Estimating Equivalent Alkane Carbon Number Using Abraham Solute Parameters
Abstract
1. Introduction
2. Materials and Methods
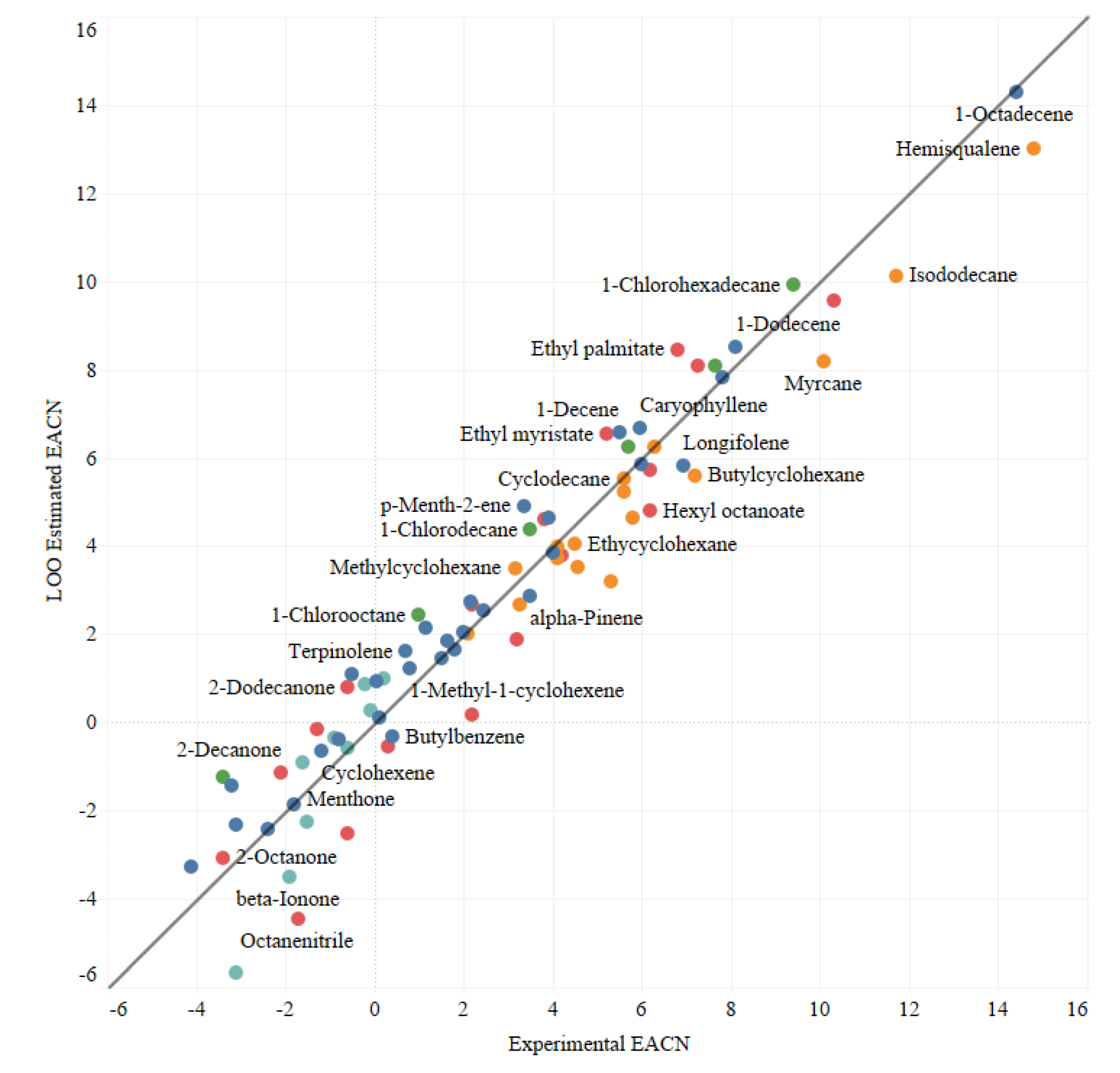
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Salager, J.L.; Morgan, J.C.; Schechter, R.S.; Wade, W.H.; Spe-Aime, M.; Vasquez, E. Optimum formulation of surfactant/water/oil systems for minimum interfacial tension or phase behavior. Soc. Pet. Eng. AIME J. 1979, 19, 107–115. [Google Scholar] [CrossRef]
- Ghayour, A.; Acosta, E. Characterizing the oil-like and surfactant-like behavior of polar oils. Langmuir 2019, 35, 15038–15050. [Google Scholar] [CrossRef] [PubMed]
- Virin Kittithammavong, V.; Charoensaeng, A.; Khaodhiar, S. A Normalized HLD (HLDN) tool for optimal salt-concentration prediction of microemulsions. Appl. Sci. 2021, 11, 9151. [Google Scholar] [CrossRef]
- Aubry, J.-M.; Ontiveros, J.F.; Salager, J.L.; Nardello-Rataj, V. Use of the normalized hydrophilic-lipophilic-deviation (HLDN) equation for determining the equivalent alkane carbon number (EACN) of oils and the preferred alkane carbon number (PACN) of nonionic surfactants by the fish-tail method (FTM). Adv. Colloid Interface Sci. 2020, 276, 102099. [Google Scholar] [CrossRef]
- Bouton, F.; Durand, M.; Nardello-Rataj, V.; Borosy, A.P.; Quellet, C.; Aubry, J.M. A QSPR model for the prediction of the “fish-tail” temperature of C i E4/water/polar hydrocarbon oil systems. Langmuir 2010, 26, 7962–7970. [Google Scholar] [CrossRef]
- Lukowicz, T.; Illous, E.; Nardello-Rataj, V.; Aubry, J.M. Prediction of the equivalent alkane carbon number (EACN) of aprotic polar oils with COSMO-RS sigma-moments. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 53–59. [Google Scholar] [CrossRef]
- Lukowicz, T.; Benazzouz, A.; Nardello-Rataj, V.; Aubry, J.-M. Rationalization and Prediction of the Equivalent Alkane Carbon Number (EACN) of Polar Hydrocarbon Oils with COSMO-RS σ-Moments. Langmuir 2015, 31, 11220–11226. [Google Scholar] [CrossRef]
- Delforce, L.; Duprat, F.; Ploix, J.-L.; Ontiveros, J.F.; Goussard, V.; Nardello-Rataj, V.; Aubry, J.-M. Fast Prediction of the Equivalent Alkane Carbon Number Using Graph Machines and Neural Networks. ACS Omega 2022, in press.
- Ulrich, N.; Endo, S.; Brown, T.N.; Watanabe, N.; Bronner, G.; Abraham, M.H.; Goss, K.-U. UFZ-LSER Database v 3.2.1 [Internet], Leipzig, Germany, Helmholtz Centre for Environmental Research-UFZ. 2017. Available online: http://www.ufz.de/lserd (accessed on 31 December 2021).
- Chung, Y.; Vermeire, F.H.; Wu, H.; Walker, P.J.; Abraham, M.H.; Green, W.H. Group Contribution and Machine Learning Approaches to Predict Abraham Solute Parameters, Solvation Free Energy, and Solvation Enthalpy. J. Chem. Inf. Modeling 2022, 62, 433–446. [Google Scholar] [CrossRef]
- Acree, W.E.; Chong, W.; Lang, A.S.I.D.; Mozafari, H. Dataset: EACN and Abraham Parameter Values. Figshare. Dataset 2022. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2022. Available online: https://www.R-project.org/ (accessed on 31 December 2021).
- Bouton, F. Influence of Terpenes and Terpenoids on the Phase Behavior of Micro-and Macro-Emulsions. Ph.D. Thesis, University of Lille, Lille, France, 2010. [Google Scholar]
- Kahlweit, M.; Strey, R.; Busse, G. Effect of alcohols on the phase behavior of microemulsions. J. Phys. Chem. 1991, 95, 5344–5352. [Google Scholar] [CrossRef]
- Burauer, S.; Sottmann, T.; Strey, R. Nonionic microemulsions with cyclic oils-Oil penetration, efficiency and monomeric solubility. Tenside Surfactants Deterg. 2000, 37, 8–16. [Google Scholar]
- Queste, S.; Salager, J.L.; Strey, R.; Aubry, J.M. The EACN scale for oil classification revisited thanks to fish diagrams. J. Colloid Interface Sci. 2007, 312, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Lukowicz, T. Synergistic Solubilisation of Fragrances in Binary Surfactant Systems and Prediction of Their EACN Value with COSMO-RS. Ph.D. Thesis, Université Lille, Lille, France, 2015. [Google Scholar]
- Tchakalova, V.; Testard, F.; Wong, K.; Parker, A.; Benczédi, D.; Zemb, T. Solubilization and interfacial curvature in microemulsions: I. Interfacial expansion and co-extraction of oil. Colloids Surf. A Physicochem. Eng. Asp. 2008, 331, 31–39. [Google Scholar] [CrossRef]
- Bouton, F.; Durand, M.; Nardello-Rataj, V.; Serry, M.; Aubry, J.M. Classification of terpene oils using the fish diagrams and the Equivalent Alkane Carbon (EACN) scale. Colloids Surf. A Physicochem. Eng. Asp. 2009, 338, 142–147. [Google Scholar] [CrossRef]
- Ontiveros, J.F.; Pierlot, C.; Catté, M.; Molinier, V.; Pizzino, A.; Salager, J.L.; Aubry, J.M. Classification of ester oils according to their Equivalent Alkane Carbon Number (EACN) and asymmetry of fish diagrams of C10E4/ester oil/water systems. J. Colloid Interface Sci. 2013, 403, 67–76. [Google Scholar] [CrossRef]
- Gradzielski, M.; Langevin, D.; Sottmann, T.; Strey, R. Droplet microemulsions at the emulsification boundary: The influence of the surfactant structure on the elastic constants of the amphiphilic film. J. Chem. Phys. 1997, 106, 8232–8238. [Google Scholar] [CrossRef]
- Tchakalova, V.; Testard, F.; Wong, K.; Parker, A.; Benczédi, D.; Zemb, T. Solubilization and interfacial curvature in microemulsions: II. Surfactant efficiency and PIT. Colloids Surf. A Physicochem. Eng. Asp. 2008, 331, 40–47. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Deen, G.R.; Pedersen, J.S. Phase behavior and microstructure of C12E5 nonionic microemulsions with chlorinated oils. Langmuir 2008, 24, 3111–3117. [Google Scholar] [CrossRef]
- Engelskirchen, S.; Elsner, N.; Sottmann, T.; Strey, R. Triacylglycerol microemulsions stabilized by alkyl ethoxylate surfactants—A basic study: Phase behavior, interfacial tension and microstructure. J. Colloid Interface Sci. 2007, 312, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Wormuth, K.R.; Kaler, E.W. Microemulsifying polar oils. J. Phys. Chem. 1989, 93, 4855–4861. [Google Scholar] [CrossRef]
- Tanford, C. The Hydrophobic Effect: Formation of Micelles and Biological Membranes, 2nd ed.; J. Wiley: Hoboken, NJ, USA, 1980. [Google Scholar]
- Kahlweit, M.; Busse, G.; Faulhaber, B.; Eibl, H. Preparing nontoxic microemulsions. Langmuir 1995, 11, 4185–4187. [Google Scholar] [CrossRef]
- van Os, N.M.; Haak, J.R.; Rupert, L.A.M. Physico-Chemical Properties of Selected Anionic, Cationic and Nonionic Surfactants; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Cayias, J.L.; Schechter, R.S.; Wade, W.H. Modeling crude oils for low interfacial tension. Soc. Pet. Eng. J. 1976, 16, 351–357. [Google Scholar] [CrossRef]
- Cash, L.; Cayias, J.L.; Fournier, G.; Macallister, D.; Schares, T.; Schechter, R.S.; Wade, W.H. The application of low interfacial tension scaling rules to binary hydrocarbon mixtures. J. Colloid Interface Sci. 1976, 59, 39–44. [Google Scholar] [CrossRef]
| Compound | EACN | E | S | A | B | V | EACN Ref. |
|---|---|---|---|---|---|---|---|
| Branched and cyclic alkanes | |||||||
| Cyclohexane | 2.4 | 0.305 | 0.10 | 0.00 | 0.00 | 0.8454 | [13] |
| Cyclohexane | 1.7 | 0.305 | 0.10 | 0.00 | 0.00 | 0.8454 | [13] |
| Cyclohexane | 1.8 | 0.305 | 0.10 | 0.00 | 0.00 | 0.8454 | [14] |
| Cyclohexane | 2.5 | 0.305 | 0.10 | 0.00 | 0.00 | 0.8454 | [15] |
| Methylcyclohexane | 3.5 | 0.244 | 0.06 | 0.00 | 0.00 | 0.9863 | [13] |
| Methylcyclohexane | 2.8 | 0.244 | 0.06 | 0.00 | 0.00 | 0.9863 | [13] |
| 1,4-Dimethylcyclohexane | 4.6 | 0.191 | 0.17 | 0.00 | 0.00 | 1.1272 | [13] |
| 1,4-Dimethylcyclohexane | 4.5 | 0.191 | 0.17 | 0.00 | 0.00 | 1.1272 | [13] |
| Ethylcyclohexane | 4.5 | 0.263 | 0.10 | 0.00 | 0.00 | 1.1272 | [13] |
| Ethylcyclohexane | 4.5 | 0.263 | 0.10 | 0.00 | 0.00 | 1.1272 | [13] |
| Ethylcyclohexane | 3.7 | 0.263 | 0.10 | 0.00 | 0.00 | 1.1272 | [16] |
| Cyclooctane | 4.1 | 0.409 | 0.10 | 0.00 | 0.00 | 1.1272 | [6] |
| 1,2-Dimethylcyclohexane | 3.9 | 0.320 | 0.23 | 0.00 | 0.00 | 1.1272 | [16] |
| 1,2-Dimethylcyclohexane | 2.6 | 0.320 | 0.23 | 0.00 | 0.00 | 1.1272 | [13] |
| Propylcyclohexane | 5.8 | 0.257 | 0.14 | 0.00 | 0.00 | 1.2681 | [13] |
| Propylcyclohexane | 6.3 | 0.257 | 0.14 | 0.00 | 0.00 | 1.2681 | [13] |
| Propylcyclohexane | 5.5 | 0.257 | 0.14 | 0.00 | 0.00 | 1.2681 | [16] |
| Isopropylcyclohexane | 5.6 | 0.283 | 0.07 | 0.00 | 0.00 | 1.2681 | [13] |
| Isopropylcyclohexane | 5.7 | 0.283 | 0.07 | 0.00 | 0.00 | 1.2681 | [13] |
| Isopropylcyclohexane | 4.5 | 0.283 | 0.07 | 0.00 | 0.00 | 1.2681 | [13] |
| Butylcyclohexane | 7.2 | 0.255 | 0.14 | 0.00 | 0.00 | 1.4090 | [13] |
| Butylcyclohexane | 7.9 | 0.255 | 0.14 | 0.00 | 0.00 | 1.4090 | [17] |
| Butylcyclohexane | 6.9 | 0.255 | 0.14 | 0.00 | 0.00 | 1.4090 | [16] |
| Cyclodecane | 5.6 | 0.474 | 0.10 | 0.00 | 0.00 | 1.4090 | [18] |
| cis-Decalin | 5.3 | 0.544 | 0.25 | 0.00 | 0.00 | 1.3004 | [18] |
| Myrcane | 10.0 | 0.000 | 0.00 | 0.00 | 0.00 | 1.5176 | [5,19] |
| Myrcane | 11.2 | 0.000 | 0.00 | 0.00 | 0.00 | 1.5176 | [6,20] |
| Myrcane | 10.1 | 0.000 | 0.00 | 0.00 | 0.00 | 1.5176 | [13] |
| Pinane | 4.3 | 0.421 | 0.12 | 0.00 | 0.13 | 1.3004 | [6,20] |
| Pinane | 3.9 | 0.421 | 0.12 | 0.00 | 0.13 | 1.3004 | [6,20] |
| p-Menthane | 6.3 | 0.270 | 0.07 | 0.00 | 0.00 | 1.4090 | [6,20] |
| p-Menthane | 6.7 | 0.270 | 0.07 | 0.00 | 0.00 | 1.4090 | [6,20] |
| p-Menthane | 4.6 | 0.270 | 0.07 | 0.00 | 0.00 | 1.4090 | [6,20] |
| Decylcyclohexane | 14.4 | 0.243 | 0.23 | 0.00 | 0.00 | 2.2544 | [21] |
| Dodecylcyclohexane | 17.5 | 0.300 | 0.23 | 0.00 | 0.00 | 2.5362 | [16] |
| Isododecane | 11.7 | 0.000 | 0.00 | 0.00 | 0.00 | 1.7994 | [7] |
| Hemisqualene | 14.8 | 0.000 | 0.00 | 0.00 | 0.00 | 2.2221 | [7] |
| Squalane | 24.5 | - | - | - | - | - | [21] |
| Squalene | 13.8 | - | - | - | - | - | [22] |
| Halogenated alkanes | |||||||
| 1-Bromo-2-methylpropane | −3.4 | 0.340 | 0.37 | 0.00 | 0.12 | 0.8472 | [18] |
| 1-Chlorooctane | 1.0 | 0.191 | 0.40 | 0.00 | 0.09 | 1.3582 | [23] |
| 1-Chlorodecane | 3.5 | 0.185 | 0.40 | 0.00 | 0.09 | 1.6400 | [18] |
| 1,10-Dichlorodecane | 6.3 | 0.366 | - | 0.00 | - | 1.7624 | [24] |
| 1-Chlorododecane | 5.6 | 0.181 | 0.40 | 0.00 | 0.10 | 1.9218 | [18] |
| 1-Chlorododecane | 5.8 | 0.181 | 0.40 | 0.00 | 0.10 | 1.9218 | [23] |
| 1-Chlorotetradecane | 8.0 | 0.176 | 0.41 | 0.00 | 0.10 | 2.2036 | [18] |
| 1-Chlorotetradecane | 7.3 | 0.176 | 0.41 | 0.00 | 0.10 | 2.2036 | [23] |
| 1-Chlorohexadecane | 9.8 | 0.173 | 0.42 | 0.00 | 0.10 | 2.4854 | [18] |
| 1-Chlorohexadecane | 9.0 | 0.173 | 0.42 | 0.00 | 0.10 | 2.4854 | [23] |
| Alkenes, terpenes, alkynes and aromatics | |||||||
| Cyclohexene | −1.2 | 0.395 | 0.28 | 0.00 | 0.09 | 0.8024 | [13] |
| 1,3-Cyclohexadiene | −3.1 | 0.515 | 0.38 | 0.00 | 0.12 | 0.7594 | [13] |
| 1,4-Cyclohexadiene | −4.1 | 0.501 | 0.46 | 0.00 | 0.16 | 0.7594 | [13] |
| 1-Methyl-1-cyclohexene | 0.8 | 0.391 | 0.18 | 0.00 | 0.10 | 0.9433 | [13] |
| 1-Methyl-1-cyclohexene | −0.8 | 0.391 | 0.18 | 0.00 | 0.10 | 0.9433 | [13] |
| 4-Methyl-1-cyclohexene | 0.6 | 0.347 | 0.22 | 0.00 | 0.10 | 0.9433 | [13] |
| 4-Methyl-1-cyclohexene | −0.5 | 0.347 | 0.22 | 0.00 | 0.10 | 0.9433 | [13] |
| 3-Methyl-1-cyclohexene | 0.4 | 0.360 | 0.20 | 0.00 | 0.10 | 0.9433 | [13] |
| 3-Methyl-1-cyclohexene | −1.4 | 0.360 | 0.20 | 0.00 | 0.10 | 0.9433 | [13] |
| 2,5-Norbornadiene | −3.2 | 0.495 | 0.32 | 0.00 | 0.11 | 0.7919 | [13] |
| 1-Octene | 3.9 | 0.094 | 0.08 | 0.00 | 0.07 | 1.1928 | [18] |
| cis-Cycloctene | 1.5 | 0.460 | 0.24 | 0.00 | 0.10 | 1.0842 | [18] |
| 1-Octyne | −1.8 | 0.155 | 0.22 | 0.09 | 0.10 | 1.1498 | [18] |
| p-Xylene | −2.4 | 0.613 | 0.52 | 0.00 | 0.16 | 0.9982 | [18] |
| 1-Decene | 5.5 | 0.093 | 0.08 | 0.00 | 0.07 | 1.4746 | [18] |
| 1-Decyne | 0.1 | 0.143 | 0.22 | 0.09 | 0.10 | 1.4316 | [18] |
| Butylbenzene | 0.4 | 0.600 | 0.51 | 0.00 | 0.15 | 1.2800 | [18] |
| Phenyl-1-butyne | −3.3 | - | - | - | - | - | [18] |
| alpha-Pinene | 3.6 | 0.438 | 0.20 | 0.00 | 0.14 | 1.2574 | [6,20] |
| alpha-Pinene | 3.4 | 0.438 | 0.20 | 0.00 | 0.14 | 1.2574 | [6,20] |
| p-Menth-2-ene | 3.1 | 0.350 | 0.12 | 0.00 | 0.07 | 1.3660 | [19] |
| p-Menth-2-ene | 3.6 | 0.350 | 0.12 | 0.00 | 0.07 | 1.3660 | [6,20] |
| Delta-3-carene | 2.9 | 0.492 | 0.22 | 0.00 | 0.14 | 1.2574 | [6,20] |
| Delta-3-carene | 2.0 | 0.492 | 0.22 | 0.00 | 0.14 | 1.2574 | [6,20] |
| beta-Pinene | 2.3 | 0.515 | 0.19 | 0.00 | 0.15 | 1.2574 | [6,20] |
| beta-Pinene | 2.0 | 0.515 | 0.19 | 0.00 | 0.15 | 1.2574 | [6,20] |
| Limonene | 2.0 | 0.501 | 0.31 | 0.00 | 0.23 | 1.3230 | [6,20] |
| Limonene | 1.6 | 0.501 | 0.31 | 0.00 | 0.23 | 1.3230 | [6,20] |
| gamma-Terpinene | 1.9 | 0.522 | 0.29 | 0.00 | 0.22 | 1.3230 | [6,20] |
| gamma-Terpinene | 1.4 | 0.522 | 0.29 | 0.00 | 0.22 | 1.3230 | [6,20] |
| alpha-Terpinene | 1.5 | 0.526 | 0.25 | 0.00 | 0.23 | 1.3230 | [6,20] |
| alpha-Terpinene | 0.8 | 0.526 | 0.25 | 0.00 | 0.23 | 1.3230 | [6,20] |
| Terpinolene | 1.3 | 0.590 | 0.31 | 0.00 | 0.20 | 1.3230 | [6,20] |
| Terpinolene | 0.1 | 0.590 | 0.31 | 0.00 | 0.20 | 1.3230 | [6,20] |
| p-Cymene | −0.3 | 0.607 | 0.49 | 0.00 | 0.19 | 1.2800 | [6,20] |
| p-Cymene | −1.3 | 0.607 | 0.49 | 0.00 | 0.19 | 1.2800 | [6,20] |
| 1-Dodecene | 8.1 | 0.089 | 0.08 | 0.00 | 0.07 | 1.7564 | [18] |
| 1-Dodecyne | 2.0 | 0.133 | 0.22 | 0.09 | 0.10 | 1.7134 | [18] |
| 1-Tetradecyne | 3.9 | - | - | - | - | - | [18] |
| Octylbenzene | 4.0 | 0.579 | 0.48 | 0.00 | 0.15 | 1.8436 | [16] |
| 2,6,10-Trimethylundecane-2,6-diene | 10.3 | - | - | - | - | - | [6,20] |
| Longifolene | 6.6 | 0.757 | 0.20 | 0.00 | 0.22 | 1.8533 | [20] |
| Longifolene | 7.3 | 0.757 | 0.20 | 0.00 | 0.22 | 1.8533 | [6,20] |
| Caryophyllene | 5.7 | 0.720 | 0.15 | 0.00 | 0.25 | 1.9189 | [6,20] |
| Caryophyllene | 6.2 | 0.720 | 0.15 | 0.00 | 0.25 | 1.9189 | [6,20] |
| Decylbenzene | 6.0 | 0.579 | 0.47 | 0.00 | 0.15 | 2.1254 | [25] |
| 1-Octadecene | 14.4 | 0.079 | 0.08 | 0.00 | 0.07 | 2.6018 | [18] |
| Dodecylbenzene | 7.8 | 0.571 | 0.47 | 0.00 | 0.15 | 2.4072 | [25] |
| Ethers, esters, nitriles and ketones | |||||||
| Diisopropyl ether | 2.2 | −0.063 | 0.17 | 0.00 | 0.57 | 1.0127 | [26] |
| Dibutyl ether | 2.4 | 0.000 | 0.25 | 0.00 | 0.45 | 1.2945 | [22] |
| Dibutyl ether | 3.3 | 0.000 | 0.25 | 0.00 | 0.45 | 1.2945 | [25] |
| Dibutyl ether | 3.2 | 0.000 | 0.25 | 0.00 | 0.45 | 1.2945 | [27] |
| 2-Octanone | –3.4 | 0.108 | 0.68 | 0.00 | 0.51 | 1.2515 | [22] |
| Octanenitrile | −1.7 | 0.162 | 0.90 | 0.00 | 0.36 | 1.2500 | [22] |
| Dipentyl ether | 4.2 | 0.000 | 0.25 | 0.00 | 0.45 | 1.5763 | [22] |
| C3-O-C4-O-C3 | 1.9 | - | - | - | - | - | [27] |
| C4-O-C2-O-C4 | 1.7 | - | 0.51 | 0.00 | - | 1.6350 | [27] |
| 2-Decanone | −2.1 | 0.108 | 0.68 | 0.00 | 0.51 | 1.5333 | [22] |
| Decanenitrile | −0.6 | 0.156 | 0.90 | 0.00 | 0.36 | 1.5320 | [22] |
| 2-Undecanone | −1.3 | 0.101 | 0.68 | 0.00 | 0.51 | 1.6742 | [22] |
| Ethyl decanoate | 1.8 | 0.013 | 0.58 | 0.00 | 0.45 | 1.8738 | [17] |
| Ethyl decanoate | 2.3 | 0.013 | 0.58 | 0.00 | 0.45 | 1.8738 | [20] |
| Ethyl decanoate | 2.2 | 0.013 | 0.58 | 0.00 | 0.45 | 1.8738 | [18] |
| Dihexyl ether | 6.2 | 0.000 | 0.25 | 0.00 | 0.45 | 1.8581 | [22] |
| 2-Dodecanone | −0.6 | 0.103 | 0.68 | 0.00 | 0.51 | 1.8151 | [22] |
| Dodecanenitrile | 0.3 | 0.132 | 0.90 | 0.00 | 0.36 | 1.8132 | [22] |
| Ethyl dodecanoate | 3.8 | 0.002 | 0.58 | 0.00 | 0.45 | 2.1556 | [6] |
| Decyl butyrate | 5.0 | - | - | - | - | - | [6] |
| Hexyl octanoate | 6.2 | 0.002 | 0.56 | 0.00 | 0.45 | 2.1556 | [6] |
| Diheptyl ether | 8.0 | - | - | - | - | - | [22] |
| Ethyl myristate | 5.2 | 0.000 | 0.58 | 0.00 | 0.45 | 2.4374 | [6] |
| Butyl dodecanoate | 7.2 | - | - | - | - | - | [6] |
| Octyl octanoate | 8.1 | −0.010 | 0.06 | 0.00 | 0.45 | 2.4374 | [6] |
| Dioctyl ether | 10.3 | 0.000 | 0.25 | 0.00 | 0.45 | 2.4217 | [22] |
| Myristyl propionate | 6.8 | - | - | - | - | - | [6] |
| Isopropyl myristate | 7.2 | −0.062 | 0.53 | 0.00 | 0.45 | 2.5783 | [6] |
| Isopropyl myristate | 7.3 | −0.062 | 0.53 | 0.00 | 0.45 | 2.5783 | [25] |
| Ethyl palmitate | 6.8 | 0.000 | 0.58 | 0.00 | 0.45 | 2.7192 | [6] |
| Hexyl dodecanoate | 9.3 | - | - | - | - | - | [20] |
| Ethyl oleate | 7.3 | - | - | - | - | - | [6] |
| Ethyl oleate | 7.1 | - | - | - | - | - | [28] |
| Bis(2-ethylhexyl) adipate | 9.7 | −0.010 | 1.10 | 0.00 | 1.13 | 3.3572 | [6] |
| Tricaprilin | 12.2 | - | - | - | - | - | [6] |
| Tricaprin | 13.8 | - | - | - | - | - | [6] |
| Tricaprin | 13.0 | - | - | - | - | - | [25] |
| Trilaurin | 15.7 | - | - | - | - | - | [5] |
| Trimyristin | 18.5 | - | - | - | - | - | [5] |
| Tripalmitin | 21.2 | - | - | - | - | - | [5] |
| Tristearin | 23.9 | −0.040 | 1.25 | 0.00 | 1.28 | 8.3631 | [5] |
| Triolein | 21.2 | - | - | - | - | - | [5] |
| Fragrances, acrylates and miscellaneous | |||||||
| Menthone | −1.5 | 0.322 | 0.61 | 0.00 | 0.62 | 1.4247 | [21] |
| Eucalyptol | −1.6 | 0.380 | 0.33 | 0.00 | 0.76 | 1.3591 | [21] |
| Rose oxide | −1.7 | - | - | - | - | - | [21] |
| D-Carvone | −3.1 | 0.674 | 0.86 | 0.00 | 0.57 | 1.3390 | [21] |
| Hexyl methacrylate | 0.4 | 0.154 | 0.49 | 0.00 | 0.45 | 1.5490 | [29] |
| Hexyl methacrylate | −0.2 | 0.154 | 0.49 | 0.00 | 0.45 | 1.5490 | [29] |
| Hexyl methacrylate | −0.1 | 0.154 | 0.49 | 0.00 | 0.45 | 1.5490 | [29] |
| Hexyl methacrylate | 0.8 | 0.154 | 0.49 | 0.00 | 0.45 | 1.5490 | [29] |
| Hexyl methacrylate | 0.7 | 0.154 | 0.49 | 0.00 | 0.45 | 1.5490 | [29] |
| Hexyl methacrylate | −0.2 | 0.154 | 0.49 | 0.00 | 0.45 | 1.5490 | [29] |
| Hexyl methacrylate | 1.5 | 0.154 | 0.49 | 0.00 | 0.45 | 1.5490 | [29] |
| Hexyl methacrylate | 0.2 | 0.154 | 0.49 | 0.00 | 0.45 | 1.5490 | [29] |
| Hexyl methacrylate | −0.4 | 0.154 | 0.49 | 0.00 | 0.45 | 1.5490 | [29] |
| Menthyl acetate | −0.1 | 0.243 | 0.65 | 0.00 | 0.54 | 1.7652 | [6,20] |
| Citronellyl acetate | −0.2 | 0.198 | 0.59 | 0.00 | 0.64 | 1.8308 | [21,22] |
| Geranyl acetate | −0.6 | 0.368 | 0.65 | 0.00 | 0.68 | 1.7878 | [21,22] |
| Linalyl acetate | −0.9 | 0.331 | 0.65 | 0.00 | 0.65 | 1.7878 | [21,22] |
| alpha-Damascone | −1.3 | - | - | - | - | - | [21] |
| Methyl dihydrojasmonate | −1.7 | 0.340 | 1.53 | 0.00 | 0.97 | 1.9218 | [21] |
| beta-Ionone | −1.9 | 0.892 | 0.78 | 0.00 | 0.76 | 1.7614 | [17] |
| Ethylene brassylate | −1.1 | - | - | - | - | - | [21] |
| Methyl cedryl ether | 3.5 | - | - | - | - | - | [21] |
| Ambrettolide | 1.0 | - | - | - | - | - | [21] |
| Compound | EACN | E | S | A | B | V | Predicted EACN |
|---|---|---|---|---|---|---|---|
| 1-Tetradecyne | 3.9 | 0.150 | 0.24 | 0.05 | 0.12 | 1.9952 | 5.7 |
| 2,6,10-Trimethylundecane-2,6-diene | 10.3 | 0.350 | 0.23 | 0.00 | 0.34 | 2.1361 | 7.7 |
| Decyl butyrate | 5.0 | 0.000 | 0.56 | 0.00 | 0.55 | 2.1556 | 4.3 |
| Butyl dodecanoate | 7.2 | 0.010 | 0.56 | 0.00 | 0.52 | 2.4374 | 6.3 |
| Myristyl propionate | 6.8 | 0.050 | 0.56 | 0.00 | 0.53 | 2.5783 | 7.2 |
| Diheptyl ether | 8.0 | 0.000 | 0.18 | 0.00 | 0.46 | 2.1399 | 8.3 |
| Hexyl dodecanoate | 9.3 | 0.010 | 0.56 | 0.00 | 0.52 | 2.7192 | 8.3 |
| Ethyl oleate | 7.1 | 0.130 | 0.72 | 0.00 | 0.68 | 2.9580 | 7.2 |
| Ethyl oleate | 7.3 | 0.130 | 0.72 | 0.00 | 0.68 | 2.9580 | 7.2 |
| Methyl cedryl ether | 3.5 | 0.650 | 0.23 | 0.00 | 0.22 | 2.0959 | 7.4 |
| Ambrettolide | 1.0 | 0.540 | 0.68 | 0.00 | 0.76 | 2.2858 | 1.8 |
| alpha-Damascone | −1.3 | 0.680 | 0.71 | 0.00 | 0.54 | 1.7614 | −1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acree, W.E., Jr.; Chong, W.-K.; Lang, A.S.I.D.; Mozafari, H. Estimating Equivalent Alkane Carbon Number Using Abraham Solute Parameters. Liquids 2022, 2, 318-326. https://doi.org/10.3390/liquids2040019
Acree WE Jr., Chong W-K, Lang ASID, Mozafari H. Estimating Equivalent Alkane Carbon Number Using Abraham Solute Parameters. Liquids. 2022; 2(4):318-326. https://doi.org/10.3390/liquids2040019
Chicago/Turabian StyleAcree, William E., Jr., Wei-Khiong Chong, Andrew S.I.D. Lang, and Hamed Mozafari. 2022. "Estimating Equivalent Alkane Carbon Number Using Abraham Solute Parameters" Liquids 2, no. 4: 318-326. https://doi.org/10.3390/liquids2040019
APA StyleAcree, W. E., Jr., Chong, W.-K., Lang, A. S. I. D., & Mozafari, H. (2022). Estimating Equivalent Alkane Carbon Number Using Abraham Solute Parameters. Liquids, 2(4), 318-326. https://doi.org/10.3390/liquids2040019








