The Baseline Gut Microbiota Enterotype Directs Lifestyle-Induced Amelioration of Pollen Allergy Severity: A Self Controlled Case-Series Study
Abstract
1. Introduction
2. Materials and Methods
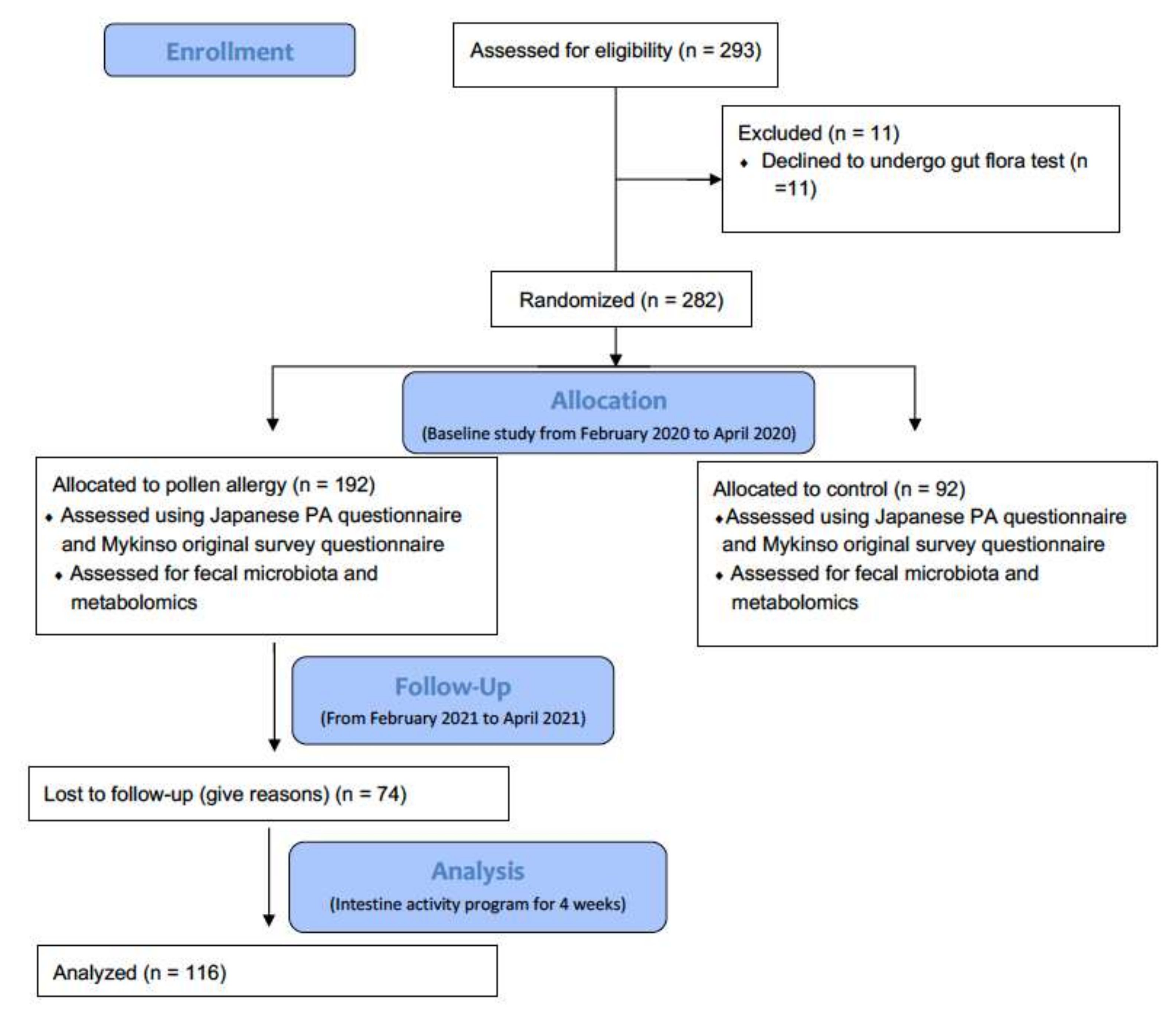
2.1. Study Design and Participant Enrollment
2.1.1. Design
2.1.2. Study Population
2.1.3. Measurement and Evaluation
2.2. Interventions
2.3. Fecal Sampling, DNA Extraction, Sequencing, and Data Analysis
2.4. Taxonomic Identification
2.5. Data Analysis
2.5.1. Enterotyping
2.5.2. Regression Analysis
2.5.3. Difference-in-Differences Analysis
3. Results
3.1. Characteristics
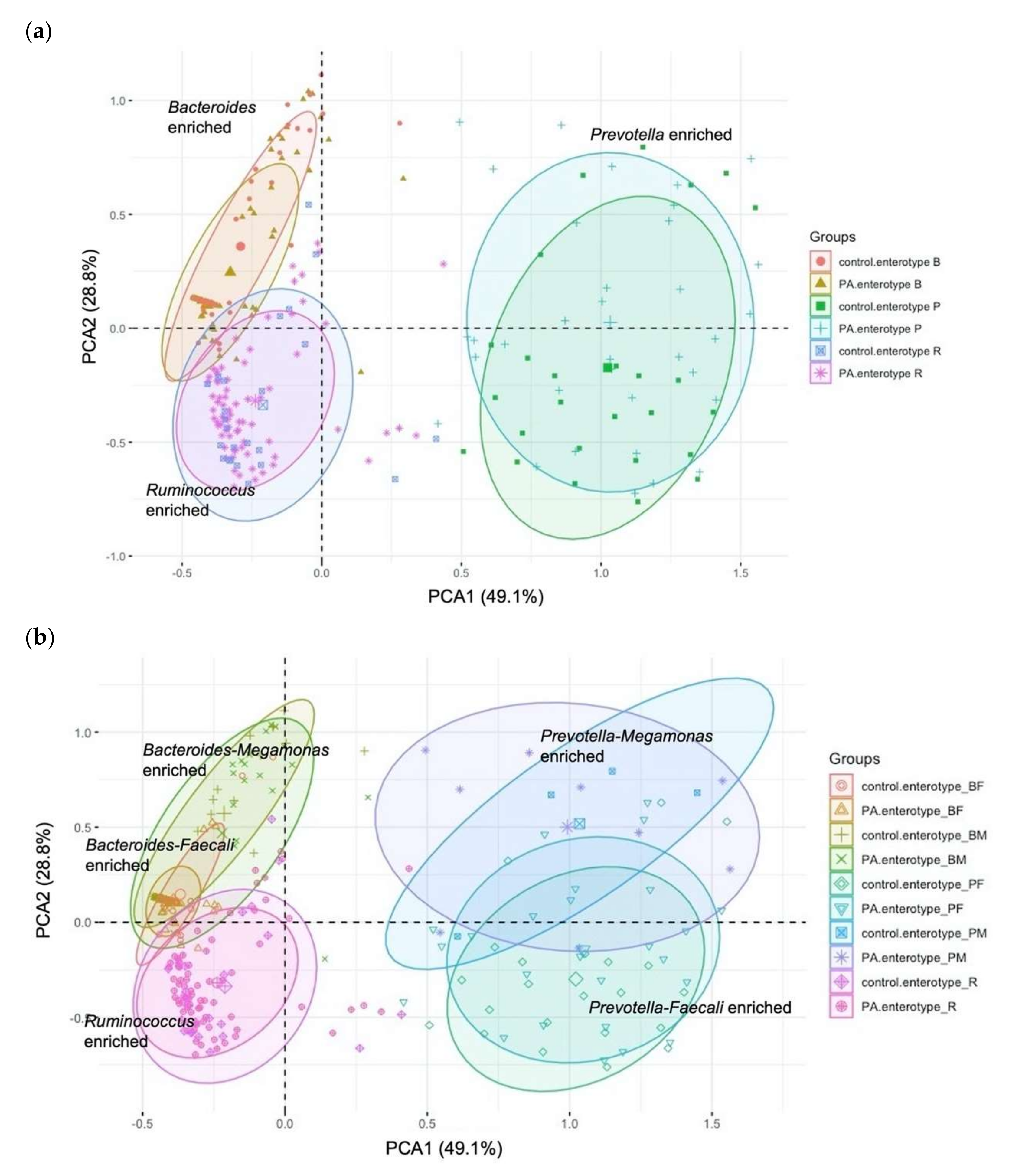
3.2. Enterotyping
3.3. Associations between Baseline Enterotype and Severity of PA
3.4. Associations between Demographic, Lifestyle Factors and Severity of PA
3.5. Interaction between Lifestyle Factors and Baseline Gut Microbiota Enterotype Is Superior as an Explanatory Factor of Severity of PA
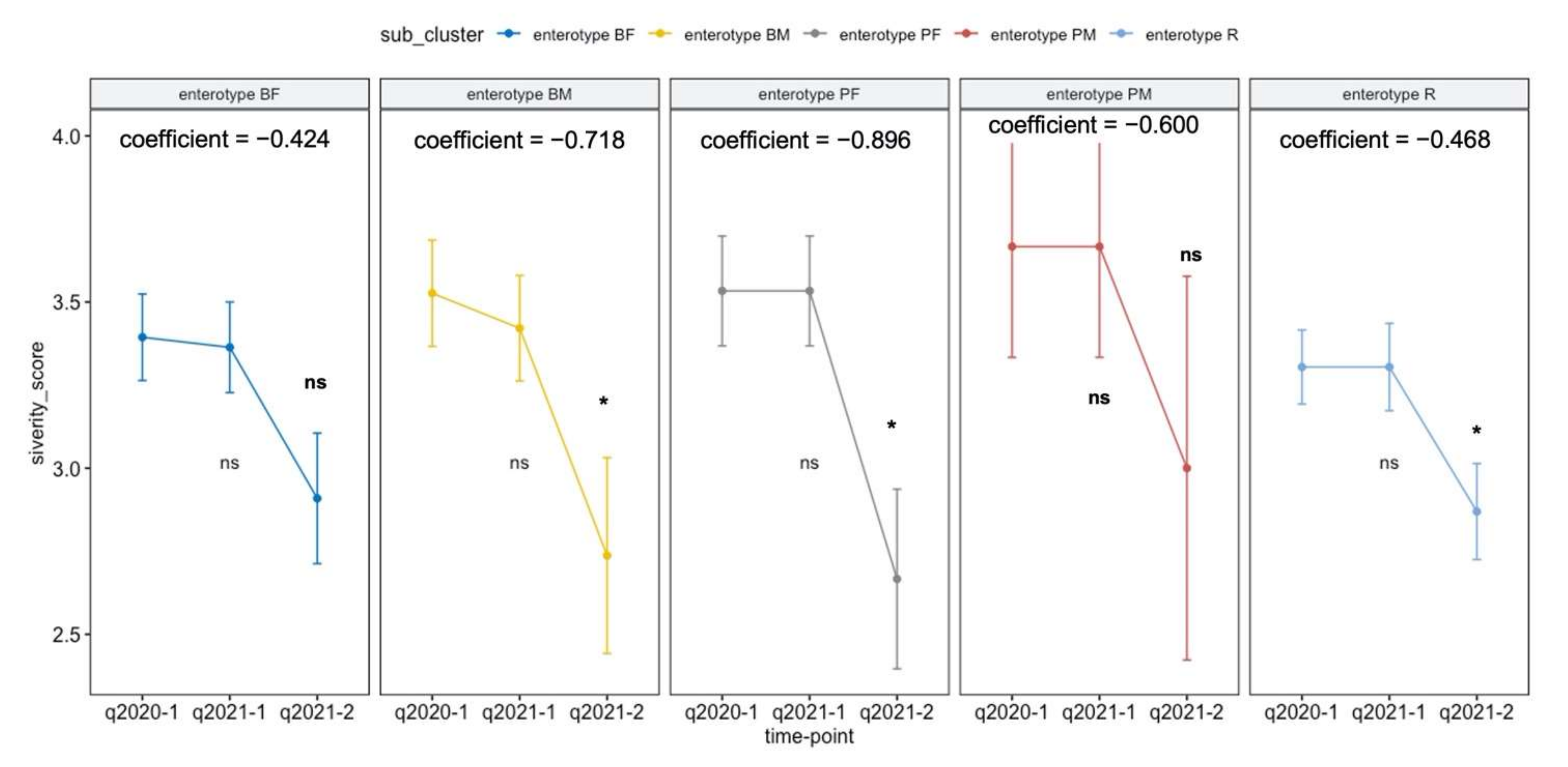
3.6. Intestinal Activity Intervention Program Depending on Enterotype Affect the PA Trajectory
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pawankar, R. Allergic rhinitis and its impacts on asthma: An evidence-based treatment strategy for allergic rhinitis. Asian Pac. J. Allergy Immunol. 2002, 20, 43–52. [Google Scholar] [PubMed]
- Seidman, M.D.; Gurgel, R.K.; Lin, S.Y.; Schwartz, S.R.; Baroody, F.M.; Bonner, J.R.; Dawson, D.E.; Dykewicz, M.S.; Hackell, J.M.; Han, J.K.; et al. Clinical practice guideline: Allergic rhinitis. Otolaryngol. Head Neck Surg. 2015, 152, S1–S43. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Kurono, Y.; Ichimura, K.; Enomoto, T.; Okamoto, Y.; Kawauchi, H.; Suzaki, H.; Fujieda, S.; Masuyama, K. Japanese guidelines for allergic rhinitis 2017. Allergol. Int. 2017, 66, 205–219. [Google Scholar] [CrossRef]
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baenacagnani, C.E.; Canonica, G.W.; Van, W.C.; et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008, 63, 8–160. [Google Scholar] [CrossRef]
- Han, M.W.; Kim, S.H.; Oh, I.; Kim, Y.H.; Lee, J. Serum IL-1beta can be a biomarker in children with severe persistent allergic rhinitis. Allergy Asthma Clin. Immunol. 2019, 15, 58. [Google Scholar] [CrossRef]
- Minami, Y.; Shiozaki, Y.; Kato, C.; Ito, M.; Takeuchi, N.; Koyanagi, M.; Ogino, S. Japanese cedar pollinosis impact on work productivity, quality of life, and symptoms 2008 vs. 2009. Nihon Jibika Gakkai Kaishi 2010, 49, 481–489. [Google Scholar] [CrossRef]
- Kakutani, C.; Ogino, S.; Ikeda, H.; Enomoto, T. Impact of allergic rhinitis on work productivity: A pilot study. Allergy 2005, 54, 627–635. [Google Scholar]
- Kakutani, C.; Ogino, S.; Ikeda, H.; Enomoto, T. Comparison of clinical efficacy and cost-quality of antihistamines in early treatment for Japanese cedar pollinosis. Arerugi = [Allergy] 2006, 55, 554–565. [Google Scholar]
- Kakli, H.A.; Riley, T.D. Allergic Rhinitis. Prim. Care 2016, 43, 465–475. [Google Scholar] [CrossRef]
- Brożek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef]
- Khan, D.A. Allergic rhinitis and asthma: Epidemiology and common pathophysiology. Allergy Asthma Proc. 2014, 35, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Skoner, D.P. Allergic rhinitis: Definition, epidemiology, pathophysiology, detection, and diagnosis. J. Allergy Clin. Immunol. 2001, 108, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- McKeever, T.M.; Britton, J. Diet and asthma. Am. J. Respir. Crit. Care Med. 2004, 170, 725–729. [Google Scholar] [CrossRef]
- Garcia-Larsen, V.; Del Giacco, S.R.; Moreira, A.; Bonini, M.; Charles, D.; Reeves, T.; Carlsen, K.H.; Haahtela, T.; Bonini, S.; Fonseca, J.; et al. Asthma and dietary intake: An overview of systematic reviews. Allergy 2016, 71, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Macias, H.; Romieu, I. Effects of antioxidant supplements and nutrients on patients with asthma and allergies. J. Allergy Clin. Immunol. 2014, 133, 1237–1244. [Google Scholar] [CrossRef]
- Nurmatov, U.; Devereux, G.; Sheikh, A. Nutrients and foods for the primary prevention of asthma and allergy: Systematic review and meta-analysis. J. Allergy Clin. Immunol. 2011, 127, 724–733. [Google Scholar] [CrossRef]
- Tamura, M.; Shikina, T.; Morihana, T.; Hayama, M.; Kajimoto, O.; Sakamoto, A.; Kajimoto, Y.; Watanabe, O.; Nonaka, C.; Shida, K.; et al. Effects of probiotics on allergic rhinitis induced by Japanese cedar pollen: Randomized, double-blind, placebo-controlled clinical trial. Int. Arch. Allergy Immunol. 2007, 143, 75–82. [Google Scholar] [CrossRef]
- Sagar, S.; Vos, A.P.; Morgan, M.E.; Garssen, J.; Georgiou, N.A.; Boon, L.; Kraneveld, A.D.; Folkerts, G. The combination of Bifidobacterium breve with non-digestible oligosaccharides suppresses airway inflammation in a murine model for chronic asthma. Biochim. Biophys. Acta 2014, 1842, 573–583. [Google Scholar] [CrossRef]
- Ege, M.J.; Mayer, M.; Schwaiger, K.; Mattes, J.; Pershagen, G.; van Hage, M.; Scheynius, A.; Bauer, J.; von Mutius, E. Environmental bacteria and childhood asthma. Allergy 2012, 67, 1565–1571. [Google Scholar] [CrossRef]
- Harata, G.; Kumar, H.; He, F.; Miyazawa, K.; Yoda, K.; Kawase, M.; Kubota, A.; Hiramatsu, M.; Rautava, S.; Salminen, S. Probiotics modulate gut microbiota and health status in Japanese cedar pollinosis patients during the pollen season. Eur. J. Nutr. 2017, 56, 2245–2253. [Google Scholar] [CrossRef]
- Fujieda, S.; Kurono, Y.; Okubo, K.; Ichimura, K.; Enomoto, T.; Kawauchi, H.; Masuyama, K.; Goto, M.; Suzaki, H.; Okamoto, Y.; et al. Examination, diagnosis and classification for Japanese allergic rhinitis: Japanese guideline. Auris Nasus Larynx 2012, 39, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Kameoka, S.; Shinozaki, N.O.; Kubo, R.; Nishida, A.; Kuriyama, M.; Takeda, A.K. A cross-sectional analysis from the Mykinso Cohort Study: Establishing reference ranges for Japanese gut microbial indices. Biosci. Microbiota Food Health 2021, 40, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Kameoka, S.; Motooka, D.; Watanabe, S.; Kubo, R.; Jung, N.; Midorikawa, Y.; Shinozaki, N.O.; Sawai, Y.; Takeda, A.K.; Nakamura, S. Benchmark of 16S rRNA gene amplicon sequencing using Japanese gut microbiome data from the V1–V2 and V3–V4 primer sets. BMC Genom. 2021, 22, 527. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Watanabe, S.; Yamasaki, H.; Sakuma, H.; Takeda, A.K.; Yamashita, T.; Hirata, K.I. Average gut flora in healthy Japanese subjects stratified by age and body mass index. Biosci. Microbiota Food Health 2022, 41, 45–53. [Google Scholar] [CrossRef]
- Kuhn, M.; Wickham, H. Tidymodels: A Collection of Packages for Modeling and Machine Learning Using Tidyverse Principles. 2020. Available online: https://www.tidymodels.org (accessed on 12 September 2022).
- Marschner, I.C. glm2: Fitting generalized linear models with convergence problems. R J. 2011, 3, 12–15. [Google Scholar] [CrossRef]
- Zenil, H.; Uthamacumaran, A.; Saeb-Parsy, K. The immune system in health and disease: The need for personalised longitudinal monitoring. In Cancer, Complexity, Computation. Emergence, Complexity and Computation; Balaz, I., Adamatzky, A., Eds.; Springer: Cham, Switzerland, 2022; Volume 46, pp. 333–348. [Google Scholar]
- Muraro, A.; Lemanske, R.F., Jr.; Hellings, P.W.; Akdis, C.A.; Bieber, T.; Casale, T.B.; Jutel, M.; Ong, P.Y.; Poulsen, L.K.; Schmid-Grendelmeier, P.; et al. Precision medicine in patients with allergic diseases: Airway diseases and atopic dermatitis-PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2016, 137, 1347–1358. [Google Scholar]
- Julia, V.; Macia, L.; Dombrowicz, D. The impact of diet on asthma and allergic diseases. Nat. Rev. Immunol. 2015, 15, 308–322. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, W.; Yung, E.; Zhong, N.; Wong, G.W.; Li, J. Frequency of food group consumption and risk of allergic disease and sensitization in schoolchildren in urban and rural China. Clin. Exp. Allergy 2015, 45, 1823–1832. [Google Scholar] [CrossRef]
- Mozaffarian, D. Foods, nutrients, and health: When will our policies catch up with nutrition science? Lancet Diabetes Endocrinol. 2017, 5, 85–88. [Google Scholar] [CrossRef]
- Fujimura, T.; Hori, A.; Torii, H.; Kishida, S.; Matsuura, Y.; Kawamoto, S. Intake of a fermented plant product attenuates allergic symptoms without changing systemic immune responses in a mouse model of Japanese cedar pollinosis. World Allergy Organ. J. 2018, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, M.; Omine, M.; Yun, Y.; Shuto, E.; Nakamoto, A.; Hata, A.; Aki, N.; Shikama, Y.; Bando, Y.; Ichihara, T.; et al. Associations of dietary diversity with allergic diseases in Japanese workers: A cross-sectional study. Asia Pac. J. Clin. Nutr. 2019, 28, 857–869. [Google Scholar] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Christensen, L.; Roager, H.M.; Astrup, A.; Hjorth, M.F. Microbial enterotypes in personalized nutrition and obesity management. Am. J. Clin. Nutr. 2018, 108, 645–651. [Google Scholar] [CrossRef]
- Watts, A.M.; West, N.P.; Zhang, P.; Smith, P.K.; Cripps, A.W.; Cox, A.J. The gut microbiome of adults with allergic rhinitis is characterised by reduced diversity and an altered abundance of key microbial taxa compared to controls. Int. Arch. Allergy Immunol. 2021, 182, 94–105. [Google Scholar] [CrossRef]
- Melli, L.C.; do Carmo-Rodrigues, M.S.; Araújo-Filho, H.B.; Solé, D.; de Morais, M.B. Intestinal microbiota and allergic diseases: A systematic review. Allergol. Immunopathol. 2016, 44, 177–188. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, F.; Wan, W.; Yu, B.; Tang, L.; Yang, Y.; Du, Y.; Chen, Z.; Xu, H. Gut microbial characteristics of adult patients with allergy rhinitis. Microb. Cell Fact. 2020, 19, 171. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Duncan, S.H.; Holtrop, G.; Lobley, G.E.; Calder, A.G.; Stewart, C.S.; Flint, H.J. Contribution of acetate to butyrate formation by human faecal bacteria. Br. J. Nutr. 2004, 91, 915–923. [Google Scholar] [CrossRef]
- Song, H.; Yoo, Y.; Hwang, J.; Na, Y.C.; Kim, H.S. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Kontula, P.; von Wright, A.; Mattila-Sandholm, T. Oat bran β-gluco- and xylo-oligosaccharides as fermentative substrates for lactic acid bacteria. Int. J. Food Microbiol. 1998, 45, 163–169. [Google Scholar] [CrossRef]
- Kubota, A.; He, F.; Kawase, M.; Harata, G.; Hiramatsu, M.; Iino, H. Diversity of intestinal Bifidobacteria in patients with Japanese cedar pollinosis and possible influence of probiotic intervention. Curr. Microbiol. 2011, 62, 71–77. [Google Scholar] [CrossRef]
- Sahoyama, Y.; Hamazato, F.; Shiozawa, M.; Nakagawa, T.; Suda, W.; Ogata, Y.; Hachiya, T.; Kawakami, E.; Hattori, M. Multiple nutritional and gut microbial factors associated with allergic rhinitis: The Hitachi Health Study. Sci. Rep. 2022, 12, 3359. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, C.; Tan, J.; Macia, L.; Mackay, C.R. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol. Rev. 2017, 278, 277–295. [Google Scholar] [CrossRef]
- Chatzi, L.; Apostolaki, G.; Bibakis, I.; Skypala, I.; Bibaki-Liakou, V.; Tzanakis, N.; Kogevinas, M.; Cullinan, P. Protective effect of fruits, vegetables and the Mediterranean diet on asthma and allergies among children in Crete. Thorax 2007, 62, 677–683. [Google Scholar] [CrossRef]
- Chang, Y.S.; Trivedi, M.K.; Jha, A.; Lin, Y.F.; Dimaano, L.; García-Romero, M.T. Synbiotics for Prevention and Treatment of Atopic Dermatitis: A Meta-analysis of Randomized Clinical Trials. JAMA Pediatr. 2016, 170, 236–242. [Google Scholar] [CrossRef]
- Allen, S.J.; Jordan, S.; Storey, M.; Thornton, C.A.; Gravenor, M.B.; Garaiova, I.; Plummer, S.F.; Wang, D.; Morgan, G. Probiotics in the prevention of eczema: A randomised controlled trial. Arch. Dis. Child. 2014, 99, 1014–1019. [Google Scholar] [CrossRef]
- Abrahamsson, T.R.; Jakobsson, T.; Böttcher, M.F.; Fredrikson, M.; Jenmalm, M.C.; Björkstén, B.; Oldaeus, G. Probiotics in prevention of IgE-associated eczema: A double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2007, 119, 1174–1180. [Google Scholar] [CrossRef]
- Nadeem, I.; Rahman, M.Z.; Ad-Dab’bagh, Y.; Akhtar, M. Effect of probiotic interventions on depressive symptoms: A narrative review evaluating systematic reviews. Psychiatry Clin. Neurosci. 2019, 73, 154–162. [Google Scholar] [CrossRef]
- Sestito, S.; D’Auria, E.; Baldassarre, M.E.; Salvatore, S.; Tallarico, V.; Stefanelli, E.; Tarsitano, F.; Concolino, D.; Pensabene, L. The Role of Prebiotics and Probiotics in Prevention of Allergic Diseases in Infants. Front. Pediatr. 2020, 8, 583946. [Google Scholar] [CrossRef] [PubMed]
- Mai, X.M.; Chen, Y.; Camargo, C.A., Jr.; Langhammer, A. Serum 25-hydroxyvitamin D levels and self-reported allergic rhinitis in Norwegian adults: The HUNT Study. Allergy 2014, 69, 488–493. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoshisue, H.; Ito, C.; Okano, M. Clinical characteristics, health care resource utilization, and prescription patterns of Japanese patients with physician-diagnosed allergic rhinitis: A secondary use of database study. Int. Arch. Allergy Immunol. 2022, 183, 953–966. [Google Scholar] [CrossRef] [PubMed]
| Normal (Control) Group (n = 92) | PA Group (n = 190) | p-Value | ||
|---|---|---|---|---|
| Gender, n (%) | 0.5 | |||
| Female | 49 (53%) | 93 (49%) | ||
| Male | 43 (47%) | 97 (51%) | ||
| Age (years) | 0.8 | |||
| 20–29 | 17 (18%) | 44 (23%) | ||
| 30–39 | 25 (27%) | 48 (25%) | ||
| 40–49 | 24 (26%) | 49 (26%) | ||
| 50–59 | 26 (28%) | 49 (26%) | ||
| Pollen allergy severity (n, %) | <0.001 | |||
| 0, none | 92 (100%) | 0 (0%) | ||
| 1, mild | 0 (0%) | 0 (0%) | ||
| 2, moderate | 0 (0%) | 32 (17%) | ||
| 3, severe | 0 (0%) | 52 (27%) | ||
| 4, very severe | 0 (0%) | 106 (56%) |
| Normal (Control) Group (n = 92) | PA Group (n = 190) | p-Value | ||
|---|---|---|---|---|
| Enterotypes [n (%)] | 0.059 | |||
| Enterotype BF | 21 (23%) | 51 (27%) | ||
| Enterotype BM | 21 (23%) | 31 (16%) | ||
| Enterotype PF | 22 (24%) | 25 (13%) | ||
| Enterotype PM | 4 (4.3%) | 9 (4.7%) | ||
| Enterotype R | 24 (26%) | 74 (39%) |
| Enterotypes | Coefficient | Covariate Adjusted p-Value |
|---|---|---|
| Enterotype BF | - | - |
| Enterotype BM | −0.161 | 0.196 |
| Enterotype PF | −0.394 | 0.005 |
| Enterotype PM | −0.004 | 0.984 |
| Enterotype R | 0.048 | 0.629 |
| Demographic, Lifestyle Factors | Coefficient | Covariate Adjusted p-Value |
|---|---|---|
| Gender: Male | 0.160 | 0.099 |
| Age | −0.002 | 0.659 |
| Three meals a day | −0.019 | 0.852 |
| Poor sleep quality | 0.195 | 0.046 |
| No weekly intake of meat | −1.333 | 0.024 |
| Daily intake of animal protein | −0.016 | 0.845 |
| Daily intake of fermented plant | −0.320 | 0.006 |
| Enterotypes | Lifestyle Factors | Coefficient | Covariate-Adjusted p-Value |
|---|---|---|---|
| BF | Three meals a day | 0.374 | 0.092 |
| BF | No weekly intake of natto | 0.303 | 0.099 |
| BM | Three meals a day | −0.395 | 0.071 |
| BM | No weekly intake of fish | 0.792 | 0.015 |
| PF | Fish daily intake | 0.859 | 0.007 |
| PF | Seaweed daily intake | 0.943 | 0.067 |
| PM | Animal protein daily intake | −1.179 | 0.029 |
| PM | Eggs daily intake | −1.286 | 0.018 |
| R | Animal protein daily intake | 0.271 | 0.098 |
| R | Dairy products daily intake | 0.357 | 0.053 |
| R | No weekly intake of dairy products | −0.456 | 0.033 |
| Interaction Factor | |||
|---|---|---|---|
| Enterotypes | Lifestyle Factors | Covariate-Adjusted Odds Ratio | Covariate-Adjusted p-Value |
| BF | Sleep quality poor | 3.36 [1.06–14.99] | 0.064 |
| BM | Three meals a day | 0.47 [0.19–1.15] | 0.101 |
| PF | Three meals a day | 0.38 [0.16–0.90] | 0.029 |
| PF | Dairy products daily intake | 0.44 [0.15–1.28] | 0.126 |
| PF | Fermented plant daily intake | 0.21 [0.05–0.72] | 0.015 |
| PM | Animal protein daily intake | 0.15 [0.01–1.28] | 0.114 |
| R | Animal protein daily intake | 2.59 [1.06–7.02] | 0.046 |
| R | Fermented plant daily intake | 0.77 [0.30–2.03] | 0.584 |
| R | No weekly intake of fermented plant | 1.98 [0.91–4.48] | 0.091 |
| Enterotype | Terms | Coefficient | Covariate-Adjusted p-Value |
|---|---|---|---|
| BF | Intervention: sleep quality × year | −0.4242 | 0.1024 |
| BM | Intervention: three meals a day × year | −0.7177 | 0.0319 |
| PF | Intervention: dairy products daily intake × year | −0.8958 | 0.0294 |
| PM | Intervention: animal protein daily intake × year | −0.6000 | 0.2108 |
| R | Intervention: fermented plant daily intake × year | −0.4637 | 0.0326 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, S.; Fukushima, T.; Matsuo, Y.; Morimoto, T.; Deguchi, T.; Fukumuro, K.; Sawai, Y. The Baseline Gut Microbiota Enterotype Directs Lifestyle-Induced Amelioration of Pollen Allergy Severity: A Self Controlled Case-Series Study. Appl. Microbiol. 2022, 2, 905-920. https://doi.org/10.3390/applmicrobiol2040069
Watanabe S, Fukushima T, Matsuo Y, Morimoto T, Deguchi T, Fukumuro K, Sawai Y. The Baseline Gut Microbiota Enterotype Directs Lifestyle-Induced Amelioration of Pollen Allergy Severity: A Self Controlled Case-Series Study. Applied Microbiology. 2022; 2(4):905-920. https://doi.org/10.3390/applmicrobiol2040069
Chicago/Turabian StyleWatanabe, Satoshi, Takayoshi Fukushima, Yusuke Matsuo, Takahito Morimoto, Tsuneo Deguchi, Keisuke Fukumuro, and Yu Sawai. 2022. "The Baseline Gut Microbiota Enterotype Directs Lifestyle-Induced Amelioration of Pollen Allergy Severity: A Self Controlled Case-Series Study" Applied Microbiology 2, no. 4: 905-920. https://doi.org/10.3390/applmicrobiol2040069
APA StyleWatanabe, S., Fukushima, T., Matsuo, Y., Morimoto, T., Deguchi, T., Fukumuro, K., & Sawai, Y. (2022). The Baseline Gut Microbiota Enterotype Directs Lifestyle-Induced Amelioration of Pollen Allergy Severity: A Self Controlled Case-Series Study. Applied Microbiology, 2(4), 905-920. https://doi.org/10.3390/applmicrobiol2040069






