Abstract
The cultivation of blueberry (Vaccinium spp.) has undergone an unprecedented global expansion, driven by its nutraceutical value and the diversification of production zones across the Americas, Europe, and Asia. Its consolidation as a strategic crop has prompted intensive scientific activity aimed at optimizing every stage of management from propagation and physiology to harvest, postharvest, and environmental sustainability. However, the available evidence remains fragmented, limiting the integration of results and the formulation of knowledge-based, comparative production strategies. The objective of this systematic review was to synthesize scientific and technological advances related to the integrated management of blueberry cultivation, incorporating physiological, agronomic, technological, and environmental dimensions. The PRISMA 2020 methodology (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) was applied to ensure transparency and reproducibility in the search, selection, and analysis of scientific literature indexed in the Scopus database. After screening, 367 articles met the inclusion criteria and were analyzed comparatively and thematically. The results reveal significant progress in propagation using hydrogel and micropropagation techniques, efficient fertigation practices, and the integration of climate control operations within greenhouses, leading to improved yield and fruit quality. Likewise, non-thermal technologies, edible coatings, and harvest automation enhance postharvest quality and reduce losses. In terms of sustainability, the incorporation of water reuse and waste biorefinery has emerged as key strategies to reduce the environmental footprint and promote circular systems. Among the main limitations are the lack of methodological standardization, the scarce economic evaluation of innovations, and the weak linkage between experimental and commercial scales. It is concluded that integrating physiology, technology, and sustainability within a unified management framework is essential to consolidate a resilient, low-carbon, and technologically advanced fruit-growing system.
1. Introduction
The cultivation of blueberries (Vaccinium spp.) has become one of the most rapidly expanding fruit crops worldwide in recent decades, driven by both its high economic value and its nutritional and functional importance [1]. Increasing international market demand has encouraged varietal diversification and the adoption of new cultivation technologies in nontraditional regions, ranging from temperate to subtropical climates [2]. This process has transformed global fruit growing, stimulating knowledge generation on physiology, agronomic management, technological innovation, and environmental sustainability. Currently, countries such as the United States, Peru, Chile, Spain, and China lead global production, while new regions in Latin America and Eastern Europe are emerging with competitive potential [3].
The integrated management of blueberries encompasses all stages of the production system from plant propagation and crop establishment to harvest, postharvest, and industrial processing. Recent scientific literature reports significant advances in in vitro and cutting propagation [4], nutritional control and fertigation management [5], phenological regulation through physiological compounds [6], the use of protected structures [7], integrated pest management, and harvest mechanization [8,9]. Likewise, postharvest stages have benefited from emerging technologies such as high hydrostatic pressure, pulsed light, thermosonication, and edible coatings, all aimed at extending shelf life and preserving the fruit’s bioactive compounds [10]. This holistic approach has enabled an understanding of blueberry as a complex biological system in which technological innovation and sustainability must operate synergistically.
Systematic reviews have become essential tools for integrating the broad and fragmented scientific evidence available on blueberry management. Unlike narrative reviews, this methodological approach allows for the identification of patterns, knowledge gaps, and emerging trends based on rigorous search, selection, and analytical criteria [11,12]. Its application in the agricultural field is particularly relevant, as it connects dispersed experimental results with real innovation needs in production systems, thus facilitating technology transfer to growers, nurseries, and agro-industries. Within this context, systematic review provides a solid scientific foundation to guide policy design, research programs, and investment decisions in sustainable and technology-driven agriculture [13].
The objective of this systematic review was to analyze the main scientific and technological approaches associated with the integrated management of blueberry cultivation, covering physiological, agronomic, technological, and environmental dimensions. To achieve this goal, the following research questions (RQ) were formulated:
RQ1. What are the most efficient and sustainable plant propagation and establishment techniques that ensure uniformity, vigor, and adaptability under different agroclimatic conditions?
RQ2. How do nutrition, fertigation, and eco-physiological regulation strategies influence productivity, fruit quality, and the efficient use of water and energy resources?
RQ3. What role do controlled-environment production technologies play in optimizing microclimate and mitigating abiotic stress?
RQ4. In what ways do sustainability and circular economy strategies (water reuse, renewable energy, life cycle assessment LCA, and biorefinery) contribute to reducing environmental impact and strengthening production system resilience?
RQ5. Which innovations in mechanization, harvesting, and postharvest handling ensure the preservation of bioactive compounds, food safety, and competitiveness of blueberries in demanding global markets?
To address these questions, the PRISMA 2020 methodology (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) was applied to ensure transparency and reproducibility throughout the search, selection, coding, and analysis processes. The review was based on documents indexed in the Scopus database, excluding grey literature and prioritizing experimental studies and specialized reviews. This integrative approach made it possible to connect the most relevant scientific evidence around technological innovation, environmental sustainability, and blueberry physiology, providing a comprehensive understanding of the current state and future challenges of this crop.
2. Global Context of Blueberry (Vaccinium spp.) Production Systems
Global blueberry production relies on a limited number of species and interspecific hybrid groups within the genus Vaccinium, whose chilling requirements and stress tolerance largely determine their geographic distribution and production systems. Northern highbush blueberry (Vaccinium corymbosum) is the dominant species in temperate regions, particularly in North America and Europe, where winter chilling requirements range from 800 to 1200 h, supporting stable production of cultivars such as ‘Duke’, ‘Bluecrop’, ‘Liberty’, ‘Aurora’, and ‘Legacy’. Rabbiteye blueberry (Vaccinium ashei), characterized by intermediate chilling requirements (500–600 h) and greater tolerance to adverse conditions, is widely used in warmer environments, including the southeastern United States and parts of South America. Southern highbush blueberries, derived from interspecific breeding programs, exhibit lower chilling requirements and higher heat tolerance, enabling commercial production in subtropical and Mediterranean regions with cultivars such as ‘Ventura’, ‘Star’, ‘Alixblue’, and ‘Gupton’. In contrast, lowbush blueberry species (Vaccinium angustifolium and Vaccinium myrtilloides) are mainly associated with cold climates and processing-oriented systems. These genetic groups underpin regional differences in production strategies, technology adoption, and research priorities across the global blueberry industry.
In line with the expansion of these genetic groups, the global area dedicated to blueberry cultivation has increased markedly over recent years (Figure 1a). By 2024, worldwide blueberry area reached approximately 282,192 hectares, with the Americas accounting for the largest share of established plantations. Between 2018 and 2024, global cultivated areas increased by 58.4%, reflecting sustained investment and market-driven expansion. The most pronounced growth occurred in the Asia–Pacific (APAC) region, which added nearly 44,687 hectares during this period, driven largely by the rapid adoption of southern highbush cultivars, intensive production systems, and protected cultivation technologies. Although area expansion continued across all regions, the annual rate of new plantation establishment slowed slightly between 2022 and 2024 in both the Americas and EMEA, suggesting a gradual transition from extensive expansion toward consolidation, intensification, and productivity-oriented strategies in more mature production systems.
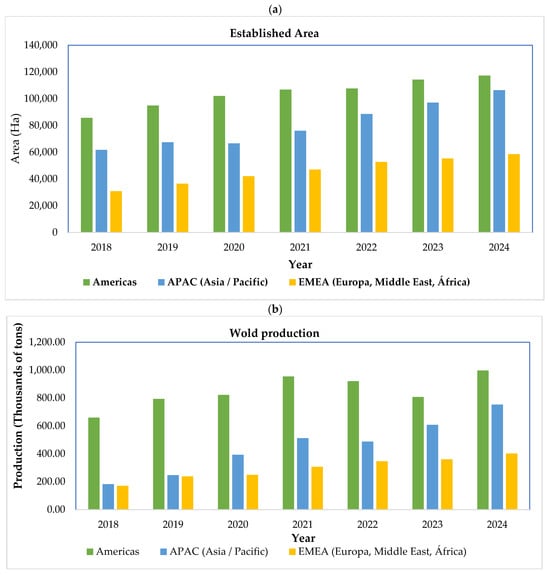
Figure 1.
(a) Global area under cultivation and (b) total blueberry production.
The expansion in cultivated area has been accompanied by a substantial increase in global blueberry production, which grew by approximately 112% between 2018 and 2024 (Figure 1b). APAC exhibited the most pronounced relative growth, with production rising from 182,380 tonnes in 2018 to 752,160 tonnes in 2024, highlighting the rapid scaling of intensive systems in the region. Nevertheless, the Americas remain the dominant contributor to cumulative global production, with an estimated total output of 5.95 million tonnes over the same period. From 2018 to 2021, global production increased steadily, supported by strong growth in APAC; however, between 2021 and 2023, production levels stabilized, reflecting contrasting regional dynamics. In 2023, a 12% decline in production in the Americas—associated with the effects of El Niño, including elevated temperatures, altered rainfall patterns, and shifts in harvest calendars was partially offset by production gains in EMEA and APAC. In 2024, global production reached record levels, with increases of 22% in APAC and 12% in EMEA, while production in the Americas returned to levels comparable to those observed in 2021. These trends, reported by the International Blueberry Organization (IBO), underscore both the resilience and vulnerability of global blueberry production systems and highlight the growing importance of region-specific adaptation strategies under increasing climatic variability.
3. Materials and Methods
The systematic review was conducted following the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) to ensure a transparent, structured, and reproducible process in the identification, selection, and analysis of scientific literature related to the integrated management, sustainability, and technological innovation of blueberry (Vaccinium spp.) cultivation [14,15]. The methodological process comprised four main stages: (i) identification of documents in scientific databases, (ii) screening and selection according to inclusion and exclusion criteria, (iii) data extraction and systematization, and (iv) qualitative and quantitative analysis of reported trends and results (Figure 2).
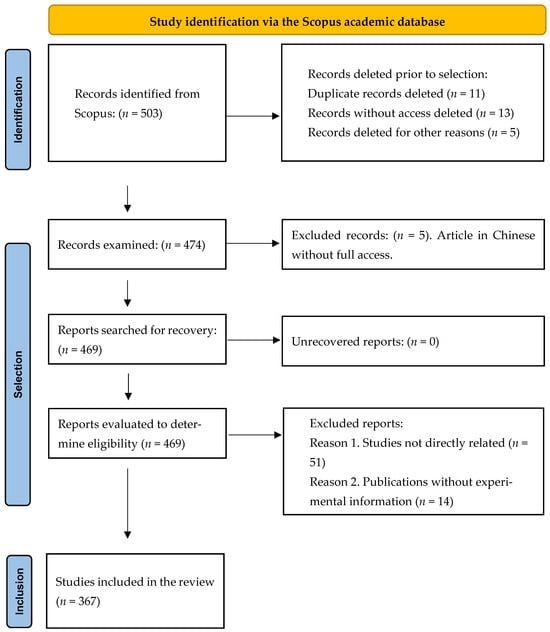
Figure 2.
PRISMA workflow for systematic reviews.
To identify relevant studies, an exhaustive search was conducted in the Scopus database, selected for its broad coverage of peer-reviewed scientific literature related to physiology, agronomic management, sustainability, and technological innovation in blueberry (Vaccinium spp.) cultivation [16]. The search strategy was developed based on Boolean logic principles, combining controlled descriptors and free terms to maximize the sensitivity and specificity of results [17]. Synonyms and related terms were considered for the study’s main analytical dimensions: propagation, nutrition, irrigation, plant health management, physiology, harvest, postharvest, sustainability, and production-applied technology. The construction of the search equation was validated with the support of specialists in protected agriculture and bibliometrics, ensuring its scientific relevance. In Scopus, the advanced search used the following query:
TITLE-ABS-KEY (blueberry OR “Vaccinium corymbosum” OR “Vaccinium spp.”) AND TITLE-ABS-KEY (“protected cultivation” OR “protected agriculture” OR “greenhouse” OR “tunnel” OR “shade net” OR “net house” OR “agribusiness” OR “fair trade” OR “processing”) AND TITLE-ABS-KEY (irrigation OR fertigation OR fertilization OR “nutrient management” OR pruning OR training OR “crop management” OR “harvest” OR “postharvest” OR “storage” OR “shelf life” OR “diseases” OR “pests” OR “crop protection” OR “biological control” OR “Transformation” OR “Commercialization” OR “Marketing” OR “export” OR “markets”).
The search covered the period 1987–2025, with no geographical restrictions, and included documents written in English, Spanish, and other languages. The retrieved records were exported in CSV (Comma-Separated Values) and RIS (Research Information Systems) formats for subsequent screening, bibliometric analysis, and systematic review.
3.1. Selection and Evaluation Process
The selection process followed the PRISMA 2020 guidelines to ensure transparency and reproducibility. Records retrieved from Scopus were imported into Microsoft Excel and Mendeley for de-duplication and reference management. In the initial phase, duplicate records (n = 11), records without full-text access (n = 13), and records excluded for other reasons (n = 5) were removed. Subsequently, three reviewers independently conducted a two-stage assessment: (i) title and abstract screening, and (ii) full-text review, applying the predefined inclusion criteria for studies on integrated management, sustainability, physiology, and technological innovation in Vaccinium spp. Each record was coded in Excel (Yes = 1; No = 0; Partial = 2), and discrepancies were resolved by consensus. The entire process, including reasons for exclusion, was documented to ensure traceability and methodological rigor.
3.2. Eligibility Criteria
Studies were considered eligible if they met the predefined criteria based on Boolean operations using search terms related to propagation, physiology, nutrition, irrigation, integrated pest and disease management, harvest, postharvest, sustainability, and technological innovation in Vaccinium spp. The eligibility criteria ensured the collection of comprehensive, high-quality evidence on integrated, sustainable, and technologically innovative blueberry management. Research from all geographic regions and production systems was included, provided it met the established methodological and thematic criteria. Peer-reviewed journal articles, conference proceedings, book chapters, and reviews published in English, Chinese, Portuguese, and Spanish between 1987 and 2025 were considered, with full-text access and verified relevance to the review objectives. At this stage, five documents in Chinese without full access were excluded.
3.3. Inclusion and Exclusion Criteria
Inclusion and exclusion criteria were defined to ensure the scientific, methodological, and thematic relevance of the analyzed documents. A total of 469 records were evaluated to determine eligibility, considering as potentially relevant those focused on the integrated management of blueberry (Vaccinium spp.) cultivation that addressed aspects such as propagation, physiology, mineral nutrition, irrigation, plant health management, sustainability, harvest, postharvest, or technological innovation. Studies that did not align with the review’s objective, lacked verifiable experimental or methodological information, or did not provide full-text access were excluded. The main reasons for exclusion from the total records evaluated were as follows: Reason 1, studies not directly related to the integrated management of Vaccinium spp. (n = 51); Reason 2, publications without sufficient experimental information (n = 14); and Reason 3, documents that did not address the main topics of the review (n = 37). The selection process, in accordance with PRISMA 2020 guidelines, ensured transparency and reproducibility of the analysis and is summarized in the flow diagram (Figure 1), which details the stages of identification, screening, and exclusion of studies
4. Results and Discussion
The present section integrates the most relevant findings derived from the systematic review, structured around six analytical axes: propagation and genetic improvement, agronomic and hydro-nutritional management, eco-physiological responses under controlled environments, harvest and postharvest processes, modeling through artificial intelligence (AI) and spectral vision, and environmental sustainability of the production system. These axes directly respond to the research questions guiding this review and reflect the main scientific and technological dimensions currently shaping blueberry production systems. To enhance interpretative coherence across these analytical dimensions, the results are contextualized within an integrated conceptual framework that highlights the functional interconnections among environmental conditions, plant material, agronomic practices, enabling technologies, postharvest logistics, and economic–market drivers. Importantly, this framework does not introduce additional analytical categories, but rather serves as a systems-level synthesis that supports the discussion of interactions, feedbacks, and trade-offs emerging from the reviewed evidence.
Overall, the results show substantial progress in yield performance, resource-use efficiency, and fruit quality, together with increased physiological resilience to thermal and water stress. Strategies based on microclimate modulation (radiation, temperature, and humidity), combined with non-thermal postharvest technologies, have proven effective in preserving texture, color, and bioactive compounds. Likewise, the integration of predictive models and AI strengthens fruit maturity monitoring and real-time decision-making, while life cycle assessment (LCA) and carbon footprint (CF) analyses identify critical environmental impact points and cost-effective pathways toward circularity and energy efficiency. Collectively, these findings consolidate a scientific–technical basis for designing more sustainable, traceable, and climate-smart blueberry production systems.
4.1. Conceptual Framework for Integrated Blueberry Production Systems
Figure 3 presents a conceptual framework that integrates the main dimensions governing contemporary blueberry (Vaccinium spp.) production, synthesizing the evidence generated across the six analytical axes addressed in this review. Rather than representing isolated management domains, the model conceptualizes blueberry production as a dynamic system in which environmental and climatic conditions, genetic and plant material, agronomic management, enabling technologies, postharvest logistics, and economic and market drivers interact continuously. This systemic perspective reflects the diversity of approaches identified in the reviewed studies and provides a unifying structure to interpret how advances in individual components contribute positively or negatively to overall system performance.
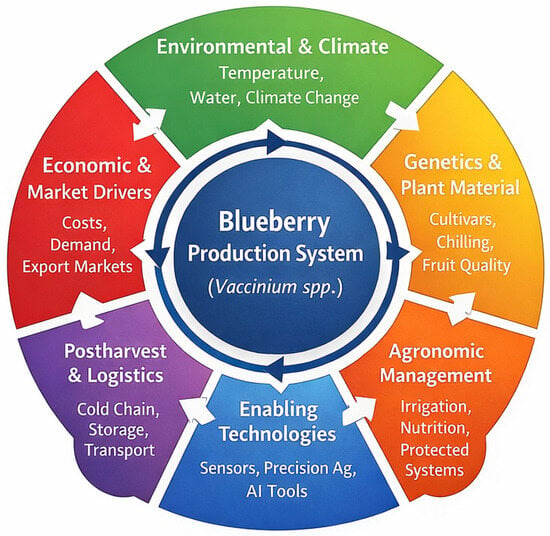
Figure 3.
Conceptual framework integrating the main components of modern blueberry (Vaccinium spp.) production systems.
A central feature of the framework is the transversal role of enabling technologies, which act as a functional bridge between biological processes, management decisions, and market requirements. Precision irrigation and fertigation systems, sensor networks, artificial intelligence tools, and spectral or imaging technologies support real-time monitoring, predictive decision-making, and resource-use optimization. As shown across multiple studies reviewed in this work, the effectiveness of these technologies depends on their alignment with cultivar characteristics, environmental constraints, and production objectives. Consequently, the framework highlights that technological innovation alone does not guarantee improved outcomes unless it is embedded within a coordinated agronomic and physiological management strategy.
The incorporation of economic and market drivers into the conceptual model emphasizes their feedback role in shaping production systems and technology adoption pathways. Market access, quality standards, postharvest requirements, and cost structures influence decisions related to cultivar selection, investment in infrastructure, and adoption of advanced management tools. At the same time, environmental sustainability considerations such as water-use efficiency, energy consumption, and carbon footprint interact with economic viability, reinforcing the need for integrated assessments. In this sense, the proposed framework provides a practical interpretative lens for understanding how productivity, quality, sustainability, and profitability converge in climate-smart blueberry production systems.
4.2. Innovations in Propagation, Plant Material Acquisition, and Genetic Improvement
Blueberry (Vaccinium spp.) is a perennial species of high economic value, whose genetic improvement aims to optimize yield, quality, and adaptation to agroclimatic conditions and market demands [18]. In the United States, studies on metaxenia (the effect of pollen on fruit characteristics) conducted with ten cultivars of highbush blueberry demonstrated that this phenomenon does not significantly alter fruit maturation or weight, except in self-fertile genotypes [19]. Variety evaluations in the Netherlands and the United States identified materials of high potential: ‘Reka’, ‘Chandler’, and ‘Brigitta Blue’ stood out for their productivity and extended postharvest life; ‘Elliott’ for its late harvest; and the MS 706, MS 271, MS 454, and MS 812 selections for their fruit size, quality, and earliness, being suitable for both fresh consumption and industrial use [20,21]. In China, the hybrid cultivar ‘Chasing Dream’ exhibited high yield and storage resistance [22], while the Polish program based on V. corymbosum focused on improving edaphoclimatic adaptation and fruit shelf life [23]. In Morocco, the cultivars ‘Gupton’, ‘Alix Blue’, and ‘Star’ showed good adaptation to local climatic conditions and significant differences in yield and quality, demonstrating the broad plasticity of the Vaccinium genus [24].
Although blueberry cultivation has traditionally relied on own-rooted plants, grafting has recently emerged as a complementary propagation strategy under specific agronomic conditions. Like other perennial fruit crops, the use of blueberry rootstocks can influence water-use efficiency, mineral nutrition, physiological performance, and fruit quality. Recent evidence reported by Zhu et al. [25], demonstrated that grafting blueberry cultivars onto different Vaccinium rootstocks significantly affected fruit quality, leaf mineral content, gas exchange parameters, xylem anatomical traits, and drought resistance. Certain rootstock enhanced photosynthetic performance and improved fruit quality while conferring greater tolerance to water stress compared with own-rooted plants. These effects were associated with differences in vascular structure and hydraulic conductivity, highlighting the importance of rootstock–scion interactions. Although grafting is not yet widely adopted in commercial blueberry production, these findings indicate its potential as a targeted strategy to improve fruit quality and stress resilience in challenging environments.
Micropropagation, an in vitro technique that allows the multiplication of genetically uniform and pathogen-free plants, has become an essential tool for producing high-quality plant material. In China, the use of Woody Plant Medium (WPM) supplemented with 0.1–2.0 mg L−1 of zeatin (ZT), partial illumination ranging from 2000 to 10,000 lux, and a temperature between 25 and 30 °C achieved up to 90% ex vitro rooting in moss treated with indole-3-butyric acid (IBA) or naphthaleneacetic acid (NAA) at concentrations of 1000–2000 mg L−1, in addition to developing a cold-storage method for viable explant conservation [26]. Another experiment, also conducted in China, using cultivars ‘Bluejay’, ‘Pink Lemonade’, ‘Sunshine Blue’, and ‘Top Hat’, showed that callus induction and shoot regeneration capacity depend on genetic constitution (polyploid hybridization), achieving survival rates above 90% in peat substrate [27]. In Austria, in vitro regeneration combined with thermotherapy proved effective in reducing viral and phytoplasma infections [28]. In Chile, cultures treated with colchicine (C22H25NO6) generated six vigorous and adaptable clones, recommended for genotype–environment evaluation across different agroclimatic zones [29].
Biotechnological advances have broadened breeding perspectives. Virus-induced gene silencing (VIGS) has proven to be an efficient method for analyzing gene function and accelerating the development of new cultivars [18]. At the University of Florida, crosses among V. corymbosum, V. darrowii, and tetraploid Southern Highbush (SHB) lines incorporated morphological traits favorable for mechanical harvesting pedicel length, cluster compactness, and growth habit thus reducing costs and labor dependence [30]. Meanwhile, the United States Department of Agriculture (USDA) promotes sanitary certification programs to prevent the spread of pathogens such as Phytophthora ramorum and Blueberry Shock Virus (BlShV). Its Vaccinium clonal collection, comprising 600 accessions, was relocated to pots under controlled conditions of substrate, pruning, and propagation [31].
Overall, innovations in blueberry propagation and breeding reflect the convergence of biotechnology, plant health, and climate adaptation. However, significant challenges remain: the limited functional characterization of genes related to fruit quality and stress resistance, the scarce multiyear validation of micro propagated clones, and the lack of international standardization of in vitro protocols. The integration of molecular tools with agronomic evaluation, phytosanitary control, and genetic traceability is essential to advance toward a globally harmonized and resilient blueberry production system.
4.3. Advances in Agronomic Practices and Cultural Management Strategies for Optimizing Blueberry (Vaccinium spp.) Cultivation
The cultural management of blueberries (Vaccinium spp.) encompasses a set of agronomic decisions that determine productivity, fruit quality, and system sustainability [32]. Factors such as substrate preparation, planting system, mulching, shading management, and phenological cycle regulation are key to the technical and economic success of commercial plantations. In Slovenia, three varieties were evaluated under raised beds and pots placed in high tunnels or under hail nets. The tunnels advanced fruit ripening by 6–18 days but reduced yield and bioactive compound content. In contrast, hail nets improved fruit quality and antioxidant concentration, while pot cultivation did not affect firmness or growth, validating its suitability for intensive systems. The study suggests that hail nets represent a sustainable management practice under climate change conditions [33].
Blueberry production occurs both in open fields and under controlled-environment greenhouses, enabling continuous fruit supply. To maintain high commercial quality, good management practices should include proper soil preparation, balanced nutrient supply, and the use of organic or mineral amendments to prevent physiological deficiencies [32]. In Oregon (USA), different substrates were compared for pot cultivation, including mixtures of peat moss, coconut fiber, and fir bark. Media containing peat (peat) or coir favored growth and moisture/nutrient retention, whereas high bark proportions increased desiccation and reduced nitrogen (N), phosphorus (P), and potassium (K) uptake, affecting vegetative development. Thus, peat and coir are consolidated as optimal substrates for intensive and greenhouse production [34].
In China, the expansion of cultivation in acidic soils has faced challenges related to inadequate site selection, limited availability of high-quality planting material, and low varietal diversity. The establishment of demonstration plantations is recommended as training and knowledge transfer spaces aimed at strengthening sustainable production systems [35]. Similarly, the introduction of biodegradable mulches such as the biopolymer Mater-Bi (derived from plant oils and starches) has shown positive effects on moisture conservation, soil fertility, and weed control, while replacing conventional plastics and reducing waste pollution [36]. This material, which naturally decomposes, represents a cultural practice aligned with circular economic principles applied to horticulture.
The use of shade nets of different colors and opacities also influences production planning. White and red nets with moderate coverage (40–60%) delayed harvest by a few days without affecting yield or quality, allowing better commercial management and preventing radiation and heat stress damage. In contrast, excessive shading reduced fruit size and sweetness [37]. Another greenhouse study evaluated the induction of flowering using hydrogen cyanamide (CH2N2) and mineral oil (MO) in three varieties. Results showed earlier and more uniform flowering in ‘Georgiagem’ and ‘Climax’, although high doses caused bud necrosis and reduced yield, highlighting the need for precise regulator management [38]. This practice is useful for synchronizing production cycles when winter chill accumulation is insufficient.
Overall, advances in planting and cultural management practices for blueberry show a trend toward technological sustainability, combining innovation in substrates, coverings, mulches, and physiological regulators. However, the systematic review reveals significant gaps: most studies lack multi-year analyses, regional comparisons, and standardized environmental performance metrics. Despite observed progress, challenges remain regarding energy balance optimization in protected crops, life-cycle assessment of biodegradable materials, and the integration of sustainability indicators into management systems. Therefore, it is proposed to advance toward a cultural management model based on quantitative and eco-physiological evidence that links productivity, water-use efficiency, and climate resilience, consolidating blueberry as a reference crop in global sustainable horticulture.
4.4. Integrated Irrigation, Fertigation, and Mineral Nutrition Strategies in Blueberry (Vaccinium spp.) Cultivation
Water and nutrient management in blueberry (Vaccinium spp.) have shifted toward more efficient and sustainable production schemes, integrating mineral nutrition, precision irrigation, and smart technologies. Among evaluated strategies, the use of mineral biostimulants such as silicon (Si) has shown notable physiological and biochemical effects. Comparative studies found that potassium silicate (K2SiO3) exhibited higher absorption than wollastonite (CaSiO3), reaching foliar concentrations of up to 370 ppm in the cultivar ‘Bluegold’, whereas ‘Earliblue’ and ‘Liberty’ reached only 34–35% of the overall average. This increase was associated with higher levels of phenolic compounds and microbial enzymatic activity, highlighting the potential of silicon to enhance fruit functional and antioxidant quality [39].
Nitrogen (N) type and source have a decisive influence on crop physiology and productivity. Exclusive ammonium (NH4+) supply promoted the accumulation of N, phosphorus (P), magnesium (Mg), sulfur (S), copper (Cu), manganese (Mn), and boron (B) in leaves and increased chlorophyll a and total sugars compared with nitrate (NO3−). In a split-root system, nitrogen use efficiency doubled: half the NH4+ dose produced yields equivalent to the full supply, confirming the combined influence of nitrogen source and application mode on crop physiology [40].
Soil and substrate management also play a key role in system productivity. In the Mekong Delta, ancient, degraded soils (AA) achieved only 81% of the yield recorded in recent fertile soils (RA). Nitrogen omission reduced yield by 47% (RA) and 39% (AA), as well as stem biomass (70% and 42%) and leaf biomass (59% and 46%), confirming the critical role of nitrogen fertilization [41]. In container systems, coconut coir showed limitations due to initial salinization: fertilization with calcium nitrate (1.75 g·L−1) or monoammonium phosphate (2.38 g·L−1) did not reduce sodium (Na) levels or improve biomass in the ‘Snowchaser’ cultivar [42]. In contrast, organic management without mineral nitrogen fertilization and with 20% organic matter in the substrate (S2) enhanced canopy volume, leaf area, and photosynthetic efficiency in the ‘Climax’ cultivar, validating organic management under protected conditions as a sustainable alternative [43].
Under hydroponic conditions, the cultivar ‘Biloxi’ achieved maximum yield at an electrical conductivity (EC) of 1.5 dS·m−1 and a NO3−/NH4+ ratio of 30/70, with soluble solids of 14.4–15.4 °Brix. Smaller fruits accumulated more anthocyanins (141 mg·100 g−1) than larger ones (85 mg·100 g−1), reinforcing the relationship between mineral nutrition and functional fruit quality [44].
Water management has been addressed through deficit irrigation and pulse optimization strategies. In sandy soils with pine bark, increasing irrigation frequency from 2 to 10 pulses per day reduced leaching fraction from 46% to 30%. The ‘Jewel’ cultivar showed losses below 20%, compared with ‘Emerald’ (>50%), due to a denser root system [45]. Applying controlled deficit irrigation (80% of evapotranspiration) under plastic tunnels improved firmness and soluble solids without reducing commercial quality, though with a slight decrease in titratable acidity [46]. Moreover, water restriction increased phenolic compound synthesis, particularly delphinidin-3-acetylhexoside, emphasizing the role of moderate water stress as a driver of functional quality [47].
Technological developments have enabled the consolidation of smart fertigation strategies. In China, the implementation of Internet of Things (IoT)-based automatic systems reduced water consumption by 35–45%, fertilizer use by 25–35%, increased yield by 10–15%, and reduced labor by over 60%, while also lowering agricultural pollution [48]. In mountain orchards, a decision-support system with moisture sensors and water balance algorithms calculated transpiration and irrigation volume in real time, activating emitters automatically without human intervention [49]. Additionally, plant bioelectrical potentials have been explored as physiological indicators: signals between −50 and −250 mV, responding within milliseconds, correlated with stomatal closure and reduced conductance, constituting an early water stress detection system for fruit crops such as blueberry, avocado, and olive [50]. Foliar nutrition also plays a decisive role in postharvest quality. Preharvest applications of 7.8 g·L−1 calcium (Ca2+) increased Ca content in leaves and fruits of blueberries grown under tunnels, establishing a direct correlation between Ca concentration and fruit firmness. This effect was associated with an increase in low-methoxyl pectin (LMP), a key component of fruit texture, demonstrating that both dosage and cultivation environment (tunnel, net, or open field) directly influence commercial quality and shelf life [51].
In summary, integrated irrigation, fertigation, and mineral nutrition strategies for blueberry demonstrate a convergence between physiological efficiency and technological sustainability. Scientific evidence confirms that synchronization between nutrient source and application method, irrigation frequency, and water management technology are key determinants of productivity and fruit quality. However, knowledge gaps remain regarding long-term evaluation of combined nutritional and water management effects on environmental footprint, energy efficiency, and system resilience. Advancing toward models based on integrated physiological indicators and real-time monitoring technologies will optimize resource use and strengthen sustainable production systems in the face of climatic and water availability constraints.
4.5. Integrated Approaches for the Sanitary and Phytosanitary Management of Blueberries (Vaccinium spp.)
Sanitary and phytosanitary management of blueberry (Vaccinium spp.) is a fundamental pillar for crop sustainability, given the rise in fungal, bacterial, and viral diseases, as well as the emergence of new pests that threaten productivity and fruit quality. Recent literature shows a global effort to identify key pathogens, develop integrated management strategies, and strengthen both molecular diagnostics and the genetic resistance of cultivars. In Chile, application of Koch’s postulates enabled the first identification of Chondrostereum purpureum as the causal agent of silver leaf disease and xylem necrosis in the cultivar ‘Brigitta Blue’. The pathogen spread rapidly between 2005 and 2006, affecting 21 orchards and causing whole-plant death with high economic impact [52]. In parallel, in China, Botryosphaeria dothidea was detected as the causal agent of shoot blight and dieback, a pathogen previously associated with species such as sycamore and olive [53]. In Georgia (USA), Xylella fastidiosa subsp. fastidiosa was confirmed as the cause of bacterial leaf scorch (BLS), while Ectophoma multirostrata was reported as a new causal agent of stem canker, establishing V. corymbosum as a global host [54,55].
Likewise, Phytophthora cinnamomi was detected in 2014 as the causal agent of root and crown rot in young blueberry plants in China [56]. Resistance screening identified tolerant cultivars (‘Aurora’, ‘Legacy’, ‘Liberty’, ‘Reka’, ‘Overtime’, and ‘Clockwork’) and susceptible ones (‘Bluetta’, ‘Bluecrop’, ‘Bluegold’, ‘Blue Ribbon’, ‘Cargo’, ‘Draper’, ‘Duke’, ‘Elliott’, ‘Last Call’, ‘Top Shelf’, and ‘Ventura’), the latter being discouraged in clayey or poorly drained soils [57]. In parallel, protocols were developed to assess germplasm and the efficacy of chemical and biological agents for controlling root rot [58,59]. Among foliar diseases, leaf rust caused by Thekospora minima was documented in Oregon (USA), affecting nurseries and greenhouses and causing early defoliation; this disease is considered a quarantine pest in the European Union, restricting international trade [60]. In Europe, Pucciniastrum vaccinii was also reported, with differences in cultivar susceptibility and natural resistance observed in V. cylindraceum [61]. In Canada, Exobasidium vaccinii, the causal agent of red leaf, showed limited incidence thanks to cultural practices such as pruning and the natural resistance of lowbush blueberry [62].
Pest control has been another major research front. In Canada, the midge Dasineura oxycoccana showed reproductive isolation between V. corymbosum and V. macrocarpon populations due to differences in sex pheromones [63]. In Poland, the management of soft scales (Parthenolecanium spp.) using mechanically acting products within Integrated Pest Management (IPM) showed efficacy equal to or greater than neonicotinoid insecticides, consolidating a sustainable strategy [64]. The bacterium Xylella fastidiosa, transmitted by the glassy-winged sharpshooter (Homalodisca vitripennis, GWSS), exhibited varietal differences in infection intensity [65]; moreover, strains of subspecies multiplex and pauca were detected in Spain and Italy with host-shift potential [66]. Advances in molecular diagnostics have enabled the identification of the distribution of these subspecies [67] and whole-genome sequencing [68]. For its control, zinc oxide (ZnO) nanoparticles Zinkicide™ TMN110 (ZnK) reduced BLS severity under controlled conditions [69].
Fungi of the genus Colletotrichum pose a growing threat. In Poland, C. fioriniae was reported for the first time as an anthracnose agent in 2016 [70], and C. acutatum showed a low correlation between foliar and fruit symptoms [71]. In China, premature defoliation caused by Corynespora cassiicola was linked to high humidity and temperature in greenhouses [72,73]. Fruit brown rot caused by Monilinia vaccinii-corymbosi remains the primary phytosanitary problem in North America. Sources of resistance have been identified in wild diploid populations [74], along with floral resistance mechanisms in the gynoecial canal [75]. Chemical control with fenbuconazole, azoxystrobin, and triforine showed variable efficacy depending on phenology [76,77], while early pollination has been proposed as a preventive measure [78]. In addition, the germination of pseudosclerotia and the development of apothecia depend on the host cultivar and its phenological stage [79,80].
In Mexico, Neofusicoccum parvum was identified as the causal agent of stem blight under tunnels and in greenhouses [81], and in China a new species, Lasiodiplodia vaccinii, adapted to warm conditions, was described [82]. In trials with Botryosphaeria, fertilization increased lesion length, whereas fungicide use significantly reduced it [58,59]. Transcriptomic analysis of Pestalotiopsis microspora revealed 727 transcription factors, of which 200 were associated with defense mechanisms and 45 with cellular regulation, highlighting seven genes involved in α-(1,3)-glucan biosynthesis, a key component in immune response [83].
Among plant-parasitic nematodes, the ring nematode Mesocriconema ornatum was found to exacerbate blueberry replant disease (RBD), with higher reproduction rates under low initial inoculum [84]. Likewise, Paratrichodorus renifer and Pratylenchus penetrans reduce yield in susceptible cultivars [85]. In biological control, Steinernema scarabaei showed effectiveness comparable to neonicotinoids against Anomala orientalis [86], while Clonostachys rosea was effective against Botrytis cinerea in V. angustifolium, including bee-vectored dissemination [87,88]. In Chile, Steinernema australe was proposed as a biocontrol agent for the weevil Aegorhinus superciliosus [89], and the endophytic fungi Codinaeella EC4 and Schizophyllum commune showed potential against Diaporthe vaccinii and Fusarium commune, respectively [90,91]. The application of organic nitrogen sources reduced wilt caused by Fusarium solani in the ‘Legacy’ cultivar [92].
For the southern red mite (Oligonychus ilicis), the predators Phytoseiulus persimilis and Neoseiulus californicus were identified as promising species under greenhouse conditions [93]. Studies of relative insecticide toxicity showed that broad-spectrum products severely affect biological control agents such as Aphidius colemani, Orius insidiosus, Chrysoperla rufilabris, and Hippodamia convergens, whereas inputs approved for organic agriculture are compatible [94]. In Canada, red sorrel pollen was observed to enhance germination of Botrytis cinerea, although pronamide application did not improve productivity [95]. Finally, spectral fluorescence analysis enabled nondestructive detection of B. cinerea in fruit during harvest and postharvest through infrared reflectance variations associated with mycelial growth [96].
Taken together, research demonstrates significant progress toward a comprehensive plant health approach that combines molecular diagnostics, genetic resistance, biological control, and the rational use of agrochemicals. However, key challenges persist limited multiyear evaluation of the efficacy of biological agents, a shortage of studies on microbiome–pathogen interactions, and the need to quantify the environmental impacts of chemical strategies. Advancing toward health management based on principles of microbial ecology, early molecular surveillance, and field pathophysiological validation will strengthen the phytosanitary resilience of blueberries in the face of climate change and new scenarios of production intensification.
4.6. Crop Physiology and Eco-Physiological Regulation Under Controlled Environments
The study of eco-physiological responses of blueberries (Vaccinium spp.) under protected systems is essential to optimize productivity, quality, and adaptation to climate change and to variations in radiation, temperature, and water availability. Light, thermal, and phenological manipulation together with biostimulants and emerging technologies has become a set of tools for synchronizing vegetative and reproductive cycles and enhancing the crop’s physiological resilience.
Within this framework, light down-conversion has proven effective in modifying the spectral quality under cover conditions. In controlled tunnels over one growing season, films enriched in red wavelengths significantly increased biomass in the ‘Duke’ cultivar and suggested a positive effect on yield, in addition to favoring the accumulation of carbohydrate reserves for the following cycle. However, its adoption requires multi-year validation with adequate replication to confirm physiological benefits and cost–benefit relationships [97]. In this context, the eco-physiological engineering framework of blueberry cultivation under controlled environments (Figure 4) integrates the dynamic interactions between microclimate and crop physiology, emphasizing how energy, water, and radiation fluxes regulate key processes such as photomorphogenesis, transpiration, and secondary metabolite synthesis, which directly influence fruit quality and production efficiency. This systemic perspective underscores the need for coordinated management of spectral radiation, ventilation, and humidity, together with thermal and water control strategies, to achieve a functional balance between growth, yield, and physiological sustainability.
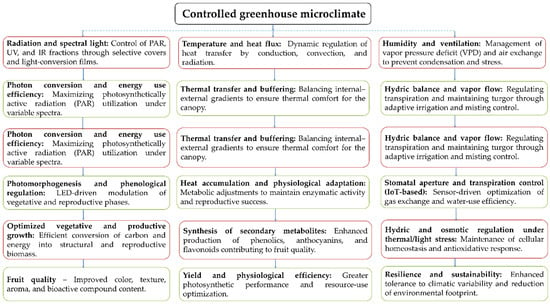
Figure 4.
Eco-physiological engineering diagram of blueberries under cover.
In the hydric dimension, the integration of electrodialysis and forward osmosis (EDFO) has emerged as an advanced strategy for nutrient recovery and wastewater reuse. In southern highbush blueberry, irrigation with EDFO-treated water did not alter plant growth or substrate ionic balance, maintaining pH within the optimal range (4.5–5.5) and an electrical conductivity (EC) slightly above 2 dS·m−1, which can be easily managed through periodic flushing. From a physiological standpoint, stable pH and EC favored the balanced uptake of macro- and micronutrients (particularly N, K, Ca, and Mg), preventing ionic competition and cell dehydration associated with excess salinity. Likewise, controlled ion concentrations may have enhanced leaf water potential efficiency, maintaining stomatal aperture and transpiration rate (E) within optimal ranges. The use of reclaimed water did not significantly modify stomatal conductance (gs) or CO2 assimilation (Aₙ), suggesting active osmotic adjustment that preserves membrane integrity and photosynthetic capacity under moderate EC conditions. Moreover, the EDFO system removed over 90% of pharmaceuticals and pesticides, thereby reducing pressure on freshwater sources and contributing to nutrient-cycle closure without compromising the physiological performance of the crop [98].
The gradual presentation of pollen in V. corymbosum can be exploited to schedule pollination, with bumblebees (Bombus spp.) proving more efficient than honeybees (Apis mellifera). In greenhouses, the combination of protected environments and managed hive placement increased yield and quality, improving fruit set and ripening uniformity compared with open-field conditions [99]. In phenological regulation, the application of 0.67% (v/v) hydrogen cyanamide (HC) during dormancy after fulfilling approximately two-thirds of the chilling requirement advanced budburst by 15–19 days, shortened flowering and harvest periods, and increased total yield by 21–42% (early yield by 43–163%), without detrimental effects on firmness, vitamin C, anthocyanins, or phenolics. The mixture of HC with gibberellic acid (GA3), ethephon, mineral oil, or KNO3 provided no advantages and sometimes reduced yield [100].Controlled trials with ‘Duke’, ‘Bluecrop’, and ‘Elliott’ confirmed that long days (16 h, 22 °C) promote vegetative flushes without floral induction or endodormancy, while short days (8 h) induce flower buds from week 2 and dormancy from week 4. After 900 h of chilling at 4 °C, budburst occurred approximately 6 days later, flowering at 26 days, and harvest between 65 and 130 days post-budburst, depending on cultivar supporting a darkening–vernalization protocol to induce buds and dormancy, followed by cultivar selection according to market windows and targeted pollination management [101,102].
Stomatal thermotolerance defines an operational window: stomatal function exhibited an optimum between 30 and 35 °C under moderate shading (20–30%), conditions that maximize CO2 assimilation, photosynthetic efficiency, and thermal dissipation, providing criteria for selecting heat-tolerant genotypes and designing greenhouses in warm or high-thermal-load regions [103]. In environments with high UV-B radiation (or coverings that transmit it), Vaccinium uliginosum accumulated more photoprotective compounds (“UV filters”) but showed a ~23% reduction in seasonal photosynthesis and impairment of photosystem II (PSII) and likely the Calvin cycle. Consequently, the use of UV-B-filtering plastics or nets, moderate shading, and irrigation and nitrogen adjustments are recommended to sustain chlorophyll and antioxidants while protecting photosynthetic capacity and yield [104].
Shade net management entails trade-offs: higher shading increases chlorophyll content (greener leaves) but reduces photosynthetic efficiency; fruits may grow slightly larger but ripen more slowly and have lower sugar content (°Brix). Dark shade nets intensify these effects, while white or light nets (25–50%) protect against excess radiation with minimal quality penalties, making them useful for adjusting harvest windows [105]. In source–sink balance, maintaining approximately five leaves per fruit enhances fruit filling, size, °Brix, and earliness; reinforcing pollination one day after the first pass increases seed number and fruit weight. These practices are implemented through selective pruning and scheduled thinning, improving uniformity and reproductive efficiency [106].
In warm climates, modulation of temperature and photoperiod combined with perennial management (pruning plus strategic fertilization) affects floral induction, budburst, and fruiting, allowing production to be advanced or concentrated in higher-value periods. The selection of low-chill cultivars and staggered production blocks are effective tools to align phenology with market demand [107]. In parallel, bio stimulants have shown anti-stress effects: seaweed extracts in greenhouse conditions increased antioxidant enzyme activity by up to 20% under drought, without altering chlorophyll or foliar nutrients [108], while applications of methyl jasmonate and abscisic acid enhanced fruit antioxidant content without affecting yield, improving the nutraceutical profile [109].
Taken together, the evidence supports an eco-physiological engineering framework for blueberry cultivation under protective covers: (i) prudent spectral management (light conversion) with multi-year verification, (ii) closed water and nutrient cycles (EDFO) with EC monitoring, (iii) phenological regulation based on short-day/vernalization/HC protocols and precise pollination management, (iv) microclimate design (moderate shading and UV-B filtering) that preserves PSII function, and (v) selective use of bio stimulants to mitigate abiotic stress. Prioritizing these elements calibrated to cultivar and market window enables gains in earliness and fruit quality without compromising the physiological sustainability of the production system.
4.7. Blueberry Harvesting: Transition to Mechanized and Assisted Systems
Harvest represents one of the most critical stages in the production chain of blueberries (Vaccinium spp.), due to its high labor demand and its direct impact on postharvest physiology and commercial fruit quality. Recent literature converges toward partial or assisted mechanization, seeking to reduce labor costs without compromising firmness, anthocyanin content, or cellular integrity of the berries. In tunnel-based systems, assisted harvesting using portable electric shakers and soft capture surfaces increased the picking rate to 81.3 berries·min−1, although with 30.6% unripe fruits. Adjusting the detachment speed to 900 rpm for 1.2 s improved overall efficiency to 80.7%, demonstrating the potential of this method to balance productivity and selectivity [110]. This hybrid approach combines the operator’s perceptual precision with the controlled force of the device, reducing physical strain and improving consistency in handling factors that directly influence the preservation of cell turgor and cuticle integrity. As shown in Table 1, electrical assistance and soft-capture mechanisms enable progress toward intermediate mechanization with minimal quality loss, while impact reduction strategies and optical sorting contribute to maintaining the structural integrity and ripeness uniformity of the fruit key elements for standardizing the harvesting system.

Table 1.
Mechanized harvesting, mechanical damage, and optical sorting.
From an applied perspective, Table 1 highlights that assisted harvesting with controlled vibration and soft-catch systems currently offers the best compromise between labor efficiency and fruit quality preservation. For mechanical damage mitigation, impact-reduction strategies (reduced drop height and padded contact surfaces) are the most effective, whereas optical selection technologies provide the highest reliability for ripeness uniformity and harvest timing in fresh-market blueberries.
The main challenge in mechanization is impact damage, which alters membrane permeability and accelerates oxidative processes. Using instrumented sensors (BIRD), it was determined that more than 30% of impacts originated from retention plates and 20% from the empty box. The inclusion of cellular silicone padding and reducing drop height from 101 to 50 cm significantly decreased bruising. In “rabbiteye” cultivars, redesigning plant architecture reduced fruit loss to the ground from 24% to 17%, highlighting the need to synchronize cluster morphology with detachment dynamics [111,112].
At the separation stage, incorporating a dual-fan plenum with in-cab speed control allowed adjustment of the airflow (~18 m·s−1), removing debris without fruit loss due to the higher terminal velocity (≈15.6 m·s−1) of berries compared with leaves and stems. This innovation reduces surface microbial contamination and, physiologically, minimizes postharvest rot risk by limiting epidermal damage and cuticular wax loss [113]. In parallel, portable optical technology has emerged as a tool for defining the optimal harvest point. A spectral index based on three wavelengths (~680, 740, and 850 nm) classified berries with >93% accuracy, correlating with physiological ripening changes: increased anthocyanins (≈1011–1060 mg·100 g−1 FW), higher soluble solids (°Brix), pH, and reducing sugars (glucose and fructose), along with decreased titratable acidity and reduced flavanols and phenolic acids, which peak in unripe fruits. These biochemical transformations reflect activation of phenylalanine ammonia-lyase (PAL) and controlled pectin degradation key processes for designing predictive algorithms of physiological maturity [114].
From an economic and operational perspective, full mechanization for the fresh market remains constrained by profitability. Simulation models indicate that adoption would be feasible with combined reductions of 20–30% in price gaps, labor costs, and yield losses [115]. Progress toward efficiency depends on the convergence of innovations in mechanical design (shock-absorbing contacts, fall control, adaptive ventilation) and mechanization-oriented genetic improvement focused on open plant architecture, loose clusters, small stem scars, persistent wax, and high firmness [116]. Additionally, harvest planning can benefit from multi-objective models based on fuzzy logic and evolutionary algorithms (NSGEA) that simultaneously optimize profitability and sustainability, considering greenhouse gas (GHG) emissions, waste, labor availability, and climatic variability. In Canadian systems, this approach outperformed traditional deterministic models, providing an analytical foundation for adaptive decision-making [117].
In summary, the blueberry production system is evolving toward progressive mechanization supported by four pillars: (i) intelligent human assistance as an intermediate phase, (ii) ergonomic redesign using impact-absorbing materials, (iii) optical diagnostics for determining the physiological harvest point, and (iv) mechanization-oriented breeding. This process not only reduces labor dependency but also integrates mechanical engineering and fruit physiology to achieve a balance between efficiency, postharvest quality, and technological sustainability.
4.8. Post-Harvest Technologies and Dynamics of Blueberry (Vaccinium spp.) Storage and Marketing
Non-thermal technologies and hybrid preservation methods represent the core of post-harvest innovation in blueberries. Table 2 summarizes the main treatments developed, their operating parameters, matrices, and validation scales, highlighting the trend toward gentle physical processes with high retention of bioactive compounds and energy efficiency. From an applied perspective, Table 2 indicates that the suitability of non-thermal technologies strongly depends on the dominant postharvest objective and the product format. For anthocyanin retention and preservation of bioactive compounds, high hydrostatic pressure (HHP/HPP) and manothermosonication emerge as the most promising approaches, as they consistently maintain or enhance anthocyanin levels while limiting enzymatic degradation. For microbial safety, HHP/HPP provides the most robust and broad-spectrum inactivation, while cold atmospheric plasma (CAPP) and pulsed light (PL) are particularly effective for surface decontamination of fresh fruit when dose–cultivar interactions are properly managed. In contrast, PEF/MPEF technologies are best suited for liquid matrices where continuous processing and nutrient retention are prioritized. Overall, Table 2 highlights the need to align technology selection with specific quality and safety goals rather than adopting a single universal postharvest solution.

Table 2.
Non-thermal technologies and process routes (post-harvest/processing).
The competitiveness of fresh and processed blueberry depends on postharvest strategies that preserve texture, color, and aroma; ensure safety; stabilize phenolic pigments; and valorize by-products within a circular-economy framework. This synthesis based on a systematic review of recent and seminal studies organizes the evidence into four axes: (i) preservation of sensory quality with minimal degradation of anthocyanins and phenolics; (ii) microbiological control through low-impact physical/chemical treatments; (iii) pigment stabilization via copigmentation, encapsulation, and biopolymeric/protein matrices; and (iv) by-product valorization into active ingredients and packaging. Figure 5 summarizes the technological flows from harvest to fruit commercialization or processing, highlighting the integration of cold chains, non-thermal treatments, and by-product valorization routes under a circular-economy approach.
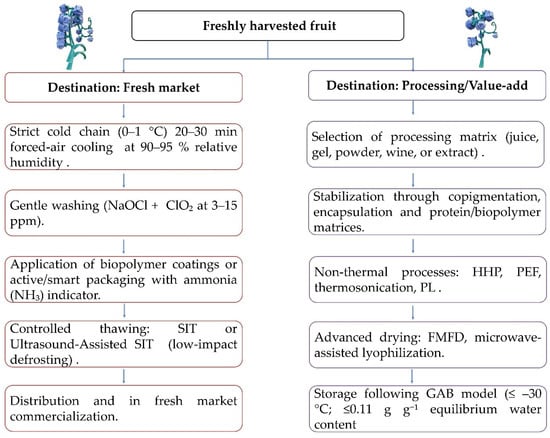
Figure 5.
Technological flow of blueberries from harvest to marketing or processing.
(i) Preservation of texture, color, and aroma with minimal degradation of anthocyanins/phenols: The sensory and nutraceutical preservation of blueberries is highly dependent on the technological route, cultivar, and maturity stage. In juice processing, the use of pectinases simultaneously increased yield (≈91%) and anthocyanin concentration (≈4287 mg/L), although with higher astringency and acidity, requiring fine balance between extraction efficiency and sensory quality [131]. In low-sugar jams, high hydrostatic pressure (HHP) preserved vitamin C and polyphenols for 68 days at 4 °C, maintaining representative levels (vitamin C ≈ 14.17 mg/mL; phenolics ≈ 1.15 mg/mL; flavonoids ≈ 4.77 mg/mL; anthocyanins ≈ 0.45 mg/mL) [132], confirming the advantage of non-thermal technologies over HTST in preserving heat-sensitive compounds [120,125,133].
The cold chain is critical: freezing at −80 °C better preserved vacuolar water and pectin, reducing drip loss compared to −20/−40 °C or liquid N2 [134], while slow thermal induction thawing (SIT) and its ultrasound-assisted variant (UASIT) maintained anthocyanin and °Brix levels, with UASIT showing higher efficiency without sensory penalties [129]. In whole fruit for fresh consumption, pulsed light (PL, 6 J/cm2) maintained firmness and reduced decay for six weeks at 0.5 °C and 90–95% relative humidity [121], while biopolymeric coatings (chitosan/pectin) decreased decay (−33%) and weight loss (−22%) after 16 days [135]. Aromatic profiling has identified 618 VOCs, with β-ionone and 2(5H)-furanone associated with sweet/fruity notes, and carotol/1-hexanol as biomarkers differentiating wild from cultivated genotypes [136].
In dried matrices, combining microwave and assisted freeze-drying (quartz or SiC) reduced processing time by >50% while retaining >80% total monomeric anthocyanins (TMA) and >75% total phenolics [137]. Freeze–microwave freeze-drying (FMFD) outperformed spray drying (SD) in TMA and TPC when using maltodextrin and whey isolate as carriers [130]. Mixed freeze-dried powders showed stability with ≈23.9% anthocyanin loss after 12 weeks [138], and GAB models defined critical moisture and temperature conditions for storage [139,140]. Moderate dehydration prior to winemaking (≈30% weight loss) increased anthocyanins (+25.9%) and phenolics (+16.1%), improving the aromatic profile [141], confirming the process-design flexibility according to product destination.
Structural and genetic factors explain differential responses: the greater firmness of the ‘Star’ cultivar compared with ‘O’Neal’ was associated with differential expression of pectin lyase (VcPL41/VcPL65) and soluble pectin treatment with 2% CaCl2 delayed softening and abscisic acid (ABA) accumulation [142]. In terms of degradation, Raman spectroscopy revealed accelerated anthocyanin losses at elevated pH, temperatures >65 °C, or prolonged UV exposure [143]; thermosonication (600 W; 60 °C) increased total phenolic content (TPC) by +139%, flavonoids by +252%, and anthocyanins by +94% [144]. These results confirm that the “triangle” of pH–temperature–light, together with cell-wall architecture and matrix–process interactions, governs chromatic stability and texture retention.
(ii) Microbiological control using low-impact physical/chemical treatments: Pathogen and spoilage control in blueberries have evolved toward mild combination treatments that minimize chemical residues while preserving sensory attributes. Washes with sodium hypochlorite (NaOCl, 200 ppm) plus chlorine dioxide (ClO2, 3–15 ppm) reduced Listeria (≈5.5 log CFU/g) and Salmonella/STEC (≈3.0/2.6 log), with freezing doubling the effect (≈5.0 log) [145]. Sequential protocols using chlorine, lactic acid, ClO2, and ozone followed by freezing at −17 °C achieved 6.6–7.2 log reductions against Listeria and Salmonella [146]. Peracetic acid (PAA) caused ≈2.2 log reductions in wild berries prior to freezing [147], and gaseous ClO2 (≈57.46 mg/L for 10 h) reduced ≈1.5 log in 50 kg batches [148].
Among physical technologies, pulsed light (PL) inactivated microorganisms without leaving residues [122]; pulsed electric fields (PEF/MPEF) preserved vitamins and anthocyanins while reducing microbial growth for up to 30 days [123]. Manothermosonication achieved a 5.85 log reduction in E. coli O157:H7 while maintaining 97.5% of anthocyanins and only 10.9% residual PPO activity [126], whereas high-pressure processing (HPP, 200–600 MPa) preserved firmness and reduced cell collapse for 28 days at 3 °C [119]. In puree matrices, HHP ≥ 550 MPa inactivated > 4 log human norovirus without affecting color or sensory properties [118]. Cold atmospheric plasma (CAPP, 5–10 min) inhibited bacteria with minimal impact on °Brix, acidity, color, or anthocyanins [127]. At harvest level, sanitation reduced aerobic plate count (APC) by 1.2 log and yeast/mold counts (YMC) by 0.5 log compared to mechanical harvest [149], underscoring the importance of on-field biosecurity.
In antimicrobial coatings, eugenol nanoemulsions (EPS from Lactiplantibacillus plantarum) reduced Salmonella/E. coli by up to 3 log [150], and almond gum + chitosan coatings extended shelf life by ≈20 days while acting against S. aureus and P. aeruginosa [151]. Although cold plasma (CP) reduced APC by 0.8–1.6 log (up to 2.0 log after 7 days), exposures beyond 60–90 s compromised firmness and anthocyanin content, confirming the need for cultivar-specific dose–response curves [128]. The integration of rapid diagnostic tools (near-infrared, hyperspectral, and thermal imaging) enables real-time monitoring of hidden damage, bruising, and overall quality through support vector machine (SVM) models [152,153].
(iii) Pigment stabilization: copigmentation, encapsulation, and protein/biopolymer matrices: The main anthocyanins responsible for color and part of the bioactivity in blueberries are stabilized through physicochemical routes and matrix-engineering strategies. Copigmentation with phenolic acids (caffeic, ferulic, and tannic acids) and CaCl2 increased anthocyanin content (3.38 vs. 2.79 mg/g) and reduced polymeric color (15.5–17.4%) [154]. In HHP-treated juices, gallic acid (2 g/L) reduced anthocyanin loss from 62.27% to 13.42% (20 days at 4 °C) and lowered polyphenol oxidase (PPO) activity [155]. Under 100–500 MPa, the λmax and color of anthocyanins (Mv 3 O gal, Mv 3 O ara) remained stable, with minor chromatic variations and improved copigmentation of caffeic acid (CA) with Mv 3 O gal and gallic acid (GA) with Mv 3 O ara [156].
In encapsulation, alginate–pectin hydrogels increased encapsulation efficiency (EEₘ), extended half-life (t½ from 58 to 630 h), and reduced photodegradation [157]. Polyphenol–protein complexes produced by spray drying (soy protein isolate) retained high levels of total phenolic content (TPC) and DPPH activity [158]. AEPU microcapsules embedded in starch films extended shelf life (>7 days) and were ≈95% biodegradable after 8 days [159]. Chitosan–κ-carrageenan nanocomplexes retained 94.4% of anthocyanins (pH 6, 25 °C) [160], while thermosonication and controlled acidification (pH 2.1) preserved anthocyanins during pasteurization and storage [144,161]. At the network scale, cyanidin 3-glucoside degradation under ultrasound followed first-order kinetics, showing higher stability in 20% ethanol [162]. In the presence of catechol and PPO, browning followed first-order kinetics dependent on concentration [163].
These pathways converge into design frameworks combining (a) pH adjustment and phenolic cofactors, (b) sequential modulation of pressure/temperature/light, and (c) biopolymeric or protein-based carriers with antioxidant and barrier capacity. In pigment stabilization, the combination of physicochemical adjustments and biopolymeric matrices has shown outstanding results in anthocyanin retention and color preservation during storage. Table 3 summarizes the main copigmentation, encapsulation, and pH control strategies applied to blueberry matrices, highlighting their effectiveness and operational conditions.

Table 3.
Pigment stabilization (copigmentation, pH, matrices).
From an applied and comparative perspective, Table 3 indicates that the most effective strategies for maximizing anthocyanin retention and long-term color stability are those that combine structural protection with enzymatic inhibition. In particular, HHP combined with gallic acid and chitosan–κ-carrageenan nanocomplexes show the highest anthocyanin preservation (>90%) while simultaneously limiting polyphenol oxidase activity, making them especially suitable for high-value beverages and semi-liquid matrices. For applications where photostability and extended shelf life are the primary objectives, alginate–pectin hydrogels provide the most robust protection by markedly increasing anthocyanin half-life. In contrast, phenolic copigmentation with CaCl2 and thermosonication offer flexible, process-compatible solutions for enhancing color intensity and antioxidant retention when full encapsulation is not technically or economically feasible. Overall, Table 3 highlights that optimal pigment stabilization depends on aligning the physicochemical strategy with the target matrix, processing intensity, and desired storage performance rather than relying on a single universal approach.
(iv) Valorization of by-products into functional ingredients and active packaging: The residual fraction (pomace, peel, seed, and process water) represents a key resource for antioxidant ingredients, natural colorants, and functional biomaterials. Blueberry Waste Material (BWM) processed through aqueous extraction and spray drying yielded powders with total phenolic content (TPC) of 25–30 mg GAE/g and total anthocyanin content (TAC) of 17–20 mg CyGE/g; alginate exhibited the highest encapsulation efficiency and prebiotic effect [164]. Pomace dried at 60–70 °C retained 66–75% of anthocyanins and >30% of fiber after 20 weeks [165], while extrusion at 180 °C/150 rpm increased procyanidin monomers (+84%) and reduced polymers (−40%) [166]. Peel/seed extracts (≈58.18 mg GAE/g) enabled edible films with ≈76% antioxidant capacity [167], and gelatin obtained from residues processed with microwaves shortened processing time by 30–50% while increasing DPPH/FRAP activity [168].
In “sensorial” packaging, HKGB films (hydroxypropyl methylcellulose, κ-carrageenan, gelatin) incorporating anthocyanins exhibited high antioxidant capacity (DPPH 82.92%; ABTS 86.44%), mechanical strength ≈ 5.85 MPa, and ammonia sensitivity ≈ 32.79%, compatible with mobile readout systems [169]. Chitosan–chestnut gum (CS:CH) coatings enriched with grape seed extract retained 84.8% antioxidant capacity and reduced decay, whereas formulations with lemon essential oil were less effective [170]. Meanwhile, natural deep eutectic solvents (NADES) combined with microwave-assisted (MAE) and ultrasound-assisted extraction (UAE) achieved 21–26 mg/g anthocyanins with antiproliferative activity against HeLa and HaCaT cells [171].
These strategies establish circular loops in which residues, ingredients, and packaging improvements contribute to enhanced product stability and safety. Within the context of functional packaging and coatings, biopolymeric and protein-based solutions have been developed with antioxidant, antimicrobial, and indicator properties designed to extend fruit shelf life and integrate freshness monitoring within the cold chain. Table 4 highlights the diversification of emerging strategies in active and intelligent packaging: antimicrobial coatings based on chitosan and natural gums, sensorial films with anthocyanins as ammonia indicators, biodegradable microcapsules with antioxidant activity, and protein matrices that enhance phenolic compound stability. These innovations strengthen the link between quality preservation and smart packaging within the circular-economy paradigm.

Table 4.
Coatings, packaging, and packaging intelligence.
From an applied perspective, Table 4 indicates that the most mature and immediately transferable strategies for extending shelf life of fresh blueberries are antimicrobial biopolymer coatings based on chitosan and natural gums, which consistently reduce decay and weight loss while remaining compatible with existing cold-chain logistics. For applications requiring real-time freshness monitoring, anthocyanin-based sensorial films offer the greatest potential due to their high antioxidant activity and reliable response to ammonia as a spoilage indicator. In contrast, active microcapsules and protein–polyphenol carriers are especially promising for processed products and functional ingredients, where controlled release, antioxidant stability, and biodegradability are prioritized over immediate antimicrobial action. Overall, Table 4 highlights that successful by-product valorization and active packaging design depend on matching material functionality with the intended product form and supply-chain requirements rather than adopting a single universal packaging solution.
Non-thermal technologies (HHP/HPP, PL, PEF/MPEF, thermosonication), combined with a strict cold chain and smart coatings/packaging, are emerging as the most robust levers to extend blueberry shelf life while preserving nutraceutical value. Anthocyanin stabilization via copigmentation/encapsulation and the use of biopolymeric/protein matrices help maintain color and functionality in juices, gels, powders, and wines. By-product valorization enables active ingredients and packaging within a circular framework. To bridge the lab–industry gap, real-environment kinetics, methodological standards, and comprehensive scale-up assessments (energy, cost, environmental footprint) are required by cultivar and processing route [120,131,164,169].
4.9. Modeling, Computer Vision, and Precision Agriculture in Blueberry Cultivation
The recent literature reveals a strong convergence between computer vision, spectroscopy, and precision agriculture as foundational pillars of intelligent modeling applied to blueberry (Vaccinium spp.). These tools address bottlenecks across the production cycle from maturity detection and yield estimation to plant health diagnostics, postharvest management, and input optimization consolidating a predictive, data-driven paradigm. For yield prediction, object-detection algorithms have proven highly effective. The YOLOv8 (You Only Look Once, version 8) model, used to differentiate between ripe and unripe berries, achieved a mean Average Precision at Intersection over Union (mAP50) of 0.708. When integrated with 42 agronomic regressors, a gradient boosting model obtained a coefficient of determination (R2) of 0.80, whereas excluding genotype reduced the fit to R2 = 0.47, confirming the genetic relevance in productivity [172]. Under low-light conditions, the YOLOv11-BSD model optimized with bidirectional attention and multiscale fusion improved accuracy to 89% (+5.4 pp) and mAP@[0.50:0.95] to 80.8% (+5.7 pp) [173]. In mechanical harvesting, YOLOv5 enhanced with saturation in CIE Lu′v′ color space increased precision (+3%), recall (+2%), and mAP (+2.6%) [174], while YOLOv3-spp trained with 4220 images classified three maturity stages (green, red, and blue) with a recall of 91% and inference times of 28.3 s [175]. Aerially, Unmanned Aerial Vehicles (UAVs) equipped with RGB cameras achieved R2 = 0.89 for yield prediction, with flights at 30 m being more efficient than at 5 or 15 m (RMSE = 542.01; MAE = 379.94) [176].
Non-destructive evaluation of internal quality using spectroscopy and deep learning has enabled accurate prediction of key physiological variables. In the visible–NIR range (400–1000 nm), Partial Least Squares (PLS) and interval PLS (iPLS) models predicted Soluble Solids Content (SSC) and Firmness Index (FI) with prediction coefficients (Rp) of 0.90 and 0.78, respectively [177]. In the NIR range (900–1700 nm), a pushbroom system applied to ‘Bluecrop’ achieved calibration and validation coefficients (RC = 0.911; RV = 0.871 for firmness; RC = 0.891; RV = 0.774 for sugars) [178]. Using FT-NIR and FT-IR (Fourier Transform Near/Mid Infrared) in ‘Brigitta’ and ‘Duke’, minimum prediction errors were obtained (RMSEP = 0.36% for SSC and 0.18 mg·g−1 for total phenolics) [179]. Portable Vis–NIR spectrophotometers (450–980 nm) achieved correlations of r = 0.80–0.92 for SSC, firmness, and functional compounds [180]. In the terahertz (THz) range (0.5–10 THz), a Gaussian-kernel Support Vector Machine (SVM) classified four ripening stages with 84.3% accuracy, confirming the submillimeter spectrum’s capacity to characterize epidermal and turgor properties [181].
In postharvest, the combination of computer vision and spectroscopy showed high sensitivity for quantifying mechanical damage. A web application based on MobileNet SSD/UNet detected berries with average precision (AP) of 0.977 and segmented bruises with Intersection over Union (IoU) of 0.773, processing 50 berries per image in under 30 s [182]. In NIR hyperspectral spectra (950–1650 nm), band ratios (R1112.6/1470.9) and the MT-COA algorithm identified mechanical damage 30 min post-impact (IoU = 70.1%) [182], correlating R2 = 0.78–0.83 with human evaluation [183]. Pulsed thermography achieved accuracies of 88% and 79% depending on cultivar (‘Farthing’, ‘Meadowlark’) [184], enabling the detection of thermal and conductivity alterations as early physiological indicators of impact damage.
In plant health, deep learning models span from leaf analysis to remote sensing. Geometric algorithms segmented herbivory damage with high robustness [185]. Through transfer learning, FCDCNN networks achieved 98% accuracy and reduced computation time by 25% [186], while EfficientNetB3 and ResNet (Residual Network) maintained 98.1% accuracy in multi-species classification, including blueberry [187,188]. Lightweight models (~2.6 M parameters) classified plants with 98.1% accuracy [189] and detected infestations with 84% accuracy [190]. For flower detection, a compact YOLOv10n (CSP_MSEE + HS-FPN + SFF + LSDECD) reached recall = 84.0% and mAP50 = 89.5% with only 1.3 M parameters, suitable for embedded systems and UAVs [191]. For internal infections, NIR spectrophotometers (650–1700 nm) exceeded 80% accuracy, while hyperspectral models (400–1000 nm) combined with Partial Least Squares Discriminant Analysis (PLSDA) identified 100% of healthy and 99% of diseased plants [192].
In precision agriculture, the guiding principle is “measure better to decide better.” In wild blueberry, yield maps based on the percentage of blue pixels achieved R2 = 0.98–0.99 with actual yield (RMSE = 277 kg·ha−1) [193]. The AYMS II system, equipped with μEye cameras and RTK-GPS (Real-Time Kinematic), enabled real-time productivity mapping, revealing spatial heterogeneity [194]. These results integrate into suitability models in ArcGIS ModelBuilder, combining satellite sensors (QuickBird, Landsat, SPOT, IRS), LiDAR (Light Detection and Ranging) technology, and agroclimatic variables (pH, drainage, chilling hours, permeability) to delineate optimal production zones [195]. In boreal regions, spatial models demonstrated that productivity increases with lower canopy density and southwest exposure, reflecting the interaction between microclimate and stand physiology [196].
Efficient input use is another direct application of intelligent modeling. Selective nozzle sprayers reduced application costs from 2052 to 1799 USD·ha−1 to 1138 USD·ha−1 [197], while multi-section equipment targeted herbicides and fungicides only to designated zones [198]. Variable-Rate Granular (VRG) and Modified Variable-Rate Granular–Variable Rate Control (MVRG–VRC) systems classified soil, weeds, and plants with 94.9% accuracy, preventing overdosing (p < 0.0001) without affecting crop efficacy (p = 0.58) [199,200]. At the territorial scale, UX5 UAVs (Trimble) mapped vegetation cover and pest pressure, significantly reducing agrochemical use [201].
In industrial quality classification, edge-to-PLC (Programmable Logic Controller) systems integrate real-time computer vision. A convolutional neural network (CNN) specifically designed for blueberries achieved 95.6% validation accuracy with 156.7 ms latency per image in a Raspberry Pi + Siemens S7-1200 PLC system [202]. A pattern-recognition model reduced 951 features to ≤20 via sequential selection and, using SVM/LDA (Support Vector Machine/Linear Discriminant Analysis), classified fungal defects, wrinkles, and mechanical damage with accuracies of 86–97% [203].
Overall, the evidence demonstrates that integrating computer vision, spectroscopy, spatial modeling, and industrial automation is transforming the blueberry supply chain into a predictive, efficient, and resilient system. Quantifiable benefits include improvements in yield (R2 up to 0.89), quality (r ≥ 0.80; RMSEP = 0.36% for SSC), plant health (accuracies of 98–100%), input efficiency (cost reductions to 1138 USD·ha−1), and operational resilience from the field to the packing line consolidating an information-driven, adaptive, and technologically convergent production model
4.10. Environmental Sustainability and Efficiency in Production Systems
The environmental sustainability of blueberry (Vaccinium spp.) cultivation represents a strategic axis within modern horticulture, aligned with the pursuit of low-carbon agricultural systems that are resource-efficient and consistent with circular economy principles [11,204]. In this context, reducing greenhouse gas (GHG) emissions, applying Life-Cycle Assessment (LCA), and improving energy efficiency emerge as key tools to quantify impacts and design sustainable mitigation strategies.
In Italy, pre-cultivation, cultivation, and post-cultivation stages of highbush blueberry production were evaluated through a GHG emission balance. The results showed that the main contributors to total emissions were the use and disposal of plastic materials, particularly agricultural packaging and postharvest containers. This highlights the urgency of implementing recycling strategies, replacing conventional plastics with biopolymers, and improving waste management to reduce the environmental footprint of the production system [205]. In Chile, a major global blueberry producer, a carbon footprint (CF) methodology based on ISO 14040 and PAS 2050-1 standards were applied to organic production orchards. The study considered inputs, machinery, services, materials, and land-use change (LUC), revealing variability depending on agricultural practices. The highest CF contributions came from organic fertilizer use and energy consumption, while the most effective mitigation strategies were fertilizer reduction, cover cropping, and replacement of inefficient tractors and irrigation pumps. Remarkably, converting annual crops to perennial systems resulted in the greatest net carbon removal, acting as a sink that partially offsets system emissions [206].
In the United States, an LCA identified cultivation, harvest, postharvest (freezing), and transport as the main environmental hotspots. Paradoxically, fresh blueberries imported from Chile showed a better environmental performance than locally produced ones due to more efficient agricultural and logistical systems in the South American country. This contrast underscores the need to consider technological and operational efficiency as determining factors in the global sustainability of supply chains [207].
In Spain, a comparative LCA of four plantations two organic and two conventional found that the most significant environmental impacts were associated with synthetic fertilizers, fossil fuels, agricultural plastics, paper, and the incineration of pruning residues. These activities represent the main intervention points for reducing environmental impacts through the adoption of biofertilizers, renewable energy, biodegradable materials, and composting of plant waste, fostering a transition toward a more circular and sustainable agricultural system [208].
From an economic perspective, a financial model based on discounted cash flow was developed in Mexico to quantify the profitability and investment risk of blueberry production. The results highlighted the importance of integrating financial and multicriteria assessment tools to simulate export scenarios, price variability, and production costs. This approach supports strategic decision-making and encourages sustainable investment in the fruit sector [209].
Finally, an LCA applied to a berry production system involving osmotic dehydration and edible coating analyzed the environmental contribution of each process stage. Osmotic dehydration had the greatest environmental impact, followed by coating and packaging. However, the high profitability margin and the process’s ability to extend shelf life and enhance the nutraceutical value of the final product justify its implementation provided compensatory measures are adopted to minimize its environmental footprint [210].
In summary, Table 5 shows that the greatest environmental impacts are concentrated in plastic use, fertilizers, and energy during cultivation, postharvest, and processing stages. Strategies such as substituting plastics with biopolymers, improving energy efficiency, and valorizing by-products emerge as cost-effective solutions to reduce the carbon footprint, while logistical efficiency and renewable energy use consolidate a comprehensive sustainability approach. From a comparative and decision-oriented perspective, Table 5 indicates that the most broadly applicable and cost-effective strategies for reducing the environmental footprint of blueberry production are those targeting plastics, energy use, and fertilizer management. Across production contexts, substitution of conventional plastics with biopolymers and improved recycling schemes emerges as the most immediate mitigation option, particularly at the postharvest stage. In parallel, energy efficiency improvements and the integration of renewable energy sources consistently reduce emissions in cultivation, cold storage, and transport, often delivering net economic benefits. In production systems where fertilizer inputs dominate the carbon footprint, as observed in organic orchards, nutrient-use optimization and cover cropping provide the highest mitigation potential. At the processing level, Table 5 shows that energy-intensive operations such as osmotic dehydration can be environmentally viable when offset by efficiency gains and high value addition. Overall, the table highlights that sustainability gains are maximized when mitigation strategies are prioritized according to system-specific hotspots rather than applied uniformly across the value chain.

Table 5.
Sustainability (LCA/HC)—critical points and mitigation.
Overall, the reviewed evidence shows that the environmental sustainability of blueberry cultivation depends on a systemic approach that integrates: (i) objective quantification of impacts through life cycle assessment (LCA) and carbon footprint (CF) analysis, (ii) technological innovation aimed at energy efficiency and greenhouse gas (GHG) reduction, and (iii) the integration of financial and environmental governance instruments that enable the closure of material and energy cycles. This framework drives the development of more resilient, competitive, and climate-smart production systems, which are fundamental pillars of sustainable fruit growing in the 21st century.
4.11. Human Health and Nutraceutical Value of Blueberry
Over the past two decades, the blueberry (Vaccinium spp.) has evolved from a high-value commercial fruit into a model species in preventive nutrition and functional medicine. Its phytochemical richness is based on anthocyanins, flavonoids, phenolic acids, and condensed tannins confers notable antioxidant, anti-inflammatory, and metabolic modulatory properties, supporting its classification as a functional and nutraceutical food. Recent literature, encompassing controlled clinical trials, molecular studies, and meta-analyses, converges on a key point: regular blueberry consumption can enhance cognition, cardiovascular function, glucolipid homeostasis, gut microbiota composition, and immune response.
In the neurocognitive domain, a synthesis of nine randomized clinical trials (n = 513) reported significant improvements in episodic and linguistic memory among older adults with mild cognitive impairment, though effects on processing speed were inconsistent, emphasizing the need for longitudinal studies with greater statistical power [211]. Similarly, a review of over 200 studies documented average reductions of 5–6% in blood pressure, improvements in lipid profile and glucose regulation, and positive effects on endothelial function and memory in older adults, recommending a practical daily intake of approximately 50 mg of anthocyanins [212]. Preclinical models have shown that blueberry flavonoids exert neuroprotective and synaptic restorative effects, with implications for preventing neurodegenerative diseases such as Alzheimer’s [213,214].
From a phytochemical perspective, blueberries stand out for their high polyphenol concentration and total antioxidant capacity [215,216]. Comparisons among ten varieties revealed wide differences in functional composition and technological suitability: Centurion’, ‘Elliott’, and ‘Yuanlan’ had high anthocyanin and polyphenol contents suitable for processing, while ‘Lanfeng’, ‘Brigitta’, and ‘Brilliant’ excelled in flavor, firmness, and appearance, ideal for fresh consumption. The variety ‘Zhaixuan No. 4′ combined both traits, offering high yield and superior sensory quality. On average, anthocyanins account for more than 70% of total polyphenols and correlate with flavonoids and ellagic acid [217]. The fruit matrix typically presents soluble solids (~10.3%), a sugar/acid ratio (~10.9), and total polyphenols (~4.9 mg·g−1), parameters crucial for industrial standardization and functional value [218].
Physiologically, numerous studies attribute antioxidant, anti-inflammatory, hypolipidemic, antidiabetic, and anticarcinogenic actions to anthocyanins—consistent with benefits against type 2 diabetes (T2DM), cardiovascular disease (CVD), cancer, and chronic inflammation [145,216]. In cell lines, blueberry polyphenols exhibit selective cytotoxicity against breast and colon cancer, modulated by processing type and simulated digestion. Non-thermal technologies such as freeze-drying and ultrasound-assisted extraction enhance the bio accessibility and stability of phenolic compounds, strengthening their nutraceutical potential [219]. In a murine model of dextran sulfate sodium (DSS)-induced colitis, cyanidin-3-glucoside/pectin complexes treated with high hydrostatic pressure (HHP-C3G-BP) reduced clinical severity, inhibited NF-κB, regulated Bcl-2/Bax and caspase-3, and restored gut microbiota, demonstrating immunomodulatory and gastroprotective activity [220].
An emerging research avenue involves blueberry extracellular vesicles (B-EVs), nanometric structures resistant to simulated digestion that remain intact in the intestinal tract. These B-EVs interact with epithelial cells to reduce pro-inflammatory mediators (IL-1β, IL-8) and modulate immune pathways linked to NF-κB and TLR5, suggesting an active role in intestinal barrier integrity and immunometabolism homeostasis [221]. In terms of bioavailability, a crossover trial in healthy adults found that consuming 250 g of whole blueberries, compared to juice, resulted in greater plasma and urinary diversity of phenolic metabolites highlighting the influence of the food matrix on bioactive compound release and absorption [222].
From a cardiovascular standpoint, bibliometric studies and meta-analyses associate the consumption of anthocyanin- and flavonoid-rich juices with reduced blood pressure and improved endothelial function [223]. Reviews focused on Vaccinium uliginosum and V. myrtillus attribute their polyphenols with antioxidant, antibacterial, anti-inflammatory, and chemo preventive properties, showing benefits for ocular and vascular health [224]. Complementarily, a review on post-pressing residues (pomace) from Vaccinium species including V. corymbosum, V. macrocarpon, V. oxycoccos, and V. vitis-idaea highlighted their potential as sources of residual phenolic compounds with preventive or adjunct functions against CVD, T2DM, cancer, and neurodegenerative diseases [225]. In diabetes, a rutin–zinc complex (antioxidant flavonoid + essential mineral) exhibited superior antidiabetic effects compared to separate extracts or mineral solutions, opening a promising avenue for developing functional natural mineral complexes [226]. In chronic kidney disease management, potassium reduction in fruits through controlled fertigation was explored to adapt blueberries for low-potassium diets [227].
The antimicrobial activity of blueberry polyphenols operates through bacterial membrane damage and loss of cell integrity. Extracts inhibit Salmonella enterica, Escherichia coli, Listeria monocytogenes, Staphylococcus aureus, and Campylobacter spp., showing potential to reduce antibiotic use in the food industry and contribute to safer, more sustainable production systems [228]. Mixed extracts from red and blue blueberries, grape seed, and oregano displayed synergistic effects against Helicobacter pylori, enhancing antioxidant and antimicrobial activity and proposing dietary adjuncts for gastrointestinal conditions [229]. Processing directly affects functional bioactivity. Blueberry jams, including low-sugar versions with white grape juice concentrate, retained high levels of phenols, flavonoids, and anthocyanins, reducing reactive oxygen species (ROS) formation and lipid peroxidation in cell models supporting their classification as functional foods [230]. Anthocyanin-rich juices maintained high antioxidant potential, though their effectiveness depends on processing and final bioavailability [231]. At the applied level, baked products enriched with 5% blueberry powder significantly increased phenolic content and antioxidant capacity, demonstrating their economic feasibility as nutraceutical ingredients [232].
Ultimately, global evidence converges on the notion that blueberries due to their abundance in anthocyanins and condensed tannins (proanthocyanidins) exert protective effects against urinary tract infections, ocular disorders, and age-related deterioration, including cognitive and muscular decline [233]. Collectively, the scientific evidence supports blueberries as comprehensive nutraceutical foods with demonstrated benefits in cardiometabolic, neurocognitive, gastrointestinal, and immunometabolism health, while emphasizing the need for larger, standardized clinical trials to consolidate dosage, matrix, and clinical biomarkers of effectiveness.
4.12. Production in Controlled Environments and Greenhouse Innovation
The use of protected structures and environmental control systems in blueberry (Vaccinium spp.) cultivation has become a key technological strategy to extend the production season, improve water-use efficiency, and mitigate climatic risks. Greenhouses and high tunnels allow the modification of the local microclimate through the regulation of radiation, temperature, and humidity, directly influencing phenology, yield, and fruit quality while facilitating the integration of smart technologies (IoT, Internet of Things) aimed at precision agriculture and sustainability [234].
In Portugal, trials under high tunnels demonstrated that polyethylene plastic covers advanced flowering, fruit set, and ripening by 4, 7, and 10 days, respectively, increasing dry matter to 18.2% and productivity to 152 fruits per plant (363 g). Conversely, silver shade nets reduced the number and weight of fruits, photosynthetically active radiation (PAR) by 51%, and photon flux density (PFD) by 54% [235]. In Chile, cultivars ‘Blue Ribbon’ and ‘Top Shelf’ under plastic covers showed higher stomatal conductance and photosynthetic efficiency under water deficit than open-field crops, confirming improved physiological adaptation [236]. In tunnels with light transmissivity between 77 and 91%, the installation of insect-proof nets (0.243 mm × 0.773 mm) significantly reduced populations of aphids and Drosophila suzukii without affecting yield or quality, validating their effectiveness within Integrated Pest Management (IPM) [237].
Smart climate control systems are driving greenhouses toward high-efficiency models. In Jiangsu (China), a Venlo-type greenhouse equipped with IoT sensors and multifactorial environmental control algorithms advanced harvest by one month and increased yield per plant by up to 94.7%, depending on the cultivar, while boosting average fruit weight by 10.9% [238]. In Central Europe, planting system configuration modified varietal performance: ‘Aurora’ yielded more in raised beds, while ‘Brigitta’ performed better in pots, with variations also observed in phenolic compounds evidence that substrate affects both yield and biochemical quality [239].
The expansion of protected cultivation has been decisive in mitigating thermal risks and stabilizing supply. In New Zealand, plastic tunnels have driven hydroponic expansion for export, leveraging the country’s fruit fly–free status [240]. In China, cultivated area increased from 681 ha (2006) to 31,210 ha (2015), with widespread adoption of tunnels and greenhouses in temperate regions, driven by cultivars such as ‘Duke’ and ‘Bluecrop’ [241]. In Mississippi, southern highbush blueberries under high tunnels advanced harvest by 4–5 weeks, achieving yields of 6.5–7.5 t·ha−1 and soluble solids of 14.2%, with a thermal gain of +3 to +10 °C compared to outdoors [242].
Positive microclimatic effects are reflected in plant physiology and water balance. In Chile, 1296 ‘O’Neal’ plants under high tunnels recorded temperatures 10–12 °C higher and 150% higher diffuse PAR, which increased stomatal conductance by up to 99% and yield by 44%, advancing harvest by 14 days [243]. In Florida, 5.5 m tunnels reduced freezing events from 61 to just 3 days per season (≤1 °C), quadrupled yield, and decreased water use for frost protection by 90% [244]. These findings validate the thermal and hydric efficiency of protected environments as a resilience mechanism.
Phenological synchronization with market windows has also improved. In Spain, unheated Italian-type tunnels yielded >1.7 kg per plant, with ‘Sharpblue’ showing consistent year-to-year performance [245]. In Japan, thermal forcing advanced flowering and harvest by 30–40 days, achieving fruit set >75%, berry weights > 2 g, and soluble solids > 12% [246]. In Canada, partial covers adjusted ripening by ±14 days while maintaining >90% market quality after controlled storage [247]. However, intensive protected systems introduce new phytosanitary challenges. In North America, high tunnels doubled productivity but increased populations of Tetranychus urticae, Aleyrodidae, and Frankliniella spp. [248]. In Georgia, winter closure advanced flowering by ≈40 days and improved anthocyanin and soluble solid content in ‘Emerald’, though frost events reduced potential yield [249]. In Europe, heated greenhouses advanced harvest by 2 months, while plastic tunnels advanced it by 5–6 weeks, and impermeable covers delayed it by 2–3 weeks, allowing harvest scheduling based on market demand [7].
In summary, the evidence confirms that blueberry production in controlled environments enhances productivity, quality, and resource-use efficiency, positioning greenhouses and tunnels as strategic platforms for sustainable intensification. However, their adoption requires precise microclimate management, integration of IoT sensors, thermal modeling, and biosecurity protocols to ensure technological advancement does not lead to phytosanitary vulnerabilities or excessive energy costs. The near future points toward hybrid passive–active climate control systems, integrated with renewable energy sources (solar thermal and photovoltaic) and artificial intelligence strategies for phenological prediction consolidating a paradigm of resilient, intelligent, and climate-adaptive protected agriculture.
5. Study Limitations
Despite the methodological rigor applied under the PRISMA 2020 guidelines, this review presents several limitations inherent to its scope and design. First, the literature search relied exclusively on the Scopus database, selected for its broad multidisciplinary coverage and rigorous editorial standards. While this approach ensured the inclusion of high-quality peer-reviewed studies, it may have excluded relevant contributions indexed in other databases or available as grey literature (theses, technical reports, institutional documents). As a result, some region-specific or practice-oriented evidence may be underrepresented, potentially affecting the global representativeness of the synthesis.
Second, a major limitation of this review arises from the high methodological heterogeneity of the included studies, which ultimately precluded the application of a quantitative meta-analysis and favored a structured narrative synthesis. Heterogeneity was evident across experimental designs, temporal and spatial scales, cultivars, environmental conditions, management practices, and the diversity of response variables evaluated. In addition, limited standardization was observed in experimental protocols and outcome reporting, particularly for physiological indicators, postharvest quality attributes, sustainability metrics, and technology performance indicators. In many cases, incomplete reporting of variance measures further constrained quantitative comparability across studies, limiting the aggregation of effect sizes and cross-study synthesis.
Beyond heterogeneity, the feasibility of meta-analytical approaches was further constrained by structural inconsistencies in data availability and reporting, including the use of non-uniform measurement units, divergent baseline definitions, variable control treatments, and inconsistent statistical summaries (means reported without dispersion metrics or effect estimates). Moreover, several studies reported results at different biological or operational levels (plant, organ, fruit, plot, or system scale), complicating data harmonization and violating key assumptions required for quantitative pooling. Under these conditions, any attempt to conduct a limited meta-analysis would risk producing biased or misleading estimates, rather than strengthening the robustness of the evidence synthesis.
Finally, although the review prioritizes recent and high-quality studies, the rapid pace of technological development in areas such as artificial intelligence, precision agriculture, biotechnology, and controlled-environment production systems means that parts of the evidence base may become outdated relatively quickly. These dynamic highlights the importance of periodic updates or living reviews to capture emerging advances. Furthermore, the predominance of studies conducted in temperate regions limits the direct applicability of findings to tropical and subtropical production systems, where climatic conditions, resource constraints, and management strategies differ substantially. Strengthening comparative, region-specific research particularly in Latin America and other emerging production regions is therefore essential to support the development of locally adapted, resilient, and technologically inclusive blueberry production systems.
6. Practical Use of Evidence-Based Recommendations
To enhance the applicability of this review for producers, consultants, and decision-makers, the following table synthesizes the main scientific findings into operational recommendations linked to specific management goals. Rather than presenting exhaustive protocols, Table 6 prioritizes decision-oriented guidance, allowing users to identify which interventions are most relevant according to their production system (open field, protected cultivation, container systems), market destination (fresh vs. processing), and prevailing constraints (water availability, labor cost, climate risk).

Table 6.
Evidence-based practical recommendations for blueberry producers and advisors.
Table 6 should be used as a diagnostic and prioritization tool, not as a prescriptive recipe. Each recommendation is associated with its expected agronomic or economic impact, an indicative cost/complexity level, and the strength of supporting evidence. Advisors are encouraged to adapt these guidelines to local conditions, cultivar behavior, and regulatory frameworks, and to validate critical thresholds (irrigation deficit levels, shading intensity, postharvest treatments) through pilot-scale trials before full implementation.
Taken together, these recommendations translate a highly diverse and multidisciplinary body of evidence into actionable guidance that supports informed decision-making at the farm and advisory levels. By linking management interventions with expected agronomic, quality, economic, and environmental outcomes, the table facilitates the prioritization of strategies according to local constraints and production objectives. Importantly, while the proposed actions are grounded in peer-reviewed evidence, their successful implementation depends on adaptive calibration to cultivar, climate, and market context. In this sense, the recommendations should be viewed as a flexible decision-support framework that bridges scientific knowledge and practical application, strengthening the role of this review as a tool for advancing sustainable, efficient, and climate-resilient blueberry production systems.
7. Economic and Regional Aspects of Technology Adoption in Blueberry Production
The adoption of agricultural technologies in blueberry (Vaccinium spp.) production is increasingly shaped by economic feasibility and regional market dynamics, particularly under a global scenario of rapid supply expansion [115]. Historically, blueberries have maintained relatively high and stable prices compared to other fruit crops, largely due to a sustained growth in global demand that outpaced production increases [250]. However, medium-term market outlooks indicate that this balance is likely to become more fragile toward 2030 as global blueberry production continues to expand rapidly, increasing the risk of market saturation and value erosion. In this context, technological adoption is no longer driven solely by yield enhancement, but by the capacity to deliver consistent quality, precise harvest timing, and market differentiation, as fruit that fails to meet these standards is increasingly excluded from premium supply chains regardless of price [251].
This evolving economic pressure is closely linked to a transformation of the global blueberry production map. While the Americas remain a central pillar of global supply, their relative contribution has declined below 50% of total world production [252]. Peru has emerged as a dominant actor due to its “flattened production curve” strategy, enabled by advanced varietal genetics, irrigation management, and data-driven scheduling, allowing year-round or extended supply windows and improved price stability [253]. However, this leadership faces growing constraints related to water availability, social pressures, and environmental sustainability [254]. In contrast, Chile confronts structural challenges associated with aging orchards and declining competitiveness in both price and quality, while Mexico must improve production efficiency and reduce its heavy dependence on the U.S. market to sustain profitability.
Economic incentives for technology adoption vary markedly across regions and market orientations. In export-driven systems supplying North America, Europe, and increasingly Asia, investments in precision irrigation, fertigation control, postharvest monitoring, and cold-chain technologies are often economically justified by the need to meet strict quality, shelf-life, and traceability requirements [255,256,257]. Conversely, in domestic or regional markets, particularly in emerging producing countries, growers tend to favor incremental, low-cost innovations such as improved irrigation scheduling or passive greenhouse structures over capital-intensive digital or automated systems [258,259]. This divergence aligns with broader evidence showing that the economic returns of digital and precision agriculture are highly context-dependent, influenced by scale, access to credit, and growers’ ability to integrate new technologies into existing management systems.
Africa and Asia are increasingly shaping the future geography of blueberry production and technology adoption [260]. According to assessments released by the International Blueberry Organization (IBO), Africa has emerged as the fastest-growing blueberry production frontier globally over the last decade, with a rapid expansion of cultivated areas across multiple countries. Countries such as Morocco exemplify this shift, combining high yields, proximity to European markets, efficient logistics, and rapid adoption of advanced genetics [261]. Neighboring countries including Zimbabwe, Zambia, Kenya, and Namibia represent a new wave of expansion, benefiting from land availability and competitive labor costs, yet facing critical challenges related to water planning, infrastructure development, and strategic market positioning. In Asia, and particularly in China, the dual role as both a major producer and a rapidly expanding consumer market reinforces the need for technologies that ensure uniform quality, efficient logistics, and brand differentiation [262,263].
Infrastructure and climate constitute additional bottlenecks that condition technology adoption. As global blueberry volumes increase, pressure on postharvest infrastructure intensifies, given the crop’s high perishability and dependence on uninterrupted cold chains and efficient logistics [264]. Regions that fail to invest in postharvest handling, storage, and transport technologies risk losing competitiveness despite agronomic potential. At the same time, climate change is reshaping production zones, enabling successful cultivation in new high-altitude or temperate regions while increasing climatic risks elsewhere. These shifts further reinforce the importance of adaptive technologies, including controlled-environment systems, improved water-use efficiency, and climate-resilient production models.
Controlled-environment agriculture illustrates the strong interaction between economics and regional context in blueberry systems. While high-tech greenhouses with active climate control can substantially reduce environmental variability and enhance fruit quality, their high capital and energy costs limit adoption to regions with favorable market access, energy prices, or policy support [265]. In contrast, low-cost passive greenhouses and tunnel systems, which dominate blueberry production in many countries, offer a more accessible pathway to technological intensification [3]. These systems provide partial microclimate regulation and water-use efficiency improvements at significantly lower investment levels, making them particularly relevant for small- and medium-scale producers in emerging regions [266].
Genetic innovation and data-driven technologies represent the structural backbone of long-term competitiveness in the blueberry industry. The widespread adoption of new cultivars with low chilling requirements, improved firmness, enhanced flavor, and extended postharvest life has been central to global expansion. Today, varietal renewal is no longer optional but a prerequisite for economic survival. Simultaneously, the sector is transitioning toward a data-intensive model, incorporating artificial intelligence, sensor networks, optical sorters, and emerging robotic harvesting solutions [267,268]. These technologies underpin the concept of the “smart orchard,” increasingly positioning blueberries among the most technologically advanced fruit crops. Ultimately, successful adoption will depend on aligning technological sophistication with economic viability, regional infrastructure, and evolving consumer expectations for quality, sustainability, and traceability.
Finally, despite the widespread recognition of circular economy strategies as a pathway to reduce the environmental footprint of blueberry production, their implementation remains constrained by multiple barriers. Technological limitations include the lack of scalable solutions for waste valorization, high energy requirements of advanced postharvest technologies, and insufficient infrastructure for by-product processing. Economic barriers are equally significant, as investments in circular systems often require high upfront capital, long return periods, and market incentives that are not consistently available, particularly in small- and medium-scale operations. Addressing these barriers requires coordinated efforts, including the development of cost-effective technologies, region-specific economic incentives, and integration of circularity principles into breeding, production, and postharvest research agendas.
8. Future Perspectives
The technological and scientific advancement of blueberry (Vaccinium spp.) cultivation demands an integrative vision that transcends traditional approaches and establishes a coherent, reproducible, and sustainability-oriented methodological framework. This review identified several persistent gaps, including strong methodological heterogeneity among studies, limited multi-year and multi-site validation, weak integration between experimental and commercial scales, and insufficient linkage between technological innovation, economic feasibility, and environmental performance. Addressing these gaps is essential to consolidate evidence-based, climate-smart blueberry production systems. Based on the findings of this review, four priority research pathways are proposed.
Experimental Standardization and International Cooperative Networks: A major limitation identified across propagation, nutrition, irrigation, and postharvest studies is the lack of standardized experimental protocols and comparable response variables, which hinders meta-analytical synthesis and cross-regional extrapolation. Future research should prioritize multi-site and multi-year experiments using harmonized methodologies, enabling robust comparison across agroclimatic zones and production systems. A minimum core set of interoperable indicators should be adopted, including physiological (gs, Aₙ, Ψ, PSII efficiency), quality (°Brix, titratable acidity, firmness, shelf life), safety (pathogen log reduction), productivity (kg·plant−1, t·ha−1), and sustainability metrics (water and energy use efficiency, LCA, CF). Establishing international cooperative networks around these indicators would significantly improve data comparability and accelerate technology transfer.
Integration of Digital Tools and Intelligent Modeling: Although numerous studies demonstrate the potential of artificial intelligence, computer vision, and sensor-based systems, this review revealed that most applications remain fragmented, cultivar-specific, or limited to short-term trials. Future research should move toward integrated digital platforms and crop digital twins that combine IoT sensors, phenomics, eco-physiological models, and real-time decision support. Priority should be given to validating these tools under commercial conditions and assessing their robustness across cultivars and environments, using standardized performance metrics (R2, RMSE, MAPE) and transparent data governance frameworks to ensure scalability and reproducibility.
Sustainability and Technological Circularity: The review highlighted that environmental impacts are consistently concentrated in plastics, fertilizer inputs, energy use, and postharvest operations, yet relatively few studies evaluate mitigation strategies in an integrated manner. Future research should explicitly link Life Cycle Assessment (LCA) and Carbon Footprint (CF) analysis with agronomic and technological interventions, such as water reuse systems (electrodialysis–forward osmosis), biodegradable coatings and packaging, renewable energy integration, and by-product valorization within agricultural biorefineries. Long-term assessments that jointly evaluate environmental performance, energy balance, and economic viability are needed to support the transition toward circular and climate-neutral blueberry production systems.
Technology Transfer and Capacity Building: A recurrent gap identified in this review is the weak connection between experimental innovation and adoption at the farm and industry levels, particularly in emerging production regions. Future research and development efforts should therefore be coupled with technology transfer programs, focusing on breeding for mechanization and climate resilience, incentives for sustainable certification, and targeted training in automation, data management, energy efficiency, and environmental control. These initiatives should be aligned with national bioeconomy strategies and the Sustainable Development Goals (SDGs) to ensure that technological advances translate into practical, inclusive, and economically viable solutions.
Together, these research pathways provide a concrete and gap-driven roadmap for advancing blueberry cultivation toward intelligent, sustainable, and climate-adaptive systems, reinforcing the role of this crop as a model for technologically advanced and environmentally responsible fruit production.
9. Conclusions
This systematic review demonstrates that the integral management of blueberry (Vaccinium spp.) cultivation requires a multidimensional approach that interlinks physiology, technology, and sustainability. The findings show that productivity, quality, and crop resilience depend on the interaction between eco-physiological regulation, mineral nutrition, water management, and environmental control, all framed within precision agriculture strategies and protected environments. The integration of sensors, automation, and intelligent modeling has optimized physiological efficiency and reduced vulnerability to thermal and water stress, establishing the foundations for more efficient and climate-adapted production systems.
Likewise, technological innovation in harvest and postharvest processes is redefining standards of quality, safety, and traceability across the blueberry value chain. Non-thermal technologies, edible coatings, smart packaging, and computer vision systems for maturity assessment and damage detection have proven effective in maintaining firmness, bioactive compounds, and sensory quality. These advances, combined with the development of digital and automated solutions, are driving a transition toward more sustainable, efficient, and market-oriented postharvest systems.
Finally, sustainability and circularity emerge as strategic pillars for the future of blueberry cultivation. The adoption of water reuse and treatment technologies (such as electrodialysis–forward osmosis), the use of renewable energy, the valorization of agro-industrial residues through biorefinery processes, and the implementation of environmental footprint metrics constitute key actions to reduce the ecological impact of the production system. These strategies, supported by scientific innovation and technological transfer, position the blueberry as a model of intelligent, profitable, and circular bioeconomy-aligned fruit production.
Author Contributions
Conceptualization, D.A.P., G.A., J.R. and E.V.; methodology, D.A.P., G.A., J.R. and E.V.; software, D.A.P., G.A., J.R. and E.V.; validation, D.A.P., G.A., J.R. and E.V.; formal analysis, D.A.P., G.A., J.R. and E.V.; investigation, D.A.P., G.A., J.R. and E.V.; resources, D.A.P., G.A., J.R. and E.V.; data curation, D.A.P., G.A., J.R. and E.V.; writing—original draft preparation, D.A.P., G.A., J.R. and E.V.; writing—review and editing, D.A.P., G.A., J.R. and E.V.; visualization, D.A.P., G.A., J.R. and E.V.; supervision, D.A.P., G.A., J.R. and E.V.; project administration, D.A.P., G.A., J.R. and E.V.; funding acquisition, E.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data are contained in the article. The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
This work is not associated with any research project; it forms part of an undergraduate thesis submitted to obtain the degree of Agricultural Management Engineer, entitled: “Bibliometric and Systematic Review of Trends, Challenges, and Opportunities in Blueberry (Vaccinium spp.) Production within Modern Agriculture for the Departments of Cundinamarca, Boyacá, and Antioquia.” Special thanks also go to Javier Quecho Mogollon, who served as a tutor at the Industrial University of Santander, Colombia.
Conflicts of Interest
Authors, Gina Amado, Jader Rodriguez and Edwin Villagran were employed by the company Corporación Colombiana de Investigación Agropecuaria. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Tundis, R.; Tenuta, M.C.; Loizzo, M.R.; Bonesi, M.; Finetti, F.; Trabalzini, L.; Deguin, B. Vaccinium Species (Ericaceae): From Chemical Composition to Bio-Functional Activities. Appl. Sci. 2021, 11, 5655. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Merca Laies, M.M.; Sărățeanu, V. Genotype and Year Effects on Some Production Characteristics in Three Blueberry Cultivars (Vaccinium corymbosum L.). Appl. Fruit Sci. 2024, 66, 1757–1765. [Google Scholar] [CrossRef]
- Fang, Y.; Nunez, G.H.; da Silva, M.N.; Phillips, D.A.; Munoz, P.R. A Review for Southern Highbush Blueberry Alternative Production Systems. Agronomy 2020, 10, 1531. [Google Scholar] [CrossRef]
- Correia, S.; Matos, M.; Leal, F. Advances in Blueberry (Vaccinium spp.) in Vitro Culture: A Review. Horticulturae 2024, 10, 533. [Google Scholar] [CrossRef]
- Messiga, A.J.; Haak, D.; Dorais, M. Blueberry Yield and Soil Properties Response to Long-Term Fertigation and Broadcast Nitrogen. Sci. Hortic. 2018, 230, 92–101. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, H.; Yang, H.; Zhang, C.; Lyu, L.; Li, W.; Wu, W. A Physiological and Metabolomic Analysis Reveals the Effect of Shading Intensity on Blueberry Fruit Quality. Food Chem. X 2022, 15, 100367. [Google Scholar] [CrossRef]
- Bal, J.J.M. Blueberry Culture in Greenhouses, Tunnels, and under Raincovers. In Proceedings of the VI International Symposium on Vaccinium Culture, Orono, ME, USA, 12–17 August 1996; Volume 446, pp. 327–332. [Google Scholar]
- Brondino, L.; Borra, D.; Giuggioli, N.R.; Massaglia, S. Mechanized Blueberry Harvesting: Preliminary Results in the Italian Context. Agriculture 2021, 11, 1197. [Google Scholar] [CrossRef]
- Cai, Y.; Takeda, F.; Foote, B.; DeVetter, L.W. Effects of Machine-Harvest Interval on Fruit Quality of Fresh Market Northern Highbush Blueberry. Horticulturae 2021, 7, 245. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, X.; Liu, Y.; Liu, C. Characterization of Postharvest Changes in Fruit Quality Traits of Highbush Blueberry (Vaccinium corymbosum L.) Cultivars. Horticulturae 2025, 11, 1250. [Google Scholar] [CrossRef]
- Salinas-Velandia, D.A.; Romero-Perdomo, F.; Numa-Vergel, S.; Villagrán, E.; Donado-Godoy, P.; Galindo-Pacheco, J.R. Insights into Circular Horticulture: Knowledge Diffusion, Resource Circulation, One Health Approach, and Greenhouse Technologies. Int. J. Environ. Res. Public Health 2022, 19, 12053. [Google Scholar] [CrossRef]
- Shehata, I.M.; Viswanath, O.; Koushik, S.S. Systematic Review: A Comprehensive. In How to Successfully Publish a Manuscript: A Step-by-Step Guide; Springer Nature: Cham, Switzerland, 2025; Volume 191. [Google Scholar]
- Villagran, E.; Espitia, J.J.; Amado, G.; Rodriguez, J.; Gomez, L.; Velasquez, J.F.; Gil, R.; Baeza, E.; Aguilar, C.E.; Akrami, M.; et al. CO2 Enrichment in Protected Agriculture: A Systematic Review of Greenhouses, Controlled Environment Systems, and Vertical Farms—Part 2. Sustainability 2025, 17, 2809. [Google Scholar] [CrossRef]
- Espitia, J.J.; Velázquez, F.A.; Rodriguez, J.; Gomez, L.; Baeza, E.; Aguilar-Rodríguez, C.E.; Flores-Velazquez, J.; Villagran, E. Solar Energy Applications in Protected Agriculture: A Technical and Bibliometric Review of Greenhouse Systems and Solar Technologies. Agronomy 2024, 14, 2791. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, Y.; Zhao, C.; Zhao, F.; Bai, S. The PRISMA 2020 Statement: A System Review of Hospital Preparedness for Bioterrorism Events. Int. J. Environ. Res. Public Health 2022, 19, 16257. [Google Scholar] [CrossRef] [PubMed]
- Villagran, E.; Ortiz, G.A.; Mojica, L.; Flores-Velasquez, J.; Aguilar, C.E.; Gomez, L.; Antolinez, E.; Numa, S. Bibliometric Study of Cut Flower Research. Ornam. Hortic. 2023, 29, 500–514. [Google Scholar] [CrossRef]
- Rocha, G.A.O.; Medina, A.N.C.; Arias, L.G.; Caita, J.F.A.; Villagran, E. Análisis Sobre La Actividad Científica Referente a Las Estrategias de Climatización Pasiva Usada En Invernaderos: Parte 2: Análisis Técnico. Cienc. Lat. Rev. Científica Multidiscip. 2022, 6, 2220–2245. [Google Scholar]
- Tian, L.; Liu, L.; Jiang, Y.; Yang, Y.; Dong, G.; Yu, H. Tobacco Rattle Virus-Induced VcANS Gene Silencing in Blueberry Fruit. Gene 2023, 852, 147054. [Google Scholar]
- Ehlenfeldt, M.K. Investigations of Metaxenia in Northern Highbush Blueberry (Vaccinium corymbosum L.) Cultivars. J. Am. Pomol. Soc. 2003, 57, 26–31. [Google Scholar] [CrossRef]
- Bal, J.J.M.; Balkhoven, J.; Peppelman, G. Results of Testing Highbush Blueberry Cultivars in The Netherlands. In Proceedings of the VIII International Symposium on Vaccinium Culture, Sevilla, Spain, 3–8 May 2004; Volume 715, pp. 157–162. [Google Scholar]
- Stringer, S.J.; Draper, A.D.; Spiers, J.M.; Marshall, D.A. Potential New Blueberry Cultivars for the Gulf Coast Region of the US. In Proceedings of the IX International Vaccinium Symposium, Corvallis, OR, USA, 14–18 July 2008; Volume 810, pp. 87–92. [Google Scholar]
- Xu, G.; An, Q.; Liu, G.; Zhao, L.; Wang, H. A New Blueberry Cultivar ‘chasing Dream’. Acta Hortic. Sin. 2021, 48, 2797–2798. [Google Scholar]
- Pluta, S.; Zurawicz, E. The High-Bush Blueberry (Vaccinium corymbosum L.) Breeding Programme in Poland. In Proceedings of the X International Symposium on Vaccinium and Other Superfruits, Maastricht, The Netherlands, 17–22 June 2012; Volume 1017, pp. 177–180. [Google Scholar]
- Hamim, A.; Lamrini, A.; Bouriah, S.; Mohammed, E. Study of Growth and Yield Parameters of Two Cultivars of Blueberry in Loukkos Region. In Proceedings of the International Conference on Advanced Intelligent Systems for Sustainable Development, Marrakech, MO, USA, 8–11 July 2019; Springer: Cham, Switzerland, 2019; pp. 357–363. [Google Scholar]
- Zhu, B.; Guo, P.; Wu, S.; Yang, Q.; He, F.; Gao, X.; Zhang, Y.; Xiao, J. A Better Fruit Quality of Grafted Blueberry than Own-Rooted Blueberry Is Linked to Its Anatomy. Plants 2024, 13, 625. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Wu, L.; Li, Y. Technical System of Blueberry Micropropagation in China. In Proceedings of the VIII International Symposium on Vaccinium Culture, Sevilla, Spain, 3–8 May 2004; Volume 715, pp. 421–426. [Google Scholar]
- Qiu, D.; Wei, X.; Fan, S.; Jian, D.; Chen, J. Regeneration of Blueberry Cultivars through Indirect Shoot Organogenesis. HortScience 2018, 53, 1045–1049. [Google Scholar] [CrossRef]
- Fernández, E.G.B.; Hanzer, V.; Lok-Lee, F.; Laimer, M. “Recovery” of Vaccinium myrtillus from Phytoplasma Infection in Vitro. Acta Hortic. 2023, 369–376. [Google Scholar] [CrossRef]
- Hernández, R.; López, A.; Valenzuela, B.; D’Afonseca, V.; Gomez, A.; Arencibia, A.D. Organogenesis of Plant Tissues in Colchicine Allows Selecting in Field Trial Blueberry (Vaccinium spp. cv Duke) Clones with Commercial Potential. Horticulturae 2024, 10, 283. [Google Scholar] [CrossRef]
- Olmstead, J.W.; Armenta, H.P.R.; Lyrene, P.M. Using Sparkleberry as a Genetic Source for Machine Harvest Traits for Southern Highbush Blueberry. Horttechnology 2013, 23, 419–424. [Google Scholar] [CrossRef]
- Postman, J.; Oliphant, J.; Hummer, K. Diseases Impact Management of USDA Clonal Vaccinium Genebank. In Proceedings of the IX International Vaccinium Symposium, Corvallis, OR, USA, 14–18 July 2008; Volume 810, pp. 319–324. [Google Scholar]
- Nestby, R.; Retamales, J.B. Diagnosis and Management of Nutritional Constraints in Berries. In Fruit Crops; Elsevier: Amsterdam, The Netherlands, 2020; pp. 567–582. [Google Scholar]
- Smrke, T.; Veberic, R.; Hudina, M.; Zitko, V.; Ferlan, M.; Jakopic, J. Fruit Quality and Yield of Three Highbush Blueberry (Vaccinium corymbosum L.) Cultivars Grown in Two Planting Systems under Different Protected Environments. Horticulturae 2021, 7, 591. [Google Scholar] [CrossRef]
- Kingston, P.H.; Scagel, C.F.; Bryla, D.R.; Strik, B. Suitability of Sphagnum Moss, Coir, and Douglas Fir Bark as Soilless Substrates for Container Production of Highbush Blueberry. HortScience 2017, 52, 1692–1699. [Google Scholar] [CrossRef]
- He, S.; Yu, H.; Gu, Y. Prospects and Problems of Blueberry Growing in China. Acta Hortic. 2009, 810, 61–64. [Google Scholar] [CrossRef]
- Girgenti, V.; Peano, C.; Giuggioli, N.R.; Giraudo, E.; Guerrini, S. First Results of Biodegradable Mulching on Small Berry Fruits. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on Berries: From Genomics to Sustainable Production, Quality and Health, Lisbon, Portugal, 22–27 August 2010; Volume 926, pp. 571–576. [Google Scholar]
- Lobos, G.A.; Retamales, J.B.; Hancock, J.F.; Flore, J.A.; Romero-Bravo, S.; Del Pozo, A. Productivity and Fruit Quality of Vaccinium corymbosum cv. Elliott under Photo-Selective Shading Nets. Sci. Hortic. 2013, 153, 143–149. [Google Scholar] [CrossRef]
- Coletti, R.; Nienow, A.A.; Calvete, E.O. Dormancy Breaking of Blueberries Cultivars in a Protected Environment with Hydrogen Cyanamide and Mineral Oil. Rev. Bras. Frutic. 2011, 33, 685–690. [Google Scholar] [CrossRef]
- Boudreau-Forgues, Ã.-M.; Gaudreau, L.; Nguyen, T.T.A.; Gosselin, A.; Thériault, L.; Brégard, A.; Dorais, M. Impact of Silicon Applications on the Agronomic and Nutritional Performance of Container-Grown Organic Highbush Blueberries. In Proceedings of the IV International Symposium on Organic Greenhouse Horticulture, Cancun, Mexico, 22–27 October 2023; Volume 1428, pp. 55–62. [Google Scholar]
- Leal-Ayala, O.G.; Sandoval-Villa, M.; Trejo-Téllez, L.I.; Sandoval-Rangel, A.; Cabrera-De la Fuente, M.; Benavides-Mendoza, A. Nitrogen Form and Root Division Modifies the Nutrimental and Biomolecules Concentration in Blueberry (Vaccinium corymbosum L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 11998. [Google Scholar] [CrossRef]
- Ngoc, N.P.; Thao, P.T.P. Yield-Limiting Nutrient Response of Lowbush Blueberry Grown in Recent and Ancient Alluvial Soils of the Mekong Delta. PeerJ 2024, 12, e17992. [Google Scholar] [CrossRef]
- Heller, C.R.; Nunez, G.H. Preplant Fertilization Increases Substrate Microbial Respiration but Does Not Affect Southern Highbush Blueberry Establishment in a Coconut Coir-Based Substrate. HortScience 2022, 57, 17–21. [Google Scholar] [CrossRef]
- Viencz, T.; Santana, K.; Ayub, R.A.; Botelho, R.V. Development, Photosynthesis and Yield of Blueberry Cultivar ‘Climax’Growth with Different Substrates and Nitrogen Fertilization under Protected Cultivation. Cienc. Rural 2021, 51, e20190367. [Google Scholar] [CrossRef]
- Bolaños-Alcántara, M.N.; Pineda-Pineda, J.; Castro-Brindis, R.; Vargas-Hernández, M.; Avitia-GarcÃa, E. Nitrate/Ammonium Ratio and Electrical Conductivity in Blueberry Quality. In Proceedings of the XXX International Horticultural Congress IHC2018: III International Berry Fruit Symposium, Istanbul, Turkey, 12–16 August 2018; Volume 1265, pp. 233–240. [Google Scholar]
- Bandaranayake, W.M.; Syvertsen, J.P.; Schumann, A.; Kadyampakeni, D.M. Leaching Losses from Blueberries Grown in Sandy Soils Amended with Pine Bark; Wiley Online Library: Hoboken, NJ, USA, 2020. [Google Scholar]
- Ordóñez-Díaz, J.L.; Pereira-Caro, G.; Cardeñosa, V.; Muriel, J.L.; Moreno-Rojas, J.M. Study of the Quality Attributes of Selected Blueberry (Vaccinium corymbosum L.) Varieties Grown under Different Irrigation Regimes and Cultivation Systems. Appl. Sci. 2020, 10, 8459. [Google Scholar] [CrossRef]
- Cardeñosa, V.; Girones-Vilaplana, A.; Muriel, J.L.; Moreno, D.A.; Moreno-Rojas, J.M. Influence of Genotype, Cultivation System and Irrigation Regime on Antioxidant Capacity and Selected Phenolics of Blueberries (Vaccinium corymbosum L.). Food Chem. 2016, 202, 276–283. [Google Scholar] [CrossRef]
- Bian, D.; Yang, F.; Zhao, G.; Zheng, K.; Lin, H. Construction of Intelligent Water and Fertilizer Integration System for Blueberry Greenhouse. J. Chinese Agric. Mech. 2022, 43, 55–61. [Google Scholar]
- Liu, L.; Zhang, Y.; Zhang, X.; Huang, X.; Huang, C. Application and Development of Agricultural Smart Irrigation in Northern Water-Scarce Areas. Agric. Eng. 2023, 13, 78–83. [Google Scholar]
- Gurovich, L.A. Real-Time Plant Water Potential Assessment Based on Electrical Signalling in Higher Plants. In Proceedings of the 7th World Congress on Computers in Agriculture Conference Proceedings, Reno, NV, USA, 22–24 June 2009; American Society of Agricultural and Biological Engineers: Saint Joseph, MI, USA, 2009; p. 1. [Google Scholar]
- Stückrath, R.; Quevedo, R.; de la Fuente, L.; Hernández, A.; Sepúlveda, V. Effect of Foliar Application of Calcium on the Quality of Blueberry Fruits. J. Plant Nutr. 2008, 31, 1299–1312. [Google Scholar] [CrossRef]
- France, A.; Santelices, C.; Buddie, A.; Kirk, P. Silver Leaf: First Worldwide Report of a New and Harmful Disease on Blueberry. In Proceedings of the IX International Vaccinium Symposium, Corvallis, OR, USA, 14–18 July 2008; Volume 810, pp. 341–344. [Google Scholar]
- Yu, L.; Rarisara, I.; Xu, S.G.; Wu, X.; Zhao, J.R. First Report of Stem Blight of Blueberry Caused by Botryosphaeria Dothidea in China. Plant Dis. 2012, 96, 1697. [Google Scholar] [CrossRef]
- Atashi Khalilabad, A.; Fotouhifar, K.; Atghia, O. Characterization and Report of Ectophoma Multirostrata, Causing Stem Canker Disease in Highbush Blueberry in Iran. J. Phytopathol. 2023, 171, 470–477. [Google Scholar] [CrossRef]
- Di Genova, D.; Lewis, K.J.; Oliver, J.E. Natural Infection of Southern Highbush Blueberry (Vaccinium corymbosum Interspecific Hybrids) by Xylella fastidiosa subsp. Fastidiosa. Plant Dis. 2020, 104, 2598–2605. [Google Scholar] [CrossRef]
- Lan, C.Z.; Ruan, H.C.; Yao, J.A. First Report of Phytophthora Cinnamomi Causing Root and Stem Rot of Blueberry (Vaccinium corymbosum) in China. Plant Dis. 2016, 100, 2537. [Google Scholar] [CrossRef]
- Yeo, J.R.; Weiland, J.E.; Sullivan, D.M.; Bryla, D.R. Susceptibility of Highbush Blueberry Cultivars to Phytophthora Root Rot. HortScience 2016, 51, 74–78. [Google Scholar] [CrossRef]
- Smith, B.J.; Miller-Butler, M.A. Effect of Nitrogen Fertilization and Fungicides on Botryosphaeria Stem Blight Lesion Development on Detached Blueberry Stems. In Proceedings of the XI International Vaccinium Symposium, Orlando, FL, USA, 10–14 April 2016; Volume 1180, pp. 61–70. [Google Scholar]
- Smith, B.J.; Miller-Butler, M.A.; Curry, K.J.; Sakhanokho, H.F. Effect of Phytophthora Cinnamomi Isolate, Inoculum Delivery Method, and Flood and Drought Conditions on Vigor, Disease Severity Scores, and Survival of Blueberry Plants. In Proceedings of the XI International Vaccinium Symposium, Orlando, FL, USA, 10–14 April 2016; Volume 1180, pp. 93–104. [Google Scholar]
- Wiseman, M.S.; Gordon, M.I.; Putnam, M.L. First Report of Leaf Rust Caused by Thekopsora Minima on Northern Highbush Blueberry in Oregon. Plant Dis. 2016, 100, 1949. [Google Scholar] [CrossRef]
- Hummer, K.; Williams, R. Pests of Blueberries on São Miguel, Açores, Portugal. In Proceedings of the IX International Vaccinium Symposium, Corvallis, OR, USA, 14–18 July 2008; Volume 810, pp. 287–292. [Google Scholar]
- Nickerson, N.L.; MacNeill, B.H. Studies on the Spread of Red Leaf Disease, Caused by Exobasidium vaccinii, in Lowbush Blueberries. Can. J. Plant Pathol. 1987, 9, 307–310. [Google Scholar] [CrossRef]
- Cook, M.A.; Ozeroff, S.N.; Fitzpatrick, S.M.; Roitberg, B.D. Host-associated Differentiation in Reproductive Behaviour of Cecidomyiid Midges on Cranberry and Blueberry. Entomol. Exp. Appl. 2011, 141, 8–14. [Google Scholar] [CrossRef]
- Tartanus, M.; Malusa, E.; Sas, D.; Łabanowska, B. Integrated Control of Lecanium Scale (Parthenolecanium sp.) on Highbush Blueberry in Open Field and Protected Crops. J. Plant Prot. Res. 2018, 58, 297–303. [Google Scholar] [CrossRef]
- Tuovinen, T. Risk of Invasive Arthropod Pests Related to Climate Change in the Northernmost Small Fruit Production Area in EU. In Proceedings of the Workshop on Berry Production in Changing Climate Conditions and Cultivation Systems. COST-Action 863: Euroberry Research: From Genomics to Sustainable Production, Quality and Health, Geisenheim, Germany, 29–31 October 2008; Volume 838, pp. 151–154. [Google Scholar]
- Shantharaj, D.; Román-Écija, M.; Velasco-Amo, M.P.; Navas-Cortés, J.A.; Landa, B.B.; De La Fuente, L. European Xylella fastidiosa Strains Can Cause Symptoms in Blueberry. Plant Dis. 2024, 108, 2658–2662. [Google Scholar] [CrossRef]
- Waliullah, S.; Di Genova, D.; Oliver, J.E.; Ali, M.E. Development of a CAPS Marker and a LAMP Assay for Rapid Detection of Xylella fastidiosa subsp. Multiplex and Differentiation from X. fastidiosa subsp. Fastidiosa on Blueberry. Int. J. Mol. Sci. 2022, 23, 1937. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.E.; Lewis, K.J.; Taylor, S.J.; Chen, J. Complete Genome Sequences of Xylella fastidiosa subsp. Fastidiosa and X. fastidiosa subsp. Multiplex Strains Causing Blueberry Bacterial Leaf Scorch Disease in Georgia, USA. Microbiol. Resour. Announc. 2023, 12, e00114-23. [Google Scholar] [PubMed]
- Shantharaj, D.; Naranjo, E.; Merfa, M.V.; Cobine, P.A.; Santra, S.; De La Fuente, L. Zinc Oxide-Based Nanoformulation Zinkicide Mitigates the Xylem-Limited Pathogen Xylella fastidiosa in Tobacco and Southern Highbush Blueberry. Plant Dis. 2023, 107, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Pszczółkowska, A.; Okorski, A.; Paukszto, Ł.; Jastrzębski, J. First Report of Anthracnose Disease Caused by Colletotrichum Fioriniae on Blueberry in Western Poland. Plant Dis. 2016, 100, 2167. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Polashock, J.J.; Stretch, A.W.; Kramer, M. Leaf Disk Infection by Colletotrichum Acutatum and Its Relation to Fruit Rot in Diverse Blueberry Germplasm. HortScience 2006, 41, 270–271. [Google Scholar] [CrossRef]
- Onofre, R.B.; Mertely, J.C.; Aguiar, F.M.; Timilsina, S.; Harmon, P.; Vallad, G.E.; Peres, N.A. First Report of Target Spot Caused by Corynespora Cassiicola on Blueberry in North America. Plant Dis. 2016, 100, 528. [Google Scholar] [CrossRef]
- Zheng, X.; Qi, X.; Xu, J.; Cui, Y.; Yu, X.; Chang, X.; Zhou, Y.; Gong, G. First Report of Corynespora Leaf Spot of Blueberry Caused by Corynespora Cassiicola in Sichuan, China. Plant Dis. 2015, 99, 1651. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Stretch, A.W. Resistance to Blighting by Monilinia vaccinii-corymbosi in Diploid and Polyploid Vaccinium Species. HortScience 2001, 36, 955–957. [Google Scholar] [CrossRef]
- Lehman, J.S.; Igarashi, S.; Oudemans, P.V. Host Resistance to Monilinia vaccinii-corymbosi in Flowers and Fruits of Highbush Blueberry. Plant Dis. 2007, 91, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Tarnowski, T.L.B.; Savelle, A.T.; Scherm, H. Activity of Fungicides against Monilinia vaccinii-corymbosi in Blueberry Flowers Treated at Different Phenological Stages. Plant Dis. 2008, 92, 961–965. [Google Scholar] [CrossRef]
- Thomas-Sharma, S.; Scherm, H. Pre-and Post-Anthesis Activity of Fenbuconazole and Triforine against Blueberry Flower Infection by Monilinia vaccinii-corymbosi. Crop Prot. 2017, 96, 180–187. [Google Scholar]
- Ngugi, H.K.; Scherm, H.; Lehman, J.S. Relationships between Blueberry Flower Age, Pollination, and Conidial Infection by Monilinia vaccinii-corymbosi. Phytopathology 2002, 92, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Lehman, J.S.; Oudemans, P.V. Phenology of Apothecium Production in Populations of Monilinia vaccinii-corymbosi from Early-and Late-Maturing Blueberry Cultivars. Phytopathology 1997, 87, 218–223. [Google Scholar]
- Lehman, J.S.; Oudemans, P.V. Variation and Heritability of Phenology in the Fungus Monilinia vaccinii-corymbosi on Blueberry. Phytopathology 2000, 90, 390–395. [Google Scholar] [CrossRef]
- Boyzo-Marin, J.; Rebollar-Alviter, A.; Silva-Rojas, H.V.; Ramirez-Maldonaldo, G. First Report of Neofusicoccum Parvum Causing Stem Blight and Dieback of Blueberry in Mexico. Plant Dis. 2016, 100, 2524. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; He, W.; Zhang, Y. Stem Blight of Blueberry Caused by Lasiodiplodia vaccinii sp. Nov. in China. Plant Dis. 2019, 103, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Yi-Lan, J.; Shi-Long, J.; Xuan-Li, J. Disease-Resistant Identification and Analysis to Transcriptome Differences of Blueberry Leaf Spot Induced by Beta-Aminobutyric Acid. Arch. Microbiol. 2021, 203, 3623–3632. [Google Scholar]
- Jagdale, G.B.; Holladay, T.; Brannen, P.M.; Cline, W.O.; Agudelo, P.; Nyczepir, A.P.; Noe, J.P. Incidence and Pathogenicity of Plant-Parasitic Nematodes Associated with Blueberry (Vaccinium spp.) Replant Disease in Georgia and North Carolina. J. Nematol. 2013, 45, 92. [Google Scholar]
- Forge, T.; Zasada, I.; Pinkerton, J.; Koch, C. Host Status and Damage Potential of Paratrichodorus Renifer and Pratylenchus penetrans (Nematoda) to Blueberry (Vaccinium spp.). Can. J. Plant Pathol. 2012, 34, 277–282. [Google Scholar]
- Polavarapu, S.; Koppenhöfer, A.M.; Barry, J.D.; Holdcraft, R.J.; Fuzy, E.M. Entomopathogenic Nematodes and Neonicotinoids for Remedial Control of Oriental Beetle, Anomala orientalis (Coleoptera: Scarabaeidae), in Highbush Blueberry. Crop Prot. 2007, 26, 1266–1271. [Google Scholar]
- Reeh, K.W.; Hillier, N.K.; Cutler, G.C. Potential of Bumble Bees as Bio-Vectors of Clonostachys Rosea for Botrytis Blight Management in Lowbush Blueberry. J. Pest Sci. 2014, 87, 543–550. [Google Scholar] [CrossRef]
- Reeh, K.W.; Cutler, G.C. Laboratory Efficacy and Fungicide Compatibility of Clonostachys Rosea against Botrytis Blight on Lowbush Blueberry. Can. J. Plant Sci. 2013, 93, 639–642. [Google Scholar] [CrossRef]
- Navarro, P.D.; Palma-Millanao, R.; Ceballos, R.; Monje, A.J. Steinernema australe Enhanced Its Efficacy against Aegorhinus nodipennis (Coleoptera: Curculionidae) Larvae in Berry Orchards after an Artificial Selection Process. Agronomy 2022, 12, 1128. [Google Scholar] [CrossRef]
- Thimmappa, B.C.; Salhi, L.N.; Forget, L.; Sarrasin, M.; Bustamante Villalobos, P.; Henrissat, B.; Lang, B.F.; Burger, G. A Biofertilizing Fungal Endophyte of Cranberry Plants Suppresses the Plant Pathogen Diaporthe. Front. Microbiol. 2024, 15, 1327392. [Google Scholar] [CrossRef]
- Li, J.; Hou, R.; Zhang, F. A New Schizophyllum commune Strain as a Potential Biocontrol Agent against Blueberry Root Rot. Arch. Microbiol. 2024, 206, 235. [Google Scholar] [CrossRef]
- Montalba, R.; Arriagada, C.; Alvear, M.; Zúñiga, G.E. Effects of Conventional and Organic Nitrogen Fertilizers on Soil Microbial Activity, Mycorrhizal Colonization, Leaf Antioxidant Content, and Fusarium Wilt in Highbush Blueberry (Vaccinium corymbosum L.). Sci. Hortic. 2010, 125, 775–778. [Google Scholar] [CrossRef]
- Marucci, R.C.; Ruber, S.E.; Pec, M.; Liburd, O.E. Are Predatory Mites Effective as Biological Control Agents to Suppress Oligonychus ilicis (Acari: Tetranychidae) in Blueberry Plantings? J. Econ. Entomol. 2024, 117, 834–842. [Google Scholar] [PubMed]
- Roubos, C.R.; Rodriguez-Saona, C.; Holdcraft, R.; Mason, K.S.; Isaacs, R. Relative Toxicity and Residual Activity of Insecticides Used in Blueberry Pest Management: Mortality of Natural Enemies. J. Econ. Entomol. 2014, 107, 277–285. [Google Scholar] [PubMed]
- Hughes, A.; White, S.N.; Boyd, N.S.; Hildebrand, P.; Christopher Cutler, G. Red Sorrel Management and Potential Effect of Red Sorrel Pollen on Botrytis Cinerea Spore Germination and Infection of Lowbush Blueberry (Vaccinium angustifolium Ait.) Flowers. Can. J. Plant Sci. 2016, 96, 590–596. [Google Scholar] [CrossRef]
- Cerna Larenas, M.; Guzmán Estrada, R.; Guerrero Contreras, J.; Meriño Gergichevich, C. Determination of the Fluorescence Spectrum of Botrytis Cinerea Pers: Fr. Isolated from Highbush Blueberry (Vaccinium corymbosum L.). J. Soil Sci. Plant Nutr. 2015, 15, 938–948. [Google Scholar]
- El Horri, H.; Bartolini, S.; Remorini, D.; Ceccanti, C.; Florio, M.; D’Asaro, L.; Jain, G.; Massai, R.; Landi, M.; Guidi, L. Light Down-Conversion Technology Improves Vegetative Growth, Berry Production, and Postharvest Quality in Tunnel-Cultivated Blueberry. Agronomy 2025, 15, 1708. [Google Scholar]
- Tran, Q.; Retano, A.; Bryla, D.R.; Garcia-Jaramillo, M.; Jin, X. Integrated Electrodialysis–Forward Osmosis Process for Sustainable Water Reuse: A Case Study in Southern Highbush Blueberry. J. Environ. Chem. Eng. 2025, 13, 117178. [Google Scholar]
- Sun, K.X.; Zhang, Y.J.; Zhou, C.Z.; Yang, S.L.; Jiang, H.X.; Cui, R.R. Effect of Gradual Pollen Presentation on Pollination Efficiency and Reproduction of Vaccinium corymbosum Berkeley in Two Habitats. Front. Ecol. Evol. 2024, 12, 1476848. [Google Scholar] [CrossRef]
- Wang, H.; Xia, X.; An, L. Effect of Hydrogen Cyanamide on Bud Break, Fruit Yield and Quality of Highbush Blueberry in Greenhouse Production. Agriculture 2021, 11, 439. [Google Scholar] [CrossRef]
- Bañados, M.P.; Strik, B. Manipulation of the Annual Growth Cycle of Blueberry Using Photoperiod. In Proceedings of the VIII International Symposium on Vaccinium Culture, Sevilla, Spain, 3–8 May 2004; Volume 715, pp. 65–72. [Google Scholar]
- Spiers, J.M.; Marshall, D.A.; Braswell, J.H. Chilling Requirement Studies in Blueberries. Small Fruits Rev. 2004, 3, 325–330. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, L.; Dang, C.; Wang, H.; Jiang, G.; Li, G.; Zhang, Z.; Lou, X.; Zheng, Y. Effects of High Temperature on Leaf Stomatal Traits and Gas Exchange Parameters of Blueberry. Trans. Chin. Soc. Agric. Eng. 2016, 32, 218–225. [Google Scholar]
- Albert, K.R.; Mikkelsen, T.N.; Ro-Poulsen, H. Ambient UV-B Radiation Decreases Photosynthesis in High Arctic Vaccinium uliginosum. Physiol. Plant. 2008, 133, 199–210. [Google Scholar] [CrossRef]
- Lobos, G.A.; Retamales, J.B.; Hancock, J.F.; Flore, J.A. Physiological Response of Vaccinium corymbosum ‘Elliott’ to Shading Nets in Michigan. In Proceedings of the IX International Vaccinium Symposium, Corvallis, OR, USA, 14–18 July 2008; Volume 810, pp. 465–470. [Google Scholar]
- Suzuki, A.; Shimizu, T.; Aoba, K. Effects of Leaf/Fruit Ratio and Pollen Density on Highbush Blueberry Fruit Quality and Maturation. J. Japanese Soc. Hortic. Sci. 1998, 67, 739–743. [Google Scholar] [CrossRef]
- Strik, B.C. Flowering and Fruiting on Command in Berry Crops. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on Berries: From Genomics to Sustainable Production, Quality and Health, Lisbon, Portugal, 22–27 August 2010; Volume 926, pp. 197–214. [Google Scholar]
- Lenart, A.; Wrona, D.; Krupa, T. Biostimulators with Marine Algae Extracts and Their Role in Increasing Tolerance to Drought Stress in Highbush Blueberry Cultivation. PLoS ONE 2024, 19, e0306831. [Google Scholar] [CrossRef]
- Percival, D.; MacKenzie, J.L. Use of Plant Growth Regulators to Increase Polyphenolic Compounds in the Wild Blueberry. Can. J. Plant Sci. 2007, 87, 333–336. [Google Scholar] [CrossRef]
- Sim, A.; Pearse, J.; McGuinness, B.; Williams, H.; Duke, M. Evaluation of a Low-Cost Selective Shaking Device and Soft Surface Catchment for Fresh Market Blueberry Harvesting. In Proceedings of the 2024 IEEE 20th International Conference on Automation Science and Engineering (CASE), Bari, Italy, 28 August–1 September 2024; IEEE: New York, NY, USA, 2024; pp. 2813–2818. [Google Scholar]
- Takeda, F.; Krewer, G.; Li, C.; MacLean, D.; Olmstead, J.W. Techniques for Increasing Machine Harvest Efficiency in Highbush Blueberry. Horttechnology 2013, 23, 430–436. [Google Scholar] [CrossRef]
- Yu, P.; Li, C.; Takeda, F.; Krewer, G.; Rains, G.; Hamrita, T. Quantitative Evaluation of a Rotary Blueberry Mechanical Harvester Using a Miniature Instrumented Sphere. Comput. Electron. Agric. 2012, 88, 25–31. [Google Scholar] [CrossRef]
- Esau, K.; Esau, T.; Zaman, Q.; Farooque, A.; Schumann, A. Effective Use of a Variable Speed Blower Fan on a Mechanical Wild Blueberry Harvester. Appl. Eng. Agric. 2018, 34, 831–840. [Google Scholar] [CrossRef]
- Gibson, L.; Rupasinghe, H.P.V.; Forney, C.F.; Eaton, L. Characterization of Changes in Polyphenols, Antioxidant Capacity and Physico-Chemical Parameters during Lowbush Blueberry Fruit Ripening. Antioxidants 2013, 2, 216–229. [Google Scholar] [CrossRef]
- Gallardo, R.K.; Zilberman, D. The Economic Feasibility of Adopting Mechanical Harvesters by the Highbush Blueberry Industry. Horttechnology 2016, 26, 299–308. [Google Scholar] [CrossRef]
- Olmstead, J.W.; Finn, C.E. Breeding Highbush Blueberry Cultivars Adapted to Machine Harvest for the Fresh Market. Horttechnology 2014, 24, 290–294. [Google Scholar] [CrossRef]
- Fathollahi-Fard, A.M.; Tian, G.; Ke, H.; Fu, Y.; Wong, K.Y. Efficient Multi-Objective Metaheuristic Algorithm for Sustainable Harvest Planning Problem. Comput. Oper. Res. 2023, 158, 106304. [Google Scholar] [CrossRef]
- Huang, R.; Ye, M.; Li, X.; Ji, L.; Karwe, M.; Chen, H. Evaluation of High Hydrostatic Pressure Inactivation of Human Norovirus on Strawberries, Blueberries, Raspberries and in Their Purees. Int. J. Food Microbiol. 2016, 223, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Scheidt, T.B.; Silva, F.V.M. High Pressure Processing and Storage of Blueberries: Effect on Fruit Hardness. High Press. Res. 2018, 38, 80–89. [Google Scholar] [CrossRef]
- Adhikari, J.; Araghi, L.R.; Singh, R.; Adhikari, K.; Patil, B.S. Continuous-Flow High-Pressure Homogenization of Blueberry Juice Enhances Anthocyanin and Ascorbic Acid Stability during Cold Storage. J. Agric. Food Chem. 2024, 72, 11629–11639. [Google Scholar] [CrossRef]
- Pratap-Singh, A.; Shojaei, M.; Singh, A.; Ye, Y.; Mandal, R.; Yan, Y.; Pico, J.; Gerbrandt, E.M.; Castellarin, S.D. Effects of Pulsed Light on the Postharvest Quality and Shelf-Life of Highbush Blueberries (cv. Draper). Appl. Food Res. 2023, 3, 100273. [Google Scholar] [CrossRef]
- Salehi, F. Application of Pulsed Light Technology for Fruits and Vegetables Disinfection: A Review. J. Appl. Microbiol. 2022, 132, 2521–2530. [Google Scholar] [CrossRef]
- Zhu, N.; Zhu, Y.; Yu, N.; Wei, Y.; Zhang, J.; Hou, Y.; Sun, A. Evaluation of Microbial, Physicochemical Parameters and Flavor of Blueberry Juice after Microchip-Pulsed Electric Field. Food Chem. 2019, 274, 146–155. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, S.; Li, J.; Qu, C.; Sun, A.; Qiao, X. Design and Optimization of a Microchip Operating at Low-Voltage Pulsed Electric Field for Juice Sterilization. Food Bioprocess Technol. 2019, 12, 1696–1707. [Google Scholar] [CrossRef]
- Barba, F.J.; Jäger, H.; Meneses, N.; Esteve, M.J.; Frígola, A.; Knorr, D. Evaluation of Quality Changes of Blueberry Juice during Refrigerated Storage after High-Pressure and Pulsed Electric Fields Processing. Innov. Food Sci. Emerg. Technol. 2012, 14, 18–24. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Li, X.; Li, B.; Liu, S.; Chang, N.; Jie, D.; Ning, C.; Gao, H.; Meng, X. Combined Effect of Ultrasound, Heat, and Pressure on Escherichia coli O157: H7, Polyphenol Oxidase Activity, and Anthocyanins in Blueberry (Vaccinium corymbosum) Juice. Ultrason. Sonochem. 2017, 37, 251–259. [Google Scholar] [CrossRef]
- Pathak, N.; Grossi Bovi, G.; Limnaios, A.; Fröhling, A.; Brincat, J.; Taoukis, P.; Valdramidis, V.P.; Schlüter, O. Impact of Cold Atmospheric Pressure Plasma Processing on Storage of Blueberries. J. Food Process. Preserv. 2020, 44, e14581. [Google Scholar] [CrossRef]
- Lacombe, A.; Niemira, B.A.; Gurtler, J.B.; Fan, X.; Sites, J.; Boyd, G.; Chen, H. Atmospheric Cold Plasma Inactivation of Aerobic Microorganisms on Blueberries and Effects on Quality Attributes. Food Microbiol. 2015, 46, 479–484. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, F.; Zhu, D.; Zhao, D.; Zhao, Y.; Li, J. Evaluation of the Effects of Immersion Thawing Methods on Quality of Blueberries. J. Food Process Eng. 2020, 43, e13538. [Google Scholar] [CrossRef]
- Darniadi, S.; Ifie, I.; Ho, P.; Murray, B.S. Evaluation of Total Monomeric Anthocyanin, Total Phenolic Content and Individual Anthocyanins of Foam-Mat Freeze-Dried and Spray-Dried Blueberry Powder. J. Food Meas. Charact. 2019, 13, 1599–1606. [Google Scholar] [CrossRef]
- Zárate-Carbajal, A.; Turgeon, S.L.; Bazinet, L.; Mcsweeney, M.; Sierra, A.D. Optimizing Wild Blueberry Juice Production: Sensory, Physicochemical, and Phytochemical Characteristics. Int. J. Gastron. Food Sci. 2025, 41, 101279. [Google Scholar] [CrossRef]
- Cao, X.M.; Bi, X.F.; Li, R.J.; Dong, P.; Hu, X.S.; Liao, X.J. Effects of Ultrahigh Pressure and Thermal Sterilization on the Quality of Strawberry Cloudy Juice and Clear Juice. J. High Press. Phys. 2014, 28, 631–640. [Google Scholar]
- Beaulieu, J.C.; Stein-Chisholm, R.E.; Lloyd, S.W.; Bett-Garber, K.L.; Grimm, C.C.; Watson, M.A.; Lea, J.M. Volatile, Anthocyanidin, Quality and Sensory Changes in Rabbiteye Blueberry from Whole Fruit through Pilot Plant Juice Processing. J. Sci. Food Agric. 2017, 97, 469–478. [Google Scholar] [CrossRef]
- Yang, L.; Feng, X.; Xu, S.; Li, Q.; Huang, B. Effects of Different Freezing Temperatures and Freeze-Thaw Cycles on the Quality Characteristics of Blueberries. Mod. Food Sci. Technol. 2023, 39, 127–136. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, L.; Li, J.; Wang, L.; Song, X. Extraction and Characterization of Pectin from Jerusalem ArtiChoke Residue and Its Application in Blueberry Preservation. Coatings 2022, 12, 385. [Google Scholar] [CrossRef]
- Xie, X.; Nie, Y.; Wang, Y.; Wen, B.; Shang, J.; Guo, X.; Tian, J.; Wang, Y.; Zhang, Y.; Li, S.; et al. Integration of GC-MS, ROAV, and Chemometrics to Characterize Key Differential Volatile Compounds in Wild and Cultivated Blueberries from Northeast China. LWT-Food Sci. Technol. 2025, 225, 11795. [Google Scholar]
- Jiahui, Y.; Wang, W.; Tang, Y.; Zhang, S.; Lin, R.; Zhang, D. Experimental Study on Microwave Freeze-Drying of Foamed Blueberry Pulp Assisted by Absorbing Materials. Trans. Chin. Soc. Agric. Eng. 2022, 38, 284–292. [Google Scholar] [CrossRef]
- Zhang, X.; Bi, J.; Chen, Q.; Wu, X.; Li, X.; Lü, Y. Quality and Processing Characteristics of a Mixed Freeze-Dried Powder of Blueberry and Blue Honeysuckle Berries during Storage. Food Sci. 2022, 43, 240–247. [Google Scholar]
- Chu, W.; Sun, Y.; Xiao, L.; Chen, A.; Tao, Y. Hygroscopic Mechanism of Blueberry Powder Based on Glass Transition Theory. Food Sci. 2023, 44, 41–47. [Google Scholar]
- Rashwan, A.K.; Osman, A.I.; Karim, N.; Mo, J.; Chen, W. Unveiling the Mechanisms of the Development of Blueberries-Based Functional Foods: An Updated and Comprehensive Review. Food Rev. Int. 2024, 40, 1913–1940. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Cui, M.-Y.; Fu, Y.; Wang, X.-H.; Yang, Q.; Zhu, Y.; Yang, X.-H.; Bi, H.-J.; Gao, X.-L. Aroma Enhancement of Blueberry Wine by Postharvest Partial Dehydration of Blueberries. Food Chem. 2023, 426, 136593. [Google Scholar] [CrossRef]
- Song, Z.; Xu, B.; Kang, S.; Dai, H.; Zhou, X.; Li, M.; Luo, M.; Ji, S.; Zhou, Q. Calcium Chloride Treatment Delayed the Softening of Postharvest Blueberries by Regulating ABA Biosynthesis and Signal Transduction. Sci. Hortic. 2025, 339, 113879. [Google Scholar]
- Duan, C.; Xiao, X.; Yu, Y.; Xu, M.; Zhang, Y.; Liu, X.; Dai, H.; Pi, F.; Wang, J. In Situ Raman Characterization of the Stability of Blueberry Anthocyanins in Aqueous Solutions under Perturbations in Temperature, UV, PH. Food Chem. 2024, 431, 137155. [Google Scholar]
- Wu, Z.; Li, H.; Wang, Y.; Tan, H.; Yang, Y.; Zhan, Y.; Wang, F. Effects of Different Thermal-Treated Temperatures on Polyphenols and Flavonoids of Blueberry Juice during Cold Storage. Food Ferment. Ind. 2019, 45, 209–215. [Google Scholar]
- Lacombe, A.; Wu, V.C.H. The Effects of Sanitizing Hurdles against Foodborne Pathogens during Blueberry Processing. Food Control 2023, 154, 109981. [Google Scholar] [CrossRef]
- Tadepalli, S.; Bridges, D.F.; Anderson, R.; Zhang, R.; Wu, V.C.H. Synergistic Effect of Sequential Wash Treatment with Two Different Low-Dosage Antimicrobial Washes in Combination with Frozen Storage Increases Salmonella typhimurium and Listeria monocytogenes Reduction on Wild Blueberries. Food Control 2019, 102, 87–93. [Google Scholar] [CrossRef]
- Callahan, S.; Perry, J.J. Survival of Listeria innocua and Native Microflora in Sanitizer-Treated Wild Blueberries (Vaccinium angustifolium). Int. J. Fruit Sci. 2020, 20, S66–S81. [Google Scholar] [CrossRef]
- Lacombe, A.; Antosch, J.G.; Wu, V.C.H. Scale-up Model of Forced Air-integrated Gaseous Chlorine Dioxide for the Decontamination of Lowbush Blueberries. J. Food Saf. 2020, 40, e12793. [Google Scholar] [CrossRef]
- Ells, T.; Tregunno, N.; Fan, L.; Elliot, M.; Doucette, C.; Lyu, H.; Jollimore, A. Microbiological Analysis of Wild Lowbush Blueberries Harvested in Nova Scotia, Canada for the Fresh Produce Market. Microorganisms 2024, 12, 2251. [Google Scholar] [CrossRef] [PubMed]
- Balyan, S.; Dadwal, V.; Jha, D.K.; Patil, B.S. Innovative Food Safety Strategy: Eugenol Nanoemulsion with Lactobacillus Derived Post-Biotic Biopolymer for Biofilm Inhibition on Food and Contact Surfaces. Food Control 2025, 176, 111348. [Google Scholar] [CrossRef]
- Suresh, S.N.; SenthilKumar, P.; Pushparaj, C.; Sarangi, P.K.; Regina, V.R.; Subramani, R. Almond Gum-chitosan Nanocomposite as Edible Formulation for Advancing Postharvest Longevity of Fruits and Vegetables. Polym. Adv. Technol. 2024, 35, e6453. [Google Scholar] [CrossRef]
- Guohong, F.; Jindong, Z.; Yujie, Z.H.U.; Tiantian, W. Comprehensive Quality Evaluation of Blueberries Based on Near-Infrared Spectroscopy and Fiber Optic Droplet Analysis. Food Sci. 2024, 45, 216–224. [Google Scholar]
- Sharma, P.; Venugopal, A.P. Bruise Damage Susceptibility of Blueberry and Strawberry. In Mechanical Damage in Fresh Horticultural Produce: Measurement, Analysis and Control; Springer: Singapore, 2024; pp. 239–267. [Google Scholar]
- Jung, J.; Lin, C.; Zhao, Y. Enhancing Anthocyanin–Phenolic Copigmentation through Epicarp Layer Treatment and Edible Coatings to Retain Anthocyanins in Thermally Processed Whole Blueberries. J. Food Sci. 2022, 87, 3809–3821. [Google Scholar] [CrossRef]
- Ning, N.; Wang, X.; Li, J.; Bi, X.; Li, M.; Xing, Y.; Che, Z.; Wang, Y. Effects of Different Antioxidants Combined with High Hydrostatic Pressure on the Color and Anthocyanin Retention of a Blueberry Juice Blend during Storage. Food Sci. Technol. Int. 2023, 29, 518–528. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, F.; Chen, Y.; Ning, N.; Hao, G.; Bi, X. The Effect of High-Pressure Processing on the Copigmentation and Storage Stability of Polyphenols with Anthocyanin Monomers. Foods 2024, 13, 3756. [Google Scholar] [CrossRef]
- Guo, J.; Giusti, M.M.; Kaletunç, G. Encapsulation of Purple Corn and Blueberry Extracts in Alginate-Pectin Hydrogel Particles: Impact of Processing and Storage Parameters on Encapsulation Efficiency. Food Res. Int. 2018, 107, 414–422. [Google Scholar] [CrossRef]
- Correia, R.; Grace, M.H.; Esposito, D.; Lila, M.A. Wild Blueberry Polyphenol-Protein Food Ingredients Produced by Three Drying Methods: Comparative Physico-Chemical Properties, Phytochemical Content, and Stability during Storage. Food Chem. 2017, 235, 76–85. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Li, L.; Deng, W.; Liu, M.; Hu, J. Biodegradable Starch-Based Packaging Films Incorporated with Polyurethane-Encapsulated Essential-Oil Microcapsules for Sustained Food Preservation. Int. J. Biol. Macromol. 2023, 235, 123889. [Google Scholar] [CrossRef]
- Xie, C.; Wang, Q.; Ying, R.; Wang, Y.; Wang, Z.; Huang, M. Binding a Chondroitin Sulfate-Based Nanocomplex with Kappa-Carrageenan to Enhance the Stability of Anthocyanins. Food Hydrocoll. 2020, 100, 105448. [Google Scholar] [CrossRef]
- Howard, L.R.; Brownmiller, C.; Mauromoustakos, A.; Prior, R.L. Improved Stability of Blueberry Juice Anthocyanins by Acidification and Refrigeration. J. Berry Res. 2016, 6, 189–201. [Google Scholar] [CrossRef]
- Yao, G.-L.; Ma, X.-H.; Cao, X.-Y.; Chen, J. Effects of Power Ultrasound on Stability of Cyanidin-3-Glucoside Obtained from Blueberry. Molecules 2016, 21, 1564. [Google Scholar] [CrossRef] [PubMed]
- Shaoqian, C.A.O.; Kai, J.; Liang, L.I.U. Coupled Oxidative Degradation of Blueberry Anthocyanins Induced by Catechol. Sci. Technol. Food Ind. 2022, 43, 58–63. [Google Scholar]
- Waterhouse, G.I.N.; Sun-Waterhouse, D.; Su, G.; Zhao, H.; Zhao, M. Spray-Drying of Antioxidant-Rich Blueberry Waste Extracts; Interplay between Waste Pretreatments and Spray-Drying Process. Food Bioprocess Technol. 2017, 10, 1074–1092. [Google Scholar] [CrossRef]
- Calabuig-Jiménez, L.; Hinestroza-Córdoba, L.I.; Barrera, C.; Seguí, L.; Betoret, N. Effects of Processing and Storage Conditions on Functional Properties of Powdered Blueberry Pomace. Sustainability 2022, 14, 1839. [Google Scholar] [CrossRef]
- Khanal, R.C.; Howard, L.R.; Brownmiller, C.R.; Prior, R.L. Influence of Extrusion Processing on Procyanidin Composition and Total Anthocyanin Contents of Blueberry Pomace. J. Food Sci. 2009, 74, H52–H58. [Google Scholar] [CrossRef]
- Griep, P.; Ferreira, J.; Fischer, B.; Fernandes, I.A.; Cansian, R.L.; Junges, A.; Backes, G.T. Development and Characterization of Active Edible Film with Blueberry Residue Extract (Vaccinium spp.). Biomass Convers. Biorefinery 2024, 14, 22087–22097. [Google Scholar]
- Ikramov, R.; Nilova, L.; Malyutenkova, S. Implementation of a Differentiated Approach to the Use of Microwaves Processing in the Production of Gelatin Jellies. In Proceedings of the E3S Web of Conferences, Chelyabinsk, Russia, 17–19 February 2021; EDP Sciences: London, UK, 2021; Volume 258, p. 12014. [Google Scholar]
- Mu, L.; Bi, J.; Zhao, H.; Li, J.; Hou, H.-M.; Zhang, G.-L.; Hao, H.; Zhou, L. Intelligent PH-Responsive Films Based on Natural Blueberry Anthocyanins: A Non-Destructive Monitoring System for the Freshness of Aquatic Products with Prospective Smartphone Compatibility. Food Chem. X 2025, 28, 102587. [Google Scholar] [CrossRef]
- Bof, M.J.; Laurent, F.E.; Massolo, F.; Locaso, D.E.; Versino, F.; García, M.A. Bio-Packaging Material Impact on Blueberries Quality Attributes under Transport and Marketing Conditions. Polymers 2021, 13, 481. [Google Scholar] [CrossRef]
- Grillo, G.; Gunjević, V.; Radošević, K.; Redovniković, I.R.; Cravotto, G. Deep Eutectic Solvents and Nonconventional Technologies for Blueberry-Peel Extraction: Kinetics, Anthocyanin Stability, and Antiproliferative Activity. Antioxidants 2020, 9, 1069. [Google Scholar] [CrossRef]
- Singh, P.; Niknejad, N.; Spiers, J.D.; Bao, Y.; Ru, S. Development of a Smartphone Application for Rapid Blueberry Detection and Yield Estimation. Smart Agric. Technol. 2025, 12, 101361. [Google Scholar] [CrossRef]
- Zhang, R.; Dong, W.; Hou, P.; Li, H.; Han, X.; Chen, Q.; Li, F.; Zhang, X. YOLOv11-BSD: Blueberry Maturity Detection Under Simulated Nighttime Conditions Evaluated with Causal Analysis. Smart Agric. Technol. 2025, 12, 101314. [Google Scholar] [CrossRef]
- Zhai, Y.; Liang, Z.; Zhang, L.; Xu, S.; Huang, C.; Zhou, X.; Li, M.; Huang, Y. Machine Learning Based Blueberry Detection Method by CIE-YOLOv5. In Proceedings of the International Conference on Computer Graphics, Artificial Intelligence, and Data Processing (ICCAID 2023), Nanchang, China, 1–3 December 2023; SPIE: Bellingham, WA, USA, 2024; Volume 13105, pp. 462–467. [Google Scholar]
- Schumann, A.W.; Mood, N.S.; Mungofa, P.D.K.; MacEachern, C.; Zaman, Q.; Esau, T. Detection of Three Fruit Maturity Stages in Wild Blueberry Fields Using Deep Learning Artificial Neural Networks. In Proceedings of the 2019 ASABE Annual International Meeting, Boston, MA, USA, 7–10 July 2019; American Society of Agricultural and Biological Engineers: Saint Joseph, MI, USA, 2019; p. 1. [Google Scholar]
- Qu, H.; Zheng, C.; Ji, H.; Barai, K.; Zhang, Y.-J. A Fast and Efficient Approach to Estimate Wild Blueberry Yield Using Machine Learning with Drone Photography: Flight Altitude, Sampling Method and Model Effects. Comput. Electron. Agric. 2024, 216, 108543. [Google Scholar] [CrossRef]
- Leiva-Valenzuela, G.A.; Lu, R.; Aguilera, J.M. Assessment of Internal Quality of Blueberry Using Hyperspectral Imaging. In Proceedings of the ASABE Annual International Meeting, Dallas, TX, USA, 29 July–1 August 2012; American Society of Agricultural and Biological Engineers: Saint Joseph, MI, USA, 2012; p. 1. [Google Scholar]
- Li, R.; Fu, L. Nondestructive Measurement of Firmness and Sugar Content of Blueberries Based on Hyperspectral Imaging. Trans. Chinese Soc. Agric. Eng. 2017, 33, 362–366. [Google Scholar]
- Sinelli, N.; Spinardi, A.; Di Egidio, V.; Mignani, I.; Casiraghi, E. Evaluation of Quality and Nutraceutical Content of Blueberries (Vaccinium corymbosum L.) by near and Mid-Infrared Spectroscopy. Postharvest Biol. Technol. 2008, 50, 31–36. [Google Scholar] [CrossRef]
- Guidetti, R.; Beghi, R.; Bodria, L.; Spinardi, A.; Mignani, I.; Folini, L. Prediction of Blueberry (Vaccinium corymbosum) Ripeness by a Portable Vis-NIR Device. In Proceedings of the IX International Vaccinium Symposium, Corvallis, OR, USA, 14–18 July 2008; Volume 810, pp. 877–886. [Google Scholar]
- Cruz, J.O. Terahertz Time-Domain Spectroscopy (THz-TDS) for Classification of Blueberries According to Their Maturity. In Proceedings of the 2020 IEEE Engineering International Research Conference (EIRCON), Lima, Peru, 21–23 October 2020; IEEE: New York, NY, USA, 2020; pp. 1–4. [Google Scholar]
- Ni, X.; Takeda, F.; Jiang, H.; Yang, W.Q.; Saito, S.; Li, C. A Deep Learning-Based Web Application for Segmentation and Quantification of Blueberry Internal Bruising. Comput. Electron. Agric. 2022, 201, 107200. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, C.; Takeda, F. Nondestructive Detection and Quantification of Blueberry Bruising Using Near-Infrared (NIR) Hyperspectral Reflectance Imaging. Sci. Rep. 2016, 6, 35679. [Google Scholar] [CrossRef]
- Kuzy, J.D.; Li, C. Blueberry Bruise Detection by Pulse-Phase Thermography and Neural Network. In Proceedings of the 2015 ASABE Annual International Meeting, New Orleans, LA, USA, 26–29 July 2015; American Society of Agricultural and Biological Engineers: Saint Joseph, MI, USA, 2015; p. 1. [Google Scholar]
- da Silva Vieira, G.; Rocha, B.M.; Fonseca, A.U.; de Sousa, N.M.; Ferreira, J.C.; Cabacinha, C.D.; Soares, F. Automatic Detection of Insect Predation through the Segmentation of Damaged Leaves. Smart Agric. Technol. 2022, 2, 100056. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Lakhan, A.; Abdulkareem, K.H.; Almujally, N.A.; Al-Attar, B.B.S.M.T.; Memon, S.; Marhoon, H.A.; Martinek, R. Edge-Cloud Remote Sensing Data-Based Plant Disease Detection Using Deep Neural Networks with Transfer Learning. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2024, 17, 11219–11229. [Google Scholar] [CrossRef]
- Bogodukhova, E.S.; Britvina, V.V.; Mukhanova, A.A.; Gelmiyarova, V.N.; Agostinho, A.C. Creating a Digital Platform with a Deep Neural Network for Detecting Plant Diseases Using Information Technology. BIO Web Conf. 2023, 71, 1117. [Google Scholar] [CrossRef]
- Kushwaha, A.; Rajesh, E. Detecting Malign in Leaves Using Deep Learning Algorithm Model ResNet for Smart Framing. In Artificial Intelligence, Blockchain, Computing and Security Volume 1; CRC Press: Boca Raton, FL, USA, 2023; pp. 407–411. ISBN 1003393586. [Google Scholar]
- Yu, B.; Zhao, H.; Zhang, X. BlueberryNet: A Lightweight CNN for Real-Time Ripeness Detection in Automated Blueberry Processing Systems. Processes 2025, 13, 2518. [Google Scholar] [CrossRef]
- Sullca, C.; Molina, C.; Rodríguez, C.; Fernández, T. Diseases Detection in Blueberry Leaves Using Computer Vision and Machine Learning Techniques. Int. J. Mach. Learn. Comput. 2019, 9, 656–661. [Google Scholar] [CrossRef]
- Jian, C.; Bingbing, W.; Long, Y.I.N.; Yanqing, L.I.; Zhaoxin, L.I.; Zhuang, L.I. The Lightweight Bee Pollination Recognition Model Based On YOLOv10n-CHL. Smart Agric. 2025, 7, 185–198. [Google Scholar]
- Huang, Y.; Wang, D.; Liu, Y.; Zhou, H.; Sun, Y. Measurement of Early Disease Blueberries Based on Vis/Nir Hyperspectral Imaging System. Sensors 2020, 20, 5783. [Google Scholar] [CrossRef] [PubMed]
- Zaman, Q.U.; Schumann, A.W.; Percival, D.C.; Gordon, R.J. Estimation of Wild Blueberry Fruit Yield Using Digital Color Photography. Trans. ASABE 2008, 51, 1539–1544. [Google Scholar] [CrossRef]
- Chang, Y.K.; Zaman, Q.; Farooque, A.A.; Schumann, A.W.; Percival, D.C. An Automated Yield Monitoring System II for Commercial Wild Blueberry Double-Head Harvester. Comput. Electron. Agric. 2012, 81, 97–103. [Google Scholar] [CrossRef]
- Panda, S.S.; Hoogenboom, G.; Paz, J.O. Remote Sensing and Geospatial Technological Applications for Site-Specific Management of Fruit and Nut Crops: A Review. Remote Sens. 2010, 2, 1973–1997. [Google Scholar]
- Reich, R.M.; Lojewski, N.; Lundquist, J.E.; Bravo, V.A. Predicting Abundance and Productivity of Blueberry Plants under Insect Defoliation in Alaska. J. Sustain. For. 2018, 37, 525–536. [Google Scholar] [CrossRef]
- Esau, T.; Zaman, Q.; Groulx, D.; Corscadden, K.; Chang, Y.; Schumann, A.; Havard, P. Economic Analysis for Smart Sprayer Application in Wild Blueberry Fields. In Proceedings of the 2015 ASABE Annual International Meeting, New Orleans, LA, USA, 26–29 July 2015; American Society of Agricultural and Biological Engineers: Saint Joseph, MI, USA, 2015; p. 1. [Google Scholar]
- Esau, T.; Zaman, Q.; Groulx, D.; Chang, Y.; Schumann, A.; Havard, P. Smart Sprayer for Spot-Application of Agrochemicals in Wild Blueberry Fields. In Proceedings of the 2014 ASABE Annual International Meeting, Montreal, QC, Canada, 13–16 July 2014; American Society of Agricultural and Biological Engineers: Saint Joseph, MI, USA, 2014; p. 1. [Google Scholar]
- Chang, Y.K.; Zaman, Q.U.; Chattha, H.; Read, S.; Schumann, A.W. Sensing System Using Digital Cameras for Spot-Application of Fertilizer in Wild Blueberry Fields. In Proceedings of the 2014 ASABE Annual International Meeting, Montreal, QC, Canada, 13–16 July 2014; American Society of Agricultural and Biological Engineers: Saint Joseph, MI, USA, 2014; p. 1. [Google Scholar]
- Chattha, H.S.; Zaman, Q.U.; Schumann, A.W.; Brewster, G.R.; Chang, Y.K.; Read, S. Evaluation of Modified Variable Rate Granular Fertilizer Spreader for Spot-Specific Fertilization in Wild Blueberry Fields. In Proceedings of the 2013 ASABE Annual International Meeting, Kansas, MO, USA, 21–24 July 2013; American Society of Agricultural and Biological Engineers: Saint Joseph, MI, USA, 2013; p. 1. [Google Scholar]
- Percival, D.C.; Gallant, D.; Harrington, T.; Brown, G. Potential for Commercial Unmanned Aerial Vehicle Use in Wild Blueberry Production. In Proceedings of the XI International Vaccinium Symposium, Orlando, FL, USA, 10–14 April 2016; Volume 1180, pp. 233–240. [Google Scholar]
- Bracamonte, D.; Chang, A.; Vinces, L.; Oliden, J. A Blueberry Classification Algorithm Using Convolutional Neural Networks Developed in Python and a Raspberry Pi 4. In Proceedings of the 2022 Congreso Internacional de Innovación y Tendencias en Ingeniería (CONIITI), Bogota, Colombia, 5–7 October 2022; IEEE: New York, NY, USA, 2022; pp. 1–5. [Google Scholar]
- Leiva-Valenzuela, G.A.; Aguilera, J.M. Automatic Detection of Orientation and Diseases in Blueberries Using Image Analysis to Improve Their Postharvest Storage Quality. Food Control 2013, 33, 166–173. [Google Scholar] [CrossRef]
- Villagrán, E.; Romero-Perdomo, F.; Numa-Vergel, S.; Galindo-Pacheco, J.R.; Salinas-Velandia, D.A. Life Cycle Assessment in Protected Agriculture: Where Are We Now, and Where Should We Go Next? Horticulturae 2024, 10, 15. [Google Scholar]
- Girgenti, V.; Peano, C.; Bounous, M.; Baudino, C. A Life Cycle Assessment of Non-Renewable Energy Use and Greenhouse Gas Emissions Associated with Blueberry and Raspberry Production in Northern Italy. Sci. Total Environ. 2013, 458, 414–418. [Google Scholar]
- Cordes, H.; Iriarte, A.; Villalobos, P. Evaluating the Carbon Footprint of Chilean Organic Blueberry Production. Int. J. Life Cycle Assess. 2016, 21, 281–292. [Google Scholar] [CrossRef]
- Chapa, J.; Kipp, S.; Cai, H.; Huang, J.-Y. A Comparative Life Cycle Assessment of Fresh Imported and Frozen Domestic Organic Blueberries Consumed in Indiana. J. Clean. Prod. 2019, 217, 716–723. [Google Scholar] [CrossRef]
- Pérez, R.; Laca, A.; Laca, A.; Díaz, M. Environmental Behaviour of Blueberry Production at Small-Scale in Northern Spain and Improvement Opportunities. J. Clean. Prod. 2022, 339, 130594. [Google Scholar] [CrossRef]
- Trejo-Pech, C.O.; Rodríguez-Magaña, A.; Briseño-Ramírez, H.; Ahumada, R. A Monte Carlo Simulation Case Study on Blueberries from Mexico. Int. Food Agribus. Manag. Rev. 2024, 27, 359–377. [Google Scholar] [CrossRef]
- Mari, A.; Kekes, T.; Boukouvalas, C.; Krokida, M. Integrating Life Cycle Assessment in Innovative Berry Processing with Edible Coating and Osmotic Dehydration. Foods 2025, 14, 1167. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.B.N.; de Oliveira, G.M.; Gallo Ruelas, M.; Gadelha, M.S.M.; de Farias Santos, A.C.F.; Zamora, F.V. Blueberries for Brainpower: A Systematic Review and Meta-Analysis with Bayesian Post Hoc Analysis of RCTS Exploring Cognitive Function in the Elderly with Prior Cognitive Decline. Biogerontology 2025, 26, 1–15. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar]
- Kalt, W. Vaccinium Berry Crops and Human Health. In Proceedings of the VIII International Symposium on Vaccinium Culture, Sevilla, Spain, 3–8 May 2004; Volume 715, pp. 533–538. [Google Scholar]
- Williams, R.J.; Spencer, J.P.E. Flavonoids, Cognition, and Dementia: Actions, Mechanisms, and Potential Therapeutic Utility for Alzheimer Disease. Free Radic. Biol. Med. 2012, 52, 35–45. [Google Scholar] [PubMed]
- Khoo, H.E.; Chew, L.; Ismail, A.; Azlan, A. Polyphenols: Chemistry, Dietary Sources and Health Benefits; Nova Science Publisher: Hauppauge, NY, USA, 2012. [Google Scholar]
- Routray, W.; Orsat, V. Blueberries and Their Anthocyanins: Factors Affecting Biosynthesis and Properties. Compr. Rev. Food Sci. Food Saf. 2011, 10, 303–320. [Google Scholar] [CrossRef]
- Zhao, F.; Fan, S.; Zhao, H.; Liu, H.; Lyu, L.; Wu, W.; Wu, Y.; Li, W. A Comparison of Fruit Quality of Ten High-Anthocyanin Blueberry Cultivars. J. Nanjing For. Univ. 2025, 49, 63. [Google Scholar]
- Wu, W.L.; Zhao, H.F.; Fang, L.; Lv, L.F.; Li, W.L. Nutritional Components in the Fruit of Different Blueberry Cultivars in Nanjing. Appl. Mech. Mater. 2012, 140, 374–378. [Google Scholar]
- Liović, N.; Čikeš-Čulić, V.; Fredotović, Ž.; Krešić, G.; Bilušić, T. The Effect of Processing Techniques on the Antiproliferative Activity of Blueberry Phenolics before and after in Vitro Digestion. J. Food Process. Preserv. 2022, 46, e16140. [Google Scholar]
- Tan, C.; Wang, M.; Kong, Y.; Wan, M.; Deng, H.; Tong, Y.; Lyu, C.; Meng, X. Anti-Inflammatory and Intestinal Microbiota Modulation Properties of High Hydrostatic Pressure Treated Cyanidin-3-Glucoside and Blueberry Pectin Complexes on Dextran Sodium Sulfate-Induced Ulcerative Colitis Mice. Food Funct. 2022, 13, 4384–4398. [Google Scholar]
- Leng, Y.; Yang, L.; Zhu, H.; Li, D.; Pan, S.; Yuan, F. Stability of Blueberry Extracellular Vesicles and Their Gene Regulation Effects in Intestinal Caco-2 Cells. Biomolecules 2023, 13, 1412. [Google Scholar] [CrossRef]
- Langer, S.; Kennel, A.; Lodge, J.K. The Influence of Juicing on the Appearance of Blueberry Metabolites 2 h after Consumption: A Metabolite Profiling Approach. Br. J. Nutr. 2018, 119, 1233–1244. [Google Scholar] [CrossRef]
- Wang, Y.; Gallegos, J.L.; Haskell-Ramsay, C.; Lodge, J.K. Effects of Chronic Consumption of Specific Fruit (Berries, Citrus and Cherries) on CVD Risk Factors: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Eur. J. Nutr. 2021, 60, 615–639. [Google Scholar] [CrossRef] [PubMed]
- Janowska, S.; Wujec, M. Bilberries as Valuable Sources of Polyphenols with Health-Promoting Properties. Farm. Pol. 2023, 79, 403–412. [Google Scholar] [CrossRef]
- Kunrade, L.; Rembergs, R.; Jēkabsons, K.; Kļaviņš, L.; Kļaviņš, M.; Muceniece, R.; Riekstiņa, U. Inhibition of NF-κ B Pathway in LPS-Stimulated THP-1 Monocytes and COX-2 Activity in Vitro by Berry Pomace Extracts from Five Vaccinium Species. J. Berry Res. 2020, 10, 381–396. [Google Scholar] [CrossRef]
- Toporkova, V.I.; Vishnyakov, E.V.; Sidorov, K.O.; Terninko, I.I.; Ivkin, D.Y. Assessment of the Influence of the Mineral Complex of Rutin on the Degree of Expression of Anti-Diabetic Activity. Drug Dev. Regist. 2021, 10, 197–205. [Google Scholar] [CrossRef]
- Takahashi, S.; Che, J.; Horiuchi, N.; Cho, H.Y.; Onwona-Agyeman, S.; Kojima, K.; Yamada, M.; Ogiwara, I. Production of Low-Potassium Fruit of Potted and Fertigated Southern Highbush Blueberry (Vaccinium corymbosum L. Interspecific Hybrid). Hortic. J. 2021, 90, 161–171. [Google Scholar] [CrossRef]
- Das, Q.; Islam, M.R.; Marcone, M.F.; Warriner, K.; Diarra, M.S. Potential of Berry Extracts to Control Foodborne Pathogens. Food Control 2017, 73, 650–662. [Google Scholar] [CrossRef]
- Vattem, D.A.; Lin, Y.-T.; Ghaedian, R.; Shetty, K. Cranberry Synergies for Dietary Management of Helicobacter pylori Infections. Process Biochem. 2005, 40, 1583–1592. [Google Scholar] [CrossRef]
- Seo, J.Y.; Jang, J.H.; Kim, J.-S.; Kim, E.-J.; Kim, J.-S. Development of Low-Sugar Antioxidant Jam by a Combination of Anthocyanin-Rich Berries. Appl. Biol. Chem. 2016, 59, 305–312. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Baduge, S.A. The Contribution of Phytochemicals to the Antioxidant Potential of Fruit Juices. In Fruit Juices; Elsevier: Amsterdam, The Netherlands, 2018; pp. 95–128. [Google Scholar]
- Aksoylu, Z.; Çağindi, Ö.; Köse, E. Effects of Blueberry, Grape Seed Powder and Poppy Seed Incorporation on Physicochemical and Sensory Properties of Biscuit. J. Food Qual. 2015, 38, 164–174. [Google Scholar] [CrossRef]
- Girard, K.K.; Sinha, N.K. Cranberry, Blueberry, Currant, and Gooseberry. In Handbook of Fruits and Fruit Processing; Wiley Online Library: Hoboken, NJ, USA, 2006; pp. 369–390. [Google Scholar]
- Villagran, E.; Toro-Tobón, G.; Velázquez, F.A.; Estrada-Bonilla, G.A. Integration of IoT Technologies and High-Performance Phenotyping for Climate Control in Greenhouses and Mitigation of Water Deficit: A Study of High-Andean Oat. AgriEngineering 2024, 6, 4011–4040. [Google Scholar] [CrossRef]
- Pereira, M.; Mota, M.; Oliveira, P.B. Extending Blueberry Production Season with Different Covering Materials. In Proceedings of the XII International Vaccinium Symposium, Debert, NS, Canada, 30 August–1 September 2021; Volume 1357, pp. 69–78. [Google Scholar]
- Calderón-Orellana, A.; Hermosilla, N.; Bastías, R.M. Impact of the Covering Material on Drought Tolerance Responses and Soil Water Content in Two Cultivars of Young Blueberry Plants under Protected Cultivation. Water 2023, 15, 2326. [Google Scholar] [CrossRef]
- Couture, A.; Gaudreau, L.; Van Sterthem, A.; Gosselin, A.; Dubé, Y.; Nguyen, T.T.A.; Brégard, A.; Dorais, M. How Can High Tunnel Coverings and an Insect-Proof Barrier Improve Productivity and Pest Management in Berry Crops? In Proceedings of the IV International Symposium on Organic Greenhouse Horticulture, Cancun, Mexico, 22–27 October 2023; Volume 1428, pp. 101–108. [Google Scholar]
- Lihong, X.; Huihui, L.; He, X.; Ruihua, W.E.I.; Wentao, C.A.I. Multi-Factor Coordination Control Technology of Promoting Early Maturing in Southern Blueberry Intelligent Greenhouse. Smart Agric. 2021, 3, 86–98. [Google Scholar]
- Smrke, T.; Veberic, R.; Hudina, M.; Jakopic, J. Pot and Ridge Production of Three Highbush Blueberry (Vaccinium corymbosum L.) Cultivars under High Tunnels. Agriculture 2022, 12, 438. [Google Scholar] [CrossRef]
- Nichols, M. Recent Changes in Berry Fruit Production in New Zealand. In Proceedings of the XI International Symposium on Protected Cultivation in Mild Winter Climates and I International Symposium on Nettings and Screens in Horticulture, Tenerife, Spain, 27–31 January 2019; Volume 1268, pp. 285–288. [Google Scholar]
- Li, Y.; Sun, H.; Chen, L. The Blueberry Industry of China: The Past 10 Years and the Future. In Proceedings of the XI International Vaccinium Symposium, Orlando, FL, USA, 10–14 April 2016; Volume 1180, pp. 531–536. [Google Scholar]
- Li, T.; Bi, G. Container Production of Southern Highbush Blueberries Using High Tunnels. HortScience 2019, 54, 267–274. [Google Scholar] [CrossRef]
- Retamal-Salgado, J.; Bastías, R.M.; Wilckens, R.; Paulino, L. Influence of Microclimatic Conditions under High Tunnels on the Physiological and Productive Responses in Blueberry ‘O’Neal’. Chil. J. Agric. Res. 2015, 75, 291–297. [Google Scholar] [CrossRef]
- Santos, B.M.; Salame-Donoso, T.P. Performance of Southern Highbush Blueberry Cultivars under High Tunnels in Florida. Horttechnology 2012, 22, 700–704. [Google Scholar]
- Ciordia, M.; Garcia, J.C.; Diaz, M.B. Off-Season Production of Southern Highbush Blueberries in the North of Spain. Acta Hortic. 2006, 715, 317–322. [Google Scholar] [CrossRef]
- Ozeki, M.; Tamada, T. The Potentials of Forcing Culture of Southern Highbush Blueberry in Japan. In Proceedings of the VIII International Symposium on Vaccinium Culture, Sevilla, Spain, 3–8 May 2004; Volume 715, pp. 241–246. [Google Scholar]
- Hicklenton, P.; Forney, C.; Domytrak, C. Use of Row Covers and Post Harvest Storage Techniques to Alter Maturity and Marketing Period for Highbush Blueberries. In Proceedings of the XXVI International Horticultural Congress: Berry Crop Breeding, Production and Utilization for a New Century, Toronto, ON, Canada, 11–17 August 2002; Volume 626, pp. 287–295. [Google Scholar]
- Demchak, K. Small Fruit Production in High Tunnels. Horttechnology 2009, 19, 44–49. [Google Scholar] [CrossRef]
- Ogden, A.B.; van Iersel, M.W. Southern Highbush Blueberry Production in High Tunnels: Temperatures, Development, Yield, and Fruit Quality during the Establishment Years. HortScience 2009, 44, 1850–1856. [Google Scholar] [CrossRef]
- Yeh, D.A.; Kramer, J.; Calvin, L.; Weber, C. The Changing Landscape of US Strawberry and Blueberry Markets: Production, Trade, and Challenges from 2000 to 2020; Bulletin Number 257; United States Department of Agriculture (USDA): Washington, DC, USA, 2023.
- Canales, E.; Gallardo, R.K.; Iorizzo, M.; Munoz, P.; Ferrao, L.F.; Luby, C.; Bassil, N.; Pottorff, M.; Perkins-Veazie, P.; Sandefur, P.; et al. Willingness to Pay for Blueberries: Sensory Attributes, Fruit Quality Traits, and Consumers’ Characteristics. HortScience 2024, 59, 1207–1218. [Google Scholar] [CrossRef]
- Brazelton, C.; Fain, C.; Ogg, M.; Riquelme, C.; Rodriguez, V.; Ilyas, S. Global State of the Blueberry Industry Report; International Blueberry Organization: Santiago, Chile, 2025. [Google Scholar]
- Pazos, N.; Janzen, J. Peru’s Blueberry Boom: More Land, More Labor, More Berries to Savor. Farmdoc Dly. 2025, 15, 1. [Google Scholar]
- Montes Ninaquispe, J.C.; Arbulú Ballesteros, M.A.; Cruz Salinas, L.E.; García Juárez, H.D.; Farfán Chilicaus, G.C.; Martel Acosta, R.; Guzmán Valle, M.d.l.Á.; Coronel Estela, C.V. A Strategy for the Sustainability of Peru’s Blueberry Exports: Diversification and Competitiveness. Sustainability 2024, 16, 6606. [Google Scholar] [CrossRef]
- Singh, R.; Singh, R.; Gehlot, A.; Akram, S.V.; Priyadarshi, N.; Twala, B. Horticulture 4.0: Adoption of Industry 4.0 Technologies in Horticulture for Meeting Sustainable Farming. Appl. Sci. 2022, 12, 12557. [Google Scholar] [CrossRef]
- Cao, S.; Qiao, L.; Wang, X.; Huang, T.; Wu, C.; Zhang, Y.; Xue, Z.; Kou, X. Discussion on Postharvest Quality, Strategies of Storage and Transportation Techniques of Blueberries: A Comprehensive Review. Food Qual. Saf. 2025, 9, fyaf038. [Google Scholar] [CrossRef]
- Chan, C.; Nelson, P.R.; Hayes, D.J.; Zhang, Y.-J.; Hall, B. Predicting Water Stress in Wild Blueberry Fields Using Airborne Visible and near Infrared Imaging Spectroscopy. Remote Sens. 2021, 13, 1425. [Google Scholar]
- Leguízamo, J.A.C. Fundamentos Técnicos Del Cultivo Del Arándano (Vaccinium corymbosum L.) En La Región Central de Colombia; Editorial de la Universidad Pedagógica y Tecnológica de Colombia—UPTC: Tunja, Colombia, 2021; Volume 43, ISBN 958660490X. [Google Scholar]
- Macha Huamán, R.; Navarro Soto, F.C.; Ramírez Ríos, A.; Alfaro Paredes, E.A. International Market Concentration of Fresh Blueberries in the Period 2001–2020. Humanit. Soc. Sci. Commun. 2023, 10, 967. [Google Scholar]
- Pinzon, D.A.; Amado, G.M.; Rodriguez, J.; Villagran, E. Global Research Trends and Thematic Evolution of Blueberry (Vaccinium spp.) Science: A Bibliometric Analysis. Horticulturae 2025, 11, 1501. [Google Scholar] [CrossRef]
- Qessaoui, R.; Chafiki, S.; Alouani, M.; Bahoch, S.; Ajerrar, A.; Tahiri, A.; Ait Aabd, N.; Wifaya, A.; Bouharroud, R. Système de Production de Fruits Rouges Dans La Région de Souss-Massa Au Maroc. Afr. Mediterr. Agric. J.-Al Awamia 2024, 145, 211–226. [Google Scholar]
- Jiang, J.; Wei, J.; Yu, H.; He, S. The Developing Blueberry Industry in China. In Modern Fruit Industry; IntechOpen: London, UK, 2019; ISBN 1789847311. [Google Scholar]
- Yu, H.; Wei, J.; Yang, S.; Yang, X.; He, S. The Development Situation and Prospect of Blueberry Industry in China. In Proceedings of the XII International Vaccinium Symposium, Debert, NS, Canada, 30 August–1 September 2021; Volume 1357, pp. 325–328. [Google Scholar]
- Ding, Y.; Ren, X.; Lv, Y. Recent Advancements in Postharvest Fruit Quality and Physiological Mechanism; MDPI-Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2024; ISBN 3725824258. [Google Scholar]
- Villagrán, E.A.; Baeza Romero, E.J.; Bojacá, C.R. Transient CFD Analysis of the Natural Ventilation of Three Types of Greenhouses Used for Agricultural Production in a Tropical Mountain Climate. Biosyst. Eng. 2019, 188, 288–304. [Google Scholar] [CrossRef]
- Rocha, G.A.O.; Pichimata, M.A.; Villagran, E. Research on the Microclimate of Protected Agriculture Structures Using Numerical Simulation Tools: A Technical and Bibliometric Analysis as a Contribution to the Sustainability of Under-Cover Cropping in Tropical and Subtropical Countries. Sustainability 2021, 13, 10433. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Mujumdar, A.S.; Yu, D. Advanced Detection Techniques Using Artificial Intelligence in Processing of Berries. Food Eng. Rev. 2022, 14, 176–199. [Google Scholar]
- Bustos, J.F.C.; Preciado, J.C. Phenological States Classification of Blueberries Using Artificial Intelligence. In Proceedings of the 2025 IEEE Conference on Artificial Intelligence (CAI), Santa Clara, CA, USA, 5–7 May 2025; IEEE: New York, NY, USA, 2025; pp. 390–393. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
