Abstract
Climate change and widespread micronutrient deficiencies threaten food security in the semi-arid tropics. Finger millet (Eleusine coracana (L.) Gaertn.) is a climate-resilient “nutri-cereal” rich in calcium, zinc, iron and dietary fiber. Finger millet is a promising crop for addressing climate stress and nutrient deficiencies. However, it remains under-explored and relatively neglected in breeding and genetic improvement programs compared to major cereals. This review synthesizes recent biotechnological advances and outlines future directions for finger millet improvement. Foundational resources now include a chromosome-scale reference genome, expanding transcriptome, diverse global germplasm panels, and growing reports of genome-wide association studies (GWAS) and quantitative trait loci (QTL) for key traits including yield, stress tolerance, blast resistance, and mineral contents. Tissue culture studies reported both somatic embryogenesis and direct regeneration. Stable genetic transformation has been achieved in finger millet via Agrobacterium-mediated methods, particularly using shoot apical meristem (SAM) and by biolistics (gene gun) methods. Genome editing has not yet been reported, but we propose a practical roadmap leveraging reported tissue culture genetic transformation protocols for applying the CRISPR/Cas system for trait improvements. Using new biotechnological methods, along with pangenome, speed breeding, and helpful microbiomes, will make finger millet a strong and reliable food source for the future.
1. Introduction
Rising global temperatures and increasingly erratic rainfall patterns have emerged as major constraints to sustainable food production in the semi-arid tropics. Climate projections indicate a global mean temperature increase of 1.5–2.0 °C by mid-century, intensifying droughts, heat waves, and irregular monsoon events []. These abiotic stresses reduce yield stability and threaten crop productivity [,]. Simultaneously, widespread micronutrient deficiencies, particularly of iron and zinc, affect more than two billion people worldwide, leading to hidden hunger and malnutrition [,]. Conventional cereals such as rice and wheat are calorie-rich but poor sources of essential minerals and are highly vulnerable to climate-induced stresses.
Finger millet (Eleusine coracana L. Gaertn.) is an allotetraploid millet cultivated across South Asia and sub-Saharan Africa. It offers a unique solution to improving climate-resilient agriculture. Finger millet tolerates high temperatures, intermittent drought, and poor soils [,] while providing exceptional levels of mineral nutrients and dietary fiber []. Its resilience and nutritional superiority make it a “climate-smart nutri-cereal” for addressing both environmental stress and micronutrient deficiencies in the context of global warming []. Nutritionally, finger millet surpasses major cereals such as rice and wheat by providing higher levels of calcium, iron, zinc, and dietary fiber []. Nutrient profiling across Asian and African germplasms shows calcium levels ranging from 240 to 450 mg 100 g−1, iron 3–14 mg 100 g−1, zinc 2–4 mg 100 g−1, and dietary fiber 15–20% of seed weight, values that surpass most major cereals [,]. Protein content varies from 5 to 9%, with balanced essential amino acids such as leucine, methionine, and phenylalanine []. Finger millet is also rich in polyphenols (0.3–3%) and antioxidants, conferring strong nutraceutical properties including anti-diabetic, anti-cancer, and cardioprotective potential [,]. Its high calcium and iron bioavailability make it particularly valuable for women, children, and elderly populations in Africa and South Asia, where micronutrient deficiencies are prevalent [,]. Its low glycemic index, gluten-free nature, and high levels of essential amino acids, especially methionine and tryptophan, further enhance its value in balanced diets []. Beyond nutrition, the crop’s deep root system, efficient water-use, and ability to perform in poor soils contribute to sustainable farming under extreme climatic conditions [,]. These features position finger millet as a strategic crop for addressing hidden hunger and ensuring climate-smart food security, particularly across sub-Saharan Africa and South Asia, where both nutrient deficiencies and environmental stress are widespread [].
Despite its nutritional value, finger millet has remained relatively neglected in modern breeding and genetic improvement programs for several decades. It is often cultivated in the semi-arid tropics of less-developed countries in Africa and Asia by subsistence farmers. Grain yields in smallholder farming systems are typically low and unstable, reflecting limited breeding gain []. Finger millet has an allotetraploid genome (2n = 4x = 36; AABB) that further complicates genetic and genomic studies such as inheritance analysis, and marker validation []. For biotechnological improvement, limited studies on genotype-dependent plant regeneration and genetic transformation studies have been reported. More robust genetic transformation protocols are needed for functional validation and trait introgression in finger millet []. Addressing these gaps with genomic resources, genome editing, and genomic selection is essential to understand climate resilience and nutrient-enrichment traits and further improve these traits in finger millet.
Advances in biotechnology now offer unprecedented opportunities to unlock the full genetic potential of finger millet. Emerging resources such as annotated genome [], transcriptomics [], proteomics [] and metabolomics provide molecular insight into stress tolerance and nutrient regulation in millets, including finger millet. Similarly, genome editing, genomic selection, and speed breeding can accelerate varietal improvement beyond the limits of traditional breeding [,]. Only a few studies have reported on genome sequencing, genomic selection, and genetic transformation in finger millet. No report is available on genome editing in finger millet till date. This review synthesizes recent progress in genomic resources, functional gene discovery, tissue culture, and genetic transformation in finger millet. It also draws inroads for CRISPR/Cas-based genome editing in finger millet. It further integrates strategies for nutrient biofortification, abiotic stress resilience, and high-throughput phenotyping for crop improvement. By combining molecular innovations, finger millet can be redefined as a climate-resilient, nutrient-dense cereal suited to future food systems.
2. Genetic and Genomic Resources
Finger millet has progressed from scaffold-level genome assemblies to a modern chromosome-scale reference genome. Early de novo genomes for two genotypes, ML-365 and PR-202, provided data on genome size (~1.1–1.5 Gb), gene catalogs, and abundant SSR/SNP resources useful for mapping and marker development [,]. This is the first report on the draft genome of finger millet. In 2023, the Devos group developed chromosome-level genome assembly for genotype KNE 796 with complete annotations []. It opened avenues for many studies, such as robust synteny analyses, genome-wide association studies (GWAS), QTL mapping, and candidate-gene discovery for stress tolerance and nutrition enrichment traits []. The reference genome with annotations is publicly available in Phytozome []. The transition from scaffold-level to chromosome-scale reference genomes marks a pivotal advancement for finger millet research. Public availability of these resources, such as through Phytozome, accelerates functional genomics, comparative studies, genome editing, and molecular breeding. Looking ahead, this genomic resource will serve as a foundation for trait pyramiding and the integration of allelic diversity from global collections, driving rapid improvement of finger millet for climate-smart and nutritionally enriched agriculture.
Gene banks at ICRISAT, Hyderabad, India and ICAR-NBPGR, India maintain reasonable accessions from global and local collections. Global finger millet accessions are maintained at ICRISAT and researchers from around the world can freely access these for their studies. As of May 2024, the ICRISAT Genebank hosts 7513 conserved accessions (global collection). From these, 622 were developed as core collections and 80 accessions as mini-core to capture maximal allelic richness with minimal redundancy [,]. India’s ICAR-NBPGR holds the largest single collection (~10,507 accessions) from India. These are widely used as a source for pre-breeding and evaluation panels across Indian agroecological conditions []. With the chromosome-scale reference genome now available for finger millet, allele discovered in core and mini-core panels can be mapped to physical coordinates, accelerating GWAS, and marker deployment []. The divided panels, along with regional diversity sets from Africa and South Asia, offer effective, statistically robust subsets for multi-location phenotyping for drought and heat tolerance, blast resistance, and the fortification of grain with Ca, Fe, and Zn. It will provide a connection from conserved diversity to marker-assisted selection and genomic selection pipelines [,]. With extensive, globally accessible finger millet germplasm and a chromosome-scale reference genome, future research will harness core and mini-core panels for high-resolution trait mapping and accelerated marker deployment. These resources will drive genomic selection, enabling rapid breeding for stress resilience, disease resistance, and enhanced nutritional quality in diverse environments. A few transcriptome resources were also developed for finger millet under biotic and abiotic stresses (Figure 1; Table 1). Transcriptome analyses in finger millet under salinity, drought, and aluminum toxicity revealed key regulators, including DREB, NAC, WRKY, and HSF transcription factors, ion-homeostasis, ABA-signaling, and transporter genes controlling stress adaptation [,,].
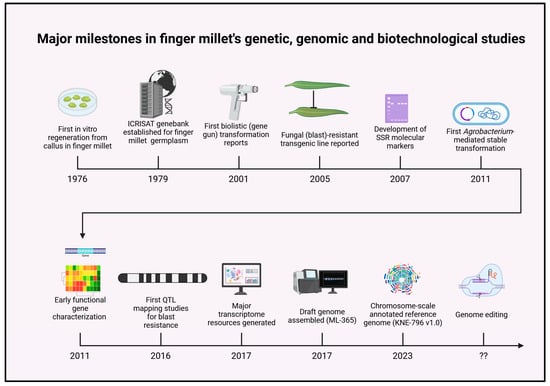
Figure 1.
Major milestones in finger millet’s genetic, genomic, and biotechnological studies. The journey began in 1976 with the first successful in vitro regeneration from callus cultures, laying the foundation for genetic manipulation. In 1979, the establishment of the ICRISAT genebank enabled global conservation and accessibility of finger millet germplasm. The first reports of biolistic (gene gun) transformation emerged in 2001, followed by the creation of blast-resistant transgenic lines in 2005, marking a breakthrough in trait improvement. By 2007, the development of SSR molecular markers facilitated genetic diversity assessment and linkage mapping. A major advance came in 2011, when Agrobacterium tumefaciens–mediated stable transformation was achieved. Subsequent years (2011–2016) saw early functional gene characterization and QTL mapping for blast resistance. In 2017, the generation of major transcriptome datasets and the draft genome assembly (ML-365) provided essential genomic resources. The release of the chromosome-scale annotated reference genome (KNE-796 v1.0) in 2023 marked a new era in functional and comparative genomics. Looking ahead, CRISPR/Cas-based genome editing represents a transformative, prospective milestone for precise trait improvement and accelerated breeding in finger millet.

Table 1.
Information about QTL, genes, transcriptomes, and molecular markers linked to abiotic stress resilience in finger millet, along with references.
Finger millet proteomics is not yet fully established and not many reports are available. An integrated RNA-seq and iTRAQ study mapped more than 3000 differentially expressed proteins under drought, implicating ABA signaling, osmolyte metabolism, chaperones, and redox enzymes as core response nodes []. Protein profiling of seed samples via LC–MS/MS analysis discovered over 450 proteins, emphasizing nutritionally significant and stress-responsiveness and defense categories in relationship with rice []. At the peptide scale, researchers reported two antioxidant peptides purified from finger-millet protein hydrolysates and validated their activities []. A recent review summarizes current methods and gaps in millet proteomics, highlighting key priorities such as improved quantification, advances in phospho-proteomics, and the establishment of standardized data protocols []. Together, these resources create a comprehensive foundation for linking genetic variation to protein expression. This integrated approach enables robust allele-to-protein validation, which is essential for accelerating the development of climate-resilient finger millet varieties through targeted breeding strategies. By facilitating a better understanding of the molecular mechanisms underlying stress tolerance, these advances support the breeding of crops better suited to withstand changing environmental conditions.
Finger millet QTL mappings have advanced significantly, evolving from initial SSR and DArT marker scans to high-resolution, SNP-dense GWAS anchored on a chromosome-scale reference genome. This progression has greatly improved the precision and efficiency of identifying genomic loci associated with important agronomic traits. Early mapping efforts using diversity panels identified loci associated with leaf blast resistance and key agronomic traits. For example, markers such as UGEP101 and UGEP95 served as proof-of-concept for the application of marker-assisted selection (MAS) in finger millet []. A subsequent high-density linkage map further refined QTL associated with yield components and stress-related indices, including canopy traits linked to senescence and stay-green characteristics []. This increased mapping resolution has enhanced the precision of breeding efforts aimed at improving these important traits. For biofortification, multi-environment GWAS pinpointed genomic regions controlling grain iron and zinc content, resulting in the identification of diagnostic SNPs for marker–trait association (MTA) validation. This progress supports more targeted breeding strategies to enhance micronutrient levels in finger millet []. Calcium enrichment in finger millet has been investigated through GWAS and association mapping, leading to the identification of candidate transporter and regulatory genes. These studies have also produced breeder-friendly markers, facilitating the selection of calcium-rich lines in breeding programs [,]. Collectively, these studies illustrate a coherent pipeline for trait improvement in finger millet. The process starts with the discovery of traits in core, minicore, or regional panels, moves forward to identify SNPs and QTLs under drought and disease stresses, and concludes with multi-location validation. This process will lead to the deployment of these markers through MAS and genomic selection. This systematic approach enables the development of stay-green, stress-resilient, and micronutrient-rich ideotypes, advancing both productivity and nutritional quality in finger millet breeding programs.
3. Physiological and Molecular Basis of Stress Resilience
Finger millet demonstrates strong resilience to abiotic stresses through mechanisms such as osmotic adjustment, enhanced antioxidant defenses, and hormonal cross-talk [,]. Physiologically, drought-tolerant genotypes maintain higher relative water content, chlorophyll content, and membrane stability, together with better photosynthetic performance under water deficit conditions [,]. Enhanced root system architecture and associated traits, together with improved water-use efficiency, enable sustained carbon assimilation even during prolonged dry spells in finger millet [,]. Osmotic adjustment via proline and soluble sugars, together with Na+ exclusion and maintenance of K+/Na+ homeostasis, preserves cellular hydration in the finger millet. This also maintains stomatal conductance and gas-exchange efficiency in finger millet during salinity stress [,,,]. Heat stress tolerance in tolerant genotypes is associated with higher membrane stability and chlorophyll content, as well as molecular responses such as (dehydration-responsive element-binding protein 2A) EcDREB2A-mediated reactive oxygen species (ROS) scavenging [,]. These together support photoprotection and reduced cellular damage during heat stress in finger millet.
During drought conditions, the accumulation of compatible solutes like proline and sugars helps maintain cellular turgor and stabilizes metabolic processes, contributing to the finger millet’s ability to tolerate water deficit stress []. Integrated transcriptome–proteome analyses have revealed the upregulation of genes involved in osmolyte biosynthesis, ROS detoxification, and the production of defense proteins in finger millet []. These molecular responses underpin finger millet’s ability to adapt and respond to abiotic stresses such as drought. Overexpression of finger millet’s calmodulin (EcCaM) in Arabidopsis plants resulted in enhanced drought tolerance, characterized by increased proline accumulation and increased sensitivity to abscisic acid (ABA) []. These traits contribute to improved stress adaptation under water-limited conditions in finger millet. Late embryogenesis abundant (LEA) proteins also play a crucial role in dehydration tolerance by helping to maintain membrane integrity during water deficit conditions []. This protective function supports cell survival under stress in finger millet. Heat stress leads to protein denaturation and the accumulation of ROS. Finger millet and related millets mitigate these effects through the action of heat-shock transcription factors (HSTFs), which activate molecular chaperones such as heat-shock protein 70 (HSP70) and HSP90, as well as antioxidant systems []. Together, these responses help sustain chloroplast integrity and support stay-green phenotypes under high-temperature conditions in finger millet. Salinity stress causes both osmotic and ionic imbalances, primarily due to increased Na+ influx. Finger millet mitigates these effects through Na+ exclusion mediated by HKT1;5 transporters and by vacuolar sequestration via NHX antiporters []. Coordinated signaling by abscisic acid (ABA), jasmonic acid (JA), and salicylic acid (SA) integrates these stress responses with ROS signaling, allowing for precise regulation and fine-tuning of adaptation to abiotic stress conditions in finger millet.
4. Biotechnological Tools for Finger Millet
4.1. In Vitro Regeneration
Tissue culture techniques have provided the foundation for genetic improvement, genome editing, and functional genomics studies in finger millet. The delivery of constructs and the regeneration of transgenic plants are achieved via tissue culture-based methods. Developing an efficient regeneration protocol is essential for the genetic transformation, genome editing, and regeneration of transgenic plants in finger millet. Early studies demonstrated that both direct organogenesis and somatic embryogenesis (SE) can be achieved from various explants of finger millet, including shoot apices, immature inflorescences, and embryos. Eapen and George (1989) first reported high-frequency somatic embryogenesis from immature inflorescences using 2,4-dichlorophenoxyacetic acid (2,4-D) and kinetin [], later refined by George and Eapen (1990) through optimized auxin–cytokinin balance []. Ceasar and Ignacimuthu (2008) achieved consistent SE and plantlet recovery across Indian genotypes []. Poddar et al. (1997) and Kothari et al. (2004) showed that modifying ammonium nitrate or inorganic salts enhanced embryogenic callus formation and shoot induction [,]. Satish et al. (2015) established direct regeneration from shoot apical meristems using 6-Benzylaminopurine (BAP), low 2,4-D and spermidine [,]. Although these studies demonstrate efficient regeneration from diverse explants, the principal limitation remains the regeneration of stable transgenic plantlets from transformed calli. As a result, the stable transformation for finger millet still exhibits low frequency.
4.2. Genetic Transformation of Finger Millet
Genetic transformation in finger millet has been accomplished using both Agrobacterium tumefaciens-mediated and biolistic (gene gun) methods (Table 2). Early Agrobacterium transformation efforts with shoot-apex explants achieved stable transformation rates of approximately 3.5–3.9%. Transient GUS expression was observed in about 16–19% of explants [] (Figure 1). These efficiencies were obtained by optimizing factors such as acetosyringone concentration, co-culture duration, and the inclusion of antioxidants like 100 µM L-cysteine. Building on earlier methods, a shoot apical meristem (SAM) protocol was developed that combined Agrobacterium infection with direct regeneration, eliminating the need for a callus phase []. This approach shortened the regeneration cycle to approximately 45 days from SAM to greenhouse-ready plant and increased stable transformation efficiency to around 11–12% in responsive cultivars. The protocol also yielded several single-copy transgenic events, with T1 progeny exhibiting the expected 3:1 segregation ratio []. These SAM-based transformation systems could be used for trait stacking and for developing genome editing works in finger millet. However, transformation efficiency still varies among genotypes, highlighting ongoing challenges related to genotype dependence.
A second Agrobacterium-mediated transformation strategy, developed by the Kothari group, utilized seed-derived regenerative callus as the target tissue []. By optimizing parameters such as infection density, acetosyringone concentration, co-culture duration, and incorporating CuSO4 into the regeneration medium, they enhanced T-DNA delivery and increased the recovery rate of stable transformants in finger millet []. Under optimized conditions, including lower incubation temperatures around 22 °C, the group achieved transformation efficiencies of up to approximately 44% at the callus stage []. However, the stable recovery of whole plants from these calli was lower, and the overall process was more time-consuming compared to the faster SAM-based protocols.
The SAM-based Agrobacterium approach offers faster transformation cycles (~45 days) and favors single-copy, Mendelian insertions, though it remains genotype-dependent. However, the Agrobacterium-mediated transformation in finger millet remains relatively inefficient compared with rice. Using shoot-apex explants, only 3.8% stable transformation was achieved [], later improved to 11.8% through protocol refinement []. The callus-based systems reached ~44% transformation frequency but with a variable stable plant recovery stage [,]. These comparisons highlight that efficient regeneration of stable transgenic plants remains a major bottleneck in finger millet, despite incremental methodological advances.
For biolistic transformation in finger millet, parameters were optimized using seed-derived callus as the target tissue, such as 1.0 µm gold particles, two shots, ~1100 psi rupture disk pressure, a 3 cm macrocarrier gap, 12 cm flight distance, and 0.4 M sorbitol treatment for osmotic conditioning before and after bombardment []. Under these conditions, a transformation efficiency of approximately 45.3% (based on selection) was achieved, with transgene integration in T0 plants confirmed by Southern blot and stable transmission observed in the T1 generation []. Biolistic transformation is less sensitive to genotype differences and accommodates larger DNA constructs but typically results in higher copy number and more variable transgene insertions compared to Agrobacterium-mediated methods.
A few traits have also been engineered in finger millet via genetic transformation. For example, blast resistance was achieved by introducing a crustacean PIN antifungal protein using biolistics []. Additionally, the rice chitinase 11 (chi11) gene was introduced via Agrobacterium-mediated transformation, resulting in 3.5–3.9% stable transformation rates []. For abiotic stress tolerance, overexpression of the sorghum sorghum bicolor Vacuolar H+-Pyrophosphatase (SbVPPase) gene in finger millet led to improved salt tolerance, enhanced ion homeostasis, and increased antioxidant enzyme activities []. In parallel, several finger millet transcription factors have been functionally validated by expression in other plants, demonstrating their regulatory roles in abiotic stress adaptation. For instance, NAC Domain-Containing Protein 67 (EcNAC67) conferred enhanced drought and salinity tolerance by improving osmotic adjustment and maintaining photosynthetic efficiency in transgenic rice []. Similarly, Basic Leucine Zipper Domain-Containing Protein 17 (EcbZIP17) expression in tobacco plants promoted improved growth, antioxidant activity, and multi-stress resilience under adverse environmental conditions [].

Table 2.
Details on genetic transformation studies reported in finger millet. The method of transformation, explants used, strain, plasmid, genes, and transformation efficiency are listed with references.
Table 2.
Details on genetic transformation studies reported in finger millet. The method of transformation, explants used, strain, plasmid, genes, and transformation efficiency are listed with references.
| Year | Method of Transformation | Explant | Strain | Plasmid | Selection Gene | Reporter Gene | Candidate Gene | Reference |
|---|---|---|---|---|---|---|---|---|
| 2001 | Biolistic method | Seed callus | - | - | - | ActI/UqI/RbcS/Ft/uidA | - | [] |
| 2005 | Biolistic method | Embryogenic calli | - | pPur | bar | uidA | PIN | [] |
| 2006 | Biolistic method | Shoot tip callus | - | TG0063 of pCAMBIA series | - | - | PcSRP | [] |
| 2011 | Agrobacterium-mediated | Shoot apex | LBA4404 | pCAMBIA1301 | HptII | uidA | - | [] |
| 2011 | Agrobacterium-mediated | Seed callus | LBA4404 | pBI-121 | nptII | uidA | - | [] |
| 2011 | Agrobacterium-mediated | Seed callus | EHA105 | pCNL-56 | nptII | uidA | - | [] |
| 2012 | Agrobacterium-mediated | Shoot apex | LBA4404 | pHyg-Chi.11 | HptII | - | Chi11 | [] |
| 2014 | Agrobacterium-mediated | Seed callus | LBA4404 | pCAMBIA1301 | HptII | uidA | SbVPPase | [] |
| 2014 | Agrobacterium-mediated | Seed callus | EHA105 | pCAMBIA1380 | HptII | uidA | mtlD | [] |
| 2017 | Agrobacterium-mediated | Shoot apex | EHA105 | pCAMBIA1301 | HptII | uidA | - | [] |
Seed-callus transformation methods expand the range of amenable genotypes, while biolistics accommodates larger genetic constructs and recalcitrant varieties, although with a greater risk of high copy-number insertions. Current priorities include developing genotype-flexible regeneration systems, expanding gene editing-ready germplasm resources, and achieving low-copy transgene delivery for complex trait stacking. Advancements such as standardized, high-throughput SAM pipelines, use of morphogenic regulators, and precise developmental-stage control hold promise for improving transformation frequencies. Looking ahead, CRISPR/Cas systems with DNA-free delivery are expected to facilitate more efficient and precise trait stacking in finger millet breeding programs.
5. Prospectus for CRISPR-Based Genome Editing in Finger Millet
Finger millet offers an excellent test material for precise gene editing because it combines nutritional promise with climate-resilience. Finger millet is a nutritionally rich grass that serves millions in semi-arid regions, yet genetic improvement has progressed slowly. No report is available to date on genome editing in finger millet. Recent advances in CRISPR/Cas technologies across foxtail millet, sorghum, maize, rice, and wheat now provide clear blueprints for precise gene editing in these crops. The following section synthesizes these lessons and maps a practical gene editing pathway for finger millet from target selection to edited line release.
The prospects for deploying CRISPR/Cas system in finger millet are highly promising. The crop’s established compatibility with Agrobacterium-mediated transformation of shoot apices, coupled with efficient direct regeneration protocols, provides a reliable entry point for the delivery of genome-editing tools such as CRISPR/Cas9, base editor, and prime editor (Figure 2) [,]. This foundation enables precise gene editing and accelerates the development of improved finger millet varieties. In sorghum, CRISPR/Cas-mediated gene edits have been successfully introduced using Agrobacterium-mediated delivery in embryo-derived tissues, resulting in reliable inheritance of the targeted modifications [,]. These studies provide valuable practical guidelines for guide RNA (gRNA) design, selectable marker strategies, and optimized tissue culture schedules, all of which can be adapted to accelerate genome editing efforts in finger millet (Figure 2).
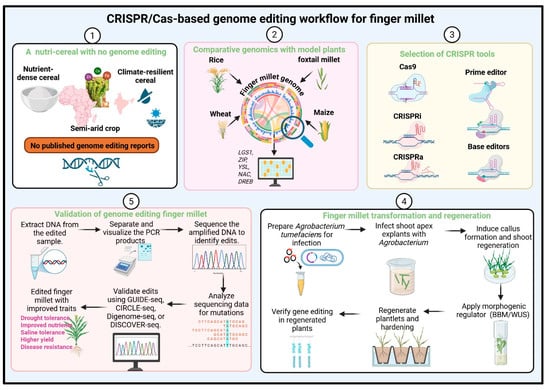
Figure 2.
Proposed CRISPR/Cas-based genome editing workflow for finger millet. This schematic outlines the stepwise strategy for establishing genome editing in finger millet, a nutrient-dense, climate-resilient cereal with no published genome editing reports. (1) Context and significance: Finger millet is a semi-arid crop rich in micronutrients (Fe, Zn, and Ca) and resilient to stress but lacks reported genome editing studies. (2) Target discovery through comparative genomics: Candidate genes (e.g., LGS1, ZIP, YSL, NAC, DREB) are identified by comparison with model cereals including foxtail millet, rice, maize, and wheat. (3) Selection of CRISPR tools: suitable editors such as Cas9, CRISPR interference (CRISPRi), CRISPR activation (CRISPRa), base editors, and prime editors are chosen depending on the desired modification. (4) Transformation and regeneration: Shoot apex explants are infected with Agrobacterium tumefaciens, followed by callus induction, shoot regeneration, and optional application of morphogenic regulators (BBM, WUS) to enhance transformation efficiency. Edited plantlets are regenerated and verified. (5) Validation and trait assessment: Edits are confirmed using PCR and sequencing-based assays (GUIDE-seq, CIRCLE-seq, Digenome-seq, or DISCOVER-seq). Edited lines are evaluated for improved traits such as drought tolerance, nutrient enrichment, salinity tolerance, higher yield, and disease resistance.
Sorghum serves as a model for trait-to-gene targeting by genome editing, particularly through the strigolactone pathway. Key genes such as LOW GERMINATION STIMULANT 1 (LGS1) have been shown to confer field-relevant resistance to Striga (a major parasitic weed), as proved by targeted gene editing [,]. These genetic pathways are preserved across grass species, including finger millet, establishing a definitive framework for adapting trait-based gene editing methodologies to enhance resistance in finger millet. In foxtail millet, researchers have achieved efficient genome editing, including base editing of herbicide target alleles and targeted modifications in the MATRILINEAL gene [,,]. Edits in MATRILINEAL have enabled the development of doubled haploid pipelines, allowing for rapid fixation of edited alleles in foxtail millet []. These advances demonstrate the potential for accelerated breeding and trait improvement using CRISPR/Cas tools in finger millet. Advances in maize and wheat have demonstrated the power of base and prime editing for highly precise genome modifications, such as promoter modifications and targeted point mutations. Notable achievements include the landmark engineering of the ARGOS8 promoter to enhance drought tolerance and robust knockout of the MLO gene for broad-spectrum disease resistance in maize, wheat and rice [,,,]. These approaches provide valuable templates for applying precise genome editing in finger millet to improve key agronomic traits. For finger millet, immediate priorities include establishing routine construct delivery systems in meristematic tissues, utilizing CRISPR/Cas tools to enhance nutritional traits, particularly in carotenoid and folate pathways, as well as to initiate pilot projects with prime editing to introduce precise promoter and coding sequence modifications. The creation and sharing of specialized toolkits by millet or cereal researchers, such as reliable CRISPR/Cas constructs tested in cereals, efficient pegRNA and base editor systems from foxtail millet, and flexible methods for transforming sorghum, could help develop efficient genome editing systems for finger millet. These collaborative efforts could make finger millet genome editing more standardized, efficient, and reproducible and offer useful models for improving finger millet. These will be instrumental in efficiently transferring targeted edits into farmer-preferred genotypes, reducing the number of breeding cycles required for trait integration. CRISPR/Cas base and prime editing technologies offer the ability to precisely refine finger millet alleles in key transporter and transcription factor genes without introducing foreign DNA. For example, cytosine or adenine base editors can induce specific amino acid changes in ZIP and YSL transporter proteins, potentially enhancing iron and zinc loading in the grain. This targeted approach enables the improvement of nutritional traits while maintaining the crop’s genetic integrity. Promoter engineering can modulate SWEET and NRT expression to balance carbon and nitrogen flux. Prime editing can install or repair functional motifs in NAC, DREB, and bZIP regulators that govern drought and grain filling in finger millet.
The gRNA expression in finger millet could begin with endogenous RNA polymerase III promoters (U6 and U3), as they yield precise small RNA transcripts with reliable initiation and termination. Evidence from rice, sorghum, and other grasses shows that U6 and U3 promoters drive strong editing and multiplexing and often work across cereals [,,]. When native U6 and U3 promoter sequences are not yet available for finger millet, orthologous promoters from rice or maize can be effectively used to drive gRNA expression. It is important to avoid long thymidine (T) tracts in the gRNA scaffold and to ensure that U6-driven gRNA begin with a guanine (G) and U3-driven gRNA start with an adenine (A) for optimal transcription efficiency [,,]. If RNA polymerase III-driven gRNA expression proves suboptimal, alternative strategies can be employed by expressing gRNAs from RNA polymerase II promoters in finger millet. This can be achieved by flanking the gRNAs with hammerhead and hepatitis delta ribozymes or by using tRNA spacers, both of which ensure accurate processing of the gRNAs [,]. These approaches also facilitate high-order multiplexing, allowing multiple gRNAs to be expressed simultaneously under strong promoters such as maize ubiquitin or rice actin, thereby increasing genome editing efficiency in finger millet.
Rigorous validation of genome edits in finger millet should establish a clear connection between edit specificity and trait-level performance. The process should begin with in silico gRNA selection to maximize on-target efficiency and minimize off-target effects. This should be followed by confirmation of edits at both on-target and potential off-target sites using amplicon deep sequencing [,]. Quantitative analysis tools such as CRISPResso2 can then be used to measure indel spectra and allele frequencies, ensuring accurate assessment of editing outcomes and their correlation with phenotypic traits. In addition to targeted validation, it is essential to incorporate unbiased discovery of genome editing outcomes in finger millet using genome-wide assays. Techniques such as GUIDE-seq in living cells, CIRCLE-seq as an in vitro screen, Digenome-seq for biochemical footprinting, and DISCOVER-seq to monitor chromatin-bound repair factor activity provide comprehensive assessment of both on-target and off-target events [,,,]. Integrating these approaches ensures a robust evaluation of edit specificity and genome integrity, supporting safe and effective deployment of genome editing technologies in finger millet improvement. Together these provide complementary coverage across sequence and chromatin contexts. Phenotyping should be conducted across both controlled and managed stress environments, utilizing standardized assays of abiotic stresses. High-throughput measurements such as canopy temperature and fluorescence imaging, combined with multi-time point assessments, will provide detailed insights into the physiological and agronomic impacts of genome edits []. This comprehensive phenotyping approach ensures robust evaluation of trait performance under relevant environmental conditions.
6. Challenges and Future Prospects for Biotechnological Improvement of Finger Millet
Finger millet research faces bottlenecks that begin with low transformation and regeneration efficiencies. These limit genotype coverage and slow the editing process, despite progress with shoot apex systems and direct regeneration. The crop is an allotetraploid with subgenomes that contain extensive duplication and high repeat content, which complicates gRNA design because paralogs and homeologs create multiple, closely related targets and increase the need for multiplexed edits to achieve complete editing. Experience from wheat shows that coordinated editing of homeoalleles is feasible but requires careful gRNA placement and delivery that supports concurrent cleavage, using tRNA or ribozyme processing to express several gRNAs from compact cassettes [,]. Base editors enable precise allele refinement without introducing double-strand breaks, but their effective use requires subgenome-level targeting and rigorous off-target assessment, especially in repetitive genomic regions []. Developing a high-quality pangenome and subgenome-level annotations for finger millet will significantly reduce false positives during in silico gRNA prediction and enhance the accuracy of linking specific edits to phenotypic outcomes, ultimately supporting more efficient and reliable genome editing efforts.
Future research in finger millet should harness artificial intelligence (AI) for guided target discovery, leveraging pangenome-based selection to identify and prioritize gene targets for editing. Despite advances in tissue culture, transformation, and genome editing, finger millet improvement still faces low regeneration efficiency, genotype dependence, and limited genomic resources. Future efforts should integrate biotechnology with AI for predictive modeling of gene–trait associations and stress responses (Figure 3). Machine-learning approaches using multi-omics and environmental data can accelerate genotype selection, optimize transformation conditions, and enhance stress-tolerance prediction [,]. Such AI-driven biotechnological frameworks will enable precision breeding and rapid trait deployment for climate-resilient finger millet cultivars. AI can accelerate CRISPR/Cas construct design pipelines for finger millet. Deep-learning models trained on large editing datasets can help predict gRNA on-target efficiency, off-target risk, and PAM-proximal base preferences, enabling in silico screening of thousands of gRNAs before wet-lab work []. This will save a lot of time, labor and cost for finger millet genome editing. For transporters and metabolic enzymes controlling nutrient use or stress tolerance, AI models can prioritize functional codons of genes or regulatory motifs in promoters for base or prime editing to enhance activity or regulation []. In finger millet, combining these AI-CRISPR tools with existing genome and transcriptome resources would rapidly pinpoint edit sites linked to drought, salinity and micronutrient traits [].
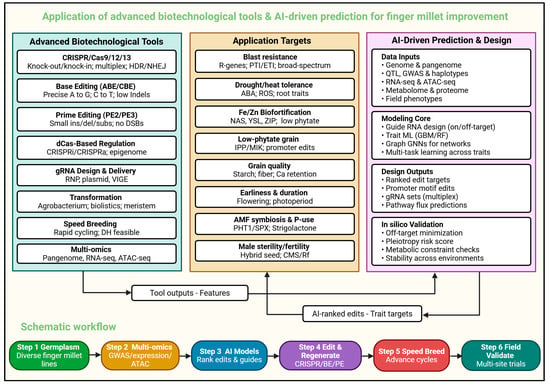
Figure 3.
Application of advanced biotechnology tools and AI-driven prediction in finger millet. The left panel compiles advanced biotechnological tools such as CRISPR/Cas nucleases, base and prime editors, CRISPRi/a regulators, RNAi/VIGS/VOX, Agrobacterium and biolistic transformation, robust regeneration pipelines, and speed breeding—which both generate actionable datasets and enable precise genome modifications. The right panel outlines an AI-driven design stack that integrates multi-omics and phenotyping like genome and pangenome resources, QTL/GWAS haplotypes, RNA-seq and ATAC-seq, metabolome/proteome profiles, and field phenotypes. Machine-learning modules such as guide on/off-target predictors, gradient-boosting and random-forest trait models, graph neural networks for regulatory and co-expression networks, and sequence transformers prioritize trait-linked genes, propose promoter-motif edits, assemble multiplex gRNA sets, and draft HDR/prime-editing donors, followed by in silico validation of off-targets, pleiotropy, metabolic constraints, and environmental stability. The central panel lists breeding targets like blast resistance; drought and heat tolerance; Fe and Zn biofortification and low-phytate grain; improved grain quality; earliness; lodging resistance; AMF symbiosis and phosphorus-use; and reproductive control for hybrid seed. The bottom schematic summarizes the schematic workflow.
Efforts should also focus on designing crop-supportive microbiomes that enhance nutrient uptake and stress tolerance and on deploying improved varieties within climate-smart agricultural systems. Importantly, aligning research outcomes with supportive policy frameworks and active farmer participation will be crucial to ensure that scientific advancements translate into tangible benefits in farmers’ fields and contribute to sustainable food systems. AI can prioritize causal genes and regulatory variants when it is trained on multi-omics data and paired with genomic prediction. Deep learning and machine learning models already improve genomic prediction across crops and they can be extended to recommend genotype-by-environment matches for diverse agroecologies where finger millet is grown [,] Pangenome resources reveal the presence or absence of variation and structural haplotypes that underlie adaptation, and a finger millet pangenome would allow for association tests that do not miss nonreference alleles [].
Microbiome engineering can complement genetics by improving phosphorus and nitrogen acquisition and by improving abiotic stress tolerance in finger millet. Core strategies include selection of phosphate-solubilizing bacteria that release mineral phosphorus and deployment of arbuscular mycorrhizal fungi that expand the effective root surface for nutrient capture and modulate water relations during drought [,,]. Future designs should treat the plant and microbiome as a key strategy and should use synthetic communities that are isolated from successful finger millet farms. Integration into climate-smart low-input systems requires agronomy that stabilizes yield while reducing external inputs. Ecological and sustainable intensification principles point to rotations with legumes, residue retention that feeds soil carbon, precise placement of small nutrient doses, and water-saving practices that fit rainfed conditions where finger millet grows well [,]. Robust phenotyping of canopy temperature, spectral indices, and ionomics should accompany variety and microbiome packages to confirm that physiological gains translate to nutrition and resilience outcomes in farmer fields. These collective efforts will ultimately place finger millet as a prominent climate-resilient and nutrient-dense crop suited for future climates.
Funding
This research received no external funding.
Data Availability Statement
No new data were created for this work.
Acknowledgments
The author thank Rajagiri College of Social Sciences for all supports.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Arias, P.; Bellouin, N.; Coppola, E.; Jones, R.; Krinner, G.; Marotzke, J.; Naik, V.; Palmer, M.; Plattner, G.-K.; Rogelj, J. Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Technical Summary; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2021. [Google Scholar]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of Extreme Weather Disasters on Global Crop Production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y. Temperature Increase Reduces Global Yields of Major Crops in Four Independent Estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2020: Transforming Food Systems for Affordable Healthy Diets; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Muthayya, S.; Rah, J.H.; Sugimoto, J.D.; Roos, F.F.; Kraemer, K.; Black, R.E. The Global Hidden Hunger Indices and Maps: An Advocacy Tool for Action. PLoS ONE 2013, 8, e67860. [Google Scholar] [CrossRef]
- Gupta, S.M.; Arora, S.; Mirza, N.; Pande, A.; Lata, C.; Puranik, S.; Kumar, J.; Kumar, A. Finger Millet: A “Certain” Crop for an “Uncertain” Future and a Solution to Food Insecurity and Hidden Hunger under Stressful Environments. Front. Plant Sci. 2017, 8, 643. [Google Scholar] [CrossRef]
- Goron, T.L.; Raizada, M.N. Genetic Diversity and Physiological Responses of Finger Millet to Abiotic Stress: Opportunities for Crop Improvement. Front. Plant Sci. 2015, 6, 157. [Google Scholar] [CrossRef]
- Devi, P.B.; Vijayabharathi, R.; Sathyabama, S.; Malleshi, N.G.; Priyadarisini, V.B. Health Benefits of Finger Millet (Eleusine coracana L.) Polyphenols and Dietary Fiber: A Review. J. Food Sci. Technol. 2014, 51, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, N.; Patro, T.S.S.K.; Singamsetti, A.; Sandhya Rani, Y.; Triveni, U.; Nirmala Kumari, A.; Govanakoppa, N.; Lakshmi Pathy, T.; Tonapi, V.A. Comparative Study of AMMI- and BLUP-Based Simultaneous Selection for Grain Yield and Stability of Finger Millet [Eleusine coracana (L.) Gaertn.] Genotypes. Front. Plant Sci. 2022, 12, 786839. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Metwal, M.; Kaur, S.; Gupta, A.K.; Puranik, S.; Singh, S.; Singh, M.; Gupta, S.; Babu, B.K.; Sood, S.; et al. Nutraceutical Value of Finger Millet [Eleusine coracana (L.) Gaertn.], and Their Improvement Using Omics Approaches. Front. Plant Sci. 2016, 7, 934. [Google Scholar] [CrossRef]
- Chandra, D.; Chandra, S.; Pallavi; Sharma, A.K. Review of Finger Millet (Eleusine coracana (L.) Gaertn): A Power House of Health Benefiting Nutrients. Food Sci. Hum. Wellness 2016, 5, 149–155. [Google Scholar] [CrossRef]
- Sharma, A.; Ceasar, S.A.; Pandey, H.; Devadas, V.S.; Kesavan, A.K.; Heisnam, P.; Vashishth, A.; Misra, V.; Mall, A.K. Millets: Nutrient-Rich and Climate-Resilient Crops for Sustainable Agriculture and Diverse Culinary Applications. J. Food Compos. Anal. 2025, 137, 106984. [Google Scholar] [CrossRef]
- Kaur, S.; Kumari, A.; Seem, K.; Kaur, G.; Kumar, D.; Verma, S.; Singh, N.; Kumar, A.; Kumar, M.; Jaiswal, S.; et al. Finger Millet (Eleusine coracana L.): From Staple to Superfood-a Comprehensive Review on Nutritional, Bioactive, Industrial, and Climate Resilience Potential. Planta 2024, 260, 75. [Google Scholar] [CrossRef] [PubMed]
- Anitha, S.; Kane-Potaka, J.; Botha, R.; Givens, D.I.; Sulaiman, N.L.B.; Upadhyay, S.; Vetriventhan, M.; Tsusaka, T.W.; Parasannanavar, D.J.; Longvah, T.; et al. Millets Can Have a Major Impact on Improving Iron Status, Hemoglobin Level, and in Reducing Iron Deficiency Anemia–A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 725529. [Google Scholar] [CrossRef] [PubMed]
- Anitha, S.; Govindaraj, M.; Kane-Potaka, J.; Sulaiman, N.L.B.; Tsusaka, T.W.; Subramaniam, K.; Rajendran, A.; Parasannanavar, D.J.; Bhandari, R.K. Calcium from Finger Millet: A Systematic Review and Meta-Analysis on Calcium Retention, Absorption and Bone Health. Sustainability 2021, 13, 8677. [Google Scholar] [CrossRef]
- Maharajan, T.; Krishna, T.P.A.; Ceasar, S.A. Finger Millet BT-Millets: Crops for Climate Resilience and for Food and Nutritional Security; Ceasar, S.A., Penna, S., Carvalho, C.W.P., Jain, S.M., Eds.; Springer: Singapore, 2025; pp. 1–32. ISBN 978-981-95-1256-0. [Google Scholar]
- Bhatt, A.; Sharma, S.; Joshi, H.C. Finger Millet: A Potential Crop for Climate Resilient Agriculture. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2190–2200. [Google Scholar]
- Bista, M.K.; Bheemanahalli, R. Finger Millet: A Climate-Resilient and Multi-Nutrient Crop for the Uncertain Future. CSA News 2024, 69, 52–56. [Google Scholar] [CrossRef]
- Maharajan, T.; Antony Ceasar, S.; Ajeesh Krishna, T.P.; Ignacimuthu, S. Finger Millet [Eleusine coracana (L.) Gaertn]: An Orphan Crop with a Potential to Alleviate the Calcium Deficiency in the Semi-Arid Tropics of Asia and Africa. Front. Sustain. Food Syst. 2021, 5, 258. [Google Scholar] [CrossRef]
- Hittalmani, S.; Mahesh, H.B.; Shirke, M.D.; Biradar, H.; Uday, G.; Yadav, M.; Aruna, Y.R.; Lohithaswa, H.C.; Mohanrao, A. Genome and Transcriptome Sequence of Finger Millet (Eleusine coracana) Provides Insights into Drought Tolerance and Nutritional Content. BMC Genom. 2017, 18, 465. [Google Scholar] [CrossRef]
- Ceasar, S.; Maharajan, T.; Ajeesh Krishna, T.P.; Ramakrishnan, M.; Victor Roch, G.; Satish, L.; Ignacimuthu, S. Finger Millet [Eleusine coracana (L.) Gaertn.] Improvement: Current Status and Future Interventions of Whole Genome Sequence. Front. Plant Sci. 2018, 9, 1054. [Google Scholar] [CrossRef]
- Maharajan, T.; Krishna, T.P.A.; Krishnakumar, N.M.; Vetriventhan, M.; Kudapa, H.; Ceasar, S.A. Role of Genome Sequences of Major and Minor Millets in Strengthening Food and Nutritional Security for Future Generations. Agriculture 2024, 14, 670. [Google Scholar] [CrossRef]
- Maharajan, T.; Ceasar, S.A.; Ajeesh Krishna, T.P. Harnessing the Transcriptomic Resources of Millets to Decipher Climate Resilience and Nutrient Enrichment Traits. CRC. Crit. Rev. Plant Sci. 2024, 43, 348–375. [Google Scholar] [CrossRef]
- Ceasar, S.A.; Prabhu, S.; Ebeed, H.T. Protein Research in Millets: Current Status and Way Forward. Planta 2024, 260, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, M.; Aluri, S.; Balachadran, M.T.; Sivarajan, S.R.; Patrignani, A.; Grüter, S.; Poveda, L.; Shimizu-Inatsugi, R.; Baeten, J.; Francoijs, K.-J.; et al. Multiple Hybrid de Novo Genome Assembly of Finger Millet, an Orphan Allotetraploid Crop. DNA Res. 2018, 25, 39–47. [Google Scholar] [CrossRef]
- Devos, K.M.; Qi, P.; Bahri, B.A.; Gimode, D.M.; Jenike, K.; Manthi, S.J.; Lule, D.; Lux, T.; Martinez-Bello, L.; Pendergast, T.H.; et al. Genome Analyses Reveal Population Structure and a Purple Stigma Color Gene Candidate in Finger Millet. Nat. Commun. 2023, 14, 3694. [Google Scholar] [CrossRef] [PubMed]
- Phytozome. Eleusine coracana KNE796-S Reference Genome Assembly (GCA_032690845.1). 2023. Available online: https://phytozome-next.jgi.doe.gov/info/EcoracanaKNE796_S_v1 (accessed on 20 September 2025).
- Upadhyaya, H.D.; Reddy, K.N.; Gowda, C.L.L.; Singh, S. Developing a Mini-Core Collection in Finger Millet Using Multilocation Data. Crop Sci. 2010, 50, 1924–1931. [Google Scholar] [CrossRef]
- International Crops Research Institute for the Semi-Arid Tropics (ICRISAT). ICRISAT Genebank Germplasm Distribution to Global Research Community (as on May 2024). Patancheru, India: ICRISAT Genebank. Available online: https://genebank.icrisat.org/IND/Distributionsummary (accessed on 19 September 2025).
- ICAR NBPGR. Annual Report 2023. New Delhi, India. 2023. Available online: https://nbpgr.org.in/nbpgr2023/wp-content/uploads/2024/07/Annual-report-2023_ICAR-NBPGR.pdf (accessed on 19 September 2025).
- Maharajan, T.; Ceasar, S.A.; Krishna, T.P.A. Finger Millet (Eleusine coracana (L.) Gaertn): Nutritional Importance and Nutrient Transporters. CRC. Crit. Rev. Plant Sci. 2022, 41, 1–31. [Google Scholar] [CrossRef]
- Brhane, H.; Haileselassie, T.; Tesfaye, K.; Ortiz, R.; Hammenhag, C.; Abreha, K.B.; Vetukuri, R.R.; Geleta, M. Finger Millet RNA-Seq Reveals Differential Gene Expression Associated with Tolerance to Aluminum Toxicity and Provides Novel Genomic Resources. Front. Plant Sci. 2022, 13, 1068383. [Google Scholar] [CrossRef]
- Parvathi, M.S.; Nataraja, K.N.; Reddy, Y.A.N.; Naika, M.B.N.; Gowda, M.V.C. Transcriptome Analysis of Finger Millet (Eleusine coracana) Exposed to Progressive Drought Reveals Novel Stress-Responsive Mechanisms. J. Genet. 2019, 98, 46. [Google Scholar] [CrossRef]
- Rahman, H.; Jagadeeshselvam, N.; Valarmathi, R.; Sachin, B.; Sasikala, R.; Senthil, N.; Sudhakar, D.; Robin, S.; Raveendran, M. Transcriptome Analysis of Salinity Responsiveness in Contrasting Genotypes of Finger Millet (Eleusine coracana L.) through RNA-Sequencing. Plant Mol. Biol. 2014, 85, 485–503. [Google Scholar] [CrossRef]
- Ramegowda, V.; Gill, U.S.; Sivalingam, P.N.; Gupta, A.; Gupta, C.; Sharma, A.; Rajamani, V.; Nataraja, K.N.; Pereira, A. GBF3 Transcription Factor Improves Heat and Drought Tolerance in Arabidopsis. Sci. Rep. 2017, 7, 9148. [Google Scholar] [CrossRef] [PubMed]
- Jamra, G.; Agarwal, A.; Singh, N.; Sanyal, S.K.; Kumar, A.; Pandey, G.K. Ectopic Expression of Finger Millet Calmodulin Confers Drought and Salinity Tolerance in Arabidopsis Thaliana. Plant Cell Rep. 2021, 40, 2205–2223. [Google Scholar] [CrossRef]
- Ramegowda, V.; Senthil-Kumar, M.; Nataraja, K.N.; Reddy, M.K.; Mysore, K.S.; Udayakumar, M. Expression of a Finger Millet Transcription Factor, EcNAC1, in Tobacco Confers Abiotic Stress-Tolerance. PLoS ONE 2012, 7, e40397. [Google Scholar] [CrossRef]
- Maharajan, T.; Ajeesh Krishna, T.P.; Rakkammal, K.; Ramakrishnan, M.; Ceasar, S.A.; Ramesh, M.; Ignacimuthu, S. Identification of QTL Associated with Agro-Morphological and Phosphorus Content Traits in Finger Millet under Differential Phosphorus Supply via Linkage Mapping. Agriculture 2023, 13, 262. [Google Scholar] [CrossRef]
- Pendergast, T.H., IV; Qi, P.; Odeny, D.A.; Dida, M.M.; Devos, K.M. A High-Density Linkage Map of Finger Millet Provides QTL for Key Agronomic Traits. Plant Genome 2022, 15, e20175. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Singh, V.K.; Raghavendrarao, S.; Phanindra, M.L.V.; Venkat Raman, K.; Solanke, A.U.; Kumar, P.A.; Sharma, T.R. Expression of Finger Millet EcDehydrin7 in Transgenic Tobacco Confers Tolerance to Drought Stress. Appl. Biochem. Biotechnol. 2015, 177, 207–216. [Google Scholar] [CrossRef]
- Nagarjuna, K.N.; Parvathi, M.S.; Sajeevan, R.S.; Pruthvi, V.; Mamrutha, H.M.; Nataraja, K.N. Full-Length Cloning and Characterization of Abiotic Stress Responsive CIPK31-like Gene from Finger Millet, a Drought-Tolerant Crop. Curr. Sci. 2016, 111, 890–894. [Google Scholar] [CrossRef]
- Rahman, H.; Ramanathan, V.; Nallathambi, J.; Duraialagaraja, S.; Raveendran, M. Over-Expression of a NAC 67 Transcription Factor from Finger Millet (Eleusine coracana L.) Confers Tolerance against Salinity and Drought Stress in Rice. BMC Biotechnol. 2016, 16, 35. [Google Scholar] [CrossRef]
- Parvathi, M.S.; Nataraja, K.N. Discovery of Stress Responsive TATA-Box Binding Protein Associated Factor6 (TAF6) from Finger Millet (Eleusine coracana (L.) Gaertn). J. Plant Biol. 2017, 60, 335–342. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Wang, L.; Zhu, J.; Deng, J.; Tang, R.; Chen, G. Integration of Transcriptomic and Proteomic Analyses for Finger Millet [Eleusine coracana (L.) Gaertn.] in Response to Drought Stress. PLoS ONE 2021, 16, e0247181. [Google Scholar] [CrossRef]
- Singh, S.; Singh, R.K.; Yadav, S.; Tiwari, R. Overexpression of EcDREB2A Transcription Factor from Finger Millet in Tobacco Enhances Tolerance to Heat Stress through ROS Scavenging. J. Biotechnol. 2021, 336, 10–24. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Singh, S.; Raghavendrarao, S.; Padaria, J.C.; Mohanty, S.; Sharma, T.R.; Solanke, A.U. The Membrane Tethered Transcription Factor EcbZIP17 from Finger Millet Promotes Plant Growth and Enhances Tolerance to Abiotic Stresses. Sci. Rep. 2018, 8, 2148. [Google Scholar] [CrossRef]
- Chopperla, R.; Singh, S.; Tomar, R.S.; Mohanty, S.; Khan, S.; Reddy, N.; Padaria, J.C.; Solanke, A.U. Isolation and Allelic Characterization of Finger Millet (Eleusine coracana L.) Small Heat Shock Protein EcHSP17. 8 for Stress Tolerance. Indian J. Genet. Plant Breed. 2018, 78, 95–103. [Google Scholar] [CrossRef]
- Pudake, R.N.; Mehta, C.M.; Mohanta, T.K.; Sharma, S.; Varma, A.; Sharma, A.K. Expression of Four Phosphate Transporter Genes from Finger Millet (Eleusine coracana L.) in Response to Mycorrhizal Colonization and Pi Stress. 3 Biotech 2017, 7, 17. [Google Scholar] [CrossRef]
- Goyal, E.; Singh, A.K.; Mahajan, M.M.; Kanika, K. Comparative Transcriptome Profiling of Contrasting Finger Millet (Eleusine coracana (L.) Gaertn) Genotypes under Heat Stress. Mol. Biol. Rep. 2024, 51, 283. [Google Scholar] [CrossRef] [PubMed]
- Mbinda, W.; Mukami, A. A Review of Recent Advances and Future Directions in the Management of Salinity Stress in Finger Millet. Front. Plant Sci. 2021, 12, 734798. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Tiwari, A.; Sood, S.; Jamra, G.; Singh, N.K.; Meher, P.K.; Kumar, A. Genome Wide Association Mapping of Agro-Morphological Traits among a Diverse Collection of Finger Millet (Eleusine coracana L.) Genotypes Using SNP Markers. PLoS ONE 2018, 13, e0199444. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Ceasar, S.A.; Vinod, K.K.; Duraipandiyan, V.; P, A.K.T.; Upadhyaya, H.D.; Al-Dhabi, N.A.; Ignacimuthu, S. Identification of Putative QTLs for Seedling Stage Phosphorus Starvation Response in Finger Millet (Eleusine coracana L. Gaertn.) by Association Mapping and Cross Species Synteny Analysis. PLoS ONE 2017, 12, e0183261. [Google Scholar] [CrossRef]
- Gaur, V.S.; Tiwari, A.; Kumar, A.; Singh, M. Comparative Proteomic Analysis for Identification and Characterization of Nutritionally and Stress-Responsive Seed Proteins of Finger Millet with Respect to Rice. Plant Cell Biotechnol. Mol. Biol. 2023, 24, 60–73. [Google Scholar] [CrossRef]
- Agrawal, H.; Joshi, R.; Gupta, M. Purification, Identification and Characterization of Two Novel Antioxidant Peptides from Finger Millet (Eleusine coracana) Protein Hydrolysate. Food Res. Int. 2019, 120, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, M.; Antony Ceasar, S.; Duraipandiyan, V.; Vinod, K.K.; Kalpana, K.; Al-Dhabi, N.A.; Ignacimuthu, S. Tracing QTLs for Leaf Blast Resistance and Agronomic Performance of Finger Millet (Eleusine coracana (L.) Gaertn.) Genotypes through Association Mapping and in Silico Comparative Genomics Analyses. PLoS ONE 2016, 11, e0159264. [Google Scholar] [CrossRef]
- Chandra, A.K.; Pandey, D.; Sood, S.; Joshi, D.C.; Tiwari, A.; Sharma, D.; Gururani, K.; Kumar, A. Uncovering the Genomic Regions Underlying Grain Iron and Zinc Content Using Genome-Wide Association Mapping in Finger Millet. 3 Biotech 2024, 14, 47. [Google Scholar] [CrossRef]
- Sharma, D.; Tiwari, A.; Sood, S.; Meher, P.K.; Kumar, A. Identification and Validation of Candidate Genes for High Calcium Content in Finger Millet [Eleusine coracana (L.) Gaertn.] through Genome-Wide Association Study. J. Cereal Sci. 2022, 107, 103517. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar, A.; Sood, S. Unraveling the Genetics of Calcium Content in Finger Millet Grains through Association Mapping. Indian J. Genet. Plant Breed. 2020, 80, 432–440. [Google Scholar] [CrossRef]
- Bhatt, D.; Negi, M.; Sharma, P.; Saxena, S.C.; Dobriyal, A.K.; Arora, S. Responses to Drought Induced Oxidative Stress in Five Finger Millet Varieties Differing in Their Geographical Distribution. Physiol. Mol. Biol. Plants 2011, 17, 347–353. [Google Scholar] [CrossRef]
- Bartwal, A.; Pande, A.; Sharma, P.; Arora, S. Intervarietal Variations in Various Oxidative Stress Markers and Antioxidant Potential of Finger Millet (Eleusine coracana) Subjected to Drought Stress. J. Environ. Biol. 2016, 37, 517–522. [Google Scholar]
- Krishnamurthy, L.; Upadhyaya, H.D.; Kashiwagi, J.; Purushothaman, R.; Dwivedi, S.L.; Vadez, V. Variation in Drought-Tolerance Components and Their Interrelationships in the Minicore Collection of Finger Millet Germplasm. Crop Sci. 2016, 56, 1914–1926. [Google Scholar] [CrossRef]
- David, H.; Ramakrishnan, M.; Maharajan, T.; BarathiKannan, K.; Atul Babu, G.; Daniel, M.A.; Agastian, P.; Antony Caesar, S.; Ignacimuthu, S. Mining QTL and Genes for Root Traits and Biochemical Parameters under Vegetative Drought in South Indian Genotypes of Finger Millet (Eleusine coracana (L.) Gaertn) by Association Mapping and in Silico Comparative Genomics. Biocatal. Agric. Biotechnol. 2021, 32, 101935. [Google Scholar] [CrossRef]
- Satish, L.; Rathinapriya, P.; Sagina Rency, A.; Antony Ceasar, S.; Prathibha, M.; Pandian, S.; Rameshkumar, R.; Ramesh, M. Effect of Salinity Stress on Finger Millet (Eleusine coracana (L.) Gaertn): Histochemical and Morphological Analysis of Coleoptile and Coleorhizae. Flora Morphol. Distrib. Funct. Ecol. Plants 2016, 222, 111–120. [Google Scholar] [CrossRef]
- Mukami, A.; Ng’etich, A.; Syombua, E.; Oduor, R.; Mbinda, W. Varietal Differences in Physiological and Biochemical Responses to Salinity Stress in Six Finger Millet Plants. Physiol. Mol. Biol. Plants 2020, 26, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Yogeesh, L.N.; Naryanareddy, A.B.; Nanjareddy, Y.A.; Gowda, M.C.; Channabyre Gowda, M.V. High Temperature Tolerant Genotypes of Finger Millet (Eleusine coracana L.). Nat. Environ. Pollut. Technol. 2016, 15, 1293–1296. [Google Scholar]
- Rakkammal, K.; Maharajan, T.; Shriram, R.N.; Ram, P.S.J.; Ceasar, S.A.; Ramesh, M. Physiological, Biochemical and Molecular Responses of Finger Millet (Eleusine coracana (L.) Gaertn.) Genotypes Exposed to Short-Term Drought Stress Induced by PEG-6000. J. Plant Physiol. 2023, 155, 45–59. [Google Scholar] [CrossRef]
- Sanku, G.; Rajasekaran, R.; Boopathi, N.M.; Krishnamoorthy, I.; Santhanakrishnan, V.P.; Mani, V. Transcriptomic Response of Minor Millets to Abiotic Stresses. Front. Sustain. Food Syst. 2024, 8, 1435437. [Google Scholar] [CrossRef]
- Eapen, S.; George, L. High Frequency Plant Regeneration through Somatic Embryogenesis in Finger Millet (Eleusine coracana Gaertn.). Plant Sci. 1989, 61, 127–130. [Google Scholar] [CrossRef]
- George, L.; Eapen, S. High Frequency Plant Regeneration through Direct Shoot Development and Somatic Embryogenesis from Immature Inflorescence Cultures of Finger Millet (Eleusine coracana Gaertn.). Euphytica 1990, 48, 269–274. [Google Scholar] [CrossRef]
- Ceasar, S.A.; Ignacimuthu, S. Efficient Somatic Embryogenesis and Plant Regeneration from Shoot Apex Explants of Different Indian Genotypes of Finger Millet (Eleusine coracana (L.) Gaertn.). In Vitro Cell. Dev. Biol.-Plant 2008, 44, 427–435. [Google Scholar] [CrossRef]
- Poddar, K.; Vishnoi, R.K.; Kothari, S.L. Plant Regeneration from Embryogenic Callus of Finger Millet (Eleusine coracana (L.) Gaertn.) on Higher Concentrations of NH4NO3 as Replacement of NAA in the Medium. Plant Sci. 1997, 129, 101–106. [Google Scholar] [CrossRef]
- Kothari, S.L.; Agarwal, K.; Kumar, S. Inorganic Nutrient Manipulation for Highly Improved in Vitro Plant Regeneration in Finger Millet (Eleusine coracana (L.) Gaertn.). In Vitro Cell. Dev. Biol.-Plant 2004, 40, 515–519. [Google Scholar] [CrossRef]
- Satish, L.; Ceasar, S.A.; Shilpha, J.; Rency, A.S.; Rathinapriya, P.; Ramesh, M. Direct Plant Regeneration from in Vitro-Derived Shoot Apical Meristems of Finger Millet (Eleusine coracana (L.) Gaertn.). In Vitro Cell. Dev. Biol.-Plant 2015, 51, 192–200. [Google Scholar] [CrossRef]
- Satish, L.; Rency, A.S.; Rathinapriya, P.; Ceasar, S.A.; Pandian, S.; Rameshkumar, R.; Rao, T.B.; Balachandran, S.M.; Ramesh, M. Influence of Plant Growth Regulators and Spermidine on Somatic Embryogenesis and Plant Regeneration in Four Indian Genotypes of Finger Millet (Eleusine coracana (L.) Gaertn). Plant Cell Tissue Organ Cult. 2015, 124, 15–31. [Google Scholar] [CrossRef]
- Ceasar, S.A.; Ignacimuthu, S. Agrobacterium-Mediated Transformation of Finger Millet (Eleusine coracana (L.) Gaertn.) Using Shoot Apex Explants. Plant Cell Rep. 2011, 30, 1759–1770. [Google Scholar] [CrossRef]
- Satish, L.; Ceasar, S.A.; Ramesh, M. Improved Agrobacterium-Mediated Transformation and Direct Plant Regeneration in Four Cultivars of Finger Millet (Eleusine coracana (L.) Gaertn.). Plant Cell Tissue Organ Cult. 2017, 131, 547–565. [Google Scholar] [CrossRef]
- Sharma, M.; Kothari-Chajer, A.; Jagga-Chugh, S.; Kothari, S.L. Factors Influencing Agrobacterium Tumefaciens-Mediated Genetic Transformation of Eleusine coracana (L.) Gaertn. Plant Cell Tissue Organ Cult. 2011, 105, 93–104. [Google Scholar] [CrossRef]
- Latha, A.M.; Rao, K.V.; Reddy, V.D. Production of Transgenic Plants Resistant to Leaf Blast Disease in Finger Millet (Eleusine coracana (L.) Gaertn.). Plant Sci. 2005, 169, 657–667. [Google Scholar] [CrossRef]
- Jagga-Chugh, S.; Kachhwaha, S.; Sharma, M.; Kothari-Chajer, A.; Kothari, S.L. Optimization of Factors Influencing Microprojectile Bombardment-Mediated Genetic Transformation of Seed-Derived Callus and Regeneration of Transgenic Plants in Eleusine coracana (L.) Gaertn. Plant Cell Tissue Organ Cult. 2012, 109, 401–410. [Google Scholar] [CrossRef]
- Ignacimuthu, S.; Ceasar, S.A. Development of Transgenic Finger Millet (Eleusine coracana (L.) Gaertn.) Resistant to Leaf Blast Disease. J. Biosci. 2012, 37, 135–147. [Google Scholar] [CrossRef]
- Anjaneyulu, E.; Reddy, P.S.; Sunita, M.S.; Kishor, P.B.K.; Meriga, B. Salt Tolerance and Activity of Antioxidative Enzymes of Transgenic Finger Millet Overexpressing a Vacuolar H+-Pyrophosphatase Gene (SbVPPase) from Sorghum Bicolor. J. Plant Physiol. 2014, 171, 789–798. [Google Scholar] [CrossRef]
- Gupta, P.; Raghuvanshi, S.; K Tyagi, A. Assessment of the Efficiency of Various Gene Promoters via Biolistics in Leaf and Regenerating Seed Callus of Millets, Eleusine coracana and Echinochloa Crusgalli. Plant Biotechnol. 2001, 18, 275–282. [Google Scholar] [CrossRef]
- Mahalakshmi, S.; Christopher, G.S.B.; Reddy, T.P.; Rao, K.V.; Reddy, V.D. Isolation of a CDNA Clone (PcSrp) Encoding Serine-Rich-Protein from Porteresia Coarctata T. and Its Expression in Yeast and Finger Millet (Eleusine coracana L.) Affording Salt Tolerance. Planta 2006, 224, 347–359. [Google Scholar] [CrossRef]
- Hema, R.; Vemanna, R.S.; Sreeramulu, S.; Reddy, C.P.; Senthil-Kumar, M.; Udayakumar, M. Stable Expression of MtlD Gene Imparts Multiple Stress Tolerance in Finger Millet. PLoS ONE 2014, 9, e99110. [Google Scholar] [CrossRef]
- Sharma, R.; Liang, Y.; Lee, M.Y.; Pidatala, V.R.; Mortimer, J.C.; Scheller, H. V Agrobacterium-Mediated Transient Transformation of Sorghum Leaves for Accelerating Functional Genomics and Genome Editing Studies. BMC Res. Notes 2020, 13, 116. [Google Scholar] [CrossRef]
- Char, S.N.; Wei, J.; Mu, Q.; Li, X.; Zhang, Z.J.; Yu, J.; Yang, B. An Agrobacterium-Delivered CRISPR/Cas9 System for Targeted Mutagenesis in Sorghum. Plant Biotechnol. J. 2020, 18, 319–321. [Google Scholar] [CrossRef]
- Shi, J.; Yu, F. Targeting the Parasite’s Lifeline: Knockout of SL Transporters Confers Durable Striga Resistance in Sorghum. Adv. Biotechnol. 2025, 3, 9. [Google Scholar] [CrossRef]
- Gobena, D.; Shimels, M.; Rich, P.J.; Ruyter-Spira, C.; Bouwmeester, H.; Kanuganti, S.; Mengiste, T.; Ejeta, G. Mutation in Sorghum LOW GERMINATION STIMULANT 1 Reduces Strigolactone Production and Confers Striga Resistance. Proc. Natl. Acad. Sci. USA 2017, 114, 4471–4476. [Google Scholar] [CrossRef]
- Cheng, Z.; Sun, Y.; Yang, S.; Zhi, H.; Yin, T.; Ma, X.; Zhang, H.; Diao, X.; Guo, Y.; Li, X.; et al. Establishing in Planta Haploid Inducer Line by Edited SiMTL in Foxtail Millet (Setaria italica). Plant Biotechnol. J. 2021, 19, 1089–1091. [Google Scholar] [CrossRef]
- Zhang, R.; Guo, R.; Zhi, H.; Tang, S.; Wang, L.; Ren, Y.; Ren, G.; Zhang, S.; Feng, J.; Diao, X.; et al. De Novo Creation of Narrowed Plant Architecture via CRISPR/Cas9-Mediated Mutagenesis of SiLGs in Foxtail Millet. Plant Biotechnol. J. 2025, 23, 2400–2402. [Google Scholar] [CrossRef]
- Liang, Z.; Wu, Y.; Ma, L.; Guo, Y.; Ran, Y. Efficient Genome Editing in Setaria italica Using CRISPR/Cas9 and Base Editors. Front. Plant Sci. 2022, 12, 815946. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, H.; Wang, H.; Lafitte, H.R.; Archibald, R.L.; Yang, M.; Hakimi, S.M.; Mo, H.; Habben, J.E. ARGOS8 Variants Generated by CRISPR-Cas9 Improve Maize Grain Yield under Field Drought Stress Conditions. Plant Biotechnol. J. 2017, 15, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Mohr, T.; Horstman, J.; Gu, Y.Q.; Elarabi, N.I.; Abdallah, N.A.; Thilmony, R. CRISPR-Cas9 Gene Editing of the Sal1 Gene Family in Wheat. Plants 2022, 11, 2259. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Zhang, R.; Gao, J.; Qi, Y.; Song, G.; Li, W.; Li, Y.; Li, G. Efficient Multiplex Genome Editing by CRISPR/Cas9 in Common Wheat. Plant Biotechnol. J. 2021, 19, 427–429. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, S.; Jiang, N.; Zhao, X.; Bai, Z.; Liu, J.; Yao, W.; Tang, Q.; Xiao, G.; Lv, C.; et al. Engineering of Rice Varieties with Enhanced Resistances to Both Blast and Bacterial Blight Diseases via CRISPR/Cas9. Plant Biotechnol. J. 2022, 20, 876–885. [Google Scholar] [CrossRef]
- Mikami, M.; Toki, S.; Endo, M. Comparison of CRISPR/Cas9 Expression Constructs for Efficient Targeted Mutagenesis in Rice. Plant Cell Rep. 2015, 88, 561–572. [Google Scholar] [CrossRef]
- Lowder, L.G.; Zhang, D.; Baltes, N.J.; Paul, J.W.; Tang, X.; Zheng, X.; Voytas, D.F.; Hsieh, T.-F.; Zhang, Y.; Qi, Y. A CRISPR/Cas9 Toolbox for Multiplexed Plant Genome Editing and Transcriptional Regulation. Plant Physiol. 2015, 169, 971–985. [Google Scholar] [CrossRef]
- Hassan, M.M.; Zhang, Y.; Yuan, G.; De, K.; Chen, J.-G.; Muchero, W.; Tuskan, G.A.; Qi, Y.; Yang, X. Construct Design for CRISPR/Cas-Based Genome Editing in Plants. Trends Plant Sci. 2021, 26, 1133–1152. [Google Scholar] [CrossRef]
- Jung, W.J.; Park, S.-J.; Cha, S.; Kim, K. Factors Affecting the Cleavage Efficiency of the CRISPR-Cas9 System. Animal Cells Syst. 2024, 28, 75–83. [Google Scholar] [CrossRef]
- Kor, S.D.; Chowdhury, N.; Keot, A.K.; Yogendra, K.; Chikkaputtaiah, C.; Sudhakar Reddy, P. RNA Pol III Promoters-Key Players in Precisely Targeted Plant Genome Editing. Front. Genet. 2022, 13, 989199. [Google Scholar] [CrossRef]
- Wang, M.; Mao, Y.; Lu, Y.; Tao, X.; Zhu, J.-K. Multiplex Gene Editing in Rice Using the CRISPR/Cpf1 System. Mol. Plant 2017, 10, 1011–1013. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Y. Self-Processing of Ribozyme-Flanked RNAs into Guide RNAs in Vitro and in Vivo for CRISPR-Mediated Genome Editing. J Integr Plant Biol. 2014, 56, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Atkins, A.; Chung, C.-H.; Allen, A.G.; Dampier, W.; Gurrola, T.E.; Sariyer, I.K.; Nonnemacher, M.R.; Wigdahl, B. Off-Target Analysis in Gene Editing and Applications for Clinical Translation of CRISPR/Cas9 in HIV-1 Therapy. Front. Genome Ed. 2021, 3, 673022. [Google Scholar] [CrossRef] [PubMed]
- Tsakirpaloglou, N.; Septiningsih, E.M.; Thomson, M.J. Guidelines for Performing CRISPR/Cas9 Genome Editing for Gene Validation and Trait Improvement in Crops. Plants 2023, 12, 3564. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Bae, S.; Park, J.; Kim, E.; Kim, S.; Yu, H.R.; Hwang, J.; Kim, J.-I. Digenome-Seq: Genome-Wide Profiling of CRISPR–Cas9 off-Target Effects in Human Cells. Nat. Methods 2015, 12, 237–243. [Google Scholar] [CrossRef]
- Clement, K.; Rees, H.; Canver, M.C.; Gehrke, J.M.; Farouni, R.; Hsu, J.Y.; Cole, M.A.; Liu, D.R.; Joung, J.K.; Bauer, D.E.; et al. CRISPResso2 Provides Accurate and Rapid Genome Editing Sequence Analysis. Nat. Biotechnol. 2019, 37, 224–226. [Google Scholar] [CrossRef]
- Wienert, B.; Wyman, S.K.; Yeh, C.D.; Conklin, B.R.; Corn, J.E. CRISPR Off-Target Detection with DISCOVER-Seq. Nat. Protoc. 2020, 15, 1775–1799. [Google Scholar] [CrossRef]
- Wienert, B.; Wyman, S.K.; Yeh, C.; Conklin, B.R.; Corn, J.E. Unbiased Detection of CRISPR Off-Targets in Vivo Using DISCOVER-Seq. Science 2019, 364, 286–289. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field High-Throughput Phenotyping: The New Crop Breeding Frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef]
- Rees, H.A.; Liu, D.R. Base Editing: Precision Chemistry on the Genome and Transcriptome of Living Cells. Nat. Rev. Genet. 2018, 19, 770–788. [Google Scholar] [CrossRef]
- Jubair, S.; Domaratzki, M. Crop Genomic Selection with Deep Learning and Environmental Data: A Survey. Front. Artif. Intell. 2022, 5, 1040295. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Li, H.; Zheng, H.; Zhang, J.; Olsen, M.S.; Varshney, R.K.; Prasanna, B.M.; Qian, Q. Smart Breeding Driven by Big Data, Artificial Intelligence, and Integrated Genomic-Enviromic Prediction. Mol. Plant 2022, 15, 1664–1695. [Google Scholar] [CrossRef] [PubMed]
- Lee, M. Deep Learning in CRISPR-Cas Systems: A Review of Recent Studies. Front. Bioeng. Biotechnol. 2023, 11, 1226182. [Google Scholar] [CrossRef]
- Chen, L.; Liu, G.; Zhang, T. Integrating Machine Learning and Genome Editing for Crop Improvement. aBIOTECH 2024, 5, 262–277. [Google Scholar] [CrossRef]
- MacNish, T.R.; Danilevicz, M.F.; Bayer, P.E.; Bestry, M.S.; Edwards, D. Application of Machine Learning and Genomics for Orphan Crop Improvement. Nat. Commun. 2025, 16, 982. [Google Scholar] [CrossRef] [PubMed]
- Crossa, J.; Perez-Rodriguez, P.; Cuevas, J.; Montesinos-Lopez, O.A.; Jarquin, D.; de los Campos, G.; Burgueño, J.; González-Camacho, J.M.; Pérez-Elizalde, S.; Beyene, Y.; et al. Genomic Selection in Plant Breeding: Methods, Models, and Perspectives. Theor. Appl. Genet. 2017, 22, 961–975. [Google Scholar] [CrossRef]
- Bellot, P.; de los Campos, G. Can Deep Learning Improve Genomic Prediction of Complex Human Traits? Genetics 2018, 210, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Golicz, A.A.; Batley, J.; Edwards, D. Towards Plant Pangenomics. Plant Biotechnol. J. 2016, 14, 1099–1105. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R. Phosphate Solubilizing Bacteria and Their Role in Plant Growth Promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Parniske, M. Arbuscular Mycorrhiza: The Mother of Plant Root Endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Sessitsch, A.; Hardoim, P.; Doring, J.; Weilharter, A.; Krause, A.; Woyke, T.; Mitter, B.; Hauberg-Lotte, L.; Friedrich, F.; Rahalkar, M.; et al. Functional Characteristics of an Endophyte Community Colonizing Rice Roots as Revealed by Metagenomic Analysis. Plant Soil. 2012, 25, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Pretty, J.; Benton, T.G.; Bharucha, Z.P.; Dicks, L.V.; Flora, C.B.; Godfray, H.C.J.; Goulson, D.; Hartley, S.; Lampkin, N.; Morris, C.; et al. Global Assessment of Agricultural System Redesign for Sustainable Intensification. Nat. Sustain. 2018, 1, 441–446. [Google Scholar] [CrossRef]
- Palombi, L.; Sessa, R. Climate Smart Agriculture Sourcebook; FAO: Rome, Italy, 2013. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).