Abstract
Diabetic foot ulcers (DFUs) and other chronic wounds are major global health challenges, often complicated by infections and delayed healing due to excessive collagen accumulation. Microbial collagenases offer an enzymatic alternative to surgical debridement by selectively degrading collagen and potentially limiting microbial colonization. In this study, an isolated and characterized thermostable collagenase from Streptomyces scabies from rhizospheric soil in Al-Lith thermal springs, Saudi Arabia, is investigated. Identification was confirmed via 16S rRNA sequencing, and enzyme production was optimized on gelatin agar. Partial purification was achieved through ammonium sulfate precipitation and dialysis, and molecular weight (~25 kDa) was determined by Sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Activity was assessed under varying temperatures, pH, substrates, and metal ions, while antibacterial potential was tested against Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas aeruginosa. The collagenase exhibited optimal activity at 80 °C and pH 9, stability under thermophilic and alkaline conditions, activation by Fe2+, and notable antibacterial effects at higher concentrations. These results demonstrate that S. scabies collagenase exhibits selective antibacterial activity in vitro, suggesting its potential as an enzymatic tool for further evaluation in diabetic foot debridement and infection control.
1. Introduction
Diabetic foot ulcers (DFUs) are among the most crippling of the many systemic consequences linked to diabetes mellitus (DM), a serious global health concern. The International Working Group on the Diabetic Foot (IWGDF) defines DFUs as open lesions or sores that develop on the foot and often spread from the deep dermal layer into the epidermis. In diabetic patients, poor glycemic management and immunological dysfunction frequently make these ulcers worse. Peripheral neuropathy, ischemia, and opportunistic infections are the usual causes of these ulcers. DFUs have a complicated and frequently multi-microbial etiology. Pathogenesis is largely influenced by both Gram-positive cocci, especially methicillin-resistant Staphylococcus aureus (MRSA), and Gram-negative bacteria, including Proteus species, Klebsiella species, and Escherichia coli [1,2].
Typically, wound dressing, metabolic stabilization, antibiotic medication, and mechanical or debridement surgery of necrotic tissue are all part of standard care for DFUs. Adjunctive techniques that enhance healing while lowering antibiotic reliance are necessary, as therapeutic success is limited by recurrent recurrence, poor vascularization, and the emergence of antimicrobial resistance [3]. Compared to surgical techniques, enzymatic debridement has drawn interest as a possible substitute, especially when using collagenase-based preparations that offer a selective, non-invasive, or preservation of tissue procedure [4]. Skin integrity and wound repair depend heavily on collagen, the main building block of tendons, skin, and connective tissue [5]. Healing is hampered in chronic wounds like DFUs by dysregulated matrix turnover and excessive collagen deposition [6]. A regulated clearance of necrotic tissue, the ejection of bioactive peptides, and the promotion of vasculature and cellular migration are all made possible by collagenase, which is a proteolytic enzyme that precisely hydrolyzes collagen fibers [7,8]. These enzymes have been used in tissue digestion and wound treatment since the initial discovery of microbial collagenase enzymes at the beginning of the 1960s [9]. Under physiological settings, the thermal stability, enzymatic efficiency, and substrate specificity of collagenases produced from bacteria like Clostridium histolyticum and actinomycetes like Streptomyces spp. have been investigated [10]. The significance of these enzymes is further highlighted by the rising antibiotic resistance among DFU infections. An investigation conducted in a Peruvian hospital revealed that E. coli and Pseudomonas aeruginosa isolates from DFUs had high levels of multidrug resistance, including resistance to β-lactam antibiotics because of the synthesis of extended-spectrum β-lactamase (ESBL) [11]. In addition, the COVID-19 pandemic made this problem worse by increasing resistance to antibiotics levels from 36% in 2019 to 63% in 2020 due to unmonitored use [12]. These results highlight how urgent it is to create substitute, non-antibiotic-based treatment options. Collagenases produced by actinomycetes have shown the most promise among microbiological sources. Streptomyces sp. isolated collagenase showed the characteristics of a “true collagenase,” breaking down both native and denatured collagen. Its molecular weight was about 75 kDa, and its catalytic activity was increased when Ca2+ and Mg2+ were present [13]. Comparative examination of various Streptomyces isolates showed that the generation of collagenase is outside the cell phase of growth, dependent, and significantly affected by the composition of the medium, especially when collagen is the only source of nitrogen [14]. Because of their quick growth, genetic adaptability, and capacity to flourish in a variety of settings, microbial enzymes provide scalable, economical, and environmentally friendly manufacturing platforms from a biotechnological standpoint. In healthcare, food processing, and pharmaceuticals, microbiological enzymes such as amylases, lipases, and collagenases already have revolutionary functions [15]. The goal of this study is to identify, isolate, and describe a heat-stable collagenase from the Streptomyces scabies strain, considering these diagnostic and industrial implications. To promote potential uses for preventing infection in chronic wounds, its biochemical characteristics, thermal endurance, and antibacterial properties against DFU-related pathogens are examined.
2. Materials and Methods
All reagents used in this study were of analytical grade to ensure experimental accuracy and reproducibility. The chemicals included Type I and Type III hydrolyzed bovine collagen (Doctor’s Best, Tustin, CA, USA) used as substrates for enzyme assays, and Tris Base (Sigma-Aldrich, Burlington, MA, USA) for Tris-HCl buffer with pH 7.5, that maintaining optimal reaction conditions, Ninhydrin reagent (Sigma-Aldrich, Burlington, MA, USA) for colorimetric detection of amino groups, and gelatin from bovine skin (Sigma, Burlington, MA, USA) employed as a substrate for enzyme induction and activity evaluation.
2.1. Microorganisms
The actinomycete strain Streptomyces scabies was isolated from rhizospheric soil collected near the Al-Lith hot spring, Saudi Arabia. Soil samples were collected in sterile tubes, air-dried, and serially diluted before being spread onto starch nitrate agar medium. Plates were incubated at 30 °C for 7 days. Morphologically distinct colonies were purified and maintained on slant agar at 4 °C for further analysis. The isolate was identified and taxonomically verified through sequence submission and confirmation in the National Collection of Industrial, Marine and Food Bacteria (NCIMB) in United Kingdom. Phylogenetic placement of the strain was illustrated based on its registered accession data. Clinical bacterial isolates associated with diabetic foot infections—Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus—were obtained under approved ethical criteria from the Clinical Microbiology Laboratory, King Abdulaziz University Hospital (Jeddah, Saudi Arabia).
2.2. Primary Screening for Collagenolytic Activity
Primary screening for collagenase production was carried out using a modified collagen agar medium that contained (g/L): 0.5 g K2HPO4, 0.2 g MgSO4·7H2O, 0.5 g NaCl, 5 g gelatin, and 15 g agar, adjusted to pH 7.0 before autoclaving. A loopful of S. scabies colony was spot inoculated onto the plates and incubated at 30 °C for 5 days. Clear hydrolysis zones surrounding colonies were visualized after flooding the plates with acidic mercuric chloride solution (1.5% w/v) to indicate collagen degradation [16,17].
2.3. Collagenase Assay
Collagenase activity was quantitatively determined using the modified ninhydrin method. The reaction mixture contained 0.2 mL of enzyme extract, 10 mg of soluble collagen (Type I or III, Doctor’s Best, Tustin, CA, USA) dissolved in 0.8 mL of 50 mM Tris-HCl containing 4 mM CaCl2, pH 7.5, and incubated at 37 °C for 30 min. The reaction was stopped by adding 1 mL of 0.1 M acetic acid (TCA), followed by centrifugation at 8000 rpm for 10 min. Supernatants were mixed with ninhydrin reagent (Sigma-Aldrich, Saint Louis, MO, USA) and heated in boiling water for 15 min. After cooling, the absorbance was measured at 570 nm. One unit (U) of enzyme activity was defined as the amount of enzyme releasing 1 µmol of L-leucine equivalents per minute under the assay conditions [18,19].
2.4. Optimization of Collagenase Production
Optimization experiments were conducted to determine the effect of different factors on enzyme yield. Each parameter varied independently while keeping others constant. Inoculum size (2%, 4%, 6%, 8%, and 10% v/v) was tested using 24 h-old S. scabies cultures in 50 mL of production medium within 250 mL Erlenmeyer flasks, incubated at 30 °C and 121 rpm for 5 days. pH optimization was performed within a range of 3.0–9.0 using appropriate buffer systems for acidic and alkaline conditions. Temperature optimization ranged from 25 °C to 80 °C. The influence of carbon (glucose, sucrose, fructose, Lactose; 1% w/v) and nitrogen sources (yeast extract, peptone, and tryptone; 0.5% w/v) was also examined to study whether they can significantly influence microbial growth and collagenase yield in some microorganisms. In addition, the day of incubation was evaluated to determine its effect on enzyme production, and each condition was conducted in triplicate.
2.5. Enzyme Purification
The crude enzyme extract was obtained by centrifugation of the culture broth at 8000 rpm for 15 min at 4 °C. The supernatant was subjected to ammonium sulfate precipitation at 30% saturation under constant stirring at 4 °C for 1 h. The precipitate was collected by centrifugation and re-dissolved in 0.05 M Tris-HCl buffer (pH 7.5). Desalting was achieved through overnight dialysis against the same buffer at 4 °C. The partially purified enzyme was used for subsequent analyses.
2.6. Molecular Weight Determination
The molecular weight and purity of the enzyme were determined using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) as described by Laemmli [20]. A 12.5% resolving gel and 5% stacking gel were used. Samples (20 µg of protein per lane) were mixed with loading buffer containing β-mercaptoethanol, heated at 95 °C for 5 min, and loaded into the gel. After electrophoresis, protein bands were stained with Coomassie Brilliant Blue R-250 and compared with pre-stained molecular weight markers (Thermo Fisher Scientific, Waltham, MA, USA).
2.7. Antibacterial Activity and Statistical Analysis
The antibacterial potential of the purified collagenase was assessed using the agar well diffusion method on Mueller–Hinton agar (MHA) plates. Bacterial cultures of P. aeruginosa, K. pneumoniae, and S. aureus were adjusted to 0.5 McFarland standard (~1.5 × 108 CFU/mL) and spread evenly on MHA plates. Wells (6 mm in diameter) were made using a sterile cork borer, and 50 µL or 100 µL of enzyme solution were added. Plates were incubated at 37 °C for 48 h, and inhibition zones were measured in millimeters. All experiments were conducted in triplicate, and the results were expressed as mean ± standard deviation (SD). Statistical analyses were performed using IBM SPSS Statistics software (version 26). Differences among experimental groups were evaluated using one-way ANOVA followed by Tukey’s post hoc test (for optimization parameters) and unpaired t-tests (for antibacterial assays). A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Morphological and Molecular Identification
The taxonomic identity of the strain was verified through sequence submission in NCIMB and phylogenetic placement within the S. scabies, showing 99.6% sequence identity with Streptomyces scabies (Figure 1a). On starch-nitrate agar medium, the isolate exhibited distinctive white aerial and substrate mycelia (Figure 1b). Extracellular collagenase production was confirmed by preliminary screening on mineral gelatin agar, where a clear hydrolytic zone surrounding the S. scabies colonies indicated enzymatic activity (Figure 1c.)
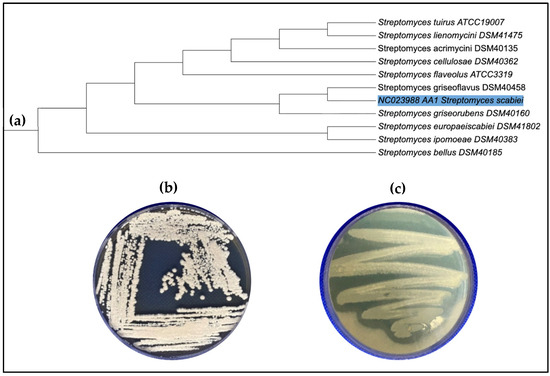
Figure 1.
(a) Phylogenetic placement of S. scabies based on 16S rRNA sequence comparison with related Streptomyces species. (b) Morphology of S. scabies isolate on Starch nitrate agar medium. (c) Collagenase activity of S. scabies showing a clear hydrolysis zone on screening gelatin agar medium.
3.2. Optimization of Collagenase Production
Utilizing a two mL inoculum and 4 percent gelatin as the substrate, collagenase synthesis was optimized throughout a 4-day incubation period at 80 °C and pH 9.0. The most efficient carbon and nitrogen sources among the nutritional sources were found to be sucrose and tryptone, respectively (Figure 2). Enzyme activity exhibited a distinct temperature-dependent pattern. Minimal activity was detected at 37 °C (control, 0.800 ± 0.009 U/mL), while the highest level was achieved at 80 °C (1.714 ± 0.022 U/mL; **** p < 0.0001) (Figure 2a) Activities at 5 °C, 28 °C, 30 °C, and 40 °C were also significantly higher than the control (*** p < 0.001–0.0001), confirming the thermostable nature of the enzyme. The enzyme showed marked pH-dependent activity. Compared with pH 7 (control, 0.569 ± 0.003 U/mL), activity significantly increased at pH 3 (1.256 ± 0.018 U/mL; **** p < 0.0001) and pH 9 (1.508 ± 0.016 U/mL; **** p < 0.0001), while a moderate increase was observed at pH 5 (* p < 0.05). These results demonstrate the enzyme’s stability and functionality in both acidic and alkaline environments (Figure 2b). Enzyme yield was influenced by inoculum concentration. The 4% inoculum resulted in the highest activity (1.360 ± 0.123 U/mL; ** p < 0.01) compared to the 2% control, while higher inoculum sizes (6% and 8%) did not improve production (p > 0.05). This indicates that excessive inoculum can deplete nutrients, limiting enzyme synthesis (Figure 2c). Collagenase production progressively increased from Day 1 (0.825 ± 0.004 U/mL) to Day 4 (1.413 ± 0.0075 U/mL; *** p < 0.0001), followed by a decline on Days 6–7 (** p < 0.05–0.01). This trend suggests that maximum secretion occurs during the mid-log growth phase, with a reduction likely due to enzyme degradation or nutrient limitation in the stationary phase (Figure 2d). Different carbon sources produced significant differences in collagenase activity. Sucrose yielded the highest activity (2.154 ± 0.004 U/mL; **** p < 0.0001) compared with glucose (control, 1.890 ± 0.009 U/mL). Fructose and lactose significantly decreased enzyme activity (**** p < 0.0001), suggesting a metabolic preference for disaccharides in supporting collagenase biosynthesis (Figure 2e). Nitrogen sources also influenced enzyme yield. Tryptone (2.238 ± 0.002 U/mL; **** p < 0.0001) and yeast extract (2.033 ± 0.003 U/mL; *** p < 0.001) both enhanced activities compared with peptone control (1.944 ± 0.004 U/mL). These complex nitrogen sources likely provide essential amino acids and cofactors that stimulate enzyme production (Figure 2f).
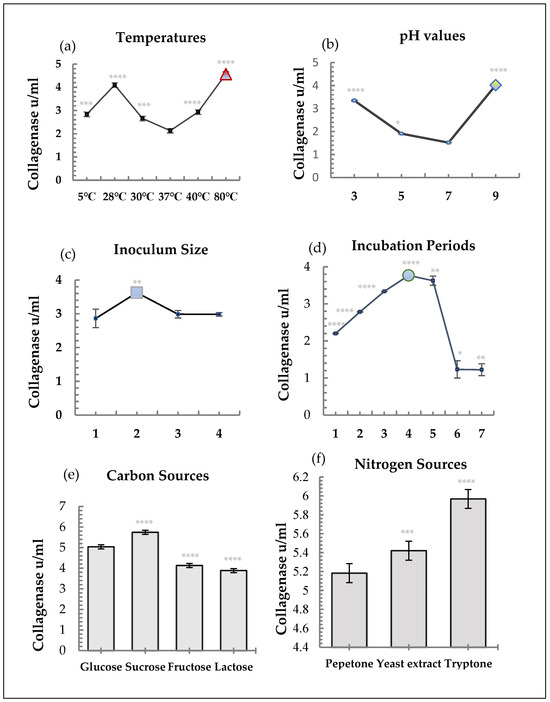
Figure 2.
Optimization factors for growth and collagenase production from S. scabies. Error bars represent mean ± SD (n = 3). p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****). (a) Effect of temperatures on collagenase production. (b) Effect of pH values on collagenase production. (c) Effect of inoculum sizes on collagenase production. (d) Effect of incubation days on collagenase production. (e) Effect of carbon sources on collagenase production. (f) Effect of nitrogen sources on collagenase production.
3.3. Biochemical Properties of Collagenase
The isolated crude collagenase maintained its activity at 20 °C and a broad pH range (3.0–9.0), with pH 5.0 showing the highest activity. Specificity and inhibition of substrates, while activation studies showed that Fe2+ significantly reduced activity, Ca2+, Hg2+, and EDTA exhibited mild inhibitory effects. Substrate preference assays, however, verified a high selectivity for gelatin (Table 1).

Table 1.
Biochemical characterization of S. scabies collagenase.
3.4. Enzyme Purification and Molecular Weight
Through dialysis and 30% ammonium sulfate precipitation, the enzyme was purified while maintaining its activity. A clear band at around 25 kDa was seen by SDS-PAGE, which is suggested to be S. scabies collagenases (Figure 3).
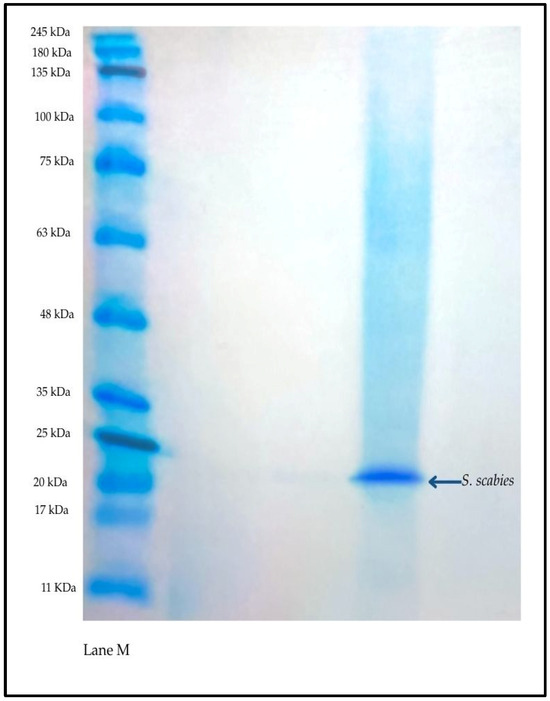
Figure 3.
SDS-PAGE showing purified collagenase from S. scabies. Left lane: molecular weight marker; right lane: purified enzyme with a distinct band at ~25 kDa.
3.5. Antibacterial Activity
The purified collagenase demonstrated remarkable antibacterial activity against the tested clinical isolates (Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas aeruginosa). A clear concentration-dependent pattern was observed, as increasing the enzyme volume from 50 µL to 100 µL resulted in larger inhibition zones for all tested bacteria. At 50 µL, inhibition zones measured 50 mm for S. aureus, 35 mm for K. pneumoniae, and 25 mm for P. aeruginosa. When the enzyme concentration was doubled to 100 µL, the inhibition zones increased to 55 mm, 40 mm, and 35 mm, respectively (Table 2; Figure 4). All antibacterial assays were performed in triplicate, and the results are expressed as mean ± standard deviation (SD). Statistical analysis using unpaired t-tests revealed significant differences between the two enzyme concentrations (p < 0.05) for each bacterial strain, confirming a concentration-dependent enhancement of inhibitory activity. These findings highlight the reproducibility and statistical reliability of the observed antibacterial effects, supporting the enzyme’s potential as a broad-spectrum antimicrobial agent. The overall inhibition trend and comparative activity profiles are further illustrated in (Figure 5).

Table 2.
Inhibition zone diameters (mm) for two collagenase concentrations (50 µL and 100 µL) against selected pathogenic bacteria.
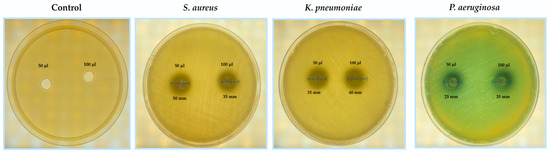
Figure 4.
Antibacterial activity of purified collagenase from S. scabies against diabetic foot pathogens. Zones of inhibition were measured on Mueller–Hinton agar using two enzyme volumes (50 µL and 100 µL).
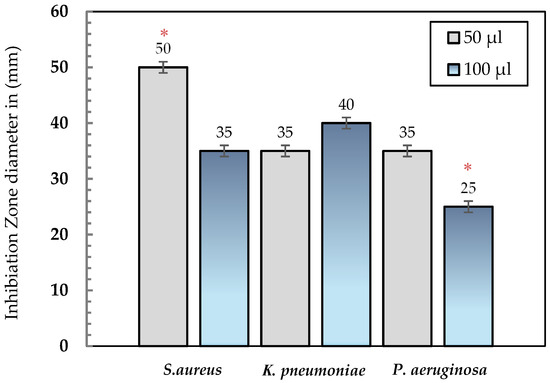
Figure 5.
Comparison of inhibition zone diameters (mm) produced by collagenase at two different volumes (50 µL and 100 µL) against S. aureus, K. pneumoniae, and P. aeruginosa. Data are expressed as mean values. Asterisks (*) indicate statistically significant differences between treatments (p < 0.05).
4. Discussion
This study identifies Streptomyces scabies as a promising microbial source of extracellular collagenase with dual therapeutic potential—enzymatic debridement and antimicrobial activity. The enzyme exhibited exceptional biochemical properties, including high thermal stability, alkaline pH tolerance, and selective inhibitory effects against Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas aeruginosa, three major pathogens commonly associated with diabetic foot ulcers (DFUs). S. scabies collagenase demonstrated remarkable thermostability, maintaining activity at elevated temperatures with an optimum at 80 °C and pH 9.0. These properties surpass those reported for several other thermostable microbial collagenases, including (60–70 °C) [21,22], Thermoactinomyces (60–65 °C) [23], Nocardiopsis dassonvillei (60 °C) [24], and Bacillus licheniformis (50 °C) [25,26,27]. Furthermore, the alkaline optimum pH of S. scabies collagenase (pH 9.0) aligns with other bacterial collagenases, such as those from Thermoactinomyces [23] and Bacillus spp. [25,26,27], while clearly exceeding the activity ranges of fungal collagenases derived from Aspergillus and Penicillium (pH 6.5–8.3) [28,29]. These features underline its suitability for industrial and biomedical applications that demand stability under harsh processing conditions. Consistent with other microbial collagenases, S. scabies collagenase activity was inhibited by Ca2+ and enhanced by heavy metals such as Fe2+, supporting its classification within the metalloprotease or serine-protease family. Notably, its relatively small molecular weight (~25 kDa) is lower than most reported collagenases, including those from Thermoactinomyces (50 kDa) [22,23], Nocardiopsis (150 kDa) [24], and Aspergillus (28.7 kDa) [28]. This reduced size may facilitate improved tissue penetration, reduced immunogenicity, and enhanced compatibility with biomedical formulations. Enzymatic debridement is increasingly favored over surgical methods in DFU management due to its precision, reduced invasiveness, and ability to promote wound healing. Although some studies report limited or biased data [30,31], enzymatic approaches have consistently demonstrated superior outcomes in tissue regeneration, epithelialization, and wound closure [32,33,34]. In line with these findings, S. scabies collagenase exhibited robust collagenolytic activity with therapeutic selectivity. Importantly, Collagenase displays direct antimicrobial action inhibition against major DFU pathogens [35,36]. Inhibition zone assays revealed strong activity against S. aureus (50 mm at 50 µL), K. pneumoniae (35–40 mm), and P. aeruginosa (25–35 mm). Interestingly, the reduced sensitivity of S. aureus at higher enzyme volumes suggests possible protein aggregation or inhibitory byproducts, a phenomenon previously observed in other proteolytic systems. The dual-action profile of S. scabies collagenase is particularly valuable. Existing enzymatic formulations, such as clostridial collagenase (Iruxol®/Iruksan), combine debridement with antimicrobial effects [37]. Similarly, bromelain-based preparations (e.g., NexoBrid®) have shown tissue selectivity in burn care [38], while clostridial collagenase remains the only FDA-approved agent for chronic wound debridement, with well-documented cost-effectiveness and therapeutic benefits [39,40]. Beyond wound care, collagenases have been applied in Dupuytren’s disease (Xiaflex®) [41] and shown to promote cell migration and tissue remodeling in alveolar and dermal wound models through the release of bioactive peptides [42,43,44]. Marine-derived peptides, such as those from Aluterus monoceros, further highlight the broad wound-healing potential of collagenolytic systems [45]. The biomedical relevance of S. scabies collagenase extends beyond wound debridement. Its gentle collagenolytic action resembles that of Vibrio alginolyticus collagenase, which has been used for high-viability mesenchymal stem cell (MSC) isolation [46]. Unlike endogenously secreted collagenases from gut flora, which can contribute to tissue pathology such as anastomotic leakage [47], S. scabies collagenase is externally applied, minimizing systemic risks. Pathogenic collagenases, such as those from Peptostreptococcus magnus in DFU isolates [48], illustrate the need to distinguish between virulence factors and controlled therapeutic enzymes. The stability, selective action, and external application of S. scabies collagenase support its clinical safety profile.
Furthermore, collagenase therapy has shown efficacy in neonatal wound care with minimal discomfort [49], suggesting broad clinical tolerability. Diabetic and infected wounds are often characterized by elevated endogenous collagenase activity and exacerbated tissue damage [50]. Controlled application of exogenous collagenase may rebalance the wound environment toward healing. Indeed, clostridial collagenases have been shown to modulate inflammatory responses by reducing TNF-α and IL-6 while enhancing anti-inflammatory mediators [39]. Precision activity, such as selective degradation of fibrotic collagen without affecting collagen VI, is particularly advantageous for chronic wound environments [51]. S. scabies collagenase’s alkaline pH preference aligns with the biochemical milieu of DFUs [52]. Overall, the findings of this study suggest that the collagenase produced by Streptomyces scabies possesses significant biotechnological potential due to its high catalytic efficiency, thermostability, and antibacterial activity under alkaline conditions. This study comprehensively characterized the enzyme’s physicochemical and functional properties, highlighting its suitability as a candidate for enzymatic debridement and infection control in chronic wounds. Nevertheless, the current work was limited to in vitro experiments. Further studies employing biofilm models and in vivo systems are essential to validate its therapeutic potential, biocompatibility, and safety before clinical translation.
5. Conclusions
In this study, a thermostable collagenase from Streptomyces scabies isolated from rhizospheric soil in Al-Lith thermal springs, Saudi Arabia, was successfully characterized. The enzyme was partially purified, and its molecular weight (~25 kDa) was determined by SDS-PAGE. Collagenase activity was optimal at 80 °C and pH 9, remained stable under thermophilic and alkaline conditions, and was enhanced by Fe2+ ions. The enzyme also exhibited selective antibacterial activity against Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas aeruginosa. These findings highlight the potential of S. scabies collagenase as a promising enzymatic tool for in vitro antibacterial applications and provide a basis for further evaluation in wound management strategies, including diabetic foot infections.
Author Contributions
Conceptualization, M.A.-K.; methodology, A.B. and A.S.; investigation, A.S.; resources, A.B.; data curation, A.S.; writing—original draft preparation, A.S.; writing—review and editing, M.A.-K. and A.B.; supervision, M.A.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Jeddah, Saudi Arabia, through the Future Researcher Program under the Research and Innovation Authority.
Data Availability Statement
All datasets generated and analyzed during the current study are fully included in this article and can be obtained from the corresponding author upon reasonable request.
Acknowledgments
The authors gratefully acknowledge King Abdulaziz University hospitals for providing the clinical bacterial isolates and King Fahad Medical Research Center for facilitating SDS-PAGE analyses. Special thanks are extended to Fayez Al-Shehri, Senior Genetics Lab Specialist at University of Jeddah, for his valuable guidance in molecular analyses specifically in SDS-PAGE method. The authors also appreciate the institutional support from the University of Jeddah and the molecular identification services provided by NCIMB Limited for the accurate characterization of the isolate.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DFUs | Diabetic foot ulcers |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| IWGDF | The International Working Group on the Diabetic Foot |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| ESBL | Extended-spectrum β-lactamase |
| SD | Standard deviation |
| MSC | Mesenchymal stem cell |
| EDTA | Ethylenediaminetetraacetic acid |
| NCIMB | The National Collection of Industrial, Marine and Food Bacteria |
References
- International Working Group on the Diabetic Foot (IWGDF). IWGDF Guidelines on the Prevention and Management of Diabetic Foot Disease; IWGDF: Maastricht, The Netherlands, 2019. [Google Scholar]
- Shaheen, M.M.A.; Al Dahab, S.; Abu Fada, M.; Idieis, R. Isolation and characterization of bacteria from diabetic foot ulcer: Amputation, antibiotic resistance and mortality rate. Int. J. Diabetes Dev. Ctries. 2022, 42, 529–537. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Amadeh, A.; Mohebbi, N.; Amadeh, Z.; Jamshidbeigi, A. Comparative efficacy of autolytic and collagenase-based enzymatic debridement in chronic wound healing: A comprehensive systematic review. Int. Wound J. 2025, 22, e70177. [Google Scholar] [CrossRef]
- Pillai, N.S.; Khan, S.A.; Mehrotra, N.; Jadhav, K. A comprehensive review on the role of collagen in health and disease. Biosci. Biotechnol. Res. Asia 2024, 21, 1329–1347. [Google Scholar] [CrossRef]
- Huang, Y.; Kyriakides, T.R. The role of extracellular matrix in the pathophysiology of diabetic wounds. Matrix Biol. Plus 2020, 6–7, 100037. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, R.D.; Ryan, T.J. Proteolytic enzymes in wound healing: The role of enzymatic debridement. Australas. J. Dermatol. 1994, 35, 35–41. [Google Scholar] [CrossRef]
- Alipour, H.; Raz, A.; Zakeri, S.; Dinparast Djadid, N. Therapeutic applications of collagenase (metalloproteases): A review. Asian Pac. J. Trop. Biomed. 2016, 6, 975–981. [Google Scholar] [CrossRef]
- Gross, J.; Lapiere, C.M. Collagenolytic activity in amphibian tissues: A tissue culture assay. Biochim. Biophys. Acta 1961, 51, 39–49. [Google Scholar] [CrossRef]
- Vachher, M.; Sen, A.; Kapila, R.; Nigam, A. Microbial therapeutic enzymes: A promising area of biopharmaceuticals. Curr. Res. Biotechnol. 2021, 3, 195–208. [Google Scholar] [CrossRef]
- Moya-Salazar, J.; Chamana, J.M.; Porras-Rivera, D.; Goicochea-Palomino, E.A.; Salazar, C.R.; Contreras-Pulache, H. Increase in antibiotic resistance in diabetic foot infections among Peruvian patients: A single-center cross-sectional study. Front. Endocrinol. 2023, 14, 1159847. [Google Scholar] [CrossRef]
- Caruso, P.; Maiorino, M.I.; Macera, M.; Signoriello, G.; Castellano, L.; Scappaticcio, L.; Longo, M.; Gicchino, M.; Campitiello, F.; Bellastella, G.; et al. Antibiotic resistance in diabetic foot infection: How it changed with COVID-19 pandemic in a tertiary care center. Diabetes Res. Clin. Pract. 2021, 175, 108815. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Chandra, A.L. Purification and characterization of a streptomycete collagenase. J. Appl. Bacteriol. 1986, 61, 331–337. [Google Scholar] [CrossRef]
- El-Sayed, S.T. Extracellular Collagenase Produced by Streptomyces Species: Optimum Culture Conditions. [Internet]. Available online: https://www.researchgate.net/publication/332268552 (accessed on 20 September 2025).
- Sharma, N.; Ahlawat, Y.K.; Stalin, N.; Mehmood, S.; Morya, S.; Malik, A.; Nellore, J.; Bhanot, D. Microbial enzymes in industrial biotechnology: Sources, production, and significant applications of lipases. J. Ind. Microbiol. Biotechnol. 2025, 52, 201–223. [Google Scholar] [CrossRef]
- Hisano, T.; Abe, S.; Wakashiro, M.; Kimura, A.; Murata, K. Isolation and properties of a collagenase with caseinolytic activity from a Pseudomonas sp. J. Ferment. Bioeng. 1989, 68, 399–403. [Google Scholar] [CrossRef]
- Endo, A.; Murakawa, S.; Sfflmizu, H.; Shiraishi, Y. Purification and properties of collagenase from a Streptomyces species. J. Biochem. 1987, 102, 941–949. [Google Scholar] [CrossRef]
- Pin, Y.A.; Takahashi, T. An improved colorimetric determination of amino acids with the use of ninhydrin. Anal. Biochem. 1966, 14, 71–77. [Google Scholar] [CrossRef]
- Tran, L.H.; Nagano, H. Isolation and characteristics of Bacillus subtilis CN2 and its collagenase production. J. Food Sci. 2002, 67, 1184–1188. [Google Scholar] [CrossRef]
- Laemmli, U.K.; Favre, M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J. Mol. Biol. 1973, 80, 575–599. [Google Scholar] [CrossRef]
- Zhang, K.; Han, Y. Thermostable bacterial collagenolytic proteases: A review. J. Microbiol. Biotechnol. 2024, 34, 1385–1394. [Google Scholar] [CrossRef]
- Petrova, D.H.; Shishkov, S.A.; Vlahov, S.S. Novel thermostable serine collagenase from Thermoactinomyces sp. 21E: Purification and some properties. J. Basic Microbiol. 2006, 46, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Petrova, D.; Vlahov, S.; Dalev, P. Purification and characterization of a thermostable alkaline collagenase from Thermoactinomyces sp. E-21 strain. Biotechnol. Biotechnol. Equip. 2001, 15, 31–38. [Google Scholar] [CrossRef]
- Abood, A.; Salman, A.M.M.; Abdelfattah, A.M.; El-Hakim, A.E.; Abdel-Aty, A.M.; Hashem, A.M. Purification and characterization of a new thermophilic collagenase from Nocardiopsis dassonvillei NRC2aza and its application in wound healing. Int. J. Biol. Macromol. 2018, 116, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Baehaki, A.; Sukarno, S.; Syah, D.; Setyahadi, S.; Suhartono, M.T. Production and characterization of collagenolytic protease from Bacillus licheniformis F11.4 originated from Indonesia. Asian J. Chem. 2014, 26, 2861–2864. [Google Scholar] [CrossRef]
- Baehaki, A. Purification and characterization of collagenase from Bacillus licheniformis F11.4. Afr. J. Microbiol. Res. 2012, 6, 2392–2398. [Google Scholar] [CrossRef]
- Natsir, H.; Dali, S. Production and characterization of collagenase from Bacillus sp. 6-2 isolated from fish liquid waste. J. Pure Appl. Microbiol. 2019, 12, 701–708. [Google Scholar]
- Ferreira, C.M.O.; Correia, P.C.; Brandão-Costa, R.M.P.; Albuquerque, W.W.C.; Lin Liu, T.P.S.; Campos-Takaki, G.M.; Porto, A.L.F. Collagenase produced from Aspergillus sp. (UCP 1276) using chicken feather industrial residue. Biomed. Chromatogr. 2017, 31, e3852. [Google Scholar] [CrossRef] [PubMed]
- Kate, S.; Pethe, A. Study of collagenase production by Penicillium sp. isolated from deteriorated leather sample. J. Adv. Sci. Res. 2022, 13, 227–234. [Google Scholar]
- Patry, J.; Blanchette, V. Enzymatic debridement with collagenase in wounds and ulcers: A systematic review and meta-analysis. Int. Wound J. 2017, 14, 1055–1065. [Google Scholar] [CrossRef]
- Ning, P.; Liu, Y.; Kang, J.; Cao, H.; Zhang, J. Comparison of healing effectiveness of different debridement approaches for diabetic foot ulcers: A network meta-analysis of randomized controlled trials. Front. Public Health 2023, 11, 1213370. [Google Scholar] [CrossRef]
- Brzyska, A.; Mozga, K.; Karabin, A.; Bojarska, M.; Domańska, N. Healing diabetic foot ulcers: A comparative review of debridement approaches. Qual. Sport 2024, 21, 44262. [Google Scholar] [CrossRef]
- Tallis, A.; Motley, T.A.; Wunderlich, R.P.; Dickerson, J.E.; Waycaster, C.; Slade, H.B. Clinical and economic assessment of diabetic foot ulcer debridement with collagenase: Results of a randomized controlled study. Clin. Ther. 2013, 35, 1805–1820. [Google Scholar] [CrossRef]
- Miller, J.D.; Carter, E.; Hatch, D.C.; Zhubrak, M.; Giovinco, N.A.; Armstrong, D.G. Use of collagenase ointment in conjunction with negative pressure wound therapy in the care of diabetic wounds: A case series of six patients. Diabet. Foot Ankle 2015, 6, 26839. [Google Scholar] [CrossRef] [PubMed]
- Rautskis, V.P.; Khimich, S.D. Role of antimicrobial activity of collagenase-based ointment in the treatment of infected wounds in an experiment. Rep. Vinnytsia Natl. Med. Univ. 2024, 28, 383–388. [Google Scholar] [CrossRef]
- Alhayek, A.; Khan, E.S.; Schönauer, E.; Däinghaus, T.; Shafiei, R.; Voos, K.; Han, M.K.; Ducho, C.; Posselt, G.; Wessler, S.; et al. Inhibition of collagenase Q1 of Bacillus cereus as a novel antivirulence strategy for the treatment of skin-wound infections. Adv. Ther. 2022, 5, 2200065. [Google Scholar] [CrossRef] [PubMed]
- Waycaster, C.; Carter, M.J.; Gilligan, A.M.; Mearns, E.S.; Fife, C.E.; Milne, C.T. Comparative cost and clinical effectiveness of clostridial collagenase ointment for chronic dermal ulcers. J. Comp. Eff. Res. 2018, 7, 149–165. [Google Scholar] [CrossRef]
- Buta, M.R.; Annand, D.; Findeisen, S.; Hickey, S.A.; Sheridan, R.L.; Friedstat, J.S.; Schulz, J.T.; Bojovic, B.; Bittner, E.A.; Goverman, J. Pain management during bromelain-based enzymatic debridement (NexoBrid®) in a USA adult burn center. Eur. Burn J. 2024, 5, 1–11. [Google Scholar] [CrossRef]
- Galperin, R.C.; Lange, D.L.; Ramsay, S.J.; Shi, L.; Weedon, K.A.; Hudson, N.M.; Dickerson, J.E.; Cargill, D.I.; Slade, H.B. Anti-inflammatory effects of clostridial collagenase result from in vitro and clinical studies. J. Am. Podiatr. Med. Assoc. 2015, 105, 509–519. [Google Scholar] [CrossRef]
- Fahie, M.A.; Shettko, D. Evidence-based wound management: A systematic review of therapeutic agents to enhance granulation and epithelialization. Vet. Clin. N. Am. Small Anim. Pract. 2007, 37, 559–577. [Google Scholar] [CrossRef] [PubMed]
- Syed, F.; Thomas, A.N.; Singh, S.; Kolluru, V.; Hart, S.G.E.; Bayat, A. In vitro study of novel collagenase (Xiaflex®) on Dupuytren’s disease fibroblasts displays unique drug related properties. PLoS ONE 2012, 7, e31430. [Google Scholar] [CrossRef]
- Banerjee, P.; Das, A.; Singh, K.; Khanna, S.; Sen, C.K.; Roy, S. Collagenase-based wound debridement agent induces extracellular matrix supporting phenotype in macrophages. Sci. Rep. 2024, 14, 53424. [Google Scholar] [CrossRef]
- Shingleton, W.D. Collagenase: A key enzyme in collagen turnover. Biochem. Cell Biol. 1996, 74, 759–775. [Google Scholar] [CrossRef] [PubMed]
- Planus, E.; Galiacy, S.; Matthay, M.; Laurent, V.; Gavrilovic, J.; Murphy, G.; Clérici, C.; Isabey, D.; Lafuma, C.; d’Ortho, M.P. Role of collagenase in mediating in vitro alveolar epithelial wound repair. J. Cell Sci. 1999, 112, 243–252. [Google Scholar] [CrossRef]
- Kumar, L.V.; Jeyashakila, R.; Dhanabalan, V.; Manivannan, M. In vitro bioaccessibility and antioxidant properties of unicorn leatherjacket fish (Aluterus monoceros) skin collagen peptides prepared using crude collagenase enzyme isolated from fish fins. Braz. J. Dev. 2024, 10, e70488. [Google Scholar] [CrossRef]
- Quintero Sierra, L.A.; Biswas, R.; Busato, A.; Conti, A.; Ossanna, R.; Conti, G.; Zingaretti, N.; Caputo, M.; Cuppari, C.; Parodi, P.C.; et al. In vitro study of a novel Vibrio alginolyticus-based collagenase for future medical application. Cells 2023, 12, 2025. [Google Scholar] [CrossRef]
- Jorgensen, A.B.; Jonsson, I.; Friis-Hansen, L.; Brandstrup, B. Collagenase-producing bacteria are common in anastomotic leakage after colorectal surgery: A systematic review. Int. J. Colorectal Dis. 2023, 38, 140. [Google Scholar] [CrossRef]
- Krepel, C.J.; Gohr, C.M.; Edmiston, C.E., Jr.; Farmer, S.G. Anaerobic pathogenesis: Collagenase production by Peptostreptococcus and its relationship to site of infection. J. Infect. Dis. 1991, 164, 54–60. [Google Scholar] [CrossRef]
- Huett, E.; Bartley, W.; Morris, D.; Reasbeck, D.; McKitrick-Bandy, B.; Yates, C. Collagenase for wound debridement in the neonatal intensive care unit: A retrospective case series. Pediatr. Dermatol. 2017, 34, 277–281. [Google Scholar] [CrossRef]
- Yadav, P.S.; Singh, M.; Vinayagam, R.; Shukla, P. Therapies and delivery systems for diabetic wound care: Current insights and future directions. Front. Pharmacol. 2025, 16, 1628252. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Ran, L.Y.; Li, C.Y.; Chen, X.L. Diversity, structures, and collagen-degrading mechanisms of bacterial collagenolytic proteases. Appl. Environ. Microbiol. 2015, 81, 6098–6107. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The effect of pH on the extracellular matrix and biofilms. Adv. Wound Care 2015, 4, 431–439. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).