Biological Management of Soil-Borne Pathogens Through Tripartite Rhizosphere Interactions with Plant Growth-Promoting Fungi
Abstract
1. Introduction
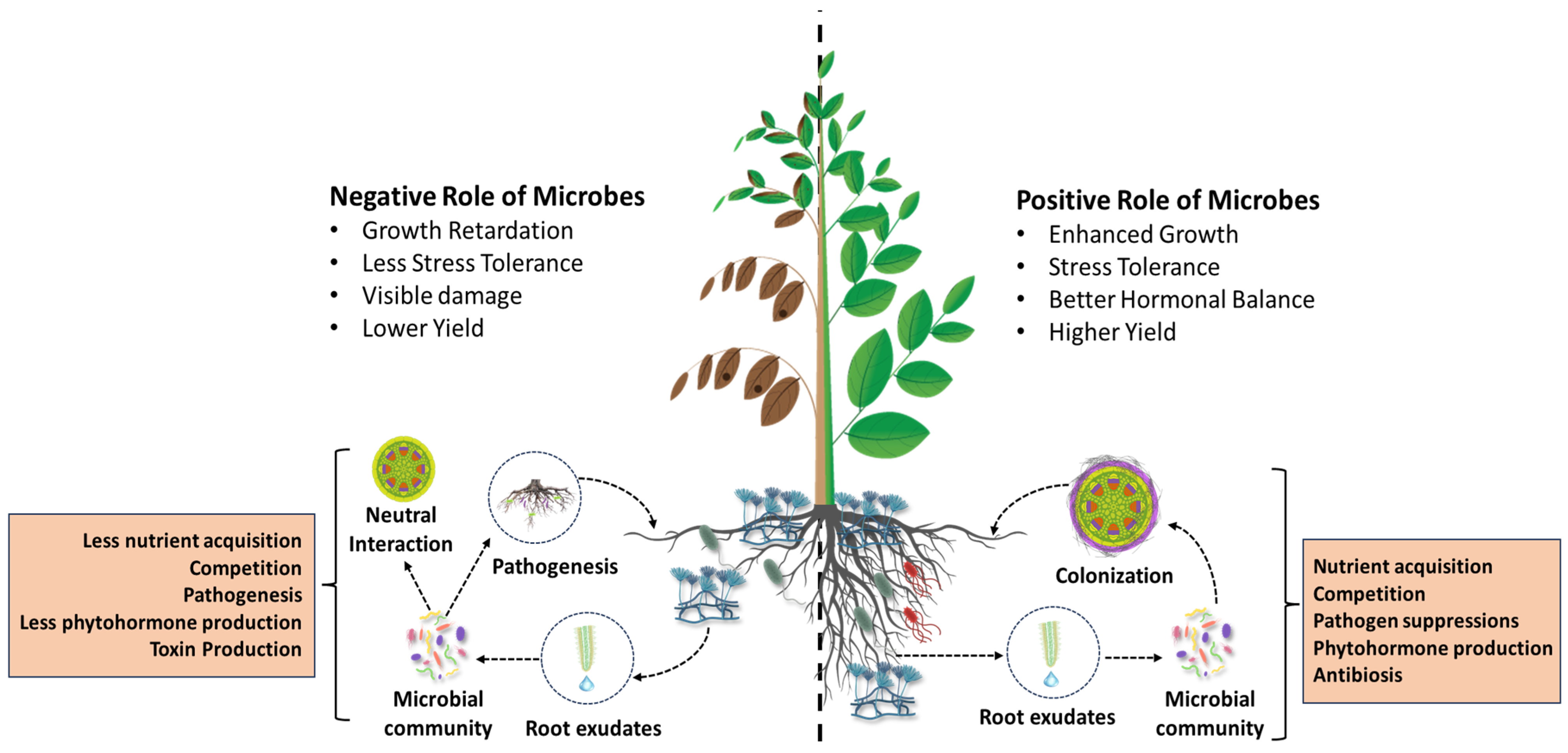
2. The Rhizosphere: A Hotspot of Microbial Interactions
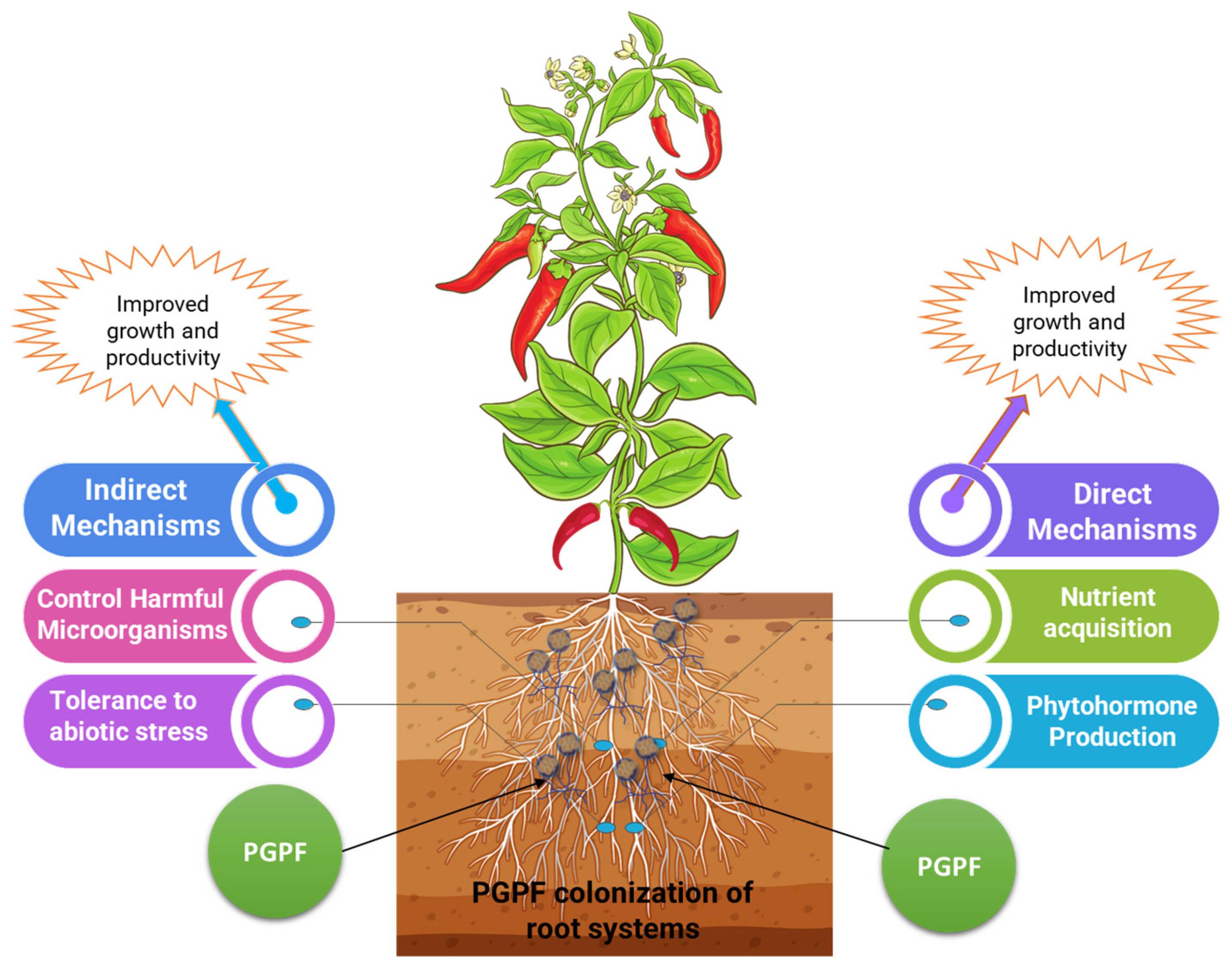
3. Functional and Ecological Roles of PGPF in the Rhizosphere
4. Rhizosphere Disturbance by Soil-Borne Plant Pathogens
5. Diversity and Characteristics of Soil-Borne Pathogens
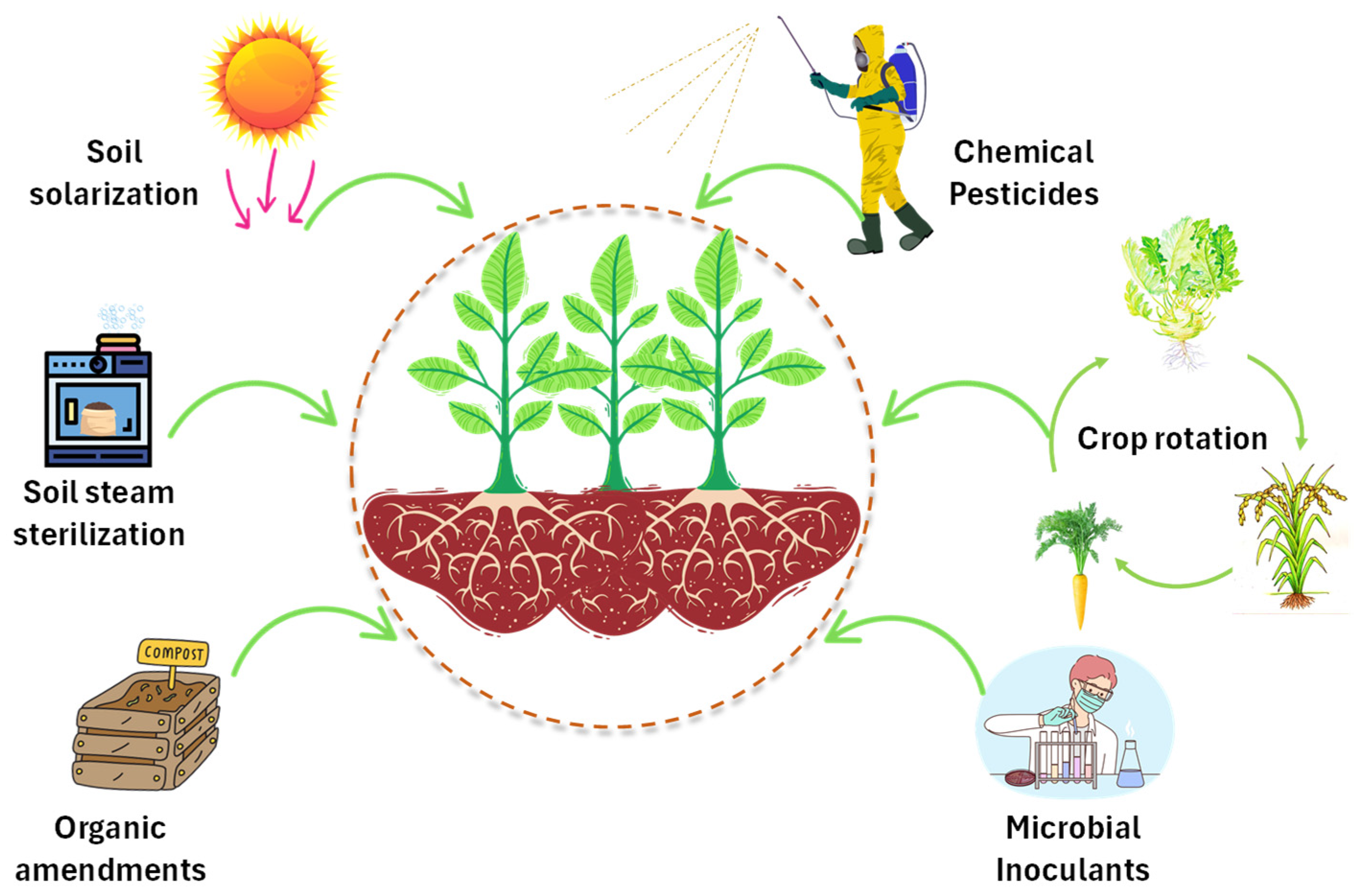
6. Conventional Approaches to Controlling Soil-Borne Pathogens
6.1. Chemical Control
6.2. Crop Rotation
6.3. Soil Sterilization
6.4. Soil Amendment
6.5. Soil Solarization
6.6. Host Resistance
6.7. Microbial Biocontrol
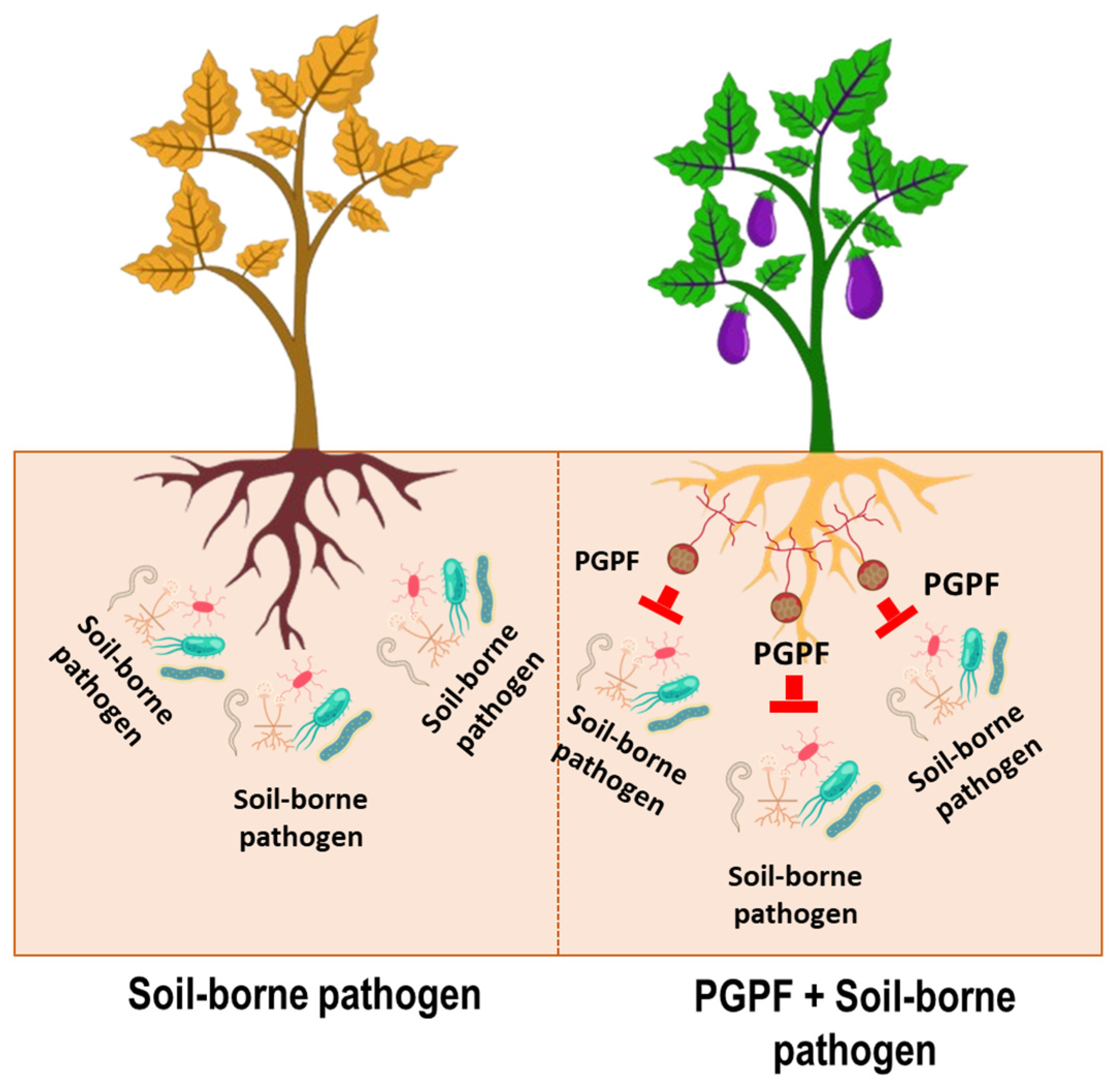
7. Plant Growth-Promoting Fungi in Soil-Borne Disease Suppression
7.1. Soil-Borne Fungal Diseases
| Disease | Pathogen Name | Host Plant | PGPF Species | Effect on Disease | Reference |
|---|---|---|---|---|---|
| Damping off | Rhizoctonia solani | Pea | Trichoderma harzianum T-3 | Inhibited 77.22% mycelial growth and reduced seedling mortality | [44] |
| Perennial ryegrass | T. atroviride T. virens | Increased seedling emergence by 60–150% (T. atroviride) and 35–212% (T. virens) | [45] | ||
| Cucumber | Penicillium viridicatum GP15-1 | Reduced damping-off by 47% and 74% at 0.5% and 1.0% inoculum levels | [43] | ||
| Sclerotinia trifoliorum | Red clover | T. atroviride T. hamatum | Enhanced seedling emergence up to 55% and growth up to 10.6 g shoot weight | [45] | |
| Sclerotium rolfsii | Tomato | T. koningii Rifai | Increased seedling emergence by 20% over untreated control | [134] | |
| Tomato | T. asperellum Tri2, Tri3, and Tri6 | Suppressed damping-off by 87–92% | [133] | ||
| Southern blight | Sclerotium rolfsii | Tomato | T. asperellum Tri2, Tri3, and Tri6 | Inhibited mycelial growth by 72.22–83.33% | [133] |
| Stem rot | Sclerotium rolfsii | Sunflower | Penicillium citrinum LWL4 Aspergillus terreus LWL5 | Reduced stem rot severity (quantitative data not reported) | [140] |
| Fusarium wilt | Fusarium udum | Pigeon pea | T. harzianum T-75 | Reduced wilt and wet root rot incidence (percentage not reported) | [160] |
| Fusarium oxysporum f. sp. ciceris | Chickpea | T. harzianum | Inhibited radial growth by 75.89% in vitro | [47,142] | |
| Chickpea | T. asperellum and T. harzianum strains 1 and 2 | Reduced disease incidence by 22.2% and 11.1% and severity by 86–92% | [143] | ||
| Fusarium oxysporum f. sp. lycopersici | Tomato | Rhizoctonia G1, L2, W1, and W7 | Reduced FCRR lesions by 74–93% depending on distance | [153,154] | |
| T. harzianum | Inhibited mycelial growth by 95.18% | [161] | |||
| T. harzianum AMUTH-1 | Enhanced plant growth by 9–28%, increased biomass by 15–21%, and reduced pathogen populations by 88% | [144] | |||
| T. virens | Reduced disease incidence by 54.66% compared to control | [8] | |||
| T. atroviride | Reduced disease incidence by 69% | [162] | |||
| Fusarium oxysporum Fo-B2 | Suppressed wilt by 14–87% under different conditions (growth chamber, greenhouse, field) | [149] | |||
| Penicillium oxalicum | Reduced wilt by 28–72% under greenhouse and field conditions | [163] | |||
| Phytophthora cryptogea | Completely suppressed wilt (100% suppression) | [146] | |||
| Fusarium oxysporum f. sp. lycopersici race 1 | Tomato, cabbage | Penicillium sp. EU0013 | Suppressed disease by 32–78% | [145] | |
| Fusarium oxysporum f. sp. cubense race 1 | Banana | T. asperellum prr2 Trichoderma sp. NRCB3 | Reduced disease incidence by 47% | [150] | |
| Fusarium oxysporum Ro-3 and Ra-1 | Reduced wilt by 80% | [164] | |||
| Fusarium oxysporum f. sp. cubense TR4 | Fusarium oxysporum isolate UPM31P1 | Delayed symptoms by 6 weeks with 95–96% plant survival | [151] | ||
| Fusarium oxysporum f. sp. spinaciae | Spinach | Rhizoctonia G1, L2, W1, and W7 | Reduced lesion development by 55–98% depending on distance | [153,155] | |
| Fusarium oxysporum f. sp. physali | Cape gooseberry | T. virens GI006 | Reduced wilt severity by up to 72% | [165] | |
| Verticillium wilt | Verticillium dahliae | Tomato | Penicillium oxalicum | Reduced wilt incidence by 72% under greenhouse and field conditions | [163] |
| Verticillium dahliae | Eggplant | T. virens HZA14 | Inhibited mycelial growth and conidial germination; reduced disease severity by 96.59% | [152] | |
| Bakanae | Gibberella fujikuroi | Rice | T. asperellum SKT-1 | Suppressed bakanae disease by 95% | [159] |
| Talaromyces flavus (Tf1, Tf2, Tf3), Fusarium equiseti, Fusarium sp., and Trichoderma sp. | Reduced disease by 67–95% depending on the isolate | [158] | |||
| Take-all | Gaeumannomyces graminis var. tritici | Wheat | T. virens (T65, T90, T96, T122), T. koningii T77 | Reduced disease severity by 25–55% | [157] |
| Dry root rot | Macrophomina Phaseolina | Chickpea | T. viride | Reduced root rot incidence by 78–86% | [42] |
7.2. Soil-Borne Oomycete Disease
| Disease | Pathogen Name | Host Type | PGPF Species | Effect on Disease | Reference |
|---|---|---|---|---|---|
| Damping off/Root rot | Pythium aphanidermatum | Tomato | Trichoderma harzianum (Th), T. asperellum (Ta), T. virens (Tvs1), T. virens (Tvs2) and T. virens (Tvs3) | Combined application increased plant survival by 74.5% in the greenhouse, a 57.2% reduction in root rot, and an 87.5% increase in survival in the field | [167] |
| Pythium aphanidermatum | Tomato | T. harzianum | Reduced damping-off up to 74% in the greenhouse and field | [166] | |
| Pythium aphanidermatum | Chinese kale | T. harzianum | Reduced disease incidence by approximately 33.6% compared to the control | [168] | |
| Pythium ultimum | White clover | T. atroviride T. hamatum | Increased seed emergence by 25–42% | [45] | |
| Pythium diclinum | Wheat | G. roseum T. harzianum | Reduced disease incidence by approximately more than 95% in both pre- and post-emergence damping-off. | [51] | |
| Pythium ultimum | Tomato | Penicillium brevicompactum Penicillium solitum strain 1 T. atroviride | Reduced disease incidence in rockwool systems by 32.9%, 54.8%, and 50.4%, respectively | [171] | |
| Phytophthora capsici | Chili | Penicillium striatisporum Pst10 | At seven days, all treatments produced total suppression (100% relative reduction compared to the 95% control). At 14 days, SLCF + Conidia + CPM produced the highest reductions (92% reduction) and SLCF + Conidia (85% reduction), but SLCF alone produced 80% and conidia alone produced 72%. | [52] | |
| Aphanomyces euteiches | Lentil | T. harzianum T-21 | 48% inhibition of Aphanomyces euteiches mycelial growth | [175] |
7.3. Suppression of Soil-Borne Bacterial Pathogens
7.4. Soil-Borne Nematode Diseases
7.5. Soil-Borne Protist Diseases
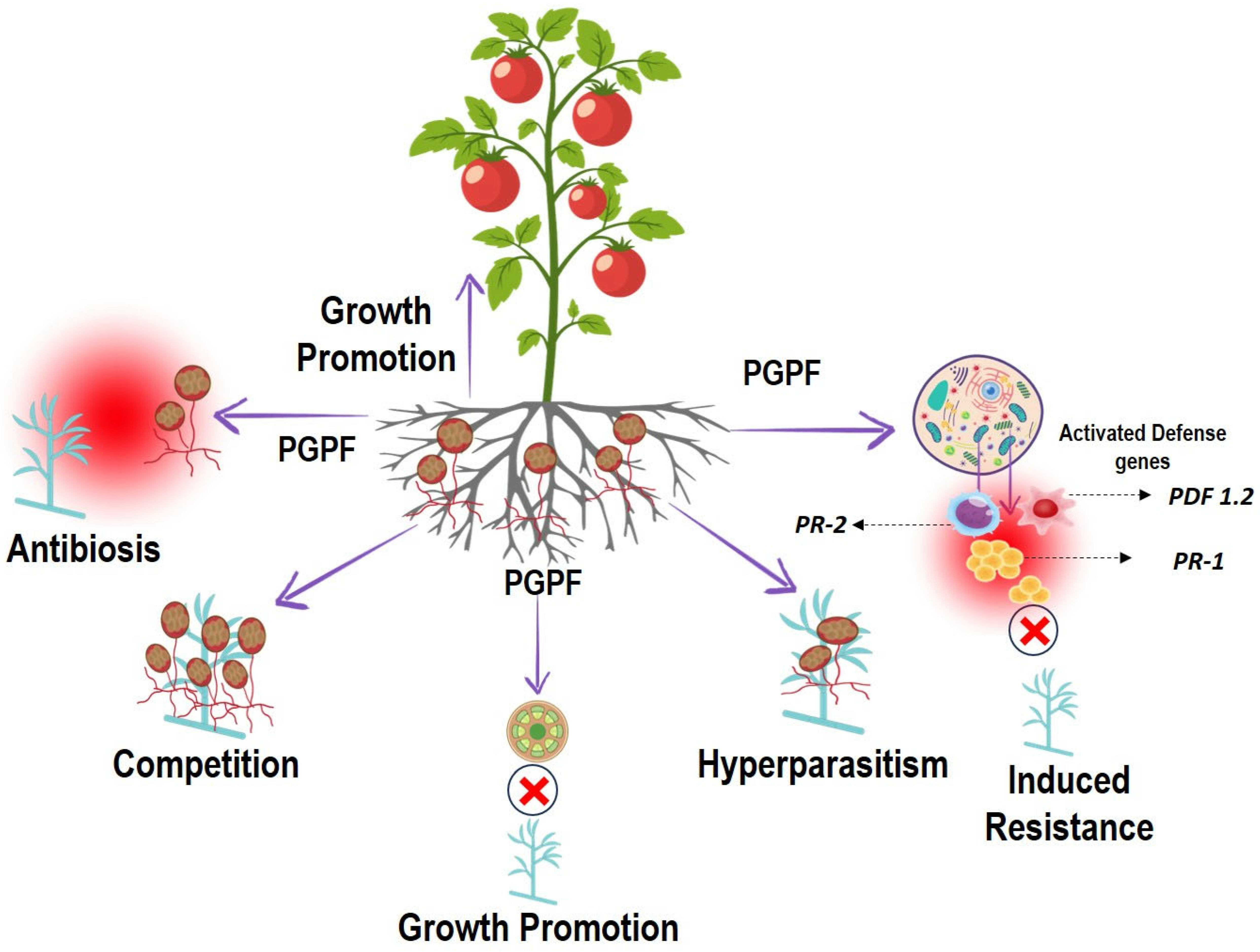
8. Mechanisms of PGPF-Mediated Soil-Borne Disease Suppression
8.1. Antibiosis
8.2. Hyperparasitism
8.3. Competition
8.4. Plant Growth Promotion
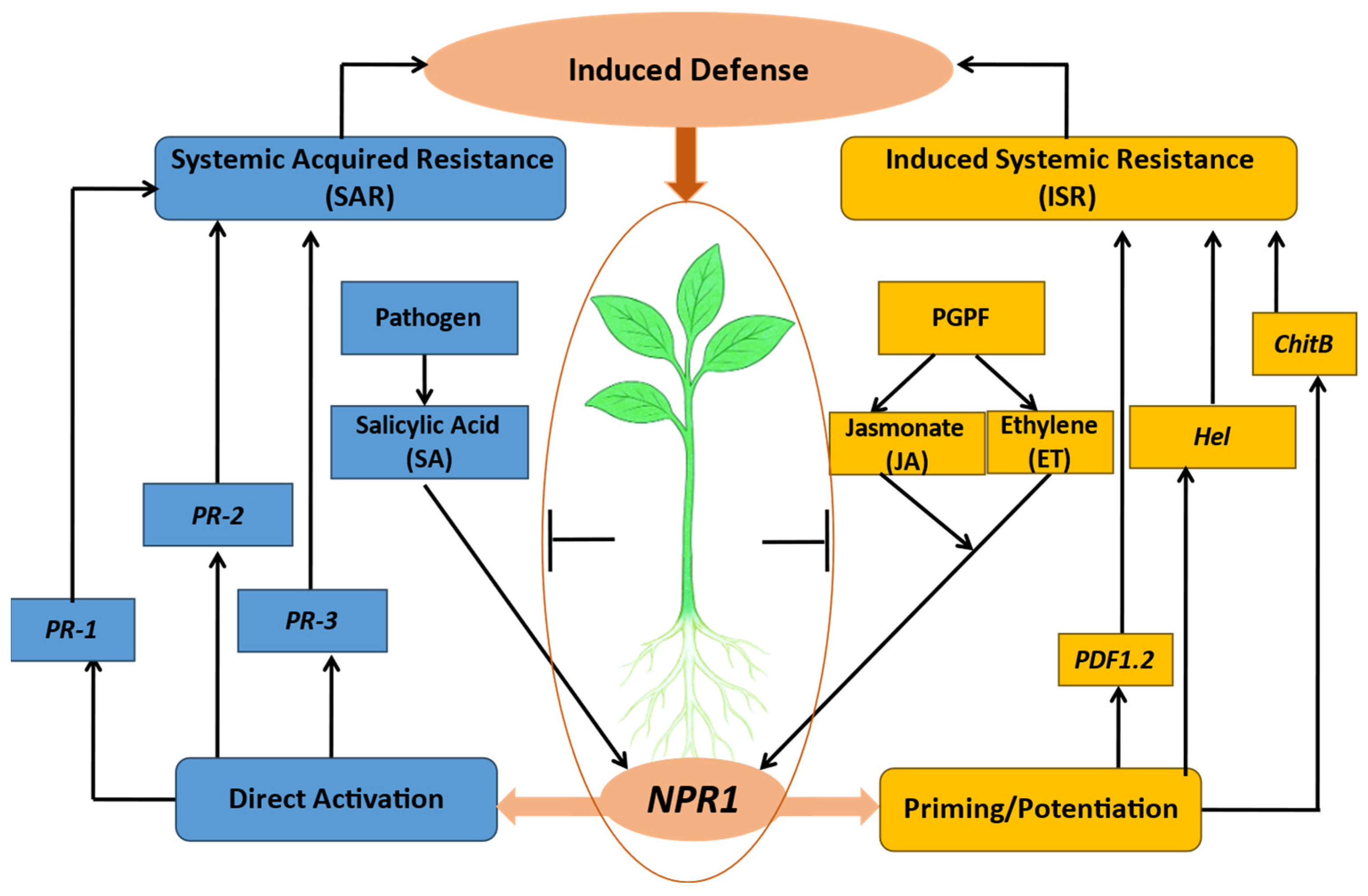
8.5. Induction of Systemic Resistance
9. Rhizosphere Competence: A Key Trait of Effective PGPF Biocontrol Agents
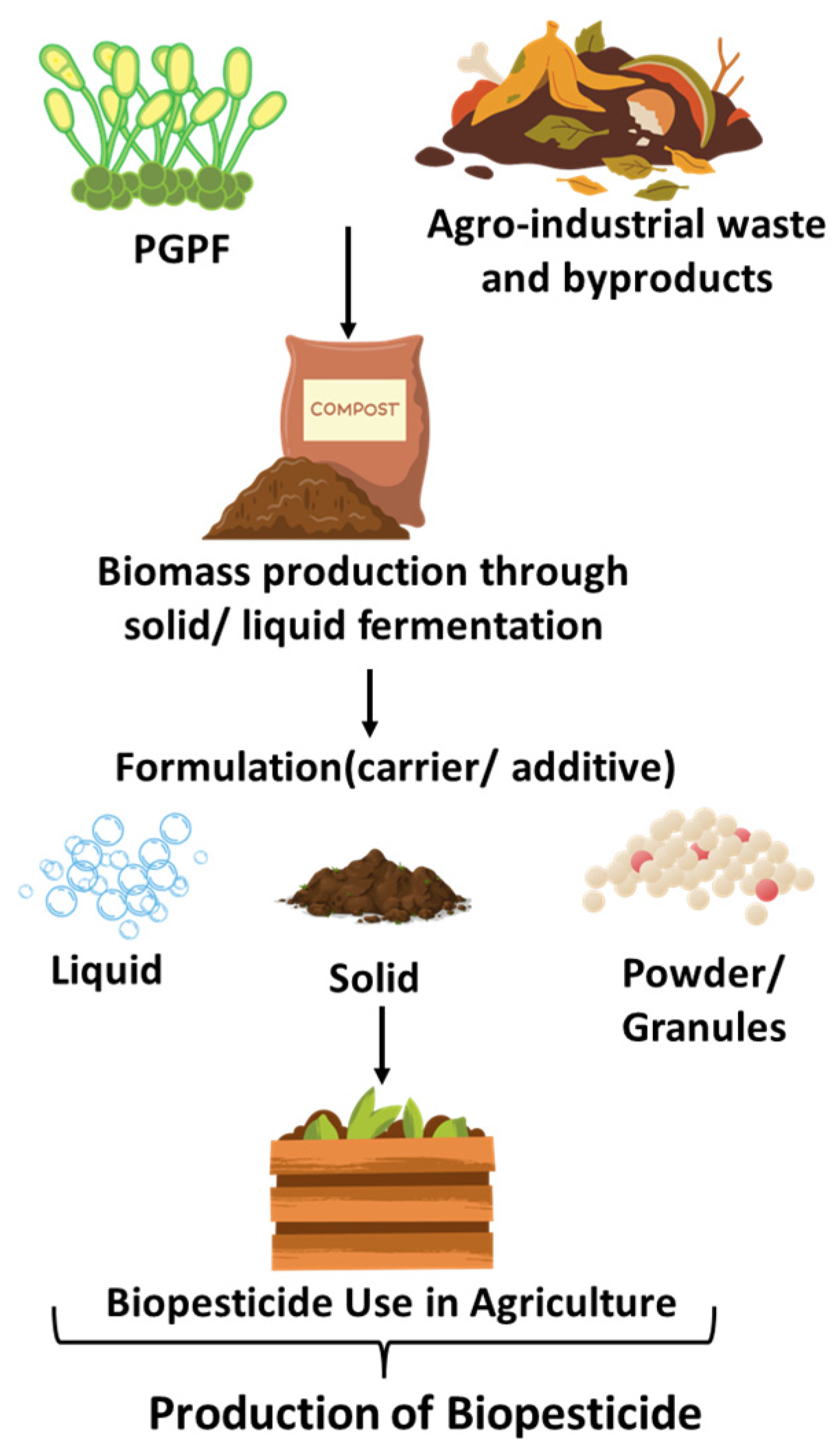
10. Mass Production and Formulation Strategies for PGPF Delivery
11. Challenges and Future Priorities
12. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. New Standards to Curb the Global Spread of Plant Pests and Diseases; Food and Agriculture Organization: Rome, Italy, 2021; Available online: https://www.fao.org/newsroom/detail/New-standards-to-curb-the-global-spread-of-plant-pests-and-diseases/en (accessed on 19 March 2025).
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evolut. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Zeng, K.; Chen, Z. Editorial: Postharvest disease management in fruits and vegetables: Recent advances and mechanisms. Front. Microbiol. 2023, 14, 1203010. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Sultana, F.; Mostafa, M.; Ferdus, H.; Rahman, M.; Rana, J.A.; Al Sabbir, M.A. Plant disease dynamics in a changing climate: Impacts, molecular mechanisms, and climate-informed strategies for sustainable management. Discov. Agric. 2024, 2, 132. [Google Scholar] [CrossRef]
- Tao, X.; Ye, W.; Vetukuri, R.R.; De Vries, S.; Kong, L.; Zhang, M. Editorial: Plant resistance to soil-borne diseases. Front. Plant Sci. 2024, 15, 1369706. [Google Scholar] [CrossRef]
- Niu, B.; Wang, W.; Yuan, Z.; Sederoff, R.R.; Sederoff, H.; Chiang, V.L.; Borriss, R. Microbial Interactions Within Multiple-Strain Biological Control Agents Impact Soil-Borne Plant Disease. Front. Microbiol. 2020, 11, 585404. [Google Scholar] [CrossRef] [PubMed]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for management of soilborne diseases in crop production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- Christopher, D.J.; Raj, T.S.; Rani, S.U.; Udhayakumar, R. Role of defense enzymes activity in tomato as induced by Trichoderma virens against Fusarium wilt caused by Fusarium oxysporum f. sp. lycopersici. J. Biopestic. 2010, 3, 158–162. [Google Scholar]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of Agrochemicals on Soil Microbiota and Management: A Review. Land 2020, 9, 34. [Google Scholar] [CrossRef]
- Rubayet, M.T.; Hossain, M.M. Bio-Exploration of Plant Growth-Promoting Fungus Trichoderma as a Potent Candidate for Plant Disease Management: An Overview. Open J. Biol. Sci. 2025, 25, 22–52. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Islam, S. Plant growth-promoting fungi (PGPF): Phytostimulation and induced systemic resistance. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Singh, D., Singh, H., Prabha, R., Eds.; Springer: Singapore, 2017; Volume 2. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F. Application and mechanisms of plant growth promoting fungi (PGPF) for phytostimulation. In Organic Agriculture; Das, S.K., Ed.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2019, 17, 621–632. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L.; Kannangara, S.D.; Promputtha, I. Fungi vs. Fungi in Biocontrol: An Overview of Fungal Antagonists Applied Against Fungal Plant Pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef]
- Fite, T.; Kebede, E.; Tefera, T.; Bekeko, Z. Endophytic fungi: Versatile partners for pest biocontrol, growth promotion, and climate change resilience in plants. Front. Sustain. Food Syst. 2023, 7, 1322861. [Google Scholar] [CrossRef]
- Jones, D.L.; Hinsinger, P. The rhizosphere: Complex by design. Plant Soil. 2008, 312, 1–6. [Google Scholar] [CrossRef]
- Wang, J.; Miao, W.; Li, S.; Yang, M.; Gao, X. Effect of Nitrogen Fertilizer on the Rhizosphere and Endosphere Bacterial Communities of Rice at Different Growth Stages. Int. J. Mol. Sci. 2024, 25, 13702. [Google Scholar] [CrossRef]
- Cui, H.; Chen, P.; He, C.; Jiang, Z.; Lan, R.; Yang, J. Soil microbial community structure dynamics shape the rhizosphere priming effect patterns in the paddy soil. Sci. Total Environ. 2022, 857, 159459. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F. Genetics of Trichoderma-plant-pathogen interactions. In CRC Press eBooks; CRC Press: Boca Raton, FL, USA, 2024; pp. 243–275. [Google Scholar] [CrossRef]
- Anzuma, A.; Hossain, M.; Mohi-Ud-Din, M.; Nazran, A.; Khan, H.I.; Islam, S.M.N.; Ghosh, T.K. Enhancing drought tolerance in common bean by plant growth promoting rhizobacterium Bacillus amyloliquefaciens tolerance of French Bean. Acta Agric. Sloven. 2024, 120, 18249. [Google Scholar] [CrossRef]
- Hyakumachi, M. Plant-growth-promoting fungi from turfgrass rhizosphere with potential for disease suppression. Soil. Microorganisms. 1994, 44, 53–68. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Kubota, M.; Koyama, H.; Hyakumachi, M. The plant growth-promoting fungus Penicillium simplicissimum GP17-2 induces resistance in Arabidopsis thaliana by activation of multiple defense signals. Plant Cell Physiol. 2007, 48, 1724–1736. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Sultana, F.; Kubota, M.; Hyakumachi, M. Differential inducible defense mechanisms against bacterial speck pathogen in Arabidopsis thaliana by plant-growth-promoting-fungus Penicillium sp. GP16-2 and its cell free filtrate. Plant Soil 2008, 304, 227–239. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Kubota, M.; Koyama, H.; Hyakumachi, M. Systemic resistance to bacterial leaf speck pathogen in Arabidopsis thaliana induced by the culture filtrate of a plant growth-promoting fungus (PGPF) Phoma sp. GS8-1. J. General. Plant Pathol. 2008, 74, 213–221. [Google Scholar] [CrossRef]
- Sultana, F.; Hossain, M.M.; Kubota, M.; Hyakumachi, M. Elicitation of systemic resistance against the bacterial speck pathogen in Arabidopsis thaliana by culture filtrates of plant growth-promoting fungi. Can. J. Plant Pathol. 2008, 30, 196–205. [Google Scholar] [CrossRef]
- Sultana, F.; Hossain, M.M.; Kubota, M.; Hyakumachi, M. Induction of systemic resistance in Arabidopsis thaliana in response to culture filtrate from a plant growth-promoting fungus, Phoma sp. GS8-3. Plant Biol. 2009, 11, 97–104. [Google Scholar] [CrossRef]
- Islam, S.; Akanda, A.M.; Prova, A.; Sultana, F.; Hossain, M.M. Growth promotion effect of Fusarium spp. PPF1 from bermudagrass (Cynodon dactylon) rhizosphere on Indian spinach (Basella alba) seedlings are linked to root colonization. Arch. Phytopathol. Plant Protect. 2014, 47, 2319–2331. [Google Scholar] [CrossRef]
- Islam, S.; Akanda, A.M.; Sultana, F.; Hossain, M.M. Chilli rhizosphere fungus Aspergillus spp. PPA1 promotes vegetative growth of cucumber (Cucumis sativus) plants upon root colonisation. Arch. Phytopathol. Plant Protect. 2014, 47, 1231–1238. [Google Scholar] [CrossRef]
- Kojima, H.; Hossain, M.M.; Kubota, M.; Hyakumachi, M. Involvement of the salicylic acid signaling pathway in the systemic resistance induced in Arabidopsis by plant growth-promoting fungus Fusarium equiseti GF19-1. J. Oleo Sci. 2013, 62, 415–426. [Google Scholar] [CrossRef]
- Shimizu, K.; Hossain, M.M.; Kato, K.; Kubota, M.; Hyakumachi, M. Induction of defense responses in cucumber plants by using the cell-free filtrate of the plant growth-promoting fungus Penicillium simplicissimum GP17-2. J. Oleo Sci. 2013, 62, 613–621. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F. Genetic variation for induced and basal resistance against leaf pathogen Pseudomonas syringae pv. tomato DC3000 among Arabidopsis thaliana accessions. SpringerPlus 2015, 4, 296. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Sultana, F.; Hyakumachi, M. Role of ethylene signalling in growth and systemic resistance induction by the plant growth-promoting fungus Penicillium viridicatum in Arabidopsis. J. Phytopathol. 2017, 165, 432–441. [Google Scholar] [CrossRef]
- Bent, E. Induced Systemic Resistance Mediated by Plant Growth-Promoting Rhizobacteria (PGPR) and Fungi (PGPF). In Multigenic and Induced Systemic Resistance in Plants; Tuzun, S., Bent, E., Eds.; Springer: Boston, MA, USA, 2006; pp. 225–258. [Google Scholar] [CrossRef]
- Katan, J. Diseases caused by soilborne pathogens: Biology, management and challenges. J. Plant Pathol. 2017, 99, 305–315. [Google Scholar] [CrossRef]
- Mihajlović, M.; Rekanović, E.; Hrustić, J.; Tanović, B. Methods for management of soilborne plant pathogens. Pestic. I Fitomed. 2017, 32, 9–24. [Google Scholar] [CrossRef]
- Baysal-Gurel, F.; Kabir, N. Comparative performance of fungicides and biocontrol products in suppression of Rhizoctonia root rot in viburnum. J. Plant Pathol. Microbiol. 2018, 9, 451. [Google Scholar] [CrossRef]
- Mokhtar, M.M.; El-Mougy, N.S. Biocompost application for controlling soilborne plant pathogens—A review. Int. J. Eng. Innov. Technol. 2014, 4, 61–68. [Google Scholar]
- Narayanasamy, P. Soilborne Microbial Plant Pathogens and Disease Management. Volume (1): Nature and Biology; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2020; 300p. [Google Scholar] [CrossRef]
- Turrà, D.; Di Pietro, A. Chemotropic sensing in fungus–plant interactions. Curr. Opin. Plant Biol. 2015, 26, 135–140. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Qi, L.; Mei, X.; Liao, J.; Ding, X.; Deng, W.; Fan, L.; He, X.; Vivanco, J.M.; et al. Plant–Plant–Microbe Mechanisms Involved in Soil-Borne Disease Suppression in a Maize and Pepper Intercropping System. PLoS ONE 2014, 9, e115052. [Google Scholar] [CrossRef] [PubMed]
- Turrà, D.; el Ghalid, M.; Rossi, F.; di Pietro, A. Fungal pathogen uses sex pheromone receptor for chemotropic sensing of host plant signals. Nature 2015, 527, 521–524. [Google Scholar] [CrossRef]
- Manjunatha, S.V.; Naik, M.K.; Khan, M.F.R.; Goswami, R.S. Evaluation of bio-control agents for management of dry root rot of chickpea caused by Macrophomina phaseolina. Crop Protect. 2013, 45, 147–150. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Miyazawa, M.; Hyakumachi, M. The plant growth-promoting fungus Penicillium spp. GP15-1 enhances growth and confers protection against damping-off and anthracnose in cucumber. J. Oleo Sci. 2014, 63, 391–400. [Google Scholar] [CrossRef]
- Akhter, W.; Bhuiyan, M.K.A.; Sultana, F.; Hossain, M.M. Integrated effect of microbial antagonist, organic amendment and fungicide in controlling seedling mortality (Rhizoctonia solani) and improving yield in pea (Pisum sativum L.). Compt. Rend. Biol. 2015, 338, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Kandula, D.R.W.; Jones, E.E.; Stewart, A.; McLean, K.L.; Hampton, J.G. Trichoderma species for biocontrol of soil-borne plant pathogens of pasture species. Biocontrol Sci. Technol. 2015, 25, 1052–1069. [Google Scholar] [CrossRef]
- Luo, X.; Yu, C. First report of damping-off disease caused by Fusarium oxysporum in Pinus massoniana in China. J. Plant Dis. Protect. 2020, 127, 401–409. [Google Scholar] [CrossRef]
- Hossain, M.M.; Hossain, N.; Sultana, F.; Islam, S.M.N.; Islam, M.S.; Bhuiyan, M.K.A. Integrated management of Fusarium wilt of chickpea (Cicer arietinum L.) caused by Fusarium oxysporum f. sp. ciceris with microbial antagonist and botanical extract. Afr. J. Biotechnol. 2013, 12, 12503. [Google Scholar] [CrossRef]
- Kader, M.A.; Mubin, M.M.U.; Rubayet, M.T.; Khan, A.A.; Hossain, M.M. First report of web blight of Lablab purpureus caused by Rhizoctonia solani AG-5 in Bangladesh. New Dis. Rep. 2022, 46, e12129. [Google Scholar] [CrossRef]
- Adhikary, S.; Rahman, M.; Kundu, M.; Hosen, A.E.; Hossain, M. Fusarium Wilt of Banana: Challenges and Resilience. OnLine J. Biol. Sci. 2024, 24, 678–694. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Rubayet, M.T.; Khan, S.; Mostafa, M.; Mishu, N.J.; Sabbir, M.A.A.; Akter, N.; Kabir, A.; Mostofa, M.G. White Mold: A Global Threat to Crops and Key Strategies for Its Sustainable Management. Microorganisms 2025, 13, 4. [Google Scholar] [CrossRef]
- Abdelzaher, H. Occurrence of damping-off of wheat caused by Pythium diclinum Tokunaga in El-Minia, Egypt and its possible control by Gliocladium roseum and Trichoderma harzianum. Arch. Phytopathol. Plant Protect. 2004, 37, 147–159. [Google Scholar] [CrossRef]
- Ma, Y.; Chang, Z.; Zhao, J.; Zhou, M. Antifungal activity of Penicillium striatisporum Pst10 and its biocontrol effect on Phytophthora root rot of chilli pepper. Biol. Control 2008, 44, 24–31. [Google Scholar] [CrossRef]
- Karppinen, E.M.; Payment, J.; Chatterton, S.; Bainard, J.D.; Hubbard, M.; Gan, Y.; Bainard, L.D. Distribution and abundance of Aphanomyces euteiches in agricultural soils: Effect of land use type, soil properties, and crop management practices. Appl. Soil. Ecol. 2020, 150, 103470. [Google Scholar] [CrossRef]
- Kageyama, K.; Asano, T. Life cycle of Plasmodiophora brassicae. J. Plant Growth Regul. 2009, 28, 203–211. [Google Scholar] [CrossRef]
- Hasan, M.; Hossain, M.; Jiang, D. New Endophytic Strains of Trichoderma Promote Growth and Reduce Clubroot Severity of Rapeseed (Brassica napus). PLoS ONE 2023, 18, e0287899. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, B.; Biosca, E.G. Bacteriophage-based bacterial wilt biocontrol for an environmentally sustainable agriculture. Front. Plant Sci. 2017, 8, 1218. [Google Scholar] [CrossRef]
- Opara, E.U.; Asuquo, A.A. An overview of characterization and identification of soft rot bacterium Erwinia in some vegetable crops. Greener J. Biol. Sci. 2016, 6, 46–55. [Google Scholar] [CrossRef]
- Kariuki, C.K.; Mutitu, E.W.; Muiru, W.M. Effect of Bacillus and Trichoderma species in the management of the bacterial wilt of tomato (Lycopersicum esculentum) in the field. Egypt. J. Biol. Pest. Control 2020, 30, 109. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier: London, UK, 2005. [Google Scholar]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.; Gaur, H.S.; Helder, J.; Jones, M.G.; Perry, R.N. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Gomes, V.M.; Souza, R.M.; Almeida, A.M.; Dolinski, C. Relationship between M. Enterolobii and F. Solani: Spatial and temporal dynamics in the occurrence of guava decline. Nematoda 2014, 1, e01014. [Google Scholar] [CrossRef]
- Björsell, P.; Edin, E.; Viketoft, M. Interactions between some plant-parasitic nematodes and Rhizoctonia solani in potato fields. Appl. Soil. Ecol. 2017, 113, 151–154. [Google Scholar] [CrossRef]
- Lot, H.; Campbell, R.N.; Souche, S.; Milne, R.G.; Roggero, P. Transmission of Olpidium brassicae of Mirafiori lettuce mosaic virus and Lettuce big-vein virus and their roles in lettuce big-vein etiology. Phytopathology 2002, 92, 288–293. [Google Scholar] [CrossRef]
- Pavli, O.I.; Prins, M.; Skaracis, G.N. Detection of Beet soil-borne virus and Beet virus Q. in sugar beet in Greece. J. Plant Pathol. 2010, 92, 793–796. [Google Scholar]
- Tan, J.L.; Trandem, N.; Fránová, J.; Hamborg, Z.; Blystad, D.-R.; Zemek, R. Known and potential invertebrate vectors of raspberry viruses. Viruses 2022, 14, 571. [Google Scholar] [CrossRef]
- Jung, T.; Pérez-Sierra, A.; Durán, A.; Horta Jung, M.; Balci, Y.; Scanu, B. Canker and decline diseases caused by soil- and airborne Phytophthora species in forests and woodlands. Persoonia 2018, 40, 182–220. [Google Scholar] [CrossRef]
- Kader, M.A.; Mian, I.H.; Hossain, M.M. Plant parasitic nematodes associated with rhizosphere soils of potato in munshigonj and Bagura districts. Ann. Bangladesh Agric. 2018, 22, 37–49. [Google Scholar]
- Koh, S.H.; Li, H.; Sivasithamparam, K.; Admiraal, R.; Jones, M.G.K.; Wylie, S.J. Low root-to-root transmission of a tobamovirus, yellow tailflower mild mottle virus, and resilience of its virions. Plant Pathol. 2018, 67, 651–659. [Google Scholar] [CrossRef]
- Hewitt, W.B.; Raski, D.J.; Goheen, A.C. Nematode vector of soilborne fan leaf virus of grapevines. Phytopathology 1958, 48, 586–595. [Google Scholar]
- Samaali, B.M.; Loulou, A.; MougouHamdane, A.; Kallel, S. Acquisition and transmission of Grapevine fanleaf virus (GFLV) by Xiphinema index and Xiphinema italiae (Longidoridae). J. Helminthol. 2024, 98, e26. [Google Scholar] [CrossRef] [PubMed]
- Budge, S.P.; Whipps, J.M. Developments in the Use of Chemical and Non-chemical Alternatives to Methyl Bromide for the Control of Soilborne Diseases. In Fumigation and Integrated Pest Management in Vegetable Production; Clay, D.S., Ed.; Springer: Dordrecht, The Netherlands, 2001; pp. 247–268. [Google Scholar] [CrossRef]
- Spasić, R. (Ed.) Pesticidi u Poljoprivredi i Šumarstvu u Srbiji; Društvo za Zaštitu Bilja Srbije: Belgrade, Serbia, 2016. [Google Scholar]
- Bubici, G.; Amenduni, M.; Colella, C.; D’Amico, M.; Cirulli, M. Efficacy of acibenzolar-S-methyl and two strobilurins, azoxystrobin and trifloxystrobin, for the control of corky root of tomato and verticillium wilt of eggplant. Crop Protect. 2006, 25, 814–820. [Google Scholar] [CrossRef]
- Mihajlović, M.; Rekanović, E.; Potočnik, I.; Lević, J. Osetljivost izolata Fusarium graminearum na difenokonazol i protiokonazol u kulturi in vitro. In Zbornik Rezimea Radova X Savetovanja o Zaštiti Bilja, Zlatibor; Društvo za Zaštitu Bilja Srbije: Belgrade, Serbia, 2010; pp. 89–90. [Google Scholar] [CrossRef]
- Rekanović, E.; Potočnik, I.; Milijašević-Marčić, S.; Stepanović, M.; Todorović, B.; Mihajlović, M. Sensitivity of Phytophthora infestans (Mont.) de Bary isolates to fluazinam, fosetyl-Al and propamocarb-hydrochloride. Pestic. Fitomed. 2011, 26, 111–116. [Google Scholar] [CrossRef]
- Windels, C.E.; Brantner, J.R. Early-season application of azoxystrobin to sugarbeet for control of Rhizoctonia solani AG 4 and AG 2-2. J. Sugar Beet Res. 2005, 42, 1–17. Available online: https://assbt.org/wp-content/uploads/2024/02/JSBRVol42No1and2p1to16EarlySeasonAppicationofAzoxystrobintoSugarbeetforControlo-Rhizoctonia.pdf (accessed on 19 March 2025). [CrossRef]
- Benigni, M.; Bompeix, G. Chemical and biological control of Sclerotinia sclerotiorum in witloof chicory culture. Pest Manag. Sci. 2010, 66, 1332–1336. [Google Scholar] [CrossRef]
- Zhou, W.; Li, M.; Achal, V. A comprehensive review on environmental and human health impacts of chemical pesticide usage. Emerg. Contam. 2025, 11, 100410. [Google Scholar] [CrossRef]
- Johnston, H.W.; Celetti, M.J.; Kimpinski, J.; Platt, H.W. Fungal pathogens and Pratylenchus penetrans associated with preceding crops of clovers, winter wheat, and annual ryegrass and their influence on succeeding potato crops on Prince Edward Island. Am. J. Potato Res. 1994, 71, 797–808. [Google Scholar] [CrossRef]
- Larkin, R.P.; Griffin, T.S.; Honeycutt, C.W. Rotation and cover crop effects on soilborne potato diseases, tuber yield, and soil microbial communities. Plant Dis. 2010, 94, 1491–1502. [Google Scholar] [CrossRef]
- Kheyrodin, H. Crop rotations for managing soil-borne plant diseases. Afr. J. Food Sci. Technol. 2010, 1, 1–9. [Google Scholar]
- Carling, D.E.; Baird, R.E.; Gitaitis, R.D.; Brainard, K.A.; Kuninaga, S. Characterization of AG-13, a newly reported anastomosis group of Rhizoctonia solani. Phytopathology 2002, 92, 893–899. [Google Scholar] [CrossRef]
- Van Loenen, M.C.A.; Turbett, Y.; Mullins, C.E.; Feilden, N.E.H. Low temperature-short duration steaming of soil kills soil-borne pathogens, nematode pests and weed. Eur. J. Plant Pathol. 2003, 109, 993–1002. [Google Scholar] [CrossRef]
- Tanaka, S.; Kobayashi, T.; Iwasaki, K.; Yamane, S.; Maeda, K.; Sakurai, K. Properties and metabolic diversity of microbial communities in soils treated with steam sterilization compared with methyl bromide and chloropicrin fumigations. Soil Sci. Plant Nutr. 2003, 49, 603–610. [Google Scholar] [CrossRef]
- Afek, U.; Orenstein, J. Disinfecting potato tubers using steam treatments. Can. J. Plant Pathol. 2002, 24, 36–39. [Google Scholar] [CrossRef]
- Fennimore, S.A.; Martin, F.N.; Miller, T.C.; Broome, J.C.; Dorn, N.; Greene, I. Evaluation of a mobile steam applicator for soil disinfestation in California strawberry. HortScience 2014, 49, 1542–1549. [Google Scholar] [CrossRef]
- Kokalis-Burelle, N.; Rosskopf, E.N.; Butler, D.M.; Fennimore, S.A.; Holzinger, J. Evaluation of steam and soil solarization for Meloidogyne arenaria control in Florida floriculture crops. J. Nematol. 2016, 48, 183–192. [Google Scholar] [CrossRef]
- Gutierrez, W.A.; Shew, H.D.; Melton, T.A. Sources of inoculum and management for Rhizoctonia solani damping-off on tobacco transplants under greenhouse conditions. Plant Dis. 1997, 81, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Minuto, G.; Gilardi, G.; Kejji, S.; Gullino, M.L.; Garibaldi, A. Effect of physical nature of soil and humidity on stream disinfestation. Acta Hortic. 2005, 698, 257–262. [Google Scholar] [CrossRef]
- Luvisi, A.; Panattoni, A.; Materazzi, A. Heat treatments for sustainable control of soil viruses. Agron. Sustain. Dev. 2015, 35, 657–666. [Google Scholar] [CrossRef]
- Samtani, J.B.; Gilbert, C.; Ben Weber, J.; Subbarao, K.V.; Goodhue, R.E.; Fennimore, S.A. Effect of steam and solarization treatments on pest control, strawberry yield, and economic returns relative to methyl bromide fumigation. HortScience 2012, 47, 64–70. [Google Scholar] [CrossRef]
- Paret, M.L.; Cabos, R.; Kratky, B.A.; Alvarez, A.M. Effect of plant essential oils on Ralstonia solanacearum race 4 and bacterial wilt of edible ginger. Plant Dis. 2010, 94, 521–527. [Google Scholar] [CrossRef]
- Auger, J.; Arnault, I.; Diwo-Allain, S.; Ravier, M.; Molia, F.; Pettiti, M. Insecticidal and fungicidal potential of Allium products and substances as biofumigants. Agroindustria 2004, 3, 367–370. [Google Scholar]
- Shafique, H.A.; Sultana, V.; Ara, J.; Ehteshamul-Haque, S.; Athar, M. Role of antagonistic microorganisms and organic amendment in stimulating the defense system of okra against root rotting fungi. Pol. J. Microbiol. 2016, 65, 195–203. [Google Scholar] [CrossRef]
- Welke, S.E. The effect of compost extract on the yield of strawberries and the severity of Botrytis cinerea. J. Sustain. Agric. 2005, 25, 57–68. [Google Scholar] [CrossRef]
- Brtnický, M.; Hojka, V.; Koutník, I.; Juřička, D.; Galiová, M.V.; Hladík, J.; Brtnická, H. Long-term effects of biochar-based organic amendments on soil microbial parameters. Agronomy 2019, 9, 747. [Google Scholar] [CrossRef]
- Jaiswal, S.; Jaiswal, S.K.; O’Toole, G.J.L. Biochar and Trichoderma harzianum for the management of damping-off caused by Pythium aphanidermatum in cucumber seedlings. Biomass Bioenergy 2019, 122, 407–417. [Google Scholar] [CrossRef]
- Yulianti, T.; Sivasithamparam, K.; Turner, D.W. Saprophytic growth of Rhizoctonia solani Kühn AG2-1 (ZG5) in soil amended with fresh green manures affects the severity of damping-off in canola. Soil. Biol. Biochem. 2006, 38, 923–930. [Google Scholar] [CrossRef]
- Klein, E.; Katan, J.; Austerweil, M.; Gamliel, A. Controlled laboratory system to study soil solarization and organic amendment effects on plant pathogens. Phytopathology 2007, 97, 1476–1483. [Google Scholar] [CrossRef]
- Colla, P.; Gilardi, G.; Gullino, M.L. A review and critical analysis of the European situation of soilborne disease management in the vegetable sector. Phytoparasitica 2012, 40, 515–523. [Google Scholar] [CrossRef]
- Sullivan, D.M.; Miller, R.O. Compost quality attributes, measurements, and variability. In Compost Utilization in Horticultural Cropping Systems; Stofella, P.J., Kahn, B.A., Eds.; Lewis Publishers: Boca Raton, FL, USA, 2001; pp. 95–120. [Google Scholar]
- Scheuerell, S.J.; Mahaffee, W.F. Compost tea: Principles and prospects for plant disease control. Compost. Sci. Utilizat. 2005, 13, 31–58. [Google Scholar] [CrossRef]
- Frenkel, O.; Jaiswal, A.K.; Elad, Y.; Lew, B.; Graber, E.R. The effect of biochar on plant diseases: What should we learn while designing biochar substrates? J. Environ. Eng. Landsc. Manag. 2017, 25, 105–113. [Google Scholar] [CrossRef]
- Termorshuizen, A.J.; van Rijn, E.; van der Gaag, D.; Alabouvette, C.; Chen, Y.; Lagerlöf, J.; Malandrakis, A.A.; Paplomatas, E.J.; Rämert, B.; Ryckeboer, J.; et al. Suppressiveness of 18 composts against 7 pathosystems: Variability in pathogen response. Soil Biol. Biochem. 2006, 38, 2461–2477. [Google Scholar] [CrossRef]
- Baysal-Gurel, F.; Gardener, B.M.; Miller, S.A. Soilborne Disease Management in Organic Vegetable Production. 2012. Available online: https://eorganic.org/node/7581 (accessed on 4 September 2025).
- Baysal-Gurel, F.; Kabir, N.; Liyanapathiranage, P. Effect of organic inputs and solarization for the suppression of Rhizoctonia solani in woody ornamental plant production. Plants 2019, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Gutkowski, D.; Terranova, S. Physical aspects of soil solarization. In Proceedings of the First International Conference on Soil Solarization, Amman, Jordan, 19–25 February 1990; FAO Plant Production and Protection Paper. DeVay, J.E., Stapleton, J.J., Elmore, C.L., Eds.; FAO: Rome, Italy, 1990; Volume 109, pp. 48–61. [Google Scholar]
- Al-Kayssi, A.W.; Al-Karaghouli, A. A new approach for soil solarization by using paraffin-wax emulsion as a mulching material. Renew. Energy. 2002, 26, 637–648. [Google Scholar] [CrossRef]
- Zheng, Y.; Yanful, E.K.; Bassi, A.S. A review of plastic waste biodegradation. Crit. Rev. Biotechnol. 2005, 25, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Watanabe, S.; Ozaki, H.; Ikeura, Y.; Kotani, A. Soil temperature and moisture environments: Lot-management water requirements associated with soil solarization. Farm. Agric. 2011, 631, 2–10. [Google Scholar]
- Dai, Y.Y.; Kondo, M.; Ito, K.; Yoshiyama, K.; Zhang, P.F.; Zhang, F.P.; Senge, M. Study on irrigation water requirements for the control of Ralstonia solanacearum via soil solarization in managing tomato cultivation. J. Irrig. Drain. Rural Eng. 2014, 294, 85–92. [Google Scholar]
- Larkin, R.P.; Honeycutt, C.W. Effects of different 3-year cropping systems on soil microbial communities and Rhizoctonia diseases of potato. Phytopathology 2006, 96, 68–79. [Google Scholar] [CrossRef]
- Larkin, R.P.; Griffin, T.S. Control of soilborne diseases of potato using Brassica green manures. Crop Prot. 2007, 26, 1067–1077. [Google Scholar] [CrossRef]
- Davis, J.R.; Pavek, J.J.; Corsini, D.L.; Sorensen, L.H. Stability of Verticillium resistance of potato clones and changes in soilborne populations with potato monoculture. In Proceedings of the Soil-Borne Diseases of 4th International Congress of Plant Pathology, Melbourne, Australia, 17–24 August 1985; pp. 165–166. [Google Scholar]
- Dong, O.X.; Ronald, P.C. Genetic engineering for disease resistance in plants: Recent progress and future perspectives. Plant Physiol. 2019, 180, 26–38. [Google Scholar] [CrossRef]
- Christou, P. Plant genetic engineering and agricultural biotechnology 1983–2013. Trends Biotechnol. 2013, 31, 125–127. [Google Scholar] [CrossRef]
- Bruton, B. Grafting watermelon onto squash or gourd rootstock makes firmer, healthier fruit. Agric. Res. 2005, 53, 8–9. [Google Scholar]
- Rouphael, Y.; Kyriacou, M.C.; Colla, G. Vegetable grafting: A toolbox for securing yield stability under multiple stress conditions. Front. Plant Sci. 2018, 8, 2255. [Google Scholar] [CrossRef]
- Stitger, H.C.M. Some aspects of the physiological functioning of the graft muskmelon/Cucurbita ficifolia. Publ./Cent. Plant Physiol. Res. 1971, 65, 223–231. [Google Scholar]
- Edelstein, M. Grafting vegetable-crop plants: Pros and Cons. Acta Hort. 2004, 659, 235–238. [Google Scholar] [CrossRef]
- Tomprefa, N.; Hill, R.; Whipps, J.; McQuilken, M. Some environmental factors affect growth and antibiotic production by mycoparasite Coniothyrium minitans. Biocontrol Sci. Technol. 2011, 21, 721–731. [Google Scholar] [CrossRef]
- Mazzola, M.; Freilich, S. Prospects for biological soilborne disease control: Application of indigenous versus synthetic microbiomes. Phytopathology 2017, 107, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.L.F.; Roberts, D.P.; Chitwood, D.J.; Carta, L.K.; Lumsden, R.D.; Mao, W. Application of Burkholderia cepacia and Trichoderma virens, alone and in combination, against Meloidogyne incognita on bell pepper. Nematropica 2001, 31, 75–86. [Google Scholar]
- Loganathan, M.; Sible, G.V.; Maruthasalam, S.; Saravanakumar, D.; Raguchander, T.; Sivakumar, M.; Samiyappan, R. Trichoderma and chitin mixture based bioformulation for the management of head rot (Sclerotinia sclerotiorum (Lib.) de Bary)–root-knot (Meloidogyne incognita Kofoid and White; Chitwood) complex diseases of cabbage. Arch. Phytopathol. Plant Prot. 2010, 43, 1011–1024. [Google Scholar] [CrossRef]
- Kowsari, M.; Motallebi, M.; Zamani, R.M. Construction of new GFP-tagged fusants for Trichoderma harzianum with enhanced biocontrol activity. J. Plant Prot. Res. 2014, 54, 122–131. [Google Scholar] [CrossRef]
- Smolińska, U.; Kowalska, B. Biological control of the soil-borne fungal pathogen Sclerotinia sclerotiorum—A review. J. Plant Pathol. 2018, 100, 1–12. [Google Scholar] [CrossRef]
- Bitsadze, N.; Siebold, M.; Koopmann, B.; von Tiedemann, A. Single and combined colonization of Sclerotinia sclerotiorum sclerotia by the fungal mycoparasites Coniothyrium minitans and Microsphaeropsis ochracea. Plant Pathol. 2015, 64, 690–700. [Google Scholar] [CrossRef]
- Henis, Y.; Papavizas, G.C. Factors affecting susceptibility of Sclerotium rolfsii sclerotia to Trichoderma harzianum in natural soil. Phytopathology 1982, 72, 1010. [Google Scholar]
- Henis, Y.; Papavizas, G.C. Factors affecting germinability and susceptibility to attack of sclerotia of Sclerotium rolfsii by Trichoderma harzianum in field soil. Phytopathology 1983, 73, 1469–1474. [Google Scholar] [CrossRef]
- Ji, S.H.; Paul, N.C.; Deng, J.X.; Kim, Y.S.; Yun, B.; Yu, S.H. Biocontrol activity of Bacillus amyloliquefaciens CNU114001 against fungal plant disease. Mycobiology 2013, 41, 234–242. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.D.; Liu, C.X.; Yuan, J.H.; Wang, X.J.; Xiang, W.S. A new prenylated indole derivative from endophytic actinobacteria Streptomyces sp. neau-D50. Nat. Prod. Res. 2014, 28, 31–437. [Google Scholar] [CrossRef]
- Berta, G.; Sampo, S.; Gamalero, E.; Massa, N.; Lemanceau, P. Suppression of Rhizoctonia root-rot of tomato by Glomus mossae BEG12 and Pseudomonas fluorescens A6RI is associated with their effect on the pathogen growth and on the root morphogenesis. Eur. J. Plant Pathol. 2005, 111, 70–288. [Google Scholar] [CrossRef]
- Mishu, N.J.; Hasan, R.; Islam, S.M.N.; Nayeema, J.; Hossain, M. Additive effects of Trichoderma isolates for enhancing growth, suppressing southern blight and modulating plant defense enzymes in tomato. PLoS ONE 2025, 20, e0329368. [Google Scholar] [CrossRef]
- Tsahouridou, P.C.; Thanassoulopoulos, C.C. Proliferation of Trichoderma koningii in the tomato rhizosphere and the suppression of damping-off by Sclerotium rolfsii. Soil. Biol. Biochem. 2002, 34, 767–777. [Google Scholar] [CrossRef]
- Lumsden, R.D.; Knauss, J.F. Commercial development of Trichoderma virens for damping-off disease. In Biological Control: A Global Perspective; Vincent, C., Goettel, M.S., Lazarovits, G., Eds.; CABI International: Wallingford, UK, 2007; pp. 203–210. [Google Scholar] [CrossRef]
- Kumar, S. Integrated management of maydis leaf blight of maize. Ann. Plant Prot. Sci. 2010, 18, 536–537. [Google Scholar]
- Kumar, S.; Upadhyay, J.P.; Rani, A. Evaluation of Trichoderma species against Fusarium udum Butler causing wilt of pigeon pea. J. Biol. Control 2009, 23, 329–332. [Google Scholar] [CrossRef]
- Reddy, K.; Krishnamma; Narayana, P. Efficacy of Trichoderma viride against Colletotrichum falcatum in sugarcane. Ind. J. Plant Protect. 2009, 37, 111–115. [Google Scholar]
- Srivastava, R.K.; Singh, R.K.; Kumar, N.; Singh, S. Management of Macrophomina disease complex in jute (Corchorus olitorius) by Trichoderma viride. J. Biol. Control 2010, 24, 77–79. [Google Scholar]
- Waqas, M.; Khan, A.L.; Hamayun, M.; Shahzad, R.; Kang, S.M.; Kim, J.G.; Lee, I.J. Endophytic fungi promote plant growth and mitigate the adverse effects of stem rot: An example of Penicillium citrinum and Aspergillus terreus. J. Plant Interact. 2015, 10, 280–287. [Google Scholar] [CrossRef]
- Pascual, C.B.; Raymundo, A.D.; Hyakumachi, M. Efficacy of hypovirulent binucleate Rhizoctonia sp. to control banded leaf and sheath blight in corn. J. General. Plant Pathol. 2000, 66, 95–102. [Google Scholar] [CrossRef]
- Dubey, S.C.; Suresh, M.; Singh, B. Evaluation of Trichoderma species against Fusarium oxysporum f. sp. ciceris for integrated management of chickpea wilt. Biol. Control 2007, 40, 118–127. [Google Scholar] [CrossRef]
- Chohan, S.A.; Akbar, M.; Iqbal, U. Trichoderma based formulations control the wilt disease of chickpea (Cicer arietinum L.) caused by Fusarium oxysporum f. sp. ciceris, better when inoculated as consortia: Findings from pot experiments under field conditions. PeerJ 2024, 12, e17835. [Google Scholar] [CrossRef]
- Haque, Z.; Pandey, K.; Zamir, S. Bio-management of Fusarium wilt of tomato (Fusarium oxysporum f. sp. lycopersici) with multifacial Trichoderma species. Discov. Agric. 2023, 1, 7. [Google Scholar] [CrossRef]
- Alam, S.S.; Sakamoto, K.; Inubushi, K. Biocontrol efficiency of Fusarium wilt diseases by a root-colonizing fungus Penicillium sp. Soil. Sci. Plant Nutr. 2011, 57, 204–212. [Google Scholar] [CrossRef]
- Attitalla, I.H.; Johnson, P.; Brishammar, S.; Quintanilla, P. Systemic resistance to Fusarium wilt in tomato induced by Phytophthora cryptogea. J. Phytopathol. 2001, 149, 373–380. [Google Scholar] [CrossRef]
- Larkin, R.P.; Fravel, D.R. Efficacy of various fungal and bacterial biocontrol organisms for control of Fusarium wilt of tomato. Plant Dis. 1998, 82, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Duijff, B.J.; Recorbet, G.; Bakker, P.A.H.M.; Loper, J.E.; Lemanceau, P. Microbial antagonism at the root level is involved in the suppression of Fusarium wilt by the combination of nonpathogenic Fusarium oxysporum Fo47 and Pseudomonas putida WCS358. Phytopathology 1999, 89, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Shishido, M.; Miwa, C.; Usami, T.; Amemiya, Y.; Johnson, K.B. Biological control efficacy of Fusarium wilt of tomato by nonpathogenic Fusarium oxysporum Fo-B2 in different environments. Phytopathology 2005, 95, 1072–1080. [Google Scholar] [CrossRef]
- Thangavelu, R.; Gopi, M. Combined application of native Trichoderma isolates possessing multiple functions for the control of Fusarium wilt disease in banana cv. Grand Naine. Biocontrol Sci. Technol. 2015, 25, 1147–1164. [Google Scholar] [CrossRef]
- Ting, A.S.Y.; Sariah, M.; Kadir, J.; Gurmit, S. Field evaluation of non-pathogenic Fusarium oxysporum isolates UPM31P1 and UPM39B3 for the control of Fusarium wilt in ‘Pisang Berangan’ (Musa, AAA). Acta Hortic. 2009, 828, 139–144. [Google Scholar] [CrossRef]
- Tomah, A.A.; Alamer, I.S.A.; Khattak, A.A.; Ahmed, T.; Hatamleh, A.A.; Al-Dosary, M.A.; Ali, H.M.; Wang, D.; Zhang, J.; Xu, L.; et al. Potential of Trichoderma virens HZA14 in Controlling Verticillium Wilt Disease of Eggplant and Analysis of Its Genes Responsible for Microsclerotial Degradation. Plants 2023, 12, 3761. [Google Scholar] [CrossRef]
- Muslim, A.; Hyakumachi, M.; Kageyama, K.; Suwandi, S.; Pratama, R. A rapid bioassay to evaluate efficacy of hypovirulent binucleate Rhizoctonia in reducing Fusarium crown and root rot of tomato. Open Agric. J. 2019, 13, 27–33. [Google Scholar] [CrossRef]
- Muslim, A.; Horinouchi, H.; Hyakumachi, M. Biological control of Fusarium wilt of tomato with hypovirulent binucleate Rhizoctonia in greenhouse conditions. Mycoscience 2003, 44, 77–84. [Google Scholar] [CrossRef]
- Muslim, A.; Horinouchi, H.; Hyakumachi, M. Suppression of Fusarium wilt of spinach with hypovirulent binucleate Rhizoctonia. J. General Plant Pathol. 2003, 69, 143–150. [Google Scholar] [CrossRef]
- Méndez, I.; Fallard, A.; Soto, I.; Tortella, G.; de la Luz Mora, M.; Valentine, A.J.; Barra, P.J.; Duran, P. Efficient Biocontrol of Gaeumannomyces graminis var. tritici in Wheat: Using bacteria Isolated from suppressive soils. Agronomy 2021, 11, 2008. [Google Scholar] [CrossRef]
- Zafari, D.; Koushki, M.M.; Bazgir, E. Biocontrol evaluation of wheat take-all disease by Trichoderma-screened isolates. Afr. J. Biotechnol. 2008, 7, 3653–3659. [Google Scholar]
- Rawat, K.; Tripathi, S.B.; Kaushik, N.; Bashyal, B.M. Management of bakanae disease of rice using biocontrol agents and insights into their biocontrol mechanisms. Arch. Microbiol. 2022, 204, 401. [Google Scholar] [CrossRef]
- Watanabe, S.; Kunakura, K.; Izawa, N.; Nagayama, K.; Mitachi, T.; Kanamori, M.; Teraoka, T.; Arie, T. Mode of action of Trichoderma asperellum SKT-1, a biocontrol agent against Gibberella fujikuroi. J. Pestic. Sci. 2007, 32, 222–228. [Google Scholar] [CrossRef]
- Prasad, R.D.; Rangeshwaran, R.; Anuroop, C.P.; Rashmi, H.J. Biological control of wilt and root rot of chickpea under field conditions. Ann. Plant Prot. Sci. 2002, 10, 72–75. [Google Scholar]
- Basco, M.J.; Bisen, K.; Keswani, C.; Singh, H.B. Biological management of fusarium wilt of tomato using biofortified vermicompost. Mycosphere 2017, 8, 467–483. [Google Scholar] [CrossRef]
- Ghazalibiglar, H.; Kandula, D.R.W.; Hampton, J.G. Biological control of fusarium wilt of tomato by Trichoderma isolates. N. Z. Plant Protect. 2016, 69, 57–63. [Google Scholar] [CrossRef]
- Larena, I.; Pascual, S.; Melgarejo, P.; De Cal, A. Biocontrol of Fusarium and Verticillium wilt of tomato by Penicillium oxalicum under greenhouse and field conditions. J. Phytopathol. 2003, 151, 507–512. [Google Scholar] [CrossRef]
- Thangavelu, R.; Jayanthi, A. RFLP analysis of rDNA-ITS regions of native non-pathogenic Fusarium oxysporum isolates and their field evaluation for the suppression of Fusarium wilt disease of banana. Australas. Plant Pathol. 2009, 38, 13–21. [Google Scholar] [CrossRef]
- Izquierdo-García, L.F.; González-Almario, A.; Cotes, A.M.; Moreno-Velandia, C.A. Trichoderma virens Gl006 and Bacillus velezensis Bs006: A compatible interaction controlling Fusarium wilt of cape gooseberry. Sci. Rep. 2020, 10, 6857. [Google Scholar] [CrossRef]
- Jayaraj, J.; Radhakrishnan, N.V.; Velazhahan, R. Development of formulations of Trichoderma harzianum strain M1 for control of damping-off of tomato caused by Pythium aphanidermatum. Arch. Phytopathol. Plant Protect. 2006, 39, 1–8. [Google Scholar] [CrossRef]
- Elshahawy, I.E.; El-Mohamedy, R.S. Biological control of Pythium damping-off and root-rot diseases of tomato using Trichoderma isolates employed alone or in combination. J. Plant Pathol. 2019, 101, 597–608. [Google Scholar] [CrossRef]
- Kanjanamaneesathian, M.; Phetcharat, V.; Pengnoo, A.; Upawan, S. Use of Trichoderma harzianum cultured on ground mesocarp fibre of oil-palm as seed treatment to control Pythium aphanidermatum, a causal agent of damping-off of Chinese kale seedling. World J. Microbiol. Biotechnol. 2003, 19, 825–829. [Google Scholar] [CrossRef]
- Neelamegam, R.; Govindarajalu, T. Integrated application of Trichoderma viride Pers: Fr. and farm yard manure to control damping-off of tomato (Lycopersicum esculentum Mill.). J. Biol. Control 2002, 16, 65–69. [Google Scholar]
- Le, H.T.; Black, L.L.; Sikora, R.A. Evaluation of Trichoderma spp. for biocontrol of tomato sudden death caused by Pythium aphanidermatum following flooding in tropical hot season. Commun. Agric. Appl. Biol. Sci. 2003, 68, 463–474. [Google Scholar]
- Gravel, V.; Martinez, C.; Antoun, H.; Tweddell, R.J. Control of greenhouse tomato root rot (Pythium ultimum) in hydroponic systems, using plant-growth-promoting microorganisms. Can. J. Plant Pathol. 2006, 28, 475–483. [Google Scholar] [CrossRef]
- Islam, M.H.; Shanta, S.S.; Hossain, M.I.; Hossain, M.A.; Hossain, M.M.; Rahaman, E.H.M.S.; Al Mahmud, A.; Akhond, M.A.Y.; Sullivan, L.; Cooke, D.E.; et al. Phenotypic and Genotypic Analysis of the Population of Phytophthora infestans in Bangladesh Between 2014 and 2019. Potato Res. 2023, 66, 255–273. [Google Scholar] [CrossRef]
- Sanchez, A.D.; Ousset, M.J.; Sosa, M.C. Biological control of Phytophthora collar rot of pear using regional Trichoderma strains with multiple mechanisms. Biol. Control 2019, 135, 124–134. [Google Scholar] [CrossRef]
- Kazerooni, E.A.; Rethinasamy, V.; Al-Sadi, A.M. Talaromyces pinophilus inhibits Pythium and Rhizoctonia-induced damping-off of cucumber. J. Plant Pathol. 2019, 101, 377–383. [Google Scholar] [CrossRef]
- Bazghaleh, N.; Prashar, P.; Woo, S.; Vandenberg, A. Effects of lentil genotype on the colonization of beneficial Trichoderma species and biocontrol of Aphanomyces root rot. Microorganisms 2020, 8, 1290. [Google Scholar] [CrossRef]
- Konappa, N.; Krishnamurthy, S.; Siddaiah, C.N.; Ramachandrappa, N.S.; Chowdappa, S. Evaluation of biological efficacy of Trichoderma asperellum against tomato bacterial wilt caused by Ralstonia solanacearum. Egypt. J. Biol. Pest. Control 2018, 28, 63. [Google Scholar] [CrossRef]
- Yendyo, S.; Ramesh, G.C.; Pandey, B.R. Evaluation of Trichoderma spp., Pseudomonas fluorescens and Bacillus subtilis for biological control of Ralstonia wilt of tomato. F1000Research 2017, 6, 1–22. [Google Scholar] [CrossRef]
- Mohamed, B.F.F.; Sallam, N.M.A.; Alamri, S.A.M. Approving the biocontrol method of potato wilt caused by Ralstonia solanacearum (Smith) using Enterobacter cloacae PS14 and Trichoderma asperellum T34. Egypt. J. Biol. Pest. Control 2020, 30, 61. [Google Scholar] [CrossRef]
- Yuan, S.; Li, M.; Fang, Z.; Liu, Y.; Shi, W.; Pan, B.; Wu, K.; Shi, J.; Shen, Q. Biological control of tobacco bacterial wilt using Trichoderma harzianum-amended bioorganic fertilizer and the arbuscular mycorrhizal fungi Glomus mosseae. Biol. Control 2016, 92, 164–171. [Google Scholar] [CrossRef]
- Alelign, S. Evaluation of the efficacy of Trichoderma and Pseudomonas species against bacterial wilt Ralstonia isolates of tomato (Lycopersicum species). Afr. J. Microbiol. Res. 2021, 15, 262–271. [Google Scholar] [CrossRef]
- Dung, D.T.; Yoshida, H.; Suyama, K. Effect of bio-factors against potato common scab disease in Vietnam. J. Int. Soc. Southeast Asian Agric. Sci. (J ISSAAS) 2010, 16, 123. [Google Scholar]
- Shiwangi, A.; Pathak, S.P. Effect of eco-friendly treatments on important fungal foliar and tuber borne diseases of potato (Solanum tuberosum L.). Int. J. Chem. Stud. 2019, 7, 06–09. [Google Scholar]
- Hu, M.J.; Zhang, X.S.; Cao, X.; Miao, Z.Q.; Zhang, Y.Z. Studies on the control effect of Trichoderma koningii against soft rot of Chinese cabbage. North. Hortic. 2009, 6, 102–103. [Google Scholar]
- Abd-El-Khair, H.; Abdel-Gaied, T.G.; Mikhail, M.S.; Abdel-Alim, A.I.; El-Nasr, H.I.S. Biological control of Pectobacterium carotovorum subsp. carotovorum, the causal agent of bacterial soft rot in vegetables, in vitro and in vivo tests. Bull. Natl. Res. Centre 2021, 45, 37. [Google Scholar] [CrossRef]
- de Medeiros, H.A.; de Araújo Filho, J.V.; De Freitas, L.G.; Castillo, P.; Rubio, M.B.; Hermosa, R.; Monte, E. Tomato progeny inherit resistance to the nematode Meloidogyne javanica linked to plant growth induced by the biocontrol fungus Trichoderma atroviride. Sci. Rep. 2017, 7, 40216. [Google Scholar] [CrossRef]
- Pocurull, M.; Fullana, A.M.; Ferro, M.; Valero, P.; Escudero, N.; Saus, E.; Gabaldón, T.; Sorribas, F.J. Commercial formulates of Trichoderma induce systemic plant resistance to Meloidogyne incognita in tomato and the effect is additive to that of the Mi-1.2 resistance gene. Front. Microbiol. 2020, 10, 3042. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Van Wees, S.C.; Pieterse, C.M. Airborne signals from Trichoderma fungi stimulate iron uptake responses in roots resulting in priming of jasmonic acid-dependent defences in shoots of Arabidopsis thaliana and Solanum lycopersicum. Plant Cell Environ. 2017, 40, 2691–2705. [Google Scholar] [CrossRef]
- Ghahremani, Z.; Escudero, N.; Saus, E.; Gabaldón, T.; Sorribas, F.J. Pochonia chlamydosporia induces plant-dependent systemic resistance to Meloidogyne incognita. Front. Plant Sci. 2019, 10, 945. [Google Scholar] [CrossRef]
- Martinuz, A.; Zewdu, G.; Ludwig, N.; Grundler, F.; Sikora, R.A.; Schouten, A. The application of Arabidopsis thaliana in studying tripartite interactions among plants, beneficial fungal endophytes and biotrophic plant-parasitic nematodes. Planta 2015, 241, 1015–1025. [Google Scholar] [CrossRef]
- Dababat, A.A.; Sikora, R.A. Induced resistance by the mutualistic endophyte, Fusarium oxysporum strain 162, toward Meloidogyne incognita on tomato. Biocontrol Sci. Technol. 2007, 17, 969–975. [Google Scholar] [CrossRef]
- Menjivar, R.; Hagemann, M.H.; Kranz, J.; Cabrera, J.A.; Dababat, A.A.; Sikora, R.A. Biological control of Meloidogyne incognita on cucurbitaceous crops by the non-pathogenic endophytic fungus Fusarium oxysporum strain 162. Int. J. Pest. Manag. 2011, 57, 249–253. [Google Scholar] [CrossRef]
- Le, H.T.T.; Padgham, J.L.; Hagemann, M.H.; Sikora, R.A.; Schouten, A. Developmental and behavioural effects of the endophytic Fusarium moniliforme Fe14 towards Meloidogyne graminicola in rice. Ann. Appl. Biol. 2016, 169, 134–143. [Google Scholar] [CrossRef]
- Tobin, J.D.; Haydock, P.P.J.; Hare, M.C.; Woods, S.R.; Crump, D.H. Effect of the fungus Pochonia chlamydosporia and fosthiazate on the multiplication rate of potato cyst nematodes (Globodera pallida and G. rostochiensis) in potato crops grown under UK field conditions. Biol. Control 2008, 46, 194–201. [Google Scholar] [CrossRef]
- Contina, J.B.; Dandurand, L.M.; Knudsen, G.R. Use of GFP-tagged Trichoderma harzianum as a tool to study the biological control of the potato cyst nematode Globodera pallida. Appl. Soil. Ecol. 2017, 115, 31–37. [Google Scholar] [CrossRef]
- Vu, T.; Sikora, R.; Hauschild, R. Fusarium oxysporum endophytes induced systemic resistance against Radopholus similis on banana. Nematology 2006, 8, 847–852. [Google Scholar] [CrossRef]
- Cheah, L.H.; Veerakone, S.; Kent, G. Biological control of clubroot on cauliflower with Trichoderma and Streptomyces spp. N. Z. Plant Protect. 2000, 53, 18–21. [Google Scholar] [CrossRef]
- Li, J.; Philp, J.; Li, J.; Wei, Y.; Wang, Y.; Hu, Y. Trichoderma harzianum inoculation reduces the incidence of clubroot disease in Chinese cabbage by regulating the rhizosphere microbial community. Microorganisms 2020, 8, 1325. [Google Scholar] [CrossRef]
- Suada, I.K. The potential of various indigenous Trichoderma spp. to suppress Plasmodiophora brassicae, the pathogen of clubroot disease on cabbage. Biodiversitas J. Biol. Divers. 2017, 18, 1424–1429. [Google Scholar] [CrossRef]
- Yu, X.X.; Zhao, Y.T.; Cheng, J.; Wang, W. Biocontrol effect of Trichoderma harzianum T4 on brassica clubroot and analysis of rhizosphere microbial communities based on T-RFLP. Biocontrol Sci. Technol. 2015, 25, 1493–1505. [Google Scholar] [CrossRef]
- Jäschke, D.; Dugassa-Gobena, D.; Karlovsky, P.; Vidal, S.; Ludwig-Müller, J. Suppression of clubroot (Plasmodiophora brassicae) development in Arabidopsis thaliana by the endophytic fungus Acremonium alternatum. Plant Pathol. 2010, 59, 100–111. [Google Scholar] [CrossRef]
- Naraghi, L.; Heydari, A.; Askari, H.; Pourrahim, R.; Marzban, R. Biological control of Polymyxa betae, fungal vector of rhizomania disease of sugar beets in greenhouse conditions. J. Plant Prot. Res. 2014, 54, 109–114. [Google Scholar] [CrossRef]
- Nouayti, F.; Madani, I.; Tahiri, A.; Blenzar, A.; Lahlali, R. Ability of Non-Pathogenic Fusarium oxysporum Strain Fo47 to Suppress Rhizomania Disease of Sugar Beets in Morocco. Notulae Sci. Biol. 2018, 10, 137–142. [Google Scholar] [CrossRef]
- Tagawa, M.; Tamaki, H.; Manome, A.; Koyama, O.; Kamagata, Y. Isolation and characterization of antagonistic fungi against potato scab pathogens from potato field soils. FEMS Microbiol. Lett. 2010, 305, 67–74. [Google Scholar] [CrossRef]
- Sharon, E.; Chet, I.; Bar-Eyal, M.; Spiegel, Y. Biocontrol of root-knot nematodes by Trichoderma–Modes of action. IOBC/WPRS Bullet. 2009, 42, 159–163. [Google Scholar] [CrossRef]
- Wann, S.B.; Borah, B.; Ahmed, R.; Gogoi, B.; Phukon, P.; Baruah, J.; Bhau, B.S. Isolation, characterization of nematode-controlling bacteria and fungi from nature. In Microbial Inoculants in Sustainable Agricultural Productivity; Volume 1: Research Perspectives; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: New Delhi, India, 2016; pp. 271–296. [Google Scholar] [CrossRef]
- Dandurand, L.M.; Knudsen, G.R. Effect of the trap crop Solanum sisymbriifolium and two biocontrol fungi on reproduction of the potato cyst nematode, Globodera pallida. Ann. Appl. Biol. 2016, 169, 180–189. [Google Scholar] [CrossRef]
- Joo, G.J.; Kim, Y.M.; Kim, J.W.; Kim, W.C.; Rhee, I.K.; Choi, Y.H.; Kim, J.H. Biocontrol of cabbage clubroot by the organic fertilizer using Streptomyces sp. AC-3. Kor. J. Microbiol. Biotechnol. 2004, 32, 172–178. [Google Scholar]
- Eziashi, E.I.; Omamor, I.B.; Odigie, E.E. Antagonism of Trichoderma viride and effects of extracted water-soluble compounds from Trichoderma species and benlate solution on Ceratocystis paradoxa. Afr. J. Biotechnol. 2007, 6, 388–392. Available online: https://www.ajol.info/index.php/ajb/article/view/56224 (accessed on 10 June 2025).
- Howell, C.R. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef]
- Lumsden, R.D.; Locke, J.C.; Adkins, S.T.; Walter, J.F.; Ridout, C.J. Isolation and localization of the antibiotic gliotoxin produced by Gliocladium virens from alginate prill in soil and soilless media. Phytopathology 1992, 82, 230–235. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.; Al-Askar, A.A.; Sayed, S.R.M.; Rady, A.M. Antagonistic activity of Trichoderma harzianum and Trichoderma viride strains against some fusarial pathogens causing stalk rot disease of maize, in vitro. J. King Saud. Univ.—Sci. 2021, 33, 101363. [Google Scholar] [CrossRef]
- Vinale, F.; Ghisalberti, E.L.; Sivasithamparam, K.; Marra, R.; Ritieni, A.; Ferracane, R.; Woo, S.; Lorito, M. Factors affecting the production of Trichoderma harzianum secondary metabolites during the interaction with different plant pathogens. Lett. Appl. Microbiol. 2009, 48, 705–711. [Google Scholar] [CrossRef]
- Vinale, F.; Nigro, M.; Sivasithamparam, K.; Flematti, G.; Ghisalberti, E.L.; Ruocco, M.; Lorito, M. Harzianic acid: A novel siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013, 347, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Barbetti, M.J.; Li, H.; Woo, S.L.; Lorito, M. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol. 2008, 72, 80–86. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Ali, D.M.I. Antimicrobial properties of 6-pentyl-α-pyrone produced by endophytic strains of Trichoderma koningii and its effect on aflatoxin B1 production. Biologia 2017, 72, 1403–1415. [Google Scholar] [CrossRef]
- Niehaus, E.M.; von Bargen, K.W.; Espino, J.J.; Pfannmüller, A.; Humpf, H.U.; Tudzynski, B. Characterization of the fusaric acid gene cluster in Fusarium fujikuroi. Appl. Microbiol. Biotechnol. 2014, 98, 1749–1762. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Wuyun, D.; Xi, X.; Dong, B.; Wang, D.; Quan, W.; Zhou, H. Application of 6-pentyl-α-pyrone in the nutrient solution used in tomato soilless cultivation to inhibit Fusarium oxysporum hf-26 growth and development. Agronomy 2023, 13, 1210. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Dong, L.; Liu, T.; Tang, Z.; Lin, R.; Xing, M. A new insight into 6-pentyl-2H-pyran-2-one against Peronophythora litchii via TOR pathway. J. Fungi. 2023, 9, 863. [Google Scholar] [CrossRef]
- Jin, X.; Guo, L.; Jin, B.; Zhu, S.; Mei, X.; Wu, J.; He, X. Inhibitory mechanism of 6-pentyl-2H-pyran-2-one secreted by Trichoderma atroviride T2 against Cylindrocarpon destructans. Pestic. Biochem. Physiol. 2020, 170, 104683. [Google Scholar] [CrossRef]
- Nicoletti, R.; Di Stefano, M.; Di Stefano, S.; Trincone, A.; Marziano, F. Antagonism against Rhizoctonia solani and fungitoxic metabolite production by some Penicillium isolates. Mycopathologia 2004, 158, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Lopez-Gresa, M.P.; Manzo, E.; Carella, A.; Ciavatta, M.L. Production and fungitoxic activity of Sch 642305, a secondary metabolite of Penicillium canescens. Mycopathologia 2007, 163, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, L.; Zhang, J.; Wu, M.; Chen, W.; Jiang, D.; Li, G. Production of antifungal volatiles by non-pathogenic Fusarium oxysporum and its efficacy in suppression of Verticillium wilt of cotton. Plant Soil 2015, 392, 101–114. [Google Scholar] [CrossRef]
- Lou, J.; Yu, R.; Wang, X.; Mao, Z.; Fu, L.; Liu, Y.; Zhou, L. Alternariol 9-methyl ether from the endophytic fungus Alternaria sp. Samif01 and its bioactivities. Braz. J. Microbiol. 2016, 47, 96–101. [Google Scholar] [CrossRef]
- Liarzi, O.; Bucki, P.; Braun Miyara, S.; Ezra, D. Bioactive volatiles from an endophytic Daldinia cf. concentrica isolate affect the viability of the plant parasitic nematode Meloidogyne javanica. PLoS ONE 2016, 11, e0168437. [Google Scholar] [CrossRef]
- Khan, B.; Yan, W.; Wei, S.; Wang, Z.; Zhao, S.; Cao, L.; Rajput, N.A.; Ye, Y. Nematicidal metabolites from endophytic fungus Chaetomium globosum YSC5. FEMS Microbiol. Lett. 2019, 366, fnz169. [Google Scholar] [CrossRef]
- Qin, S.; Krohn, K.; Schulz, B. Two new metabolites, epoxydine A and B, from Phoma sp. Helv. Chim. Acta 2009, 93, 169–174. [Google Scholar] [CrossRef]
- Wang, L.W.; Xu, B.G.; Wang, J.Y.; Su, Z.Z.; Lin, F.C.; Zhang, C.L.; Kubicek, C.P. Bioactive metabolites from Phoma species, an endophytic fungus from the Chinese medicinal plant Arisaema erubescens. Appl. Microbiol. Biotechnol. 2012, 93, 1231–1239. [Google Scholar] [CrossRef]
- Huang, S.; Xu, J.; Li, F.; Zhou, D.; Xu, L.; Li, C. Identification and antifungal activity of metabolites from the mangrove fungus Phoma sp. L28. Chem. Nat. Compd. 2017, 53, 237–240. [Google Scholar] [CrossRef]
- Hoffman, A.M.; Mayer, S.G.; Strobel, G.A.; Hess, W.M.; Sovocool, G.W.; Grange, A.H.; Kelley-Swift, E.G. Purification, identification and activity of phomodione, a furandione from an endophytic Phoma species. Phytochemistry 2008, 69, 1049–1056. [Google Scholar] [CrossRef]
- Mousa, W.K.; Schwan, A.; Davidson, J.; Strange, P.; Liu, H.; Zhou, T.; Auzanneau, F.I.; Raizada, M.N. An endophytic fungus isolated from finger millet (Eleusine coracana) produces anti-fungal natural products. Front. Microbiol. 2015, 6, 1157. [Google Scholar] [CrossRef]
- Gorai, P.S.; Barman, S.; Gond, S.K.; Mandal, N.C. Trichoderma. In Beneficial Microbes in Agro-Ecology; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Almeida, F.B.R.; Cerqueira, F.M.; Silva, R.N.; Ulhoa, C.J.; Lima, A.L. Mycoparasitism studies of Trichoderma harzianum strains against Rhizoctonia solani: Evaluation of coiling and hydrolytic enzyme production. Biotechnol. Lett. 2007, 29, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Ezziyyani, M.; Requena, M.E.; Egea-Gilabert, C.; Candela, M.E. Biological control of phytophthora root rot of pepper using Trichoderma harzianum and Streptomyces rochei in combination. J. Phytopathol. 2007, 155, 342–349. [Google Scholar] [CrossRef]
- Gomes, E.; Costa, M.; de Paula, R.; Ricci de Azevedo, R.; da Silva, F.L.; Noronha, E.F.; Nascimento Silva, R. The Cerato-Platanin protein Epl-1 from Trichoderma harzianum is involved in mycoparasitism, plant resistance induction and self cell wall protection. Sci. Rep. 2016, 5, 17998. [Google Scholar] [CrossRef]
- Szabó, M.; Csepregi, K.; Gálber, M.; Virányi, F.; Fekete, C. Control of plant-parasitic nematodes with Trichoderma species and nematode-trapping fungi: The role of chi18-5 and chi18-12 genes in nematode egg-parasitism. Biol. Control 2012, 63, 121–128. [Google Scholar] [CrossRef]
- Sharon, E.; Chet, I.; Viterbo, A.; Bar-Eyal, M.; Nagan, H.; Samuels, G.J.; Spiegel, Y. Parasitism of Trichoderma on Meloidogyne javanica and role of the gelatinous matrix. Eur. J. Plant Pathol. 2007, 118, 247–258. [Google Scholar] [CrossRef]
- Singh, U.B.; Singh, S.; Malviya, D.; Chaurasia, R.; Imran, M.; Rai, J.; Sharma, A.K. Harnessing biocontrol potential of Trichoderma harzianum for control of Meloidogyne incognita in tomato. Ind. Phytopathol. 2017, 70, 331–335. [Google Scholar] [CrossRef]
- Yao, Y.R.; Tian, X.L.; Shen, B.M.; Mao, Z.C.; Chen, G.H.; Xie, B.Y. Transformation of the endophytic fungus Acremonium implicatum with GFP and evaluation of its biocontrol effect against Meloidogyne incognita. World J. Microbiol. Biotechnol. 2015, 31, 549–556. [Google Scholar] [CrossRef]
- Mwaura, P.; Dubois, T.; Losenge, T.; Coyne, D.; Kahangi, E. Effect of endophytic Fusarium oxysporum on paralysis and mortality of Pratylenchus goodeyi. Afr. J. Biotechnol. 2010, 9, 1130–1134. [Google Scholar] [CrossRef]
- Tjamos, E.C.; Papavizas, G.C.; Cook, R.J. Biological Control of Plant Diseases: Progress and Challenges for the Future; Plenum Press: New York, NY, USA, 1922; p. 222. [Google Scholar]
- Chet, I.; Inbar, J. Biological control of fungal pathogens. Appl. Biochem. Biotechnol. 1994, 48, 37–43. [Google Scholar] [CrossRef]
- Shivanna, M.B.; Meera, M.S.; Hyakumachi, M. Role of root colonization ability of plant growth-promoting fungi in the suppression of take-all and common root rot of wheat. Crop Protect. 1996, 15, 497–504. [Google Scholar] [CrossRef]
- Hyakumachi, M. Fungi as plant growth promoter and disease suppressor. In Proceedings of the 46th Annual Meeting and the 8th International Symposium (Part I) of the Mycological Society of Japan, Nagano, Japan, 21–25 August 2002; pp. 32–35. [Google Scholar]
- Howell, C.R.; Hanson, L.E.; Stipanovic, R.D.; Puckhaber, L.S. Induction of terpenoid synthesis in cotton roots and control of Rhizoctonia solani by seed treatment with Trichoderma virens. Phytopathology 2000, 90, 248–252. [Google Scholar] [CrossRef]
- Guzmán-Valle, P.; Bravo-Luna, L.; Montes-Belmont, R.; Sepúlveda-Jiménez, G. Induction of resistance to Sclerotium rolfsii in different varieties of onion by inoculation with Trichoderma asperellum. Eur. J. Plant Pathol. 2014, 138, 223–229. [Google Scholar] [CrossRef]
- de Rezende, L.C.; de Andrade Carvalho, A.L.; Costa, L.B.; de Almeida Halfeld-Vieira, B.; Silva, L.G.; Pinto, Z.V.; Morandi, M.A.B.; de Medeiros, F.H.V.; Mascarin, G.M.; Bettiol, W. Optimizing mass production of Trichoderma asperelloides by submerged liquid fermentation and its antagonism against Sclerotinia sclerotiorum. World J. Microbiol. Biotechnol. 2020, 36, 113. [Google Scholar] [CrossRef] [PubMed]
- Larena, I.; Melgarejo, P.; De Cal, A. Production, survival, and evaluation of solid-substrate inocula of Penicillium oxalicum, a biocontrol agent against Fusarium wilt of tomato. Phytopathology 2002, 92, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.S.; Panja, B.; Shah, J. Evaluation of Suitable Organic Substrates-Based Trichoderma harzianum Formulation for Managing Rhizoctonia solani Causing Collar Rot Disease of Cowpea. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 127–134. [Google Scholar]
- Srivastava, R.; Khalid, A.; Singh, U.S.; Sharma, A.K. Evaluation of arbuscular mycorrhizal fungus, fluorescent Pseudomonas and Trichoderma harzianum formulation against Fusarium oxysporum f. sp. lycopersici for the management of tomato wilt. Biol. Control 2010, 53, 24–31. [Google Scholar] [CrossRef]
- Sriram, S.; Roopa, K.P.; Savitha, M.J. Extended shelf life of liquid fermentation derived talc formulations of Trichoderma harzianum with the addition of glycerol. Crop Prot. 2011, 30, 1334–1339. [Google Scholar] [CrossRef]
- Jakubíková, L.; Farkas, V.; Kolarova, N.; Nemcovic, M. Conidiation of Trichoderma atroviride isolate during submerged cultivation in a laboratory stirred-tank fermenter. Folia Microbiol. 2006, 51, 209–213. [Google Scholar] [CrossRef]
- Watanabe, S.; Kato, H.; Kumakura, K.; Ishibashi, E.; Nagayama, K. Properties and biological control activities of aerial and submerged spores in Trichoderma asperellum SKT-1. J. Pesticide Sci. 2006, 31, 375–379. [Google Scholar] [CrossRef]
- Jaronski, S.T. Mass production of entomopathogenic fungi: State of the art. In Mass Production of Beneficial Organisms; Morales-Ramos, J.A., Rojas, M.G., Shapiro-Ilan, D., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 357–413. [Google Scholar] [CrossRef]
- Kobori, N.N.; Mascarin, G.M.; Jackson, M.A.; Schisler, D.A. Liquid culture production of microsclerotia and submerged conidia by Trichoderma harzianum active against damping-off disease caused by Rhizoctonia solani. Fungal. Biol. 2015, 119, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.R.O.; Locatelli, G.O.; Barbosa, R.M.; Lobo Junior, M.; Mascarin, G.M.; Finkler, C.L.L. Preparation, characterization and cell viability of encapsulated Trichoderma asperellum in alginate beads. J. Microencapsulat. 2020, 37, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Summerell, B.A.; Salleh, B.; Leslie, J.F. A utilitarian approach to Fusarium identification. Plant Dis. 2003, 87, 117–128. [Google Scholar] [CrossRef]
- Farr, D.F.; Rossman, A.Y. Fungal databases, U.S. National Fungus Collections. U.S. Department of Agriculture, Agricultural Research Service. 2023. Available online: https://fungi.ars.usda.gov/ (accessed on 20 March 2025).
- Martin, F.N.; Abad, Z.G.; Balci, Y.; Ivors, K. Identification and detection of Phytophthora: Reviewing our progress, identifying our needs. Plant Dis. 2012, 96, 1080–1103. [Google Scholar] [CrossRef] [PubMed]
- Henricot, B.; Pérez Sierra, A.; Jung, T. Phytophthora pachypleura sp. nov., a new species causing root rot of Aucuba japonica and other ornamentals in the United Kingdom. Plant Pathol. 2014, 63, 1095–1109. [Google Scholar] [CrossRef]
- Zitnick-Anderson, K.K.; Nelson, B.D. Identification and pathogenicity of Pythium on soybean in North Dakota. Plant Dis. 2014, 99, 31–38. [Google Scholar] [CrossRef]
- Bik, H.M.; Porazinska, D.L.; Creer, S.; Caporaso, J.G.; Knight, R.; Thomas, W.K. Sequencing our way towards understanding global eukaryotic biodiversity. Trends Ecol. Evolut. 2016, 27, 233–243. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Venturi, V. Synergisms between microbial pathogens in plant disease complexes: A growing trend. Front. Plant Sci. 2015, 6, 385. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, Y.; Asano, T.; Otsubo, K.; Kageyama, K. Simultaneous detection by multiplex PCR of the high-temperature-growing Pythium species: P. aphanidermatum, P. helicoides, and P. myriotylum. J. General. Plant Pathol. 2013, 79, 350–358. [Google Scholar] [CrossRef]
- Schroeder, K.L.; Martin, F.N.; de Cock, A.W.A.M.; Paulitz, T.C. Molecular detection and quantification of Pythium species: Evolving taxonomy, new tools, and challenges. Plant Dis. 2012, 97, 4–20. [Google Scholar] [CrossRef] [PubMed]
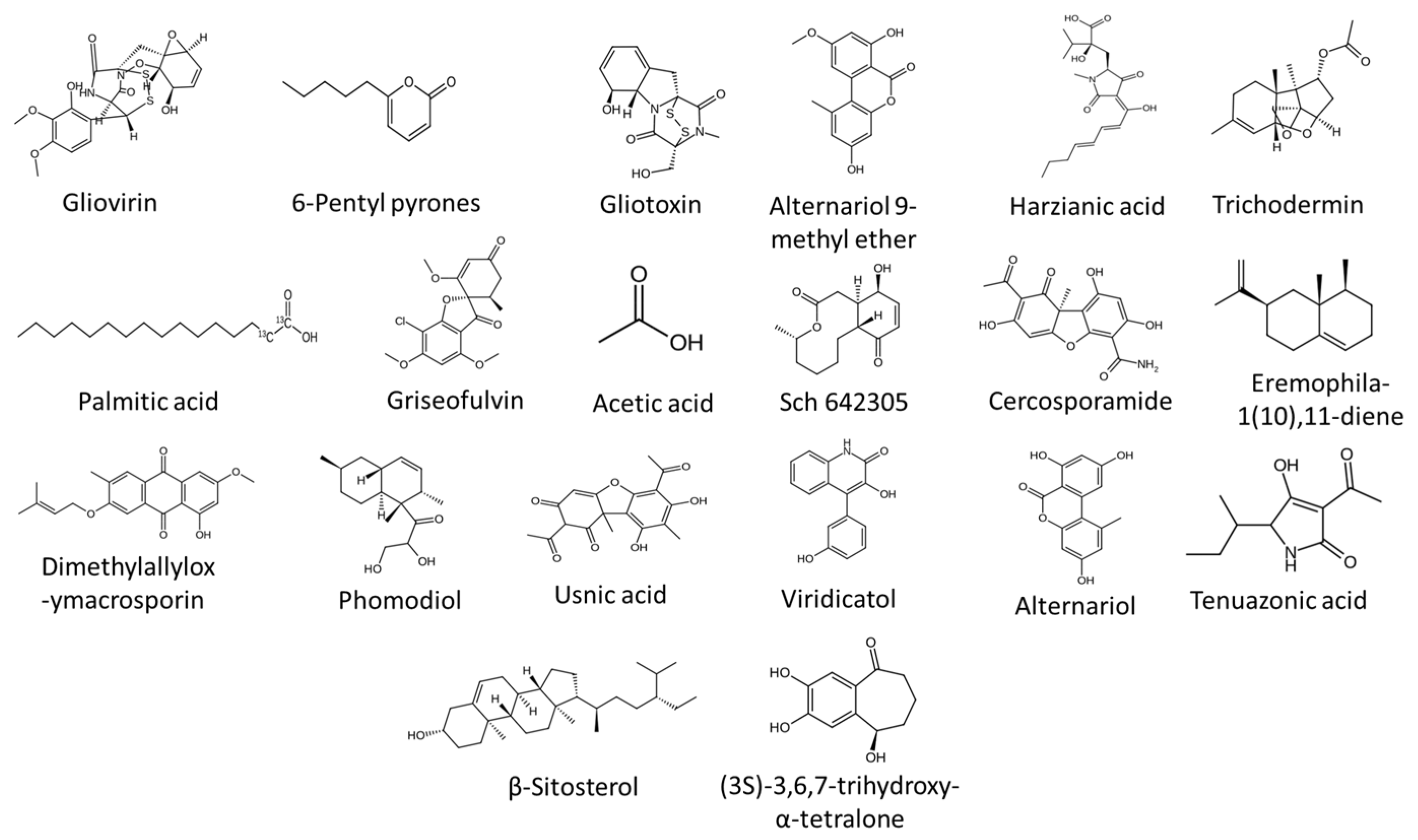
| Pathogen Group | Common Genera/Species | Disease(s) Caused | Key Features | References |
|---|---|---|---|---|
| Fungi | Sclerotium, Rhizoctonia, Sclerotinia, Fusarium, Macrophomina, Verticillium, Gaeumannomyces, Gibberella, Colletotrichum | Damping-off, root/stem/collar/tuber rots, wilts, take all, bakanae, anthracnose | Most destructive group; produces resting structures (sclerotia, chlamydospores); affects a wide range of crops | [42,43,44,45,46,47,48,49,50] |
| Oomycetes | Pythium, Phytophthora, Aphanomyces | Damping-off, crown rot, root rot, polycyclic leaf diseases | Produce durable oospores; Phytophthora species can also infect aerial parts | [45,51,52,53] |
| Protists | Plasmodiophora brassicae | Clubroot in crucifers | Obligate biotroph; survives as resting spores; infects root hairs and cortex | [54,55] |
| Bacteria | Ralstonia solanacearum, Pectobacterium carotovorum, Agrobacterium tumefaciens, Xanthomonas, Streptomyces | Wilts, soft rot, crown gall, scab, blackleg | Highly destructive; Ralstonia infects >200 species; soft rot affects many vegetables | [56,57,58] |
| Nematodes | Heterodera, Globodera, Meloidogyne, Pratylenchus, Radopholus | Cyst, root-knot, lesion, and burrowing nematode diseases | Feed on roots; impair water/nutrient uptake; often cause secondary infections | [59,60,61,62] |
| Viruses | Tobamovirus, Potexvirus, Tombusvirus, BNYVV, LBVV, GFLV | TMV, ToMV, rhizomania, big-vein, fan leaf, etc. | Some are transmitted abiotically or by soil-borne vectors (fungi, protists, nematodes) | [4,63,64,65] |
| Disease | Pathogen Name | Host Type | PGPF Species | Effect on Disease | Reference |
|---|---|---|---|---|---|
| Bacterial wilt | Ralstonia solanacearum | Tomato | Trichoderma spp. isolate T1 | Reduced bacterial wilt incidence by more than 61.66% and decreased the Ralstonia solanacearum population in the soil by over 92% | [58] |
| Trichoderma spp. AA2 | Prevented 92–97% of the infection in the field | [177,178] | |||
| Potato | T. asperellum T34 | Reduced disease severity in the greenhouse and field | |||
| T. asperellum (T4 and T8) | Reduced about 46–52% across years and locations | [176] | |||
| Common scab | Streptomyces scabies | Potato | T. viride | Reduced potato common scab incidence by about 41% | [181] |
| T. viride | Reduced early blight disease incidence by 65.48% | [182] | |||
| Soft rot | Pectobacterium carotovorum subsp. carotovorum | Chinese cabbage | T. pseudokoningii SMF2 | Reduced infection in the field, with up to 82.08% protection | [183] |
| Potato | T. viride T. virens T. harzianum | Reduced soft rot incidence by up to 96.8% with T. viride and T. virens and 73.6–90.4% with T. harzianum) | [184] | ||
| Root-knot | Meloidogyne javanica | Tomato | T. atroviride | Reduced RKN incidence by 53.5–91.7% | [185] |
| Meloidogyne incognita | Tomato | T. asperellum T34 T. harzianum T22 | Reduced by 71% and 54% by T34, respectively, while T22 reduced 48% of the number of eggs per plant | [186] | |
| Meloidogyne incognita | Tomato | T. harzianum T-78 | Reduced severe disease incidence in Arabidopsis from ~20% to 0%, representing a 100% reduction | [187] | |
| Meloidogyne incognita | Tomato | Pochonia chlamydosporia isolates M10.43.21 | Reduced infection (32–43%), reproduction (44–59%), and fecundity (14.7–27.6%) | [188] | |
| Meloidogyne incognita | Arabidopsis | Fusarium oxysporum, strain Fo162 | Reduced disease incidence approximately 35–53% | [189] | |
| Meloidogyne incognita | Tomato | Fusarium oxysporum strain Fo162 | Showed 26–45% less nematode penetration, 21–36% less galls and a 22–26% reduction in the number of egg masses in the roots | [190] | |
| Meloidogyne incognita race 3 | Melon, Squash | Fusarium oxysporum strain Fo162 | Reduced early root penetration up to 69–73% | [191] | |
| M. graminicola | Rice | Fusarium graminicola | Reduced nematode penetration (55%) and increased the male-to-female ratio (nine times) | [192] | |
| Cyst nematodes | Globodera pallida G. rostochiensis | Potato | Pochonia chlamydosporia | Reduced the multiplication rate of potato cyst nematodes by approximately 48–51% in field conditions | [193] |
| G. pallida | Potato | T. harzianum ThzID1-M3 | Reduced Globodera pallida infection and reproduction by 49% and 60%, respectively | [194] | |
| Burrowing nematode | Radopholus similis | Banana | Fusarium oxysporum, Fusarium diversisporum | Reduced disease (nematode) incidence by approximately 29–39% after 5 days and 22–45% after 15 days of inoculation | [195] |
| Club root | Plasmodiophora brassicae | Cauliflower | Trichoderma spp. isolate TC32 TC45 and TC63 | In the glasshouse experiment, Trichoderma isolates TC32, TC45, and TC63 reduced clubroot disease severity in Chinese cabbage seedlings by approximately 56.76%, 83.78%, and 59.46%, respectively, while in the field trial, the same isolates reduced disease incidence by about 58.33%, 27.78%, and 27.78%, respectively, compared to the untreated control | [196] |
| Plasmodiophora brassicae | Rapeseed | Trichoderma strains ReTk1 and ReTv2 | Reduced clubroot disease incidence in rapeseed by approximately 32.82–52.52% | [55] | |
| Plasmodiophora brassicae | Chinese cabbage | T. harzianum LTR-2 | Reduced disease incidence (45.4%) and pathogen abundance | [197] | |
| Plasmodiophora brassicae | Cabbage | T. hamatum T. harzianum | Reduced the incidence of clubroot disease by 45.4% | [198] | |
| Plasmodiophora brassicae | Chinese cabbage | T. harzianum T4 | Reduced the incidence of clubroot disease by 79.3% | [199] | |
| Plasmodiophora brassicae | Arabidopsis | Acremonium alternatum | Reduced gall formation and the disease index by up to 50% | [200] | |
| Rhizomania | Polymyxa betae | Sugar beet | T. harzianum | Reduced pathogen population by approximately 46–68% | [201] |
| Polymyxa betae | Sugar beet | Fusarium oxysporum Strain Fo47 | Reduced the incidence of disease by 44.7% | [202] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.M.; Sultana, F.; Mostafa, M.; Rubayet, M.T.; Mishu, N.J.; Khan, I.; Mostofa, M.G. Biological Management of Soil-Borne Pathogens Through Tripartite Rhizosphere Interactions with Plant Growth-Promoting Fungi. Appl. Microbiol. 2025, 5, 123. https://doi.org/10.3390/applmicrobiol5040123
Hossain MM, Sultana F, Mostafa M, Rubayet MT, Mishu NJ, Khan I, Mostofa MG. Biological Management of Soil-Borne Pathogens Through Tripartite Rhizosphere Interactions with Plant Growth-Promoting Fungi. Applied Microbiology. 2025; 5(4):123. https://doi.org/10.3390/applmicrobiol5040123
Chicago/Turabian StyleHossain, Md. Motaher, Farjana Sultana, Mahabuba Mostafa, Md. Tanbir Rubayet, Nusrat Jahan Mishu, Imran Khan, and Mohammad Golam Mostofa. 2025. "Biological Management of Soil-Borne Pathogens Through Tripartite Rhizosphere Interactions with Plant Growth-Promoting Fungi" Applied Microbiology 5, no. 4: 123. https://doi.org/10.3390/applmicrobiol5040123
APA StyleHossain, M. M., Sultana, F., Mostafa, M., Rubayet, M. T., Mishu, N. J., Khan, I., & Mostofa, M. G. (2025). Biological Management of Soil-Borne Pathogens Through Tripartite Rhizosphere Interactions with Plant Growth-Promoting Fungi. Applied Microbiology, 5(4), 123. https://doi.org/10.3390/applmicrobiol5040123






