Abstract
Drought, high temperature, salinity, waterlogging, and nutrient deficiency, along with metal toxicity, are among the environmental factors that have resulted in much alteration of many ecosystems by climate change. Such stresses have dramatically lowered the global average human harvest of core crops, which, in turn, has driven an overall decrease in worldwide agricultural productivity. Plants have developed a variety of defense strategies against biotic and abiotic stress. Evidence of the successful roles of phytohormone-like neurotransmitters in ameliorating the response to stress has already been established. One neurotransmitter accumulated by the plants is gamma-aminobutyric acid (GABA), a non-protein amino acid that is essential for signaling in plant growth regulation and development via the control of physiological and biochemical processes. Plant tissues demonstrate rapid accumulation of GABA when exposed to various abiotic stresses. Consequently, it is imperative to understand how this accumulation affects the resistance and productivity of crops in challenging environmental conditions. Previously, different application methods and doses of GABA on different plant species were used under various abiotic stress conditions. The research findings exhibited that the method and concentration of GABA depend on the type of crop. Furthermore, the GABA dose depends on the methods of GABA application. The present review summarizes the potential doses and methods of applications of GABA under different abiotic stress conditions to ameliorate deficiencies in plant growth, yield, and stress tolerance through the avoidance of oxidative damage and maintenance of cell organelle structures. This review will also describe the complex mechanism by which GABA contributes to the attenuation of the effects of abiotic stresses by regulating some important physiological, molecular, and biochemical processes in crops.
1. Introduction
Global agricultural production is significantly reduced by abiotic stress conditions such as drought, salinity, extreme temperatures, and nutrient deficiencies [1,2,3]. Before the pandemic, the Food and Agriculture Organization (FAO) claimed that 135 million individuals faced severe food shortages. The situation has worsened, creating a “crisis within a crisis”. According to the Global Nutrition Report in 2020, several nations experience a significant prevalence of stunted growth in children as a result of inadequate nutrition. In order to address these difficulties, it is essential to build a regulatory framework that encourages sustainable production and consumption practices [4,5].
Food-crop-based production methods have the potential to make a substantial contribution in this context by providing many services that promote sustainability principles [6]. Environmental factors such as heat, cold, salt, drought, and heavy metals can damage plants and reduce their growth, yield, and quality [7]. With ongoing changes in the Earth’s climate, the factors influencing plant growth and survival are becoming increasingly intricate. Plants have many defenses against both living and non-living stresses, including drought, salt, and high and low temperatures. Recent discoveries highlight the effectiveness of phytohormone-like substances such as polyamines (PAs), sugars, neurotransmitters (NTs), and strigolactones in mitigating both biotic and abiotic stresses in plants [8,9]. Neuroregulatory chemicals produced by plants are crucial for organ development in flowering, photosynthesis, reproduction, and environmental adaptation in plants [10]. Common NTs such as melatonin, histamine, GABA, acetylcholine (ACh), and glutamate are found in various species [11,12]. NTs play an important role in plants and crops, affecting many biologically occurring processes, such as root and shoot growth, fruit ripening, cell aging, seed germination and pollen, embryo development, protection of germ tissues, and ion flow control [13,14].
GABA is an amino acid with a four-carbon structure that occurs in mammals, plants, and microorganisms [15]. Integrating GABA into climate change adaptation strategies aligns with the assumption of sustainability in agricultural work by promoting efficient usability in resources, minimizing environmental impacts, and enhancing resilience [16]. This review provides a comprehensive overview of GABA biosynthesis, function, and its application methods under abiotic stress. This model systematically integrates experimental data from several crop species under various stress conditions to characterize the GABA function in enhancing crop resilience. Studies investigating various GABA application methods and the optimization of GABA concentrations to improve yields under abiotic stress conditions were analyzed to allow meaningful comparisons between different crops. The primary objectives of this review were 1. to understand the metabolic biosynthesis pathway of GABA; 2. to investigate GABA function under abiotic stress; 3. to identify the ways in which GABA interacts with chemical compounds to promote tolerance; and 4. to identify the relationship between the methods and doses of GABA application in different plant species.
2. Metabolic Pathway for GABA Biosynthesis
The synthesis of GABA has two pathways, as shown in Figure 1. GABA’s synthesis pathway, the GABA shunt, is the metabolic pathway of GABA synthesis. This path of synthesis in plants involves the tricarboxylic acid (TCA) cycle. GABA is supplied through a feedback loop called the GABA shunt, which is both produced and sustained. Glutamate is produced when glutamate dehydrogenase aminates α-ketoglutarate. Glutamic acid decarboxylase (GAD) then converts the glutamate into GABA and carbon dioxide (CO2). The mitochondrial enzyme GABA transaminase (GABA-T) reversibly transaminates GABA to succinic semialdehyde, utilizing pyruvate or α-ketoglutarate as amino group acceptors [17,18,19]. The second pathway to synthesize GABA is through metabolite remodeling like proline and polyamine. The proline converts to GABA via a non-enzymatic pathway, while polyamine converts to GABA by pyrroline decarboxylase [20,21,22].
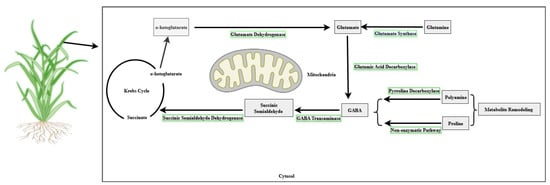
Figure 1.
A Schematic representation of the pathways of biosynthesis of GABA in plants. The Figure demonstrates the two ways of GABA production. Source: authors’ own compilation, unpublished (created with BioRender.com).
3. GABA Functions Under Stress Conditions
In plants, GABA is a signaling molecule that is essential for growth, development, physiological process, and stress response. This section of the review describes the main functions of GABA:
3.1. Regulation of Cytosolic pH
The first role of GABA is to control the pH of the cytosol in the plant cells, particularly under stressful conditions (Figure 2). Through the GABA shunt pathway, GABA synthesis is quickly triggered when plant cells’ cytosolic pH drops (becomes more acidic) [18]. GAD plays an important role in lessening acidity by utilizing protons (H+) in the conversion of glutamate into GABA and regulating cytosolic pH. Thus, the generation of GABA must be vital for controlling intracellular pH. This buffering ability aids in preventing acidification-induced damage to plant cells [23,24]. According to experimental data, GABA levels rise in response to abrupt drops in cytosolic pH, such as those that occur under hypoxic conditions or after the addition of weak acids [25]. It also aids in stress adaptation during flooding, salinity, and heavy metal exposure and improved the quality of apple fruit by enhancing drought tolerance when exogenously applied in a dose of 1 mM [26]. A small amount of GABA at a high concentration will lower the pH to about 7.0 when a concentration of nutrients with buffer capacity such as ammonium (NH4+), dihydrogen phosphate (H2PO4−), boric acid (H3BO3), bicarbonate (HCO3−), and carbonate (CO32−) is added to see how the pH value changes [27]. This result was also demonstrated in a research study performed by Xu et al. [28]. According to studies, plants exhibit the maximum levels of GAD activity in the acidic pH range, with a pH of 5.8 being ideal for GAD activity. When cell pH rises above the typical physiological pH, GAD binds to intracellular Ca2+/CaM to control enzyme activity. This method reduces the amount of damage to plant cells during hypoxic stress by activating plant GAD, which leads to increased H+ consumption and GABA production [29,30]. When weak acid with cell membrane permeability was applied to asparagus cells, real-time monitoring of GABA concentration and pH revealed that GABA content rose in tandem with a drop in intracellular pH. Furthermore, 50% of the H+ present in the weak acid was used during the GABA production process after 45 s of treatment. This suggests that GAD is activated and GABA production is increased when the intracellular pH falls. A portion of the H+ is used up in the production of GABA [31]. In a similar vein, the synthesis of GABA can prevent the intracellular acidification process in plant cells, and the change in intracellular pH in carrot cells and the change in GABA content during ammonium assimilation are mutually regulated [32].
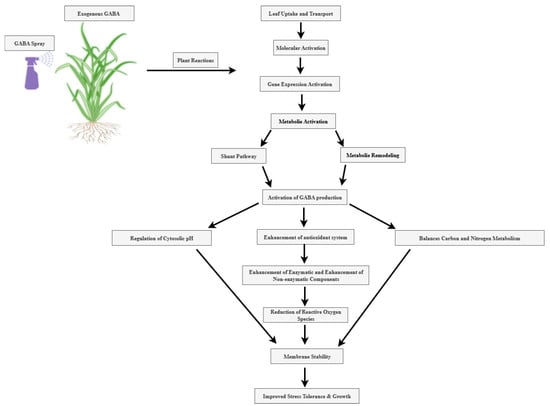
Figure 2.
Summary of molecular and biochemical functions of GABA-mediated stress tolerance in crops. The Figure shows ways for boosting stress tolerance and growth in plants via GABA application. Source: authors’ own compilation, unpublished (created with BioRender.com).
3.2. Scavenging Reactive Oxygen Species
GABA plays an important role in scavenging reactive oxygen species (ROS) in plants, particularly under stress conditions, such as heavy metal toxicity, salinity, drought, and high temperatures (Figure 2). It improves plant photosynthesis and activates enzymes that protect against oxidative stress under stressed conditions. This leads to a reduction in malondialdehyde (MDA) levels, a marker of oxidative injury, and lowered levels synthesis of ROS [33]. Studies indicated that GABA triggers plant growth and reduces stress by boosting their antioxidant defenses [34]. The GABA-induced activity of enzymes in the GABA shunt, such as GABA transaminase and succinic semialdehyde dehydrogenase, is associated with improved ROS detoxification and redox balance in the plant cells [35]. GABA can directly contribute to ROS scavenging by upregulating key antioxidant enzymes, including peroxidase (POD), catalase (CAT), and superoxide dismutase (SOD) [36]. This cooperative relationship enhances maize resistance to drought and salinity, highlighting GABA’s integrative role in antioxidative defense and stress resilience [37]. Exogenous GABA application on tomato plants improves photosynthetic performance and activates protective antioxidant enzymes under chilling stress. Consequently, it reduces malondialdehyde (MDA), a marker of oxidative damage, and lowers the synthesis/accumulation of ROS [38], which is consistent with the findings of Abd Elbar et al. [33]. Under salt stress, GABA-treated barley exhibited reduced hydrogen peroxide accumulation and a better-balanced redox state relative to controls, indicating enhanced antioxidative capacity and stress tolerance controls [39]. In rice, GABA reduces oxidative damage in the shoots under heat-stress conditions [40]. These results illustrate that GABA’s stress-mitigating function is mediated through its interactions with critical signaling molecules and enzymes, notably GAD and ROS-scavenging enzymes.
3.3. Balancing of Carbon and Nitrogen Metabolism
By its participation in the GABA shunt pathway, which links the carbon and nitrogen metabolic pathways and integrates with the TCA cycle, GABA plays a crucial role in maintaining a balance between carbon and nitrogen metabolism in plants (Figure 2). Carbon skeletons for energy metabolism are produced by synthesizing succinate, a TCA cycle intermediate, from glutamate, an amino acid that is rich in nitrogen. Particularly in stressful situations like drought, this delicate balancing act enables plants to efficiently redistribute carbon and nitrogen resources [22,41].
Research works have proven that exogenous GABA administration increases root development and nutrient uptake efficiency under stressful situations [42]. This enhancement is fundamental for sustaining crop production in the face of unfavorable environmental circumstances [43]. GABA functions as a compatible solute and aids in osmotic equilibrium (osmoregulation) by maintaining the turgor and osmotic adjustment through which cell water balance occurs. Like other osmolytes, such as proline and glycine betaine, it assists in stabilizing cellular turgor pressure in the presence of salinity or dehydration [44]. This stabilization protects against damage to enzymes and cellular components [45]. GABA enables plants to uptake nutrients and assimilate nitrogen, which reduces the need for synthetic fertilizers [46]. It also causes increases in the water content in leaves and the improved stomatal conductance associated with the accumulation of osmolytes such as proline and trehalose [47]. The increase in stomatal conductance and net photosynthetic rate suggests that GABA-mediated regulation supports higher carbon assimilation under water-limited conditions. GABA application helps maintain the integrity of the plant cell membranes in bentgrass and black gram crops under drought and salt stress, respectively [48,49].
3.4. Maintenance of Ion Homeostasis
The maintenance of proper concentrations of inorganic ions in living cells is called ion homeostasis. It is vital for physiological functions like membrane potential, signaling, and enzyme activity. Ion channels, pumps, and transporters take part in this process and are responsible for the regulation of ions such as sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl−) across the membranes [50]. This ion homeostasis complements the other functions of GABA in tolerance mechanisms, such as redox balance and osmotic adjustment, leading to a combinational defense system against environmental challenges. GABA maintains ion homeostasis by inducing the expression of Na+ and K+ transport-related genes, especially when subjected to salt stress [51]. This process decreases the intake and delivery of sodium to the shoots and leaves, while simultaneously increasing the retention and uptake of potassium (Figure 3). This is crucial for plant health and can help plants cope with salt stress. GABA accumulation also causes H+-ATPase to be turned on. This boosts ion transport while facilitating pH regulation and the maintenance of the electrochemical gradients necessary for ion transport. By offering protection against Na+ toxicity and promoting K+ uptake, GABA is able to maintain membrane integrity osmoregulation and cellular life under stress conditions [22,52].
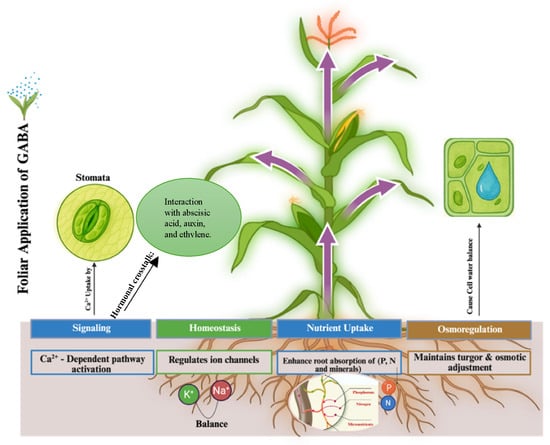
Figure 3.
Summarizing cellular and physiological mechanisms of GABA-mediated stress tolerance. Source: authors’ own compilation, unpublished (created with BioRender.com).
GABA aids in the regulation of guard cell anion and ion channels, such as the aluminum malate transporter (ALMT), in saline environments, preserving the equilibrium between K+ and Na+ ions [53]. For crops growing in soils influenced by salt, this control ensures that cellular activities are maintained by preventing Na+ toxicity. GABA mediates plant stress adaptation by signaling, regulating ion balance (homeostasis), supporting osmoregulation, and enhancing growth of the root and uptake of nutrient under adverse circumstances [45]. GABA promotes K+ retention and Na+ exclusion in salinity, most likely through the control of ion transporters such as salt overly sensitive 1 (SOS1) and sodium/hydrogen exchange [54]. This is consistent with research on black gram, where GABA decreased oxidative damage and enhanced K+/Na+ ratios [49]. In saline conditions, maintaining ion balance is critical to avoid toxicity and sustain growth. GABA application in crops, such as rice and quinoa, improves ionic regulation, likely by influencing transporter proteins and root permeability [55,56]. This results in reduced accumulation of harmful ions like Na+, and better uptake of beneficial ions, such as K+ and Ca2+, highlighting GABA’s role in modulating ion transport processes. Another way to maintain ion homeostasis in plants under stress conditions is the integration of GABA with phytohormones, such as abscisic acid (ABA), auxins (IAA), cytokinins, gibberellins, and ethylene, which frequently increases their synthesis in response to stress to express stress-responsive genes [57,58]. For example, exogenous GABA increases the levels of ABA, IAA, and jasmonic acid in citrus, which helps the fruit adapt to salinity and drought [59]. These interactions link GABA to phytohormone-regulated functions such as ROS scavenging and root growth.
4. Agricultural Methods for the Application of GABA Under Different Stress Conditions
GABA can be used in agriculture in a number of ways, each of which is appropriate for particular crops and to achieve the intended results (Table 1).
4.1. Foliar Application
One of the most popular and efficient techniques is that of foliar application. After dissolving in water, GABA was sprayed directly onto the leaves at concentrations between 0.5 and 5.0 mM. This approach enables prompt uptake and brings about a direct influence on the metabolism of the stressed leaves. Plants treated with foliar GABA often exhibit enhanced resilience to drought and saline conditions. Hence, the activation of POD, CAT, and SOD can neutralize ROS. Thus, GABA treatment can help detoxify ROS by increasing ROS removal, thereby decreasing the levels of MDA in rice as a marker of lipid peroxidation levels [55,60]. This implies that GABA helps maintain the integrity of membranes during stress [40]. In Arabidopsis, heat stress leads to higher levels of GABA because heat activates the enzyme that transforms glutamate into GABA with the help of calcium [61]. Applying GABA externally to plants under heat stress significantly improves their growth and chances of survival. This is achieved by reducing damage to the plant’s membranes, enhancing the viability of the cells, increasing the amount of chlorophyll present, and improving the plant’s ability to carry out photosynthesis in its leaves [62]. The cumulative effect of these protective and restorative mechanisms is a significant promotion of overall plant development under stress conditions. For instance, research on wheat demonstrated that foliar application of 1.0 mM GABA significantly improved seedling length and fresh weight under drought conditions [16]. This correlates directly with the physiological markers of improved plant vigor and water retention, suggesting that GABA plays a fundamental role in mitigating the growth inhibitory effects of water deficit. The consistency of this response is further highlighted in another key crop, common bean (Phaseolus vulgaris L.), where a foliar treatment with 2.0 mM GABA under drought conditions resulted in a notable increase in shoot weight and leaf area [63]. The growth-promoting effects of GABA is powerfully demonstrated in bitter gourd fruit, where a GABA application at 1.5 mg/L was found to be the most effective concentration for improving a comprehensive suite of traits, including vegetative growth (vine length and diameter and branch number), flowering (reducing days to anthesis and increasing flower number), and, most significantly, final yield components (fruit set, fruit size, weight, and seed number) [64]. Further research was performed on the effect of GABA on vegetables as a study on Allium species (garlic) confirmed that a 1 mM GABA foliar spray optimally enhances overall plant performance, boosting growth, yield, mineral content, and key biochemical markers like chlorophyll and phenols [65]. This was unequivocally demonstrated in mustard, where GABA application at 2 and 3 mg/L significantly increased seed yield owing to improved yield components like siliqua number and seed count [66].

Table 1.
Application methods and dose of GABA used in different types of crops.
Table 1.
Application methods and dose of GABA used in different types of crops.
| Application Method | Typical Dose Range (mM) | Type of Crops | References |
|---|---|---|---|
| Foliar spray | 0.50–5.00 | Field, fruit, and vegetable crops | [16,61,63,64,65,66] |
| Seed soaking | 0.001–5.00 | Cereals, legumes, and vegetable seeds | [17,67,68,69] |
| Fertigation | 0.05-0.08 | Fruit, root crops | [70] |
| Hydroponic solution | 0.10–5.00 | Field and vegetable crops | [60] |
| Postharvest dip | 1.00–10.00 | Fruit and vegetable | [71,72,73,74,75] |
| Fertilizer blending | 20.00–30.00 | All major field crops | [76] |
4.2. Seed Soaking
Before being planted, seeds are soaked in a diluted GABA solution (0.001–5 mM) or coated with the solution. This promotes young seedlings’ stress resilience, vigor, and early germination. Conventional protocols usually use low millimolar concentrations, which are significantly diluted with respect to commercial stock solutions. Optimal rates are crop- or stress-specific but seed priming with GABA consistently improves germination indices and early growth under saline conditions in several crops [17,68]. In addition, soaking white clover seeds in 1 μM GABA significantly mitigated the salt-induced reductions in endogenous GABA content, germination percentage, shoot and root length, biomass, and root activity [67,69].
4.3. Fertigation
Depending on the crop stage, GABA can be applied by fertigation systems, which deliver it to the roots along with irrigation water at a dose of 0.05–0.08 mM, or by soaking the soil. To achieve synergistic effects on nutrient absorption and stress tolerance, GABA can be added to fertilizers (urea, NPK, and biofertilizers) at a rate of 50–75 g/hectare, which enhances nutrient uptake, root growth, ion homeostasis, and early vigor under salinity, drought osmotic, and hypoxia-related stresses [41,70].
4.4. Hydroponic Solution
GABA is commonly sprayed on plants or added directly to the nutrient solution in hydroponic systems. It has been demonstrated to enhance plant growth and stress tolerance in situations like drought and salinity. Depending on the crop type and stress level, GABA was directly added to the hydroponic nutrient solution at concentrations ranging from 0.1 to 5.0 mM [60,77]. Exogenous GABA enhances the salt tolerance of hydroponically grown rice seedlings at the three-leaf stage, and this is accompanied by sustained shoot growth, antioxidant action, root activity, and minimization of leaf aging [60]. However, the application of GABA led to a continuous effect on the upregulation of POD, SOD, and APX, a decrease in dead leaves, and an increase in plant height and leaf area under mild to moderate salinity conditions. Collectively, these results indicate that GABA plays a key role in enhancing yield resilience in hydroponic systems; however, the optimal concentrations for different plant species need to be determined.
4.5. Postharvest Dip
A postharvest GABA dip refers to a treatment in which fruits and vegetables are dipped in GABA solution for quality improvement and the extension of shelf life. This application prevents softening, helps to retain nutrients such as phenols and vitamins, and reduces chilling injury and weight loss during storage [78,79]. The application of GABA improved the postharvest quality of zucchini, grapes, and peaches primarily by reducing weight loss, maintaining firmness, and enhancing phenolic content and antioxidant activity during cold storage, with 50 mM generally outperforming other doses. Meanwhile, biochemical content was largely unaffected, indicating that GABA delays senescence without altering basic taste attributes, thus extending marketability and shelf life [71,72,73]. Similar postharvest benefits of exogenous GABA have been documented across fruit species: reduced chilling injury and weight loss with improved quality in zucchini during 4 °C storage [71], improved cold-stored grape quality with lower decay and stem browning [73], enhanced chilling tolerance in peaches via upregulation of ascorbic acid and glutathione pathways [72], and increased phenolics/antioxidant systems and chilling tolerance in blood oranges under prolonged cold storage [74]. Mechanistically, exogenous GABA is linked to strengthened antioxidant defenses and phenylpropanoid activation (such as raised phenylalanine ammonia-lyase activity and lower polyphenol oxidase), improved energy status under cold stress, and mitigation of ROS damage, which together explain its role in slowing softening and dehydration during storage [74]. A postharvest application of 10 mM GABA significantly slowed the loss of organic acids, soluble sugars, firmness, and color and it also reduced decay and weight loss in strawberries [75].
4.6. Fertilizer Blending
The blending of different raw materials enables the user to formulate custom blends that provide the specific nutrients needed for a given crop or soil type. This synergistic combination is able to enhance plant nutrition and production even under a lower total fertilizer rate. The mixing of GABA with synthetic fertilizers and biofertilizers boosts and improves a plant’s resistance to unfavorable conditions. The optimal doses of GABA for blending with fertilizers range between 2 and 3 kg/ton or 20 and 30 mM per ton fertilizer [70]. Incorporating amino acids, particularly GABA, into such blends can enhance nutrient uptake and assimilation, stimulate root growth, and bolster stress tolerance via osmotic adjustment, ion homeostasis, and antioxidant defense, thereby supporting growth and yield under adverse conditions [76].
5. Beneficial-Effect Applications of GABA Under Various Stress Conditions
Under different stress conditions, different responses were observed in plants treated with GABA, as shown in Table 2.
5.1. Drought-Stress Tolerance
Drought is a dominant abiotic stressor that substantially affects the entire agroecosystem. In addition, drought stress significantly disrupts the physiological, anatomical, and biochemical functions of crops, greatly affecting their growth and yields [80]. To minimize the effects of drought stress, the neurotransmitter GABA is frequently used. Empirical evidence suggests that the use of GABA results in a significant enhancement in the development and yield of crops that are being subjected to stressful circumstances [81]. For instance, applying 1.0 mM GABA extends germination time and enhances seedling growth in wheat under drought conditions [16]. Similarly, under drought conditions external GABA application (2.0 mM) increased shoot weight and leaf area in common bean crops [63]. However, GABA’s effects can vary; for example, a concentration of 1.54 μmol/g either promotes or inhibits pollen tube growth in Arabidopsis thaliana, depending on the specific conditions [81]. In addition, applying GABA to sunflowers at a dose of 2.0 mg/L increased plant height and the fresh and dry weight of shoots and roots, and improved osmolyte metabolism, expression of genes, and antioxidant enzyme activity [82]. In white clover, 8 mM GABA improves drought tolerance by increasing water content, reducing electrolyte leakage and lipid peroxidation, and preventing leaf wilting [69]. This dose increased water content, decreased electrolyte leakage, reduced lipid peroxidation, and prevented leaf wilting. Using GABA during drought conditions also boosts the activities of enzymes such as GABA transaminase and alpha ketone glutarate dehydrogenase, while decreasing the activity of GAD. Consequently, this leads to increased levels of endogenous glutamate and GABA. The necessity of species and stress specific optimization is highlighted by this discrepancy.

Table 2.
Beneficial effects of GABA in different crops under different stress conditions.
Table 2.
Beneficial effects of GABA in different crops under different stress conditions.
| Field of Application | Beneficial Effects | Example Crops | References |
|---|---|---|---|
| Drought tolerance |
| Wheat, rice, and sunflower | [16,63,69,82] |
| Salt tolerance |
| Maize, lentil, bean, rice, soybean, mung bean, and rapeseed | [49,55,56,83,84,85,86,87] |
| Cold tolerance |
| Crops | [88,89] |
| Heat tolerance |
| Mung bean and bentgrass | [48,90] |
| Waterlogging resilience |
| Cotton | [91,92] |
| Postharvest quality |
| Strawberry, cherry, tomato, and mango | [73,75,93,94,95,96] |
5.2. Salt-Stress Tolerance
GABA promotes K+ retention and Na+ exclusion in salinity, most likely through the control of ion transporters such as SOS1. Moreover, treating lentil seedlings with GABA significantly increased MDA levels [85]. This shows that the GABA shunt is important for signaling and metabolism, helping seedlings adapt to salty conditions and improving germination rates. Research on black gram plants exposed to salt stress showed that treating these plants with 70 mM of GABA significantly improved their height, dry weight, and pod production per plant [49]. Consequently, this led to a substantial improvement in crop output across all phases of growth. A study conducted on rice examined the impact of applying 0.5 mM GABA to seeds exposed to osmotic stress [55]. As a result, the cell cycle development of rice seedlings was prolonged, which impeded their growth. Studies have also found that salt, osmotic stress, and their combination can change cell structure and development, leading to longer cell cycles and slower growth of seedlings [97]. Additionally, the use of GABA at a concentration of 1 mM significantly enhanced the growth of maize, particularly the development of fresh shoots and roots [87]. In saline environments, mung bean and rapeseed crops were evaluated for morphological and biochemical modifications upon GABA treatment [83,98]. Mung beans exposed to 1.5 mM GABA demonstrated a preserved morpho-physiological profile [83]. This led to a significant reduction in ion toxicity by inhibiting NaCl accumulation and concomitant increases in cellular osmolytes, such as proline. The enhancement of enzymatic antioxidants leads to a decrease in lipid peroxidation, thereby preserving the integrity of cellular membranes. In a similar vein, rapeseed plants that were exposed to 1.5 mM GABA demonstrated improved germination rates and vigorous seedling growth in conditions of salt stress [98]. These antioxidant enzyme activities were evident, as indicated by the upregulation of enzymes involved in ROS detoxification. In another study quinoa plants subjected to salinity stress and treated with a higher GABA dose (10 mM) generated the highest number of spikes per plant compared with those treated with lower doses and untreated controls [56]. A notable observation is the variability in crop responses to specific GABA doses, for example, quinoa under salinity stress showed optimal results with a 10 mM dose [56], whereas rice responded better to a much lower dose of 0.5 mM [99]. Quinoa is naturally much more salt-tolerant, and its antioxidant systems, ion homeostasis (Na+/K+ balance), and osmotic adjustment are already strong. These findings indicate that GABA application allows plants to better regulate ion homeostasis and activate defense mechanisms that alleviate saline stress [100].
5.3. Cold-Stress Tolerance
GABA application enhances cold tolerance by modulating antioxidant enzyme activity, stabilizing chloroplasts, and maintaining photosynthetic pigments [34]. GABA contributes to chilling stress mitigation by preserving cellular integrity and improving overall plant vigor in cold environments. Medicago ruthenica plants treated with exogenous GABA at concentrations between 1 and 10 mmol/L demonstrated improved growth traits, such as plant height, root length, stem diameter, leaf area, and dry biomass under cold stress [88]. Additionally, based on the provided study, the optimal dose for improving cold tolerance in wheat was determined to be 4 mmol/L GABA, applied via a 12-h root soak before cold stress [89].
5.4. Heat-Stress Tolerance
Heat stress affects plant functions, slowing their growth, development, and overall productivity, resulting in increased ROS production and oxidative stress. Plants face challenges in managing heat stress alongside other environmental stressors [101]. Owing to its structural resemblance to glutamate, GABA can interact with Ca receptors and channels to affect the Ca2+-dependent signaling cascades that are essential for stress reactions. For instance, calmodulin-dependent proteins may be activated by GABA-induced Ca2+ flow, which, in turn, controls osmotic adjustment and stomatal closure [102]. In mung beans, using 1 mM GABA greatly helped with important reproductive processes like pollen germination, pollen health, stigma readiness, and ovule survival [90]. This treatment also minimized membrane damage, increased the efficiency of the photosynthetic apparatus, boosted carbon assimilation processes, such as sucrose production and utilization, and promoted osmolyte accumulation. In addition, application of 0.5 mM of GABA to creeping bentgrass contributed to maintaining stable cell membranes, slowed down leaf aging, and improved osmotic adjustment under heat stress [48].
5.5. Waterlogging Tolerance
The term “waterlogging” refers to the saturation of soil with water, which prevents plant roots from accessing oxygen and encourages anaerobic conditions that negatively impact crop growth, nutrient uptake, and yield [103]. The addition of GABA to the soil mitigates these negative effects of waterlogging in plants by boosting root vitality, antioxidant enzyme activity, and proline accumulation [104]. Under waterlogging stress, maize plants treated with 1 mmol/L GABA demonstrated significant seedling growth [92]. The upregulation of ROS-scavenging enzymes under GABA treatment reduced the accumulation of toxic metabolites, thereby creating a more favorable physiological state that supported reproductive development in plants. According to Li Wang et al. [91], the application of 10 mM of GABA reactivated the GABA shunt pathway in cotton, and it created a new form of location for energy production, which helped seedlings to survive after waterlogging stress.
5.6. Improving Postharvest Quality
Preharvest application of GABA improves postharvest quality, primarily by slowing deterioration and significantly reducing weight loss. Application of 50 mM GABA to pomegranates at both 2 °C and 10 °C caused lowering dehydration, maintained firmness by slowing softening, and elevated total phenolics in both peel and juice, along with total antioxidant activity in the juice, which was especially evident at 2 °C during the first 4 weeks, while leaving total soluble solids and titratable acidity largely unchanged [105]. Collectively, these effects delayed senescence, preserved visual and textural quality, and extended practical shelf life. In strawberries, application of 10 mM GABA delayed the decreases in organic acids, soluble sugars, firmness, and color after preharvest treatment [75]. In pears, exogenous GABA treatment delayed peel browning by preserving mitochondrial structure and enhancing the efficiency of the mitochondrial oxidative defense system [96]. In addition, GABA application improved sensory and nutritional quality in cornelian cherry via delayed softening and phenolic accumulation [93]. In a study on tomatoes, preharvest foliar application of 20 mmol/L GABA, particularly when sprayed three days before harvest, significantly enhanced fruit quality and yield-related parameters by reprogramming the primary metabolism [95,106]. Studies on olive fruits have demonstrated that a 1.0 mM GABA treatment effectively reduces chilling injury and maintains quality during storage [107]. In grapes, postharvest GABA application at 1 °C increased the levels of phenols, soluble sugars, and organic acids, which helped reduce mildew and chilling damage, thereby maintaining fruit quality and prolonging storage life [73]. Furthermore, in citrus fruit, exogenous GABA significantly increased the levels of various amino acids and enhanced the activity of GABA shunt enzymes, facilitating its conversion into succinate to integrate with the primary metabolism [59]. For instance, the postharvest application of 20 mM GABA can play a significant role in alleviating chilling injury in banana fruit peel [108].
6. Conclusions and Perspectives
Strategic research, its harmonization with other technologies, and field-level validation are crucial for making GABA an important tool for climate-resilient agriculture. GABA application seems to improve the physiological and biochemical capacity in plants through the enhancement of antioxidant defense, osmotic regulation, and nutrient uptake. Optimizing the dose and method of application of GABA to crops is a promising means of alleviating the detrimental effects of abiotic stresses. The findings concluded that the GABA dose depends on the crop species and method of application. Furthermore, the methods of GABA application depend on the crop species. The proposed practices, like seed priming, foliar spray, and soil amendment, are innovative delivery systems for targeted stress alleviation and ensuring yield sustainability.
The potential for synergy between GABA applications and other biostimulant factors may, additionally, improve crop performance and sustainability. In real farming, the application of GABA innovations needs to be supported by active extension education on the right use, cost–benefit, and risk. When applying GABA commercially, regulatory measures and environmental assessments should be parallelly conducted to track long-term soil health, ecosystem impacts, and food safety in relation to widespread use of GABA.
Author Contributions
Conceptualization, N.A.-r.T. and S.S.A.; data curation, N.A.-r.T. and S.S.A.; formal analysis, N.A.-r.T. and S.S.A.; writing original draft preparation, N.A.-r.T. and S.S.A.; and writing—review and editing, N.A.-r.T. and S.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
The article contains all data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.B.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Lesk, C.; Anderson, W.; Rigden, A.; Coast, O.; Jägermeyr, J.; McDermid, S.; Davis, K.F.; Konar, M. Compound heat and moisture extreme impacts on global crop yields under climate change. Nat. Rev. Earth Environ. 2022, 3, 872–889. [Google Scholar] [CrossRef]
- Mulyani, A.T.; Khairinisa, M.A.; Khatib, A.; Chaerunisaa, A.Y. Understanding stunting: Impact, causes, and strategy to accelerate stunting reduction—A narrative Review. Nutrients 2025, 17, 1493. [Google Scholar] [CrossRef]
- Parajuli, J.; Prangthip, P. Adolescent Nutrition and Health: A Critical Period for Nutritional Intervention to Prevent Long Term Health Consequences. Curr. Nutr. Rep. 2025, 14, 116. [Google Scholar] [CrossRef]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple benefits of legumes for agriculture sustainability: An overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef]
- Bukhari, S.A.H.; Peerzada, A.M.; Javed, M.H.; Dawood, M.; Hussain, N.; Ahmad, S. Growth and Development Dynamics in Agronomic Crops Under Environmental Stress. In Agronomic Crops: Volume 1: Production Technologies; Hasanuzzaman, M., Ed.; Springer: Singapore, 2019; pp. 83–114. ISBN 978-981-32-9151-5. [Google Scholar]
- Altaf, M.A.; Shahid, R.; Kumar, R.; Altaf, M.M.; Kumar, A.; Khan, L.U.; Saqib, M.; Nawaz, M.A.; Saddiq, B.; Bahadur, S. Phytohormones mediated modulation of abiotic stress tolerance and potential crosstalk in horticultural crops. J. Plant Growth Regul. 2023, 42, 4724–4750. [Google Scholar] [CrossRef]
- Tarkowski, Ł.P.; Signorelli, S.; Höfte, M. γ-Aminobutyric acid and related amino acids in plant immune responses: Emerging mechanisms of action. Plant Cell Environ. 2020, 43, 1103–1116. [Google Scholar] [CrossRef]
- Tanveer, M.; Yousaf, U. Chapter 23—Plant single-cell biology and abiotic stress tolerance. In Plant Life Under Changing Environment; Tripathi, D.K., Singh, V.P., Chauhan, D.K., Sharma, S., Prasad, S.M., Dubey, N.K., Ramawat, N., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 611–626. ISBN 978-0-12-818204-8. [Google Scholar]
- Konovalov, D.A. Neurotransmitters in Medicinal Plants. In Neurotransmitters in Plants; CRC Press: Boca Raton, FL, USA, 2018; pp. 331–356. [Google Scholar]
- Raza, A.; Salehi, H.; Rahman, M.A.; Zahid, Z.; Madadkar Haghjou, M.; Najafi-Kakavand, S.; Charagh, S.; Osman, H.S.; Albaqami, M.; Zhuang, Y.; et al. Plant hormones and neurotransmitter interactions mediate antioxidant defenses under induced oxidative stress in plants. Front. Plant Sci. 2022, 13, 961872. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Malakar, P.; Gupta, S.K.; Chattopadhyay, D. Role of plant neurotransmitters in salt stress: A critical review. Plant Physiol. Biochem. 2024, 11, 108601. [Google Scholar] [CrossRef]
- Puttegowda, D.; Jayaram, L.; Firdose, N.; Lobo, R.O.; Stalekar, N.; Ramu, R. Plant-derived gamma-aminobutyric acid (GABA): Role in stress responses, growth, metabolism, and therapeutic potential for neuropsychiatric disorders. Physiol. Mol. Plant Pathol. 2025, 139, 102807. [Google Scholar] [CrossRef]
- Zhao, Q.; Ma, Y.; Huang, X.; Song, L.; Li, N.; Qiao, M.; Li, T.; Hai, D.; Cheng, Y. GABA application enhances drought stress tolerance in wheat seedlings (Triticum aestivum L.). Plants 2023, 12, 2495. [Google Scholar] [CrossRef]
- Ahmad, S.; Fariduddin, Q. Deciphering the enigmatic role of gamma-aminobutyric acid (GABA) in plants: Synthesis, transport, regulation, signaling, and biological roles in interaction with growth regulators and abiotic stresses. Plant Physiol. Biochem. 2024, 208, 108502. [Google Scholar] [CrossRef]
- Adamipour, N.; Nazari, F.; Teixeira da Silva, J.A. GABA Biosynthesis Pathways and its Signaling in Plants. In GABA in Plants; Singh, S., Tripathi, D.K., Singh, V.P., Eds.; Wiley: Hoboken, NJ, USA, 2025; pp. 19–41. ISBN 9781394217755. [Google Scholar]
- Al-Khayri, J.M.; Abdel-Haleem, M.; Khedr, E.H. Harnessing GABA pathways to improve plant resilience against salt stress. Horticulturae 2024, 10, 1296. [Google Scholar] [CrossRef]
- Abdullah; Wani, K.I.; Naeem, M.; Aftab, T. From neurotransmitter to plant protector: The intricate world of GABA signaling and its diverse functions in stress mitigation. J. Plant Growth Regul. 2025, 44, 403–418. [Google Scholar] [CrossRef]
- Yuan, D.; Wu, X.; Gong, B.; Huo, R.; Zhao, L.; Li, J.; Lü, G.; Gao, H. GABA metabolism, transport and their roles and mechanisms in the regulation of abiotic stress (Hypoxia, Salt, Drought) resistance in plants. Metabolites 2023, 13, 347. [Google Scholar] [CrossRef]
- Zarbakhsh, S.; Saleem, A.; Fayezizadeh, M.R.; Hafeez, M.B. Interplay between γ-aminobutyric acid metabolism and other crucial amino acid pathways in modulating plant growth and stress conditions. Plant Stress 2025, 16, 100883. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Tyerman, S.D.; Xu, B.; Bose, J.; Kaur, S.; Conn, V.; Domingos, P.; Ullah, S.; Wege, S.; Shabala, S.; et al. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat. Commun. 2015, 6, 7879. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Jalil, S.U.; Chopra, P.; Chhillar, H.; Ferrante, A.; Khan, N.A.; Ansari, M.I. Role of GABA in plant growth, development and senescence. Plant Gene 2021, 26, 100283. [Google Scholar] [CrossRef]
- Wu, Q.; Su, N.; Huang, X.; Cui, J.; Shabala, L.; Zhou, M.; Yu, M.; Shabala, S. Hypoxia-induced increase in GABA content is essential for restoration of membrane potential and preventing ROS-induced disturbance to ion homeostasis. Plant Commun. 2021, 2, 100188. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Yue, Q.; Zhang, Y.; Zhao, S.; Khan, A.; Yang, X.; He, J.; Wang, S.; Shen, W.; Qian, Q.; et al. Application of γ-aminobutyric acid (GABA) improves fruit quality and rootstock drought tolerance in apple. J. Plant Physiol. 2023, 280, 153890. [Google Scholar] [CrossRef]
- Kamran, M.; Ramesh, S.A.; Gilliham, M.; Tyerman, S.D.; Bose, J. Role of TaALMT1 malate-GABA transporter in alkaline pH tolerance of wheat. Plant Cell Environ. 2020, 43, 2443–2459. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Sai, N.; Gilliham, M. The emerging role of GABA as a transport regulator and physiological signal. Plant. Physiol. 2021, 187, 2005–2016. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, H.; Song, Y.; Gu, Z. Effects of soaking and aeration treatment on γ-aminobutyric acid accumulation in germinated soybean (Glycine max L.). Eur. Food Res. Technol. 2011, 232, 787–795. [Google Scholar] [CrossRef]
- Yin, Y.; Cheng, C.; Fang, W. Effects of the inhibitor of glutamate decarboxylase on the development and GABA accumulation in germinating fava beans under hypoxia-NaCl stress. RSC Adv. 2018, 8, 20456–20461. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Gong, J.; Luo, S.; Zuo, Y.; Shen, Y. Role of gamma-aminobutyric acid in plant defense response. Metabolites 2023, 13, 741. [Google Scholar] [CrossRef]
- Carroll, A.D.; Fox, G.G.; Laurie, S.; Phillips, R.; Ratcliffe, R.G.; Stewart, G.R. Ammonium assimilation and the role of [gamma]-aminobutyric acid in pH homeostasis in carrot cell suspensions. Plant Physiol. 1994, 106, 513–520. [Google Scholar] [CrossRef]
- Abd Elbar, O.H.; ElKelish, A.; Niedbała, G.; Farag, R.; Wojciechowski, T.; Mukherjee, S.; Abou-Hadid, A.F.; El-Hennawy, H.M.; Abou El-Yazied, A.; Abd El-Gawad, H.G. Protective effect of γ-aminobutyric acid against chilling stress during reproductive stage in tomato plants through modulation of sugar metabolism, chloroplast integrity, and antioxidative defense systems. Front. Plant Sci. 2021, 12, 663750. [Google Scholar] [CrossRef]
- Ramos-Ruiz, R.; Martinez, F.; Knauf-Beiter, G. The effects of GABA in plants. Cogent Food Agric. 2019, 5, 1670553. [Google Scholar] [CrossRef]
- Zarbakhsh, S.; Shahsavar, A.R.; Afaghi, A.; Hasanuzzaman, M. Predicting and optimizing reactive oxygen species metabolism in Punica granatum L. through machine learning: Role of exogenous GABA on antioxidant enzyme activity under drought and salinity stress. BMC Plant Biol. 2024, 24, 65. [Google Scholar] [CrossRef]
- Li, D.; Zhang, D.; Zhang, Z.; Xing, Y.; Sun, N.; Wang, S.; Cai, H. Exogenous application of GABA alleviates alkali damage in alfalfa by increasing the activities of antioxidant enzymes. Agronomy 2022, 12, 1577. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Bernard, F.; Seif, M.; Latifi, M.; Hassani, B.; Didaran, F.; Bosacchi, M.; Rezadoost, H.; Li, T. γ-Aminobutyric acid confers cadmium tolerance in maize plants by concerted regulation of polyamine metabolism and antioxidant defense systems. Sci. Rep. 2020, 10, 3356. [Google Scholar] [CrossRef]
- Malekzadeh, P.; Khara, J.; Heydari, R. Alleviating effects of exogenous Gamma-aminobutiric acid on tomato seedling under chilling stress. Physiol. Mol. Biol. Plants 2014, 20, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, P.; Chen, Z.; Gu, Z.; Yang, R. GABA enhances physio-biochemical metabolism and antioxidant capacity of germinated hulless barley under NaCl stress. J. Plant Physiol. 2018, 231, 192–201. [Google Scholar] [CrossRef]
- Nayyar, H.; Kaur, R.; Kaur, S.; Singh, R. γ-Aminobutyric acid (GABA) imparts partial protection from heat stress injury to rice seedlings by improving leaf turgor and upregulating osmoprotectants and antioxidants. J. Plant Growth Regul. 2014, 33, 408–419. [Google Scholar] [CrossRef]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The versatile GABA in plants. Plant Signal. Behav. 2021, 16, 1862565. [Google Scholar] [CrossRef]
- Hao, X.H.; Liu, K.X.; Zhang, M.Y. Effect of exogenous γ-aminobutyric acid on physiological property, antioxidant activity, and cadmium uptake of quinoa seedlings under cadmium stress. Biosci. Rep. 2024, 44, BSR20240215. [Google Scholar] [CrossRef]
- Hayat, F.; Khan, U.; Li, J.; Ahmed, N.; Khanum, F.; Iqbal, S.; Altaf, M.A.; Ahmad, J.; Javed, H.U.; Peng, Y. γ Aminobutyric acid (GABA): A key player in alleviating abiotic stress resistance in horticultural crops: Current insights and future directions. Horticulturae 2023, 9, 647. [Google Scholar] [CrossRef]
- Qian, Z.; Lu, L.; Zihan, W.; Qianyue, B.; Chungang, Z.; Shuheng, Z.; Jiali, P.; Jiaxin, Y.; Shuang, Z.; Jian, W. Gamma-aminobutyric acid (GABA) improves salinity stress tolerance in soybean seedlings by modulating their mineral nutrition, osmolyte contents, and ascorbate-glutathione cycle. BMC Plant Biol. 2024, 24, 365. [Google Scholar] [CrossRef] [PubMed]
- Kaspal, M.; Kanapaddalagamage, M.H.; Ramesh, S.A. Emerging roles of γ aminobutyric acid (GABA) gated channels in plant stress tolerance. Plants 2021, 10, 2178. [Google Scholar] [CrossRef]
- Huang, X.-J.; Jian, S.-F.; Wan, S.; Miao, J.-H.; Zhong, C. Exogenous γ-aminobutyric acid (GABA) alleviates nitrogen deficiency by mediating nitrate uptake and assimilation in Andrographis paniculata seedlings. Plant Physiol. Biochem. 2023, 198, 107700. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Isayenkov, S.V. The Role of the γ-aminobutyric acid (GABA) in plant salt stress tolerance. Horticulturae 2023, 9, 230. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y.; Huang, B. Physiological effects of γ-aminobutyric acid application on improving heat and drought tolerance in creeping bentgrass. J. Exp. Bot. 2016, 141, 76–84. [Google Scholar] [CrossRef]
- Kumar, S.; Jangde, S.; Priya, T.; Yashu, B.R. Effect of GABA on morphology, yield and yield attributes in black gram (Vigna mungo (L.) Hepper) under salt stress condition. Int. J. Pure App. Biosci. 2017, 5, 1223–1228. [Google Scholar] [CrossRef]
- Mulet, J.M.; Campos, F.; Yenush, L. Editorial: Ion homeostasis in plant stress and development. Front. Plant Sci. 2020, 11, 618273. [Google Scholar] [CrossRef] [PubMed]
- Shelp, B.J.; Aghdam, M.S.; Flaherty, E.J. γ-Aminobutyrate (GABA) regulated plant defense: Mechanisms and opportunities. Plants 2021, 10, 1939. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Liu, T.; Guo, C.; Liang, W.; Ma, F.; Li, C. γ-Aminobutyric acid enhances salt tolerance by sustaining ion homeostasis in apples. Plant Physiol. Biochem. 2024, 206, 108306. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.A.; Kamran, M.; Sullivan, W.; Chirkova, L.; Okamoto, M.; Degryse, F.; McLaughlin, M.; Gilliham, M.; Tyerman, S.D. Aluminum-activated malate transporters can facilitate GABA transport. Plant Cell 2018, 30, 1147–1164. [Google Scholar] [CrossRef]
- Su, N.; Wu, Q.; Chen, J.; Shabala, L.; Mithöfer, A.; Wang, H.; Qu, M.; Yu, M.; Cui, J.; Shabala, S. GABA operates upstream of H+-ATPase and improves salinity tolerance in Arabidopsis by enabling cytosolic K+ retention and Na+ exclusion. J. Exp. Bot. 2019, 70, 6349–6361. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; Shao, H.; Qi, W.; Hamoud, Y.A.; Shaghaleh, H.; Khan, N.U.; Yang, R.; Tang, B. GABA-alleviated oxidative injury induced by salinity, osmotic stress and their combination by regulating cellular and molecular signals in rice. Int. J. Mol. Sci. 2019, 20, 5709. [Google Scholar] [CrossRef] [PubMed]
- Hatami, A.-A.; Aminian, R.; Mafakheri, S.; Soleimani Aghdam, M. Effect of gamma amino butyric acid on morpho-physiological traits and seed yield of quinoa under salinity stress. Plant Prod. 2021, 44, 559–572. [Google Scholar]
- Podlešáková, K.; Ugena, L.; Spíchal, L.; Doležal, K.; De Diego, N. Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. New Biotechnol. 2019, 48, 53–65. [Google Scholar] [CrossRef]
- Islam, S.N.U.; Kouser, S.; Hassan, P.; Asgher, M.; Shah, A.A.; Khan, N.A. Gamma-aminobutyric acid interactions with phytohormones and its role in modulating abiotic and biotic stress in plants. Stress Biol. 2024, 4, 36. [Google Scholar] [CrossRef]
- Hijaz, F.; Nehela, Y.; Killiny, N. Application of gamma-aminobutyric acid increased the level of phytohormones in Citrus sinensis. Planta 2018, 248, 909–918. [Google Scholar] [CrossRef]
- Feng, D.; Gao, Q.; Sun, X.; Ning, S.; Qi, N.; Hua, Z.; Tang, J. Effects of foliage-applied exogenous γ-aminobutyric acid on seedling growth of two rice varieties under salt stress. PLoS ONE 2023, 18, e0281846. [Google Scholar] [CrossRef]
- Locy, R.D.; Wu, S.J.; Bisnette, J.; Barger, T.W.; McNabb, D.; Zik, M.; Fromm, H.; Singh, N.K.; Cherry, J.H. The regulation of GABA accumulation by heat stress in Arabidopsis. In Plant Tolerance to Abiotic Stresses in Agriculture: Role of Genetic Engineering; Springer: Dordrecht, The Netherlands, 2000; pp. 39–52. [Google Scholar]
- Zandalinas, S.I.; Balfagón, D.; Gómez-Cadenas, A.; Mittler, R. Plant responses to climate change: Metabolic changes under combined abiotic stresses. J. Exp. Bot. 2022, 73, 3339–3354. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Gawad, H.G.; Mukherjee, S.; Farag, R.; Abd Elbar, O.H.; Hikal, M.; Abou El-Yazied, A.; Abd Elhady, S.A.; Helal, N.; ElKelish, A.; El Nahhas, N. Exogenous γ-aminobutyric acid (GABA)-induced signaling events and field performance associated with mitigation of drought stress in Phaseolus vulgaris L. Plant Signal. Behav. 2021, 16, 1853384. [Google Scholar] [CrossRef] [PubMed]
- Ashrafuzzaman, M.; Ismail, M.; Fazal, K.M.; Uddin, M.; Prodhan, A.K.M.A. Effect of GABA application on the growth and yield of bitter gourd (Momordica charantia). Int. J. Agric. Biol. 2010, 12, 129–132. [Google Scholar]
- Nasef, I.N.; Yousef, E.A.A. Growth and yield response of garlic genotypes to foliar application of γ-aminobutyric acid. Int. J. Agric. Biol. 2019, 8, 35–43. [Google Scholar]
- Saied, M.A.; Momjurul, A.M.M.; Shahidur, R.M. Foliar application of GABA improve growth and yield of mustard. MOJ Curr. Res. Rev. 2018, 1, 119–122. [Google Scholar] [CrossRef]
- Cheng, B.; Li, Z.; Liang, L.; Cao, Y.; Zeng, W.; Zhang, X.; Ma, X.; Huang, L.; Nie, G.; Liu, W.; et al. The γ-aminobutyric acid (GABA) alleviates salt stress damage during seeds germination of white clover associated with Na+/K+ transportation, Dehydrins Accumulation, and Stress-Related Genes Expression in White Clover. Int. J. Mol. Sci. 2018, 19, 2520. [Google Scholar] [CrossRef]
- Jia, Q.; Wu, X.; Ji, S.; Chu, X.; Zhao, F.; Gong, B.; Li, J.; Gao, H. Physiological regulation of γ-aminobutyric acid on the salt tolerance of grafted tomato seedlings. J. Plant Nutr. Fertil. 2021, 27, 122–134. [Google Scholar]
- Yong, B.; Xie, H.; Li, Z.; Li, Y.-P.; Zhang, Y.; Nie, G.; Zhang, X.-Q.; Ma, X.; Huang, L.-K.; Yan, Y.-H.; et al. Exogenous application of GABA improves PEG-induced drought tolerance positively associated with GABA-shunt, polyamines, and proline metabolism in white clover. Front. Physiol. 2017, 8, 1107. [Google Scholar] [CrossRef] [PubMed]
- Cathy, L. GABA: The Remarkable Role in Plant Growth and Stress Resistance. Wellyou Tech [Online]. 14 July 2025. Available online: https://wellyoutech.com/gaba-the-remarkable-role-in-plant-growth-and-stress-resistance/ (accessed on 30 December 2025).
- Palma, F.; Carvajal, F.; Jiménez-Muñoz, R.; Pulido, A.; Jamilena, M.; Garrido, D. Exogenous γ-aminobutyric acid treatment improves the cold tolerance of zucchini fruit during postharvest storage. Plant Physiol. Biochem. 2019, 136, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Dong, W.; Jin, S.; Liu, Q.; Shi, L.; Cao, S.; Li, S.; Chen, W.; Yang, Z. γ-Aminobutyric acid treatment induced chilling tolerance in postharvest peach fruit by upregulating ascorbic acid and glutathione contents at the molecular level. Front. Plant Sci. 2022, 13, 1059979. [Google Scholar] [CrossRef]
- Asgarian, Z.S.; Karimi, R.; Ghabooli, M.; Maleki, M. Biochemical changes and quality characterization of cold-stored ‘Sahebi’grape in response to postharvest application of GABA. Food Chem. 2022, 373, 131401. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Rahemi, M.; Eshghi, S.; Guillén, F.; Serrano, M.; Valero, D. Postharvest treatments with γ-aminobutyric acid, methyl jasmonate, or methyl salicylate enhance chilling tolerance of blood orange fruit at prolonged cold storage. J. Sci. Food Agric. 2019, 99, 6408–6417. [Google Scholar] [CrossRef]
- Zheng, Y.; Han, X.; Zhang, Y.; Qiu, W.; Tao, T.; Xu, Y.; Li, M.; Xie, X.; Sun, P.; Zheng, G.; et al. Preharvest and postharvest γ-aminobutyric acid treatment enhance quality and shelf life in strawberry (Fragaria × ananassa) fruits. J. Plant Growth Regul. 2025, 44, 3900–3915. [Google Scholar] [CrossRef]
- Bhadu, A.; Singh, B.; Gulshan, T.; Kumawat, S.N.; Choudhary, R.K.; Farooq, F. Customized fertilizer: A key for enhanced crop production. Int. J. Plant Soil Sci. 2022, 34, 954–964. [Google Scholar] [CrossRef]
- Palabıyık, Ş.; Çetinkaya, İ.; Öztürk, T.A.; Bor, M. Flagellin Induced GABA-shunt improves drought stress tolerance in Brassica napus L. BMC Plant Biol. 2024, 24, 864. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Anjum, M.A.; Nawaz, A.; Ejaz, S.; Anwar, R.; Khaliq, G.; Hussain, S.; Ullah, S.; Hussain, R.; Saleem, M.S.; et al. Postharvest γ-aminobutyric acid application mitigates chilling injury of aonla (Emblica officinalis Gaertn.) fruit during low temperature storage. Postharvest Biol. Technol. 2022, 185, 111803. [Google Scholar] [CrossRef]
- Ngaffo Mekontso, F.; Duan, W.; Cisse, E.H.M.; Chen, T.; Xu, X. Alleviation of postharvest chilling injury of Carambola fruit by γ-aminobutyric acid: Physiological, biochemical, and structural characterization. Front. Nutr. 2021, 8, 752583. [Google Scholar] [CrossRef]
- Kumar, S.; Sindhu, S.S. Drought stress mitigation through bioengineering of microbes and crop varieties for sustainable agriculture and food security. Curr. Res. Microb. Sci. 2024, 7, 100285. [Google Scholar] [CrossRef]
- Palanivelu, R.; Brass, L.; Edlund, A.F.; Preuss, D. Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 2003, 114, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Abdel Razik, E.S.; Alharbi, B.M.; Pirzadah, T.B.; Alnusairi, G.S.H.; Soliman, M.H.; Hakeem, K.R. γ-Aminobutyric acid (GABA) mitigates drought and heat stress in sunflower (Helianthus annuus L.) by regulating its physiological, biochemical and molecular pathways. Physiol. Plant. 2021, 172, 505–527. [Google Scholar] [CrossRef]
- Ullah, A.; Ali, I.; Noor, J.; Zeng, F.; Bawazeer, S.; Eldin, S.M.; Asghar, M.A.; Javed, H.H.; Saleem, K.; Ullah, S. Exogenous γ-aminobutyric acid (GABA) mitigated salinity-induced impairments in mungbean plants by regulating their nitrogen metabolism and antioxidant potential. Front. Plant Sci. 2023, 13, 1081188. [Google Scholar] [CrossRef]
- Zhang, R.; Hussain, S.; Wang, Y.; Liu, Y.; Li, Q.; Chen, Y.; Wei, H.; Gao, P.; Dai, Q. Comprehensive evaluation of salt tolerance in rice (Oryza sativa L.) germplasm at the germination stage. Agronomy 2021, 11, 1569. [Google Scholar] [CrossRef]
- Al-Quraan, N.A.; Al-Omari, H.A. GABA accumulation and oxidative damage responses to salt, osmotic and H2O2 treatments in two lentil (Lens culinaris Medik) accessions. Plant Biosyst. 2017, 151, 148–157. [Google Scholar]
- Jalil, S.U.; Ansari, M.I. Physiological Role of Gamma-Aminobutyric Acid in Salt Stress Tolerance. In Salt and Drought Stress Tolerance in Plants: Signaling Networks and Adaptive Mechanisms; Hasanuzzaman, M., Tanveer, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 337–350. ISBN 978-3-030-40277-8. [Google Scholar]
- Aljuaid, B.S.; Ashour, H. Exogenous γ-aminobutyric acid (GABA) application mitigates salinity stress in maize plants. Life 2022, 12, 1860. [Google Scholar] [CrossRef]
- Li, Y.; Yu, X.; Ma, K. Physiological effects of γ-aminobutyric acid application on cold tolerance in Medicago ruthenica. Front. Plant Sci. 2022, 13, 958029. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Yuan, Z. Gamma-aminobutyric acid improves cold tolerance of wheat seedlings. Plant Soil Environ. 2025, 71, 441–452. [Google Scholar] [CrossRef]
- Priya, M.; Sharma, L.; Kaur, R.; Bindumadhava, H.; Nair, R.M.; Siddique, K.H.M.; Nayyar, H. GABA (γ-aminobutyric acid), as a thermo-protectant, to improve the reproductive function of heat-stressed mungbean plants. Sci. Rep. 2019, 9, 7788. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Zheng, S.; Li, L.; Liu, X.; Feng, L.; Huang, Q.; Ma, C.; Zhang, Y.; Yan, G.; et al. The GABA-modulated energy metabolism reconfiguration positively regulates cotton (Gossypium hirsutum L.) responses during post-waterlogging recovery. Ind. Crop. Prod. 2025, 231, 121223. [Google Scholar] [CrossRef]
- Salah, A.; Zhan, M.; Cao, C.; Han, Y.; Ling, L.; Liu, Z.; Li, P.; Ye, M.; Jiang, Y. γ-aminobutyric acid promotes chloroplast ultrastructure, antioxidant capacity, and growth of waterlogged maize seedlings. Sci. Rep. 2019, 9, 484. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Kakavand, F.; Rabiei, V.; Zaare-Nahandi, F.; Razavi, F. γ-Aminobutyric acid and nitric oxide treatments preserve sensory and nutritional quality of cornelian cherry fruits during postharvest cold storage by delaying softening and enhancing phenols accumulation. Sci. Hortic. 2019, 246, 812–817. [Google Scholar] [CrossRef]
- Rastegar, S.; Khankahdani, H.H.; Rahimzadeh, M. Effect of γ-aminobutyric acid on the antioxidant system and biochemical changes of mango fruit during storage. J. Food Meas. Charact. 2020, 14, 778–789. [Google Scholar] [CrossRef]
- Wu, X.; Huo, R.; Yuan, D.; Zhao, L.; Kang, X.; Gong, B.; Lü, G.; Gao, H. Exogenous GABA improves tomato fruit quality by contributing to regulation of the metabolism of amino acids, organic acids and sugars. Sci. Hortic. 2024, 338, 113750. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Wei, B.; Cheng, S.; Zhou, Q.; Ji, S. GABA application improves the mitochondrial antioxidant system and reduces peel browning in ‘Nanguo’ pears after removal from cold storage. Food Chem. 2019, 297, 124903. [Google Scholar] [CrossRef]
- Pace, R.; Benincasa, P. Effect of salinity and low osmotic potential on the germination and seedling growth of rapeseed cultivars with different stress tolerance. Ital. J. Agron. 2010, 5, 69–77. [Google Scholar] [CrossRef]
- Zhang, S.; Khan, A.; Zhao, L.; Feng, N.; Zheng, D.; Shen, X. Effect of GABA on seed germination and seedling growth of rapeseed under salt stress. Research Square 2023. [Google Scholar] [CrossRef]
- Kumar, N.; Gautam, A.; Dubey, A.K.; Ranjan, R.; Pandey, A.; Kumari, B.; Singh, G.; Mandotra, S.; Chauhan, P.S.; Srikrishna, S. GABA mediated reduction of arsenite toxicity in rice seedling through modulation of fatty acids, stress responsive amino acids and polyamines biosynthesis. Eur. Food Res. Technol. 2019, 173, 15–27. [Google Scholar] [CrossRef]
- Adolf, V.I.; Jacobsen, S.-E.; Shabala, S. Salt tolerance mechanisms in quinoa (Chenopodium quinoa Willd.). Environ. Exp. Bot. 2013, 92, 43–54. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Tyerman, S.D.; Gilliham, M.; Xu, B. γ-Aminobutyric acid (GABA) signalling in plants. Cell. Mol. Life Sci. 2017, 74, 1577–1603. [Google Scholar] [CrossRef]
- Baum, G.; Lev-Yadun, S.; Fridmann, Y.; Arazi, T.; Katsnelson, H.; Zik, M.; Fromm, H. Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO J. 1996, 15, 2988–2996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Geng, S.; Zhang, X. A review of soil waterlogging impacts, mechanisms, and adaptive strategies. Front. Plant Sci. 2025, 16, 1545912. [Google Scholar] [CrossRef] [PubMed]
- Li, L.J.; Jia, L.T.; Zhang, P.; Lyu, D.G.; Qin, S.J. γ -Aminobutyric acid promotes aerobic respiration and antioxidant capacity of waterlogged Cerasus sachalinensis roots. Acta Hortic. 2024, 1408, 397–406. [Google Scholar] [CrossRef]
- Lorente-Mento, J.M.; Valero, D.; Martínez-Romero, D.; Badiche, F.; Serrano, M.; Guillén, F. Preharvest multiple applications of GABA improve quality traits and antioxidant compounds of pomegranate fruit during storage. Horticulturae 2023, 9, 534. [Google Scholar] [CrossRef]
- Wu, X.; Jia, Q.; Ji, S.; Gong, B.; Li, J.; Lü, G.; Gao, H. Gamma-aminobutyric acid (GABA) alleviates salt damage in tomato by modulating Na+ uptake, the GAD gene, amino acid synthesis and reactive oxygen species metabolism. BMC Plant Biol. 2020, 20, 465. [Google Scholar] [CrossRef]
- Fan, Z.; Lin, B.; Lin, H.; Lin, M.; Chen, J.; Lin, Y. γ-Aminobutyric acid treatment reduces chilling injury and improves quality maintenance of cold-stored Chinese olive fruit. Food Chem. 2022, 13, 100208. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Huang, X.; Yang, K.; Gao, S.; Du, R. Effect of exogenous γ-aminobutyric acid (GABA) treatment on chilling injury and antioxidant capacity in banana peel. Sci. Hortic. 2014, 168, 132–137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.