Abstract
In the case of a notifiable animal disease like salmonellosis, manure is contaminated and must be disinfected. This can be performed using heat measures, chemical disinfectants, or long-term storage. All these measures bring along severe economic, ecological, and logistical problems. The aim of this study was to evaluate lactic acid fermentation (LAF) as an alternative disinfection method. Fermentation was started by adding a carbohydrate source to the manure and creating anaerobic conditions. For testing, cattle manure was enriched with different carbohydrate (CHO) sources and spiked with Salmonella Typhimurium (S. Ty.). The samples were incubated at 10 °C and 21 °C for 111 days (Exp1) and at 21 °C for 50 days (Exp2). The microbial shift was determined using cultural methods and MALDI-TOF. Both the change in pH and Enterococcus spp. were tested as suitable indicators. The results showed the different suitability of the selected CHO for hygienization by LAF. Using squeezed oat as an additive, S. Ty was reduced to below the detection limit under both temperature conditions within 21 days and 14 days. Additional saccharose decreased the reduction time. This study showed that LAF is a valuable alternative for disinfecting cattle manure in the case of bovine salmonellosis. Using this method, both manure and feed residues can be treated in one approach and afterwards be used as fertilizer.
1. Introduction
Livestock is an important pillar of agriculture. In Germany alone, about 3.9 million dairy cows were registered in 54,300 livestock farms in 2023 [1]. The resulting amount of manure is considerable. On average, one dairy cow produces 1.5–1.8 m3 of manure per month [2]. This equates to about 70,000 tons of manure per year for dairy cattle in Germany. Usually, manure is returned to the nutrient cycle as fertilizer. Cattle manure is an important source of carbon (C) and nitrogen (N) for agriculture. Nevertheless, there is a potential risk of spreading foodborne diseases [3,4].
Cattle excrement, and thus manure, provides a remarkable substrate for the survival and spread of a wide range of pathogenic and saprophytic bacteria [4,5]. Salmonella are one of the most significant bacterial pathogens in foodborne diseases, and therefore they are of public concern [6,7]. In Germany, from 2016 to 2024, between 68 and 109 proven cases per year were described in cattle livestock [8]. These Gram-negative rods survive and multiply extensively in ileal and colon fluids [4,9], and they are finally excreted in cattle feces [10]. Factors such as temperature, lack of nutrients, osmotic stress, and exposure to UV light are known to affect their survival [11,12,13]. Salmonella have high tenacity and are capable of surviving in chicken manure compost for approximately 200 days [9]. Additionally, in aquatic environments, salmonellae have a high survival rate, particularly in highly eutrophicated areas [14]. Therefore, understanding their behavior in agricultural environments is crucial for improving risk evaluations and guidelines for the safe handling of pathogen-contaminated manure, compost, and soil [9,14].
In the case of a salmonellosis outbreak, it is almost impossible to identify the source of the infection [15]. It is necessary to consider the fundamental principles of veterinary medicine, namely the separation of sick from healthy cattle, the maintenance of a high hygienic standard, the treatment and monitoring of sick animals, and the disinfection of contaminated material. During an outbreak of a notifiable animal disease in livestock farms, a large amount of contaminated manure accumulates. Hence, it is important to establish a system of monitoring and decontamination [5]. The standard decontamination methods for biomass according to disinfection guidelines are chemical disinfection, long-term storage for 3 to 6 months, aerobic thermophilic stabilization (T > 50 °C for 3 days, pH > 8.5), and, for certain diseases, the injection of liquid manure into the soil [16]. These methods require different amounts of time and technical equipment.
In this study, lactic acid fermentation (LAF) is tested as an alternative method. Lactic acid bacteria (LAB), like Lactobacillus or Enterococcus, produce lactic acid by the fermentation of organic matter like food, e.g., [17,18]. Lactic acid as a product is widely used in various industries such as food and animal feed [17,18,19], textiles and cosmetics [18], and pharmacy [18,19] and in the production of bioplastics [20]. LAF is also used for the production of volatile fatty acids (VFAs) [21]. Here, the initial pH and fermentation time had an important effect on the production of metabolites [21]. Cow manure contains an abundant lignocellulosic fraction, which can be valorized as the raw material for several fermentative processes, including the production of lactic acid (LA) using LAB [20,22].
To activate LAF on animal farm manure for the purpose of hygienization, anaerobic conditions and a carbon source are needed [3]. An increasing number of LAB produce lactic acid and other VFAs so that cattle manure is acidified. Depending on the pH, VFAs can be bactericidal [23,24,25]. This prevents oxidation and thus the growth of pathogens [26]. Earlier studies showed that pathogens were significantly reduced by LAF, and cattle manure can be returned to nutrient circulation [3]. Enterococci were discussed as an indicator organism for the fermentation process. Furthermore, it was shown that LAF has a good N and C outcome [3]. The mass loss during fermentation (<2.45%) is much lower compared to the mass loss through composting (15–57%), making it a cost-effective and ecologically beneficial approach [3]. Knowledge of the microbial community is necessary to improve the fermentation process [21].
The current study was conducted to determine the effect of temperature and carbon source on the inactivation of S. Typhimurium (S. Ty.) in cattle manure by LAF. The influence of outside conditions during the year were simulated by different temperatures according to the testing guidelines of the German Veterinary Medical Society [27]. Different carbohydrates (CHO) were compared, and the shift in the microbial community was investigated with both cultural methods and MALDI-TOF. The hypothesis that enterococci can be monitored as an indicator organism for the fermentation process was tested.
2. Materials and Methods
2.1. Sample Source and Preparation
The cattle manure (CM) used in this study was collected from a slurry store (cattle) from University of Hohenheim, Stuttgart, Germany. For sample preparation, hay cobs (Sniff Spezialdiäten GmbH, Soest, Germany), squeezed oat (Mühle Richter, Mockrehna, Germany), and saccharose (Raffinadezucker Diadem, Nordzucker, Braunschweig, Germany) were added to the cattle manure, according to Table 1, as sources of carbohydrates (CHO). The content of squeezed oat differs between the two setups. After mixing thoroughly, aliquots of 27 g were weighed into 50 mL tubes (Sarstedt, Nümbrecht, Germany) and frozen at −18 °C until further processing.

Table 1.
Overview of kind and amount of added carbohydrates in different approaches in Exp1 (a 21 °C, b 10 °C) and Exp2. Temperatures are indicated in italics. Abrv.: CM—cattle manure; HCs—hay cobs; SO—squeezed oat; Sac—saccharose.
2.2. Experimental Design
To test the influence of temperature and different CH sources, two types of setups were chosen. In Exp1 the dependence of LAF on temperature and hay cobs and squeezed oat as carbon sources was investigated. The effect of sugar and squeezed oat on the fermentation process was investigated in Exp2.
For Exp1 and Exp2, an inoculum of Salmonella enterica subsp. enterica ser. Typhimurium (S. Ty.) was grown in an overnight culture in tryptone soy broth (Oxoid, Wesel, Germany) at 36 °C. To determine the initial cell concentration, a dilution series to 10−12 was prepared and spread out on Xylose–Lysin–Desoxycholat agar plates (Roth, Karlsruhe, Germany; in the following, it is called XLD agar) [28] and Salmonella-Shigella agar (Sifin, Berlin, Germany; in the following, it is called SS agar) (Exp1) [28,29] or water-blue–metachrome-yellow lactose agar acc. to Gassner (modified, Sifin; Exp2; in the following, it is called Gassner agar) [30] in triplicate. The colonies of S. Ty. on XLD agar plates were characterized by a black dot in the center [31]. Unspecific colonies were not counted. After incubation at 36 °C overnight, colony-forming units (CFU) were counted, and cell concentration was calculated to log10 (CFU/g sample).
For every approach, seven replicates of prepared cattle manure were thawed, as shown in Table 1. Three milliliters of a cell suspension (5 × 109 CFU/mL) of S. Ty. was added to 27 g of each aliquot of the sample and mixed thoroughly. PBS was added to negative controls instead of the Salmonella suspension to achieve a comparable moisture content. For anaerobic conditions, the samples were placed into an anaerobic jar (Oxoid) with an anaerobic kit (Anaerocult A, Millipore, Merck, Darmstadt, Germany) and incubated at 21 °C or 10 °C according to Table 1.
The concentrations of the microorganisms were determined immediately after inoculation (0 d) and after 3, 7, 14, 21, 30, 50, 63, 84, and 111 days for Exp1 and after 0, 3, 7, 14, 21, 28, and 50 days of incubation for Exp2 by taking 0.5 g of each replicate and diluting it in 4.5 mL PBS. A dilution series was prepared from each sample on a 48-well plate (Corning Inc., Corning, NY, USA). In every dilution step, 20 µL was plated in duplicate on the following agar plates: Columbia agar with 5% sheep blood (ISG Intermed, Geesthacht, Germany) for the identification of aerobic and simultaneously anaerobic organisms [32], Gassner agar for detecting Gram-negative organisms, XLD agar for the detection of Salmonella and Shigella, Citrate–azide–tween–carbonate agar (CATC, Sifin) to identify Enterococcus [33,34], and MRS agar (Oxoid) to screen the growth of lactic acid bacteria [35,36]. The plates were incubated under aerobic conditions, except the MRS agar plates and one of the blood agar plates, which were incubated under anaerobic conditions as described above. All agar plates were incubated for 24 h, except the CATC and MRS plates which were incubated for 48 h.
For the detection of sulfite-reducing microorganisms such as clostridia, 0.5 g of the treated sample was diluted in 4.5 mL PBS. A dilution series 10−2 to 10−7 on liquid differential reinforced clostridia medium (DRCM, Roth, Germany) was performed on a 96-well plate (U-well, Corning) and cultivated at 37 °C under anaerobic conditions [37,38]. For the estimation of sporulated sulfate reducers, the samples were heated for 10 min at 80 °C on a thermocycler (thermomixer comfort, Eppendorf, Hamburg, Germany). Samples were treated as described above. All plates were incubated for six days. The results were analyzed as described by Scheinemann et al. [3].
2.3. pH Measurement
In Exp2 for pH measurement, three sample tubes of each approach were incubated according to Table 1 without the addition of Salmonella. On all sampling days, pH was measured with an Orion 3 star pH Benchtop (Thermo Electron Corporated, Karlsruhe, Germany).
2.4. Salmonella Enrichment
To confirm the removal of S. Ty. from samples, an enrichment of Salmonella according to the Biowaste Ordinance was performed [39,40,41]. Briefly, 270 g of prepared cattle manure according to Exp1 was inoculated with S. Ty. and incubated at 10° or 21 °C for 80 days. Then, 50 g of each bottle was sampled for further processing. From all sample tubes of one approach from Exp2, 7 g of each aliquot was pooled to obtain about 50 g per approach. All samples were diluted in 450 mL peptone water (buffered, Roth) with novobiocin (Roth) and incubated at 37° overnight. From each sample bottle, 100 µL of suspension was transferred into ten tubes with 9.9 mL Rappaport–Vassiliadis Bouillon (Roth). Five tubes were then incubated at 36 °C and five tubes at 42 °C overnight. From each tube, 200 µL was placed on three XLD agar plates and three Gassner agar plates. Plates were incubated at 36 °C for 48 h and monitored for growth. The test was considered successful if no salmonellae were detectable in the collection samples.
2.5. MALDI-TOF
For the detection of the dominant groups of microorganisms, MALDI-TOF measurements were carried out [42]. Fourteen tubes of each of CM, CM + SO, and CM + HC were incubated, seven at 21 °C, seven at 10 °C, without the addition of Salmonella serovars. Samples were taken after 0, 3, 7, 14, 21, 30, 50, 63, and 100 days and treated as described above. Colonies from blood agar, CATC agar, XLD agar, Gassner agar, and MRS agar plates were picked randomly for MALDI-TOF MS analysis. Cells of each strain were aseptically transferred to microtubes with 300 µL of deionized water and mixed thoroughly. Subsequently, 900 µL of ethanol was added, mixed thoroughly, and centrifuged at the maximum speed (21,100× g) for two minutes. The supernatant was discarded, and the tubes were centrifuged again. The remaining ethanol was discarded by pipetting, and the pellet was air-dried for two to three minutes. Then, 70% formic acid was added and resuspended by pipetting. Pure ACN was added, softly mixed, and centrifuged at the maximum speed for two minutes. After that, 1 µL of supernatant was transferred to a MALDI target plate and air-dried. The samples were spotted with 1 µL HCCA solution within 1 h and air-dried. Each MALDI-TOF sample was spotted in triplicate to evaluate reproducibility. Samples were then analyzed in a MALDI-TOF ultraflextreme LT spectrometer (Bruker Daltonics, Bremen, Germany), using the MALDI Biotyper 3.0 automatic system. Bruker-Database with MBT-Compass 4.1-Software was used for analyses.
3. Results
3.1. Temperature Experiment
The inactivation time of S. Ty. depends on the temperature and the carbon source (Figure 1). At 21 °C, the process of LAF and the decrease in Salmonella were achieved much faster in all of the different test conditions (Figure 1). In the approach with squeezed oat (OA), the number CFU/g sample of S. Ty. dropped below the detection limit after 15 days and remained there. In the approach enriched with hay cobs (HA), the CFU/g of S. Ty. decreased to log 1.2 ± 2 after 30 days and was not detectable after 50 days. The numbers of S. Ty. fell to log 1.0 ± 1.8 after 50 days in the non-enriched approach CHO-A. The enrichment of S. Ty. in OA after 80 days was negative. Thus, it was shown successfully that S. Ty. bacterial count was reduced in the samples with OA (Table 2).
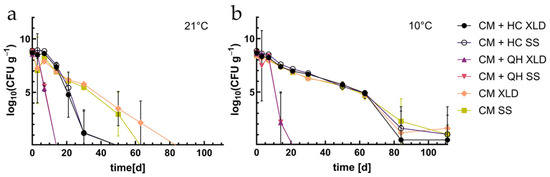
Figure 1.
EXP1: Survival of S. Ty. in cattle manure in different CHO approaches in log10 on XLD and SS agar (a) at 21 °C and (b) at 10 °C. Abrv.: CM—cattle manure; HCs—hay cobs; SO—squeezed oat.

Table 2.
Overview of results of Salmonella enrichment: (+)—detection of Salmonella in sample; (−)—negative result. Abrev. as in Figure 1.
At 10 °C, in approaches with hay cobs (HA) and without additional carbohydrates (CHO-A), S. Ty decreased from log 9 to log 5 within 60 days without significant differences between the approaches. After 111 days they were at the detection limit but still detectable on agar plates. OA decreased to log 2.1 ± 0.2 after 21 days and was undetectable afterwards. Nevertheless, the enrichment of S. Ty. of OA resulted in one positive sample after 80 days and showed that S. Ty. was still present in small numbers in the sample.
3.2. Saccharose Experiment
Changes in the microbial community depending on the carbon source (Exp2) are shown in Figure 2. Aerobically and anaerobically incubated blood agar plates provided a general overview of the microbial community and its development (Figure 2a,b). After 3 days, an increase in CFU/g was observed in all approaches, followed by a steady decrease to log 6 to log 7. The increase and decrease were visible in all approaches and under both incubation conditions.
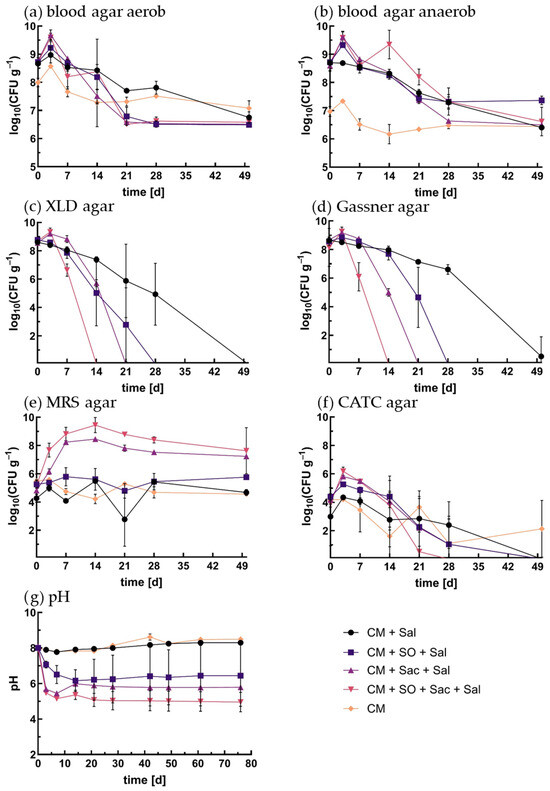
Figure 2.
Results of Exp2 CFU/g of microbial community in log10 at 21 °C: (a) blood agar incubated under aerobic conditions, (b) blood agar incubated under anaerobic conditions, (c) XLD agar, (d) Gassner agar, (e) MRS agar, (f) CATC agar, (g) pH. Abrev.: CM—cattle manure; Sal—S. Ty.; SO—squeezed oat; Sac—saccharose.
The initial CFU/g of the non-spiked approach (NSA) of aerobic and anaerobic incubations were log 8.0 ± 0.2 and 7.0 ± 0.2, respectively, while the S. Ty.-enriched approaches were at log 8.7 and log 8.6 under aerobic and anaerobic conditions, respectively. The significant difference in log CFU/g at T0 was due to the addition of Salmonella. Under aerobic conditions, the CFU values of the CHO-enriched approaches dropped below those of the NSA after 14 days, while under anaerobic conditions, the CFU/g of the NSA remained the lowest.
The saccharose approach (SA) and squeezed oat–saccharose approach (OSA) reached the maximum values of the total microbial abundance up to log 9.6 ± 0.2 and 9.7 ± 0.2 CFU/g, respectively. CHO-A showed the same trends with lower values. The anaerobically incubated blood agar plates revealed the same pattern of increase and decrease in CFU/g. In contrast to the aerobic incubation, the OSA had a second peak after 14 days before a constant decrease to log 7.
In the NSA, all specific colonies of S. Ty. grew on both agar types. In the spiked approaches, the initial concentrations were about log 8.5 ± 0.2 (Figure 2c,d). In the OSA the number of colonies increased slightly after three days, followed by a rapid decrease, and they were not detectable after two weeks anymore. Similarly, S. Ty. was not detectable in SA after three weeks and in OA after four weeks. In CHO-A, the number of CFU also decreased but was still detectable until the end of sampling.
The enrichment of S. Ty in all approaches of Exp2 after 50 days showed negative results for SA and OA but positive results for OA and CHO-A (Table 2). The repetition on day 75 showed negative results for OA but still remained positive for CH-A.
The initial concentrations of lactobacilli were between log 4.2 and 5.2 CFU/g (Figure 2e). The maximum values of log 8.5 ± 0.1 for SA and log 9.4 ± 0.5 for the OSA were reached after 14 days. Afterwards, the number of CFU/g decreased to log 7.2 ± 0.0 and 7.3 ± 1.6 at the end of the experiment, respectively. In OA the number of CFU/g increased slightly and dropped to a minimum of log 4.8 ± 0.5 after 21 days and increased again to 5.8 ± 0.1 after 50 days. The two non-enriched approaches showed oscillating numbers of CFU/g between log 4.2 ± 0.3 and 5.6 ± 0.2 for the NSA and log 5.5 ± 0.1 and 2.8 ± 1.9 for CHO-A.
The occurrence of Enterococcus spp. (CATC agar) showed different numbers of CFU between the approaches at day 0 (Figure 2f). The initial CFU/g of CHO-A (log 3.0 ± 2.0) was significantly lower than the initial CFU/g of other approaches (OSA log 3.9 ± 0.3 and OA 4.4 ± 0.2). In all approaches except CHO-A, the number of CFU increased by about half a log after three days and decreased constantly. In the OSA, Enterococcus spp. were not detectable on day 28 or later. After 50 days in both approaches, enterococci were not detectable by cultivation anymore.
The non-enriched approaches (NSA and CHO-A) had a constant pH of about 8 with a minimum of pH 7.8 ± 0.1 after seven days (Figure 2g). Henceforth, pH increased constantly to 8.5 ± 0.1 after 76 days of incubation. In the enrichment approaches, pH decreased immediately. OA and SA reached a minimum value of 6.2 ± 0.6 and 5.5 ± 0.0 after 14 and 7 days, respectively. The OSA decreased to pH 5.2 ± 0.1 after seven days and decreased further to a final pH of 4.96 on day 76. Of note is the difference in the course of SA (Supplementary Figure S1). While two of the replicates decreased to pH 5.5 after 21 days and stably remained there, one approach reached a minimum after 14 days at 6.8 and then increased again to a final pH of 8.5. Obviously, fermentation did not work in this sample for reasons that were not further explored. To be sure that fermentation worked in the spiked approach samples, pH was measured after the last sampling. No such difference in pH was measurable, and therefore it can be assumed that fermentation worked in all samples.
For the enumeration of sulfite-reducing anaerobes, like Clostridium spp. and their spores, DRCM bouillon was used. In all approaches, the minimum was observed after seven days (Figure 3). OA showed a minimum of log 1 TCID50/mL, while in the other approach, minimum values between log 4 and 5 TCID50/mL were measured. Afterwards, the values rose to the previous level. The highest values were measured in CHO-A, whereas the other approaches showed no significant difference.
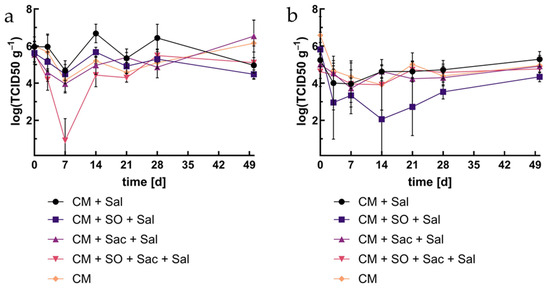
Figure 3.
Occurrence of Clostridium spp. and other spore-forming sulfite reducers: (a) living cells, (b) after inactivation. Abrev. as in Figure 1.
For all inactivated approaches, TCID50 decreased by 2 to 3 log levels during the first three days. OA showed significantly lower values during the whole period of investigation, while no significant difference was observed for any of the other approaches.
MALDI-TOF is a qualitative method for the detection of species based on proteins. The results represent the most dominant culturable microorganisms but not the absolute abundance or completeness of the microbial community. Keeping this in mind, in Table 3, detected microorganisms are presented on the genus level with the number of detections per sampling day, approach, and incubation temperature in Exp1. The species of one genus were summarized. The full list of detections is presented in Supplementary Table S1.

Table 3.
Overview of results of MALDI-TOF; in column “next organism”, results were summed to genus level and indicated in bold. Numbers in brackets are numbers of detected species. In column “CM”, “CM + HC” and “CM + SO” numbers represent number of detected species at time of sampling. Tempera-tures are shown in italics. Abrv.: temp.—temperature; CM—cattle manure; HCs—hay cobs; SO—squeezed oat.
At both incubation temperatures, comparable numbers of detected species were observed, 39 at 21 °C vs. 38 species at 10 °C. In CHO-A at 10 °C, the most abundant genera were Bacillus spp., Clostridium spp., and Enterococcus spp. In HA, most results were comparable. The most obvious difference was the detection of Lactobacillus spp. after 14 d until the end, while in CHO-A, it was not detected anymore after 14 days. In OA, lactobacilli were already detectable from day 3. Here, no enterococci were detected anymore after 14 days.
At 21 °C E. coli, Enterococcus spp. and Bacillus spp. were the most abundant microorganisms in CHO-A and HA. Enterococcus spp. were present all the time. Lactobacilli were detected in low numbers and without a visible trend. On the other hand, in OA, lactobacilli were detected over the whole measurement period and with five species. Bacteria of the species Bacillus spp. were present every day of sampling, while enterococci were not detectable after 14 days.
4. Discussion
In the case of a salmonellosis outbreak, a large amount of potentially contaminated manure accumulates. To break the infection cycle, this study tested LAF as a low-tech and easy-to-use method for the hygienization of CM in low-scale experiments. The results showed that during the fermentation process, Salmonella enterica serovar enterica Typhimurium was degraded to below the detection limit.
4.1. The Role of the C Source
The enrichment of the CHO source is mandatory for the successful hygienization of contaminated cattle manure. The results showed a higher impact with the addition of squeezed oat than with that of hay cobs, but this was exceeded by saccharose. Also, the MALDI-TOF results confirm that at both temperatures, the amount and the diversity of lactobacillus detections were significantly lower in HA than in OA. Similar results were described by Scheinemann and colleagues [3].
Without treatment, Salmonella survived in the approach until the end of sampling at 10 °C and for at least 80 days at 21 °C. Salmonella species are known to survive in stored slurries and dirty waters for up to three months and for less than one month in solid manure heaps with temperatures greater than 55 °C [43]. The survival rate of S. Ty. depends on pH and medium composition, the chain length of the acid, and the concentration of VFAs and temperature [24].
Between the two setups, the content of OA was reduced from 18.75% in the temperature experiment to 10% in the saccharose experiment to keep enough manure material for a representative reaction when saccharose is added. As a result of reduction, the reaction time of degradation in OA was twice as long as that in Exp1. Therefore, the amount of CHO addition has an impact on the reaction speed.
With the addition of sugar, the degradation of Salmonella was enhanced considerably. The combination of both sources of CHO adds up to half of the rate of SA. Salmonella enrichments proved the complete removal of S. Ty. in the CHO-enriched samples after fermentation. With the addition of sugar or squeezed oat as CHO sources, the degradation of Salmonella was reliably successful. Hence, the amount of available C sources has an impact on the speed of the fermentation reaction as well as the type. Pradhan and colleagues [44] showed comparable results with glucose and other sugars in lactic acid production. Among the carbon sources, glucose was found to be the best-performing carbon source with 2.2 times more lactic acid synthesis. Harlow and colleagues [45] compared the effect of oat, wheat, and corn with positive results on LAF and pH decrease. Therefore, it is conceivable that more grains are a suitable source of CHO with similar achievable results. More studies are necessary to improve this method.
4.2. Temperature Dependence of Salmonella Degradation During LAF
The effectiveness of LAF was thereby also temperature-dependent. The non-enriched approach showed higher survival rates of S. Ty at 10 °C than at 21 °C. Such temperature dependence of S. Ty. survival was described before. There might be several factors responsible such as the temperature-dependent activity of other microorganisms or bacteriophages [46,47].
The addition of hay cobs as a CHO source accelerated the degradation of S. Ty. at 21 °C compared to CHO-A, but there was no difference compared to CHO-A at 10 °C. Hay cobs contain cell wall fiber cellulose and hemicellulose, which requires the synergistic activity of multiple enzymes to degrade [48]. Ruminants have a wide range of symbiont microflora and enzymes to degrade organic matter of hay and grass [49]. In a slurry, however, microorganisms must provide these enzymes themselves. Glycolytic activity and other metabolic pathways of lactobacilli are strongly temperature-dependent [50,51]. Hay cobs were tested as a C source, because they may already be available in livestock in the case of an animal disease. Obviously, hay cobs are a less efficient source of energy for the microbial community of cattle manure at low temperatures.
Squeezed oat on the other hand is an easy-to-use substrate, so the degradation of salmonellae in OA was more efficient. S. Ty. were below the detection limit after two weeks at 21 °C and after three weeks at 10 °C. The results showed that LAF worked at different temperatures and may be an appropriate method for degrading Salmonella serovars much faster and more efficiently than without the addition of any carbon source during the winter season. Similar results were observed in large-scale experiments at summer temperatures (publ. in prep.).
4.3. Lactobacillus and pH
The shift in the microbial community was detectable in all approaches. Lactobacillae, as the main responsible organisms for LAF, showed a different growth pattern between the approaches. The addition of sugar promoted the growth of lactobacilli, which was enhanced by the addition of squeezed oat (Figure 2e). The increase in the cell numbers of lactobacillae corresponded with the decrease in the pH value. The increase in lactobacillae dependent on different grains was described before [45].
The results of MALDI-TOF revealed a high diversity in OA at 10 °C and 21 °C with the detected amount and occurrence of five out of six species (Table S1 and Table 3). In a study by Hrubant [52], the peaks of different species of Lactobacillus were shown at different points of time during the fermentation process. Indeed, the size and shape of colonies on the MRS plates differed during the fermentation process and may be an explanation for the fluctuation. Nevertheless, the decrease to pH 6 and reduction in Salmonella support the assumption that LAF proceeded. Therefore, the CFU count alone is not the only measure for the activity of lactobacillae.
Salmonella spp. are known to prefer a pH above 5.5 and survive until pH 4 [53]. Therefore, the pH cannot be the only reason for the reduction in Salmonella by LAF. Lactobacillae are known to produce antimicrobial agents, e.g., [54]. Volatile fatty acids appear to have a toxic effect on S. Ty. [23]. In a study with L. plantarum, which is known to produce lactic and acetic acid, the antimicrobial effects of VFAs and the reduction in the pH were responsible for Salmonella reduction in fermented feed [55]. Therefore, it is conceivable that pathogen organisms with a known pH sensitivity like FMD virus [56,57] may be successfully inactivated by LAF as well as other bacterial pathogens.
4.4. Enterococci as Indicator Organisms
The proliferation of enterococci corresponds to the fermentation process and can be seen as a pioneer of fermentation and as a bioindicator. The MALDI-TOF results suggested the same at 10 °C. Species of Enterococcus are a part of common genera of LAB and often play a role in lactic acid fermentation [58,59]. The increase and following decrease went along with the start and end of the fermentation process. The genus Enterococcus is easy to detect and can be used to confirm the end of fermentation. Additionally, the tenacity of this genus was higher than that of Salmonella and is therefore a reliable indicator of the hygienisation process.. Therefore, Enterococcus ssp. can be approved as a suitable indicator organism for the fermentation process also at 10 °C and 21 °C as suggested earlier and approved for higher temperatures [3].
4.5. Sporulating Organisms
The sulfite-reducing and spore-forming clostridia fluctuated in all approaches but without a visible trend. In contrast to other organism groups, there was a decrease in the detection of clostridia at the beginning of fermentation. In particular, SOA dropped to the detection limit after seven days and regenerated afterwards. On the other hand, CHO-A revealed the highest detection numbers. The sporulation numbers are 1–2 log lower than those of the living cells. This corresponded with the results by Scheinemann et al. [3]. There were no differences between the results of the enriched approaches, except SOA which revealed the lowest results but without statistical significance.
It is known that Salmonella can coexist with Clostridium in guts and digesters [60]. Clostridia strains play an important role in the anaerobic degradation of dairy cattle manure during mesophilic and thermophilic digestion [48,61]. Additionally, a novel cold-tolerant Clostridium strain is suggested for potential applications in efficient manure fermentation [48]. However, the presence in the digestate of biogas plants can be controversial due to the potential growth of pathogenic species like C. tyrobutyricum and C. butyricum [61]. A mesophilic anaerobic digestion approach in an agricultural population reduced the clostridial population abundance and also spores significantly [61]. In contrast, our results showed that the population of Clostridium species remained stable throughout LAF.
4.6. LAF as Alternative
Treating pathogen-contaminated manure is essential to break the infection cycle within the agricultural cycle. Other fermentation methods were already shown to be the most efficient [5,62,63]. The simplest method is the extended storage of contaminated waste [5]. On the other hand, it was shown that without further treatment, S. enterica survived for about 130 d in pig manure and for over 200 d in chicken manure at 25 °C [9]. The sanitizing effect of a full-scale thermophilic high-solid anaerobic digestion process resulted in the undetectability of Salmonella and other pathogens after 24–48 h under anaerobic conditions [63]. Mesophilic fermentation as a hygienization method uses the microbiotic shift to inactivate pathogens such as Salmonella [48,62]. Also, aerated waste microcosms of manure flush lagoons resulted in a significant decrease in Salmonella [6].
Among this methods, LAF was already described as an efficient hygienization method [3]. The results of this study showed its efficiency at different temperatures and with different CHO sources. Fermentation degrades S. Ty. in cattle manure to below the detection limit within two to four weeks by agar plates or within 50 days by Salmonella enrichment according to Biowaste Ordinance [39]. Degradation was shown at 10 °C and 21 °C.
The same applies to other pathogens such as E. coli. The MALDI results showed the presence of E. coli mainly in manure without additives at 21 °C, while in HA, it was detected only to a small extent after 21 days and not at all in OA. An earlier study also showed that E. coli was inactivated by LAF within three days [3]. It can therefore be assumed that LAF is also suitable for inactivating non-sporulating pathogens such as non-O157 STEC and E. coli O157.
Which fermentation method is used depends on the aim of treatment and the technical possibilities on site and the availability of disinfectants. The main advantage of LAF lies in the fact that the method does not require harmful chemicals or high technical effort. One ethical objection is the use of edible food for disinfection purposes. Here, further studies can investigate the suitability of contaminated, inedible food or food waste as a CHO source.
There are more possible ways to apply LAF. First tests showed that LAF also works with pig manure (data not published). Jung and colleagues [9] showed the long survival of pathogens in pig and chicken manure, while Scheinemann and colleagues hygienized human sludge by LAF [3]. Further investigations are necessary for the transferability to other types of animal manure or sludge to establish the method.
5. Conclusions
The LAF process was shown to be able to degrade Salmonella Typhimurium in cattle manure. The speed and quality of the process thereby depend on the temperature and the available C source. The shift in the Enterococcus spp. populations confirmed that they are suitable indicator organisms for LAF. The method does not require any harmful chemicals or technical effort. In addition, disinfected manure can be used as fertilizer afterwards. Further studies should investigate alternative CHO sources and the transferability to other animal or human disease-related pathogens. This study confirms LAF as an alternative method for the decontamination of pathogen-contaminated liquid manure.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/applmicrobiol5030088/s1, Figure S1: Results of pH measurement of SA (Exp 2); Table S1: Overview of results of MALDI-TOF.
Author Contributions
Conceptualization, H.H. and H.A.S.; methodology, H.H., T.M., and H.A.S.; formal analysis and investigation, H.H. and S.W.; writing—original draft preparation, H.H.; review and editing, S.W., C.S., H.A.S., I.L., T.S. and L.E.H.; visualization, H.H.; supervision, H.A.S.; funding acquisition, H.A.S. and L.E.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Bundesministerium für Forschung, Technologie und Raumfahrt, grant number 03VP09081, and Bundeswehr Research Institute for Protective Technologies and CBRN Protection (WIS) contributed in kind.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
We thank Wiebke Lange and Yolanda Marschner from the FLI and Antje Rußmann and Kristina Sonnwald from WIS for technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| C | Carbon |
| CATC agar | Citrate–azide–Tween®–carbonate agar |
| CFU | Colony-forming unit |
| CHO-A | Approach without carbohydrates |
| CM | Cattle manure |
| DRCM | Differential reinforced clostridial broth |
| HA | Hay cob approach |
| HCs | Hay cobs |
| LA | Lactic acid |
| LAB | Lactic acid bacteria |
| LAF | Lactic acid fermentation |
| N | Nitrogen |
| NSA | Non-spiked sample |
| OA | Squeezed oat approach |
| OSA | Squeezed oat and saccharose approach |
| SA | Saccharose approach |
| Sac | Saccharose |
| Sal | Salmonella enterica subsp. enterica ser. Typhimurium |
| SS agar | Salmonella Shigella agar |
| S. Thy | Salmonella enterica subsp. enterica ser. Typhimurium |
| SO | Squeezed oat |
| TCID50 | Tissue culture infection dose 50 |
| VFA | Volatile fatty acid |
| XLD agar | Xylose–lysine–deoxycholate agar |
References
- Anzeigepflichtige Tierseuchen. Available online: https://www.bmel.de/DE/themen/tiere/tiergesundheit/tierseuchen/anzeigepflichtige-tierseuchen.html (accessed on 28 March 2025).
- Düngemittelverordnung Anlage 9. Available online: https://www.gesetze-im-internet.de/d_v_2017/anlage_9.html (accessed on 28 February 2025).
- Scheinemann, H.A.; Dittmar, K.; Stöckel, F.S.; Müller, H.; Krüger, M.E. Hygienisation and Nutrient Conservation of Sewage Sludge or Cattle Manure by Lactic Acid Fermentation. PLoS ONE 2015, 10, e0118230. [Google Scholar] [CrossRef]
- Mindžáková, I.; Gregová, G.; Szabóová, T.; Sasáková, N.; Venglovský, J. Devitalization of Bacteria in Composted Cattle Manure with Natural Additives and Risk for Environment. Life 2024, 14, 490. [Google Scholar] [CrossRef] [PubMed]
- Blaiotta, G.; Di Cerbo, A.; Murru, N.; Coppola, R.; Aponte, M. Persistence of bacterial indicators and zoonotic pathogens in contaminated cattle wastes. BMC Microbiol. 2016, 16, 87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ravva, S.; Sarreal, C. Survival of Salmonella enterica in Aerated and Nonaerated Wastewaters from Dairy Lagoons. Int. J. Environ. Res. Public Health 2014, 11, 11249–11260. [Google Scholar] [CrossRef] [PubMed]
- Igue, P. Survival of Salmonella Typhimurium in Simulated Intestinal Fluids. Master’s Thesis, McGill University, Montreal, QC, Canada, 2001. [Google Scholar][Green Version]
- Animal Disease Situation. Available online: https://www.fli.de/en/news/animal-disease-situation/ (accessed on 28 February 2025).[Green Version]
- Jung, K.-S.; Heu, S.-G.; Roh, E.-J.; Kim, M.-H.; Gil, H.-J.; Choi, N.-Y.; Lee, D.-H.; Lim, J.-A.; Ryu, J.-G.; Kim, K.-H. Survival of Salmonella enterica and Listeria monocytogenes in Chicken and Pig Manure Compost. Korean J. Soil Sci. Fertil. 2013, 46, 469–473. [Google Scholar] [CrossRef]
- Ravva, S.V.; Sarreal, C.Z.; Mandrell, R.E. Identification of Protozoa in Dairy Lagoon Wastewater that Consume Escherichia coli O157:H7 Preferentially. PLoS ONE 2010, 5, e15671. [Google Scholar] [CrossRef]
- Mejri, S.; Boukef Ben Omrane, I.; Mraouna, R.; Amara, A.; Got, P.; Boudabous, A.; El Bour, M. Effect of Environmental Factors on Salmonella thyphimurium in Marine Water Microcosms. Rapp. Comm. Int. Mer Médit. 2013, 40, 405. [Google Scholar]
- Aljarallah, K.M. Physiological Responses of Salmonella Typhimurium Under Combined Osmotic and Heat Stress. Ph.D. Thesis, University of Surrey, Guildford, UK, 2006. [Google Scholar]
- Szejniuk, B.; Budzińska, K.; Jurek, A.; Traczykowski, A.; Berleć, K.; Michalska, M.; Piątkowski, J.K. Przeżywalność bakterii Salmonella Enteritidis w wodach powierzchniowych. Annu. Set Environ. Prot. 2013, 15, 2738–2749. [Google Scholar]
- Semenov, A.V.; Van Overbeek, L.; Van Bruggen, A.H.C. Percolation and Survival of Escherichia coli O157:H7 and Salmonella enterica Serovar Typhimurium in Soil Amended with Contaminated Dairy Manure or Slurry. Appl. Environ. Microbiol. 2009, 75, 3206–3215. [Google Scholar] [CrossRef]
- Methner, U. Salmonellose der Rinder—Empfehlungen zur Vorgehensweise nach Feststellung eines Ausbruchs. Amtstierärztlicher Dienst Leb. 2012, 19, 4. [Google Scholar]
- Richtlinie über Mittel und Verfahren Für die Durchführung der Desinfektion Bei Bestimmten Tierseuchen. Available online: https://desinfektions-rl.fli.de/de/home (accessed on 25 March 2025).
- Zapaśnik, A.; Sokołowska, B.; Bryła, M. Role of Lactic Acid Bacteria in Food Preservation and Safety. Foods 2022, 11, 1283. [Google Scholar] [CrossRef]
- Narayanan, N.; Roychoudhury, P.K.; Srivastava, A. L (+) lactic acid fermentation and its product polymerization. Electron. J. Biotechnol. 2004, 7, 168–179. [Google Scholar]
- Castillo Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Domínguez González, J.M.; Converti, A.; Oliveira, R.P.D.S. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Garrido, R.; Cabeza, L.F.; Falguera, V.; Pérez Navarro, O. Potential Use of Cow Manure for Poly(Lactic Acid) Production. Sustainability 2022, 14, 16753. [Google Scholar] [CrossRef]
- Castro-Ramos, J.J.; Solís-Oba, A.; Solís-Oba, M.; Calderón-Vázquez, C.L.; Higuera-Rubio, J.M.; Castro-Rivera, R. Effect of the initial pH on the anaerobic digestion process of dairy cattle manure. AMB Express 2022, 12, 162. [Google Scholar] [CrossRef]
- Sun, Z.H.; Liu, S.M.; Tayo, G.O.; Tang, S.X.; Tan, Z.L.; Lin, B.; He, Z.X.; Hang, X.F.; Zhou, Z.S.; Wang, M. Effects of cellulase or lactic acid bacteria on silage fermentation and in vitro gas production of several morphological fractions of maize stover. Anim. Feed Sci. Technol. 2009, 152, 219–231. [Google Scholar] [CrossRef]
- Levine, A.S.; Fellers, C.R. Action of Acetic Acid on Food Spoilage Microörganisms. J. Bacteriol. 1940, 39, 499–515. [Google Scholar] [CrossRef]
- Goepfert, J.M.; Hicks, R. Effect of Volantile Fatty Acids on Salmonella thyphimurium. J. Bacteriol. 1969, 97, 956–958. [Google Scholar] [CrossRef]
- Knarreborg, A.; Miquel, N.; Granli, T.; Jensen, B.B. Establishment and application of an in vitro methodology to study the effects of organic acids on coliform and lactic acid bacteria in the proximal part of the gastrointestinal tract of piglets. Anim. Feed Sci. Technol. 2002, 99, 131–140. [Google Scholar] [CrossRef]
- Hammes, W.P.; Hertel, C. The Genera Lactobacillus and Carnobacterium. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 320–403. [Google Scholar]
- Desinfektion in der Veterinärmedizin- DVG-Prüfrichtlinien Tierhaltung (Kapitel V), Bakterizidie; Stand 26.12.2024. Available online: https://www.desinfektion-dvg.de/infos-fuer-hersteller-und-gutachter/pruefrichtlinien (accessed on 28 February 2025).
- Taylor, W.I.; Harris, B. Isolation of Shigellae. II. Comparison of Plating Media and Enrichment Broths. Am. J. Clin. Pathol. 1965, 44, 476–479. [Google Scholar] [CrossRef]
- Leifson, E. New culture media based on sodium desoxycholate for the isolation of intestinal pathogens and for the enumeration of colon bacilli in milk and water. J. Pathol. Bacteriol. 1935, 40, 581–599. [Google Scholar] [CrossRef]
- Gassner, G. Ein neuer Dreifarbnährboden zur Typhus-Ruhr Diagnose. Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. Abt. 1 Orig. Reihe A 1918, 80, 219–222. [Google Scholar]
- Taylor, W.I.; Schelhart, D. Isolation of Shigellae: VIII. Comparison of Xylose Lysine Deoxycholate Agar, Hektoen Enteric Agar, Salmonella-Shigella Agar, and Eosin Methylene Blue Agar with Stool Specimens. Appl. Microbiol. 1971, 21, 32–37. [Google Scholar]
- OXOID GmbH, W. Oxoid Handbuch, 6th edDruckerei und Verlag VVA Vereinigte Verlagsanstalten: Düsseldorf, Germany, 2003; Available online: https://www.analisisavanzados.com/modules/mod_tecdata/manuales/oxoid-manual-9th-edition.pdf (accessed on 25 March 2025).
- Burkwall, M.K.; Hartman, P.A. Comparison of Direct Plating Media for the Isolation and Enumeration of Enterococci in Certain Frozen Foods. Appl. Microbiol. 1964, 12, 18–23. [Google Scholar] [CrossRef]
- Devriese, L.A.; Pot, B.; Collins, M.D. Phenotypic identification of the genus Enterococcus and differentiation of phylogenetically distinct enterococcal species and species groups. J. Appl. Bacteriol. 1993, 75, 399–408. [Google Scholar] [CrossRef]
- Briggs, M. 497. An improved medium for lactobacilli. J. Dairy Res. 1953, 20, 36–40. [Google Scholar] [CrossRef]
- Reuter, G. Elective and selective media for lactic acid bacteria. Int. J. Food Microbiol. 1985, 2, 55–68. [Google Scholar] [CrossRef]
- Gibbs, B.M.; Freame, B. Methods for the Recovery of Clostridia from Foods. J. Appl. Bacteriol. 1965, 28, 95–111. [Google Scholar] [CrossRef]
- Gibbs, P.A. The Detection of Clostridium welchii in the Differential Reinforced Clostridial Medium Technique. J. Appl. Bacteriol. 1973, 36, 23–33. [Google Scholar] [CrossRef]
- Verordnung über die Verwertung von Bioabfällen auf Böden1,2, (Bioabfallverordnung—BioAbfV). 1998. Available online: https://www.gesetze-im-internet.de/bioabfv/ (accessed on 2 January 2025).
- Harvey, R.W.S.; Price, T.H. Comparison of selenite F, Muller-Kauffmann tetrathionate and Rappaport’s medium for salmonella isolation from chicken giblets after pre-enrichment in buffered peptone water. J. Hyg. 1981, 87, 219–224. [Google Scholar] [CrossRef]
- Schothorst, M.V.; Renaud, A.M. Dynamics of salmonella isolation with modified Rappaport’s medium (R10). J. Appl. Bacteriol. 1983, 54, 209–215. [Google Scholar] [CrossRef]
- Alizadeh, M.; Yousefi, L.; Pakdel, F.; Ghotaslou, R.; Rezaee, M.A.; Khodadadi, E.; Oskouei, M.A.; Soroush Barhaghi, M.H.; Kafil, H.S. MALDI-TOF Mass Spectroscopy Applications in Clinical Microbiology. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 9928238. [Google Scholar] [CrossRef]
- Nicholson, F.A.; Groves, S.J.; Chambers, B.J. Pathogen survival during livestock manure storage and following land application. Bioresour. Technol. 2005, 96, 135–143. [Google Scholar] [CrossRef]
- Pradhan, N.; d’Ippolito, G.; Dipasquale, L.; Esposito, G.; Panico, A.; Lens, P.N.L.; Fontana, A. Simultaneous synthesis of lactic acid and hydrogen from sugars via capnophilic lactic fermentation by Thermotoga neapolitana cf capnolactica. Biomass Bioenergy 2019, 125, 17–22. [Google Scholar] [CrossRef]
- Harlow, B.E.; Lawrence, L.M.; Harris, P.A.; Aiken, G.E.; Flythe, M.D. Exogenous lactobacilli mitigate microbial changes associated with grain fermentation (corn, oats, and wheat) by equine fecal microflora ex vivo. PLoS ONE 2017, 12, e0174059. [Google Scholar] [CrossRef]
- Olszewska, H.; Skowron, K. Effect of storage temperature and type of slurry on survivability of Salmonella. J. Cent. Eur. Agric. 2013, 14, 369–375. [Google Scholar] [CrossRef]
- Yaziz, M.I. The Effect of Temperature on the Destruction of Salmonellas in Activated Sludge. Pertanika 1985, 8, 343–346. [Google Scholar]
- Akila, G.; Chandra, T.S. A novel cold-tolerant Clostridium strain PXYL1 isolated from a psychrophilic cattle manure digester that secretes thermolabile xylanase and cellulase. FEMS Microbiol. Lett. 2003, 219, 63–67. [Google Scholar] [CrossRef]
- Ismajli, I.; Fetoshi, O.; Abazi Shala, A.; Bytyçi, P.; Hyseni, A.; Ramshaj, Q. Bacterial, Fungal, and Protozoal Microflora of Hay. Int. J. Adv. Study Res. Work. 2019, 2, 22–26. [Google Scholar]
- Wouters, J.A.; Kamphuis, H.H.; Hugenholtz, J.; Kuipers, O.P.; De Vos, W.M.; Abee, T. Changes in Glycolytic Activity of Lactococcus lactis Induced by Low Temperature. Appl. Environ. Microbiol. 2000, 66, 3686–3691. [Google Scholar] [CrossRef]
- McLeod, A.; Mosleth, E.F.; Rud, I.; Branco Dos Santos, F.; Snipen, L.; Liland, K.H.; Axelsson, L. Effects of glucose availability in Lactobacillus sakei; metabolic change and regulation of the proteome and transcriptome. PLoS ONE 2017, 12, e0187542. [Google Scholar] [CrossRef]
- Hrubant, G.R. Changes in Microbial Population During Fermentation of Feedlot Waste with Corn. Appl. Microbiol. 1975, 30, 113–119. [Google Scholar] [CrossRef]
- Foster, J.W. Low pH Adaptation and the Acid Tolerance Response of Salmonella typhimurium. Crit. Rev. Microbiol. 2008, 21, 215–237. [Google Scholar] [CrossRef]
- Niku-Paavola, M.-L.; Laitila, A.; Mattila-Sandholm, T.; Haikara, A. New types of antimicrobial compounds produced by Lactobacillus plantarum. J. Appl. Microbiol. 1999, 86, 29–35. [Google Scholar] [CrossRef]
- Van Winsen, R.L.; Lipman, L.J.A.; Biesterveld, S.; Urlings, B.A.P.; Snijders, J.M.A.; Van Knapen, F. Mechanism of Salmonella reduction in fermented pig feed. J. Sci. Food Agric. 2001, 81, 342–346. [Google Scholar] [CrossRef]
- Bachrach, H.L.; Breese, S.S.; Callis, J.J.; Hess, W.R.; Patty, R.E. Inactivation of Foot-and-Mouth Disease Virus by pH and Temperature Changes and by Formaldehyde. Exp. Biol. Med. 1957, 95, 147–152. [Google Scholar] [CrossRef]
- Caridi, F.; Vázquez-Calvo, A.; Sobrino, F.; Martín-Acebes, M.A. The pH Stability of Foot-and-Mouth Disease Virus Particles Is Modulated by Residues Located at the Pentameric Interface and in the N Terminus of VP1. J. Virol. 2015, 89, 5633–5642. [Google Scholar] [CrossRef]
- Min, K.H.; Yin, F.H.; Amin, Z.; Mansa, R.F.; Ling, C.M.W.V. An Overview of the Role of Lactic Acid Bacteria in Fermented Foods and Their Potential Probiotic Properties. Borneo Int. J. Biotechnol. 2023, 2, 65–83. [Google Scholar]
- Graham, K.; Stack, H.; Rea, R. Safety, beneficial and technological properties of enterococci for use in functional food applications—A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3836–3861. [Google Scholar] [CrossRef]
- Rivera-Chávez, F.; Zhang, L.F.; Faber, F.; Lopez, C.A.; Byndloss, M.X.; Olsan, E.E.; Xu, G.; Velazquez, E.M.; Lebrilla, C.B.; Winter, S.E.; et al. Depletion of Butyrate-Producing Clostridia from the Gut Microbiota Drives an Aerobic Luminal Expansion of Salmonella. Cell Host Microbe 2016, 19, 443–454. [Google Scholar] [CrossRef]
- Fontana, A.; Soldano, M.; Bellassi, P.; Fabbri, C.; Gallucci, F.; Morelli, L.; Cappa, F. Dynamics of Clostridium genus and hard-cheese spoiling Clostridium species in anaerobic digesters treating agricultural biomass. AMB Express 2020, 10, 102. [Google Scholar] [CrossRef]
- Machnicka, A.; Grübel, K. The effect of pre-treatment and anaerobic digestion for pathogens reduction in agricultural utilization of sewage sludge. Environ. Sci. Pollut. Res. 2022, 30, 13801–13810. [Google Scholar] [CrossRef]
- Carraturo, F.; Panico, A.; Giordano, A.; Libralato, G.; Aliberti, F.; Galdiero, E.; Guida, M. Hygienic assessment of digestate from a high solids anaerobic co-digestion of sewage sludge with biowaste by testing Salmonella Typhimurium, Escherichia coli and SARS-CoV-2. Environ. Res. 2022, 206, 112585. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).