Microplastics in Freshwater River in Rio de Janeiro and Its Role as a Source of Microplastic Pollution in Guanabara Bay, SE Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Preparation and Analyses
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lithner, D.; Larsson, Å.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- PlasticsEurope. 2018. Annual Review 2017–2018. Available online: https://www.plasticseurope.org/download_file/force/1830/181 (accessed on 28 December 2022).
- Galgani, F.; Hanke, G.; Maes, T. Global Distribution, Composition and Abundance of Marine Litter. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Waring, R.; Harris, R.; Mitchell, S. Plastic contamination of the food chain: A threat to human health? Maturitas 2018, 115, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Ding, Y.; Zou, X.; Wang, C.; Feng, Z.; Wang, Y.; Fan, Q.; Chen, H. The abundance and characteristics of atmospheric microplastic deposition in the northwestern South China Sea in the fall. Atmos. Environ. 2021, 253, 118389. [Google Scholar] [CrossRef]
- PlasticEurope. Plastics—The Facts 2020 An Analysis of European Plastics Production, Demand and Waste Data; Plastic Europe: Brussels, Belgium, 2020. [Google Scholar]
- Neves, C.V.; Gaylarde, C.C.; Neto, J.A.B.; Vieira, K.S.; Pierri, B.; Waite, C.C.; Scott, D.C.; da Fonseca, E.M. The transfer and resulting negative effects of nano- and micro-plastics through the aquatic trophic web—A discreet threat to human health. Water Biol. Secur. 2022, 1, 100080. [Google Scholar] [CrossRef]
- GESAMP; Anderson, A.; Andrady, A.; Arthur, C.; Baker, J.; Bouwman, H.; Gall, S.; Hildalgo-Ruz, V.; Köhler, A.; Lavender Law, K.; et al. Sources, Fate and Effects of Microplastics in the Environment: A Global Assessment; GESAMP Reports&Studies Series; No. 90; International Maritime Organization: London, UK, 2015; Available online: http://www.gesamp.org/data/gesamp/files/media/Publications/Reports_and_studies_90/gallery_2230/object_2500_large.pdf (accessed on 28 December 2022).
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Persistence of Plastic Litter in the Oceans Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 57–72. [Google Scholar]
- Xu, C.; Zhang, B.; Gu, C.; Shen, C.; Yin, S.; Aamir, M.; Li, F. Are we underestimating the sources of microplastic pollution in terrestrial environment? J. Hazard. Mater. 2020, 400, 123228. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Santos, I.R.; Friedrich, A.C.; Sul, J.A.I.D. Marine debris contamination along undeveloped tropical beaches from northeast Brazil. Environ. Monit. Assess. 2009, 148, 455–462. [Google Scholar] [CrossRef]
- Hirai, H.; Takada, H.; Ogata, Y.; Yamashita, R.; Mizukawa, K.; Saha, M.; Kwan, C.; Moore, C.; Gray, H.; Laursen, D.; et al. Organic micropollutants in marine plastics debris from the open ocean and remote and urban beaches. Mar. Pollut. Bull. 2011, 62, 1683–1692. [Google Scholar] [CrossRef]
- Ivar Do Sul, J.A.; Costa, M.F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef]
- Laglbauer, B.J.; Franco-Santos, R.M.; Andreu-Cazenave, M.; Brunelli, L.; Papadatou, M.; Palatinus, A.; Grego, M.; Deprez, T. Macrodebris and microplastics from beaches in Slovenia. Mar. Pollut. Bull. 2014, 89, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Amador, E.S. Bacia da Baía de Guanabara: Características Geoambientais, Formação e Ecossistemas; Interciência: Rio de Janeiro, Brazil, 2012; 432p. [Google Scholar]
- Soares-Gomes, A.; da Gama, B.; Neto, J.B.; Freire, D.; Cordeiro, R.; Machado, W.; Bernardes, M.; Coutinho, R.; Thompson, F.; Pereira, R. An environmental overview of Guanabara Bay, Rio de Janeiro. Reg. Stud. Mar. Sci. 2016, 8, 319–330. [Google Scholar] [CrossRef]
- Baptista Neto, J.A.; Gingele, F.X.; Leipe, T.; Brehme, I. Spatial distribution of trace elements in surficial sediments from Guanabara Bay–Rio de Janeiro/Brazil. Environ. Geol. 2006, 49, 1051–1063. [Google Scholar] [CrossRef]
- Baptista Neto, J.A.; Peixoto, T.C.S.; Smith, B.J.; McAlister, J.J.; Patchineelam, S.M.; Patchineelam, S.R.; Fonseca, E.M. Geochronology and heavy metal flux to Guanabara Bay, Rio de Janeiro state: A preliminary study. Ann. Braz. Acad. Sci. 2013, 85, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, V.M.D.C.; Abuchacra, P.F.F.; Neto, J.A.B.; de Oliveira, A.S. Environmental assessment concerning trace metals and ecological risks at Guanabara Bay, RJ, Brazil. Environ. Monit. Assess. 2018, 190, 448. [Google Scholar] [CrossRef] [PubMed]
- Baptista Neto, J.; Fonseca, E.M. Variação sazonal, espacial e composicional de lixo ao longo das praias da margem oriental da Baía de Guanabara (Rio de Janeiro) no período de 1999–2008. Rev. Gestão Costeira Integr. 2011, 11, 31–39. [Google Scholar] [CrossRef]
- Bernardino, D.; Franz, B. Lixo flutuante na Baía de Guanabara: Passado, presente e perspectivas para o futuro. Desenvolv. Meio Ambient. 2016, 38, 231–252. [Google Scholar] [CrossRef]
- Castro, R.O.; Silva, M.L.; Marques, M.R.C.; de Araújo, F.V. Evaluation of microplastics in Jurujuba Cove, Niterói, RJ, Brazil, an area of mussels farming. Mar. Pollut. Bull. 2016, 110, 555–558. [Google Scholar] [CrossRef]
- Figueiredo, G.M.; Vianna, T.M.P. Suspended microplastics in a highly polluted bay: Abundance, size, and availability for mesozooplankton. Mar. Pollut. Bull. 2018, 135, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.E.; Figueiredo, G.M. Microplastic in the sediments of a highly eutrophic tropical estuary. Mar. Pollut. Bull. 2019, 146, 326–335. [Google Scholar] [CrossRef]
- Olivatto, G.P.; Martins, M.C.T.; Montagner, C.C.; Henry, T.B.; Carreira, R.S. Microplastic contamination in surface waters in Guanabara Bay, Rio de Janeiro, Brazil. Mar. Pollut. Bull. 2019, 139, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Maldonado, G.C.; Castro, R.O.; Felizardo, J.D.S.; Cardoso, R.P.; dos Anjos, R.M.; de Araújo, F.V. Dispersal of potentially pathogenic bacteria by plastic debris in Guanabara Bay, RJ, Brazil. Mar. Pollut. Bull. 2019, 141, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.G.; Baptista Neto, J.A. Microplastic pollution of the beaches of Guanabara Bay, Southeast Brazil. Ocean Coast. Manag. 2016, 128, 10–17. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vieira, L.R.; Guilhermino, L. Single and combined effects of microplastics and mercury on juveniles of the European seabass (Dicentrarchus labrax): Changes in behavioural responses and reduction of swimming velocity and resistance time. Environ. Pollut. 2018, 236, 1014–1019. [Google Scholar] [CrossRef]
- Rochman, C.M. Microplastics research—From sink to source. Science 2018, 360, 28–29. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Kjerfve, B.; Ribeiro, C.H.A.; Dias, G.T.M.; Filippo, A.M.; Da Silva Quaresma, V. Oceanographic characteristics of an impacted coastal bay: Baía de Guanabara, Rio de Janeiro, Brazil. Cont. Shelf Res. 1997, 17, 1609–1643. [Google Scholar] [CrossRef]
- Catanzaro, L.F.; Neto, J.A.B.; Guimarães, M.S.D.; Silva, C.G. Distinctive sedimentary processes in Guanabara Bay—SE/Brazil, based on the analysis of echo-character (7.0 kHz). Braz. J. Geophys. 2004, 22, 69–83. [Google Scholar] [CrossRef]
- Fonseca, E.; Neto, J.B.; Pereira, M.; Silva, C.; Junior, J.A. Study of pollutant distribution in the Guaxindiba Estuarine System—SE Brazil. Mar. Pollut. Bull. 2014, 82, 45–54. [Google Scholar] [CrossRef]
- Borges, R.C.; Caldas, V.G.; Filho, F.F.L.S.; Ferreira, M.M.; Lapa, C.M.F. Use of GIS for the evaluation of heavy metal contamination in the Cunha Canal watershed and west of the Guanabara Bay, Rio de Janeiro, RJ. Mar. Pollut. Bull. 2014, 89, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Rezende, O.M.; Franco, A.B.R.D.C.D.; de Oliveira, A.K.B.; Jacob, A.C.P.; Miguez, M.G. A framework to introduce urban flood resilience into the design of flood control alternatives. J. Hydrol. 2019, 576, 478–493. [Google Scholar] [CrossRef]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C.; Herring, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters And Sediments; NOAA Technical Memorandum NOS-OR&R-48; NOAA Marine Debris Division: Siver Spring, MD, USA, 2015; p. 31. [CrossRef]
- McCormick, A.R.; Hoellein, T.J.; London, M.G.; Hittie, J.; Scott, J.W.; Kelly, J.J. Microplastic in surface waters of urban rivers: Concentration, sources, and associated bacterial assemblages. Ecosphere 2016, 7, e01556. [Google Scholar] [CrossRef]
- Cowger, W.; Steinmetz, Z.; Gray, A.; Munno, K.; Lynch, J.; Hapich, H.; Primpke, S.; De Frond, H.; Rochman, C.; Herodotou, O. Microplastic Spectral Classification Needs an Open Source Community: Open Specy to the Rescue! Anal. Chem. 2021, 93, 7543–7548. [Google Scholar] [CrossRef]
- Napper, I.E.; Baroth, A.; Barrett, A.C.; Bhola, S.; Chowdhury, G.W.; Davies, B.F.; Duncan, E.M.; Kumar, S.; Nelms, S.E.; Niloy, N.H.; et al. The abundance and characteristics of microplastics in surface water in the transboundary Ganges River. Environ. Pollut. 2021, 274, 116348. [Google Scholar] [CrossRef]
- Ding, L.; Mao, R.F.; Guo, X.; Yang, X.; Zhang, Q.; Yang, C. Microplastics in surface waters and sediments of the Wei River, in the northwest of China. Sci. Total Environ. 2019, 667, 427–434. [Google Scholar] [CrossRef]
- Bertoldi, C.; Lara, L.Z.; Mizushima, F.A.D.L.; Martins, F.C.; Battisti, M.A.; Hinrichs, R.; Fernandes, A.N. First evidence of microplastic contamination in the freshwater of Lake Guaíba, Porto Alegre, Brazil. Sci. Total Environ. 2020, 759, 143503. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Li, Y.; Powell, T.; Wang, X.; Wang, G.; Zhang, P. Microplastics as contaminants in the soil environment: A mini-review. Sci. Total Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A.; Machado, A.A.D.S.; Yang, G. Microplastic effects on plants. New Phytol. 2019, 223, 1066–1070. [Google Scholar] [CrossRef]
- Helmberger, M.S.; Tiemann, L.K.; Grieshop, M.J. Towards an ecology of soil microplastics. Funct. Ecol. 2019, 34, 550–560. [Google Scholar] [CrossRef]
- Mani, T.; Burkhardt-Holm, P. Seasonal microplastics variation in nival and pluvial stretches of the Rhine River—From the Swiss catchment towards the North Sea. Sci. Total Environ. 2020, 707, 135579. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Abrantes, N.; Gonçalves, F.; Nogueira, H.; Marques, J.; Gonçalves, A. Spatial and temporal distribution of microplastics in water and sediments of a freshwater system (Antuã River, Portugal). Sci. Total Environ. 2019, 633, 1549–1559. [Google Scholar] [CrossRef]
- Gago, J.; Carretero, O.; Filgueiras, A.V.; Viñas, L. Synthetic microfibers in the marine environment: A review on their occurrence in seawater and sediments. Mar. Pollut. Bull. 2018, 127, 365–376. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in soil: A review on methods, occurrence, sources, and potential risk. Sci. Total Environ. 2021, 780, 146546. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Imhof, H.; Sanchez, W.; Gasperi, J.; Galgani, F.; Tassin, B.; Laforsch, C. Beyond the ocean: Contamination of freshwater ecosystems with (micro-)plastic particles. Environ. Chem. 2015, 12, 539–550. [Google Scholar] [CrossRef]
- Browne, M.A. Sources and Pathways of Microplastics to Habitats. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Kapp, K.J.; Yeatman, E. Microplastic hotspots in the Snake and Lower Columbia rivers: A journey from the Greater Yellowstone Ecosystem to the Pacific Ocean. Environ. Pollut. 2018, 241, 1082–1090. [Google Scholar] [CrossRef]
- Wang, C.; Xing, R.; Sun, M.; Ling, W.; Shi, W.; Cui, S.; An, L. Microplastics profile in a typical urban river in Beijing. Sci. Total Environ. 2020, 743, 140708. [Google Scholar] [CrossRef]
- Harpah, N.; Ageng, P.; Addauwiyah, R.; Rizki, A.; Perdana, Z.; Suryati, I.; Leonardo, R.; Husin, A.; Faisal, M. Microplastic pollution in Deli River Medan. IOP Conf. Series Earth Environ. Sci. 2021, 802, 012019. [Google Scholar] [CrossRef]
- Gaylarde, C.; Baptista-Neto, J.A.; da Fonseca, E.M. Plastic microfibre pollution: How important is clothes’ laundering? Heliyon 2021, 7, e07105. [Google Scholar] [CrossRef]
- Wong, G.; Lowemark, L.; Kunz, A. Microplastic pollution of the Tamsui River and its tributaries in northern Taiwan: Spatial heterogeneity and correlation with precipitation. Environ. Pollut. 2020, 260, 113935. [Google Scholar] [CrossRef] [PubMed]
- Pariatamby, A.; Hamid, F.S.; Bhatti, M.S.; Anuar, N.; Anuar, N. Status of Microplastic Pollution in Aquatic Ecosystem with a Case Study on Cherating River, Malaysia. J. Eng. Technol. Sci. 2020, 52, 222. [Google Scholar] [CrossRef]
- Eo, S.; Hong, S.H.; Song, Y.K.; Han, G.M.; Shim, W.J. Spatiotemporal Distribution and Annual Load of Microplastics in the Nakdong River, South Korea. Water Res. 2019, 160, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T.; Nihei, Y.; Kudou, K.; Hinata, H. Assessment of the sources and inflow processes of microplastics in the river environments of Japan. Environ. Pollut. 2019, 244, 958–965. [Google Scholar] [CrossRef]
- Mccormick, A.R.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Microplastic is an Abundant and Distinct Microbial Habitat in an Urban River. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef]
- Hogue, C. Microplastic Beads Pollute Great Lakes. Chem. Eng. News 2013, 91, 23–25. [Google Scholar] [CrossRef]
- Weithmann, N.; Möller, J.N.; Löder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic fertilizer as a vehicle for the entry of microplastic into the environment. Sci. Adv. 2018, 4, eaap8060. [Google Scholar] [CrossRef]
- Akkajit, P.; Khongsang, A. Distribution of Microplastics along Mai Khao Coastline, Phuket. J. Eng. Technol. Sci. 2022, 54, 220105. [Google Scholar] [CrossRef]
- Rummel, C.D.; Löder, M.G.J.; Fricke, N.F.; Lang, T.; Griebeler, E.-M.; Janke, M.; Gerdts, G. Plastic ingestion by pelagic and demersal fish from the North Sea and Baltic Sea. Mar. Pollut. Bull. 2016, 102, 134–141. [Google Scholar] [CrossRef]
- Rios-Fuster, B.; Alomar, C.; Compa, M.; Guijarro, B.; Deudero, S. Anthropogenic particles ingestion in fish species from two areas of the western Mediterranean Sea. Mar. Pollut. Bull. 2019, 144, 325–333. [Google Scholar] [CrossRef]
- Thetford, D.; Chorlton, A.; Hardman, J. Synthesis and properties of some polycyclic barbiturate pigments. Dye. Pigment. 2003, 59, 185–191. [Google Scholar] [CrossRef]
- Duncan, E.M.; Arrowsmith, J.; Bain, C.; Broderick, A.C.; Lee, J.; Metcalfe, K.; Pikesley, S.K.; Snape, R.T.; van Sebille, E.; Godley, B.J. The true depth of the Mediterranean plastic problem: Extreme microplastic pollution on marine turtle nesting beaches in Cyprus. Mar. Pollut. Bull. 2018, 136, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wong, C.; Chen, D.; Lu, X.; Wang, F.; Zeng, E.Y. Interaction of toxic chemicals with microplastics: A critical review. Water Res. 2018, 139, 208–219. [Google Scholar] [CrossRef]
- Fries, E.; Dekiff, J.H.; Willmeyer, J.; Nuelle, M.-T.; Ebert, M.; Remy, D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci. Process. Impacts 2013, 15, 1949–1956. [Google Scholar] [CrossRef]
- Nascimento, M.; Oliveira-Santos, A.; Cunha, D.; Felix, L.; Gomes, G.; Rangel, C.; Hauser-Davis, R.; Fonseca, E.; Maia-Bila, D.; Baptista-Neto, J.A. Endocrine disruptors, estrogenic activity by the YES bioassay, and acute toxicity in Southeastern Brazil metropolitan surface waters. Geochim. Bras. 2022, 36, 1–14. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total Environ. 2018, 651, 3253–3268. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Song, Z.; Wei, N.; Li, D. Terrestrial plants as a potential temporary sink of atmospheric microplastics during transport. Sci. Total Environ. 2020, 742, 140523. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Liu, M.; Zhou, Q.; He, H.; Chen, K.; Zhang, H.; Hu, J.; Huang, Q.; Luo, Y.; Ke, H.; et al. Microplastics and polycyclic aromatic hydrocarbons (PAHs) in Xiamen coastal areas: Implications for anthropogenic impacts. Sci. Total Environ. 2018, 634, 811–820. [Google Scholar] [CrossRef]
- Hüffer, T.; Weniger, A.-K.; Hofmann, T. Sorption of organic compounds by aged polystyrene microplastic particles. Environ. Pollut. 2018, 236, 218–225. [Google Scholar] [CrossRef]
- Zhou, Q.; Tian, C.; Luo, Y. Various forms and deposition fluxes of microplastics identified in the coastal urban atmosphere. Chin. Sci. Bull. 2017, 62, 3902–3909. [Google Scholar] [CrossRef]
- Hodson, M.E.; Duffus-Hodson, C.A.; Clark, A.; Prendergast-Miller, M.T.; Thorpe, K.L. Plastic Bag Derived-Microplastics as a Vector for Metal Exposure in Terrestrial Invertebrates. Environ. Sci. Technol. 2017, 51, 4714–4721. [Google Scholar] [CrossRef] [PubMed]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Adsorption of trace metals to plastic resin pellets in the marine environment. Environ. Pollut. 2012, 160, 42–48. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Hentschel, B.T.; Kaye, S. Long-Term Field Measurement of Sorption of Organic Contaminants to Five Types of Plastic Pellets: Implications for Plastic Marine Debris. Environ. Sci. Technol. 2012, 47, 1646–1654. [Google Scholar] [CrossRef]
- Debroas, D.; Mone, A.; Ter Halle, A. Plastics in the North Atlantic garbage patch: A boat-microbe for hitchhikers and plastic degraders. Sci. Total Environ. 2017, 599, 1222–1232. [Google Scholar] [CrossRef]
- Murphy, L.; Germaine, K.; Dowling, D.N.; Kakouli-Duarte, T.; Cleary, J. Association of Potential Human Pathogens with Microplastics in Freshwater Systems. In Proceedings of the 2nd International Conference on Microplastic Pollution in the Mediterranean Sea, Capri, Italy, 15–18 September 2019; ICMPMS 2019; Cocca, M., Di Pace, E., Errico, M.E., Gentile, G., Montarsolo, A., Mossotti, R., Avella, M., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lu, S.; Song, Y.; Lei, L.; Hu, J.; Lv, W.; Zhou, W.; Cao, C.; Shi, H.; Yang, X.; et al. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ. Pollut. 2018, 242, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Graca, B.; Szewc, K.; Zakrzewska, D.; Dołęga, A.; Szczerbowska-Boruchowska, M. Sources and fate of microplastics in marine and beach sediments of the Southern Baltic Sea—A preliminary study. Environ. Sci. Pollut. Res. 2017, 24, 7650–7661. [Google Scholar] [CrossRef] [PubMed]
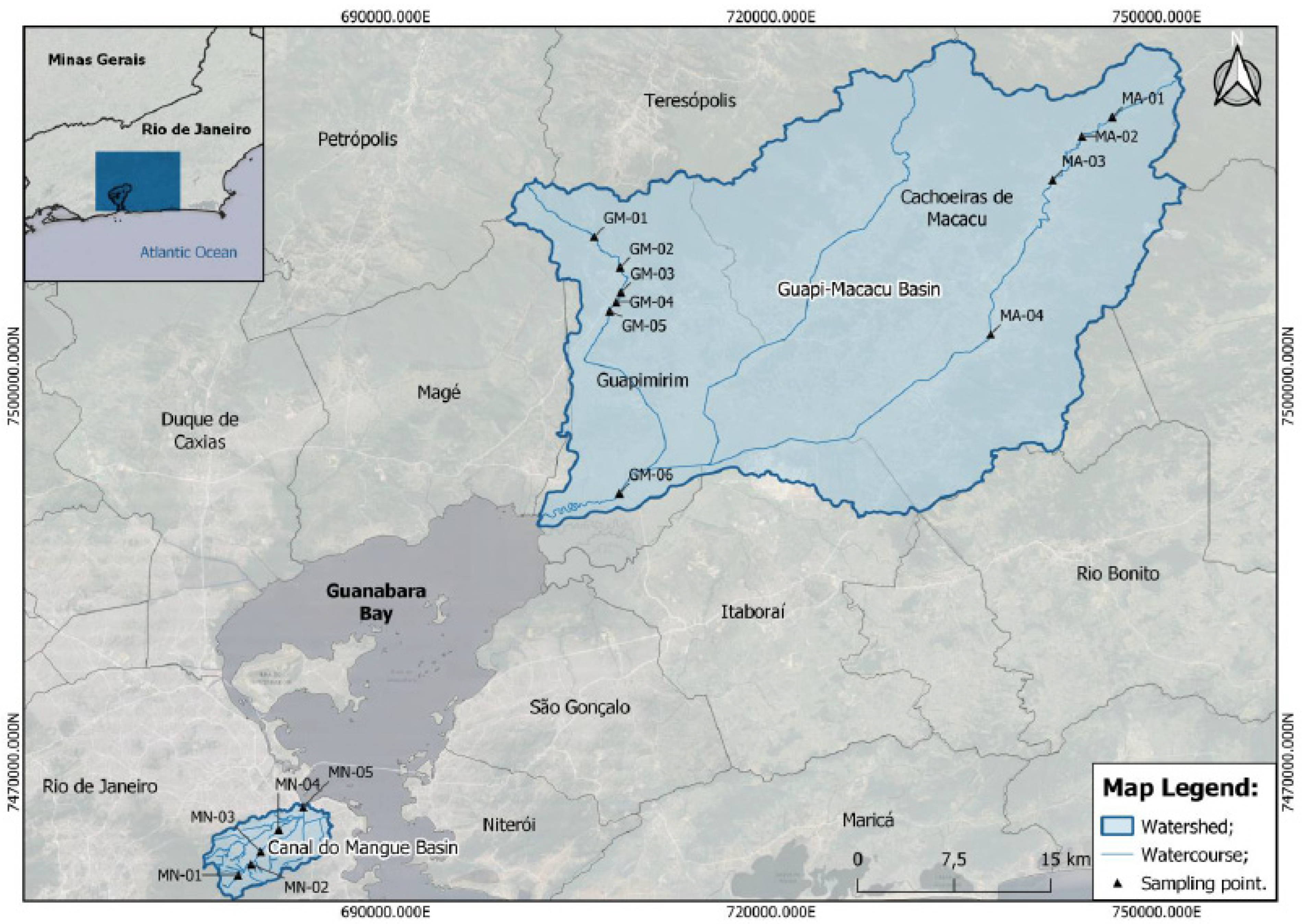

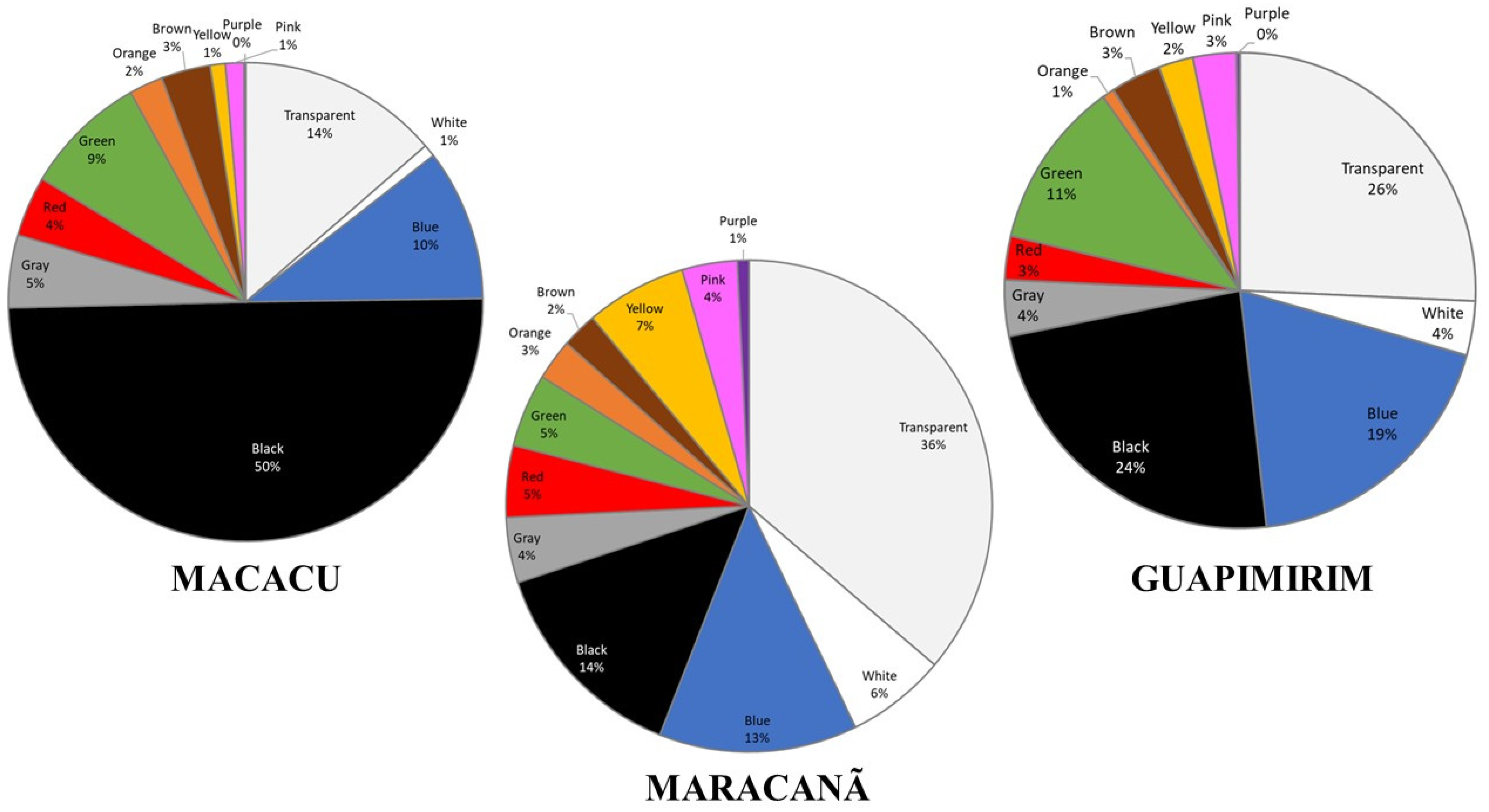
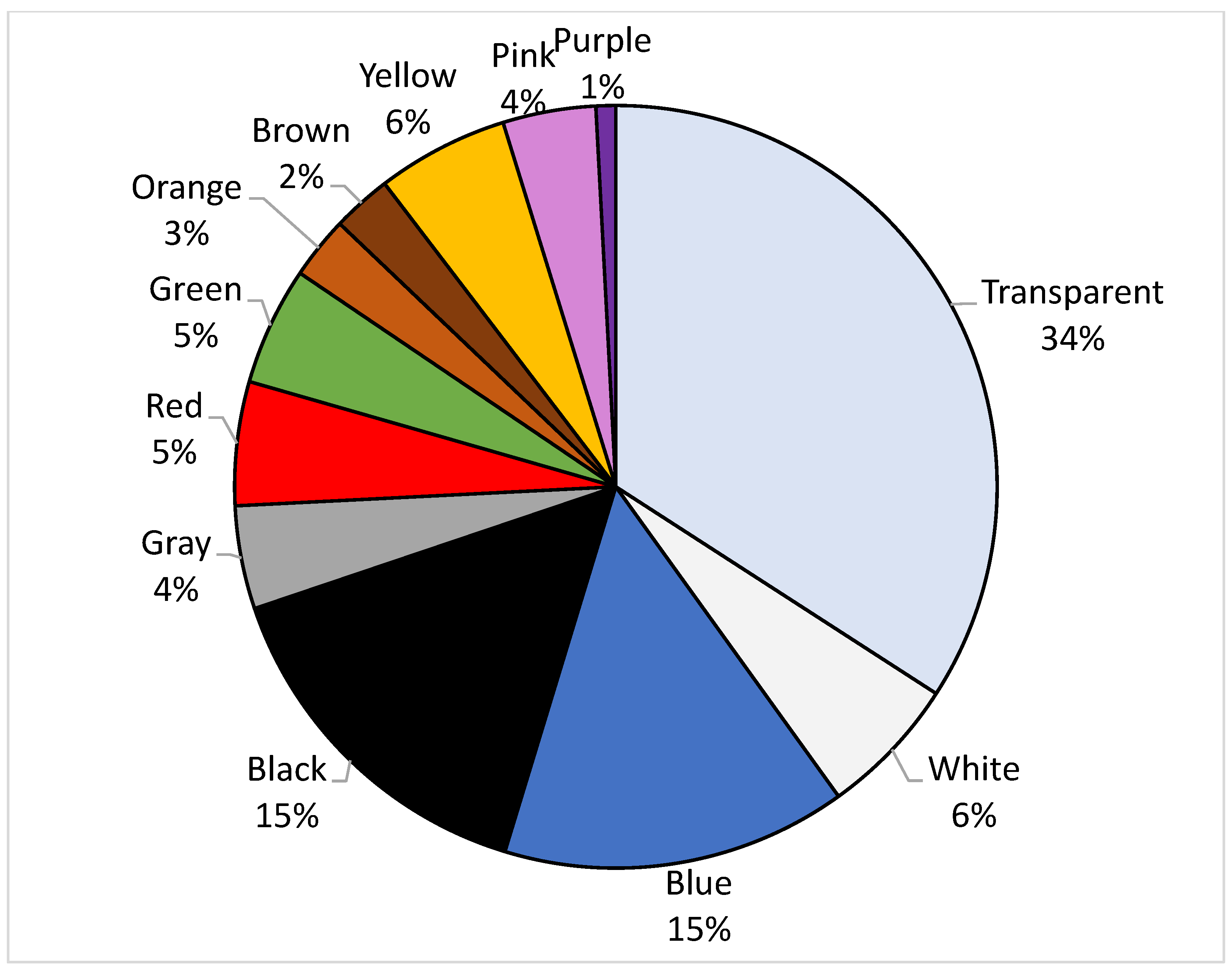
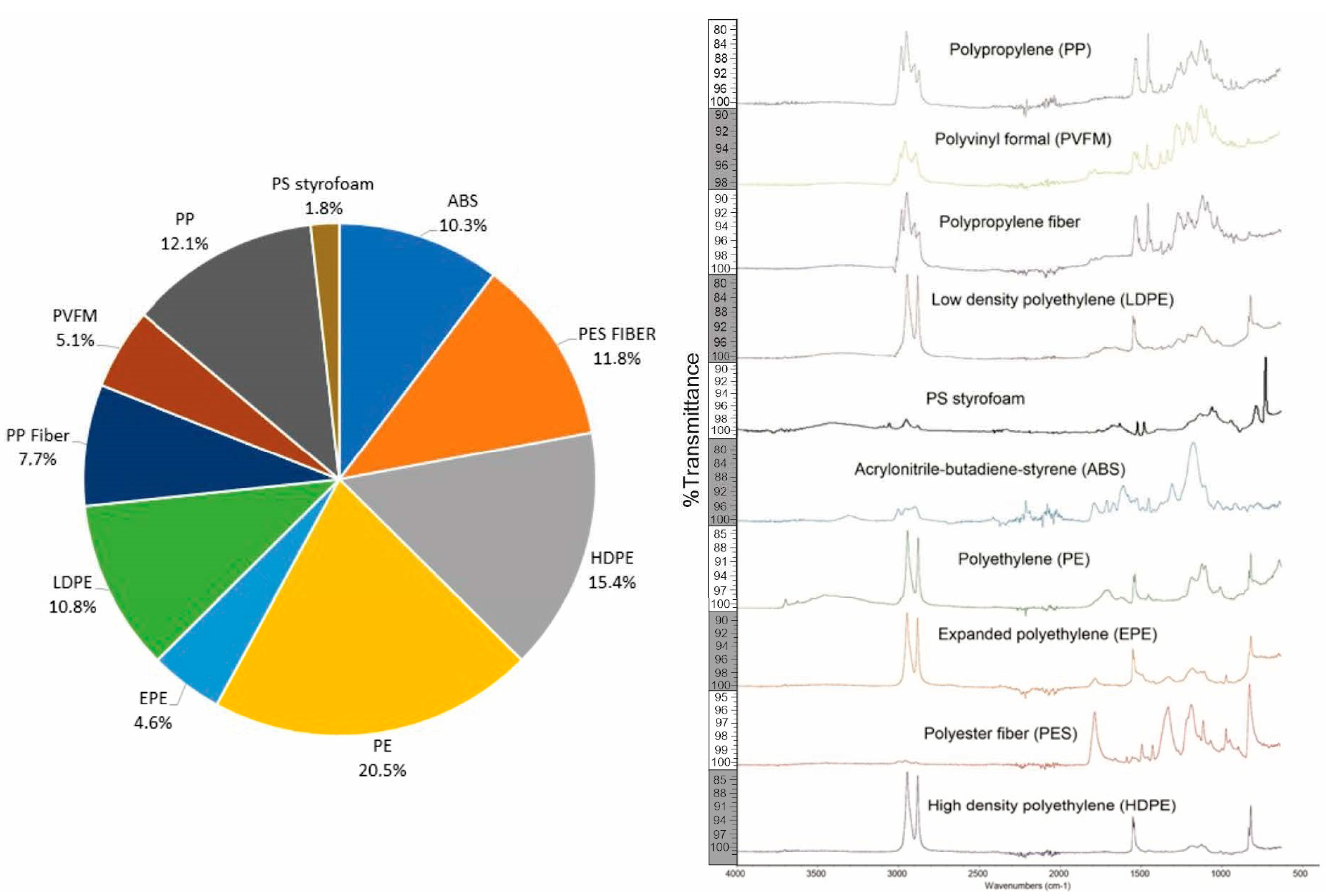
| Stations | Longitude | Latitude |
|---|---|---|
| MA-01 | −42.605 | −22.4069 |
| MA-02 | −42.6278 | −22.4209 |
| MA-03 | −42.6494 | −22.4517 |
| MA-04 | −42.6943 | −22.5608 |
| GM-01 | −42.9953 | −22.4964 |
| GM-02 | −42.9755 | −22.5176 |
| GM-03 | −42.9745 | −22.5351 |
| GM-04 | −42.978 | −22.5418 |
| GM-05 | −42.983 | −22.5485 |
| GM-06 | −42.9737 | −22.6762 |
| MN-01 | −43.2591 | −22.9475 |
| MN-02 | −43.2494 | −22.9397 |
| MN-03 | −43.2423 | −22.9309 |
| MN-04 | −43.2288 | −22.9152 |
| MN-05 | −43.2104 | −22.8991 |
| Stations | Styrofoam | Fragment | Film | Fiber | Pellet | Total |
|---|---|---|---|---|---|---|
| MA-01 | 0 | 3 | 14 | 71 | 0 | 88 |
| MA-02 | 0 | 3 | 15 | 379 | 0 | 397 |
| MA-03 | 0 | 27 | 49 | 164 | 0 | 240 |
| MA-04 | 0 | 17 | 41 | 174 | 0 | 232 |
| GM-01 | 0 | 13 | 18 | 107 | 0 | 138 |
| GM-02 | 0 | 9 | 22 | 156 | 0 | 187 |
| GM-03 | 0 | 21 | 37 | 81 | 3 | 142 |
| GM-04 | 0 | 15 | 1 | 60 | 0 | 76 |
| GM-05 | 0 | 27 | 27 | 132 | 0 | 186 |
| GM-06 | 0 | 5 | 14 | 91 | 1 | 111 |
| MN-01 | 8 | 38 | 181 | 280 | 0 | 507 |
| MN-02 | 16 | 275 | 2168 | 1547 | 1 | 4007 |
| MN-03 | 35 | 89 | 277 | 479 | 4 | 884 |
| MN-04 | 94 | 215 | 326 | 2572 | 1 | 3208 |
| MN-05 | 82 | 190 | 280 | 1755 | 3 | 2310 |
| No. | Study Area | Abundance (Items m−3) | Reference |
|---|---|---|---|
| 1. | Koshi River, China | 50–325 | [52] Yang et al. (2021) |
| 2. | Dahan River, Taiwan | 6.7–83.7 | [59] Wong et al. (2020) |
| 3. | Cherating River, Malaysia | 0.005–0.007 | [60] Pariatamby et al. (2020) |
| 4. | Nakdong River, Republic of Korea | 293–4760 | [61] Eo et al. (2019) |
| 5. | Japanese Rivers, Japan | 0-12 | [62] Kataoka et al. (2019) |
| 6. | Columbia Rivers, USA | 0–13.7 | [59] Kapp e Yeatman (2018) |
| 7. | Antuã River, Portugal | 58–1265 | [50] Rodrigues et al. (2019) |
| 8. | Sena and Marne Rivers, France | 4–108 | [53] Dris et al. (2015) |
| 9. | Chicago River, USA | 1.94–17.93 | [63] McCormick et al. (2014) |
| 10. | Macacu River, Brazil | 7.1–32.5 | present study |
| 11. | Guapimirm River, Brazil | 3.6–1179.6 | present study |
| 12. | Maracanã River, Brazil | 17.6–51,166.5 | present study |
| Stations | Volume (m³) | Items | Items m−3 |
|---|---|---|---|
| GM-01 | 35.272460 | 138 | 3.9 |
| GM-02 | 51.418850 | 187 | 3.6 |
| GM-03 | 36.497870 | 142 | 3.9 |
| GM-04 | 0.235408 | 76 | 322.8 |
| GM-05 | 0.157674 | 186 | 1179.6 |
| GM-06 | 0.306353 | 111 | 362.3 |
| MA-01 | 3.714927 | 88 | 23.7 |
| MA-02 | 12.228302 | 397 | 32.5 |
| MA-03 | 20.551416 | 240 | 11.7 |
| MA-04 | 32.592682 | 232 | 7.1 |
| MN-01 | 28.816484 | 507 | 17.6 |
| MN-02 | 6.694608 | 4007 | 598.5 |
| MN-03 | 4.840370 | 884 | 182.6 |
| MN-04 | 3.747175 | 3208 | 856.1 |
| MN-05 | 0.045147 | 2310 | 51,166.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drabinski, T.L.; de Carvalho, D.G.; Gaylarde, C.C.; Lourenço, M.F.P.; Machado, W.T.V.; da Fonseca, E.M.; da Silva, A.L.C.; Baptista Neto, J.A. Microplastics in Freshwater River in Rio de Janeiro and Its Role as a Source of Microplastic Pollution in Guanabara Bay, SE Brazil. Micro 2023, 3, 208-223. https://doi.org/10.3390/micro3010015
Drabinski TL, de Carvalho DG, Gaylarde CC, Lourenço MFP, Machado WTV, da Fonseca EM, da Silva ALC, Baptista Neto JA. Microplastics in Freshwater River in Rio de Janeiro and Its Role as a Source of Microplastic Pollution in Guanabara Bay, SE Brazil. Micro. 2023; 3(1):208-223. https://doi.org/10.3390/micro3010015
Chicago/Turabian StyleDrabinski, Thiago L., Diego G. de Carvalho, Christine C. Gaylarde, Marcos F. P. Lourenço, Wilson T. V. Machado, Estefan M. da Fonseca, André Luiz Carvalho da Silva, and José Antônio Baptista Neto. 2023. "Microplastics in Freshwater River in Rio de Janeiro and Its Role as a Source of Microplastic Pollution in Guanabara Bay, SE Brazil" Micro 3, no. 1: 208-223. https://doi.org/10.3390/micro3010015
APA StyleDrabinski, T. L., de Carvalho, D. G., Gaylarde, C. C., Lourenço, M. F. P., Machado, W. T. V., da Fonseca, E. M., da Silva, A. L. C., & Baptista Neto, J. A. (2023). Microplastics in Freshwater River in Rio de Janeiro and Its Role as a Source of Microplastic Pollution in Guanabara Bay, SE Brazil. Micro, 3(1), 208-223. https://doi.org/10.3390/micro3010015






