Abstract
Pathogenic bacteria have developed different ways to cause infections. One strategy involves using components from host cells. This study looks at the role of the cytoskeleton in the human colon adenocarcinoma Caco-2 and neonatal non-transformed epithelial H4 cell lines during bacterial invasion. The bacteria studied include Cronobacter malonaticus, Cronobacter sakazakii, and E. coli K1, as they are associated with known diseases. Salmonella enteritidis 358 served as a positive control and E. coli K12 as a negative control for the invasion experiments. Before the invasion experiments, cell lines were treated with microfilament inhibitors, specifically Cytochalasin D, and microtubule inhibitors, such as Colchicine, Nocodazole, Vinblastine, and Taxol. The results showed that Cytochalasin D reduced about 60–80% of Cronobacter invasion into H4 cells and 50% of E. coli K1 invasion. In contrast, Colchicine reduced the invasion of some strains to just 2% compared to untreated cells. Meanwhile, Nocodazole and Taxol increased the invasion of C. sakazakii 709 and C. malonaticus 1569 into H4 cells by about 140% and 160%, respectively, while slightly inhibiting other strains. In Caco-2 cells, certain strains exhibited increased invasion due to Cytochalasin D, Vinblastine, and Colchicine treatment. This led to increases of up to 500%, 227%, and 248% compared to untreated cells. However, Nocodazole and Taxol decreased invasion into Caco-2 cells, with only E. coli K1 showing an increase of about 150% in Taxol-treated cells. The findings with eukaryotic cytoskeleton inhibitors on neonatal H4 cells suggest that bacterial invasion mainly relies on microfilaments or microfilament-dependent. No specific dependence on the cytoskeleton was seen in Caco-2 cells. In conclusion, cytoskeletal inhibitors significantly affected bacterial invasion, specifically Cronobacter, compared to untreated cells. This suggests that invasion methods may vary by strain and are influenced by how each inhibitor alters cytoskeleton behavior. Therefore, the invasion process, both with and without cytoskeletal inhibitors, is crucial for understanding how bacteria manipulate cell components during infection.
1. Introduction
The increasing challenge of bacterial infections, particularly due to the rise in antibiotic resistance, highlights the need for innovative therapeutic strategies. One promising approach among these strategies is to target the mechanisms that enable bacteria to invade human cells. Dynamic cytoskeletal networks in eukaryotic cells have been implicated in bacterial adhesion and host cell entry, though the precise mechanisms remain unclear [1]. Eukaryotic cytoskeleton inhibitors have emerged as key players in this process [2]. For example, Vinblastine, Colchicine, Nocodazole, and Taxol have been shown to disrupt microtubule (MT) dynamics. At the same time, Cytochalasin D was found to work as a microfilament (MF) depolymerization agent, thereby impeding bacterial invasion [3]. These compounds interfere with the cytoskeletal framework, crucial for the pathogen’s ability to adhere to and penetrate host cells [2].
Cytochalasin D targets actin microfilaments and binds to the ends of barbed actin filaments. This process inhibits both polymerization and depolymerization, leading to disruption of actin filaments, which finally affects the cell motility, shape and other cellular processes that rely on actin dynamics [4,5]. Alternatively, Colchicine, Vinblastine, Taxol and Nocodazole target the MT structures in the eukaryotic cytoskeletons, and can disrupt various cellular processes, particularly those related to cell division and intracellular transport [6]. These inhibitors have variable modes of action [3,7]. Vinblastine and Taxol, inhibiting MT assembly, leading to mitotic arrest and interfering with normal cell division by preventing MT depolymerization, while Nocodazole inhibits MT polymerization through binding to tubulin, disrupting the MT dynamics. Colchicine binds to tubulin, inhibiting MT polymerization, and at higher concentrations, it can promote MT depolymerization [6]. Several pathogens can use this alteration in the host’s cytoskeleton to accomplish infection [8].
In terms of bacterial infection, several pathogenic bacteria have been found to manipulate host cell components to facilitate their adhesion and entry into host cells [9]. Rearrangement of actin cytoskeleton in host cells is one of the common strategies that can be used by various enteropathogenic bacteria such as Salmonella, Listeria, Shigella, Yersinia and Cronobacter spp. for the internalization process [9,10,11].
The genus Cronobacter has been involved in many outbreaks worldwide and has resulted in series neonatal deaths due to meningitis and necrotizing enterocolitis (NEC) [12,13,14,15]. Some species, such as C. sakazakii, C. malonaticus are more likely linked to life-threatening infections in neonates and adults, respectively [16,17,18,19].
The use of human and other eukaryotic cell lines for host–pathogen interaction studies is very common. Cell lines simplify the complex in vivo processes and may considerably shorten the period of research in contrast to the in vivo models [20]. An intensive amount of research was conducted in the investigation of bacterial pathogenicity mechanisms, with a primary focus on host–pathogen interaction experiments. The human colorectal adenocarcinoma epithelial cell line (Caco-2) is one of the most widely used in vitro cell lines in investigations of host–pathogen interactions. However, Caco-2 cells are an abnormal cell line derived from an adult colon carcinoma [21,22], which may not simulate the pathogenesis mechanisms in neonates [21,23]. Non-malignant neonatal cell lines, H4 cells, are a non-transformed cell line obtained from the fetal intestine, and limited data are available about using this cell line in investigating the host–pathogen interactions. Compression studies of inflammatory responses have shown that H4 cells are more responsive and produce higher levels of inflammatory cytokines than adult cells [24]. We have conducted separate studies that confirmed differences in immune responses between neonatal and adult cell lines during pathogen co-culturing, which highlights the importance of using these cell lines in this area of research [25]. Based on the sideline research of the recent study, bacterial strains may exhibit different behaviors depending on the cell line used. Notably, the H4 cell line represents a non-transformed neonatal epithelial cell line and differs from genetically modified and adult-derived cell lines. These suggestions encouraged us to investigate the possible role of host cell components in the invasion process. In this study, we investigated the role of host cells in this process, examining how eukaryotic cytoskeleton inhibitors influence the invasion mechanism and the number of internalized bacteria, with a special emphasis on Cronobacter.
2. Materials and Methods
2.1. Bacterial Isolates
Four Cronobacter isolates from the NTU collection were selected based on their clinical history and invasion levels in a previous assay (Table A1). Additionally, E. coli K1 strain 939 from Nottingham Trent University (NTU) was included as a comparative strain from a different genus, given its strong association with neonatal meningitis. Salmonella enteritidis 358 and E. coli K12 were used as positive and negative controls, respectively, for the invasion assay.
2.2. Human Cell Lines
Two cell lines were used in this study. Human colorectal adenocarcinoma epithelial cell line Caco-2 and the non-malignant human fetal primary small intestinal cell line H4. Both cell lines were sourced from the biosciences department at Nottingham Trent University. Caco-2 and H4 cells were used at low passages and maintained according to established protocols in Minimum Essential Medium Eagle and Dulbecco’s Eagle’s medium (Life Technologies, Gibco, UK) [26,27]. Once confluent, cells were trypsinized, resuspended in a growth medium, and diluted in the appropriate medium at the desired concentration. Then, 1 mL seeded into 24-well plates to achieve a concentration of ~104 cell/mL for the Caco-2 cell line and ~105 cell/mL for the H4 cell line.
2.3. Eukaryotic Cytoskeleton Inhibitors
The inhibitors used in the recent research include Cytochalasin D, a microfilament (MF) inhibitor, as well as Colchicine, Vinblastine, Taxol and Nocodazole, which are microtubule (MT) inhibitors. All inhibitors were obtained from Sigma, UK.
2.4. Invasion Experiments
For internalized bacteria, confluent cells in 24-well plates were washed three times with Dulbecco’s Phosphate-Buffered Saline (PBS) and incubated with selected bacterial strains at 0.05 of OD600 for 3 h at 37 °C under 5% CO2. Thereafter, the media was removed, and the cells washed three times with PBS and incubated for another hour in sterile media containing 125 μg/mL gentamicin to kill non-internalized bacteria. Subsequently, the media was removed, and cells were washed 3 times and permeabilized with 1% (v/v) Triton X-100. Lysates containing internalized bacteria were serially diluted in PBS. The viable count, measured as Colony Forming Units (CFUs), was conducted on Tryptone Soy Agar (TSA) plates using the Miles and Misra technique [28,29], and the results are presented as log10 CFU/mL.
2.5. Investigating the Role of Inhibitors in Bacterial Invasion
To examine the role of inhibitors in the bacterial invasion, human cell lines were pre-incubating with the selected inhibitors as follows: Cytochalasin D (2 µM) for 30 min at 37 °C, Vinblastine and Taxol (20 µM) each preincubated for an hour at 37 °C, before infection, while Nocodazole (20 µM) and Colchicine (10 µM) were pre-incubated with epithelial cells for 60 min at 4 °C followed by 30 min incubation at 37 °C [11]. After the incubation period, the cells were washed with PBS and then incubated with the selected bacterial strains as described in Section 2.4. For the control, cells were incubated with the selected bacterial strains under the same conditions without pre-incubation with any inhibitor. The assays were run in triplicate and replicated three times. The percentage of the invasion is calculated using the following formula.
2.6. Statistical Analysis
Statistical significance of data generated in this study was determined using two-tailed Student t and two-way ANOVA (GraphPad Prism Software, Version 10). Data were expressed by mean ± standard deviation and all experiments were carried out in independent at least twice. Differences were considered to be significant when p ≤ 0.05.
3. Results
3.1. Bacterial Invasion
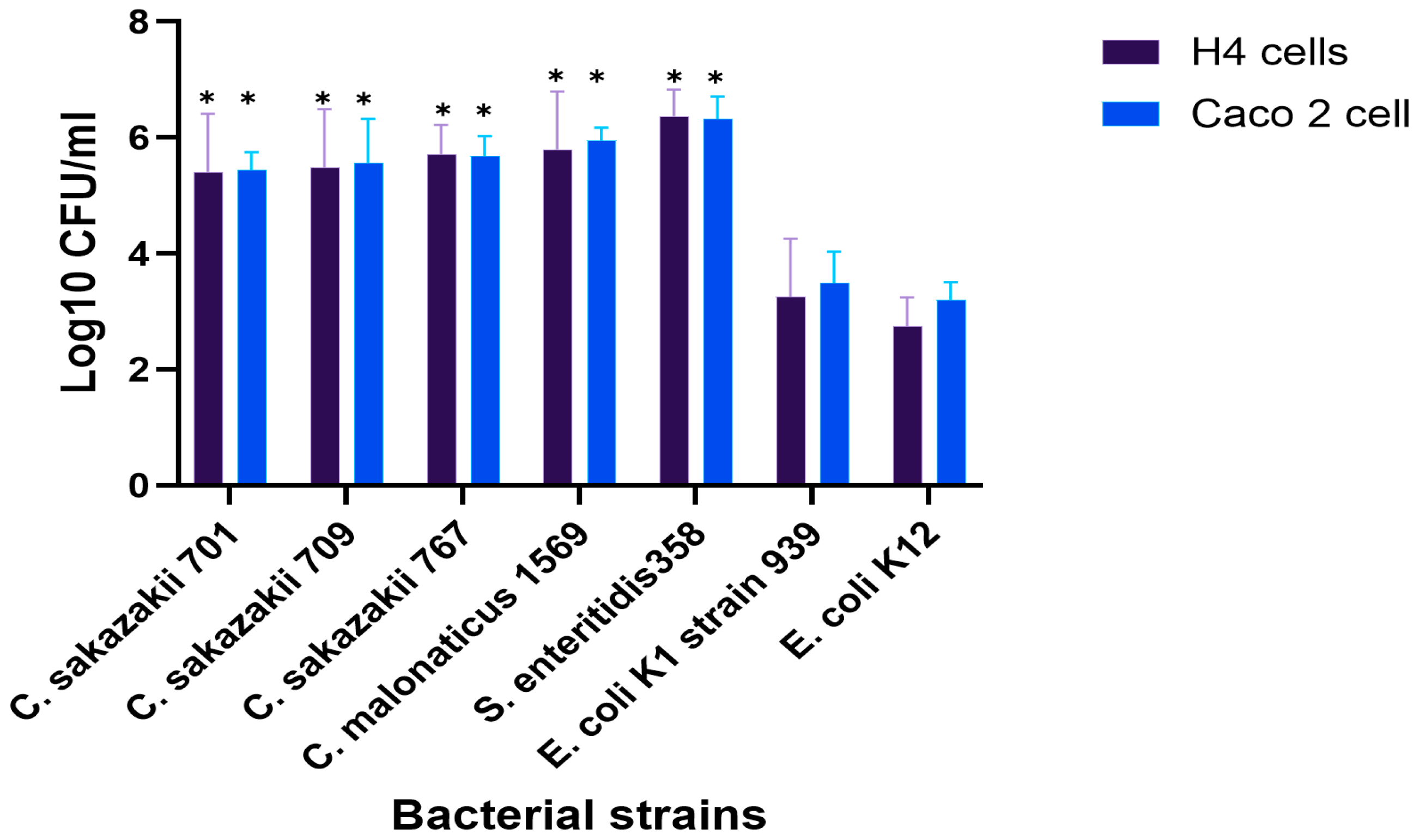
The invasion of clinically important isolates from the genus Cronobacter into H4 and Caco-2 cells, mainly linked to fatal blood infections in neonates, was investigated. In general, the differences between the two cell lines were not substantial. However, the invasion of Cronobacter and E. coli K1 isolates was significantly higher in both cell lines compared with the non-pathogenic, E. coli K12 isolate as negative control, which showed only 3.2 and 2.7 log10 CFU, compared to other strains (p < 0.05), as shown in Figure 1.
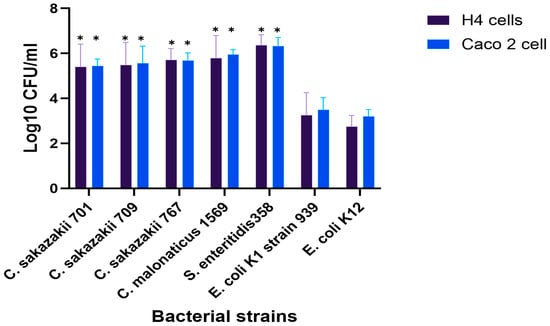
Figure 1.
Bacterial invasion of Caco-2 and H4 cells after 3 h of incubation at 37 °C with 5% CO2. Results showing the differences in invasion levels between strains. The displayed data are the mean ± standard deviation of invasion efficiency and were analyzed using an unpaired Student’s t-test, p < 0.05. The asterisk (*) indicates a statistically significant difference compared to E. coli K12. For more information about the strains, see Table A2.
3.2. Effect of Inhibitors on Bacterial Invasion of H4 Cell Line
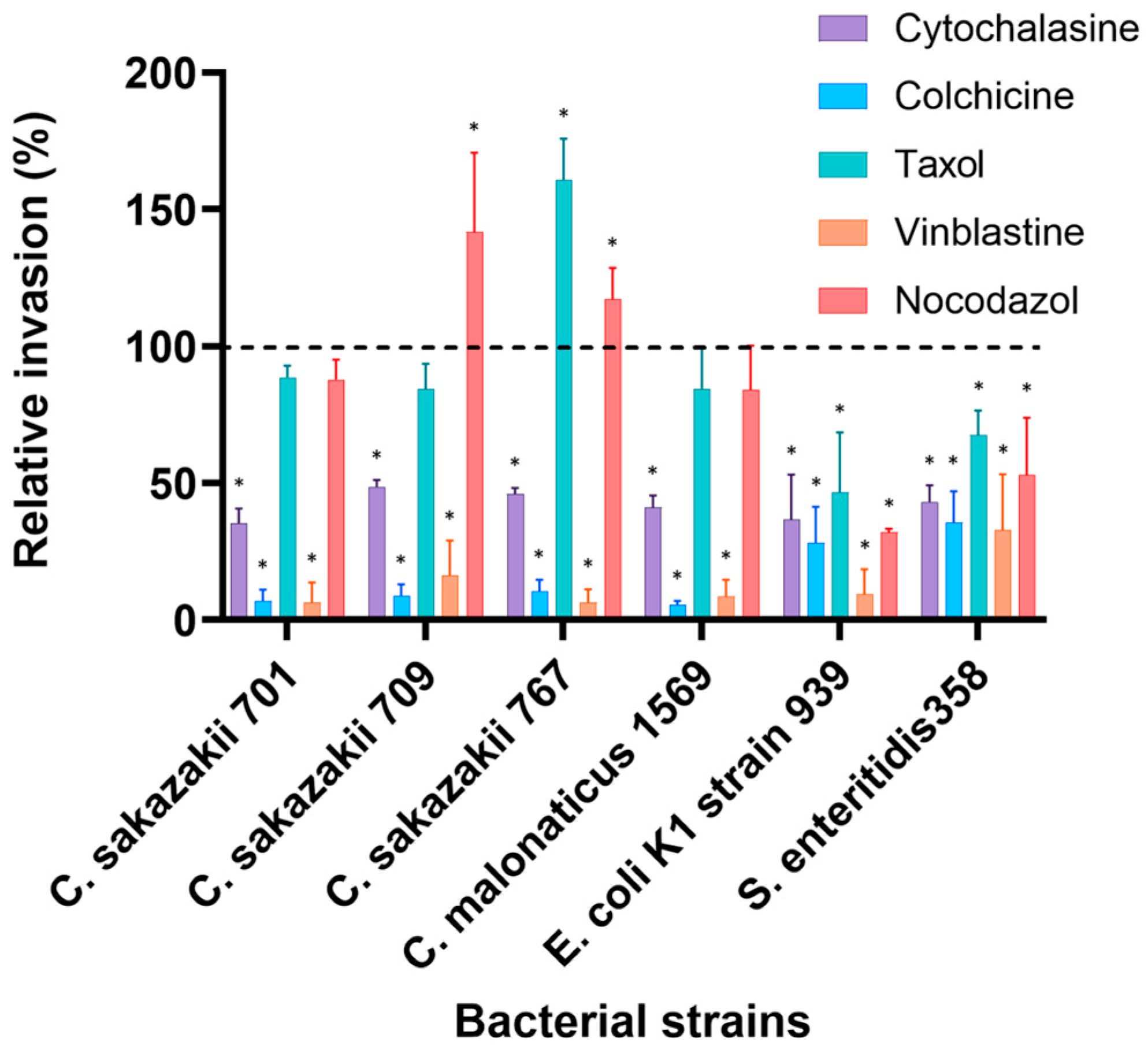
Pre-treatment of neonatal H4 cells with cytoskeleton inhibitors resulted in a significant reduction in bacterial invasion (p < 0.05), except for Taxol and Nocodazol. Cytochalasin D has decreased the number of invasive bacteria to levels ranging from about 15% for C. malonaticus to 58% for Salmonella enteritidis 358 compared to 100% presented by non-treated cells. Other C. sakazakii isolates were significantly inhibited (p < 0.05) and showed 21%, 25% and 28% by strains 701, 767 and 709, while E. coli K1 strain 939 was inhabited by 52% compared to non-treated cells (Figure 2).
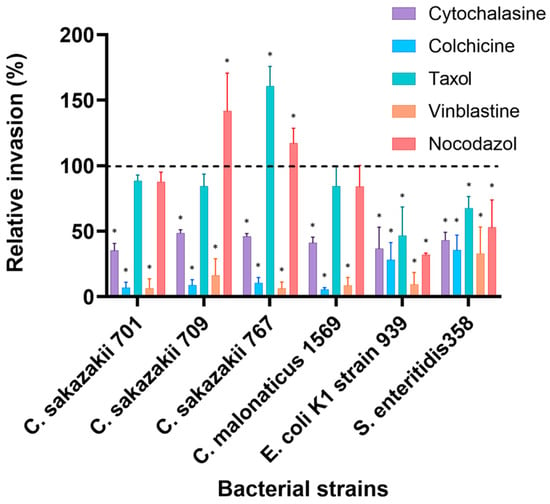
Figure 2.
Effect of cytoskeleton inhibitors on the invasion of selected bacterial isolates into H4 cells. Cells were pre-incubated with stated inhibitors (Cytochalasin D (2 µM), Nocodazole (20 µM), Colchicine (10 µM), Taxol (20 µM) and Vinblastine (20 µM)) as described by [11] and then infected with bacteria for 3 h. The data presented is the mean ± standard deviation of two independent experiments. Data are presented as a percentage of invasion compared to 100% control (untreated cells) for each strain, where no inhibitors were added to human cells (dashed line). The asterisk (*) indicates a statistically significant difference (p < 0.05) compared to control.
The microtubules inhibitor Colchicine significantly decreased the invasion of all Cronobacter isolates, with maximum reduction of 91% to 94% compared to the invasion without inhibitors, while the invasion of S. enteritidis 358 and E.coli K1 strain 939 was reduced to about 46% and 35%, respectively. However, the MT inhibitor Nocodazole resulted in increased invasion of C. sakazakii strains 709 (42%) and 767 (17%), which was higher than that of untreated cells. However, the invasion of other strains was variably affected by Nocodazole-treated cells. C. sakazakii strain 701 and C. malonaticus strain 1569 were slightly inhibited to 88% and 84%, respectively, while invasion of E. coli K1 strain 939 and Salmonella enteritidis 358 was reduced to 32% and 53%, respectively. Taxol stimulated the invasion of only C. sakazakii 767 to 61% higher than untreated cells and showed a minor reduction in other Cronobacter’ isolates (14–15%). Invasion of E. coli K1 and Salmonella enteritidis 358 was inhibited by about 50% and 35%, respectively. Remarkably, 98% to 77% of bacterial invasion was disrupted by the MT inhibitor Vinblastine (Figure 2).
3.3. Effect of Inhibitors on Bacterial Invasion of Caco-2 Cell Line
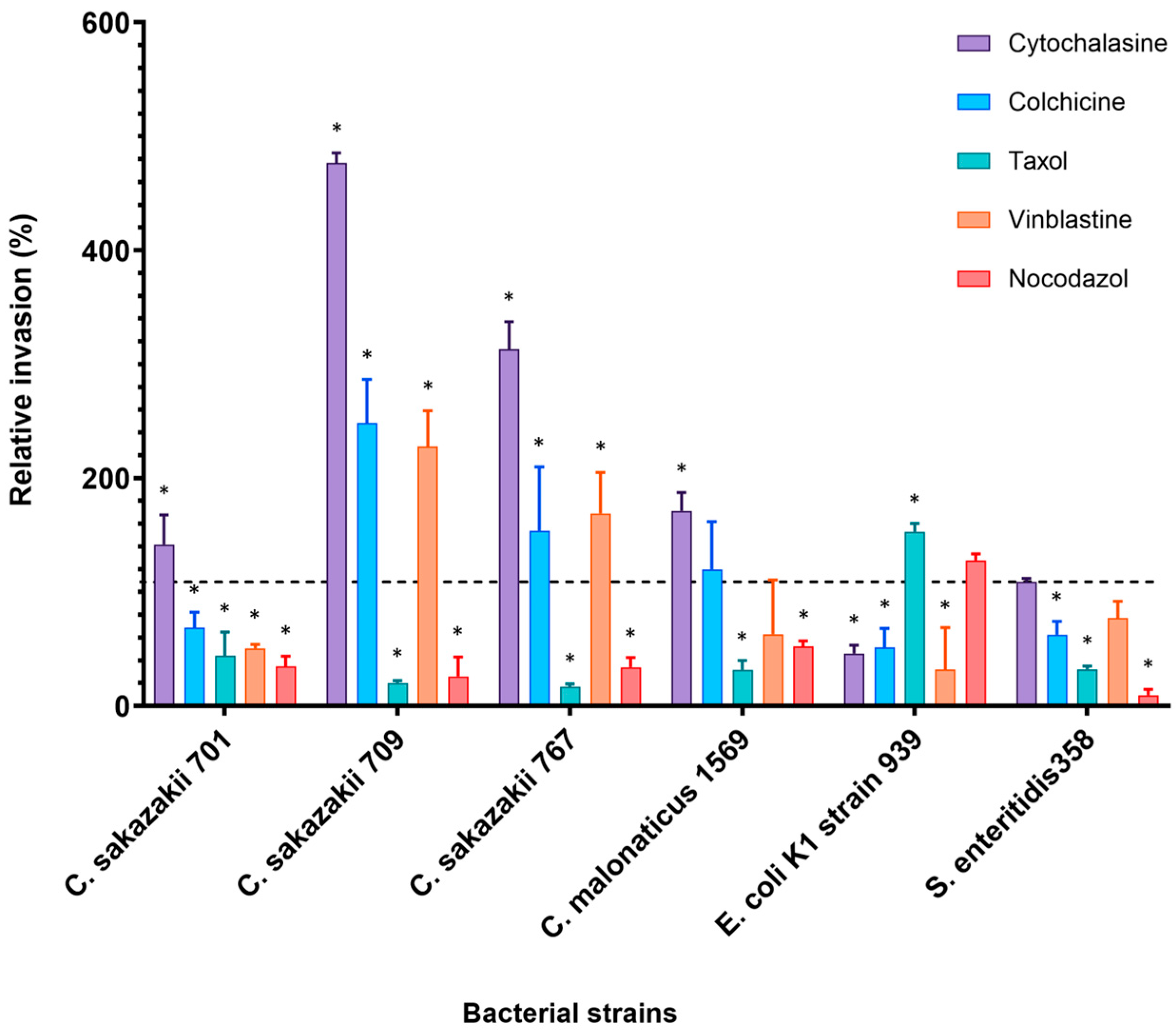
The effect of cytoskeleton inhibitors on the bacterial invasion of Caco-2 cells was completely different to the results obtained from H4 cell line as shown in Figure 3.
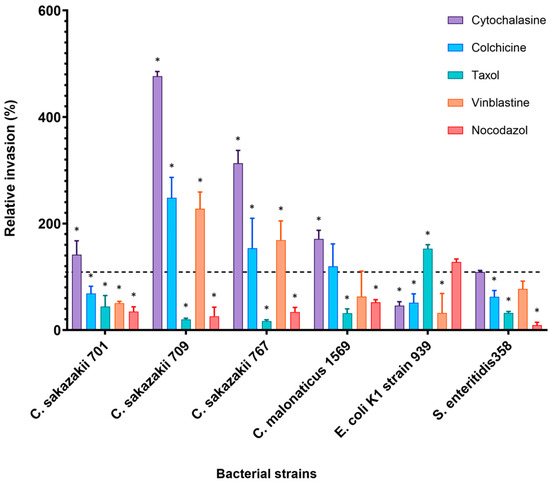
Figure 3.
Effect of cytoskeleton inhibitors on the invasion of selected bacterial isolates into Caco-2 cell line. Cells were pre-incubated with stated inhibitors (Cytochalasin D (2 µM), Nocodazole (20 µM), Colchicine (10 µM), Taxol (20 µM) and Vinblastine (20 µM)) as described by [11] and then infected with bacteria for 3 h. The data presented is the mean ± standard deviation of two independent experiments. Data are presented as a percentage of invasion compared to 100% control (untreated cells) for each strain, where no inhibitors were added to human cells (dashed line). The asterisk (*) indicates a statistically significant difference (p < 0.05) compared to control.
Cytochalasin D enhanced bacterial invasion in Caco-2 five-fold higher than the controls for C. sakazakii strain 709 (476%) and about three-fold for C. sakazakii strains 767 (313%). Invasion of C. sakazakii strains 701 and C. malonaticus strain 1569 increased by about 41% and 71% of relative invasion, respectively. An insignificant increase in the invasion was noted by S. enteritidis 358 (110%). In contrast, a 54% inhibition was observed in the E. coli K1 strain 939 (Figure 3). Similarly, preincubation of human cells with MT inhibitor Colchicine resulted in increased invasion of some bacterial isolates. C. sakazakii strains 709, 767, 1557, and C. malonaticus strain 1569 showed an increase of 148.6%, 53.8%, 38% and 19.7% higher than in untreated cells, respectively. Interestingly, C. sakazakii ST4 strain 701, although it is from the same sequence type as strains 709 and 767, was inhibited by 32% compared with the invasion in the absence of any inhibitor.
In contrast to H4 cell line, bacterial invasion of Caco-2 cells decreased when cells were pre-incubated with MT depolymerization, Nocodazole. For example, the invasion of C. sakazakii strains 709 and 767 was inhibited to only 40% and 54% of that in nontreated cells, respectively. In general, the overall invasion in the presence of Nocodazole ranged from 40 to 80% of relative invasion (Figure 3).
MT inhibitor Vinblastine variably affected the bacterial invasion into Caco-2 cells. The number of internalized C. sakazakii strains 709 and 767 increased by 127% and 70%, respectively, more than that in untreated cells. Alternately, Nocodazole inhibited the invasion of C. sakazakii 701 by 50% and S. enteritidis 358 by up to 23%, which is used as the positive control for the invasion experiment.
Pre-incubation of the Caco-2 cell line with MT inhibitor Taxol resulted in decreased invasion of all Cronobacter isolates. C. sakazakii strains 767 and 709 were significantly inhibited with up to 85% and 80% reduction, respectively. Only the invasion of E. coli K1 was increased by this inhibitor.
4. Discussion
Host cell cytoskeletons of eukaryotic cells have been previously found to be involved in bacterial adhesion and invasion by pathogenic bacteria, such as Salmonella, Shigella, Yersinia, and Listeria [9,10,30]. Several studies investigated the effect of cytoskeleton inhibitors on bacterial invasion into different cell lines, including the invasion of E. coli to brain microvascular endothelial cells (BMEC) [31], Plesiomonas shigelloides to Caco-2 cells [32] and the invasion of Pseudomonas aeruginosa into human middle ear epithelial cells (HMEECs) [33]. Mohan Nair and Venkitanarayanan [11], investigated the effect of several cytoskeleton inhibitors on Enterobacter sakazakii (Cronobacter spp.) invasion into human intestinal (INT407) cells, and their findings of Cytochalasin D and Vinblastine were in parallel with H4 results in our study. However, according to the conclusions of most previous studies, the inhibition of bacterial invasion is more likely to be strain-dependent.
Sun et al., (2020) have used Cytochalasin D, the F-actin inhibitor, to investigate the role of Salmonella plasmid virulence SpvB in bacterial pathogenicity. SpvB acts in redistributing some of the junctional protein and results in increased bacterial entry and translocation by both wild-type and mutant SpvB salmonella strains into Caco-2 cells [30]. This result supports our findings when Cytochalasin D increased the number of internalized Cronobacter and salmonella isolates in Caco-2 cell line. However, this is in contrast with H4 cells results, as the invasion of all tested strains was inhibited by the MF inhibitor Cytochalasin D. In general, the utilization of MF in neonatal H4 cells in bacterial invasion demonstrates the possible role of cell component development in infection control.
Although several studies have investigated the role of MF and MT in bacterial invasion into human cell lines, this is the first report to use cytoskeleton inhibitors with the H4 cell line in examining the invasion of selected bacterial isolates associated with serious pathology in neonates from the genus Cronobacter. In addition, due to the historical association of E. coli K1 with neonatal meningitis, one strain from the NTU collection serotype (O-393) was used in this study to compare with Cronobacter.
All strains showed remarkable levels of invasion in both cell lines. Cronobacter strains showed invasion levels in H4 and Caco-2 cell lines that ranged from 0.2% to 0.8% of the initial inoculum, which is considerably higher compared with the non-invasive strain E. coli K12 (Figure 1). However, although E. coli K1 strain 939 showed noticeably low levels of invasion compared to Cronobacter isolates, this strain was among the highest invasive E. coli strains to Caco-2, Human brain cell line (HBMEC) and rat brain cell line (rBCEC4) and constantly persisted in U937 macrophages for about 48 h [34]. These virulence traits constitute serious pathological ability, enabling this particular strain to reach the spinal fluid and infect the human brain.
Overall, the findings of this study indicate that the invasion of most strains was largely affected by cytoskeleton inhibitors. As we summarized in Figure 4, the invasion of all bacterial strains, especially Cronobacter, into neonatal H4 cells was reduced by Cytochalasin D and Colchicine, including E. coli and Salmonella. This result suggests that bacterial strains facilitate their uptake into this cell line by a mechanism of redistributing MF and MT, including F-actin. Nocodazole enhanced the invasion of C. sakazakii strains 709 and 767 and suppressed the others, including C. sakazakii 701 from the same sequence type (ST4), which was responsible for neonatal death due to necrotizing enterocolitis type three (NECIII). This variation is possibly due to differences in strain selectivity of the invasion mechanism. These two strains (709 and 767) were able to enter the bloodstream and develop septicaemia and fatal meningitis, respectively. Wide range of inhibition exposed by other inhibitors ranged from only 2% and 3% of relative invasion shown by strains 701 and 767 as a result of preincubation of cell lines with vinblastine, respectively, and up to only 5% inhibition showed by C. sakazakii 767 and C. malonaticus 1569 as an effect of Taxol. In contrast, the effect of eukaryotic cytoskeleton inhibitors on the bacterial invasion of Caco-2 was different to that of H4 cells. Cytochalasin D enhanced the invasion of most bacterial strains, and C. sakazakii strain 709 showed about five-fold the untreated cells (Figure 3). Kim and Loessner reported an increase of about 700% invasion to Cytochalasin D treated Caco-2 cells by Cronobacter spp. isolate, which is previously known as Enterobacter sakazakii [35]. The increase in bacterial invasion to Caco2 cells is seemingly due to the disruption of human actin filament network, which is necessary for bacterial uptake and internalization, and the increase in bacterial invasion suggests that these structural proteins play an important role in impeding microbial infection in adult cells. In contrast, bacterial invasion was significantly reduced in Cytochalasin D-treated H4 cells, indicating a possible expansion of this cell component.
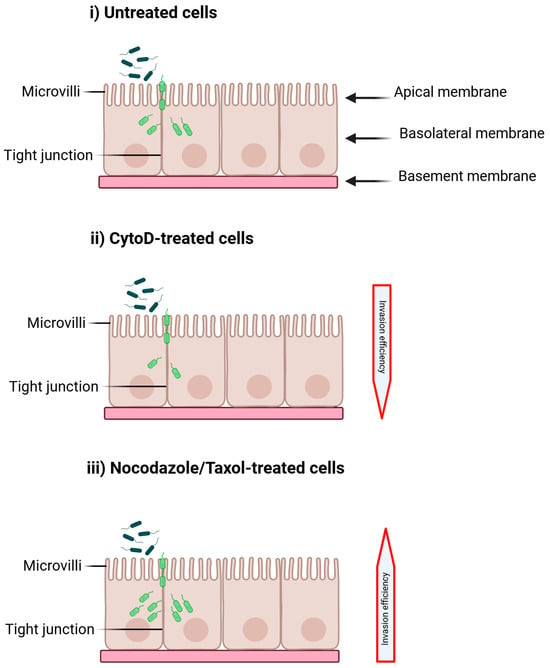
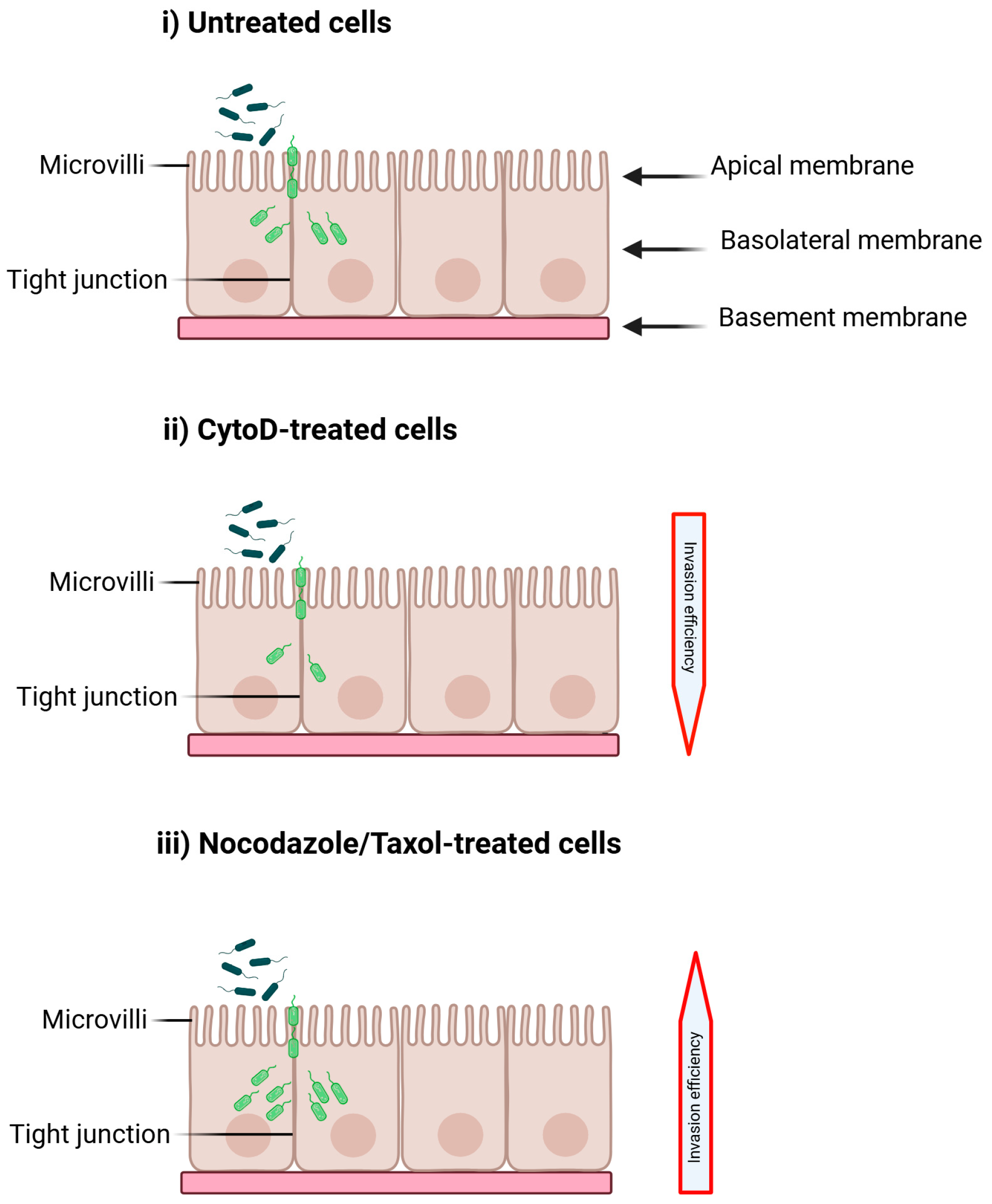
Figure 4.
The effect of cytoskeleton inhibitors on Cronobacter invasion is examined under three different conditions: (i) untreated H4 cells, (ii) cells treated with Cytochalasin D (CytoD), and (iii) cells treated with Nocodazole or Taxol. Alterations in cytoskeleton function may impede invasion (or decrease the invasion efficiency (ii)) by blocking pseudopodia formation and engulfment, or they may enhance invasion (or increase the invasion efficiency (iii)) by disrupting tight junctions or outer membrane proteins. Created with BioRender. https://BioRender.com/kdr6hg5 (18 August 2025).
Previous findings by Kim and Loesser have also shown a variable result, with a clear strain-specific role of bacterial invasion in Caco-2 Cells and suggested that the increase in bacterial invasion is likely due to the disruption of tight junction development by cytoskeleton inhibitors in Caco-2 cells [35].
Invasion of E. coli K1 into both cell lines was reduced by most inhibitors. However, Taxol revealed an increased invasion of Caco-2 cells, which warrants further investigation, which may include microtubule-mediated invasion pathways. Similarly, Ayibieke et al. (2024) found that Cytochalasin D inhibits E. coli invasion and observed F-actin accumulation at bacterial entry sites, implicating actin polymerization in internalization [36].
5. Conclusions
Our findings highlight the role of the eukaryotic cytoskeleton in the bacterial invasion of host cells. The varying effects of inhibitors on bacterial invasion may stem from differences in their impact on cytoskeleton rearrangement and the genetic diversity of bacteria. This diversity reflects the presence of different effector proteins that can modify the host cytoskeleton to facilitate bacterial uptake and induce infection. This can be observed in the differing levels of invasion exhibited by the Cronobacter ST4 isolates, specifically strains 701, 709, and 767, in response to the inhibitors used in this study.
Importantly, we hypothesize that the mechanisms of invasion in neonatal tissues may be different from those in adults. There may be a greater utilization of neonatal cell components during the invasion process compared to what is observed in adult cells. Consequently, using adult-derived cell lines to study neonatal infections may not accurately represent the infection mechanisms at play. Additionally, it is worth noting that Caco-2 cells are derived from a 74-year-old adult with colon cancer, while H4 cells originate from the normal intestine of a neonate. These two critical factors, age and the source of the cell line, make H4 cells more suitable than Caco-2 cells for investigating the neonatal infection process.
Author Contributions
M.B.A., designed the research, methodology, formal analysis, validation and writing—original draft preparation. K.M.I., A.M.A. and M.T.S., methodology, formal analysis, and writing—review and editing. B.A.E., supervision, validation, project administration, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries may be directed to the corresponding authors.
Acknowledgments
We thank the Libyan Ministry of Higher Education and Zawia University for their support.
Conflicts of Interest
All authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
Appendix A

Table A1.
Showing the details of the strains used in this study. NTU: Nottingham Trent university, NA: Not Applicable; USA: United States of America; UK: United Kingdom; UN: Unknown. ST: sequence type, NECIII: necrotizing enterocolitis stage: 3.
Table A1.
Showing the details of the strains used in this study. NTU: Nottingham Trent university, NA: Not Applicable; USA: United States of America; UK: United Kingdom; UN: Unknown. ST: sequence type, NECIII: necrotizing enterocolitis stage: 3.
| NTU No | Species | ST | Country | Source | Year | Disease | Site of Isolation |
|---|---|---|---|---|---|---|---|
| 701 | C. sakazakii | 4 | France | Clinical | 1994 | Fatal neonatal infection, NECIII | Infant, peritoneal fluid |
| 709 | C. sakazakii | 4 | France | Clinical | 1994 | Septicaemia | Infant trachea isolate |
| 767 | C. sakazakii | 4 | France | Clinical | 1994 | Fatal neonatal meningitis | Infant trachea isolate |
| 1569 | C. malonaticus | 307 | USA | Clinical | 1994 | Fatal infant meningitis | Blood isolate |
| 939 | E. coli K1 | 95 | UK | Clinical | NA | Clinical | Enteral Feeding Tube |
| 358 | Salmonella enteritidis 358 | NA | Positive control in tissue culture experiments | ||||
| 1230 | E. coli K12 | NA | Negative control in tissue culture experiments | ||||

Table A2.
Changes in the bacterial invasion of pre-incubated human cells with different cytoskeleton inhibitors compared with non-treated cells, with changes in CFU. CFU: colony forming unit, CytoD: Cytochalasin D, Vin: Vinblastine, Colch: Colchicine, Tax; Taxol and Noco: Nocodazole.
Table A2.
Changes in the bacterial invasion of pre-incubated human cells with different cytoskeleton inhibitors compared with non-treated cells, with changes in CFU. CFU: colony forming unit, CytoD: Cytochalasin D, Vin: Vinblastine, Colch: Colchicine, Tax; Taxol and Noco: Nocodazole.
| Isolate | Cell Line | Without Inhibitor (CFU/mL) | CytoD (CFU/mL) | Vin (CFU/mL) | Colch (CFU/mL) | Tax (CFU/mL) | Noco (CFU/mL) |
|---|---|---|---|---|---|---|---|
| C. sakazakii 701 | H4 | 1.5 × 105 | 3.25 × 104 | 3.15 × 103 | 1.32 × 104 | 1.43 × 105 | 1.32 × 105 |
| Caco-2 | 5.77 × 104 | 8.17 × 104 | 2.92 × 104 | 3.96 × 104 | 3.10 × 104 | 3.39 × 104 | |
| C. sakazakii 709 | H4 | 1.64 × 105 | 4.53 × 104 | 3.86 × 104 | 1.44 × 104 | 1.40 × 105 | 2.33 × 105 |
| Caco-2 | 1.23 × 105 | 5.84 × 105 | 2.79 × 105 | 3.05 × 105 | 2.45 × 104 | 4.89 × 104 | |
| C. sakazakii 767 | H4 | 2.29 × 105 | 5.8 × 104 | 8.36 × 103 | 1.83 × 104 | 3.71 × 105 | 2.69 × 105 |
| Caco-2 | 2.18 × 105 | 6.84 × 105 | 3.69 × 105 | 3.36 × 105 | 3.31 × 104 | 1.19 × 105 | |
| C. malonaticus 1569 | H4 | 3.3 × 105 | 4.87 × 104 | 3.94 × 104 | 1.79 × 104 | 3.11 × 105 | 2.77 × 105 |
| Caco-2 | 3.66 × 105 | 6.26 × 105 | 2.31 × 105 | 4.38 × 105 | 1.44 × 105 | 2.67 × 105 | |
| E.coli 939 | H4 | 8.78 × 103 | 4.61 × 103 | 3.51 × 102 | 3.10 × 103 | 4.44 × 103 | 2.81 × 103 |
| Caco-2 | 8.22 × 103 | 3.79 × 103 | 4.46 × 103 | 4.22 × 103 | 1.23 × 104 | 6.58 × 103 | |
| Salmonell enteritidis 358 | H4 | 2.40 × 106 | 1.4 × 106 | 5.03 × 105 | 1.11 × 106 | 1.58 × 106 | 1.27 × 106 |
| Caco-2 | 7.29 × 105 | 7.96 × 105 | 5.62 × 105 | 4.57 × 105 | 3.01 × 105 | 3.54 × 105 |
References
- Colonne, P.M.; Winchell, C.G.; Voth, D.E. Hijacking host cell highways: Manipulation of the host actin cytoskeleton by obligate intracellular bacterial pathogens. Front. Cell. Infect. Microbiol. 2016, 6, 107. [Google Scholar] [CrossRef]
- Sarkar, P.; Kontsedalov, S.; Lebedev, G.; Ghanim, M. The actin cytoskeleton mediates transmission of “Candidatus Liberibacter solanacearum” by the Carrot Psyllid. Appl. Environ. Microbiol. 2021, 87, e02393-20. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, H.; Feng, Y.; Hou, X. Development of anticancer drugs: From mechanical properties of tumor stiffness to cytoskeleton-targeting natural products. Preprints 2021. [Google Scholar] [CrossRef]
- Kim, M.; Song, K.; Jin, E.J.; Sonn, J. Staurosporine and cytochalasin D induce chondrogenesis by regulation of actin dynamics in different way. Exp. Mol. Med. 2012, 44, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Schmidt, K.; Karger, M.; Stadler, M.; Stradal, T.E.B.; Rottner, K. Cytochalasans and their impact on actin filament remodeling. Biomolecules 2023, 13, 1247. [Google Scholar] [CrossRef]
- Laisne, M.-C.; Michallet, S.; Lafanechère, L. Characterization of microtubule destabilizing drugs: A quantitative cell-based assay that bridges the gap between Tubulin based- and cytotoxicity assays. Cancers 2021, 13, 5226. [Google Scholar] [CrossRef]
- Meyer, D.H.; Rose, J.E.; Lippmann, J.E.; Fives-Taylor, P.M. Microtubules are associated with intracellular movement and spread of the periodontopathogen Actinobacillus actinomycetemcomitans. Infect. Immun. 1999, 67, 6518–6525. [Google Scholar] [CrossRef]
- Naydenov, N.G.; Marino-Melendez, A.; Campellone, K.G.; Ivanov, A.I. Cytoskeletal mechanisms regulating attaching/effacing bacteria interactions with host cells: It takes a village to build the pedestal. BioEssays 2024, 46, 2400160. [Google Scholar] [CrossRef]
- de Souza Santos, M.; Orth, K. Subversion of the cytoskeleton by intracellular bacteria: Lessons from Listeria, Salmonella and Vibrio. Cell. Microbiol. 2015, 17, 164–173. [Google Scholar] [CrossRef]
- Navarro-Garcia, F.; Serapio-Palacios, A.; Ugalde-Silva, P.; Tapia-Pastrana, G.; Chavez-Dueñas, L. Actin cytoskeleton manipulation by effector proteins secreted by diarrheagenic Escherichia coli pathotypes. BioMed Res. Int. 2013, 2013, 374395. [Google Scholar] [CrossRef]
- Mohan Nair, M.K.; Venkitanarayanan, K. Role of bacterial OmpA and host cytoskeleton in the invasion of human intestinal epithelial cells by Enterobacter sakazakii. Pediatr. Res. 2007, 62, 664–669. [Google Scholar] [CrossRef]
- Healy, B.; Cooney, S.; O’Brien, S.; Iversen, C.; Whyte, P.; Nally, J.; Callanan, J.J.; Fanning, S. Cronobacter (Enterobacter sakazakii): An opportunistic foodborne pathogen. Foodborne Pathog. Dis. 2010, 7, 339–350. [Google Scholar] [CrossRef]
- Hariri, S.; Joseph, S.; Forsythe, S.J. Cronobacter sakazakii ST4 strains and neonatal meningitis, United States. Emerg. Infect. Dis. 2013, 19, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.M.; Alsonosi, A.M.; Agena, M.B.; Elgamoudi, B.A.; Forsythe, S.J. Multiplex determination of K-antigen and colanic acid capsule variants of Cronobacter sakazakii. Genes 2024, 15, 1282. [Google Scholar] [CrossRef] [PubMed]
- Alsonosi, A.M.; Ibrahim, K.M.; Elgamoudi, B.A.; Agena, M.B.; Forsythe, S.J. The potential role of rpoS and ompR in the acid resistance and desiccation tolerance of Cronobacter malonaticus Strains. Microbiol. Res. 2025, 16, 53. [Google Scholar] [CrossRef]
- Holý, O.; Cruz-Córdova, A.; Xicohtencatl-Cortes, J.; Hochel, I.; Parra-Flores, J.; Petrželová, J.; Fačevicová, K.; Forsythe, S.; Alsonosi, A. Occurrence of virulence factors in Cronobacter sakazakii and Cronobacter malonaticus originated from clinical samples. Microb. Pathog. 2019, 127, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Alsonosi, A.; Hariri, S.; Kajsík, M.; Oriešková, M.; Hanulík, V.; Röderová, M.; Petrželová, J.; Kollárová, H.; Drahovská, H.; Forsythe, S.; et al. The speciation and genotyping of Cronobacter isolates from hospitalised patients. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1979–1988. [Google Scholar] [CrossRef]
- Joseph, S.; Sonbol, H.; Hariri, S.; Desai, P.; McClelland, M.; Forsythe, S.J. Diversity of the Cronobacter genus as revealed by multilocus sequence typing. J. Clin. Microbiol. 2012, 50, 3031–3039. [Google Scholar] [CrossRef]
- Joseph, S.; Desai, P.; Ji, Y.; Cummings, C.A.; Shih, R.; Degoricija, L.; Rico, A.; Brzoska, P.; Hamby, S.E.; Masood, N.; et al. Comparative analysis of genome sequences covering the seven Cronobacter species. PLoS ONE 2012, 7, e49455. [Google Scholar] [CrossRef]
- Law, R.J.; Gur-Arie, L.; Rosenshine, I.; Finlay, B.B. In vitro and in vivo model systems for studying enteropathogenic Escherichia coli infections. Cold Spring Harb. Perspect. Med. 2013, 3, a009977. [Google Scholar] [CrossRef]
- Buhrke, T.; Lengler, I.; Lampen, A. Analysis of proteomic changes induced upon cellular differentiation of the human intestinal cell line Caco-2. Dev. Growth Differ. 2011, 53, 411–426. [Google Scholar] [CrossRef]
- Huang, X.; Huang, C. Fructose shields human colorectal cancer cells from hypoxia-induced necroptosis. Npj Sci. Food 2024, 8, 71. [Google Scholar] [CrossRef]
- Alsonosi, A.M.; Holy, O.; Forsythe, S.J. Characterization of the pathogenicity of clinical Cronobacter malonaticus strains based on the tissue culture investigations. Antonie Van Leeuwenhoek 2018, 112, 435–450. [Google Scholar] [CrossRef]
- Claud, E.C.; Savidge, T.; Walker, W.A. Modulation of human intestinal epithelial cell IL-8 secretion by human milk factors. Pediatr. Res. 2003, 53, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Agena, M.B. Neonatal Exposure to Pathogens: Determining Key Virulence Factors. Ph.D. Thesis, Nottingham Trent University, Nottingham, UK, 2017. Available online: https://irep.ntu.ac.uk/id/eprint/32859 (accessed on 18 August 2025).
- Lněničková, K.; Šadibolová, M.; Matoušková, P.; Szotáková, B.; Skálová, L.; Boušová, I. The modulation of phase II drug-metabolizing enzymes in proliferating and differentiated CaCo-2 cells by hop-derived prenylflavonoids. Nutrients 2020, 12, 2138. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Park, H.G.; Xu, C.; Lawrence, P.; Su, X.; Wijendran, V.; Walker, W.A.; Kothapalli, K.S.; Brenna, J.T. Human fetal intestinal epithelial cells metabolize and incorporate branched chain fatty acids in a structure specific manner. Prostaglandins Leukot. Essent. Fat. Acids 2017, 116, 32–39. [Google Scholar] [CrossRef]
- Uapipatanakul, B. A study of the effects of waste egg and shrimp shells on the toxicity immobilisation of chemicals. Orient. J. Chem. 2018, 34, 1926–1929. [Google Scholar] [CrossRef]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The estimation of the bactericidal power of the blood. J. Hyg. 1938, 38, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yang, S.; Deng, Q.; Dong, K.; Li, Y.; Wu, S.; Huang, R. Salmonella Effector SpvB disrupts Intestinal epithelial barrier integrity for bacterial translocation. Front. Cell. Infect. Microbiol. 2020, 10, 606541. [Google Scholar] [CrossRef]
- Rudrabhatla, R.S.; Selvaraj, S.K.; Prasadarao, N.V. Role of Rac1 in Escherichia coli K1 invasion of human brain microvascular endothelial cells. Microbes Infect. 2006, 8, 460–469. [Google Scholar] [CrossRef]
- Tsugawa, H.; Ono, T.; Murakami, H.; Okawa, Y. Invasive phenotype and apoptosis induction of Plesiomonas shigelloides P-1 strain to Caco-2 cells. J. Appl. Microbiol. 2005, 99, 1435–1443. [Google Scholar] [CrossRef]
- Mittal, R.; Grati, M.; Gerring, R.; Blackwelder, P.; Yan, D.; Li, J.D.; Liu, X.Z. In vitro interaction of Pseudomonas aeruginosa with human middle ear epithelial cells. PLoS ONE 2014, 9, e91885. [Google Scholar] [CrossRef] [PubMed]
- Alkeskas, A.; Ogrodzki, P.; Saad, M.; Masood, N.; Rhoma, N.R.; Moore, K.; Farbos, A.; Paszkiewicz, K.; Forsythe, S. The molecular characterisation of Escherichia coli K1 isolated from neonatal nasogastric feeding tubes. BMC Infect. Dis. 2015, 15, 449. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.P.; Loessner, M.J. Enterobacter sakazakii invasion in human intestinal Caco-2 cells requires the host cell cytoskeleton and is enhanced by disruption of tight junction. Infect. Immun. 2008, 76, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Ayibieke, A.; Wajima, T.; Kano, S.; Chatterjee, N.S.; Hamabata, T. The colonization factor CS6 of enterotoxigenic Escherichia coli contributes to host cell invasion. Microb. Pathog. 2024, 190, 106636. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).