Unraveling Potential Compounds of Uncaria gambir (W.Hunter) Roxb. as Antikeloid Agent: In Silico, In Vitro and Ex Vivo Experimental Validation
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Fractionation Procedure
2.2. Phytochemical Analysis
2.3. Gambir Bioactive Compound Screening Process
2.4. In Silico Analysis
2.5. Cytotoxic and Bioactivity Effect
2.6. Ex Vivo Assay Analysis
3. Results
3.1. Phytochemical and Bioactive Compounds Analysis of Gambir
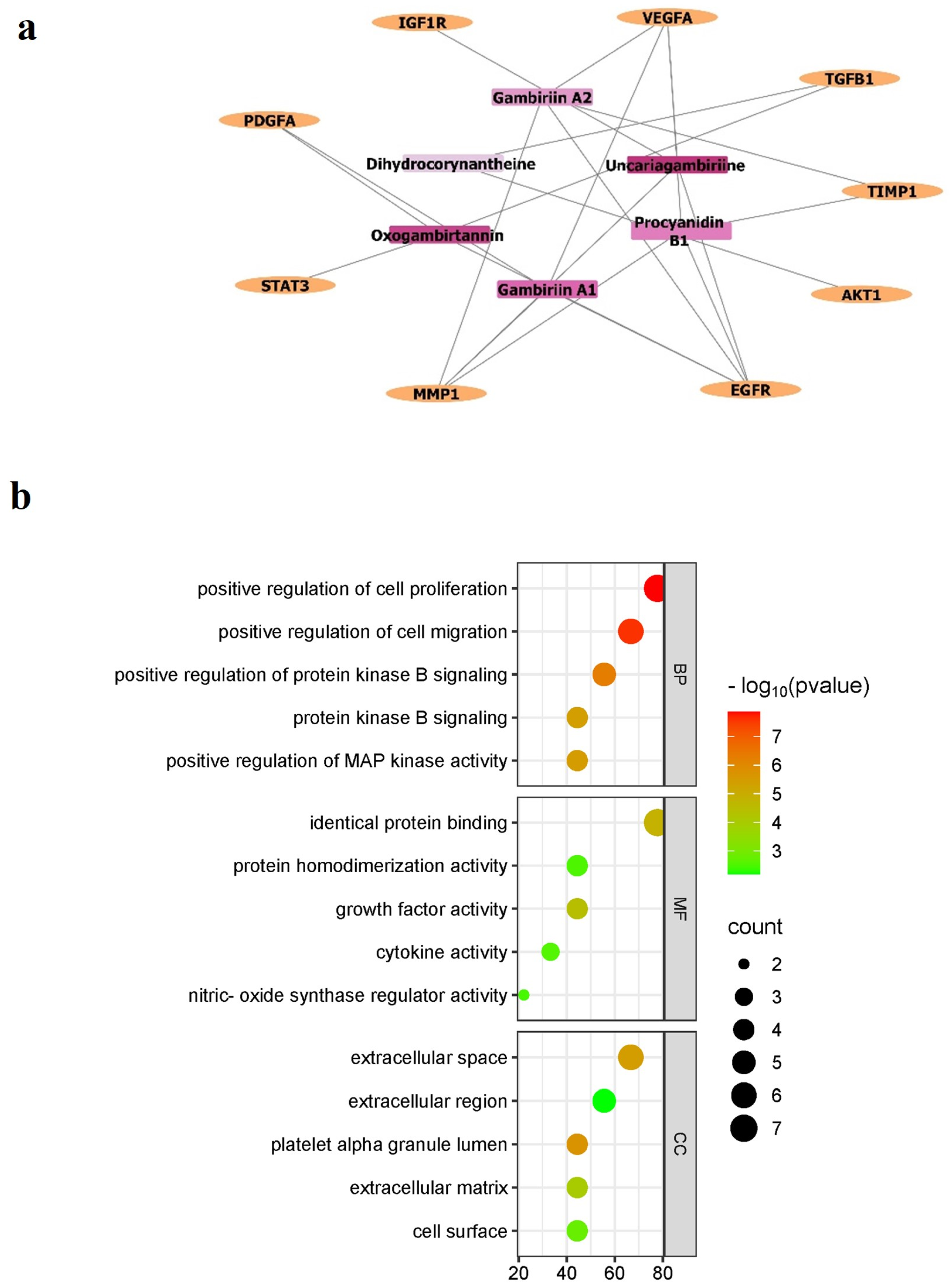
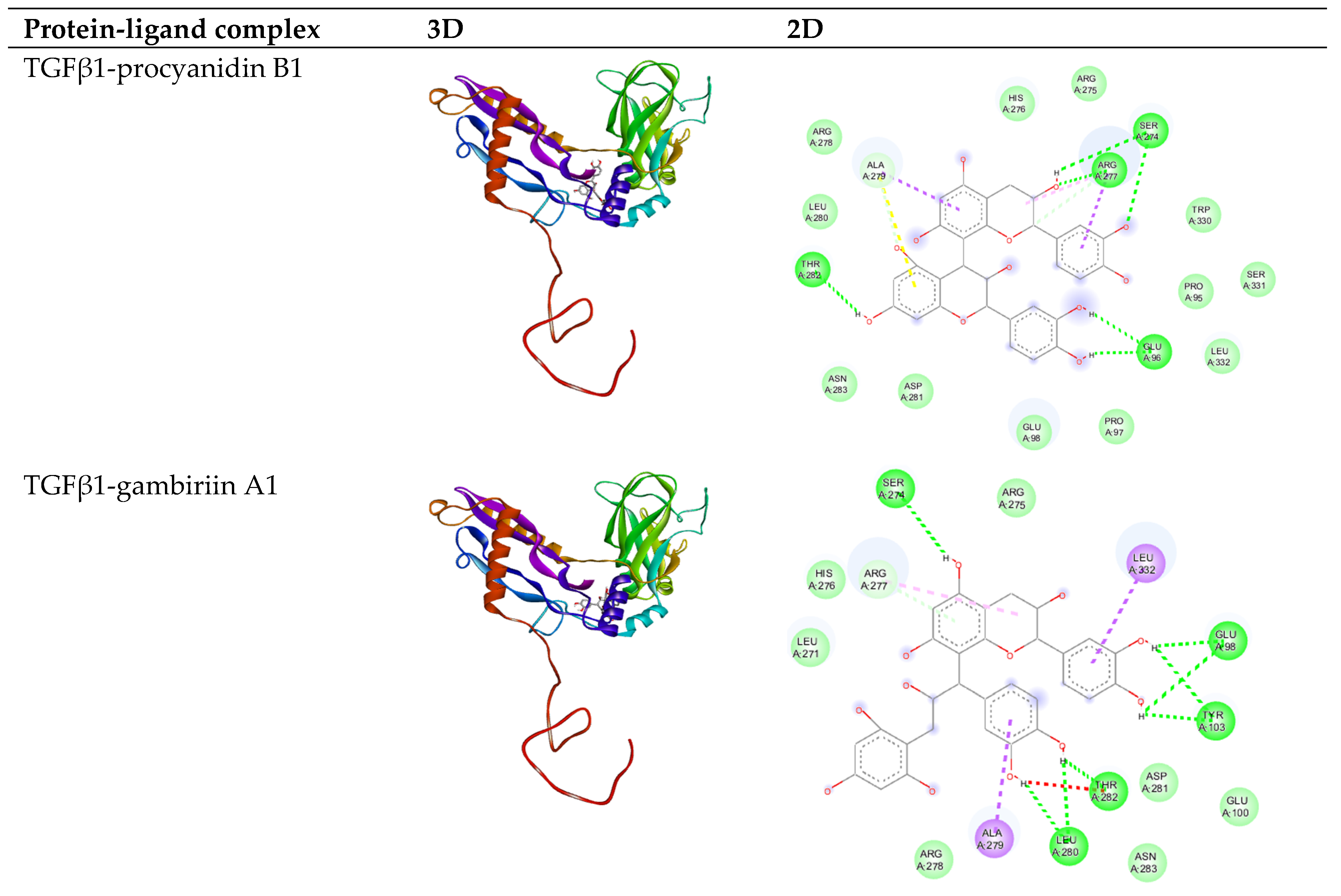
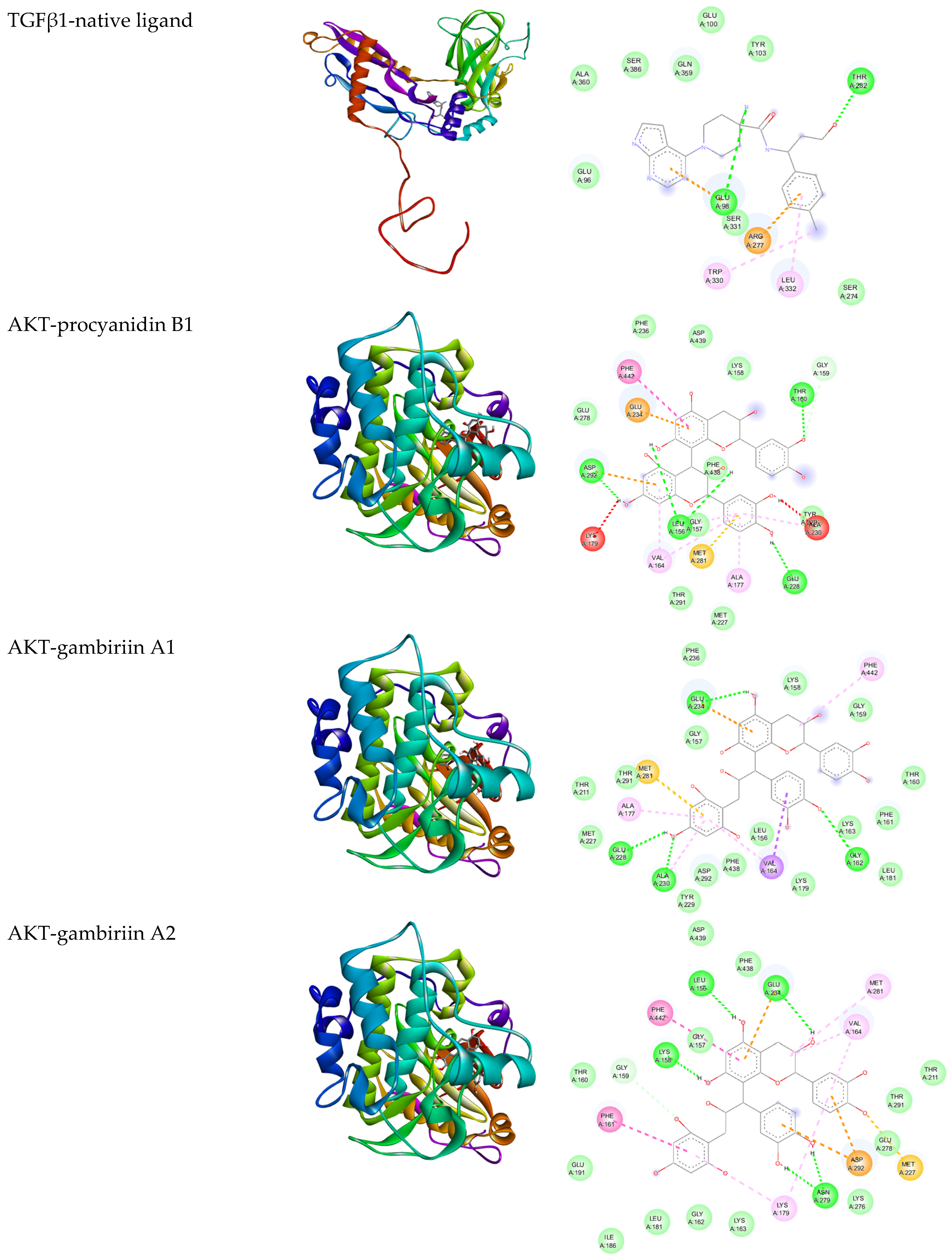
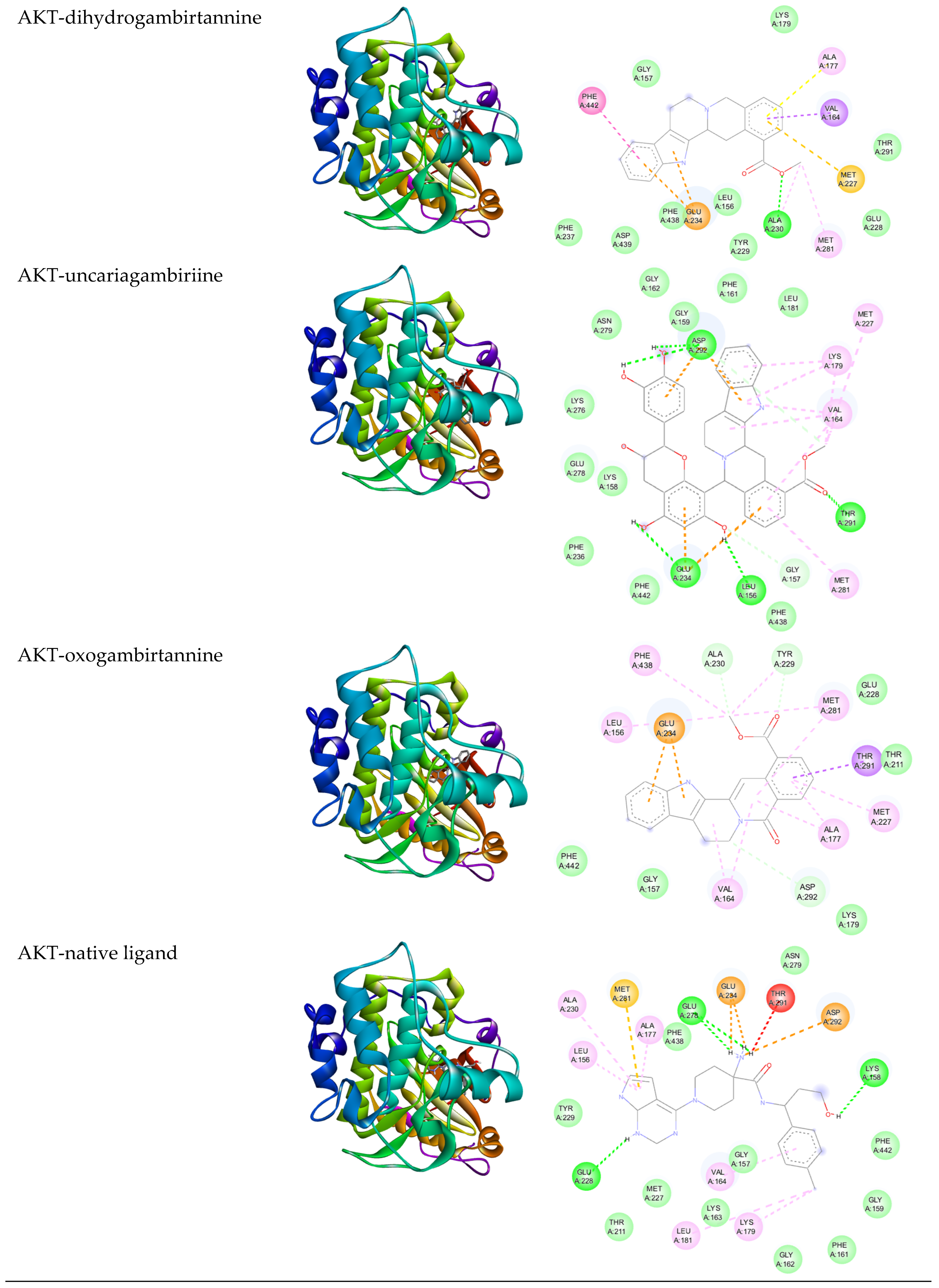
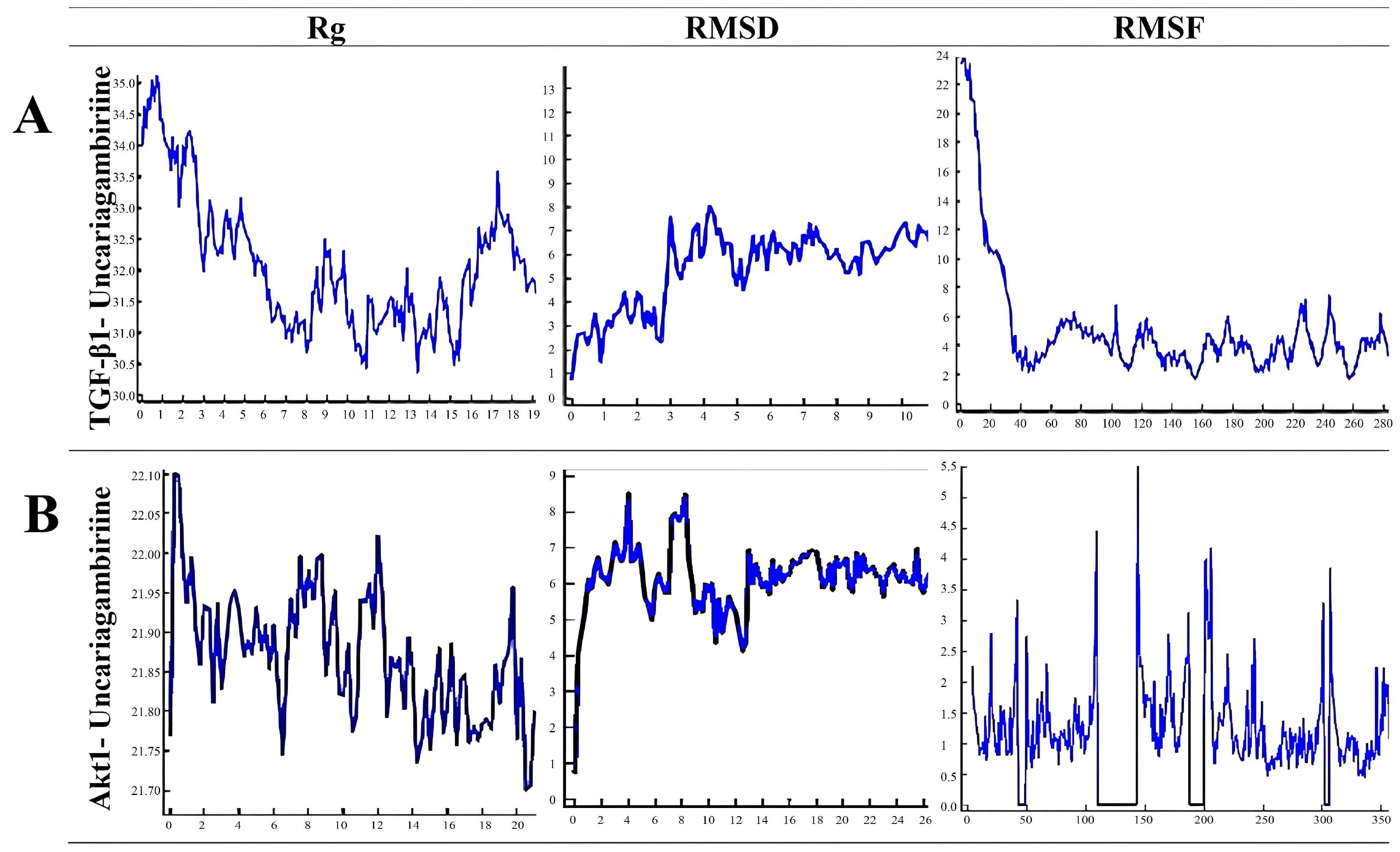
3.2. In Silico Analysis
3.3. Cytotoxic and Bioactivity Effect of Gambir Extract and Fraction Towards Keloid Fibroblast
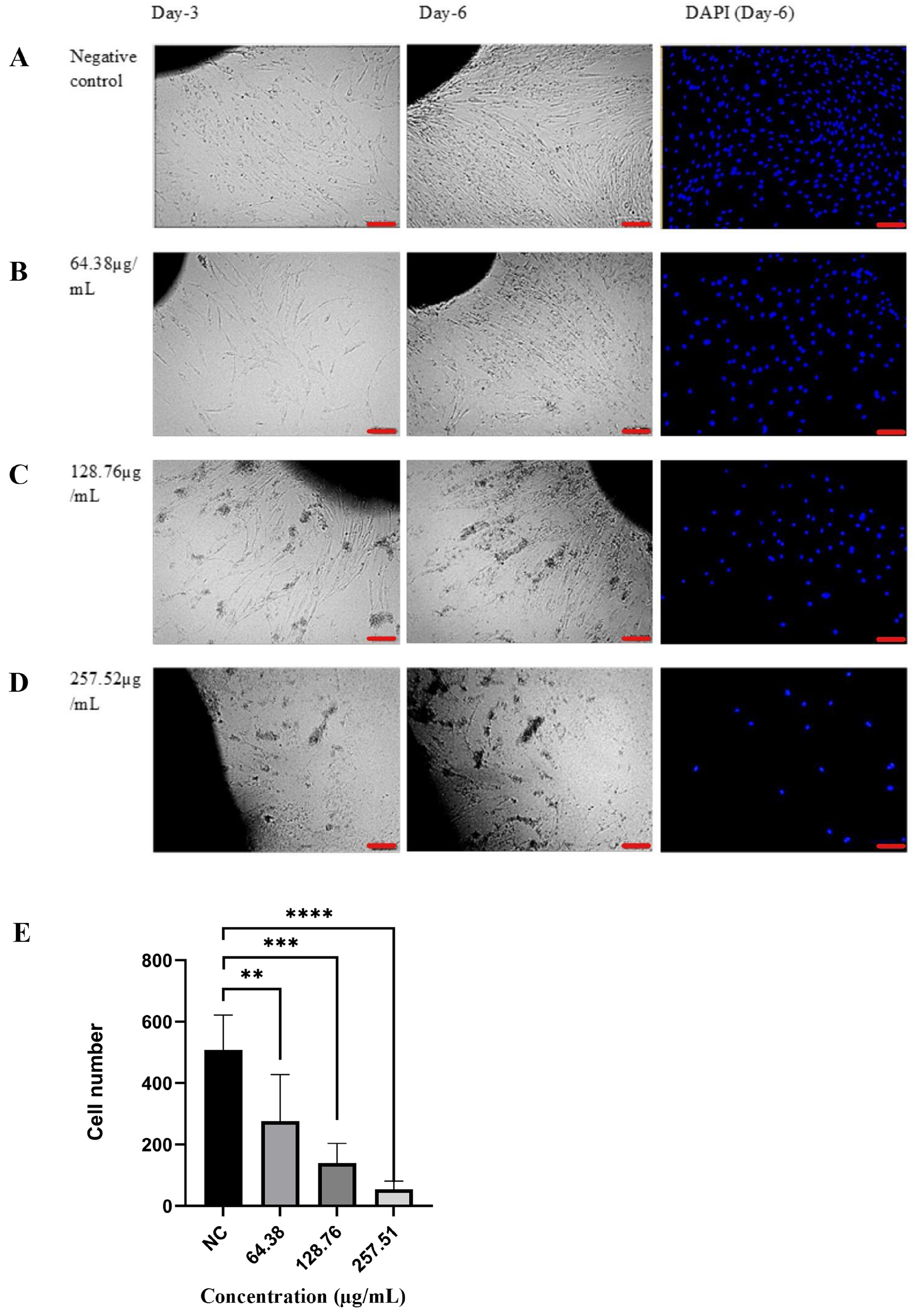
3.4. Ex Vivo Analysis of Gambir Ethanol Fraction Effect Towards Keloid Fibroblast
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LCMS/MS | liquid chromatography–mass spectrometry |
| ECM | extracellular matrix |
| TGFβ1 | transforming growth factor beta 1 |
| AKT1 | AKT serine/threonine kinase 1 |
| MMP1 | matrix metallopeptidase 1 |
| UPLC | ultra-performance liquid chromatography |
| ESI | electrospray ionization |
| SMILE | canonical simplified molecular-input line-entry |
| DAVID | database for annotation, visualization, and integrated discovery |
| PDB | protein data bank |
| Rg | radius of gyration |
| RMSD | root mean square deviation |
| RMSF | root mean square fluctuation |
| PBS | phosphate-buffered saline |
| BSC | biosafety cabinet |
| DAPI | 4′,6-diamidino-2-phenylindole |
| GO | gene ontology |
| BP | biological processes |
| MF | molecular functions |
| CC | cellular components |
| SI | selectivity index |
References
- Ekstein, S.F.; Wyles, S.P.; Moran, S.L.; Meves, A. Keloids: A review of therapeutic management. Int. J. Dermatol. 2021, 60, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Pezzani, R.; Salehi, B.; Vitalini, S.; Iriti, M.; Zuñiga, F.A.; Sharifi-Rad, J.; Martorell, M.; Martins, N. Synergistic effects of plant derivatives and conventional chemotherapeutic agents: An update on the cancer perspective. Medicina 2019, 55, 110. [Google Scholar] [CrossRef]
- Yimam, M.; Lee, Y.C.; Kim, T.W.; Moore, B.; Jiao, P.; Hong, M.; Kim, H.-J.; Nam, J.-B.; Kim, M.-R.; Oh, J.-S.; et al. UP3005, a botanical composition containing two standardized extracts of Uncaria gambir and Morus alba, improves pain sensitivity and cartilage degradations in monosodium iodoacetate-induced rat OA disease model. Evid. Based Complement. Altern. Med. 2015, 2015, 785638. [Google Scholar] [CrossRef][Green Version]
- Syarifah, S.; Widyawati, T.; Rita Anggraini, D.; Sari Wahyuni, A.; Indah Sari, M. Anticancer activity of Uncaria gambir roxb on T47D breast cancer cells. J. Phys. Conf. Ser. 2019, 1317, 012106. [Google Scholar] [CrossRef]
- Musdja, M.Y.; Rahman, H.A.; Hasan, D. Antioxidant Activity of Catechins Isolate of Uncaria gambir Roxb in Male Rats. LIFE Int. J. Health Life-Sci. 2018, 4, 34–46. [Google Scholar] [CrossRef]
- Mat Saad, M.F.; Goh, H.H.; Rajikan, R.; Tuan Yusof, T.R.; Baharum, S.N.; Bunawan, H. From phytochemical composition to pharmacological importance. Trop. J. Pharm. Res. 2020, 19, 1767–1773. [Google Scholar] [CrossRef]
- Munggari, I.P.; Kurnia, D.; Deawati, Y.; Julaeha, E. Current Research of Phytochemical, Medicinal and Non-Medicinal Uses of Uncaria gambir Roxb.: A Review. Molecules 2022, 27, 6551. [Google Scholar] [CrossRef]
- Nur Sazwi, N.; Nalina, T.; Rahim, Z.H.A. Antioxidant and cytoprotective activities of Piper betle, Areca catechu, Uncaria gambir and betel quid with and without calcium hydroxide. BMC Complement. Altern. Med. 2013, 13, 1–12. [Google Scholar] [CrossRef]
- Yimam, M.; Lee, Y.C.; Kim, T.W.; Moore, B.; Jiao, P.; Hong, M.; Kim, H.-J.; Nam, J.-B.; Kim, M.-R.; Oh, J.-S.; et al. Analgesic and anti-inflammatory effect of UP3005, a botanical composition Containing two standardized extracts of Uncaria gambir and Morus alba. Pharmacogn. Res. 2015, 7, S39–S46. [Google Scholar] [CrossRef]
- Desdiani, D.; Rengganis, I.; Djauzi, S.; Setiyono, A.; Sadikin, M.; Jusman, S.W.A.; Siregar, N.C.; Suradi, S.; Eyanoer, P.C. Fibropreventive and Antifibrotic Effects of Uncaria gambir on Rats with Pulmonary Fibrosis. Evid. Based Complement. Altern. Med. 2022, 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ningsih, S. Efek Hepatoprotektor Gambir (Uncaria gambir (Hunter) Roxb.) Dalam Menghambat Pembentukan Pembentukan Kolagen Dengan Menekan TiMP-1 In Vivo. Ph.D. Thesis, Universitas Indonesia, Kota Depok, Indonesia, 2015. [Google Scholar]
- Jusman, S.W.A.; Amalia, M.F.; Paramita, R.; Ningsih, S.; Fadilah, F. Structure-based screening of inhibitor platelet-derived growth factor from ethanol extract of Uncaria gambir (Hunter) Roxb. as an antifibrotic in keloid fibroblast cells. J. Appl. Pharm. Sci. 2023, 13, 036–043. [Google Scholar] [CrossRef]
- Andrews, J.P.; Marttala, J.; Macarak, E.; Rosenbloom, J.; Uitto, J. Keloids: The paradigm of skin fibrosis—Pathomechanisms and treatment. Matrix Biol. 2016, 51, 37–46. [Google Scholar] [CrossRef]
- Ningsih, S.S.; Fadilah, F.; Jusman, S.W.A.; Syaidah, R.; Yashiro, T. Profibrotic Inflammatory Cytokines and Growth Factors Are Predicted as the Key Targets of Uncaria gambir (Hunter) Roxb. in Keloids: An Epistatic and Molecular Simulation Approach. Pharmaceuticals 2024, 17, 662. [Google Scholar] [CrossRef] [PubMed]
- Strobel, O.; Dadabaeva, N.; Felix, K.; Hackert, T.; Giese, N.A.; Jesenofsky, R.; Werner, J. Isolation and culture of primary human pancreatic stellate cells that reflect the context of their tissue of origin. Langenbeck’s Arch. Surg. 2016, 401, 89–97. [Google Scholar] [CrossRef]
- Berman, B.; Maderal, A.; Raphael, B. Keloids and hypertrophic scars: Pathophysiology, classification, and treatment. Dermatol. Surg. 2017, 43, S3–S18. [Google Scholar] [CrossRef]
- Hasan, M.; Zafar, A.; Shahzadi, I.; Luo, F.; Hassan, S.G.; Tariq, T.; Iqbal, F.; Shu, X. Fractionation of biomolecules in Withania coagulans extract for bioreductive nanoparticle synthesis, antifungal and biofilm activity. Molecules 2020, 25, 3478. [Google Scholar] [CrossRef]
- Gueboudji, Z.; Kadi, K.; Mahmoudi, M.; Hannachi, H.; Nagaz, K.; Addad, D.; Yahya, L.B.; Lachehib, B.; Hessini, K. Maceration and liquid–liquid extractions of phenolic compounds and antioxidants from Algerian olive oil mill wastewater. Environ. Sci. Pollut. Res. 2023, 30, 3432–3439. [Google Scholar] [CrossRef]
- Srivastava, N.; Bansal, A.; Aggarwal, K.; Nagpal, K. Development and Validation of UV Spectrophotometric Method for the Quantitative Estimation of Quercetin in Bulk Followed by Its Solubility Studies. J. Appl. Spectrosc. 2024, 91, 700–708. [Google Scholar] [CrossRef]
- Ismed, F.; Desti, W.N.; Arifa, N.; Rustini, R.; Putra, D.P. TLC-Bioautographic and LC-MS/MS Detection of Antimicrobial Compounds from Four Semipolar Extracts of Cladonia Species. In Proceedings of the 2nd International Conference on Contemporary Science and Clinical Pharmacy 2021 (ICCSCP 2021); Atlantis Press International B.V.: Dordrecht, The Netherlands, 2022; Volume 40, pp. 49–59. [Google Scholar]
- Fadilah, F.; Kezia, I.; Erlina, L. Uncovering potential neuroprotective flavonoids for Alzheimer’s disease using cutting-edge molecular simulation and in vitro SHSY-5Y analysis. J. Pharm. Pharmacogn. Res. 2024, 12, 204–217. [Google Scholar] [CrossRef]
- Ningsih, S.S. Efficient protocol for isolating human fibroblast from primary skin cell cultures: Application to keloid, hypertrophic scar, and normal skin biopsies. Biol. Methods Protoc. 2024, 9, bpae082. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Li, L.; Hu, Z. Exploring the Molecular Mechanism of Action of Yinchen Wuling Powder for the Treatment of Hyperlipidemia, Using Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation. Biomed. Res. Int. 2021, 2021, 9965906. [Google Scholar] [CrossRef]
- Graidist, P.; Martla, M.; Sukpondma, Y. Cytotoxic activity of Piper cubeba extract in breast cancer cell lines. Nutrients 2015, 7, 2707–2718. [Google Scholar] [CrossRef]
- Huang, C.; Wu, Z.; Du, Y.; Ogawa, R. Textbook on Scar Management. In Textbook on Scar Management; Springer: Cham, Switzerland, 2020; pp. 29–35. [Google Scholar]
- Elsaie, M.L. Update on management of keloid and hypertrophic scars: A systemic review. J. Cosmet. Dermatol. 2021, 20, 2729–2738. [Google Scholar] [CrossRef]
- Abubakar, A.R.; Haque, M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioallied Sci. 2020, 7, 1–5. [Google Scholar] [CrossRef]
- Macarak, E.J.; Wermuth, P.J.; Rosenbloom, J.; Uitto, J. Keloid disorder: Fibroblast differentiation and gene expression profile in fibrotic skin diseases. Exp. Dermatol. 2021, 30, 132–145. [Google Scholar] [CrossRef]
- Dowling, C.M.; Kiely, P.A. Targeting protein Kinase C downstream of growth factor and adhesion signalling. Cancers 2015, 7, 1271–1291. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.F.; Wang, Z.C.; Lou, D.; Fang, Q.Q.; Hu, Y.Y.; Zhao, W.-Y.; Zhang, L.-Y.; Wu, L.-H.; Tan, W.-Q. Current potential therapeutic strategies targeting the TGF-β/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed. Pharmacother. 2020, 129, 110287. [Google Scholar] [CrossRef]
- Zhang, Y.E. Non-Smad pathways in TGF-β signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef]
- Yang, J.F.; Wang, F.; Chen, Y.Z.; Hao, G.F.; Yang, G.F. LARMD: Integration of bioinformatic resources to profile ligand-driven protein dynamics with a case on the activation of estrogen receptor. Brief. Bioinform. 2020, 21, 2206–2218. [Google Scholar] [CrossRef]
- Lica, J.J.; Wieczór, M.; Grabe, G.J.; Heldt, M.; Jancz, M.; Misiak, M.; Gucwa, K.; Brankiewicz, W.; Maciejewska, N.; Stupak, A.; et al. Effective drug concentration and selectivity depends on fraction of primitive cells. Int. J. Mol. Sci. 2021, 22, 4931. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Insights into How Plant-Derived Extracts and Compounds Can Help in the Prevention and Treatment of Keloid Disease: Established and Emerging Therapeutic Targets. Int. J. Mol. Sci. 2024, 25, 1235. [Google Scholar] [CrossRef] [PubMed]

| Gambir’s Sample | Yield (%) | Total Phenolic (mg GAE/g) | Total Flavonoid (mg QE/g) |
|---|---|---|---|
| Crude extract | 44 | 33.4 | 19.59 |
| Ethanol Fraction | 10.95 | 34.1 | 17.09 |
| Hexane Fraction | 25.61 | 34.0 | 12.39 |
| Ethyl Acetate Fraction | 1.14 | 33.4 | 13.33 |
| Sample | RT (min) | Abundance (%) | m/z | Formula | Suspect Compound |
|---|---|---|---|---|---|
| Crude extract | 3.41 | 1.91 | 579.15 | C30H26O12 | Procyanidin B1 |
| 4.27 | 0.35 | 581.17 | C30H28O12 | Gambiriin A1 | |
| 6.95 | 2.23 | 333.16 | C21H20N2O2 | Dihydrogambirtannine | |
| 7.28 | 14.46 | 621.22 | C36H32N2O8 | Uncariagambiriine | |
| Ethanol fraction | 1.28 | 0.97 | 579.15 | C30H26O12 | Procyanidin B1 |
| 4.29 | 0.36 | 581.16 | C30H28O12 | Gambiriin A1 | |
| 7.01 | 4.50 | 333.16 | C21H20N2O2 | Dihydrogambirtannine | |
| 7.26 | 8.27 | 621.22 | C36H32N2O8 | Uncariagambiriine | |
| 8.53 | 1.96 | 345.12 | C21H16N2O3 | Oxogambirtannine | |
| Ethyl acetate fraction | 1.28 | 0.85 | 579.15 | C30H26O12 | Procyanidin B1 |
| 4.29 | 0.34 | 581.16 | C30H28O12 | Gambiriin A2 | |
| 7.01 | 3.61 | 333.16 | C21H20N2O2 | Dihydrogambirtannine | |
| 7.26 | 7.10 | 621.22 | C36H32N2O8 | Uncariagambiriine | |
| 8.51 | 2.73 | 345.12 | C21H16N2O3 | Oxogambirtannine | |
| Hexne fraction | 6.99 | 4.30 | 333.16 | C21H20N2O2 | Dihydrogambirtannine |
| 7.24 | 8.19 | 621.22 | C36H32N2O8 | Uncariagambiriine |
| Gambir Bioactive Compounds | Binding Affinity (Kcal/mol) | |
|---|---|---|
| TGFβ1 | Akt | |
| Procyanidin B1 | −8.05 | −9.33 |
| Gambiriin A1 | −6.03 | −9.17 |
| Gambiriin A2 | −5.41 | −10.51 |
| Dihydrogambirtannine | −6.75 | −9.18 |
| Uncariagambiriine | −8.24 | −11.02 |
| Oxogambirtannine | −7.16 | −8.84 |
| Native ligand | −7.21 | −10.73 |
| Sample | IC50 KF | IC50 NF | SI (IC50 KF/NF) |
|---|---|---|---|
| Crude extract | 249.56 ± 0.22 | 1096.42 ± 0.52 | 4.39 |
| Ethanol fraction | 128.76 ± 0.24 | 780.58 ± 0.19 | 6.06 |
| Ethyl acetate fraction | 238.79 ± 0.23 | 1012.03 ± 0.48 | 4.24 |
| Hexane fraction | 162.07 ± 0.26 | 570.23 ± 0.49 | 3.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ningsih, S.S.; Jusman, S.W.A.; Syaidah, R.; Budiman, M.A.; Khatib, A.; Fadilah, F. Unraveling Potential Compounds of Uncaria gambir (W.Hunter) Roxb. as Antikeloid Agent: In Silico, In Vitro and Ex Vivo Experimental Validation. Biologics 2025, 5, 18. https://doi.org/10.3390/biologics5030018
Ningsih SS, Jusman SWA, Syaidah R, Budiman MA, Khatib A, Fadilah F. Unraveling Potential Compounds of Uncaria gambir (W.Hunter) Roxb. as Antikeloid Agent: In Silico, In Vitro and Ex Vivo Experimental Validation. Biologics. 2025; 5(3):18. https://doi.org/10.3390/biologics5030018
Chicago/Turabian StyleNingsih, Sri Suciati, Sri Widia A. Jusman, Rahimi Syaidah, Muhamad Arif Budiman, Alfi Khatib, and Fadilah Fadilah. 2025. "Unraveling Potential Compounds of Uncaria gambir (W.Hunter) Roxb. as Antikeloid Agent: In Silico, In Vitro and Ex Vivo Experimental Validation" Biologics 5, no. 3: 18. https://doi.org/10.3390/biologics5030018
APA StyleNingsih, S. S., Jusman, S. W. A., Syaidah, R., Budiman, M. A., Khatib, A., & Fadilah, F. (2025). Unraveling Potential Compounds of Uncaria gambir (W.Hunter) Roxb. as Antikeloid Agent: In Silico, In Vitro and Ex Vivo Experimental Validation. Biologics, 5(3), 18. https://doi.org/10.3390/biologics5030018






