Insights Gained from the Immune Response and Screening of Healthcare Workers After COVID-19 Vaccination
Abstract
1. Introduction
2. Materials and Methods
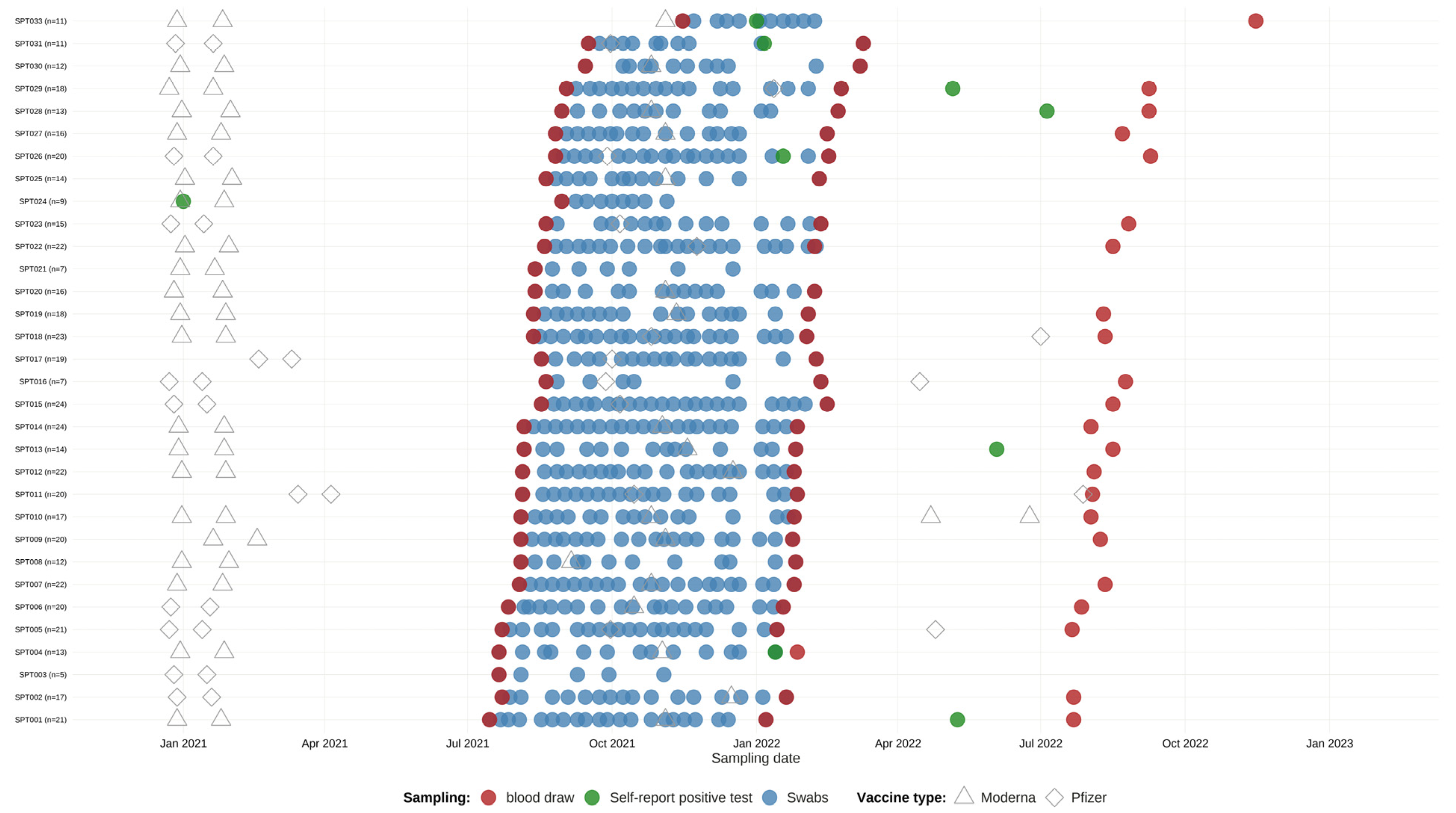
2.1. Screening for Healthcare Workers
2.2. Detection of Antibody Levels: Multi-Antigen Serology Panel [Genalyte]
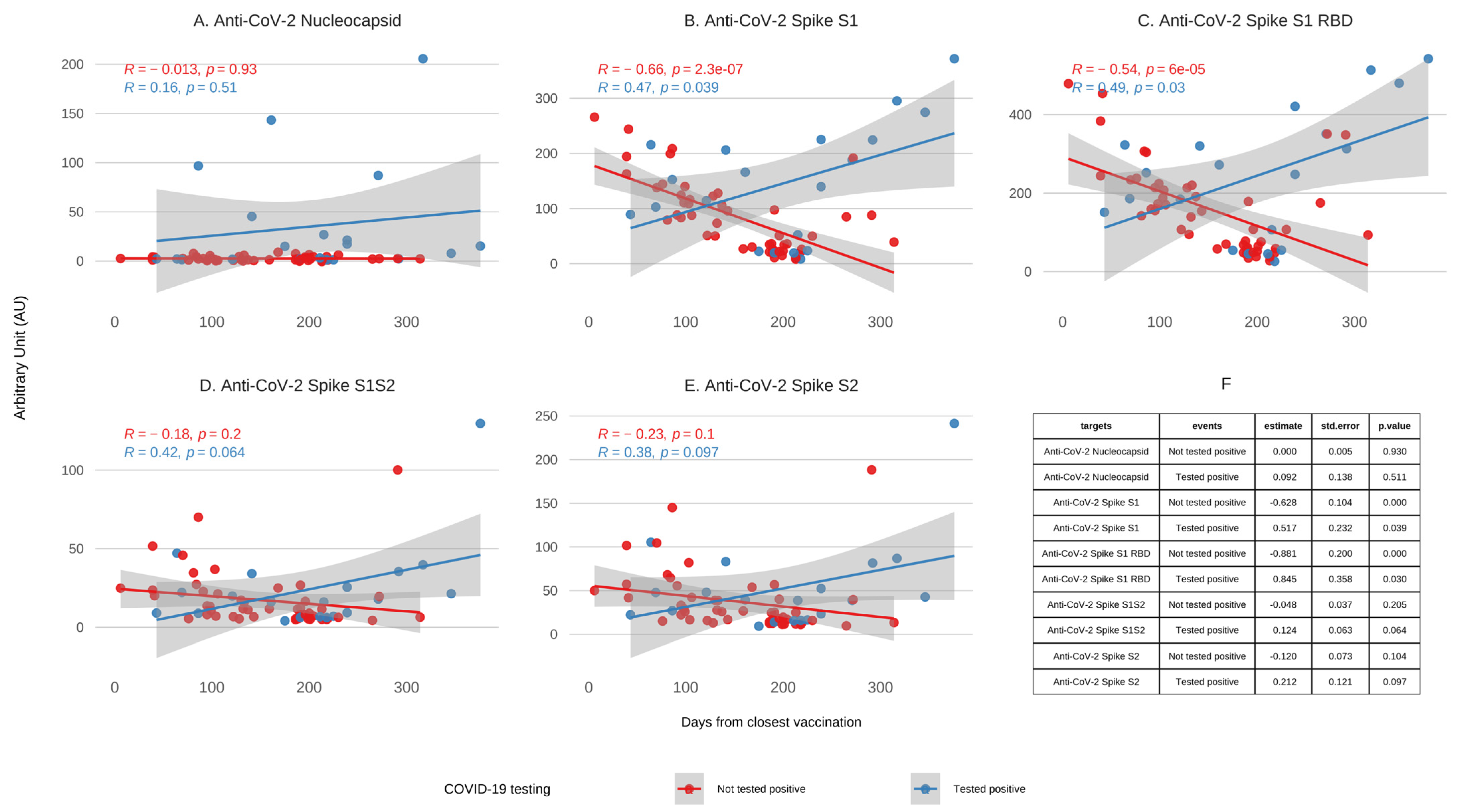
2.3. Antibody Half-Life Estimation
- Ct is the antibody concentration at time t,
- Co is the estimated concentration at time zero (intercept),
- k is the elimination rate constant (slope of the regression),
- t is time since injection.
3. Results
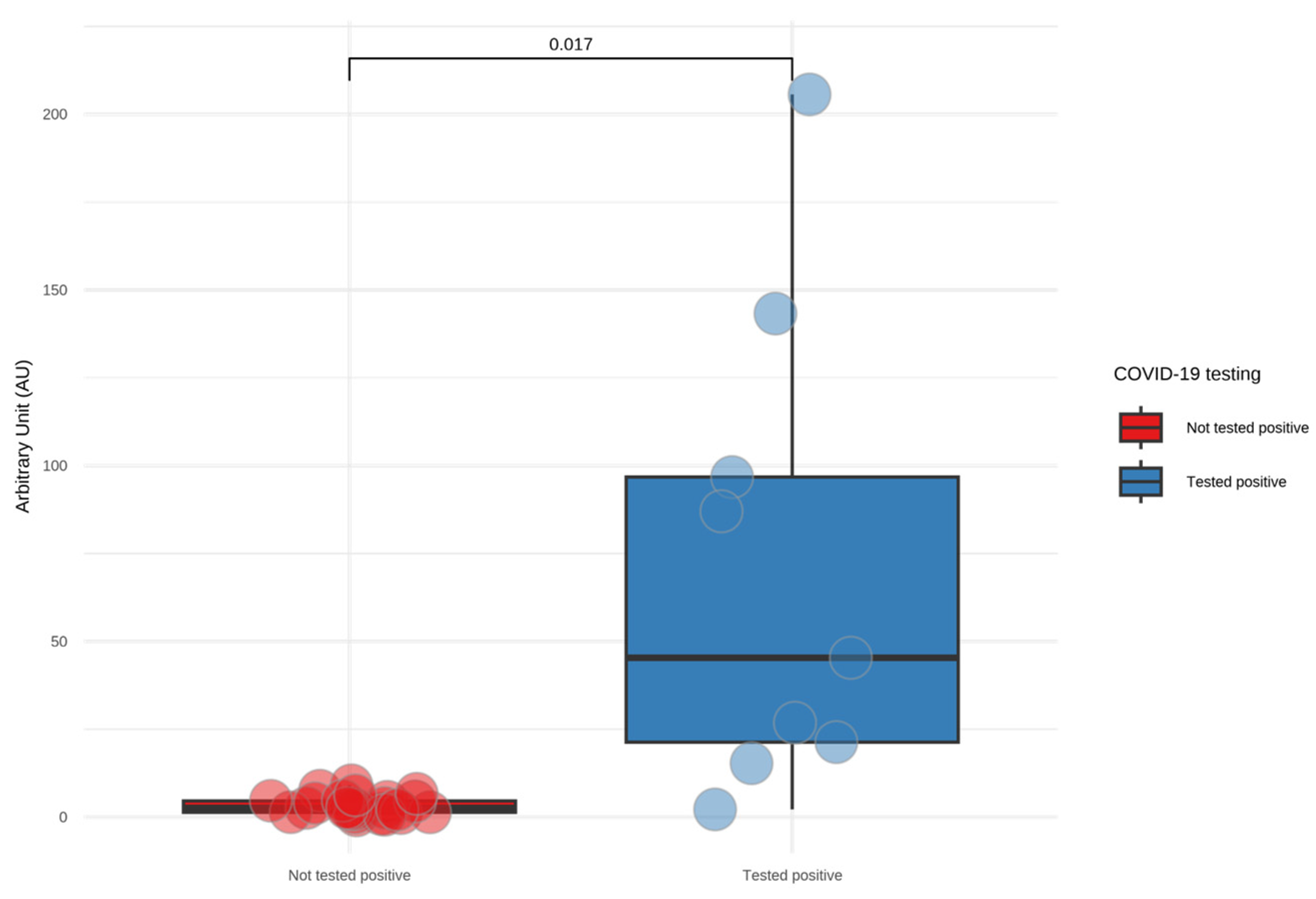
3.1. Screening HCWs for SARS-CoV-2 Infection
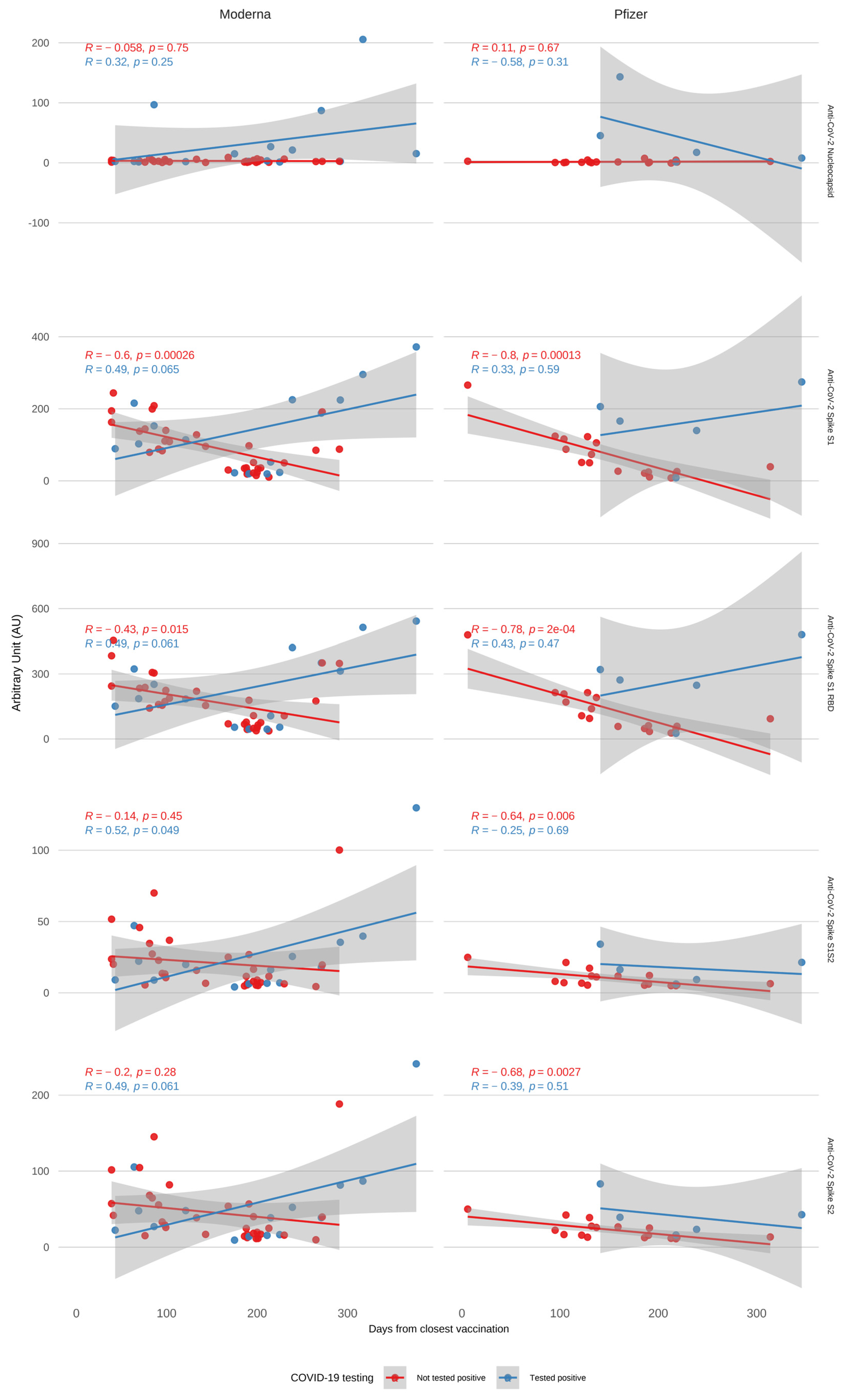
3.2. Antibody Kinetics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HCW | Healthcare Worker |
| RDB | Receptor-Binding Domain |
| NC | Nucleocapsid |
| PPE | Personal Protective Equipment |
| CDC | Centers for Disease Control and Prevention |
| VTM | Virus Transport Media |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| AU | Arbitrary Units |
| GRU | Genalyte Response Units |
| OLS | Ordinary Least Squares |
| IQR | Interquartile Range |
| BMI | Body Mass Index |
| VOI | Variants of Interest |
Appendix A
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 12 March 2025).
- World Health Organization. Health and Care Worker Deaths During COVID-19. Available online: https://www.who.int/news/item/20-10-2021-health-and-care-worker-deaths-during-covid-19 (accessed on 12 March 2025).
- Prévost, J.; Finzi, A. The great escape? SARS-CoV-2 variants evading neutralizing responses. Cell Host. Microbe 2021, 29, 322–324. [Google Scholar] [CrossRef]
- Stokel-Walker, C. COVID-19: The countries that have mandatory vaccination for health workers. BMJ 2021, 373, n1645. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.; Meng, L.; Barbre, K.; Wong, E.; Lape-Newman, B.; Koech, W.; Soe, M.M.; Woods, A.; Kuhar, D.T.; Stuckey, M.J.; et al. Influenza and COVID-19 Vaccination Coverage Among Health Care Personnel—National Healthcare Safety Network, United States, 2023–2024 Respiratory Virus Season. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 966–972. [Google Scholar] [CrossRef]
- Axios Less than 1 in 6 Health Workers Got COVID Booster. Available online: https://www.axios.com/2024/11/04/health-workers-covid-booster-rates?utm_source=chatgpt.com (accessed on 12 March 2025).
- Vaishya, R.; Sibal, A.; Malani, A.; Prasad, K.H. SARS-CoV-2 infection after COVID-19 immunization in healthcare workers: A retrospective, pilot study. Indian J. Med. Res. 2021, 153, 550–554. [Google Scholar] [CrossRef]
- Kasztelewicz, B.; Skrok, K.; Burzyńska, J.; Migdał, M.; Dzierżanowska-Fangrat, K. Incidence of SARS-CoV-2 infection among healthcare workers before and after COVID-19 vaccination in a tertiary paediatric hospital in Warsaw: A retrospective cohort study. PLoS ONE 2024, 19, e0301612. [Google Scholar] [CrossRef]
- Xiang, T.; Liang, B.; Fang, Y.; Lu, S.; Li, S.; Wang, H.; Li, H.; Yang, X.; Shen, S.; Zhu, B.; et al. Declining Levels of Neutralizing Antibodies Against SARS-CoV-2 in Convalescent COVID-19 Patients One Year Post Symptom Onset. Front. Immunol. 2021, 12, 708523. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, S.A.; Scott, B.; Layman, L.; Naranatt, P.; Heltsley, R.; Ignacio, C.; Porrachia, M.; Gianella, S.; Smith, D.; Chaillon, A. Can’t Work From Home: Pooled Nucleic Acid Testing of Laboratory Workers During the COVID-19 Pandemic. Open Forum Infect. Dis. 2021, 8, ofab129. [Google Scholar] [CrossRef]
- Donato, L.J.; Theel, E.S.; Baumann, N.A.; Bridgeman, A.R.; Blommel, J.H.; Wu, Y.; Karon, B.S. Evaluation of the genalyte maverick SARS-CoV-2 multi-antigen serology panel. J. Clin. Virol. Plus 2021, 1, 100030. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administraiton (FDA) Maverick™ SARS-CoV-2 Multi-Antigen Serology Panel v2; 2021. Available online: https://www.fda.gov/media/142915/download (accessed on 12 June 2025).
- RStudio Team. RStudio: Integrated Development for R; Version 2024.12.1.563; RStudio: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 12 June 2025).
- Marcotte, H.; Piralla, A.; Zuo, F.; Du, L.; Cassaniti, I.; Wan, H.; Kumagai-Braesh, M.; Andréll, J.; Percivalle, E.; Sammartino, J.C.; et al. Immunity to SARS-CoV-2 up to 15 months after infection. iScience 2022, 25, 103743. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention Science Brief: SARS-CoV-2 Infection-Induced and Vaccine-Induced Immunity. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/vaccine-induced-immunity.html (accessed on 12 March 2025).
- Dai, Y.C.; Lin, Y.C.; Ching, L.L.; Tsai, J.J.; Ishikawa, K.; Tsai, W.Y.; Chen, J.J.; Nerurkar, V.R.; Wang, W.K. Determining the Time of Booster Dose Based on the Half-Life and Neutralization Titers against SARS-CoV-2 Variants of Concern in Fully Vaccinated Individuals. Microbiol. Spectr. 2023, 11, e0408122. [Google Scholar] [CrossRef]
- European Medicines Agency Regulatory Requirements for Vaccines Intended to Provide Protection Against Variant Strain(s) of SARS-CoV-2-Scientific Guideline 2021. Available online: https://www.ema.europa.eu/en/regulatory-requirements-vaccines-intended-provide-protection-against-variant-strains-sars-cov-2-scientific-guideline (accessed on 12 June 2025).
- Centers for Disease Control and Prevention Interim Clinical Considerations for Use of COVID-19 Vaccines in the United States. 2025. Available online: https://www.cdc.gov/covid/hcp/vaccine-considerations/index.html (accessed on 12 June 2025).
- U.S. Food and Drug Administration Emergency Use Authorization for Vaccines to Prevent COVID-19. 2022. Available online: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization (accessed on 12 June 2025).
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.B.; Fong, Y.; Benkeser, D.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022, 375, 43–50. [Google Scholar] [CrossRef]
- Burckhardt, R.M.; Dennehy, J.J.; Poon, L.L.M.; Saif, L.J.; Enquist, L.W. Are COVID-19 Vaccine Boosters Needed? The Science behind Boosters. J. Virol. 2022, 96, e0197321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, S.; Liu, Y.; Wang, S.; Peng, H.; Hao, Y.; Hong, K.; Li, D.; Shao, Y. Sequential Administration of SARS-CoV-2 Strains-Based Vaccines Effectively Induces Potent Immune Responses against Previously Unexposed Omicron Strain. Pathogens 2023, 12, 655. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Hao, Y.; Wang, S.; Li, D.; Ren, L.; Zhu, M.; Wang, S.; Li, J.; Tang, W.; Fu, Y.; et al. Sequential heterologous immunization with COVID-19 vaccines induces broader neutralizing responses against SARS-CoV-2 variants in comparison with homologous boosters. Vaccine 2023, 41, 6645–6653. [Google Scholar] [CrossRef]
- World Health Organization Tracking SARS-COV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 12 March 2025).
- Padoan, A.; Cosma, C.; Della Rocca, F.; Barbaro, F.; Santarossa, C.; Dall’Olmo, L.; Galla, L.; Cattelan, A.; Cianci, V.; Basso, D.; et al. A cohort analysis of SARS-CoV-2 anti-spike protein receptor binding domain (RBD) IgG levels and neutralizing antibodies in fully vaccinated healthcare workers. Clin. Chem. Lab. Med. 2022, 60, 1110–1115. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Tseng, H.F.; Ackerson, B.K.; Luo, Y.; Sy, L.S.; Talarico, C.A.; Tian, Y.; Bruxvoort, K.J.; Tubert, J.E.; Florea, A.; Ku, J.H.; et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat. Med. 2022, 28, 1063–1071. [Google Scholar] [CrossRef]
- Skrzat-Klapaczyńska, A.; Bieńkowski, C.; Kowalska, J.; Paciorek, M.; Puła, J.; Krogulec, D.; Stengiel, J.; Pawełczyk, A.; Perlejewski, K.; Osuch, S.; et al. The Beneficial Effect of the COVID-19 Vaccine Booster Dose among Healthcare Workers in an Infectious Diseases Center. Vaccines 2022, 10, 552. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, Y.R.; Heo, S.T.; Oh, H.; Kim, M.; Lee, H.R.; Yoo, J.R. Healthcare Workers in South Korea Maintain a SARS-CoV-2 Antibody Response Six Months After Receiving a Second Dose of the BNT162b2 mRNA Vaccine. Front. Immunol. 2022, 13, 827306. [Google Scholar] [CrossRef] [PubMed]
- Davis-Gardner, M.E.; Lai, L.; Wali, B.; Samaha, H.; Solis, D.; Lee, M.; Porter-Morrison, A.; Hentenaar, I.T.; Yamamoto, F.; Godbole, S.; et al. Neutralization against BA.2.75.2, BQ.1.1, and XBB from mRNA Bivalent Booster. N. Engl. J. Med. 2023, 388, 183–185. [Google Scholar] [CrossRef]
- Chalkias, S.; Harper, C.; Vrbicky, K.; Walsh, S.R.; Essink, B.; Brosz, A.; McGhee, N.; Tomassini, J.E.; Chen, X.; Ying, C.; et al. Three-month antibody persistence of a bivalent Omicron-containing booster vaccine against COVID-19. Nat. Commun. 2023, 14, 5125. [Google Scholar] [CrossRef]
- Collier, A.Y.; Miller, J.; Hachmann, N.P.; McMahan, K.; Liu, J.; Bondzie, E.A.; Gallup, L.; Rowe, M.; Schonberg, E.; Thai, S.; et al. Immunogenicity of BA.5 Bivalent mRNA Vaccine Boosters. N. Engl. J. Med. 2023, 388, 565–567. [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant—United States, December 1–8, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1731–1734. [Google Scholar] [CrossRef]
- Lambrou, A.S.; Shirk, P.; Steele, M.K.; Paul, P.; Paden, C.R.; Cadwell, B.; Reese, H.E.; Aoki, Y.; Hassell, N.; Zheng, X.Y.; et al. Genomic Surveillance for SARS-CoV-2 Variants: Predominance of the Delta (B.1.617.2) and Omicron (B.1.1.529) Variants—United States, June 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 206–211. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.C.; Tiu, C.; Hu, Z.; Chen, V.C.-W.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Tan, C.W.; Chia, W.N.; Zhu, F.; Young, B.; Chantasrisawad, N.; Hwa, S.H.; Yeoh, A.Y.; Lim, B.L.; Yap, W.C.; et al. Differential escape of neutralizing antibodies by SARS-CoV-2 Omicron and pre-emergent sarbecoviruses. Res. Sq. 2022, rs.3.rs-1362541. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef] [PubMed]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847–859.e11. [Google Scholar] [CrossRef]
| Variables (N) | Values |
|---|---|
| Age (32): | 44 [40.9;47.1] |
| Gender (32): | |
| Female | 21 (65.6%) |
| Male | 10 (31.2%) |
| Non-Binary | 1 (3.12%) |
| Ethnicity (32): | |
| Hispanic or Latino/a | 9 (28.1%) |
| Not Hispanic or Latino/a | 23 (71.9%) |
| Race (32): | |
| Asian | 7 (21.9%) |
| Black or African American | 1 (3.12%) |
| Native American | 1 (3.12%) |
| White Caucasian | 23 (71.9%) |
| BMI (30): | 24.4 [19.5;34.3] |
| Prior COVID Diagnosis (32) | 2 (6.25%) |
| Vaccination (32): | |
| Yes | 32 (100%) |
| Initial Vaccine Type (32): | |
| Moderna | 21 (65.6%) |
| Pfizer | 11 (34.3%) |
| Vaccine Dosage (32): | |
| First Dose | 32 (100%) |
| Second Dose | 32 (100%) |
| Third Dose | 29 (90.6%) |
| Fourth Dose | 5 (15.6%) |
| Fifth Dose | 1 (3.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, D.M.; Huynh, J.; Pham, B.; Porrachia, M.; Ignacio, C.; Mudumba, S.; Kuizon, C.N.; Gianella, S.; Chaillon, A. Insights Gained from the Immune Response and Screening of Healthcare Workers After COVID-19 Vaccination. COVID 2025, 5, 129. https://doi.org/10.3390/covid5080129
Smith DM, Huynh J, Pham B, Porrachia M, Ignacio C, Mudumba S, Kuizon CN, Gianella S, Chaillon A. Insights Gained from the Immune Response and Screening of Healthcare Workers After COVID-19 Vaccination. COVID. 2025; 5(8):129. https://doi.org/10.3390/covid5080129
Chicago/Turabian StyleSmith, Davey M., Jonathan Huynh, Bryan Pham, Magali Porrachia, Caroline Ignacio, Sasi Mudumba, Cristina N. Kuizon, Sara Gianella, and Antoine Chaillon. 2025. "Insights Gained from the Immune Response and Screening of Healthcare Workers After COVID-19 Vaccination" COVID 5, no. 8: 129. https://doi.org/10.3390/covid5080129
APA StyleSmith, D. M., Huynh, J., Pham, B., Porrachia, M., Ignacio, C., Mudumba, S., Kuizon, C. N., Gianella, S., & Chaillon, A. (2025). Insights Gained from the Immune Response and Screening of Healthcare Workers After COVID-19 Vaccination. COVID, 5(8), 129. https://doi.org/10.3390/covid5080129






