Impacts of Tillage Practices on Growth, Phosphorus Uptake, and Yield of Maize in Controlled and Field-Based Studies in Relation to Arbuscular Mycorrhizal Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1
2.1.1. Effect of Rotary Tillage on Growth, Yield, and AMF Colonization in the Roots of Maize (Field Experiments)
2.1.2. Maize Sampling and Determination of Biomass, P Concentration, and Grain Weight
2.1.3. AMF Colonization within Maize Roots
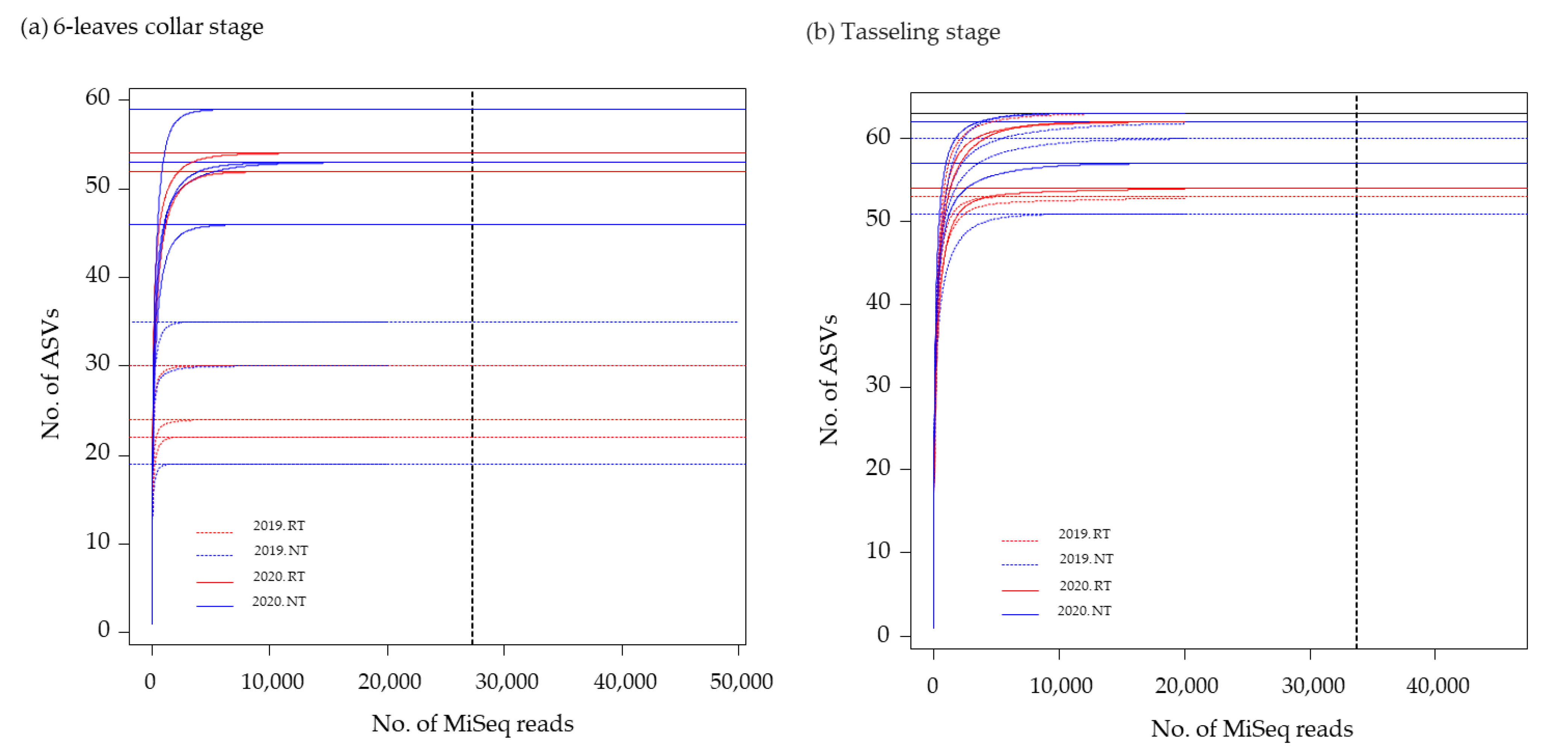
2.1.4. DNA Extraction from Maize Roots and Amplicon Sequence Analysis
2.2. Experiment 2
Effect of Different Colonized AMF Species on Maize Growth and P Uptake (Pot Experiment)
2.3. Statistical Analysis
3. Results
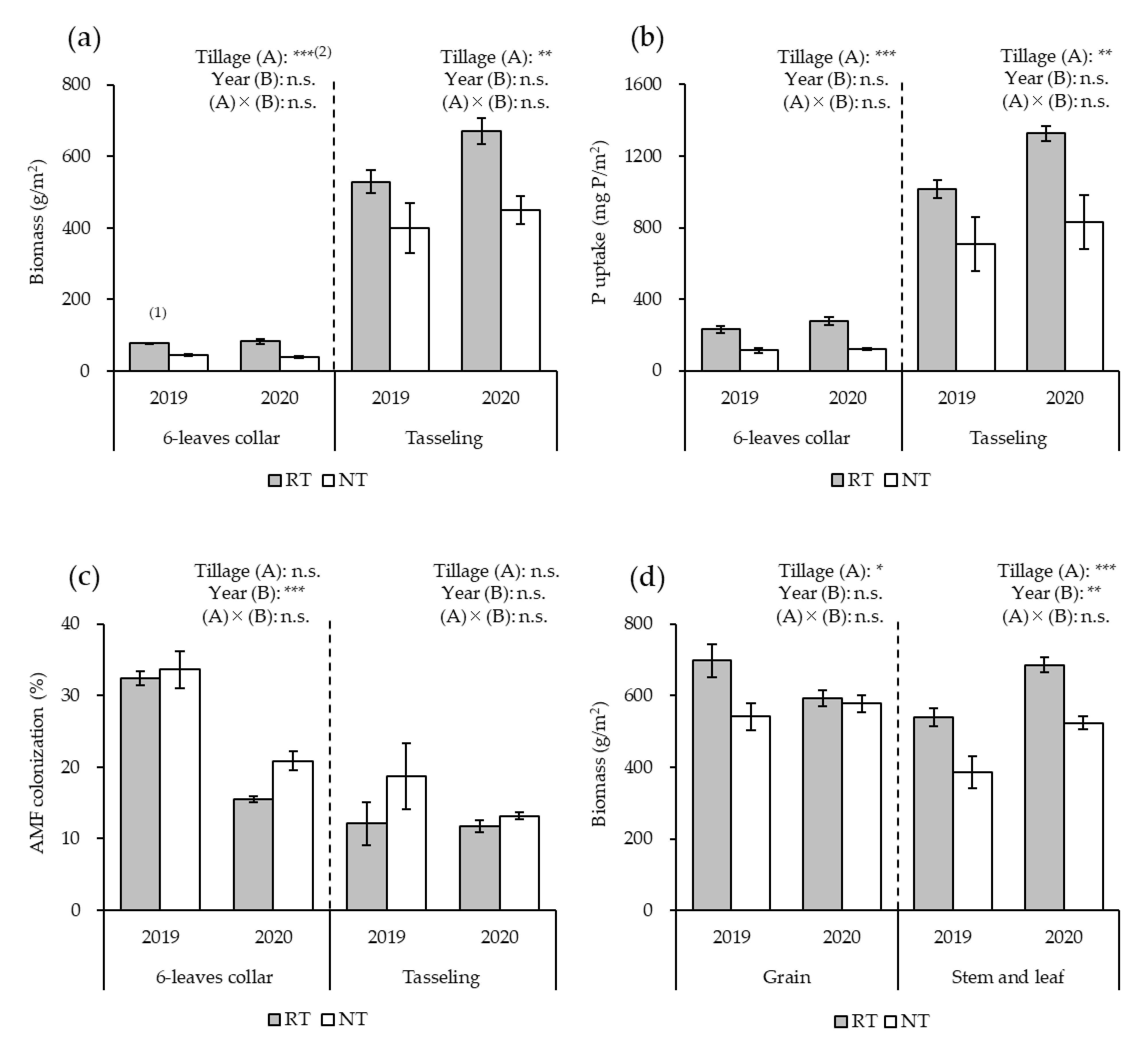
3.1. Growth and Yield of Maize
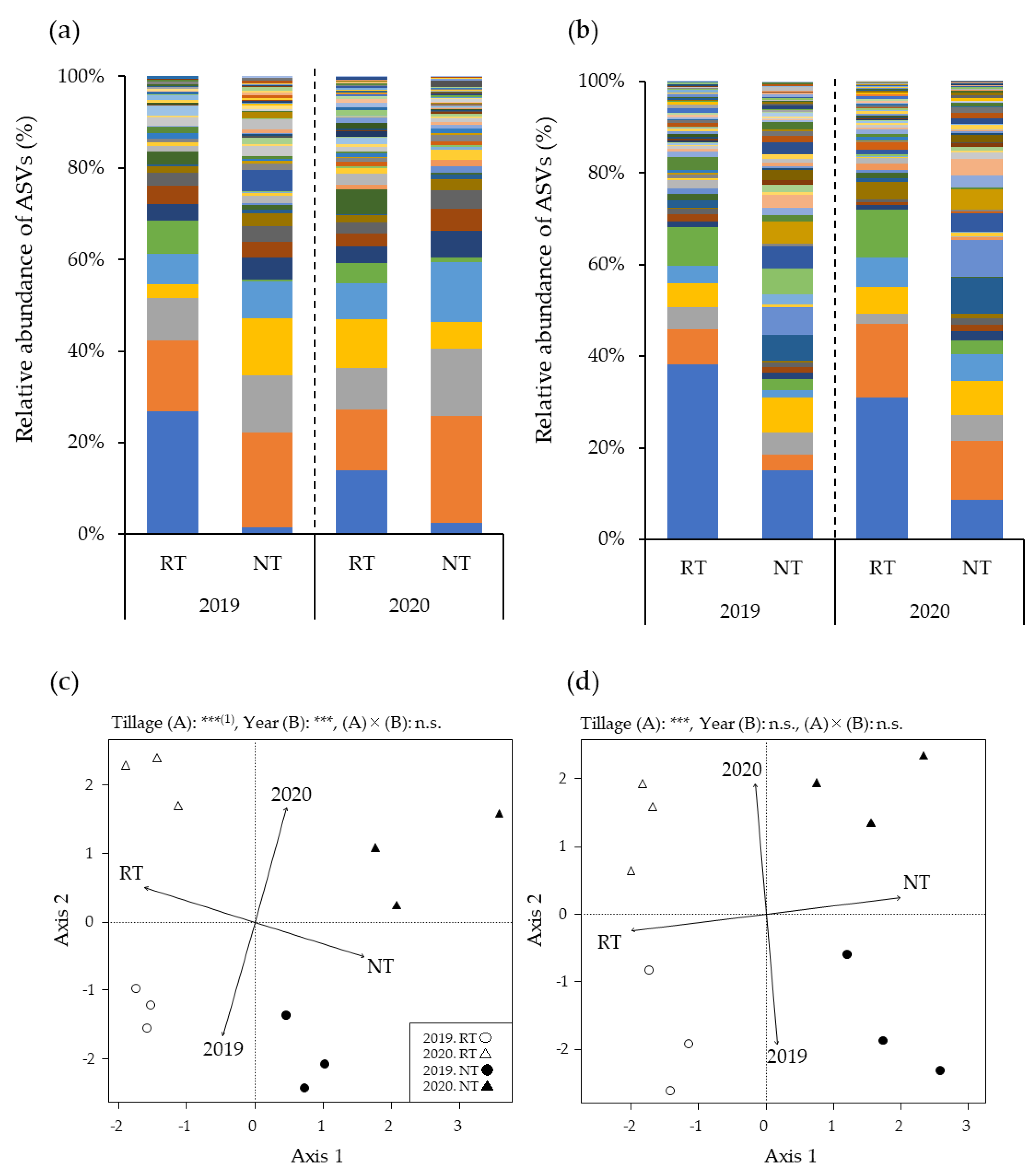
3.2. Relative AMF Abundance in Maize Roots
3.3. Growth and P Uptake of Maize in Pot Inoculation Experiment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huynh, H.T.; Hufnagel, J.; Wurbs, A.; Bellingrath-Kimura, S.D. Influences of soil tillage, irrigation and crop rotation on maize biomass yield in a 9-year field study in Müncheberg, Germany. Field Crops Res. 2019, 241, 107565. [Google Scholar] [CrossRef]
- Büchi, L.; Wendling, M.; Amossé, C.; Jeangros, B.; Sinaj, S.; Charles, R. Long and short term changes in crop yield and soil properties induced by the reduction of soil tillage in a long term experiment in Switzerland. Soil Tillage Res. 2017, 174, 120–129. [Google Scholar] [CrossRef]
- da Silva, G.F.; Calonego, J.C.; Luperini, B.C.O.; Chamma, L.; Alves, E.R.; Rodrigues, S.A.; Putti, F.F.; da Silva, V.M.; de Almeida Silva, M. Soil—Plant Relationships in Soybean Cultivated under Conventional Tillage and Long-Term No-Tillage. Agronomy 2022, 12, 697. [Google Scholar] [CrossRef]
- Ren, B.; Li, X.; Dong, S.; Liu, P.; Zhao, B.; Zhang, J. Soil physical properties and maize root growth under different tillage systems in the North China Plain. Crop J. 2018, 6, 669–676. [Google Scholar] [CrossRef]
- Ma, G.; Kang, J.; Wang, J.; Chen, Y.; Lu, H.; Wang, L.; Wang, C.; Xie, Y.; Ma, D.; Kang, G. Bacterial community structure and predicted function in wheat soil from the North China plain are closely linked with soil and plant characteristics after seven years of irrigation and nitrogen application. Front. Microbiol. 2020, 11, 506. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Arbuscular mycorrhizaes. In Mycorrhizal Symbiosis, 3rd ed.; Smith, S.E., Read, D.J., Eds.; Academic Press: London, UK, 2008; pp. 13–187. [Google Scholar]
- Rozpądek, P.; Rąpała-Kozik, M.; Wężowicz, K.; Grandin, A.; Karlsson, S.; Ważny, R.; Anielska, R.; Turnau, K. Arbuscular mycorrhiza improves yield and nutritional properties of onion (Allium cepa). Plant Physiol. Biochem. 2016, 107, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Guo, H.; Zhang, Q.; Guo, H.; Zhang, L.; Zhang, C.; Gou, Z.; Liu, Y.; Wei, J.; Chen, A.; et al. Arbuscular mycorrhizal fungi (AMF) enhanced the growth, yield, fiber quality and phosphorus regulation in upland cotton (Gossypium hirsutum L.). Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Watts-Williams, S.J.; Gill, A.R.; Jewell, N.; Brien, C.J.; Berger, B.; Tran, B.T.T.; Mace, E.; Cruickshank, A.W.; Jordan, D.R.; Garnett, T.; et al. Enhancement of sorghum grain yield and nutrition: A role for arbuscular mycorrhizal fungi regardless of soil phosphorus availability. Plants People Planet 2022, 4, 143–156. [Google Scholar] [CrossRef]
- Yu, F.; Goto, B.T.; Magurno, F.; Błaszkowski, J.; Wang, J.; Ma, W.; Feng, H.; Liu, Y. Glomus chinense and Dominikia gansuensis, two new Glomeraceae species of arbuscular mycorrhizal fungi from high altitude in the Tibetan Plateau. Mycol. Prog. 2022, 21, 1–11. [Google Scholar] [CrossRef]
- Munkvold, L.; Kjøller, R.; Vestberg, M.; Rosendahl, S.; Jakobsen, I. High functional diversity within species of arbuscular mycorrhizal fungi. New Phytol. 2004, 164, 357–364. [Google Scholar] [CrossRef]
- Thonar, C.; Schnepf, A.; Frossard, E.; Roose, T.; Jansa, J. Traits related to differences in function among three arbuscular mycorrhizal fungi. Plant Soil 2011, 339, 231–245. [Google Scholar] [CrossRef]
- Thingstrup, I.; Kahiluoto, H.; Jakobsen, I. Phosphate transport by hyphae of field communities of arbuscular mycorrhizal fungi at two levels of P fertilization. Plant Soil 2000, 221, 181–187. [Google Scholar] [CrossRef]
- Jakobsen, I.; Gazey, C.; Abbott, L.K. Phosphate transport by communities of arbuscular mycorrhizal fungi in intact soil cores. New Phytol. 2001, 149, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Jansa, J.; Mozafar, A.; Frossard, E. Phosphorus acquisition strategies within arbuscular mycorrhizal fungal community of a single field site. Plant Soil 2005, 276, 163–176. [Google Scholar] [CrossRef]
- Mensah, J.; Koch, A.; Antunes, P.; Kiers, E.; Hart, M.; Bucking, H. High functional diversity within species of arbuscular mycorrhizal fungi is associated with differences in phosphate and nitrogen uptake and fungal phosphate metabolism. Mycorrhiza 2015, 25, 533–546. [Google Scholar] [CrossRef]
- Säle, V.; Palenzuela, J.; Azcón-Aguilar, C. Ancient lineages of arbuscular mycorrhizal fungi provide little plant benefit. Mycorrhiza 2021, 31, 559–576. [Google Scholar] [CrossRef] [PubMed]
- Moukarzel, R.; Ridgway, H.J.; Waller, L.; Guerin-Laguette, A.; Cripps-Guazzone, N.; Jones, E.E. Soil Arbuscular Mycorrhizal Fungal Communities Differentially Affect Growth and Nutrient Uptake by Grapevine Rootstocks. Microb. Ecol. 2022, 1–15. [Google Scholar] [CrossRef]
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.A.; Hart, M.M.; Bago, A.; et al. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef]
- Verbruggen, E.; Toby Kiers, E. Evolutionary ecology of mycorrhizal functional diversity in agricultural systems. Evol. Appl. 2010, 3, 547–560. [Google Scholar] [CrossRef]
- Ma, M.; Ongena, M.; Wang, Q.; Guan, D.; Cao, F.; Jiang, X.; Li, J. Chronic fertilization of 37 years alters the phylogenetic structure of soil arbuscular mycorrhizal fungi in Chinese Mollisols. Amb Express 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Castelli, M.; Urcoviche, R.C.; Gimenes, R.M.T.; Alberton, O. Arbuscular mycorrhizal fungi diversity in maize under different soil managements and seed treatment with fungicide. J. Food Agric. Environ. 2014, 12, 486–491. [Google Scholar]
- Tatewaki, Y.; Higo, M.; Isobe, K. Community structure of arbuscular mycorrhizal fungi in the roots of maize under different of preceding winter cropping and tillage systems in south Kanto region of Japan. Soil Microorg. 2021, 75, 23–31. (In Japanese) [Google Scholar] [CrossRef]
- Liu, W.; Ma, K.; Wang, X.; Wang, Z.; Negrete-Yankelevich, S. Effects of no-tillage and biologically-based organic fertilizer on soil arbuscular mycorrhizal fungal communities in winter wheat field. Appl. Soil Ecol. 2022, 178, 104564. [Google Scholar] [CrossRef]
- Lu, X.; Lu, X.; Liao, Y. Effect of tillage treatment on the diversity of soil arbuscular mycorrhizal fungal and soil aggregate-associated carbon content. Front. Microbiol. 2018, 9, 2986. [Google Scholar] [CrossRef]
- Gu, S.; Wu, S.; Guan, Y.; Zhai, C.; Zhang, Z.; Bello, A.; Guo, X.; Yang, W. Arbuscular mycorrhizal fungal community was affected by tillage practices rather than residue management in black soil of northeast China. Soil Tillage Res. 2020, 198, 104552. [Google Scholar] [CrossRef]
- Martinez, T.N.; Johnson, N.C. Agricultural management influences propagule densities and functioning of arbuscular mycorrhizas in low-and high-input agroecosystems in arid environments. Appl. Soil Ecol. 2010, 46, 300–306. [Google Scholar] [CrossRef]
- Zhao, C.; Fu, S.; Mathew, R.P.; Lawrence, K.S.; Feng, Y. Soil microbial community structure and activity in a 100-year-old fertilization and crop rotation experiment. J. Plant Ecol. 2015, 8, 623–632. [Google Scholar] [CrossRef]
- Higo, M.; Kang, D.J.; Isobe, K. First report of community dynamics of arbuscular mycorrhizal fungi in radiocesium degradation lands after the Fukushima-Daiichi Nuclear disaster in Japan. Sci. Rep. 2019, 9, 8240. [Google Scholar] [CrossRef] [PubMed]
- Davison, J.; Garcia de Leon, D.; Zobel, M.; Moora, M.; Bueno, C.G.; Barceló, M.; Gerz, M.; León, D.; Meng, Y.; Pillar, V.D.; et al. Plant functional groups associate with distinct arbuscular mycorrhizal fungal communities. New Phytol. 2020, 226, 1117–1128. [Google Scholar] [CrossRef]
- Higo, M.; Tatewaki, Y.; Iida, K.; Yokota, Y.; Isobe, K. Amplicon sequencing analysis of arbuscular mycorrhizal fungal communities colonizing maize roots in different cover cropping and tillage systems. Sci. Rep. 2020, 10, 6093. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Huang, G.; Li, Y.; Zhang, X.; Lei, Y.; Li, Y.; Xiong, J.; Sun, Y. Illumina miSeq sequencing reveals correlations among fruit ingredients, environmental factors, and AMF Communities in three Lycium barbarum producing regions of China. Microbiol. Spectr. 2022, 10, e02293-21. [Google Scholar] [CrossRef]
- Sekiya, K. Standard Methods for Analysis of Soil Nutrients. In Committee of Standard Methods for Analysis of Soil Nutrients; Yokendo: Tokyo, Japan, 1970; pp. 229–238. [Google Scholar]
- Kobae, Y.; Ohtomo, R. An improved method for bright-field imaging of arbuscular mycorrhizal fungi in plant roots. Soil Sci. Plant Nutr. 2016, 62, 27–30. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Helgason, T.; Daniell, T.J.; Husband, R.; Fitter, A.H.; Young, J.P.W. Ploughing up the wood-wide web? Nature 1998, 394, 431. [Google Scholar] [CrossRef]
- Simon, L.; Lalonde, M.; Bruns, T. Specific amplification of 18S fungal ribosomal genes from vesicular-arbuscular endomycorrhizal fungi colonizing roots. Appl. Environ. Microbiol. 1992, 58, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Suyama, Y.; Saito, M.; Sugawara, K. A new primer for discrimination of arbuscular mycorrhizal fungi with polymerase chain reaction-denature gradient gel electrophoresis. Grassl. Sci. 2005, 51, 179–181. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Stefani, F.; Bencherif, K.; Sabourin, S. Taxonomic assignment of arbuscular mycorrhizal fungi in an 18S metagenomic dataset: A case study with saltcedar (Tamarix aphylla). Mycorrhiza 2020, 30, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 848–857. [Google Scholar] [CrossRef]
- Sayers, E.W.; Cavanaugh, M.; Clark, K. GenBank. Nucleic Acids Res. 2019, 47, D94–D99. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Öpik, M.; Vanatoa, A.; Vanatoa, E. The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol. 2010, 188, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-6. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 21 March 2023).
- Bowles, T.M.; Jackson, L.E.; Loeher, M.; Cavagnaro, T.R. Ecological intensification and arbuscular mycorrhizas: A meta-analysis of tillage and cover crop effects. J. Appl. Ecol. 2017, 54, 1785–1793. [Google Scholar] [CrossRef]
- Mariotte, P.; Mehrabi, Z.; Bezemer, T.M.; De Deyn, G.B.; Kulmatiski, A.; Drigo, B.; Veen, G.C.; Van der Heijden, M.G.A.; Kardol, P. Plant–soil feedback: Bridging natural and agricultural sciences. Trends Ecol. Evol. 2018, 33, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Sommermann, L.; Geistlinger, J.; Wibberg, D.; Deubel, A.; Zwanzig, J.; Babin, D.; Schlüter, A.; Schellenberg, I. Fungal community profiles in agricultural soils of a long-term field trial under different tillage, fertilization and crop rotation conditions analyzed by high-throughput ITS-amplicon sequencing. PLoS ONE 2018, 13, e0195345. [Google Scholar] [CrossRef] [PubMed]
- Battie-Laclau, P.; Taschen, E.; Plassard, C.; Dezette, D.; Abadie, J.; Arnal, D.; Benezech, P.; Duthoit, M.; Pablo, A.L.; Jourdan, C.; et al. Role of trees and herbaceous vegetation beneath trees in maintaining arbuscular mycorrhizal communities in temperate alley cropping systems. Plant Soil 2019, 453, 153–171. [Google Scholar] [CrossRef]
- Patanita, M.; Campos, M.D.; Félix, M.D.R.; Carvalho, M.; Brito, I. Effect of tillage system and cover crop on maize mycorrhization and presence of Magnaporthiopsis maydis. Biology 2020, 9, 46. [Google Scholar] [CrossRef]
- Kurm, V.; Schilder, M.T.; Haagsma, W.K.; Bloem, J.; Scholten, O.E.; Postma, J. Reduced tillage increases soil biological properties but not suppressiveness against Rhizoctonia solani and Streptomyces scabies. Appl. Soil Ecol. 2023, 181, 104646. [Google Scholar] [CrossRef]
- Holden, J.; Grayson, R.; Berdeni, D.; Bird, S.; Chapman, P.; Edmondson, J.; Firbank, L.; Helgason, T.; Hodson, M.E.; Hunt, S. The role of hedgerows in soil functioning within agricultural landscapes. Agric. Ecosyst. Environ. 2019, 273, 1–12. [Google Scholar] [CrossRef]
- Hirakubo, T.; Uozumi, S.; Kawahata, S.; Saiga, S.; Sano, H. Effect of continuous no-tillage cropping for five years on corn yield in the northern Tohoku region. Jpn. J. Grassl. Sci. 2011, 57, 73–79. [Google Scholar]
- Dai, Z.; Hu, J.; Fan, J.; Fu, W.; Wang, H.; Hao, M. No-tillage with mulching improves maize yield in dryland farming through regulating soil temperature, water and nitrate-N. Agric. Ecosyst. Environ. 2021, 309, 107288. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, T.; Cui, J.; Chen, S.; Han, H.; Ning, T. Increase in maize yield and soil aggregate-associated carbon in North China due to long-term conservation tillage. Exp. Agric. 2021, 57, 270–281. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Du, Y.; Niu, W. Conservation tillage improves the yield of summer maize by regulating soil water, photosynthesis and inferior kernel grain filling on the semiarid Loess Plateau, China. J. Sci. Food Agric. 2022, 102, 2330–2341. [Google Scholar] [CrossRef] [PubMed]
- Simić, M.; Dragičević, V.; Mladenović Drinić, S.; Vukadinović, J.; Kresović, B.; Tabaković, M.; Brankov, M. The contribution of soil tillage and nitrogen rate to the quality of maize grain. Agronomy 2020, 10, 976. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Shao, X.; Kong, Y.; Lyu, Y.; Wang, Y. Deep Tillage Improves the Grain Yield and Nitrogen Use Efficiency of Maize (Zea mays L.) Under a Wide–Narrow Row Alternative System in Northeast China. Int. J. Plant Prod. 2022, 16, 63–76. [Google Scholar] [CrossRef]
- Sun, B.; Gao, Y.; Wu, X.; Ma, H.; Zheng, C.; Wang, X.; Zhang, H.; Li, Z.; Yang, H. The relative contributions of pH, organic anions, and phosphatase to rhizosphere soil phosphorus mobilization and crop phosphorus uptake in maize/alfalfa polyculture. Plant Soil 2020, 447, 117–133. [Google Scholar] [CrossRef]
- Kuppe, C.W.; Kirk, G.J.; Wissuwa, M.; Postma, J.A. Rice increases phosphorus uptake in strongly sorbing soils by intra-root facilitation. Plant Cell Environ. 2022, 45, 884–899. [Google Scholar] [CrossRef]
- Li, M.; Cai, L. Biochar and Arbuscular mycorrhizal fungi play different roles in enabling maize to uptake phosphorus. Sustainability 2021, 13, 3244. [Google Scholar] [CrossRef]
- Long, J.; Chen, B.; Zhu, Y.; Li, X.; Yue, X.; Zhang, N.; Xia, Y. Mycorrhiza and Iron Tailings Synergistically Enhance Maize Resistance to Arsenic on Medium Arsenic-Polluted Soils Through Increasing Phosphorus and Iron Uptake. Bull. Environ. Contam. Toxicol. 2021, 107, 1155–1160. [Google Scholar] [CrossRef]
- Qi, S.; Wang, J.; Wan, L.; Dai, Z.; da Silva Matos, D.M.; Du, D.; Egan, S.; Bonser, S.P.; Thomas, T.; Moles, A.T. Arbuscular mycorrhizal fungi contribute to phosphorous uptake and allocation strategies of Solidago canadensis in a phosphorous-deficient environment. Front. Plant Sci. 2022, 13, 831654. [Google Scholar] [CrossRef]
- Anusha, K.; Gopal, K.S.; Ajithkumar, B. Funneliformis mosseae enhanced growth, tuber yield and P-uptake of Solenostemon rotundifolius under acidic and lateritic soil of Kerala. J. Trop. Agric. 2022, 59, 236–244. [Google Scholar]
- Juntahum, S.; Ekprasert, J.; Boonlue, S. Efficiency of Arbuscular Mycorrhizal Fungi for the Growth Promotion of Sugarcane Under Pot Conditions. Sugar Tech. 2022, 24, 1738–1747. [Google Scholar] [CrossRef]
- Biermann, B.; Linderman, R.G. Use of vesicular-arbuscular mycorrhizal roots, intraradical vesicles and extraradical vesicles as inoculum. New Phytol. 1983, 95, 97–105. [Google Scholar] [CrossRef]
- Hart, M.M.; Reader, R.J. Do arbuscular mycorrhizal fungi recover from soil disturbance differently? Trop. Ecol. 2004, 45, 97–111. [Google Scholar]
- Soteras, F.; Grilli, G.; Cofré, M.N.; Marro, N.; Becerra, A. Arbuscular mycorrhizal fungal composition in high montane forests with different disturbance histories in central Argentina. Appl. Soil Ecol. 2015, 85, 30–37. [Google Scholar] [CrossRef]
- Jasper, D.A.; Abbott, L.K.; Robson, A.D. Hyphae of a vesicular—Arbuscular mycorrhizal fungus maintain infectivity in dry soil, except when the soil is disturbed. New Phytol. 1989, 112, 101–107. [Google Scholar] [CrossRef]
- Li, L.F.; Li, T.; Zhao, Z.W. Differences of arbuscular mycorrhizal fungal diversity and community; between a cultivated land, an old field, and a never cultivated field in a hot and arid ecosystem of southwest China. Mycorrhiza 2007, 17, 655–665. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Jasper, D.A.; Ashwath, N. Glomalean mycorrhizal fungi from tropical Australia: II. The effect of nutrient levels and host species on the isolation of fungi. Mycorrhiza 1999, 8, 315–321. [Google Scholar] [CrossRef]
- Jasper, D.A.; Abbott, L.K.; Robson, A.D. The effect of soil disturbance on vesicular-arbuscular mycorrhizal fungi in soils from different vegetation types. New Phytol. 1991, 118, 471–476. [Google Scholar] [CrossRef]
- Hempel, S.; Renker, C.; Buscot, F. Differences in the species composition of arbuscular mycorrhizal fungi in spore, root and soil communities in a grassland ecosystem. Environ. Microbiol. 2007, 9, 1930–1938. [Google Scholar] [CrossRef]
- Li, Z.F.; Lü, P.P.; Wang, Y.L.; Yao, H.; Maitra, P.; Sun, X.; Zheng, Y.; Guo, L.D. Response of arbuscular mycorrhizal fungal community in soil and roots to grazing differs in a wetland on the Qinghai-Tibet plateau. PeerJ 2020, 8, e9375. [Google Scholar] [CrossRef] [PubMed]
- Guardiola-Marquez, C.E.; Pacheco, A.; Mora-Godinez, S.; Schüßler, A.; Gradilla-Hernández, M.S.; Senés-Guerrero, C. Septoglomus species dominate the arbuscular mycorrhiza of five crop plants in an arid region of northern Mexico. Symbiosis 2022, 87, 93–106. [Google Scholar] [CrossRef]
- Xu, J.; Liu, S.; Song, S.; Guo, H.; Tang, J.; Yong, J.W.H.; Ma, Y.; Chen, X. Arbuscular mycorrhizal fungi influence decomposition and the associated soil microbial community under different soil phosphorus availability. Soil Biol. Biochem. 2018, 120, 181–190. [Google Scholar] [CrossRef]
- Higo, M.; Tatewaki, Y.; Gunji, K.; Kaseda, A.; Isobe, K. Cover cropping can be a stronger determinant than host crop identity for arbuscular mycorrhizal fungal communities colonizing maize and soybean roots? PeerJ 2019, 7, e6403. [Google Scholar] [CrossRef] [PubMed]
- Higo, M.; Azuma, M.; Kamiyoshihara, Y.; Kanda, A.; Tatewaki, Y.; Isobe, K. Impact of phosphorus fertilization on tomato growth and arbuscular mycorrhizal fungal communities. Microorganims 2020, 8, 178. [Google Scholar] [CrossRef]
- Higo, M.; Isobe, K.; Yamaguchi, M.; Drijber, R.A.; Jeske, E.S.; Ishii, R. Diversity and vertical distribution of indigenous arbuscular mycorrhizal fungi under two soybean rotational systems. Biol. Fertil. Soils 2013, 48, 1085–1096. [Google Scholar] [CrossRef]
- Higo, M.; Isobe, K.; Drijber, R.A.; Kondo, T.; Yamaguchi, M.; Takeyama, S.; Suzuki, Y.; Niijima, D.; Matsuda, Y.; Ishii, R.; et al. Impact of a 5-year winter cover crop rotational system on the molecular diversity of arbuscular mycorrhizal fungi colonizing roots of subsequent soybean. Biol. Fertil. Soils 2014, 50, 913–926. [Google Scholar] [CrossRef]
- Higo, M.; Isobe, K.; Kondo, T.; Yamaguchi, M.; Takeyama, S.; Drijber, R.A.; Torigoe, Y. Temporal variation of the molecular diversity of arbuscular mycorrhizal communities in three different winter cover crop rotational systems. Biol. Fertil. Soils 2015, 51, 21–32. [Google Scholar] [CrossRef]
- Niwa, R.; Koyama, T.; Sato, T.; Adachi, K.; Tawaraya, K.; Sato, S.; Hirakawa, H.; Yoshida, S.; Ezawa, T. Dissection of niche competition between introduced and indigenous arbuscular mycorrhizal fungi with respect to soybean yield responses. Sci. Rep. 2018, 8, 7419. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; Ferrocino, I.; Rantsiou, K.; Cocolin, L. Metataxonomic comparison between internal transcribed spacer and 26S ribosomal large subunit (LSU) rDNA gene. Int. J. Food Microbiol. 2019, 290, 132–140. [Google Scholar] [CrossRef]
- Suzuki, K.; Takahashi, K.; Harada, N. Evaluation of primer pairs for studying arbuscular mycorrhizal fungal community compositions using a MiSeq platform. Biol. Fertil. Soils 2020, 56, 853–858. [Google Scholar] [CrossRef]
- Jansa, J.; Smith, F.A.; Smith, S.E. Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol. 2008, 177, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Tchabi, A.; Coyne, D.; Hountondji, F.; Lawouin, L.; Wiemken, A.; Oehl, F. Efficacy of indigenous arbuscular mycorrhizal fungi for promoting white yam (Dioscorea rotundata) growth in West Africa. Appl. Soil Ecol. 2010, 45, 92–100. [Google Scholar] [CrossRef]
- Gosling, P.; Jones, J.; Bending, G.D. Evidence for functional redundancy in arbuscular mycorrhizal fungi and implications for agroecosystem management. Mycorrhiza 2016, 26, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Higo, M.; Isobe, K.; Kang, D.J.; Ujiie, K.; Drijber, R.A.; Ishii, R. Inoculation with arbuscular mycorrhizal fungi or crop rotation with mycorrhizal plants improves the growth of maize in limed acid sulfate soil. Plant Prod. Sci. 2010, 13, 74–79. [Google Scholar] [CrossRef]
- Ortas, I.; Sari, N.; Akpinar, C.; Yetisir, H. Selection of Arbuscular Mycorrhizal Fungi Species for Tomato Seedling Growth Mycorrhizal Dependency and Nutrient Uptake. Europ. J. Hort. Sci. 2013, 78, 209–218. [Google Scholar]
- Hart, M.; Forsythe, J. Using arbuscular mycorrhizal fungi to improve the nutrient quality of crops; nutritional benefits in addition to phosphorus. Sci. Hortic. 2012, 148, 206–214. [Google Scholar] [CrossRef]
- Lendenmann, M.; Thonar, C.; Barnard, R.; Salmon, Y.; Werner, R.; Frossard, E.; Jansa, J. Symbiont identity matters: Carbon and phosphorus fluxes between Medicago truncatula and different arbuscular mycorrhizal fungi. Mycorrhiza 2011, 21, 689–702. [Google Scholar] [CrossRef]
- Waddell, H.A.; Simpson, R.J.; Ryan, M.H.; Lambers, H.; Garden, D.L.; Richardson, A.E. Root morphology and its contribution to a large root system for phosphorus uptake by Rytidosperma species (wallaby grass). Plant Soil 2017, 412, 7–19. [Google Scholar] [CrossRef]
- Burleigh, S.; Cavagnaro, T.; Jakobsen, I. Functional diversity of arbuscular mycorrhizas extends to the expression of plant genes involved in P nutrition. J. Exp. Bot. 2002, 53, 1593–1601. [Google Scholar] [CrossRef]
- Dodd, J.; Boddington, C.; Rodriguez, A.; Gonzalez-Chavez, C.; Mansur, I. Mycelium of Arbuscular Mycorrhizal fungi (AMF) from different genera: Form function and detection. Plant Soil 2000, 226, 131–151. [Google Scholar] [CrossRef]
- Solaiman, M.; Saito, A. Phosphate efflux from intraradical hyphae of Gigaspora margarita in vitro and its implication for phosphorus translocation. New Phytol. 2001, 151, 525–533. [Google Scholar] [CrossRef]
- Smith, S.; Smith, F.; Jakobsen, I. Functional diversity in arbuscular mycorrhizal (AM) symbioses: The contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol. 2004, 162, 511–524. [Google Scholar] [CrossRef]
- Ndoye, F.; Kane, A.; Bakhoum, N.; Sanon, A.; Fall, D.; Diouf, D.; Sylla, S.N.; Bâ, A.M.; Sy, M.O.; Noba, K. Response of Acacia senegal (L.) Willd. to inoculation with arbuscular mycorrhizal fungi isolates in sterilized and unsterilized soils in Senegal. Agrofor. Syst. 2013, 87, 941–952. [Google Scholar] [CrossRef]
- Hart, M.M.; Reader, R.J. Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol. 2002, 153, 335–344. [Google Scholar] [CrossRef]
- Maherali, H.; Klironomos, J.N. Phylogenetic and trait-based assembly of arbuscular mycorrhizal fungal communities. PLoS ONE 2012, 7, e36695. [Google Scholar] [CrossRef] [PubMed]
- Chagnon, P.L.; Bradley, R.L.; Maherali, H.; Klironomos, J.N. A trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci. 2013, 18, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Crossay, T.; Majorel, C.; Redecker, D.; Gensous, S.; Medevielle, V.; Durrieu, G.; Cavalok Amir, H. Is a mixture of arbuscular mycorrhizal fungi better for plant growth than single-species inoculants? Mycorrhiza 2019, 29, 325–339. [Google Scholar] [CrossRef]
- Van Geel, M.; De Beenhouwer, M.; Lievens, B.; Honnay, O. Crop-specific and single-species mycorrhizal inoculation is the best approach to improve crop growth in controlled environments. Agron. Sustain. Dev. 2016, 36, 1–10. [Google Scholar] [CrossRef]
- Verbruggen, E.; Van der Heijden, M.G.A.; Rillig, M.C.; Kiers, E.T. Mycorrhizal fungal establishment in agricultural soils: Factors determining inoculation success. New Phytol. 2013, 197, 1104–1109. [Google Scholar] [CrossRef]
- Baum, C.; El-Tohamy, W.; Gruda, N. Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: A review. Sci. Hortic. 2015, 187, 131–141. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular mycorrhizal fungi as natural biofertilizers: Let’s benefit from past successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef]
- Köhl, L.; Lukasiewicz, C.E.; Van der Heijden, M.G.A. Establishment and effectiveness of inoculated arbuscular mycorrhizal fungi in agricultural soils. Plant Cell Environ. 2016, 39, 136–146. [Google Scholar] [CrossRef]
- Bender, S.F.; Schlaeppi, K.; Held, A.; Van der Heijden, M.G.A. Establishment success and crop growth effects of an arbuscular mycorrhizal fungus inoculated into Swiss corn fields. Agric. Ecosyst. Environ. 2019, 273, 13–24. [Google Scholar] [CrossRef]
- Emam, T. Local soil, but not commercial AMF inoculum, increases native and non-native grass growth at a mine restoration site. Restor. Ecol. 2016, 24, 35–44. [Google Scholar] [CrossRef]
- Loján, P.; Senés-Guerrero, C.; Suárez, J.P.; Kromann, P.; SCHÜßLER, A.; Declerck, S. Potato field-inoculation in Ecuador with Rhizophagus irregularis: No impact on growth performance and associated arbuscular mycorrhizal fungal communities. Symbiosis 2017, 73, 45–56. [Google Scholar] [CrossRef]
- Basiru, S.; Hijri, M. Does Commercial Inoculation Promote Arbuscular Mycorrhizal Fungi Invasion? Microorganisms 2022, 10, 404. [Google Scholar] [CrossRef]
- Thomsen, C.; Loverock, L.; Kokkoris, V.; Holland, T.; Bowen, P.A.; Hart, M. Commercial arbuscular mycorrhizal fungal inoculant failed to establish in a vineyard despite priority advantage. PeerJ 2021, 9, e11119. [Google Scholar] [CrossRef]
- Chaudhary, V.B.; Nolimal, S.; Sosa-Hernandez, M.A.; Egan, C.; Kastens, J. Trait-based aerial dispersal of arbuscular mycorrhizal fungi. New Phytol. 2020, 228, 238–252. [Google Scholar] [CrossRef]
- Islam, M.N.; Germida, J.J.; Walley, F.L. Survival of a commercial AM fungal inoculant and its impact on indigenous AM fungal communities in field soils. Appl. Soil Ecol. 2021, 166, 103979. [Google Scholar] [CrossRef]
- Paz, C.; Opik, M.; Bulascoschi, L.; Bueno, C.G.; Galetti, M. Dispersal of Arbuscular Mycorrhizal Fungi: Evidence and Insights for Ecological Studies. Microb. Ecol. 2021, 81, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Bainard, L.D.; Bainard, J.D.; Hamel, C.; Gan, Y. Spatial and temporal structuring of arbuscular mycorrhizal communities is differentially influenced by abiotic factors and host crop in a semi-arid prairie agroecosystem. FEMS Microbiol. Ecol. 2014, 88, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Renaut, S.; Daoud, R.; Masse, J.; Vialle, A.; Hijri, M. Inoculation with Rhizophagus irregularis does not alter arbuscular mycorrhizal fungal community structure within the roots of corn, wheat, and soybean crops. Microorganisms 2020, 8, 83. [Google Scholar] [CrossRef]
- Alguacil, M.D.M.; Torres, M.P.; Montesinos-Navarro, A.; Roldán, A. Soil characteristics driving arbuscular mycorrhizal fungal communities in semiarid Mediterranean soils. Appl. Environ. Microbiol. 2016, 82, 3348–3356. [Google Scholar] [CrossRef]
- Vieira, L.C.; Silva, D.K.A.D.; Escobar, I.E.C.; Silva, J.M.D.; Moura, I.A.D.; Oehl, F.; Silva, G.A.D. Changes in an arbuscular mycorrhizal fungi community along an environmental gradient. Plants 2020, 9, 52. [Google Scholar] [CrossRef]
- Bainard, L.D.; Dai, M.; Gomez, E.F.; Torres-Arias, Y.; Bainard, J.D.; Sheng, M.; Eilers, W.; Hamel, C. Arbuscular mycorrhizal fungal communities are influenced by agricultural land use and not soil type among the Chernozem great groups of the Canadian Prairies. Plant Soil 2015, 387, 351–362. [Google Scholar] [CrossRef]
- Albornoz, F.E.; Ryan, M.H.; Bending, G.D.; Hilton, S.; Dickie, I.A.; Gleeson, D.B.; Standish, R.J. Agricultural land-use favours Mucoromycotinian, but not Glomeromycotinian, arbuscular mycorrhizal fungi across ten biomes. New Phytol. 2022, 233, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, M.G.A.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T.; Wiemken, A.; Sanders, I.R. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]

| Treatments | AMF Colonization (%) | Aboveground Biomass (g/Plant) | Aboveground P Concentration (mg P/g) | Aboveground P Uptake (mg P/Plant) | ||||
|---|---|---|---|---|---|---|---|---|
| Control | - | 0.75 ± 0.03 | 0.68 ± 0.00 | 0.60 ± 0.05 | ||||
| A. morrowiae AP-5 A. longula F-1 D. cerradensis TK-1 C. Pellucida SZ-3 | 4.97 ± 3.27 (1) | a (2) | 0.99 ± 0.11 | n.s. (3) | 1.23 ± 0.24 | n.s. | 1.20 ± 0.22 | * |
| 0.15 ± 0.15 | a | 0.87 ± 0.04 | n.s. | 0.88 ± 0.13 | n.s. | 0.76 ± 0.13 | n.s. | |
| 2.88 ± 1.57 | a | 0.75 ± 0.07 | n.s. | 1.54 ± 0.18 | ** | 1.14 ± 0.08 | n.s. | |
| 5.52 ± 2.34 | a | 0.89 ± 0.07 | n.s. | 1.52 ± 0.05 | ** | 1.35 ± 0.12 | ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatewaki, Y.; Higo, M.; Isobe, K. Impacts of Tillage Practices on Growth, Phosphorus Uptake, and Yield of Maize in Controlled and Field-Based Studies in Relation to Arbuscular Mycorrhizal Fungi. Appl. Microbiol. 2023, 3, 358-374. https://doi.org/10.3390/applmicrobiol3020025
Tatewaki Y, Higo M, Isobe K. Impacts of Tillage Practices on Growth, Phosphorus Uptake, and Yield of Maize in Controlled and Field-Based Studies in Relation to Arbuscular Mycorrhizal Fungi. Applied Microbiology. 2023; 3(2):358-374. https://doi.org/10.3390/applmicrobiol3020025
Chicago/Turabian StyleTatewaki, Yuya, Masao Higo, and Katsunori Isobe. 2023. "Impacts of Tillage Practices on Growth, Phosphorus Uptake, and Yield of Maize in Controlled and Field-Based Studies in Relation to Arbuscular Mycorrhizal Fungi" Applied Microbiology 3, no. 2: 358-374. https://doi.org/10.3390/applmicrobiol3020025
APA StyleTatewaki, Y., Higo, M., & Isobe, K. (2023). Impacts of Tillage Practices on Growth, Phosphorus Uptake, and Yield of Maize in Controlled and Field-Based Studies in Relation to Arbuscular Mycorrhizal Fungi. Applied Microbiology, 3(2), 358-374. https://doi.org/10.3390/applmicrobiol3020025






