Abstract
In a continuation and extension of an earlier publication, the calculation of the refractivity and polarizability of organic molecules at standard conditions is presented, applying a commonly applicable computer algorithm based on an atom group additivity method, where the molecules are broken down into their constituting atoms, these again being further characterized by their immediate neighbor atoms. The calculation of their group contributions, carried out by means of a fast Gauss–Seidel fitting calculus, used the experimental data of 5988 molecules from literature. An immediate subsequent ten-fold cross-validation test confirmed the extraordinary accuracy of the prediction of the molar refractivity, indicated by a correlation coefficient R2 and a cross-validated analog Q2 of 0.9997, a standard deviation σ of 0.38, a cross-validated analog S of 0.41, and a mean absolute deviation of 0.76%. The high reliability of the predictions was exemplified with three classes of molecules: ionic liquids and silicon- and boron-containing compounds. The corresponding molecular polarizabilities were calculated indirectly from the refractivity using the inverse Lorentz–Lorenz relation. In addition, it could be shown that there is a close relationship between the “true” volume and the refractivity of a molecule, revealing an excellent correlation coefficient R2 of 0.9645 and a mean absolute deviation of 7.53%.
1. Introduction
In continuation of an earlier paper [1], which used a generally applicable atom groups additivity method for the prediction of various molecular descriptors including the refractivity and the polarizability of molecules, the present work puts the focus on the latter two descriptors, for which on the one hand, an extended number of further experimental refractivity data has been included in the atom group parameters calculation, and on the other hand, a different method for the prediction of the polarizability has been introduced, this time based on the former descriptor. The main goal of the present work was to not only increase the reliability of the atom group parameters already published in [1], but in particular to extend the number of atom groups for which as yet no parameter values have been available, with the main interest aimed at atom groups found in ionic liquids. In addition to these, parameters for a large number of additional groups with boron and silicon as central atom could be generated, thus enabling the prediction of the refractivities and polarizabilities of many boranes and silanes.
Earlier calculations of the refractivity and polarizability have been based on the bond refraction and bond polarizability, respectively, on the assumption that the molar refraction and polarizability is the sum of all the bonds in the molecule [2]. The average error between experiment and calculation was 0.7% over a number of less than 100 sample molecules. Later on, Ghose and Crippen [3] developed a method based on 110 atom types, characterized by the polarizing effect of the heteroatoms and the effect of overlapping with non-hydrogen atoms, again assuming that the sum of all the atom parameters defines the molecular descriptor value. Applying a quadratic, constrained least squares technique for the evaluation of the atom type parameters for 504 molecules, they reported a correlation coefficient of 0.994 and a standard deviation of 1.269. Except for the parameters calculation approach, Ghose and Crippen’s method compares closely with the present one, since their atom types follow a similar principle and therefore the present results may best be compared with theirs. Another group additivity approach was chosen by Miller [4,5] for the calculation of the molecular polarizability, whereby the atoms are defined by their state of hybridization, neglecting their neighbor atoms.
The importance of the knowledge of the refractivity and polarizability for the modeling of the dispersive and hydrophobic interactions was outlined in detail by Ghose and Crippen [3]. The attractive forces between nonpolar compounds, also known as dispersive forces, are the result of the correlated motions of their electrons. These forces are evidently closely related to the polarizability of the molecules. Their polarizability again is linearly proportional to their refractivity, given by the Lorentz–Lorenz relation R = 4/3πNα, where R is the molar refractivity, N is Avogadro’s constant, and α is the polarizability. Accordingly, and in contrast to our earlier calculations of the polarizability by means of the group additivity method in [1], the present polarizabilities are directly evaluated from the molar refractivities, with the added bonus that the amount of experimental refractivity data is much larger than that of the polarity, thus enabling the prediction of molecular polarities for which in its atom groups parameter set in [1], no atom groups are defined. It has also been shown that the molecular polarizability is directly proportional to the molecular volume [6]. Hence, on combining the polarizability/volume and polarizability/refractivity correlations, there should be a direct correlation of the refractivity with the molecular volume as postulated by Ghose and Crippen [3]. It would therefore be interesting to see if there is indeed a direct correlation of the refractivity with the “true” molecular volume as applied for the prediction of the heat capacity of solids and liquids in an earlier paper [7].
2. Method
The calculations were carried out on a set of 5988 compounds for which the experimental refractivity or polarizability data have been published, collected from a database of at present 35,952 molecules in their geometry-optimized 3D conformation, encompassing pharmaceuticals, plant protectors, dyes, ionic liquids, liquid crystals, metal-organics, intermediates, and many more, including many further experimentally determined and calculated molecular descriptors. The structural presentations were standardized before storage by a special algorithm, ensuring that all six-membered aromatic ring systems are defined by six aromatic bonds in order to avoid structural ambiguities. In addition and for the same reason, the positive charge in amidinium, pyrazolium, and guanidinium fragments of the ionic liquids was manually positioned on the carbon atom between the nitrogen atoms and their C(+)-N bonds were assumed to be aromatic, which incidentally is in better conformance with the true charge distribution in these cations, as exemplified in, e.g., Figure 1 in [8]. The analogous treatment of the carboxylate and nitro groups is not necessary, as within the present concept of atom groups definitions, they are unambiguously defined.
2.1. Definition of the Atom Groups
Details of the definition of the atom groups for use in a computer-readable form were outlined in [1]. In Table 1 of [1], their namings and meanings were explained; they have been retained in all the subsequent papers including the present one. However, in order to cover the successively increasing amount of additional, structurally variable molecules, several further atom groups had to be added to the parameters list. In particular, the inclusion of ordinary salts and ionic liquids as well as a number of boron- and silicon-containing molecules required the corresponding atom groups listed and explained in Table 1 on some examples. These new atom groups were interpreted and processed by the computer algorithm in the same way as the remaining ones. In fact, some of these have already been applied in the calculation of the liquid viscosity of molecules in [8].

Table 1.
Examples of charged or boron- or silicon-containing atom groups and their meaning.
2.2. Calculation of the Atom Group Contributions
As outlined in [1], the parameter values of the atom groups are evaluated in four steps: in the first step, those compounds for which the experimental refractivities are known are stored in a temporarily generated help list. In the second step, each molecule in the help list is broken down into its constituting “backbone” atoms (i.e., atoms bound to at least two directly bound neighbor atoms), their atom types and neighbor terms defined according to the rules detailed in [1], and then their occurrences counted. The third step involves the generation of an M × (N + 1) matrix, wherein M is the number of molecules, N + 1 is the complete number of atom groups occurring plus the molecules’ refractivity value, and where each matrix element (i,j) receives the number of occurrences of the jth atom group in the ith molecule. The final step comprises the normalization of this matrix into an Ax = B matrix and its subsequent balancing by means of a fast Gauss–Seidel calculus [9] to receive the atom group contributions x, which are stored and shown in Table 2, together with the corresponding statistics data at the bottom in lines A to H.

Table 2.
Atom groups and their contribution in refractivity calculations.
2.3. Calculation of the Refractivity
The calculation of the refractivity of a molecule, based on the atom group parameters compiled in Table 2, is a simple summing up of the contribution of each atom group found in a molecule, as exemplified in Table 3 for 1-butyl-3-methylimidazolium tetrafluoroborate (Figure 1), for which the experimentally evaluated refractivity value was 47.81 [10]. The parameters for the monoatomic anions found among some ILs are given under the respective “group” names “Chloride” and “Bromide”. Any further halogenide anion can be taken into account analogously as soon as the experimental data of at least three representative compounds are available.

Table 3.
Example calculation of the refractivity of 1-butyl-3-methylimidazolium tetrafluoroborate.
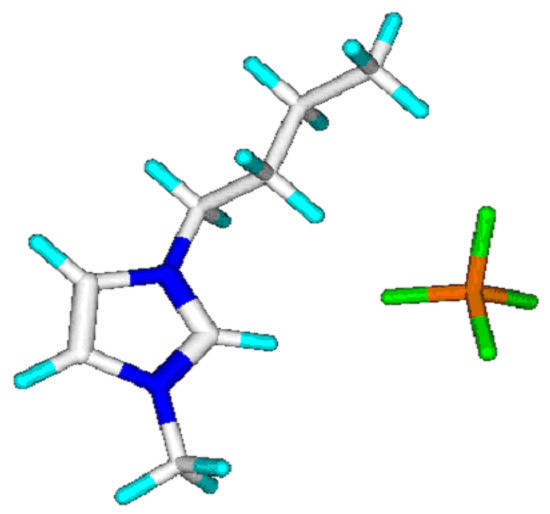
Figure 1.
1-Butyl-3-methylimidazolium tetrafluoroborate.
It goes without saying that this calculation method is limited to compounds for which each atom group is defined by a parameter value in Table 2. In addition, as the reliability of these parameter values increases with the number of independent molecules upon which they are based, only atom groups should be considered for which the number of molecules in the rightmost column of Table 2 is three or more, which are henceforth called “valid”. (It could be shown by means of several cross-validation calculations that the decrease of the cross-validated standard deviation on going from three to four molecules per atom group is insignificant compared with the decrease observed when going from two to three molecules per atom group.) Consequently, the number of molecules for which the refractivity values have been calculated (lines B, C, and D in Table 2) is necessarily smaller than the number upon which the calculation of the complete set of parameters is based (line A in Table 2).
2.4. Cross-Validation Calculations
The calculations of the atom group parameters are immediately followed by a plausibility test applying a 10-fold cross-validation algorithm comprising 10 recalculations omitting in each case a different tenth of the complete set of compounds, ensuring that each compound has been used once as a test sample. The resulting training and test data are added to the molecule’s datafiles. Finally, the corresponding statistics data are evaluated and collected at the bottom of Table 2. Due to the smaller number of training molecules in the cross-validation calculations and the condition that only atom group parameters should be considered in the calculation of the individual refractivities for which the number of molecules in the rightmost column is three or more, the number of molecules with calculated refractivities (lines E, F, G, and H) is again lower in the test set than in the training set (lines B, C, and D). Atom group parameters with molecule numbers below three in the rightmost column, which are accordingly at present not applicable for refractivity calculations, have deliberately been left in Table 2 for future use in this continuing project and not least in the hope that interested scientists may assist in increasing the number of “valid” groups in this parameters list by compounds carrying the underrepresented atom groups. At present, the list of elements for refractivity/polarizability calculations is limited to H, B, C, N, O, P, S, Si, and/or halogen, but is easily extendable to enable the parametrization of atom groups containing additional elements for which experimental densities and refractive indices are available.
2.5. Calculation of the Polarizability
According to the Lorentz–Lorenz relation R = 4/3πNα, N being Avogadro’s number, the refractivity R of a molecule can be translated into its polarizability α by simply multiplying its refractivity value with the reciprocal value of 4/3πN, which is 0.3964, if the refractivity is expressed in mL and the polarizability in A3. Therefore, in this study, for each input experimental refractivity value, the corresponding polarizability value was also evaluated and stored as experimental value in the database, and vice versa. The latter is all the more justified as in many (if not most) cases, the polarizability value was evaluated via the refractivity value. Accordingly, the number of experimental data for these two descriptors is identical, and so is their list of atom group parameters. As a consequence, calculation of a molecule’s refractivity value by means of the group additivity method, based on the refractivity parameters in Table 2, immediately enabled the calculation of its polarizability value by simply multiplying it by 0.3964.
3. Sources of Refractivity and Polarizability Data
In most cases, it was not the refractivity value itself that was published in the following references but the refractive index (nd) and the density (d) of the molecules, which then had to be translated into the refractivity (R) according to the equation R = (nd2 − 1)/(nd2 + 2) × (M/d), where M is the molecular weight. The primary sources of the refractivity data for the earlier [1] as well as the present study were the comprehensive CRC Handbook of Chemistry and Physics [11] and the collective work of Ghose and Crippen [12]. Within the last 7 years since the first publication dealing with the present subject however, a large number of further papers has been collected producing additional refractivity and polarizability data which helped to extend the scope of applicability of the atom group additivity method, particularly for boron- and silicon-containing compounds and ionic liquids. In the following, they have been sorted by their dominant functional features. Within the last ca. 85 years, many papers have been published producing the refractive indices and densities to characterize various hydrocarbons [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34], alcohols [35,36,37,38,39,40,41,42], ethers [43,44,45,46,47], acids [48], (ortho)esters and carbonates [49,50,51,52,53,54,55,56,57,58,59,60,61,62], acetals [63,64], ketones [65,66], peroxides [67,68,69,70,71], amines, hydrazines, nitriles, and nitro compounds [72,73,74,75,76,77,78], and various boron- [79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96], phosphorus- [97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136], and sulfur-containing compounds [137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160]. Many of the compounds mentioned so far also carried halogens [161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196]. An interesting extension to the parameters database was provided by papers presenting results of silicon-containing compounds [197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240]. Beyond the refractivity data of the various mentioned functional groups, those for a number of hetarenes and heterocycles have been published [241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260]. Another important extension that was not covered in the earlier paper [1] is the class of ionic liquids [10,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323,324,325,326,327,328,329,330,331,332,333,334,335,336,337,338,339,340,341,342,343,344,345,346,347,348,349,350,351,352,353,354,355,356,357,358,359,360,361,362,363,364,365,366]. In addition, several papers have been added which contributed various subjects that could not be assigned to any specific subject of the aforementioned ones [2,367,368,369,370,371,372,373,374,375,376,377,378,379,380,381,382,383,384,385]. Finally, a number of papers published experimental data of the polarizability of molecules, in many cases derived from their refractivity values [6,386,387,388,389,390,391,392,393,394,395].
4. Results
4.1. Refractivity
In the paper of Ghose and Crippen [3] mentioned earlier, it was stated that the molar refractivity is directly related to the molecule’s volume, expressed in the refractivity’s unit “mL”, their atom group parameters accordingly being associated with the volume of the molecule’s constituting atoms. The present approach, on the other hand, does not care about the theoretical background of the refractivity as it is a purely mathematical method to adjust the calculated to the experimental data, and therefore the resulting atom group parameters must not be assigned with any physical meaning. Consequently, as can be seen in Table 2 and Table 3, negative parameter values are not unusual.
While in the earlier paper [1], generally no limit was given concerning the deviation of the experimental data from the calculated ones for the evaluation of the atom group parameters, in the present work, the atom group parameters in Table 2 and the statistics data at its bottom (Lines A to H) are the result after a stepwise elimination of outliers, defined as their experimental value deviating from the calculated one by more than three times the cross-validated standard error Q2. The final list of discarded outliers is available in the Supplementary Materials. As a consequence, 5988 of the originally 6501 compounds with experimental data remained for the evaluation of said parameters, leaving ca. 7.9% as outliers. Due to the elimination of the outliers, the statistical data significantly improved in comparison with those in the earlier paper: not only is the present set of parameters based on a significantly larger number of molecules (5988 vs. 4300, rows A in present Table 2 vs. corresponding Table 13 of [1]) and a larger number of atom groups (562 vs. 364), but also their standard deviation (0.38 vs. 0.66, rows D) and the cross-validated deviation (0.41 vs. 0.7, rows H) drastically improved. Together with the corresponding correlation coefficients R2 and Q2 of 0.9997 (rows B and F in Table 2) in the present work, they compare very favorably with the correlation coefficient of 0.994 and the standard deviation of 1.269 published by Ghose and Crippen [3]. The mean absolute percentage deviation (MAPD) of the finally calculated refractivities from the experimental values of 5988 molecules is 0.76%. The increased number of “valid” atom group parameters enabled the calculation of the refractivities and polarizabilities of ca. 80% of the close to 36,000 molecules in the present database, which can be viewed as representative for the entire chemical realm. The excellent correlation between experiment and prediction is visualized in the correlation diagram of Figure 2. The corresponding histogram in Figure 3 confirms the uniform distribution of the deviations between the experimental and calculated refractivities, their experimental values ranging from 8.23 (methanol) to 271.13 (glycerol tristearate). The complete set of compounds with experimental refractivities used for the atom group parameters of Table 2 are available in the Supplementary Materials.
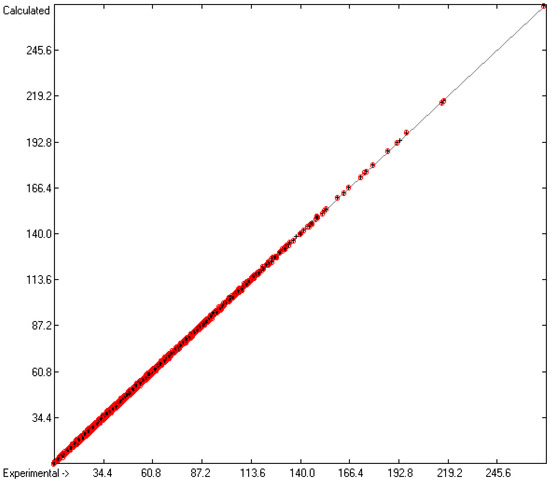
Figure 2.
Correlation diagram of the refractivity data. Cross-validation data are superpositioned as red circles. (10-fold cross-valid.: N = 5763, Q2 = 0.9997, regression line: intercept = 0.0292; slope = 0.9995, MAPD = 0.76%).
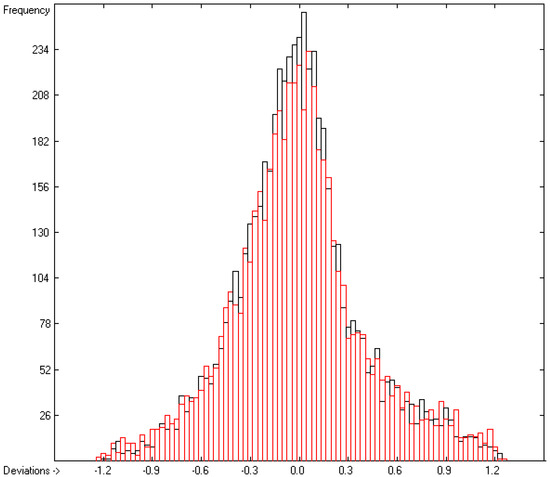
Figure 3.
Histogram of the refractivity data. Cross-validation data are superpositioned as red bars. (σ = 0.38; S = 0.41; experimental values range from 8.23 to 271.13).
An interesting observation can be made with respect to the outliers in that many of them are solids. Cao et al. [396] showed that solid compounds can exhibit up to three differing refractive indices, depending on their crystal symmetry. A typical example is Ibuprofen, a non-steroidal antirheumatic, which shows the three refractive index values 1.522, 1.572, and 1.644. With its reported density of 1.119 g/cm3 and a refractivity of 60.95, calculated by means of our group additivity model, we calculated a refractive index of 1.575, which is pretty close to the mean of the three experimental values. Analogous results have been found with several other outliers, confirming the assessment that in cases where the experimental refractive index strongly deviates from the predicted one, the reason might be that a specific crystal form of the compound was examined. Since these mean refractive index values usually do not represent real crystalline forms, they were not included in the group parameters optimization procedure.
Since the last paper [1] of 2015, the systematic screening of the chemical literature has provided a number of refractivities data of previously under-represented classes of molecules enabling, as mentioned earlier, a substantial increase of the number of atom group parameters in the present Table 2 compared with the one of Table 13 in [1]. In particular, three classes of compounds have experienced an extended representation and will in the following be discussed in more detail: ionic liquids and silicon- and boron-containing compounds.
4.1.1. Ionic Liquids
In the last ca. 25 years, ionic liquids (ILs) have experienced increasing attention as potential replacements for volatile solvents as they are non-volatile, non-inflammable, and can easily be recycled. Their physical properties are easily tunable by suitable choice of their cation and anion, making them favorable candidates as media for chemical syntheses. The enormous variety of potential cation–anion combinations, however, obliges one to put the focus on those candidates with the most promising properties. In the last few years, a substantial number of ILs have been synthesized and their physical properties have been examined. Based on these results, few attempts have been made so far to utilize these results for the prediction of the physical properties of as yet unknown cation–anion combinations, and if yes, then for a narrow scope within the scientists’ range of experience (see, e.g., Almeida et al. [317]), or as in other cases, as in the papers of Sattari et al. [335] or of Venkatraman et al. [359], for the prediction of a specific property based on a fairly large range of ILs by either applying quantitative structure–property relationship (QSPR) technique or machine learning. The present atom group additivity approach, on the other hand, has proven its versatility in that it is able to predict a number of properties of nearly any type of compound by means of an identical algorithm, simply using the appropriate atom group parameters tables. Accordingly, based on the updated parameters tables in this ongoing project, we have been able to calculate the heat of combustion [397] for 30 ILs with a correlation coefficient R2 of 1.0 and a mean average percentage deviation from experimental values (MAPD) of 0.21% and a standard deviation σ of 17.75 kJ/mol, the heat of vaporization [398] of 61 ILs (R2 = 0.9615, MAPD = 2.12%, σ = 4.22 kJ/mol), the liquid viscosity [8] for 113 ILs (R2 = 0.9830, MAPD = 3.43%, σ = 0.11 J/mol/K), the surface tension [399] of 161 ILs (R2 = 0.8413, MAPD = 5.17%, σ = 2.40 dyn/cm), and the liquid heat capacity at 298 K [400] of 140 ILs (R2 = 0.9986, MAPD = 1.05%, σ = 7.50 J/mol/K). In analogy to these results, the refractivity values of 228 ILs calculated by means of the atom group parameters of Table 2 were compared with their experimental data and collected alphabetically in Table 4, revealing a MAPD of only 0.44% and a σ of 0.38. For comparison: the statistics for the 203 ILs for which, while serving as test samples in the cross-validation calculations, the test results could be calculated, yielded an only slightly inferior MAPD of 0.51% and a σ of 0.44.

Table 4.
Calculated and experimental refractivity of ionic liquids.
4.1.2. Silanes, Silanols, Siloxanes, Silazanes, and Silicates
Silicon-containing compounds have found use in synthetic processes as intermediates as well as in commercial products, e.g., in detergents, cosmetics, deodorants, soaps, as water-resistant coatings, as defoaming agents, or as coolants. Despite the large variety of applications, the number of physico-chemical data for this class of molecules is fairly limited within the chemical realm. Nevertheless, a thorough scan of the literature of the last ca. 80 years delivered a sufficient number of data to enable the creation of a basis for the prediction of several chemical descriptors of interest based on the present atom group additivity principle. Accordingly, in analogy to the previous section, the updated parameters tables provided the group parameters for the heat of combustion [397], enabling its calculation for 99 silicon compounds with a correlation coefficient R2 of 1.0, a MAPD of 0.19% and a σ of 17.67 kJ/mol, for the heat of vaporization [398] for 106 (R2 = 0.7936, MAPD = 10.91%, σ = 6.17 kJ/mol), for the surface tension [399] of 18 (R2 = 0.9835, MAPD = 2.62%, σ = 0.66 dyn/cm), for the liquid heat capacity at 298 K [400] of 26 (R2 = 0.9981, MAPD = 2.32%, σ = 9.02 J/mol/K), for the solid heat capacity at 298 K [400] of 14 (R2 = 0.9925, MAPD = 2.77%, σ = 18.35 J/mol/K), for the standard entropy of fusion [398] of 45 (R2 = 0.7251, MAPD = 15.09%, σ = 15.56 J/mol/K), and even for the vapor pressure at 298 K [401] of 9 silicon compounds (R2 = 0.9897, MAPD = 7.92%, σ = 0.16). In addition to these descriptors, the present work now provides the refractivity data for 351 silicon derivatives, alphabetically sampled in Table 5. They prove the reliability of the calculated values with a MAPD of only 0.39% and a standard deviation σ of 0.31, compared with their experimental values. Analogously, when used as test samples in the cv calculations, 324 of these silicon compounds yielded a MAPD of 0.47% and a σ of 0.37.

Table 5.
Calculated and experimental refractivity of silicon-containing compounds.
4.1.3. Boranes, Borines, Borazines, Boronates, and Borates
In contrast to the prior two sections, boron-containing compounds are essentially important intermediates in chemical syntheses, and therefore experimental physico-chemical data are scarce. The large number of refractivity data, on the other hand, is primarily owed to the need to characterize the newly synthetized molecules by some easily accessible physical data, such as elemental analysis, melting point, density, and refractive index. With a few exceptions (e.g., Christopher et al. [80]) however, most authors have not shown any interest in using the latter two values for the calculation of the molecules’ refractivity or polarizability. The present collection of refractivity data for 137 boron compounds listed in Table 6, although perhaps of merely academic interest, nevertheless confirms—by the strong linearity over the complete set—the overall correctness of the experimental data and at the same time proves the versatility of the present group additivity approach, revealing a MAPD of just 0.46% and a σ of 0.37. Similarly, the test data of 127 of these boron derivatives, when applied as test samples in the cv calculations, resulted in a MAPD of 0.54% and a σ of 0.45.

Table 6.
Calculated and experimental refractivity of boron-containing compounds.
4.2. Polarizability
The calculation of the molecular polarizabilities was carried out indirectly via the calculated refractivities applying the inverse Lorentz–Lorenz relation. In order to include the relatively limited number of experimentally determined polarizability data in the atom group parameters and any further calculations, they were translated into the corresponding refractivity values and henceforth treated just like the remaining experimental refractivities. Conversely, all the experimental refractivity values were analogously converted into “experimental” polarizabilities. The complete set of true and indirectly determined experimental polarizability values is compared in Figure 4 with the indirectly calculated polarizability values, mirroring the excellent correlation of Figure 2, which at first sight is not surprising as both value sets are multiplied with the same factor. However, we should not forget that the truly experimentally determined polarizability values were evaluated by various methods that differ from those for the experimental determination of the refractivity. In fact, as the histogram in Figure 5 reveals, it turned out that 23 compounds should be viewed as outliers because their experimental refractivity values deviated by more than three times the standard deviation σ of 0.15 A3 from calculations. They are collected in a separate list, available in the Supplementary Materials, together with the complete set of compounds with experimental and calculated polarizabilities.
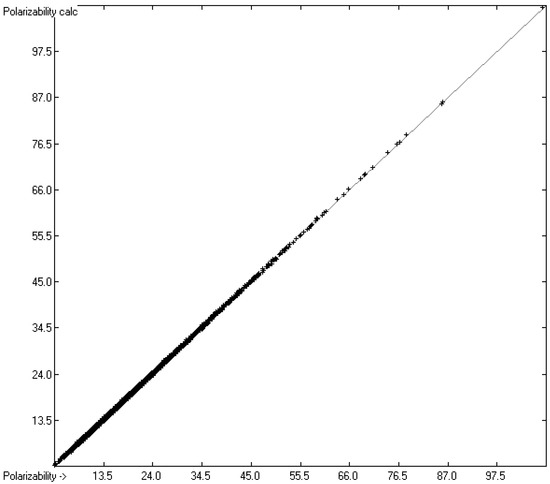
Figure 4.
Correlation diagram of the polarizability data (in A3). (N = 5763, R2 = 0.9997, regression line: intercept = 0.0115; slope = 0.9995, MAPD = 0.72%).
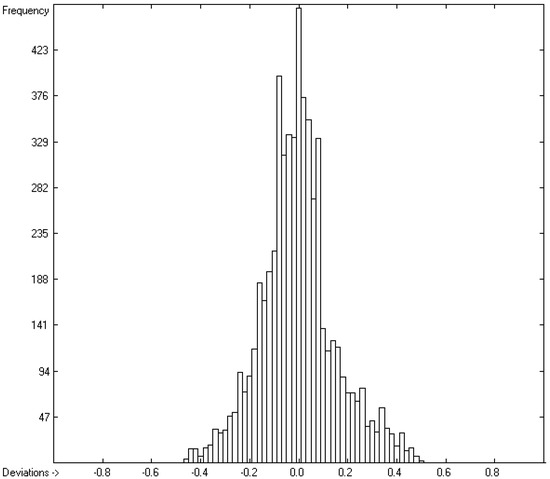
Figure 5.
Histogram of the polarizability data (σ = 0.15 A3; exp. values range from 3.23 to 107.53 A3).
In a paper by Tariq et al. [273], the applicability of the Lorentz–Lorenz relation was questioned for ILs because it is based on the assumption of the compounds being “isotropic fluids composed of spherical and non-interacting particles” which is not given with this class of salts, since at least one of its ions is non-spherical, and they are clearly non-isotropic fluids as they consist of polar centers surrounded by non-polar moieties. These considerations are certainly justified with respect to the relationship between refractivity and polarizability of ILs. In the following section, however, we will demonstrate that the non-spherical character of the ILs is no obstacle for a reasonable correlation between molecular volume and refractivity.
4.3. Refractivity/Polarizability and Molecular Volume
A paper of Brinck et al. [6] discussed the relationship between the polarizability of a molecule and its volume, arguing that physically “the polarizability α of a conducting sphere of radius R is equal to R3”, its relation expressed by the equation α = 3V/4π, where V is the volume. This relation is true on condition that the electrostatic potential is uniform within this sphere, which is certainly not the case in a molecule. An approximate equation, known as the Clausius–Mossotti equation, proposes for nonpolar molecules the polarizability as being directly proportional to their volume and a function of their dielectric constant. Several approaches for the calculation of the molecular volumes have been chosen in order to assess their applicability for polarizability predictions. Gough [402], Laidig and Bader [403], and Brinck et al. [6] used various Hartree–Fock self-consistent field methods to compute the volumes of a limited number of small molecules and achieved good linearity with their polarizability, depending on the size of the contour of the electronic density defining the molecule’s surface. In an earlier paper [7], we presented a fast numerical method for the calculation of the “true” molecular volume (in A3) of molecules of any size and type, including ILs, based on the atoms’ Van-der-Waals radii. Since these “true” (elsewhere also called “hardcore”) volumes are automatically generated on entering a new compound to the database, it was obvious to examine their potential linearity with their experimental polarizability or refractivity as far as available. In Figure 6, the correlation between the “true” molecular volume and the experimental refractivity of 6069 molecules is shown, revealing an excellent correlation coefficient R2 of 0.9645 and a MAPD of 7.53%. Figure 7 presents the same correlation diagram, but restricted to the class of ILs, indicating that the path of prediction of their refractivity R via their molecular volume V as calculated in [7] and applying the simple linear equation R = intercept + (V × slope) provides a reliable refractivity value with a MAPD of little more than 5% within the ILs class over a large range of molecular volumes, if the atom group additivity method does not allow a calculation due to the limitations mentioned earlier. The complete list of molecules with their “true” molecular volume and experimental and volume-derived refractivity is available in the Supplementary Materials.
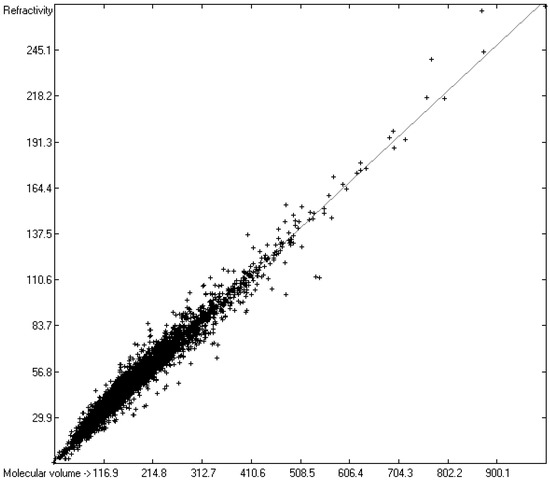
Figure 6.
Correlation diagram of “true” molecular volume (in A3) [7] vs. experimental refractivity. (N = 6069, R2 = 0.9645, regression line: intercept = 1.4354; slope = 0.2743, MAPD = 7.53%).
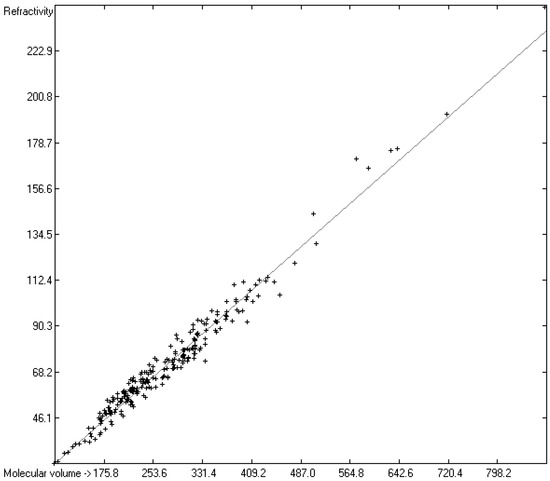
Figure 7.
Correlation diagram of “true” molecular volume (in A3) [7] vs. experimental refractivity of ILs. (N = 247, R2 = 0.9700, regression line: intercept = −2.4557; slope = 0.2686, MAPD = 5.35%).
5. Conclusions
In several earlier papers [1,7,8,397,398,399,400,401], the present atom groups additivity algorithm, outlined in [1], proved its formidable versatility for the reliable prediction of up to 17 physical, thermodynamic, solubility-, optics-, charge-, and environment-related descriptors. In the present work, which is part of an ongoing project, the results of the present refractivity/polarizability calculations again demonstrate its as-yet unsurpassed accuracy and easy expandability. The nearly 6000 molecules providing their experimental refractivity or polarizability values, either directly or via their refractive index and density, enabled the calculation of a large set of atom group parameters allowing the refractivity/polarizability of nearly 80% of the compounds listed in a database of presently approaching 36,000 of nearly any molecular structure, size, and application. The big advantage of the present method is the basic possibility to calculate the refractivity simply by means of paper and pencil applying the parameters set listed in Table 2. In addition, we have shown that optional refractivity/polarizability calculations are possible via the molecular volume route—although with lower accuracy—in cases where the group additivity method is disabled.
The mentioned project’s software is called ChemBrain IXL, available from Neuronix Software (www.neuronix.ch, 1.1.2015, Rudolf Naef, Lupsingen, Switzerland).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/liquids2040020/s1, The list of compounds used in the present work, their experimental data, and 3D structures are available online as standard SDF files, accessible for external chemistry software, under the name of “S01. Compounds List for Refractivity-Parameters Calculations.sdf”. The list of the compounds used in the correlation diagrams and histograms containing their names and their experimental and calculated values are available under the corresponding names of “S02. Experimental vs. Calculated Refractivities.doc”, “S03. Experimental vs. Calculated Polarizabilities.doc” and “S04. Molecular Volume vs. Refractivity Data Table.doc”. Separate analogous lists are available for ionic liquids under the name of “S05. Experimental vs. Calculated Refractivities of Ionic Liquids.doc”, for silicon compounds under the name of “S06. Experimental vs. Calculated Refractivities of Silicon Compounds.doc”, and for boron compounds under the name of “S07. Experimental vs. Calculated Refractivities of Boron Compounds.doc”. In addition, two lists containing the outliers in the calculations of the refractivity and polarizability of molecules are available under the names of “S08. Refractivity Outliers.doc” and “S09. Polarizability outliers.doc”. Finally, the figures are available as .tif files and the tables as .doc files under the names given in the text.
Author Contributions
R.N. developed project ChemBrain and its software upon which this paper is based, and also fed the database, calculated, and analyzed the results, and wrote the paper. W.E.A.J. suggested the extension of ChemBrain’s tool and contributed experimental data and the great majority of the literature references. Beyond this, R.N. is indebted to W.E.A.J. for the many valuable discussions. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in Supplementary Material.
Acknowledgments
R.N. is indebted to the library of the University of Basel for allowing him full and free access to the electronic literature database.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Naef, R. A Generally Applicable Computer Algorithm Based on the Group Additivity Method for the Calculation of Seven Molecular Descriptors: Heat of Combustion, LogPO/W, LogS, Refractivity, Polarizability, Toxicity and LogBB of Organic Compounds; Scope and Limits of Applicability. Molecules 2015, 20, 18279–18351. [Google Scholar] [CrossRef] [PubMed]
- Denbigh, K.G. The Polarizabilities of Bonds—I. Trans. Faraday Soc. 1940, 36, 936–948. [Google Scholar] [CrossRef]
- Ghose, A.K.; Crippen, G.M. Atomic physicochemical parameters for three-dimensional structure-directed quantitative structure-activity relationships I. Partition coefficients as a measure of hydrophobicity. J. Comput. Chem. 1986, 7, 565–577. [Google Scholar] [CrossRef]
- Miller, K.J.; Savchik, J.A. A new empirical Method to calculate Average Molecular Polarizabilities. J. Am. Chem. Soc. 1979, 101, 7206–7213. [Google Scholar] [CrossRef]
- Miller, K.J. Additivity methods in molecular polarizability. J. Am. Chem. Soc. 1990, 112, 8533–8542. [Google Scholar] [CrossRef]
- Brink, T.; Murray, J.S.; Politzer, P. Polarizability and volume. J. Chem. Phys. 1993, 98, 4305–4306. [Google Scholar] [CrossRef]
- Naef, R. Calculation of the isobaric heat capacities of the liquid and solid phase of organic compounds at and around 298.15 K based on their “True” molecular volume. Molecules 2019, 24, 1626. [Google Scholar] [CrossRef] [PubMed]
- Naef, R.; Acree, W.E. Application of a General Computer Algorithm Based on the Group-Additivity Method for the Calculation of Two Molecular Descriptors at Both Ends of Dilution: Liquid Viscosity and Activity Coefficient in Water at Infinite Dilution. Molecules 2018, 23, 5. [Google Scholar] [CrossRef] [PubMed]
- Hardtwig, E. Fehler- Und Ausgleichsrechnung; Bibliographisches Institut AG: Mannheim, Germany, 1968. [Google Scholar]
- Kim, K.-S.; Shin, B.-K.; Lee, H.; Ziegler, F. Refractive index and heat capacity of 1-butyl-3-methylimidazolium bromide and 1-butyl-3-methylimidazolium tetrafluoroborate, and vapor pressure of binary systems for 1-butyl-3-methylimidazolium bromide + trifluoroethanol and 1-butyl-3-methylimidazolium tetrafluoroborate + trifluoroethanol. Fluid Phase Equil. 2004, 218, 215–220. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) Physical Constants of Organic Compounds. In CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2005; pp. 3-1–3-740. [Google Scholar]
- Ghose, A.K.; Crippen, G.M. Atomic Physicochemical Parameters for Three-Dimensional-Structure-Directed Quantitative Structure-Activity Relationships. 2. Modeling Dispersive and Hydrophobic Interactions. J. Chem. Inf. Comput. Sci. 1987, 27, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Perlman, D.; Davidson, D.; Bogert, M.T. The Synthesis of Phenanthrenes from Hydroxyl Derivatives of beta-Phenylethylcyclohexanes and the Nature of the By-Product. J. Org. Chem. 1936, 01, 288–299. [Google Scholar] [CrossRef]
- Drake, N.L.; Welsh, L.H. 2,2,3,4-Tetramethylhexane and 3,3,5-trimethylheptane. J. Am. Chem. Soc. 1938, 60, 488–489. [Google Scholar] [CrossRef]
- Petrov, A.D.; Baidanov, A.P.; Zakotin, N.N.; Suntsov, P.I. The synthesis and properties of α-methylheptylbenzene, α-butylamylbenzene and α-hexylheptylbenzene. Zh. Obsh. Khim. 1939, 9, 509–512. [Google Scholar]
- Matui, E. Styrene substitutes and their polymers. II. p-Ethylstyrene and its polymers. Kogyo Kagaku Zasshi 1941, 44, 107–108. [Google Scholar]
- Matui, E. Substituted styrenes and their polymers. III. p-Isopropylstyrene and its polymer. Kogyo Kagaku Zasshi 1941, 44, 284–286. [Google Scholar]
- Petrov, A.D.; Pavlov, A.M.; Makarov, Y.A. Synthesis of 3-ethyldecane and of 2,5-dimethylhendecane. Zh. Obsh. Khim. 1941, 2, 1104–1106. [Google Scholar]
- Petrov, A.D.; Chel’tsova, M.A. Synthesis and properties of hydrocarbons of aromatic and naphthenic series of the composition C19-C26. II. Zh. Obsh. Khim. 1942, 12, 87–94. [Google Scholar]
- Petrov, A.D.; Kaplan, E.P. Synthesis and properties of isoparaffin hydrocarbons of the composition C12–C22. II. Zh. Obsh. Khim. 1942, 12, 99–103. [Google Scholar]
- Petrov, A.D.; Shchupina, Z.K.; Ol’dekop, Y.A. Synthesis of 9,10-dimethyloctadecane and 9,10-di-propyloctadecane. Zh. Obsh. Khim. 1944, 14, 490–500. [Google Scholar]
- Petrov, A.D.; Vittikh, M.V. Synthesis and properties of isoparaffinic hydrocarbons of the composition C13–C24. Izvest. Akad. Nauk SSSR Ser. Khim. 1944, 238–242. [Google Scholar]
- Petrov, A.D.; Krutov, K.M.; Khrenov, I.M. Synthesis and properties of cyclohexylhexylmethanol and 3-cyclohexyl-2-methylnonane. Zh. Obsh. Khim. 1945, 15, 799–801. [Google Scholar]
- Lunshof, H.J.; van Stenis, J.; Waterman, H.I. Preparation of some Physical Constants of 2-Methyltetradecane and 3-Methylpentadecane. Rec. Trav. Chim. Pays Bas 1947, 66, 348–352. [Google Scholar] [CrossRef]
- Petrov, A.D.; Ol’dekop, Y.A. Synthesis and properties of higher isoparaffin hydrocarbons of composition C20-C34 (7,8-diisopropyltetradecane, 7,8-diisoamyltetradecane, 10,11-dipropyleicosane, 11,12-dipropyldocosane, 9,10-dioctyloctadecane, and 9,10,11,12-tetrapropyleicosane). Zh. Obsh. Khim. 1948, 18, 859–864. [Google Scholar]
- Petrov, A.D.; Kaplan, E.P. The synthesis and the physical properties of C22-branched hydrocarbons. Izvest. Akad. Nauk SSSR Ser. Khim. 1949, 539–544. [Google Scholar]
- Romadane, I. Alkylation of naphthalene with iso alcohols in the presence of boron trifluoride. Zh. Obsh. Khim. 1957, 27, 1939–1941. [Google Scholar]
- Petrov, A.A.; Mingaleva, K.S.; Kupin, B.S. Dipole moments and reactivity of vinylacetylenic hydrocarbons. Dokl. Akad. Nauk SSSR 1958, 123, 298–300. [Google Scholar]
- Terres, E.; Brinkmann, L.; Fischer, D.; Hüllstrung, D.; Lorz, W.; Weisbrod, G. Synthese und physikalische Daten einiger Isoparaffinreihen mit 11 bis 24 C-Atomen. Brennstoff Chem. 1959, 40, 279–280. [Google Scholar]
- Petrov, A.A.; Sergienko, S.R.; Nechitailo, N.A.; Tsedilina, A.L. Synthesis and properties of C12—C16 monomethylalkanes. Russ. Chem. Bull. 1959, 8, 1091–1097. [Google Scholar] [CrossRef]
- Leibnitz, E.; Hager, W.; Winkler, R. Studien zur Chemie der Paraffine und Paraffingatsche. V. Synthese und physikalische Eigenschaften einiger 4n-Propyl- und 2-Methyl-3-isopropyl-Alkane mit mehr als 10 C-Atomen. J. Prakt. Chem. 1959, 9, 275–288. [Google Scholar] [CrossRef]
- Levina, R.Y.; Kostin, V.N.; Gembitskii, P.A.; Treshchova, E.G. Cyclopropanes and cyclobutanes. XVII. Reduction of arylcyclopropanes with metals in liquid ammonia and with methyl alcohol. Zh. Obsh. Khim. 1960, 31, 829–836. [Google Scholar]
- Terres, E.; Paulsen, S.R.; Huellstrung, D. Isoparaffins. Erdoel Kohle 1960, 13, 323–325. [Google Scholar]
- Xiaomei, Q.; Xiaofang, C.; Yongsheng, G.; Li, X.; Shenlin, H.; Wenjun, F. Density, Viscosity, Surface Tension, and Refractive Index for Binary Mixtures of 1,3-Dimethyladamantane with Four C10 Alkanes. J. Chem. Eng. Data 2014, 59, 775–783. [Google Scholar]
- Skita, A.; Faust, W. Velocities of formation of the stereomeric methylcyclohexanols. Ber. Dt. Chem. Ges. 1931, 64B, 2878–2892. [Google Scholar] [CrossRef]
- Viktorova, E.A.; Shuikin, N.I.; Karakhanov, E.A. Catalytic alkylation of p-cresol by dipropenyl. Izvest. Akad. Nauk SSSR Ser. Khim. 1963, 12, 2226–2227. [Google Scholar] [CrossRef]
- Viktorova, E.A.; Karakhanov, E.A.; Shuikin, A.N.; Shuikin, N.I. Alkylation of phenols by compounds with two functions. II. Alkylation of p-cresol by diene hydrocarbons with isolated double bonds. Izvest. Akad. Nauk SSSR Ser. Khim. 1966, 3, 523–527. [Google Scholar]
- Viktorova, E.A.; Shuikin, N.I.; Karakahanov, E.A. Alkylation of phenols with bifunctional compounds. XII. Catalytic alkenylation of o- and p-cresols with butadiene. Izvest. Akad. Nauk SSSR Ser. Khim. 1966, 5, 915–918. [Google Scholar]
- Mori, S. Response Correction of Differential Refractometer for Polyethylene Glycols in Size Exclusion Chromatography. Anal. Chem. 1978, 50, 1639–1643. [Google Scholar] [CrossRef]
- Teregulova, G.T. Synthesis of 1,3-dioxolanes containing aromatic fragments. Zh. Priklad Khim. 1990, 63, 1383–1386. [Google Scholar]
- Crespo, E.A.; Costa, J.M.L.; Hanafiah, Z.B.M.A.; Kurnia, K.A.; Oliveira, M.B.; Lovell, F.; Vega, L.F.; Carvalho, P.J.; Coutinho, J.A.P. New measurements and modeling of high pressure thermodynamic properties of glycols. Fluid Phase Equil. 2017, 436, 113–123. [Google Scholar] [CrossRef]
- Chaudhary, N.; Nain, A.K. Densities, speeds of sound, refractive indices, excess and partial molar properties of polyethylene glycol 200 + benzyl methacrylate binary mixtures at temperatures from 293.15 to 318.15 K. J. Mol. Liq. 2021, 346, 117923. [Google Scholar] [CrossRef]
- Mottier, M. Sur La Méthylene-Pyrocatéchine. Arch. Sci. Phys. Nat. 1935, 17, 289–291. [Google Scholar]
- Jacobs, T.L.; Cramer, R.; Hanson, J.E. Acetylenic ethers. II. Ethoxy- and butoxyacetylene. J. Am. Chem. Soc. 1942, 64, 223–226. [Google Scholar] [CrossRef]
- Dandegaonker, S.H.; Gerrard, W.; Lappert, M.F. Reactions of phenylboron dichloride with ethers. J. Chem. Soc. 1957, 2893–2897. [Google Scholar] [CrossRef]
- Kalabina, A.V.; Shergina, S.I.; Shergina, N.I. Synthesis and properties of the cis and trans isomers of β-bromo vinyl aryl ethers. Izvest. Vyssnikh Ucheb. Zavedenii Khim Khim. Tekhnol. 1959, 2, 545–549. [Google Scholar]
- Dai, F.; Xin, K.; Song, Y.; Shi, M.; Yu, Y.; Li, Q. Liquid-liquid equilibria for the ternary system containing 1-Butanol + methoxy-(methoxymethoxy)methane + water at temperatures of 303.15, 323.15 and 343.15 K. Fluid Phase Equil. 2016, 409, 466–471. [Google Scholar] [CrossRef]
- Berinde, Z.M. QSPR Models for the Molar Refraction, Polarizability and Refractive Index of Aliphatic Carboxylic Acids Using the ZEP Topological Index. Symmetry 2021, 13, 2359. [Google Scholar] [CrossRef]
- West, C.D. Crystal Form of Sucrose Octaacetate. J. Am. Chem. Soc. 1941, 63, 630. [Google Scholar] [CrossRef]
- Rehberg, C.E.; Faucette, W.A. Preparation and Polymerization of Cycloalkyl Acrylates. J. Am. Chem. Soc. 1950, 72, 4307. [Google Scholar] [CrossRef]
- Alexander, E.R.; Busch, H.M. A convenient synthesis of orthoformic esters. J. Am. Chem. Soc. 1952, 74, 554–555. [Google Scholar] [CrossRef]
- Satta, V.; Fein, M.L.; Filachtone, E.M. Some Esters of Unsaturated Acids. J. Am. Chem. Soc. 1953, 75, 4101. [Google Scholar] [CrossRef]
- Arbuzov, B.A.; Shavsha-Tolkacheva, T.G. Dipole moments of esters of orthopropionic and orthoformic acids. Russ. Chem. Bull. 1954, 3, 525–530. [Google Scholar] [CrossRef]
- Shigley, J.W.; Bonhorst, C.W.; Liang, C.C.; Althouse, P.M.; Triebold, H.O. Physical Characterization of a) a Series of Ethyl Esters and b) a Series of Ethanoate Esters. J. Am. Oil Chem. Soc. 1955, 32, 213–215. [Google Scholar] [CrossRef]
- Grzeskowiak, R.; Jeffrey, G.H.; Vogel, A.I. Physical Properties and Chemical Constitution. Part XXIX. Acetylenic Compounds. J. Chem. Soc. 1960, 4719–4722. [Google Scholar] [CrossRef]
- Mekhtiev, S.D.; Sharifova, S.M.; Smirnova, V.P. Esterification of terephthalic and isophthalic acids by aliphatic alcohols. Azerbaidzhan. Khim. Zh. 1965, 3, 67–72. [Google Scholar]
- Freidlin, G.N.; Bushinskii, V.I. Physical Properties of Monoalkyl Esters of Adipic Acid. Zh. Prikl. Khim. 1971, 44, 944–945. [Google Scholar]
- Ortega, J. Measurements of Excess Enthalpies of {a Methyl n-Alkanoate (from n-Hexanoate to n-Pentadecanoate) + n-Pentadeane} at 298.15 K. J. Chem. Thermodyn. 1990, 22, 1165–1170. [Google Scholar] [CrossRef]
- De Lorenzi, L.; Fermeglia, M.; Torriani, G. Density, Refractive Index, and Kinematic Viscosity of Diesters and Triesters. J. Chem. Eng. Data 1997, 42, 919–923. [Google Scholar] [CrossRef]
- De Lorenzi, L.; Fermeglia, M.; Torriani, G. Density, Kinematic Viscosity, and Refractive Index for Bis(2-ethylhexyl) Adipate, Tris(2-ethylhexyl) Trimellitate, and Diisononyl Phthalate. J. Chem. Eng. Data 1998, 43, 183–186. [Google Scholar] [CrossRef]
- Oswal, S.L.; Oswal, P.; Modi, P.S.; Dave, J.P.; Gardas, R.L. Acoustic, volumetric, compressibility and refractivity properties and Flory’s reduction parameters of some homologous series of alkyl alkanoates from 298.15 to 333.15 K. Thermochim. Acta 2004, 410, 1–14. [Google Scholar] [CrossRef]
- Anton, V.; Munoz-Embid, J.; Gascon, I.; Artal, M.; Lafuente, C. Thermophysical Characterization of Furfuryl Esters: Experimental and Modeling. Energy Fuels 2017, 31, 4143–4154. [Google Scholar] [CrossRef]
- Bogdanova, A.V.; Shostakovskii, M.F.; Plotnikova, G.I. Synthesis of unsaturated ether acetals, thioether acetals, and mercaptals. Dokl Akad. Nauk SSSR 1960, 134, 587–590. [Google Scholar]
- Makin, S.M.; Sudakova, V.S. Telomerization of vinyl ethyl ether with acetaldehyde acetal. Synthesis of 1-alkoxypolyenes. Zh. Obsh. Khim. 1962, 32, 3161–3166. [Google Scholar]
- Vogel, A.I. Physical Properties and Chemical Constitution. Part XI. Ketones. J. Chem. Soc. 1948, 610–615. [Google Scholar] [CrossRef]
- Overberger, C.G.; Frazier, C.; Mandelman, J.; Smith, H.F. The Preparation and Polymerization of p-Alkylstyrenes. Effect of Structure on the Transition Temperatures of the Polymers. J. Am. Chem. Soc. 1953, 75, 3326–3330. [Google Scholar] [CrossRef]
- Medwedew, S.S.; Alexejewa, E.N.; Organische Peroxyde, I. Mitteil.: Propyl- und Isopropyl-hydroperoxyd. Ber. Dt. Chem. Ges. 1932, 65, 133–137. [Google Scholar] [CrossRef]
- Harris, E.J. Decomposition of alkyl peroxides: Propyl peroxide, ethyl hydrogen peroxide and propyl hydrogen peroxide. Proc. R. Soc. A 1939, 173, 126–146. [Google Scholar] [CrossRef]
- Milas, N.A.; Surgenor, D.M. Organic peroxides. X. t-Amyl hydroperoxide and di-t-amyl peroxide. J. Am. Chem. Soc. 1946, 68, 643–644. [Google Scholar] [CrossRef]
- Lindstrom, E.G. Preparation of normal and secondary butyl hydroperoxides. J. Am. Chem. Soc. 1953, 75, 5123–5124. [Google Scholar] [CrossRef]
- Williams, H.R.; Mosher, H.S. Peroxides. I. n-Alkyl Hydroperoxides. J. Am. Chem. Soc. 1954, 76, 2984–2987. [Google Scholar] [CrossRef]
- Sanz, L.F.; Gonzalez, J.A.; de la Garcia Fuente, I.; Cobos, J.C. Thermodynamics of mixtures with strongly negative deviations from Raoult’s law. XII. Densities, viscosities and refractive indices at T = (293.15 to 303.15) K for (1-heptanol, or 1-decanol + cyclohexylamine) systems. Application of the ERAS model to (1-alkanol + cyclohexylamine) mixtures. J. Chem. Thermodyn. 2015, 80, 161–171. [Google Scholar] [CrossRef]
- Ioffe, B.V. Synthesis of unsymmetric dialkylhydrazines. Zh. Obsh. Khim. 1958, 28, 1296–1302. [Google Scholar]
- Legrand, R. Dimethylaminobenzylidenemalononitrile. Bull. Soc. Chim. Belges 1944, 53, 166–177. [Google Scholar]
- Paul, R.; Tchelitcheff, S. Synthesis of w-dinitriles and w-chlorinated nitriles from acetonitrile. Bull. Soc. Chim. Fr. 1949, 16, 470–475. [Google Scholar]
- Kuhn, L.P.; DeAngelis, L. The thermal decomposition of dinitrites. I. Vicinal dinitrites. J. Am. Chem. Soc. 1954, 76, 328–329. [Google Scholar] [CrossRef]
- Toops, E.E., Jr. Physical Properties of Eight High-Purity Nitroparaffins. J. Phys. Chem. 1956, 60, 304–306. [Google Scholar] [CrossRef]
- Oswal, S.L.; Oswal, P.; Gardas, R.L.; Patel, S.G.; Shinde, R.G. Acoustic, volumetric, compressibility and refractivity properties and reduction parameters for the ERAS and Flory models of some homologous series of amines from 298.15 to 328.15 K. Fluid Phase Equil. 2004, 216, 33–45. [Google Scholar] [CrossRef]
- McCusker, P.A.; Ashby, E.C.; Makowski, H.S. Organoboron compounds. III. Preparation and properties of alkyldichloroboranes. J. Am. Chem. Soc. 1957, 79, 5182–5184. [Google Scholar] [CrossRef]
- Christopher, P.M.; Tully, T.J. Some Octet and Bond Refractivities Involving Boron. J. Am. Chem. Soc. 1958, 80, 6516–6519. [Google Scholar] [CrossRef]
- Aubrey, D.W.; Lappert, M.F. 586. Cyclic organic boron compounds. Part IV. B-amino- and B-alkoxy-borazoles and their precursors the tris(primary amino)borons and (primary amino)boron alkoxides. J. Chem. Soc. 1959, 2927–2931. [Google Scholar] [CrossRef]
- Mikhailov, B.M.; Bazhenova, A.V. Organoboron compounds. 29. Cyclohexaneboronic acid and its derivatives. Russ. Chem. Bull. 1959, 8, 68–71. [Google Scholar] [CrossRef]
- Mikhailov, B.M.; Bubnov, Y.N. Organoboron compounds. XXXVIII. Reaction of trialkylboron with sulfur. Synthesis of esters of dialkylthioboronic acids. Zh. Obsh. Khim. 1959, 29, 1648–1650. [Google Scholar]
- Mikhailov, B.M.; Aronovich, P.M. Orgonoboron compounds. XXXV. Alkylphenylboronic acids and their anhydrides. Zh. Obsh. Khim. 1959, 29, 1257–1262. [Google Scholar]
- Mikhailov, B.M.; Shchegoleva, T.A. Synthesis of bis(alkylthio) boranes and trialkyl thioborates. Izvest. Akad. Nauk SSSR Ser. Khim. 1959, 8, 1868. [Google Scholar]
- Mikhailov, B.M.; Shchegoleva, T.A.; Blokhina, A.N. Reaction of tetra-n-butylmercaptodiborane with unsaturated compounds. Russ. Chem. Bull. 1960, 9, 1218–1219. [Google Scholar] [CrossRef]
- Mikhailov, B.M.; Shchegoleva, T.A. Synthesis and some transformations of alkylthiodiboranes. Dokl. Akad. Nauk SSSR 1960, 131, 834–836. [Google Scholar]
- Mikhailov, B.M.; Dorokhov, V.A. Organoboron compounds. LXXXVI. Alkylthio(diethylamino) boranes. Zh. Obsh. Khim. 1961, 31, 3750–3756. [Google Scholar]
- Mikhailov, B.M.; Bubnov, Y.N. Organoboron compounds. LXV. Synthesis of esters of dialkylthioboronic acids by the action of mercaptans on trialkylboron. Zh. Obsh. Khim. 1961, 31, 160–166. [Google Scholar]
- Shchegoleva, T.A.; Belyavskaya, E.M. Organoboron compounds. Synthesis and some properties of tris(ethylthio) diborane. Dokl Akad. Nauk SSSR 1961, 136, 638–641. [Google Scholar]
- Mikhailov, B.M.; Shchegoleva, T.A.; Shashkova, E.M. The synthesis of esters of alkyl thioboronic acids from trialkylboron and thioborates. Russ. Chem. Bull. 1961, 10, 845–847. [Google Scholar] [CrossRef]
- Mikhailov, B.M.; Kozminskaya, T.K. Organoboron Compounds. 90. Alkanehalothioboronic esters. Russ. Chem. Bull. 1962, 11, 234–237. [Google Scholar] [CrossRef]
- Mikhailov, B.M.; Dorokhov, V.A. Organoboron compounds. XCIV. Bis(dialkylamino) boranes, and bis(monoalkylamino) boranes. Zh. Obsh. Khim. 1962, 32, 1511–1514. [Google Scholar]
- Mikhailov, B.M.; Fedotov, N.S. The mechanism of nucleophilic substitution at the boron atom in organoboron compounds. Dokl. Akad. Nauk SSSR 1964, 154, 1128–1131. [Google Scholar]
- Mikhailov, B.M.; Vasil’ev, L.S. Organoboron compounds. CLII. Mutual exchange of alkoxy and alkylthio groups in organoboron compounds. Zh. Obsh. Khim. 1965, 35, 1073–1078. [Google Scholar]
- Zakharkin, L.I.; Kovredov, A.I. Compounds produced from products of 1,3-butadiene hydroboronation. Zh. Obsh. Khim. 1966, 36, 2153–2170. [Google Scholar]
- Jackson, I.K.; Davies, W.C.; Jones, W.J. Tertiary arylalkylphosphines. I. J. Chem. Soc. 1930, 2298–2301. [Google Scholar] [CrossRef]
- Kosolapoff, G.M. Isomerization of alkyl phosphites. III. Synthesis of alkylphosphonic acids. J. Am. Chem. Soc. 1945, 67, 1180–1182. [Google Scholar] [CrossRef]
- Jones, W.J.; Davies, W.C.; Bowden, S.T.; Edwards, C.; Davis, V.E.; Thomas, L.H. Preparation and properties of allyl phosphines, arsines, and stannanes. J. Chem. Soc. 1947, 1446–1450. [Google Scholar] [CrossRef]
- Knunyants, I.L.; Sterlin, R.N. Reactions between organic oxides and phosphine. Comptes Rendus (Dokl.) Acad. Des Sci. URSS 1947, 56, 49–52. [Google Scholar]
- Fox, R.B. Organophosphorus compounds. Alkyldichlorophosphines. J. Am. Chem. Soc. 1950, 72, 4147–4149. [Google Scholar] [CrossRef]
- Razumov, A.I.; Mukhacheva, O.A.; Khen, S.-D. Certain alkylphosphonothionic, alkylphosphonoselenonic, dialkylphosphinic, and alkylphosphonous esters, and the mechanism of addition to alkylphosphonous esters. Russ. Chem. Bull. 1952, 1, 797–802. [Google Scholar] [CrossRef]
- Pudovik, A.N.; Yarmukhametova, D.K. New synthesis of esters of phosphonic and thiophosphonic acids. XV. Addition of esters of phenyl- and alkylphosphonous acids to esters of methacrylic and acrylic acids. Izvest. Akad. Nauk SSSR Ser. Khim. 1952, 902–907. [Google Scholar]
- Yakubovich, A.Y.; Motsarev, G.V. Synthesis of hetero-organic compounds of the aromatic series by the reaction of arylsilanes with aluminum chloride and halides of various elements. I. Organophosphorus compounds. Zh. Obsh. Khim. 1953, 23, 1547–1552. [Google Scholar]
- Arbuzov, B.A.; Rizpolozhenskii, N.I. Esters of diethylphosphinous acid. Dokl. Akad. Nauk SSSR 1953, 89, 291–292. [Google Scholar]
- Anlsimov, K.N.; Nesmeyanov, A.N. Derivatives of unsaturated phosphonic acids. Russ. Chem. Bull. 1955, 4, 915–917. [Google Scholar] [CrossRef]
- Anisimov, K.N.; Kolobova, N.E.; Nesmeyanov, A.N. Derivatives of unsaturated phosphonic acids. IX. Neutral esters of 2-alkoxy (or phenoxy)vinylthiophosphonic acids. Izvest. Akad. Nauk SSSR Ser. Khim. 1955, 669–671. [Google Scholar]
- Razumov, A.I.; Mukhacheva, O.A. Derivatives of alkylphosphonous and dialkylphosphinic acids. III. Atomic refraction of phosphorus in esters of alkylphosphonous acids. Zh. Obsh. Khim. 1956, 26, 1436–1440. [Google Scholar]
- Razumov, A.I.; Mukhacheva, O.A. Derivatives of alkylphosphonous and dialkylphosphinic acids. IV. Reactions of addition and isomerization of esters of alkylphosphonous acids. Zh. Obsh. Khim. 1956, 26, 2463–2468. [Google Scholar]
- Anisimov, K.N.; Nesmeyanov, A.N. Derivatives of unsaturated phosphonic acids. XVII. Derivatives of β-phenylvinylphosphonic acid. Izvest. Akad. Nauk SSSR Ser. Khim. 1956, 19–22. [Google Scholar]
- Anisimov, K.N.; Kolobova, N.E.; Nesmeyanov, A.N. Derivatives of unsaturated phosphonic acids. XVIII. Chlorides of alkylthiovinylphosphonic acids and their derivatives. Izvest. Akad. Nauk SSSR Ser. Khim. 1956, 23–26. [Google Scholar]
- Lenard-Borecka, B.; Michalski, J. Organophosphorus compounds of sulfur and selenium. VII. Dialkoxyphosphinylsulfenyl chlorides. Rocz. Chem. 1957, 31, 1167–1176. [Google Scholar]
- Razumov, A.I.; Mukhacheva, O.A.; Markovich, E.A. Derivatives of alkylphosphonous and phosphonic acids. VIII. Synthesis and properties of some alkylated amides of alkylphosphonic chlorides. Zh. Obsh. Khim. 1958, 28, 194–197. [Google Scholar]
- Yamasaki, T. Preparation and properties of alkyl phosphonothionates, (RO)2P(S)H. Sci. Repts. Res. Insts. Tohoku Univ. Ser. A 1959, 11, 73–79. [Google Scholar]
- Kukhtin, V.A.; Abramov, V.S.; Orekhova, K.M. Rearrangement of esters of x-hydroxyalkylphosphonic acids into isomeric phosphates. Dokl. Akad. Nauk SSSR 1959, 128, 1198–1200. [Google Scholar]
- Grechkin, N.P.; Shagidullin, R.R. Organophosphorus derivatives of ethylenimine. Russ. Chem. Bull. 1960, 9, 1978–1982. [Google Scholar] [CrossRef]
- Grechkin, N.P.; Shagidullin, R.R. Organophosphorus derivatives of ethylenimine. III. Addition of acids to ethylenamides of phosphorus acids. Izvest. Akad. Nauk SSSR Ser. Khim. 1960, 2135–2139. [Google Scholar]
- Stolzer, C.; Simon, A. Fluorophosphorus compounds. III. Symmetrical diphosphoryl difluoride dichloride, P2O3Cl2F2. Chem. Ber. 1961, 94, 1976–1979. [Google Scholar] [CrossRef]
- Pass, F.; Steininger, E.; Zorn, H. Organic phosphorus compounds III. A new method for the preparation of primary phosphines. Monats. Chem. 1962, 93, 230–236. [Google Scholar] [CrossRef]
- Pass, F.; Steininger, E.; Zorn, H. Eine neue Methode zur Darstellung primärer Phosphine. Mon. Chem. Und Verwandte Teile And. Wiss. 1962, 93, 230–236. [Google Scholar] [CrossRef]
- Kabachnik, M.I.; Tsvetkov, E.N. Lower dialkylphosphinous acids (secondary phosphine oxides) and some of their properties. Russ. Chem. Bull. 1963, 12, 1120–1124. [Google Scholar] [CrossRef]
- Boerner, K.B.; Stoelzer, C.; Simon, A. Fluorophosphorus compounds. IX. The catalytic hydrogenation of fluorophosphoric acid phenyl esters. Chem. Ber. 1963, 96, 1328–1334. [Google Scholar]
- Stoelzer, C.; Simon, A. Fluorophosphorus compounds. VI. Alkylamides of fluorodiphosphoric acids. Chem. Ber. 1963, 96, 881–895. [Google Scholar]
- Stoelzer, C.; Simon, A. Fluorophosphorus compounds. X. Results of refractometric investigations of fluorophosphoric acid derivatives. Chem. Ber. 1963, 96, 1335–1340. [Google Scholar]
- Arbuzov, B.A.; Vinokurova, G.M. Synthesis of bffunctional organophosphorus compounds. II. Addition of butylphosphine to unsaturated compounds. Izvest. Akad. Nauk SSSR Ser. Khim. 1963, 3, 502–506. [Google Scholar]
- Voigt, D.; Labarre, M.C. Synthesis and magneto-optical study of some trialkylated trithiophosphites. Compt. Rend. 1964, 259, 4632–4634. [Google Scholar]
- Zhmurova, I.N.; Voitsekhovskaya, I.Y. Alkyltetrachlorophosphoranes. Zh. Obsh. Khim. 1965, 35, 2197–2200. [Google Scholar]
- Grishina, O.N.; Bezzubova, L.M. Alkylthiophosphine sulfides. III. O-Alkyl alkylphosphonodithioates. Izvest. Akad. Nauk SSSR Ser. Khim. 1966, 9, 1617–1620. [Google Scholar]
- Neimysheva, A.A.; Knunyants, I.L. Nucleophilic displacement in the series of derivatives of acids of phosphorus. I. Kinetics of hydrolysis of chlorides of di-alkylphosphinic acids. Zh. Obsh. Khim. 1966, 36, 1090–1098. [Google Scholar]
- Foxton, A.A.; Jeffrey, G.H.; Vogel, A.I. Physical properties and chemical constitution. Part XLIX. The refractivities, densities, and surface tensions of some organophosphorus compounds. J. Chem. Soc. A 1966, 249–253. [Google Scholar] [CrossRef]
- Akamsin, V.D.; Rizpolozhenskii, N.I. Esters of phosphorus(III) thioacids. V. New method for preparation of thiophosphinous acid esters. Izvest. Akad. Nauk SSSR Ser. Khim. 1967, 9, 1987–1989. [Google Scholar]
- Buina, N.A.; Nuretdinov, I.A.; Grechkin, N.P. Ethylenimides of arylphosphorous and thiophosphoric acids. Izvest. Akad. Nauk SSSR Ser. Khim. 1967, 1, 217–220. [Google Scholar] [CrossRef]
- Voigt, D.; Turpin, R.; Torres, M. Magnetooptical study of some dialkylphosphines. Comptes Rendus. Seances Acad. Sci. Ser. C Sc. Chim. 1967, 265, 884–887. [Google Scholar]
- Grishina, O.N.; Potekhina, M.I. Synthesis of O-alkylalkyldithiophosphinic aids from products of oxidative phosphination of hydrocarbons of petroleum fractions. Neftekhim 1968, 8, 111–117. [Google Scholar]
- Kas’yanova, E.F.; Gurvich, S.M. Synthesis of some derivatives of monothiophosphoric acid for the flotation of ores of heavy nonferrous metals. Zh. Obsh. Khim. 1969, 39, 365–366. [Google Scholar]
- Fushimi, T.; Allcock, H.R. Cyclotriphosphazenes with sulfur-containing side groups: Refractive index and optical dispersion. Dalton Trans. 2009, 14, 2477–2481. [Google Scholar] [CrossRef]
- Noller, C.R.; Gordon, J.J. The Preparation of Some Higher Aliphatic Sulfonic Acids. J. Am. Chem. Soc. 1933, 55, 1090–1094. [Google Scholar] [CrossRef]
- Allen, P., Jr. The Preparation of Some Normal Aliphatic Thiocyanates. J. Am. Chem. Soc. 1935, 57, 198–199. [Google Scholar] [CrossRef]
- Post, H.W. The reaction of certain orthoesters with aldehydes. J. Org. Chem. 1940, 5, 244–249. [Google Scholar] [CrossRef]
- Hall, W.P.; Reid, E.E. A Series of α,w-Dimercaptans. J. Am. Chem. Soc. 1943, 65, 1466–1468. [Google Scholar] [CrossRef]
- Whitehead, E.V.; Dean, R.A.; Fidler, F.A. The preparation and properties of sulfur compounds related to petroleum. II. Cyclic sulfides. J. Am. Chem. Soc. 1951, 73, 3632–3635. [Google Scholar] [CrossRef]
- Cairns, T.L.; Evans, G.L.; Larchar, A.W.; McKusick, B.C. Gem-Dithiols. J. Am. Chem. Soc. 1952, 74, 3982–3989. [Google Scholar] [CrossRef]
- Birch, S.F.; Cullum, T.V.; Dean, R.A. The preparation and properties of dialkyl di- and polysulfides. Some disproportionation reactions. J. Inst. Pet. 1953, 39, 206–219. [Google Scholar]
- Cope, A.C.; Farkas, E. Cleavage of carbon-sulfur bonds by catalytic hydrogenation. J. Org. Chem. 1954, 19, 385–390. [Google Scholar] [CrossRef]
- Backer, H.J.; Kloosterziel, H. Thiolsulfinic esters. Rec. Trav. Chim. Pays Bas Belg. 1954, 73, 129–139. [Google Scholar] [CrossRef]
- Kabachnik, M.I.; Golubeva, E.I. Addition of sulfur to dialkyl phosphites. Dokl. Akad. Nauk SSSR 1955, 105, 1258–1261. [Google Scholar]
- Haines, W.E.; Helm, B.V.; Cook, G.L.; Ball, J.S. Purification and Properties of Ten Organic Sulfur Compounds—Second Series. Phys. Chem. 1956, 60, 549–555. [Google Scholar] [CrossRef]
- Freidlina, R.K.; Chukovskaya, E.T. Reaction of mercuric acetate with esters of xanthic acids. Izvest. Akad. Nauk SSSR Ser. Khim. 1957, 6, 187–193. [Google Scholar]
- Boonstra, H.J.; Brandsma, L.; Wiegman, A.M.; Arens, J.F. Chemistry of acetylenic ethers. XXXVI. Preparation and properties of some 1-alkylthio-1-alkynes. Rec. Trav. Chim. Pays Bas Belg. 1959, 78, 252–264. [Google Scholar] [CrossRef]
- Jeffrey, G.H.; Parker, R.; Vogel, A.I. 113. Physical properties and chemical constitution. Part XXXII. Thiophen compounds. J. Chem. Soc. 1961, 570–575. [Google Scholar] [CrossRef]
- Mathias, S.; de Carvalho, E., Jr.; Cecchini, R.G. The Dipole Moments of Cyclohexanethiol, a-Toluenethiol and Benzenethiol. J. Phys. Chem. 1961, 65, 425–427. [Google Scholar] [CrossRef]
- Hine, J.; Bayer, R.P.; Hammer, G.G. Formation of Bis-(Methylthio)-Methylene from Methyl Orthothioformate and Potassium Amide. J. Am. Chem. Soc. 1962, 84, 1751–1752. [Google Scholar] [CrossRef]
- Volynskii, N.P.; Gal’pern, G.D.; Smolyaninov, V.V. Synthesis of 2-substituted thiacyclohexanes. Neftekhim 1963, 3, 482–487. [Google Scholar] [CrossRef]
- Shostakovskii, M.F.; Atavin, A.S.; Dmitrieva, L.P.; Vasil’ev, N.P.; Gladkova, G.A. Reaction of 2,2-dialkyl-4-vinyloxymethyl-1,3-dioxolanes with thiols. Zh. Obsh. Khim. 1966, 2, 209–212. [Google Scholar]
- Shostakovskii, M.F.; Atabin, A.S.; Mikhaleva, A.I.; Vasil’ev, N.P.; Dmitrieva, L.P. Synthesis of 2,2-bis(alkthio)propyl vinyl ethers. Russ. Chem. Bull. 1967, 16, 1337–1338. [Google Scholar] [CrossRef]
- Nakhmanovich, A.S.; Skvortsova, G.G.; Shostakovskii, M.F.; Shulyak, L.A. Synthesis of vinyl esters of α-thienylcarbinols. Khim. Atset. Dokl. Vsesoyuz. Nauch. Konf. Khim. Atset. Ego Proiz. 1968, 256–259. [Google Scholar]
- Bittell, J.E.; Speier, J.L. Synthesis of Thiols and Polysulfides from Alkyl Halides, Hydrogen Sulfide, Ammonia, and Sulfur. J. Org. Chem. 1978, 43, 1687–1689. [Google Scholar] [CrossRef]
- Taganliev, A.; Rol’nik, L.Z.; Lapuka, L.F.; Rol’nik, L.Z.; Kirilyuk, G.G.; Pastushchenko, E.V.; Khekimov, Y.K. Structure and physicochemical properties of thioorthoformates. Izvest Akad. Nauk Turkm. SSR Ser. Fiz. Tekhn. Khim. Geolog. Nauk 1986, 1, 60–64. [Google Scholar]
- Khekimov, Y.K.; Taganlyev, A.; Kurbanov, D.; Khodzhalyev, T.K.; Kurbanov, I. Homolytic isomerization of 1,1,1-tris(ethylthio) ethane. Izvest Akad. Nauk Turkm. SSR Ser. Fiz. Tekhn. Khim. Geolog. Nauk 1987, 4, 105–106. [Google Scholar]
- Gilani, H.G.; Gilani, A.G.; Shekarsaree, S. Solubility and tie line data of the water–phosphoric acid–solvents at T = 303.2, 313.2, and 323.2 K: An experimental and correlational study. Thermochim. Acta 2013, 558, 36–45. [Google Scholar] [CrossRef]
- Vaughn, T.H. 1-Propyl-2-iodoacetylene. J. Am. Chem. Soc. 1933, 55, 1293. [Google Scholar] [CrossRef]
- Vaughn, T.H. Direct iodination of monosubstituted acetylenes. J. Am. Chem. Soc. 1933, 55, 2150–2153. [Google Scholar] [CrossRef]
- Bachman, G.B. Dehalogenation of aliphatic bromo acids. The bromo- and dibromo lefins. J. Am. Chem. Soc. 1933, 55, 4279–4284. [Google Scholar] [CrossRef]
- Vaughn, T.H.; Nieuwland, J.A. Synthesis and properties of 2-iodo-1-vinylacetylene. J. Chem. Soc. 1933, 741–743. [Google Scholar] [CrossRef]
- Desreux, V. Further study of alkyl fluorides. Bull. Cl. Sci. Acad. Royale Belg. 1934, 20, 457–476. [Google Scholar]
- Audsley, A.; Goss, F.R. The magnitude of the solvent effect in dipole-moment measurements. V. The solvent-effect constant and the moments of alkyl iodides. J. Chem. Soc. 1942, 358, 358–366. [Google Scholar] [CrossRef]
- Schmerling, L. Condensation of saturated halides with unsaturated compounds. II. Condensation of alkyl halides with monohaloo lefins. J. Am. Chem. Soc. 1946, 68, 1650–1654. [Google Scholar] [CrossRef]
- Mousseron, M.; Winternitz, F.; Jacquier, R. Some alicyclic chloro epoxides. Compt. Rend. 1946, 223, 1014–1015. [Google Scholar]
- Luciens, H.W.; Mason, C.T. The Preparation and Properties of Some Branched-Chain Alkyl Bromomethyl Ethers. J. Am. Chem. Soc. 1949, 71, 258–260. [Google Scholar] [CrossRef]
- Hoffmann, F.W. Aliphatic fluorides. I. ω, ω’-Difluoroalkanes. J. Org. Chem. 1949, 14, 105–110. [Google Scholar] [CrossRef]
- Coffman, D.D.; Raasch, M.S.; Rigby, G.W.; Barrick, P.L.; Hanford, W.E. Addition reactions of tetrafluoroethylene. J. Org. Chem. 1949, 14, 747–753. [Google Scholar] [CrossRef]
- Roe, A.; Cheek, P.H.; Hawkins, G.F. The Synthesis of 2-Fluoro-4- and 2-Fluoro-6-pyridinecarboxylic Acid and Derivatives. J. Am. Chem. Soc. 1949, 71, 4152–4153. [Google Scholar] [CrossRef]
- Stone, H.; Shechter, H. A new method for the preparation of organic iodides. J. Org. Chem. 1950, 15, 491–495. [Google Scholar] [CrossRef]
- Norton, T.R. New synthesis of ethyl trifluoroacetate. J. Am. Chem. Soc. 1950, 72, 3527–3528. [Google Scholar] [CrossRef]
- Hauptschein, M.; Grosse, A.V. Perfluoroalkyl Halides Prepared from Silver Perfluoro-fatty Acid Salts. I. Perfluoroalkyl Iodides. J. Am. Chem. Soc. 1951, 73, 2461–2463. [Google Scholar] [CrossRef]
- Douglass, I.B.; Martin, F.T.; Addor, R. Sulfenyl Chloride Studies. II. Mono-, Di-, and Tri-Chloromethanesulfenyl Chlorides and Certain of their Derivatives. J. Org. Chem. 1951, 16, 1297–1302. [Google Scholar] [CrossRef]
- Hauptschein, M.; Stokes, C.S.; Grosse, A.V. The properties and reactions of perfluorobutyrolactone. J. Am. Chem. Soc. 1952, 74, 1974–1976. [Google Scholar] [CrossRef]
- Douglass, I.B.; Osborne, C.E. The anhydrous chlorination of thioesters and related compounds. J. Am. Chem. Soc. 1953, 75, 4582–4583. [Google Scholar] [CrossRef]
- Yagupol’skii, L.M. Synthesis of derivatives of phenyl trifluoromethyl ether. Dokl. Akad. Nauk SSSR 1955, 105, 100–102. [Google Scholar]
- Stevens, C.L.; Mukherjee, T.K.; Traynelis, V. gem-Dihalides from the Hofmann degradation of α-haloamides. J. Am. Chem. Soc. 1956, 78, 2264–2267. [Google Scholar] [CrossRef]
- Douglass, I.B.; Warner, G.H. Methyl and ethyl trichloromethyl ethers. J. Am. Chem. Soc. 1956, 78, 6070–6071. [Google Scholar] [CrossRef]
- Douglass, I.B.; Poole, D.R. A New Method for the Preparation of Sulfinyl Chlorides. J. Org. Chem. 1957, 22, 536–537. [Google Scholar] [CrossRef]
- Yarovenko, N.N.; Vasil’eva, A.S. New method of introduction of the trihalomethyl group into organic compounds. Zh. Obsh. Khim. 1958, 28, 2502–2504. [Google Scholar]
- Soborovskii, L.Z.; Gladshtein, B.M.; Kiseleva, M.I.; Chernetskii, V.N. Organic compounds of sulfur. I. Synthesis of fluorides of alkanesulfonic acids and their halogen derivatives. Zh. Obsh. Khim. 1958, 28, 1866–1870. [Google Scholar]
- Bissell, E.R.; Spengler, R.E. Styrene-p-carboxylic acid. J. Org. Chem. 1959, 24, 1146–1147. [Google Scholar] [CrossRef]
- Macey, W.A.T. The Physical Properties of Certain Organic Fluorides. J. Phys. Chem. 1960, 64, 254–257. [Google Scholar] [CrossRef]
- Sadykh-Zade, S.I.; Sultanov, N.T. A new synthesis of α- and β-chlorostyrenes by direct chlorination of styrene. Azerbaid. Khim. Zh. 1960, 5, 33–36. [Google Scholar]
- Stolzer, C.; Simon, A. Fluorophosphorus compounds. I. Chem. Ber. 1960, 93, 1323–1331. [Google Scholar]
- Stolzer, C.; Simon, A. Fluorophosphorus compounds. II. Esters of fluorodiphosphoric acids. Chem. Ber. 1960, 93, 2578–2590. [Google Scholar]
- Bergel’son, L.D. Stereochemistry of addition reactions at a triple bond. VII. Stereochemistry of hydrobromination of bromoacetylenes under radical conditions. Izvest. Akad. Nauk SSSR Ser. Khim. 1960, 9, 1235–1240. [Google Scholar]
- Kost, V.N.; Freidlina, R.K. Telomerization of ethylene with polychloroalkanes containing the CCl2Br group. Izvest. Akad. Nauk SSSR Ser. Khim. 1961, 10, 1252–1256. [Google Scholar] [CrossRef]
- Gubanov, V.A.; Tumanova, A.V.; Dolgopol’skii, I.M.; Shcherbakov, V.A. Reaction of perfluoromethyl perfluorovinyl ether with hydrogen halides. Zh. Obsh. Khim. 1964, 34, 2802–2803. [Google Scholar]
- Ol’dekop, Y.A.; Kaberdin, R.V. Acyl peroxides. IX. Reaction of acetyl peroxide with cis-1-2dichloroethylene. Zh. Organ. Khim. 1965, 1, 873–876. [Google Scholar]
- Knunyants, I.L.; Krasuskaya, M.P.; Del’tsova, D.P. Di (1, 2, 4-oxadiazolyl) polydifluoromethylenes. Izvest. Akad. Nauk SSSR Ser. Khim. 1966, 577–579. [Google Scholar]
- Tataurov, G.P.; Sokolov, S.V. Synthesis and properties of octafluoroanisole. Zh. Obsh. Khim. 1966, 36, 537–540. [Google Scholar]
- Vipinchandra, A.R.; Hemalkumar, P.V.; Hemant, A.C. Static Permittivity and Refractive Index of Binary Mixtures of 3-Bromoanisole and 1-Propanol at Different Temperatures. J. Chem. Eng. Data 2015, 60, 3113–3119. [Google Scholar] [CrossRef]
- Gierut, J.A.; Sowa, F.J.; Nieuwland, J.A. Organic reactions with silicon compounds. II. The reaction of silicon tetrafluoride with the Grignard reagent. J. Am. Chem. Soc. 1936, 58, 897–898. [Google Scholar] [CrossRef]
- Gilman, H.; Clark, R.N. Some steric effects of the isopropyl group in organosilicon compounds. J. Am. Chem. Soc. 1947, 69, 1499–1500. [Google Scholar] [CrossRef]
- Petrov, A.D.; Shchukovskaya, L.L. Synthesis and properties of symmetric acetylenic disilanes. Dokl. Akad. Nauk SSSR 1952, 86, 551–553. [Google Scholar]
- Takatani, T. Silicic acid esters. V. Some physical properties of the silicates of aliphatic alcohols. Nippon Kagaku Zasshi 1953, 74, 948–950. [Google Scholar] [CrossRef]
- Petrov, A.D.; Ponomarenko, V.A. Synthesis and properties of disilylmethane, 1,2-disilylethane, 1,3-disilylpropane, and 1,3,5-trisilacyclohexane. Dokl. Akad. Nauk SSSR 1953, 90, 387–390. [Google Scholar]
- Zimmermann, W. Stability of chlorinated methylchlorosilane and chlorinated methylsiloxanes. Chem. Ber. 1954, 87, 887–891. [Google Scholar] [CrossRef]
- Batuev, M.I.; Shostakovskii, M.F.; Belyaev, V.I.; Matveeva, A.D.; Dubrova, E.V. Chemical and physical properties of the hydroxyl group in trimethylsilanol. Dokl. Akad. Nauk SSSR 1954, 95, 531–534. [Google Scholar]
- Dolgov, B.N.; Kharitonov, N.P.; Voronkov, M.G. Reaction of triethylsilane with ammonia and amines. Zh. Obsh. Khim. 1954, 24, 678–683. [Google Scholar]
- Voronkov, M.G.; Dolgov, B.N. Isothiocyano-substituted silanes. Zh. Obsh. Khim. 1954, 24, 1082–1087. [Google Scholar]
- Petrov, A.D.; Chernysheva, T.I. Synthesis of tetraisobutyl, tetraisopropyl, tetracyclohexyl, and tetra-1-naphthylsilanes. Zh. Obsh. Khim. 1954, 24, 1189–1192. [Google Scholar]
- Shostakovskii, M.F.; Shikhiev, I.A.; Kochkin, D.A.; Belyaev, V.I. Oxygen-containing organosilicon compounds. III. Preparation of trimethyl- and triethylsilanols and their transformations. Zh. Obsh. Khim. 1954, 24, 2202–2206. [Google Scholar]
- Petrov, A.D.; Shchukovskaya, L.L. Behavior toward chemical reagents of the silicon-carbon bond in α-alkynyl- and β-alkenylsilanes. Zh. Obsh. Khim. 1955, 25, 1128–1136. [Google Scholar]
- Shostakovskii, M.F.; Malinovskii, M.S.; Romantsevich, M.K.; Kochkin, D.A. Synthesis and transformations of oxygen-containing organosilicon compounds. III. Reactions of propylene oxide with alkyl(aryl)chlorosilanes. Izvest. Akad. Nauk SSSR Ser. Khim. 1956, 632–634. [Google Scholar]
- Voronkov, M.G.; Khudobin, Y.I. Reaction of trialkylsilanes with iodine and hydrogen iodide. Akad. Nauk SSSR Ser. Khim. 1956, 5, 805–810. [Google Scholar] [CrossRef]
- Shostakovskii, M.F.; Kochkin, D.A.; Rogov, V.M. 102. Synthesis and transformation of oxygen-containing organosilicon compounds. VI. Preparation of secondary dialkyl(aryl)chlorosilanes, dialkyl(aryl)silanols, and some of their transformations. Akad. Nauk SSSR Ser. Khim. 1956, 1062–1069. [Google Scholar]
- Shostakovskii, M.F.; Kochkin, D.A.; Vinogradov, V.L.; Neterman, V.A. Synthesis and transformation of oxygen-containing organosilicon compounds. VI. Reaction of hydrogen containing alkyl(aryl)dichlorosilanes with alcohols. Akad. Nauk SSSR Ser. Khim. 1956, 1269–1271. [Google Scholar]
- Dolgov, B.N.; Borisov, S.N.; Voronkov, M.G. Reaction of alkylhalosilanes with trialkylsilanes. Zh. Obsh. Khim. 1957, 27, 2692–2697. [Google Scholar]
- Kaufman, H.C.; Douthett, O.R. Preparaton and Comparison of the Physical Properties of Alkyl and Alkforyl Silicates. Ind. Eng. Chem. 1958, 3, 324–327. [Google Scholar]
- Voronkov, M.G.; Shabarova, Z.I. Alkoxysilanes. XIV. Cleavage of organosiloxanes by alcohols as a method of synthesis of organoalkoxysilanes. Zh. Obsh. Khim. 1959, 29, 1528–1534. [Google Scholar]
- Duffaut, N.; Calas, R.; Mace, J.C. The oxidation of trialkyl and triaryl silanes by oxygen containing silver compounds. Bull. Soc. Chim. Fr. 1959, 1971–1973. [Google Scholar]
- Wilson, G.R.; Smith, A.G. Preparation of Decamethyltetrasilane and Its Lower Homologs. J. Org. Chem. 1961, 26, 557–559. [Google Scholar] [CrossRef]
- Sergeeva, S.I.; Chien, H.-C.; Tsitovich, D.D. Synthesis of alkyl- and dialkylbis(1,1-dialkylhydrazino)silane. Zh. Obsh. Khim. 1960, 30, 694–695. [Google Scholar]
- Voronkov, M.G.; Rabkina, S.M. Alkoxysilanes. XVI. Reaction of tetraalkoxysilanes with ketones. Zh. Obsh. Khim. 1961, 31, 1259–1265. [Google Scholar]
- Sergeeva, Z.I.; Hsieh, C.-L. A new method of synthesis of organosilicon hydrazines. Zh. Obsh. Khim. 1962, 32, 1987–1993. [Google Scholar]
- Stolberg, U.G. Octamethyltrisilane and decamethyltetrasilane. Angew. Chem. 1962, 74, 696. [Google Scholar]
- Sergeeva, Z.I.; Hsieh, C.-L. Reaction of nonsymmetric dialkylhydrazines with alkylchloro-silanes. Zh. Obsh. Khim. 1963, 33, 1874–1878. [Google Scholar]
- Shostakovskii, M.F.; Sokolov, B.A.; Dmitrieva, G.V.; Alekseeva, G.M. Addition reaction of hydrosilanes with vinyl ethers. Zh. Obsh. Khim. 1964, 34, 2839–2842. [Google Scholar]
- Cudlin, J.; Schraml, J.; Chvalovsky, V. Organosilicon compounds. XXXV. Addition of dichloromethylene to isomeric bis(trimethylsilyl) ethylenes. Coll. Czech. Chem. Comm. 1964, 29, 1476–1483. [Google Scholar] [CrossRef]
- Voronkov, M.G.; Skorik, Y.I. Synthesis of organofluorosilanes. Akad. Nauk SSSR Ser. Khim. 1964, 7, 1215–1221. [Google Scholar] [CrossRef]
- Giao, D.-H. New cleavage method for tetrabutylated derivatives of Si, Ge, and Sn. Compt. Rend. 1965, 260, 6937–6938. [Google Scholar]
- Prejzner, J. Organoxyisocyanatosilanes. I. Preparation and properties of phenoxyisocyanatosilanes. Rocz. Chem. 1965, 39, 747–753. [Google Scholar]
- Bolotov, B.A.; Kharitonov, N.P.; Batyaev, E.A.; Rumyantseva, E.G. Destructive hydrogenation of trialkylalkoxysilanes. Zh. Obsh. Khim. 1967, 37, 2113–2117. [Google Scholar]
- Cer, L.; Vaisarova, V.; Chvalovsky, V. Organosilicon compounds. LIII. Dipole moments of benzylmethylethoxysilanes. Coll. Czech. Chem. Comm. 1967, 32, 3784–3786. [Google Scholar] [CrossRef]
- Kannengiesser, G.; Damm, F. Preparation of some tetrakis(dialkylamino)silanes. Bull. Soc. Chim. Fr. 1967, 7, 2492–2495. [Google Scholar]
- Khudobin, Y.I.; Voronkov, M.G.; Kharitonov, N.P. Trialkyl(aryl)bromosilanes. Latv. PSR Zinat. Akad. Vest. Kim. Ser. 1967, 5, 595–600. [Google Scholar]
- Zhinkin, D.Y.; Mal’nova, G.N.; Gorislavskaya, Z.V. Ammonolysis of triorganochlorosilanes, their coammonolysis with trimethylchlorosilane, and coammonolysis of some tri- and diorganochlorosilanes. Zh. Obsh. Khim. 1968, 38, 2800–2807. [Google Scholar]
- Feher, F.; Hädicke, P.; Frings, H. Beiträge zur Chemie des Siliciums und Germaniums, XXIII (1) Physikalisch-Chemische Eigenschaften der Silane von Trisilan bis Heptasilan. Inorg. Nucl. Chem. Lett. 1973, 9, 931–936. [Google Scholar] [CrossRef]
- Boredau, M.; Dédier, J.; Frainnet, E. Etude Structurale d’Hexaalkyldisiloxanes et de Trialkylalkoxy- ou Aryloxysilanes. I. Determination par Dipolemetrie d’Angles SiOC et SiOSi. J. Organomet. Chem. 1973, 59, 125–139. [Google Scholar]
- Moerlein, S.M. Synthesis and Spectroscopic Characteristics of Aryltrimethyl-Silicon, -Germanium, and Tin Compounds. J. Organomet. Chem. 1987, 319, 29–39. [Google Scholar] [CrossRef]
- Alagar, M.; Krishnasami, V. Ultrasonic Properties of Tetraalkoxysilanes. Ultrasonics 1987, 25, 283–287. [Google Scholar] [CrossRef]
- Alagar, M.; Ponnusamy, M.; Amsavel, A. Studies on Dielectric Behaviour of Dimethyldialkoxysilanes. Hung. J. Ind. Chem. 1993, 21, 19–22. [Google Scholar]
- Yu, L.; Hu, Y.; Dong, H.; Wu, C. The physicochemical properties of 1,3,5-trimethyl-1,3,5-tris(3,3,3-trifluoropropyl)cyclotrisiloxane with various aromatic hydrocarbons at T = (308.15 to 323.15) K. J. Chem. Thermodyn. 2016, 96, 117–126. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, C.; Dong, H.; Wu, C. Excess molar volume along with refractive index for binary systems of dimethoxymethylphenylsilane with dimethyldimethoxysilane, dimethyldiethoxylsilane, methylvinyldiethoxysilane and ethenyltrimethoxysilane. J. Chem. Thermodyn. 2017, 109, 82–90. [Google Scholar] [CrossRef]
- Jin, J.; Shao, F.; Dong, H.; ·Wu, C. Volumetric and Optical Properties of Dimethylvinylethoxysilane with n-Alkanes (C7 to C10) at Temperatures in the Range (298.15 to 318.15) K. J. Sol. Chem. 2019, 48, 82–103. [Google Scholar] [CrossRef]
- Terent’ev, A.P.; Yanovskaya, L.A. Sulfonation and sulfonic acids of acidophobic substances. XVI. Sulfonation of some indole derivatives. Zh. Obsh. Khim. 1951, 21, 1295–1297. [Google Scholar]
- Leonard, N.J.; Ryder, B.L. The stereoisomers of vic-dialkylpiperidines. The 2-butyl-3-methylpiperidines. J. Org. Chem. 1953, 18, 598–608. [Google Scholar] [CrossRef]
- Mikhailov, G.I. 2-Bromopyridine. Zh. Prikl. Khim. 1954, 27, 349–351. [Google Scholar]
- Shuikin, N.I.; Bel’skii, I.F.; Skobtsova, G.E. Catalytic synthesis of higher homologs of pyrrole and pyrrolidine from α-furylalkylamines. Izvest. Akad. Nauk SSSR Ser. Khim. 1963, 9, 1678–1680. [Google Scholar] [CrossRef]
- Bel’skii, I.F.; Shuikin, N.I.; Skobtsova, G.E. Conjugated hydrogenolysis in the synthesis of pyrrolidine homologs. Izvest. Akad. Nauk SSSR Ser. Khim. 1963, 9, 1675–1678. [Google Scholar] [CrossRef]
- Shuikin, N.I.; Bel’skii, I.F.; Karakhanov, R.A.; Kozma, B.; Bartok, M. Investigations in the field of diols and cyclic ethers. V. Preparation of 2-monosubstituted derivatives of tetrahydropyran. Acta Phys. Chem. 1963, 9, 37–42. [Google Scholar]
- Bel’skii, I.F.; Shuikin, N.I.; Skobtsova, G.E. Catalytic conversion of furan amines into 2,4-dialkylpyrroles. Izvest. Akad. Nauk SSSR Ser. Khim. 1964, 6, 1118–1120. [Google Scholar]
- Shuikin, N.I.; Bel’skii, I.F.; Karakhanov, R.A.; Kozma, B.; Bartok, M. Isomerization of tetrahydropyrans. Izvest. Akad. Nauk SSSR Ser. Khim. 1964, 4, 747–750. [Google Scholar] [CrossRef]
- Shuikin, N.I.; Bel’skii, I.F.; Barkovskaya, L.Y.; Dronov, V.I.; Alalykina, L.A. Synthesis of 2,4- and 2,5-dialkylthiophanes. Khim. Sera Organ. Soedin. Soderzhashch. Neff. Nefteprod. Akad. Nauk SSSR Bashkirsk. Filial 1964, 6, 197–200. [Google Scholar]
- Shuikin, N.I.; Bel’skii, I.F.; Barkovskaya, L.Y.; Gerasimov, M.M. Synthesis of some cyclic sulfides. Khim. Sera Organ. Soedin. Soderzhashch. Neff. Nefteprod. Akad. Nauk SSSR Bashkirsk. Filial 1964, 7, 58–60. [Google Scholar]
- Bel’skii, I.F.; Shuikin, N.I.; Karakhanov, R.A. Synthesis of γ-oxo alcohols and dihydrofurans from 1-furyl-3alkanols. Izvest. Akad Nauk SSSR Ser. Khim. 1964, 2, 326–331. [Google Scholar]
- Bel’skii, I.F.; Shuikin, N.I.; Skobtsova, G.E. Catalytic synthesis of pyrrole and pyrrolidine homologs. Probl. Organ. Sinteza Akad. Nauk SSSR Otd. Obshch. Tekhn. Khim. 1965, 186–189. [Google Scholar]
- Shuikin, N.I.; Bel’skii, I.F.; Abgaforova, G.E. Conjugated hydrogenolysis in the synthesis of 2,5-dialkylpyrroles. Izvest. Akad. Nauk SSSR Ser. Khim. 1965, 1, 163–165. [Google Scholar]
- Bel’skii, I.F.; Khar’kov, S.N.; Shuikin, N.I. Synthesis of homologs of 1,4-dioxane and 1,4-dioxene. Dokl. Akad. Nauk SSSR 1965, 165, 821–823. [Google Scholar]
- Abgaforova, G.E.; Shuikin, N.I.; Bel’skii, I.F. Synthesis of trialkyl derivatives of pyrrole and pyrrolidine. Izvest. Akad. Nauk SSSR Ser. Khim. 1965, 4, 734–736. [Google Scholar] [CrossRef]
- Shuikin, N.I.; Lebedev, B.L. Alkylation of thiophene by isobutylene. Izvest. Akad. Nauk SSSR Ser. Khim. 1967, 5, 1154–1155. [Google Scholar] [CrossRef]
- Shuikin, N.I.; Lebedev, B.L.; Nikol’skii, V.G.; Korytina, O.A.; Kessenikh, A.V.; Prokof’ev, E.P. Direction of furan alkylation with isobutylene. Izvest. Akad. Nauk SSSR Ser. Khim. 1967, 7, 1618–1620. [Google Scholar]
- Fedorov, E.I.; Mikhant’ev, B.I. Crotylation of sodium 2-pyridinolate. Khim. Geterotsikl. Soedin. 1969, 6, 1022–1023. [Google Scholar]
- Anton, V.; Munoz-Embid, J.; Artigas, H.; Artal, M.; Lafuente, C. Thermophysical properties of oxygenated thiophene derivatives: Experimental data and modelling. J. Chem. Thermodyn. 2017, 113, 330–339. [Google Scholar] [CrossRef]
- Baird, Z.S.; Dahlberg, A.; Uusi-Kyyny, P.; Osmanbegovic, N.; Witos, J.; Helminen, J.; Cederkrantz, D.; Hyväri, P.; Alopaeus, V.; Kilpeläinen, I.; et al. Physical Properties of 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (mTBD). Int. J. Thermophys. 2019, 40, 71. [Google Scholar] [CrossRef]
- Gomez, E.; Gonzalez, B.; Dominguez, A.; Tojo, E.; Tojo, J. Dynamic Viscosities of a Series of 1-Alkyl-3-methylimidazolium Chloride Ionic Liquids and Their Binary Mixtures with Water at Several Temperatures. J. Chem. Eng. Data 2006, 51, 696–701. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Tojo, E.; Rodriguez, A.; Canosa, J.; Tojo, J. Properties of ionic liquid HMIMPF6 with carbonates, ketones and alkyl acetates. J. Chem. Thermodyn. 2006, 38, 651–661. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Santamarta, F.; Tojo, E.; Rodriguez, A.; Tojo, J. Temperature Dependence of Physical Properties of Ionic Liquid 1,3-Dimethylimidazolium Methyl Sulfate. J. Chem. Eng. Data 2006, 51, 952–954. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Verdia, P.; Tojo, E.; Rodriguez, A. Physical Properties of 1-Butyl-3-methylimidazolium Methyl Sulfate as a Function of Temperature. J. Chem. Eng. Data 2007, 52, 377–380. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Legido, J.L.; Rodriguez, A. Physical properties of ionic liquids based on 1-alkyl-3-methylimidazolium cation and hexafluorophosphate as anion and temperature dependence. J. Chem. Thermodyn. 2007, 39, 1168–1175. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.; Zhang, J.; Zhang, S.; Zhu, L.; Yang, J.; Zhang, X.; Deng, Y. Physicochemical Properties of Nitrile-Functionalized Ionic Liquids. J. Phys. Chem. B 2007, 111, 2864–2872. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Rodriguez, A. Thermodynamic Properties of Ionic Liquids in Organic Solvents from (293.15 to 303.15) K. J. Chem. Eng. Data 2007, 52, 600–608. [Google Scholar] [CrossRef]
- Mokhtarain, B.; Mojtahedi, M.M.; Mortaheb, H.R.; Mafi, M.; Yazdani, F.; Sadeghian, F. Densities, Refractive Indices, and Viscosities of the Ionic Liquids 1-Methyl-3-octylimidazolium Tetrafluoroborate and 1-Methyl-3-butylimidazolium Perchlorate and Their Binary Mixtures with Ethanol at Several Temperatures. J. Chem. Eng. Data 2008, 53, 677–682. [Google Scholar] [CrossRef]
- Ortega, J.; Vreekamp, R.; Penco, E.; Marrero, E. Mixing thermodynamic properties of 1-butyl-4-methylpyridinium tetrafluoroborate [b4mpy][BF4] with water and with an alkan-1ol (methanol to pentanol). J. Chem. Thermodyn. 2008, 40, 1087–1094. [Google Scholar] [CrossRef]
- Gonzalez, B.; Calvar, N.; Gomez, E.; Macedo, E.A.; Dominguez, A. Synthesis and Physical Properties of 1-Ethyl 3-methylpyridinium Ethylsulfate and Its Binary Mixtures with Ethanol and Water at Several Temperatures. J. Chem. Eng. Data 2008, 53, 1824–1828. [Google Scholar] [CrossRef]
- Muhammad, A.; Mutalib, M.I.A.; Wilfred, C.D.; Murugesn, T.; Shafeeq, A. Thermophysical properties of 1-hexyl-3-methyl imidazolium based ionic liquids with tetrafluoroborate, hexafluorophosphate and bis(trifluoromethylsulfonyl)imide anions. J. Chem. Thermodyn. 2008, 40, 1433–1438. [Google Scholar] [CrossRef]
- Malham, I.B.; Turmine, M. Viscosities and refractive indices of binary mixtures of 1-butyl-3-methylimidazolium tetrafluoroborate and 1-butyl-2,3-dimethylimidazolium tetrafluoroborate with water at 298 K. J. Chem. Thermodyn. 2008, 40, 718–723. [Google Scholar] [CrossRef]
- Tariq, M.; Forte, P.A.S.; Gomes, M.F.C.; Lopes, J.N.C.; Rebelo, L.P.N. Densities and refractive indices of imidazolium- and phosphonium-based ionic liquids: Effect of temperature, alkyl chain length, and anion. J. Chem. Thermodyn. 2009, 41, 790–798. [Google Scholar] [CrossRef]
- Torrecilla, J.S.; Palomar, J.; Garcia, J.; Rodriguez, F. Effect of Cationic and Anionic Chain Lengths on Volumetric, Transport, and Surface Properties of 1-Alkyl-3-methylimidazolium Alkylsulfate Ionic Liquids at (298.15 and 313.15) K. J. Chem. Eng. Data 2009, 54, 1297–1301. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Veiga, H.I.M.; Esperanca, J.M.S.S.; Rodriguez, A. Effect of temperature on the physical properties of two ionic liquids. J. Chem. Thermodyn. 2009, 41, 1419–1423. [Google Scholar] [CrossRef]
- Calvar, N.; Gomez, E.; Gonzalez, B.; Dominguez, A. Experimental Determination, Correlation, and Prediction of Physical Properties of the Ternary Mixtures Ethanol and 1-Propanol + Water + 1-Ethyl-3-methylpyridinium Ethylsulfate at 298.15 K. J. Chem. Eng. Data 2009, 54, 2229–2234. [Google Scholar] [CrossRef]
- Navas, A.; Ortega, J.; Vreekamp, R.; Marrero, E.; Palomar, J. Experimental Thermodynamic Properties of 1-Butyl-2-methylpyridinium Tetrafluoroborate [b2mpy][BF4] with Water and with Alkan-1-ol and Their Interpretation with the COSMO-RS Methodology. Ind. Eng. Chem. Res. 2009, 48, 2678–2690. [Google Scholar] [CrossRef]
- Soriano, A.N.; Doma, B.T., Jr.; Li, M.-H. Measurements of the density and refractive index for 1-n-butyl-3-methylimidazolium-based ionic liquids. J. Chem. Thermodyn. 2009, 41, 301–307. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, S.; Li, Z.; Li, J.; Chen, Z.; Wang, R.; Lu, L.; Deng, Y. Novel Cyclic Sulfonium-Based Ionic Liquids: Synthesis, Characterization, and Physicochemical Properties. Chem. Eur. J. 2009, 15, 765–778. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Rodriguez, A. Separation of Ethanol-Heptane Azeotropic Mixtures by Solvent Extraction with an Ionic Liquid. Ind. Eng. Chem. Res. 2009, 48, 1579–1585. [Google Scholar] [CrossRef]
- Gonzalez, R.; Calvar, N.; Gomez, E.; Dominguez, I.; Dominguez, A. Synthesis and Physical Properties of 1-Ethylpyridinium Ethylsulfate and its Binary Mixtures with Ethanol and 1-Propanol at Several Temperatures. J. Chem. Eng. Data 2009, 54, 1353–1358. [Google Scholar] [CrossRef]
- Singh, T.; Kumar, A. Temperature Dependence of Physical Properties of Imidazolium Based Ionic Liquids: Internal Pressure and Molar Refraction. J. Solut. Chem. 2009, 38, 1043–1053. [Google Scholar] [CrossRef]
- Bandres, I.; Giner, B.; Artigas, H.; Lafuente, C.; Royo, F.M. Thermophysical Properties of N-Octyl-3-methylpyridinium Tetrafluoroborate. J. Chem. Eng. Data 2009, 54, 236–240. [Google Scholar] [CrossRef]
- Bandres, I.; Pera, G.; Martin, S.; Castro, M.; Lafuente, C. Thermophysical Study of 1-Butyl-2-Methylpyridinium Tetrafluoroborate Ionic Liquid. J. Phys. Chem. B 2009, 113, 11936–11942. [Google Scholar] [CrossRef] [PubMed]
- Bandres, I.; Royo, F.; Gascon, I.; Castro, M.; Lafuente, C. Anion Influence on Thermophysical Properties of Ionic Liquids: 1-Butylpyridinium Tetrafluoroborate and 1-Butylpyridinium Triflate. J. Phys. Chem. B 2010, 114, 3601–3607. [Google Scholar] [CrossRef] [PubMed]
- Arce, A.; Francisco, M.; Soto, A. Evaluation of the polysubstituted pyridinium ionic liquid [hmmpy][Ntf2] as a suitable solvent for desulfurization: Phase equilibria. J. Chem. Thermodyn. 2010, 42, 712–718. [Google Scholar] [CrossRef]
- Shekaari, H.; Armanfar, E. Physical Properties of Aqueous Solutions of Ionic Liquid, 1-Propyl-3-methylimidazolium Methyl Sulfate, at T) (298.15 to 328.15) K. J. Chem. Eng. Data 2010, 55, 765–772. [Google Scholar] [CrossRef]
- Brigouleix, C.; Arnouti, M.; Jacquemin, J.; Caillon-Caravanier, M.; Galiano, H.; Lemordant, D. Physicochemical Characterization of Morpholinium Cation Based Protic Ionic Liquids Used As Electrolytes. J. Phys. Chem. B 2010, 114, 1757–1766. [Google Scholar] [CrossRef]
- Alvarez, V.H.; Mattedi, S.; Martin-Pastor, M.; Aznar, M.; Iglesias, M. Synthesis and thermophysical properties of two new protic long-chain ionic liquids with the oleate anion. Fluid Phase Equil. 2010, 299, 42–50. [Google Scholar] [CrossRef]
- Yunus, N.M.; Mutalib, M.I.A.; Man, Z.; Bustam, M.A.; Murugesan, T. Thermophysical properties of 1-alkylpyridinum bis(trifluoromethylsulfonyl)imide ionic liquids. J. Chem. Thermodyn. 2010, 42, 491–495. [Google Scholar] [CrossRef]
- Ziyada, A.K.; Wilfred, C.D.; Bustam, M.A.; Man, Z.; Murugesan, T. Thermophysical Properties of 1-Propyronitrile-3-alkylimidazolium Bromide Ionic Liquids at Temperatures from (293.15 to 353.15) K. J. Chem. Eng. Data 2010, 55, 3886–3890. [Google Scholar] [CrossRef]
- Yu, Z.; Gao, H.; Wang, H.; Chen, L. Densities, Excess Molar Volumes, and Refractive Properties of the Binary Mixtures of the Amino Acid Ionic Liquid [bmim][Gly] with 1-Butanol or Isopropanol at T = (298.15 to 313.15) K. J. Chem. Eng. Data 2011, 56, 4295–4300. [Google Scholar] [CrossRef]
- Kurnia, K.A.; Taib, M.M.; Mutalib, M.I.A.; Murugesan, T. Densities, refractive indices and excess molar volumes for binary mixtures of protic ionic liquids with methanol at T = 293.15 to 313.15 K. J. Mol. Liq. 2011, 159, 211–219. [Google Scholar] [CrossRef]
- Ziyada, A.K.; Bustam, M.A.; Wilfred, C.D.; Murugesan, T. Densities, Viscosities, and Refractive Indices of 1-Hexyl-3-propanenitrile Imidazolium Ionic Liquids Incorporated with Sulfonate-Based Anions. J. Chem. Eng. Data 2011, 56, 2343–2348. [Google Scholar] [CrossRef]
- Yu, Z.; Gao, H.; Wang, H.; Chen, L. Densities, Viscosities, and Refractive Properties of the Binary Mixtures of the Amino Acid Ionic Liquid [bmim][Ala] with Methanol or Benzylalcohol at T = (298.15 to 313.15) K. J. Chem. Eng. Data 2011, 56, 2877–2883. [Google Scholar] [CrossRef]
- Taib, M.M.; Murugesan, T. Density, Refractive Index, and Excess Properties of 1-Butyl-3-methylimidazolium Tetrafluoroborate with Water and Monoethanolamine. J. Chem. Eng. Data 2012, 57, 120–126. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, X.; Liu, S.; Yang, B.; Lu, L.; He, Y.; Deng, Y. Hydrophobic 1-allyl-3-alkylimidazolium dicyanamide ionic liquids with low densities. J. Mater. Chem. 2011, 21, 6864–6868. [Google Scholar] [CrossRef]
- de Hadlich Oliveira, L.; Aznar, M. Liquid_Liquid Equilibria for {1-Ethyl-3-methylimidazolium Diethylphosphate or 1-Ethyl-3-methylimidazolium Ethylsulfate} + 4,6-Dimethyldibenzothiophene + Dodecane Systems at 298.2 K and 313.2 K. J. Chem. Eng. Data 2011, 56, 2005–2012. [Google Scholar] [CrossRef]
- Tsunashima, K.; Kawabata, A.; Matsumiya, M.; Kodama, S.; Enomoto, R.; Sugiya, M.; Kunugi, Y. Low viscous and highly conductive phosphonium ionic liquids based on bis(fluorosulfonyl)amide anion as potential electrolytes. Electrochem. Comm. 2011, 13, 178–181. [Google Scholar] [CrossRef]
- Gonzalez, B.; Gomez, E.; Dominguez, A.; Vilas, M.; Tojo, E. Physicochemical Characterization of New Sulfate Ionic Liquids. J. Chem. Eng. Data 2011, 56, 14–20. [Google Scholar] [CrossRef]
- Vercher, E.; Llopis, F.J.; Gonzalez-Alfaro, V.; Miguel, P.J.; Martinez-Andreu, A. Refractive Indices and Deviations in Refractive Indices of Trifluoromethanesulfonate-Based Ionic Liquids in Water. J. Chem. Eng. Data 2011, 56, 4499–4504. [Google Scholar] [CrossRef]
- Wu, T.Y.; Su, S.G.; Gung, S.T.; Lin, M.W.; Ouyang, W.C.; Sun, I.W.; Lai, C.A. Synthesis and Characterization of Protic Ionic liquids Containing Cyclic Amine Cations and Tetrafluoroborate Anion. J. Iran. Chem. Soc. 2011, 8, 149–165. [Google Scholar] [CrossRef]
- Muhammad, N.; Man, Z.B.; Bustam, M.A.; Mutalib, M.I.A.; Wilfred, C.D.; Rafiq, S. Synthesis and Thermophysical Properties of Low Viscosity Amino Acid-Based Ionic Liquids. J. Chem. Eng. Data 2011, 56, 3157–3162. [Google Scholar] [CrossRef]
- Hossain, M.I.; Babaa, M.-R.; El-Harbawi, M.; Man, Z.; Hefter, G.; Yin, C.-Y. Synthesis, Characterization, Physical Properties, and Cytotoxicities of 1-(6-Hydroxyhexyl)-3-alkylimidazolium Chloride Ionic Liquids. J. Chem. Eng. Data 2011, 56, 4188–4193. [Google Scholar] [CrossRef]
- Freire, M.G.; Teles, A.R.R.; Rocha, M.A.A.; Schröder, B.; Neves, C.M.S.S.; Carvalho, P.J.; Evtuguin, D.V.; Santos, L.M.N.B.F.; Coutinho, J.A.P. Thermophysical Characterization of Ionic Liquids Able To Dissolve Biomass. J. Chem. Eng. Data 2011, 56, 4813–4822. [Google Scholar] [CrossRef]
- Larriba, M.; Garcia, S.; Garcia, J.; Torrecilla, J.S.; Rodriguez, F. Thermophysical Properties of 1-Ethyl-3-methylimidazolium 1,1,2,2-Tetrafluoroethanesulfonate and 1-Ethyl-3-methylimidazolium Ethylsulfate Ionic Liquids as a Function of Temperature. J. Chem. Eng. Data 2011, 56, 3589–3597. [Google Scholar] [CrossRef]
- Deive, F.J.; Rivas, M.A.; Rodriguez, A. Thermophysical properties of two ionic liquids based on benzyl imidazolium cation. J. Chem. Thermodyn. 2011, 43, 487–491. [Google Scholar] [CrossRef]
- Seki, S.; Tsuzuki, S.; Hayamizu, K.; Umebayashi, Y.; Serizawa, N.; Takei, K.; Miyashiro, H. Comprehensive Refractive Index Property for Room-Temperature Ionic Liquids. J. Chem. Eng. Data 2012, 57, 2211–2216. [Google Scholar] [CrossRef]
- Xu, W.-G.; Li, L.; Ma, X.-X.; Wei, J.; Duan, W.-B.; Guan, W.; Yang, J.-Z. Density, Surface Tension, and Refractive Index of Ionic Liquids Homologue of 1-Alkyl-3-methylimidazolium Tetrafluoroborate [Cnmim][BF4] (n = 2,3,4,5,6). J. Chem. Eng. Data 2012, 57, 2177–2184. [Google Scholar] [CrossRef]
- Gomez, E.; Calvar, N.; Macedo, E.A.; Dominguez, A. Effect of the temperature on the physical properties of pure 1-propyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide and characterization of its binary mixtures with alcohols. J. Chem. Thermodyn. 2012, 45, 9–15. [Google Scholar] [CrossRef]
- Gonzalez, E.J.; Dominguez, A.; Macedo, E.A. Physical and Excess Properties of Eight Binary Mixtures Containing Water and Ionic Liquids. J. Chem. Eng. Data 2012, 57, 2165–2176. [Google Scholar] [CrossRef]
- Larriba, M.; Garcia, S.; Navarro, P.; Garcia, J.; Rodriguez, F. Physical Properties of N-Butylpyridinium Tetrafluoroborate and N-Butylpyridinium Bis(trifluoromethylsulfonyl)imide Binary Ionic Liquid Mixtures. J. Chem. Eng. Data 2012, 57, 1318–1325. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Bharmoria, P.; Singh, T.; Kumar, A. Temperature Dependence of Physical Properties of Amino Acid Ionic Liquid Surfactants. J. Chem. Eng. Data 2012, 57, 317–323. [Google Scholar] [CrossRef]
- Bandres, I.; Lopez, M.C.; Castro, M.; Barbera, J.; Lafuente, C. Thermophysical properties of 1-propylpyridinium tetrafluoroborate. J. Chem. Thermodyn. 2012, 44, 148–153. [Google Scholar] [CrossRef]
- Machanova, K.; Boisset, A.; Sedlakova, Z.; Anouti, M.; Bendova, M.; Jacquemin, J. Thermophysical Properties of Ammonium-Based Bis{(trifluoromethyl)sulfonyl}imide Ionic Liquids: Volumetric and Transport Properties. J. Chem. Eng. Data 2012, 57, 2227–2235. [Google Scholar] [CrossRef]
- Muhammad, N.; Man, Z.; Ziyada, A.K.; Bustam, M.A.; Mutalib, M.I.A.; Wilfred, C.D.; Rafiq, S.; Tan, I.M. Thermophysical Properties of Dual Functionalized Imidazolium-Based Ionic Liquids. J. Chem. Eng. Data 2012, 57, 737–743. [Google Scholar] [CrossRef]
- Almeida, H.F.D.; Passos, H.; Lopes-da-Silva, J.A.; Fernandes, A.M.; Freire, M.G.; Coutinho, J.A.P. Thermophysical Properties of Five Acetate-Based Ionic Liquids. J. Chem. Eng. Data 2012, 57, 3005–3013. [Google Scholar] [CrossRef]
- Reddy, P.; Siddiqi, M.A.; Atakan, B.; Diedenhofen, M.; Ramjugernath, D. Activity coefficients at infinite dilution of organic solutes in the ionic liquid PEG-5 cocomonium methylsulfate at T = (313.15, 323.15, 333.15, and 343.15) K: Experimental results and COSMO-RS predictions. J. Chem. Thermodyn. 2013, 58, 322–329. [Google Scholar] [CrossRef]
- Ma, X.-X.; Wei, J.; Zhang, Q.-B.; Tian, F.; Feng, Y.-Y.; Guan, W. Prediction of Thermophysical Properties of Acetate-Based Ionic Liquids Using Semiempirical Methods. Ind. Eng. Chem. Res. 2013, 52, 9490–9496. [Google Scholar] [CrossRef]
- Neves, C.M.S.S.; Kurnia, K.A.; Coutinho, J.A.P.; Marrucho, I.M.; Lopes, J.N.C.; Freire, M.G.; Rebelo, L.P.N. Systematic Study of the Thermophysical Properties of Imidazolium-Based Ionic Liquids with Cyano-Functionalized Anions. J. Phys. Chem. B 2013, 117, 10271–10283. [Google Scholar] [CrossRef]
- Rocha, M.A.A.; Ribeiro, F.M.S.; Loobo Ferreira, A.I.M.C.; Coutinho, J.A.P.; Santos, L.M.N.B.F. Thermophysical properties of [CN − 1C1im][PF6] ionic liquids. J. Mol. Liq. 2013, 188, 196–202. [Google Scholar] [CrossRef]
- Koller, T.M.; Rausch, M.H.; Ramos, J.; Schulz, P.S.; Wasserscheid, P.; Economou, I.G.; Fröba, A.P. Thermophysical Properties of the Ionic Liquids [EMIM][B(CN)4] and [HMIM][B(CN)4]. J. Phys. Chem. B 2013, 117, 8512–8523. [Google Scholar] [CrossRef]
- Koller, T.M.; Schmid, S.R.; Sachnov, S.J.; Rausch, M.H.; Wasserscheid, P.; Fröba, A.P. Measurement and Prediction of the Thermal Conductivity of Tricyanomethanide- and Tetracyanoborate-Based Imidazolium Ionic Liquids. Int. J. Thermophys. 2014, 35, 195–217. [Google Scholar] [CrossRef]
- Oezdemir, M.C.; Oezgün, B. Phenyl/alkyl-substituted-3,5-dimethylpyrazolium ionic liquids. J. Mol. Liq. 2014, 200, 129–135. [Google Scholar] [CrossRef]
- Seki, S.; Tsuzuki, S.; Hayamizu, K.; Serizawa, N.; Ono, S.; Takei, K.; Doi, H.; Umebayashi, Y. Static and Transport Properties of Alkyltrimethylammonium Cation-Based Room-Temperature Ionic Liquids. J. Phys. Chem. B 2014, 118, 4590–4599. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Ma, T.; Ma, X.; Guan, W.; Liu, Q.; Yang, J. Study on thermodynamic properties and estimation of polarity of ionic liquids {[Cnmmim]-[NTf2] (n = 2, 4)}. RSC Adv. 2014, 4, 30725–30732. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, J.; Li, Z.; Wei, H. Synthesis and Physicochemical Properties of Two SO3H-Functionalized Ionic Liquids with Hydrogen Sulfate Anion. J. Chem. Eng. Data 2014, 59, 2186–2195. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Carvalho, P.J.; Coutinho, J.A.P. The effect of the cation aromaticity upon the thermophysicalproperties of piperidinium- and pyridinium-based ionic liquids. Fluid Phase Equil. 2014, 375, 80–88. [Google Scholar] [CrossRef]
- Umapathi, R.; Attri, P.; Venkatesu, P. Thermophysical Properties of Aqueous Solution of Ammonium-Based Ionic Liquids. J. Phys. Chem. B 2014, 118, 5971–5982. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Luis, A.; Santos, J.H.; Lopes-da-Silva, J.A.; Freire, M.G.; Carvalho, P.J.; Coutinho, J.A.P. Thermophysical properties of sulfonium- and ammonium-based ionic liquids. Fluid Phase Equil. 2014, 381, 36–45. [Google Scholar] [CrossRef]
- Pinto, R.R.; Santos, D.; Mattedi, S.; Aznar, M. Density, refractive index, apparent volumes and excess molar Volumes of four protic ionic liquids + water at T = 298.15 and 323.15 K. Braz. J. Chem. Eng. 2015, 32, 671–682. [Google Scholar] [CrossRef]
- Rodriguez, A.S.M.C.; Rocha, M.A.A.; Almeida, H.F.D.; Neves, C.M.S.S.; Lopes-da-Silva, J.A.; Freire, M.G.; Coutinho, J.A.P.; Santos, L.M.N.B.F. Effect of the Methylation and N−H Acidic Group on the Physicochemical Properties of Imidazolium-Based Ionic Liquids. J. Phys. Chem. B 2015, 119, 8781–8792. [Google Scholar] [CrossRef]
- Gonfa, G.; Bustam, M.A.; Muhammad, N.; Khan, A.S. Evaluation of Thermophysical Properties of Functionalized Imidazolium Thiocyanate Based Ionic Liquids. Ind. Eng. Chem. Res. 2015, 54, 12428–12437. [Google Scholar] [CrossRef]
- Rao, S.G.; Mohan, T.M.; Krishna, T.V.; Raju, K.T.S.S.; Rao, B.S. Excess thermodynamic properties of ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate and N-octyl-2-pyrrolidone from T = (298.15 to 323.15) K at atmospheric pressure. J. Chem. Thermodyn. 2015, 89, 286–295. [Google Scholar]
- Sattari, M.; Kamari, A.; Mohammadi, A.H.; Ramjugernath, D. Prediction of refractive indices of ionic liquids—A quantitative structure-property relationship based model. J. Taiwan Inst. Chem. Eng. 2015, 52, 165–180. [Google Scholar] [CrossRef]
- Soucknova, M.; Klomfar, J.; Patek, J. Surface tension and 0.1 MPa density data for 1-Cn-3-methylimidazolium iodides with n = 3, 4, and 6, validated using a parachor and group contribution model. J. Chem. Thermodyn. 2015, 83, 52–60. [Google Scholar] [CrossRef]
- Vatascin, E.; Dohnal, V. Thermophysical properties of aqueous solutions of the 1-ethyl-3-methylimidazolium tricyanomethanide ionic liquid. J. Chem. Thermodyn. 2015, 89, 169–176. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Lopes-da-Silva, J.A.; Freire, M.G.; Coutinho, J.A.P.; Carvalho, P.J. Thermophysical properties of phosphonium-based ionic liquids. Fluid Phase Equil. 2015, 400, 103–113. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Coutinho, J.A.P.; Freire, M.G.; Carvalho, P.J. Thermophysical Properties of Two Ammonium-Based Protic Ionic Liquids. J. Solut. Chem. 2015, 44, 703–717. [Google Scholar] [CrossRef]
- Akbar, M.M.; Chemat, F.; Arunagiri, A.; Murugesan, T. Density and excess properties of N-methyldiethanolamine (MDEA) with 1-hexyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate [hmim][FAP]. J. Therm. Anal. Calorim. 2016, 123, 785–791. [Google Scholar] [CrossRef]
- Gouveia, A.S.L.; Tome, L.C.; Marrucho, I.M. Density, Viscosity, and Refractive Index of Ionic Liquid Mixtures Containing Cyano and Amino Acid-Based Anions. J. Chem. Eng. Data 2016, 61, 83–93. [Google Scholar] [CrossRef]
- Xue, L.; Gurung, E.; Tamas, G.; Koh, Y.P.; Shadeck, M.; Simon, S.l.; Maroncelli, M.; Quitevis, E.L. Effect of Alkyl Chain Branching on Physicochemical Properties of Imidazolium-Based Ionic Liquids. J. Chem. Eng. Data 2016, 61, 1078–1091. [Google Scholar] [CrossRef]
- Kumar, A.V.; Redhi, G.G.; Gengan, R.M. Influence of Epoxy Group in 2-Pyrrolidonium Ionic Liquid Interactions and Thermo-Physical Properties with Ethanoic or Propanoic Acid at Various Temperatures. ACS Sustain. Chem. Eng. 2016, 4, 4951–4964. [Google Scholar] [CrossRef]
- Chen, F.-F.; Dong, Y.; Sang, X.-Y.; Zhou, Y.; Tao, D.-J. Physicochemical Properties and CO2 Solubility of Tetrabutylphosphonium Carboxylate Ionic Liquids. Acta Phys. Chim. Sin. 2016, 32, 605–610. [Google Scholar] [CrossRef]
- Ye, F.; Zhu, J.; Yu, K.; Zhu, R.; Xu, Y.; Chen, J.; Chen, L. Physicochemical properties of binary mixtures of 1,1,3,3-tetramethylguanidine imidazolide ionic liquid with water and alcohols. J. Chem. Thermodyn. 2016, 97, 39–47. [Google Scholar] [CrossRef]
- Segade, L.; Cabanas, M.; Dominguez-Perez, M.; Rilo, E.; Garcia-Garabal, S.; Turmine, M.; Varela, L.M.; Gomez-Gonzalez, V.; Docampo-Alvarez, B.; Cabeza, O. Surface and bulk characterisation of mixtures containing alkylammonium nitrates and water or ethanol: Experimental and simulated properties at 298.15 K. J. Mol. Liq. 2016, 222, 663–670. [Google Scholar] [CrossRef]
- Rodrigues, A.S.M.C.; Almeida, H.F.D.; Freire, M.G.; Lopes-da-Silva, J.A.; Coutinho, J.A.P.; Santos, L.M.N.B.F. The effect of n vs. iso isomerization on the thermophysical properties of aromatic and non-aromatic ionic liquids. Fluid Phase Equil. 2016, 423, 190–202. [Google Scholar] [CrossRef]
- Xing, N.; Dai, B.; Ma, X.; Wei, J.; Pan, Y.; Guan, W. The molar surface Gibbs energy and prediction of surface tension of [Cnpy][DCA] (n = 3,4,5). J. Chem. Thermodyn. 2016, 95, 21–25. [Google Scholar] [CrossRef]
- Shekaari, H.; Zafarani-Moattar, M.T.; Niknam, M. Thermodynamic evaluation of imidazolium based ionic liquids with thiocyanate anion as effective solvent to thiophene extraction. J. Mol. Liq. 2016, 219, 975–984. [Google Scholar] [CrossRef]
- Garcia-Andreu, M.; Castro, M.; Gascon, I.; Lafuente, C. Thermophysical characterization of 1-ethylpyridinium triflate and comparison with similar ionic liquids. J. Chem. Thermodyn. 2016, 103, 395–402. [Google Scholar] [CrossRef]
- Xu, Y. CO2 absorption behavior of azole-based protic ionic liquids: Influence of the alkalinity and physicochemical properties. J. CO2 Utiliz. 2017, 19, 1–8. [Google Scholar] [CrossRef]
- Nazet, A.; Sokolov, S.; Sonnleitner, T.; Friesen, S.; Buchner, R. Densities, Refractive Indices, Viscosities, and Conductivities of Non-Imidazolium Ionic Liquids [Et3S][TFSI], [Et2MeS][TFSI], [BuPy][TFSI], [N8881][TFA], and [P14][DCA]. J. Chem. Eng. Data 2017, 62, 2549–2561. [Google Scholar] [CrossRef]
- Lee, K.-H.; Park, S.-J.; Choi, Y.-Y. Density, refractive index and kinematic viscosity of MIPK, MEK and phosphonium-based ionic liquids and the excess and deviation properties of their binary systems. Korean J. Chem. Eng. 2017, 34, 214–224. [Google Scholar] [CrossRef]
- Sardar, S.; Wilfred, C.D.; Mumtaz, A.; Leveque, J.-M. Investigation of the Thermophysical Properties of AMPS-Based Aprotic Ionic Liquids for Potential Application in CO2 Sorption Processes. J. Chem. Eng. Data 2017, 62, 4160–4168. [Google Scholar] [CrossRef]
- Zhang, D.; Gong, Y.-Y.; Tong, J.; Fang, D.-W.; Tong, J. Physicochemical properties of [C n mim][Thr](n = 3,5,6) amino acid ionic liquids. J. Chem. Thermodyn. 2017, 115, 47–51. [Google Scholar] [CrossRef]
- Arosa, Y.; Fernandez, C.D.R.; Lago, E.L.; Amigo, A.; Varela, L.M.; Cabeza, O.; de la Fuente, R. Refractive index measurement of imidazolium based ionic liquids in the Vis-NIR. Opt. Mat. 2017, 73, 647–657. [Google Scholar] [CrossRef]
- Arumugam, V.; Redhi, G.G.; Gengan, R.M. Synthesis, characterization and thermophysical properties of novel 2′,3′-N-epoxypropyl-N-methyl-2-oxopyrrolidinium acetate ionic liquid and their binary mixture with water or methanol. J. Mol. Liq. 2017, 242, 1215–1227. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Llovell, F.; Araujo, J.M.M.; Santos, A.S.S.; Rebelo, L.P.N.; Pineiro, M.M.; Vega, L.F. Thermophysical Characterization of Ionic Liquids Based on the Perfluorobutanesulfonate Anion: Experimental and Soft-SAFT Modeling Results. ChemPhysChem 2017, 18, 2012–2023. [Google Scholar] [CrossRef]
- Venkatraman, V.; Raj, J.J.; Evjen, S.; Lethesh, K.C.; Fiksdahl, A. In silico prediction and experimental verification of ionic liquid refractive indices. J. Mol. Liq. 2018, 264, 563–570. [Google Scholar] [CrossRef]
- Marcinkowski, L.; Szepiński, E.; Milewska, M.J.; Kloskowski, A. Density, sound velocity, viscosity, and refractive index of new morpholinium ionic liquids with amino acid-based anions: Effect of temperature, alkyl chain length, and anion. J. Mol. Liq. 2019, 284, 557–568. [Google Scholar] [CrossRef]
- Ferreira, M.L.; Araújo, J.M.M.; Vega, L.F.; Llovell, F.; Pereiro, A.B. Functionalization of fluorinated ionic liquids: A combined experimental-theoretical study. J. Mol. Liq. 2020, 302, 112489. [Google Scholar] [CrossRef]
- Wu, X.-L.; Sang, X.-Y.; Li, Z.-M.; Tao, D.-J. Study on physicochemical properties and basicity of carbanionfunctionalized ionic liquids. J. Mol. Liq. 2020, 312, 113405. [Google Scholar] [CrossRef]
- Song, Z.; Yan, Q.; Xia, M.; Qi, X.; Zhang, Z.; Wei, J.; Fang, D.; Ma, X. Physicochemical properties of N-alkylpyridine trifluoroacetate ionic liquids [Cn Py][TFA] (n = 2–6). J. Chem. Thermodyn. 2021, 155, 106366. [Google Scholar] [CrossRef]
- Fei, Y.; Chen, Z.; Zhang, J.; Yu, M.; Kong, J.; Wu, Z.; Cao, J.; Zhang, J. Thiazolium-based ionic liquids: Synthesis, characterization and physicochemical properties. J. Mol. Liq. 2021, 342, 117553. [Google Scholar] [CrossRef]
- Aljasmi, A.; Aljimaz, A.S.; Alkhaldi, K.H.A.E.; AlTuwaim, M.S. Dependency of Physicochemical Properties of Imidazolium Bis(Trifluoromethylsulfonyl)Imide-Based Ionic Liquids on Temperature and Alkyl Chain. J. Chem. Eng. Data 2022, 67, 858–868. [Google Scholar] [CrossRef]
- Kong, J.; Liu, L.; Li, X.; Yang, Y.; Chen, X.; Fei, Y.; Xu, L.; Chen, Z. Dimethylthioformamide-derived ionic liquids: Synthesis, characterization and application as supercapacitor electrolyte. J. Mol. Liq. 2022, 365, 120114. [Google Scholar] [CrossRef]
- Vogel, A.I. 369. Physical properties and chemical constitution. Part XXIII. Miscellaneous compounds. Investigation of the so-called co-ordinate or dative link in esters of oxy-acids and in nitro-paraffins by molecular refractivity determinations. atomic, structural, and group parachors and refractivities. J. Chem. Soc. 1948, 1833–1855. [Google Scholar] [CrossRef]
- Vogel, A.I.; Cresswell, W.T.; Jeffrey, G.H.; Leicester, J. 98. Physical properties and chemical constitution. Part XXIV. Aliphatic aldoximes, ketoximes, and ketoxime O-alkyl ethers, NN-dialkylhydrazines, aliphatic ketazines, mono- and di-alkylaminopropionitriles, alkoxypropionitriles, dialkyl azodiformates, and dialkyl carbonates. Bond parachors, bond refractions, and bond refraction coefficients. J. Chem. Soc. 1952, 514–549. [Google Scholar] [CrossRef]
- Shuikin, N.I.; Bel’skii, I.F. Catalytic hydrogenolysis in the series of furan compounds. Zh. Obsh. Khim. 1957, 27, 402–406. [Google Scholar]
- Yur’ev, Y.K.; Novitskii, K.Y.; Bolesov, I.G. Furan series. I. Synthesis of N-(β-hydroxyalkyl)furfurylamines. Zh. Obsh. Khim. 1959, 29, 2951–2954. [Google Scholar]
- Petrov, A.A.; Mingaleva, K.S.; Zavgorodnii, V.S. Chemistry of unsaturated stannyl hydrocarbons. IV. Dipole moments of alkyl-, alkenyl-, and phenylacetylenic stannyl hydrocarbons. Zh. Obsh. Khim. 1964, 34, 533–535. [Google Scholar]
- Bel’skii, I.F.; Shuikin, N.I.; Vol’nova, Z. K Synthesis and isomerization of 2,2-dialkyl-5-propyltetrahydrofurans. Izvest Akad. Nauk SSSR Ser Khim. 1964, 2, 369–371. [Google Scholar] [CrossRef]
- Shuikin, N.I.; Lebedev, B.L.; Nikol’skii, V.G. Vinylation of cyclanes and cyclic ethers. Dokl. Akad. Nauk SSSR 1964, 158, 692–693. [Google Scholar]
- Arrowsmith, G.B.; Jeffery, G.H. Vogel, Physical Properties and Chemical Constitution. Part XLI. Naphthalene Compounds. J. Chem. Soc. 1965, 2072–2078. [Google Scholar] [CrossRef]
- Bel’skii, I.F.; Khar’kov, S.N.; Shuikin, N.I. Synthesis of 3-oxa-5-oxo alcohols and a study of their tautomeric transformations to form dioxenes. Dokl. Akad. Nauk SSSR 1965, 165, 1071–1074. [Google Scholar]
- Wiswanadhan, V.N.; Ghose, A.K.; Revankar, G.R.; Robins, R.K. Atomic Physicochemical Parameters for Three Dimensional Structure Directed Quantitative Structure-Activity Relationships. 4. Additional Parameters for Hydrophobic and Dispersive Interactions and Their Application for an Automated Superposition of Certain Naturally Occurring Nucleoside Antibiotics. J. Chem. Inf. Comput. Sci. 1989, 29, 163–172. [Google Scholar]
- Pacak, P. Refractivity and density of some organic solvents. Chem. Pap. 1991, 45, 227–232. [Google Scholar]
- Oswal, S.; Patel, A.T. Speeds of Sound, Isentropic Compressibilities, and Excess Volumes of Binary Mixtures. 1. Tri-n-alkylamines with Cyclohexane and Benzene. J. Chem. Eng. Data 1994, 39, 366–371. [Google Scholar] [CrossRef]
- Gao, D.; Zhu, D.; Zhang, L.; Guan, H.; Sun, H.; Chen, H.; Si, J. Isobaric Vapor-Liquid Equilibria for Binary and Ternary Mixtures of Propanal, Propanol, and Propanoic Acid. J. Chem. Eng. Data 2010, 55, 5887–5895. [Google Scholar] [CrossRef]
- Chen, K.-D.; Lin, Y.-F.; Tu, C.-H. Densities, Viscosities, Refractive Indexes, and Surface Tensions for Mixtures of Ethanol, Benzyl Acetate, and Benzyl Alcohol. J. Chem. Eng. Data 2012, 57, 1118–1127. [Google Scholar] [CrossRef]
- Kumar, S.; Jeevanandham, P. Densities, viscosities, refractive indices and excess properties of aniline and o-anisidine with 2-alkoxyethanols at 303.15 K. J. Mol. Liq. 2012, 174, 34–41. [Google Scholar] [CrossRef]
- Jeevanandham, P.; Kumar, S.; Periyasamy, P. Densities, viscosities, refractive indices and excess properties of ortho- and meta-chloroaniline with 2-alkoxyethanols at 303.15 K. J. Mol. Liq. 2013, 188, 203–209. [Google Scholar] [CrossRef]
- Cai, C.; Marsh, A.; Zhang, Y.-H.; Reid, J.P. Group Contribution Approach To Predict the Refractive Index of Pure Organic Components in Ambient Organic Aerosol. Environ. Sci. Technol. 2017, 51, 9683–9690. [Google Scholar] [CrossRef] [PubMed]
- Dantas, C.E.S.; Ceriani, R. Liquid−Liquid Equilibria of Ternary Mixtures Containing n-Tetradecane + γ-Valerolactone + Aldehyde [Butanal or Pentanal or (E)-2-Undecenal] at 298.15 K. J. Chem. Eng. Data 2022, 67, 393–403. [Google Scholar] [CrossRef]
- Sema, T.; Gao, H.; Liang, Z.; Tontiwachwuthikul, P.; Idem, R.O. New Insights and Updated Correlations for Density, Viscosity, Refractive Index, and Associated Properties of Aqueous 4-Diethyl-Amino-2-Butanol Solution. Fluid Phase Equil. 2022, 562, 113565. [Google Scholar] [CrossRef]
- Applequist, J.; Carl, J.R.; Fung, K.-K. An Atom Dipole Interaction Model for Molecular Polarizability. Application to Polyatomic Molecules and Determination of Atom Polarizabilities. J. Am. Chem. Soc. 1972, 94, 2952–2960. [Google Scholar] [CrossRef]
- Thole, B.T. Molecular polarizabilities calculated with a modified dipole interaction. Chem. Phys. 1981, 59, 341–350. [Google Scholar] [CrossRef]
- Nagle, J.K. Atomic Polarizability and Electronegativity. J. Am. Chem. Soc. 1990, 112, 4741–4747. [Google Scholar] [CrossRef]
- Miller, K.J. Calculation of the Molecular Polarizability Tensor. J. Am. Chem. Soc. 1990, 112, 8543–8551. [Google Scholar] [CrossRef]
- Cheng, L.-T.; Tam, W.; Stevenson, S.H.; Meredith, G.R.; Rikken, G.; Marder, S.R. Experimental Investigations of Organic Molecular Nonlinear Optical Polarizabilities. 1. Methods and Results on Benzene and Stilbene Derivatives. J. Phys. Chem. 1991, 95, 10631–10643. [Google Scholar] [CrossRef]
- Lu, Y.-J.; Lee, S.-L. Semi-empirical calculations of the nonlinear optical properties of polycyclic aromatic compounds. Chem. Phys. 1994, 179, 431–444. [Google Scholar] [CrossRef]
- Schürer, G.; Gedeck, P.; Gottschalk, M.; Clark, T. Accurate Parametrized Variational Calculations of the Molecular Electronic Polarizability by NDDO-Based Methods. Intern. J. Quantum Chem. 1999, 75, 17–31. [Google Scholar] [CrossRef]
- Bosque, R.; Sales, J. Polarizabilities of Solvents from the Chemical Composition. J. Chem. Inf. Comput. Sci. 2002, 42, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, X.-Q.; Hou, T.; Xu, X. Fast Approaches for Molecular Polarizability Calculations. Phys. Chem. A 2007, 111, 4443–4448. [Google Scholar] [CrossRef] [PubMed]
- Murthy, A.R.; Giridhar, G.; Rangacharyulu, M. Molecular Polarizabilities of Some Liquid Crystal Compounds. Chin. J. Phys. 2008, 46, 54–62. [Google Scholar]
- Cao, X.; Hancock, B.C.; Leyva, N.; Becker, J.; Yu, W.; Masterson, V.M. Estimating the refractive index of pharmaceutical solids using predictive methods. Int. J. Pharm. 2009, 368, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Naef, R.; Acree, W.E., Jr. Revision and Extension of a Generally Applicable Group-Additivity Method for the Calculation of the Standard Heat of Combustion and Formation of Organic Molecules. Molecules 2021, 26, 6101. [Google Scholar] [CrossRef] [PubMed]
- Naef, R.; Acree, W.E., Jr. Calculation of Five Thermodynamic Molecular Descriptors by Means of a General Computer Algorithm Based on the Group-Additivity Method: Standard Enthalpies of Vaporization, Sublimation and Solvation, and Entropy of Fusion of Ordinary Organic Molecules and Total Phase-Change Entropy of Liquid Crystals. Molecules 2017, 22, 1059. [Google Scholar] [CrossRef]
- Naef, R.; Acree, W.E., Jr. Calculation of the Surface Tension of Ordinary Organic and Ionic Liquids by Means of a Generally Applicable Computer Algorithm Based on the Group-Additivity Method. Molecules 2018, 23, 1224. [Google Scholar] [CrossRef]
- Naef, R. Calculation of the Isobaric Heat Capacities of the Liquid and Solid Phase of Organic Compounds at 298.15K by Means of the Group-Additivity Method. Molecules 2020, 25, 1147. [Google Scholar] [CrossRef]
- Naef, R.; Acree, W.E., Jr. Calculation of the Vapour Pressure of Organic Molecules by Means of a Group-Additivity Method and Their Resultant Gibbs Free Energy and Entropy of Vaporization at 298.15 K. Molecules 2021, 26, 1045. [Google Scholar] [CrossRef]
- Gough, K.M. Theoretical analysis of molecular polarizabilities and polarizability derivatives in hydrocarbons. J. Chem. Phys. 1989, 91, 2424. [Google Scholar] [CrossRef]
- Laidig, K.E.; Bader, R.F.W. Properties of atoms in molecules: Atomic polarizabilities. J. Chem. Phys. 1990, 93, 7213. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).