. Ethnopharmacol. 2019, 232, 30–38. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Theoduloz, C.; Franco, L.; Ferro, E.; de Arias, A.R. Preliminary pharmacological studies on Eugenia uniflora leaves: Xanthine oxidase inhibitory activity. J. Ethnopharmacol. 1987, 21, 183–186. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Aranha, E.S.P.; de Azevedo, S.G.; dos Reis, G.G.; Silva Lima, E.; Machado, M.B.; de Vasconcellos, M.C. Essential oils from Eugenia spp.: In vitro antiproliferative potential with inhibitory action of metalloproteinases. Ind. Crops Prod. 2019, 141, 111736. [Google Scholar] [CrossRef]
- de Oliveira, G.G.F.V.; Longue, M.F.; Pescinelli, L.M.R.; Charret, T.S.; Nogueira, T.S.R.; Pereira, M.T.M.; Vieira, I.J.C.; Abreu, L.S.; Pascoal, V.D.B.; Pascoal, A.C.R.F. Eugenia brasiliensis: Analysis of the Chemical Profile and Evaluation of Cytotoxic Potential. Chem. Biodivers. 2025, e202500429. [Google Scholar] [CrossRef]
- Ismiyati, N.; Putri, D.; Kusumastuti, S.A.; Febriansyah, R. Antiproliferative Effect of Ethanolic Extract Eugenia uniflora Lam. Leaves on T47D Cells. Indones. J. Cancer Chemoprev. 2012, 3, 370. [Google Scholar] [CrossRef]
- Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Lampri, E.; Fitsiou, E.; Vasileiadis, S.; Vamvakias, M.; Bardouki, H.; Goussia, A.; Malamou-Mitsi, V.; Panayiotidis, M.I.; et al. Dietary mastic oil extracted from Pistacia lentiscus var. chia suppresses tumor growth in experimental colon cancer models. Sci. Rep. 2017, 7, 3782. [Google Scholar] [CrossRef]
- Agus, H.H.; Sarp, C.; Cemiloglu, M. Oxidative stress and mitochondrial impairment mediated apoptotic cell death induced by terpinolene in Schizosaccharomyces pombe. Toxicol. Res. 2018, 7, 848–858. [Google Scholar] [CrossRef]
- Jia, S.S.; Xi, G.P.; Zhang, M.; Chen, Y.B.; Lei, B.; Dong, X.S.; Yang, Y.M. Induction of apoptosis by D-limonene is mediated by inactivation of Akt in LS174T human colon cancer cells. Oncol. Rep. 2013, 29, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Yoshida, H.; Nishimura, Y.; Kitagishi, Y.; Matsuda, S. Terpinolene, a component of herbal sage, downregulates AKT1 expression in K562 cells. Oncol. Lett. 2012, 3, 321–324. [Google Scholar] [CrossRef]
- Menezes, I.O.; Scherf, J.R.; Martins, A.O.B.P.B.; Ramos, A.G.B.; Quintans, J.d.S.S.; Coutinho, H.D.M.; Ribeiro-Filho, J.; de Menezes, I.R.A. Biological properties of terpinolene evidenced by in silico, in vitro and in vivo studies: A systematic review. Phytomedicine 2021, 93, 153768. [Google Scholar] [CrossRef]
- Zhong, Z.-C.; Zhao, D.-D.; Liu, Z.-D.; Jiang, S.; Zhang, Y.-L. A New Human Cancer Cell Proliferation Inhibition Sesquiterpene, Dryofraterpene A, from Medicinal Plant Dryopteris fragrans (L.) Schott. Molecules 2017, 22, 180. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.M.; Speakman, J.B.; Zapp, J.; Becker, H. Bioactivity guided isolation of antifungal compounds from the liverwort Bazzania trilobata (L.) S.F. Gray. Phytochemistry 2004, 65, 2583–2588. [Google Scholar] [CrossRef]
- Hui, L.M.; Zhao, G.D.; Zhao, J.J. δ-Cadinene inhibits the growth of ovarian cancer cells via caspase-dependent apoptosis and cell cycle arrest. Int. J. Clin. Exp. Pathol. 2015, 8, 6046–6056. [Google Scholar] [PubMed]
- Chien, T.C.; Lo, S.F.; Ho, C.L. Chemical composition and anti-inflammatory activity of Chamaecyparis obtusa f. formosana wood essential oil from Taiwan. Nat. Prod. Commun. 2014, 9, 723–726. [Google Scholar]
- Zhu, Q.; Wang, S.; Fu, G.; Guo, F.; Huang, W.; Zhang, T.; Dong, H.; Jin, Z.; Zhang, D. Highly flexible cell membranes are the key to efficient production of lipophilic compounds. J. Lipid Res. 2024, 65, 100597. [Google Scholar] [CrossRef]
- Kundu, A.; Supradip, S.; Suresh, W.; Vivek, A.; Kaur, C. Antioxidant potential of essential oil and cadinene sesquiterpenes of Eupatorium adenophorum. Toxicol. Environ. Chem. 2013, 95, 127–137. [Google Scholar] [CrossRef]
- Olano, C.; Méndez, C.; Salas, J.A. Antitumor compounds from marine actinomycetes. Mar. Drugs 2009, 7, 210–248. [Google Scholar] [CrossRef]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; ME, L.L. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, F.A.B.; Wallau, G.L.; Pinho, A.I.; Nunes, M.E.M.; Leite, N.F.; Tintino, S.R.; da Costa, G.M.; Athayde, M.L.; Boligon, A.A.; Coutinho, H.D.M.; et al. Eugenia uniflora leaves essential oil induces toxicity in Drosophila melanogaster: Involvement of oxidative stress mechanisms. Toxicol. Res. 2015, 4, 634–644. [Google Scholar] [CrossRef]
- de Carvalho, N.R.; Rodrigues, N.R.; Macedo, G.E.; Bristot, I.J.; Boligon, A.A.; de Campos, M.M.; Cunha, F.A.B.; Coutinho, H.D.; Klamt, F.; Merritt, T.J.S.; et al. Eugenia uniflora leaf essential oil promotes mitochondrial dysfunction in Drosophila melanogaster through the inhibition of oxidative phosphorylation. Toxicol. Res. 2017, 6, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.-S.; Thakur, K.; Hussain, S.S.; Zhang, J.-G.; Xiao, G.-R.; Wei, Z.-J. Licochalcone A from licorice root, an inhibitor of human hepatoma cell growth via induction of cell apoptosis and cell cycle arrest. Food Chem. Toxicol. 2018, 120, 407–417. [Google Scholar] [CrossRef]
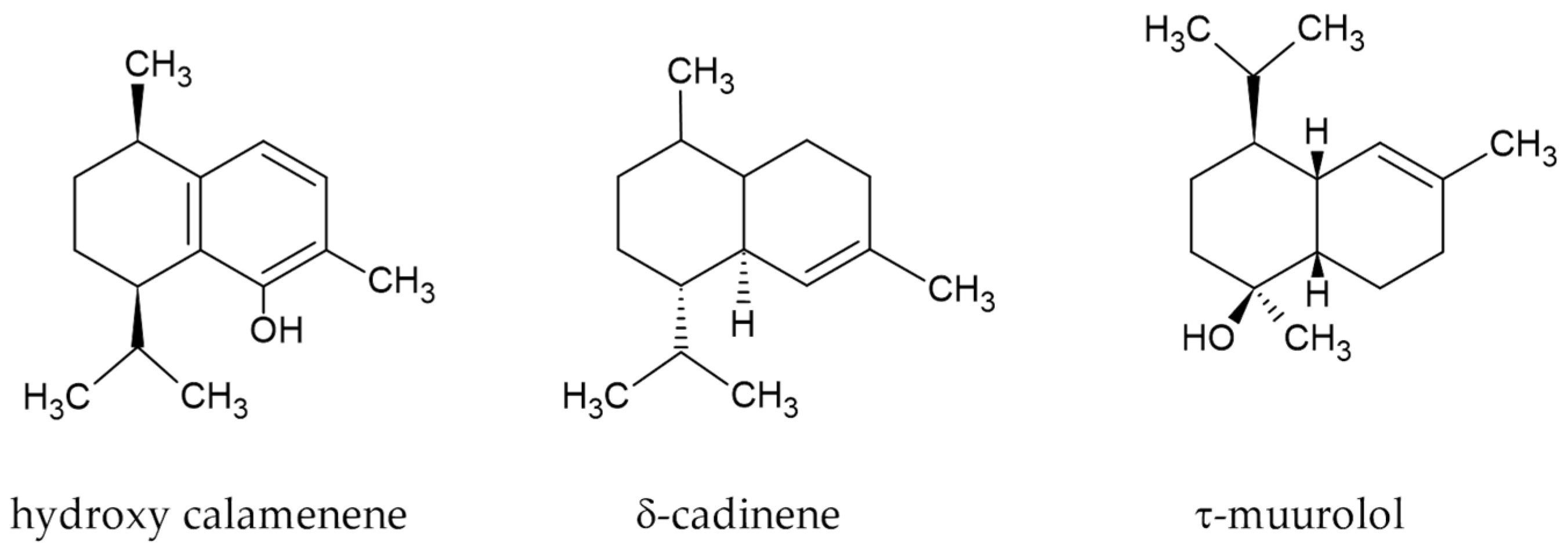








| LRI 1 | Compound 2 | % | LRI | Compound | % |
|---|---|---|---|---|---|
| 1082 | Hexanal | 0.01 | 1666 | α-Humulene | 0.07 |
| 1110 | β-Pinene | 0.01 | 1668 | γ-Gurjunene | 0.06 |
| 1122 | Sabinene | 0.01 | 1699 | Bicyclogermacrene | 0.02 |
| 1162 | α-Terpinene | 0.01 | 1682 | β-Humulene | 0.13 |
| 1167 | α-Phellandrene | 0.01 | 1684 | trans-β-Guaiene | 0.01 |
| 1169 | Myrcene | 1.10 | 1686 | β-Chamigrene | 3.71 |
| 1198 | Limonene | 0.25 | 1688 | Zonarene | 0.48 |
| 1209 | β-Phellandrene | 0.26 | 1689 | γ-Muurolene | 5.22 |
| 1239 | 2-Pentylfuran | 0.01 | 1708 | Germacrene D | 0.22 |
| 1250 | trans-β-Ocimene | 0.71 | 1720 | α-Cadinene | 0.41 |
| 1234 | cis-β-Ocimene | 1.82 | 1722 | Viridiflorene | 0.71 |
| 1270 | p-Cymene | 0.03 | 1723 | α-Muurolene | 0.55 |
| 1282 | Terpinolene | 0.03 | 1746 | cis-Muurola-3,5-diene | 0.41 |
| 1351 | 1-Hexanol | 0.01 | 1755 | d-Cadinene | 18.73 |
| 1351 | allo-Ocimene | 0.01 | 1806 | Germacrene B | 0.55 |
| 1380 | (Z)-3-Hexen-1-ol | 0.07 | 1808 | trans-Calamenene | 0.02 |
| 1460 | α-Cubebene | 0.06 | 1818 | cis-Calamenene | 0.27 |
| 1523 | β-Bourbonene | 0.03 | 1918 | β-Calacorene | 0.06 |
| 1600 | Guaia-6,9-diene | 0.03 | 1901 | α-Colacorene | 0.05 |
| 1626 | β-Copaene | 0.59 | 1930 | Palustrol | 0.01 |
| 1636 | trans-Caryophyllene | 0.23 | 2082 | Globulol | 0.54 |
| 1637 | Aromadendrene | 0.17 | 2089 | Viridiflorol | 3.85 |
| 1642 | trans-Muurola-3,5-diene | 0.17 | 2185 | τ-Muurolol | 12.72 |
| 1652 | α-Guaiene | 0.01 | 2325 | Hydroxy calamenene | 41.04 |
| 1658 | γ-Elemene | 0.05 | |||
| Total | 95.51 |
| Nmax (Cells/mL) | DT (h) | t1/2 (h) | tlag (h) | |
|---|---|---|---|---|
| Control CCD 841 CoN | 1.0 × 106 ± 1.5 × 105 | 57.9 ± 8.9 | 309.5 ± 30.8 | 66.0 ± 3.0 |
| Control Caco-2 | 8.2 × 106 ± 2.8 × 105 | 17.1 ± 3.0 | 168.0 ± 5.1 | 12.0 ± 0.6 |
| Treated Caco-2 | 5.2 × 106 ± 2.6 × 105 | 60.0 ± 3.0 | 225.0 ± 11.3 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, A.G.; Menoni, M.; Lombardo, P.; Dellacassa, E.; Severi, M.A.; Ferragut, G.; Vignale, B.; Cedano, J.; Zuluaga, M.J.; Keszenman, D.J. Antiproliferative Potential of Eugenia uniflora L. Leaf Essential Oil in Normal and Tumoral Human Colon Cells. Biologics 2025, 5, 19. https://doi.org/10.3390/biologics5030019
Sánchez AG, Menoni M, Lombardo P, Dellacassa E, Severi MA, Ferragut G, Vignale B, Cedano J, Zuluaga MJ, Keszenman DJ. Antiproliferative Potential of Eugenia uniflora L. Leaf Essential Oil in Normal and Tumoral Human Colon Cells. Biologics. 2025; 5(3):19. https://doi.org/10.3390/biologics5030019
Chicago/Turabian StyleSánchez, Ana G., Macarena Menoni, Pamela Lombardo, Eduardo Dellacassa, María Angélica Severi, Gabriela Ferragut, Beatriz Vignale, Juan Cedano, María José Zuluaga, and Deborah J. Keszenman. 2025. "Antiproliferative Potential of Eugenia uniflora L. Leaf Essential Oil in Normal and Tumoral Human Colon Cells" Biologics 5, no. 3: 19. https://doi.org/10.3390/biologics5030019
APA StyleSánchez, A. G., Menoni, M., Lombardo, P., Dellacassa, E., Severi, M. A., Ferragut, G., Vignale, B., Cedano, J., Zuluaga, M. J., & Keszenman, D. J. (2025). Antiproliferative Potential of Eugenia uniflora L. Leaf Essential Oil in Normal and Tumoral Human Colon Cells. Biologics, 5(3), 19. https://doi.org/10.3390/biologics5030019








