Abstract
Drastic changes in temperature, salinity of soils and drought are some of the most studied abiotic stressors in important crops. Plants have developed various biochemical mechanisms to counteract these conditions. Transcription factors play a significant role in regulating stress responses. Previously, in our lab, it was identified that the CpRap2.4a protein, which belongs to the AP2/ERF superfamily, is related to the response to abiotic stress from extreme temperature, and confers thermal tolerance to Carica papaya CV. This study presents a randomized experimental strategy for the analysis of the physiological and biochemical responses of Nicotiana tabacum plants subjected to heat stress, and how the foliar application of the recombinantly expressed CpRap2.4a can modulate beneficial responses. Plants subjected to heat stress present a healthier physiology, as clearly shown by biochemical parameters. Moreover, physiological parameters also suggest an improvement of heat tolerance compared with the control group. Scanning electron microscopy suggests that stomatal aperture and conductance are the key mechanisms for how recombinantly expressed CpRap2.4a can act as a regulatory player to heat stress.
1. Introduction
Plants tend to be exposed to several challenging conditions, due to their sessile nature. Generally, these conditions can be divided into (a) abiotic and (b) biotic stressors. Several studies have described the nature of biotic stressors and their impact on plant development and growth; however, the abiotic stressors and how the plant response is controlled and regulated are still obscure [1,2,3,4]. Major abiotic factors representing challenges to crops and forest plants are drought, flooding, low temperatures and recently, high temperatures [5,6,7,8]. In order to respond to heat and warming environmental conditions, plants tend to modulate the expression and activities of several heat response factors (HRFs), which include transcription factors (TF), functioning as key regulators of the heat response (HR), and uncharged HR proteins (HRP), to drive enzymatic and molecular activities that subsequently respond to heat [9,10,11,12].
TF are vastly known for acting as key players of the transcriptional response to several abiotic stressors, including heat and warm seasons. They tend to act by regulating the expression of several HRP, by interacting with their cis-element DNA, the heat response element (HRE) upstream of the coding region, and consequently, driving the expression of genes [13,14,15]. A particular family of TF implicated in the HR at the transcriptional level are the AP2/ERF transcription factors, which bind to cis-DRE/CTR (A/GCCGAC) sites, located in order to regulate the expression of stress-related genes [16,17,18,19]. Previously, the CpRap2.4a TF was demonstrated to confer tolerance to detrimental thermal exposure, and more interestingly, its transcript and potential protein can be transported to the vascular system of papaya [20]. Moreover, CpRap2.4a over-expression in tobacco plants provides resistance under high temperatures of 40 °C, as demonstrated by Figueroa-Yañez et al., 2016 [20]. The movement ability of TF transcripts and other types of RNA molecules have been described in the literature for at least twenty years. However, its mechanistic and molecular drivers, which are coupled to stimulate the movement through the phloem of vascular plants, are important material for plant researchers to study [21,22,23,24]. Moreover, it seems to be an evolutionary conserved mechanism driving the movement of several RNA molecules through the phloem, particularly where active translation and protein regulation are required for further distances in the whole plant [23,25].
Heat and thermal stress, on the other hand, have been major in the field, but most of the studies rely on the over-expression of HRP and/or HRF improving the tolerance to heat, and in some cases, promoting low yield and phenotype variables that are not suitable for consumption or commercialization [26,27,28]. Other alternatives need to improve heat and thermal resistance without negatively influencing the yield and development of fruits and vegetables used for consumption. One of these comes from the direct application of protein extracts by foliar and topic spray to crops or cultivars [29,30]. However, only a few studies have been conducted in order to generate knowledge on the mechanisms of how tolerance can be modulated by the exogenous application of protein or amino acid preparation. Recently, the exogenous application of a combination of amino acids on soybean plants was described, and it is suggested that this application provides the salt–stress tolerance under this study’s treatment. Mainly, the application was conducted through a foliar application of a mixture of glycine (2.5%), arginine (2.4%), aspartic acid (1.6%) and tryptophan (0.5%). The study suggests that mechanistically, the salt tolerance was mediated by modifying different physiological and biochemical processes inside the plant; however, no molecular mechanisms have been described here [31].
Another study from Matysiak et al., 2020 [32], conducted on maize with the foliar application of L-arginine and glycine, was performed to evaluate the effect on temperature stress. The author shows that with L-Arg and Gly, at concentrations of 6 mM and 3 mM, respectively, the physiological conditions were improved in the maize plants used in the experimental treatment. Particularly, the mass of roots and shoots were increased in the L-Arg treatment, and with the Gly treatment, the heights of plants subjected to high temperatures between 30 and 38 °C were improved. Intriguingly, no biochemical experiments were conducted to evaluate the effects of both treatments. Moreover, no insight was presented to support the nature of molecular mechanisms regarding the positive effect observed.
Only a few studies have been conducted with protein extracts and other protein preparations to evaluate their effects on plant tolerance to abiotic stresses. For example, a study conducted by Ismail et al., 2017 [33] tested the effect of hydrolyzed egg albumin on Pisum sativum L. plants. The authors suggested that with a concentration of 2 mL/L, increased chlorophyll a, b and carotenoids were observed. The study was limited by biochemical and more detailed physiological data for the foliar application of albumin.
The application of exogenous substances and other bio-stimulants have been documented in reviews compiling the information regarding the possible molecular mechanisms underlying the effects of foliar application. Particularly, a good example of a review is referenced in Feng et al., 2023 [34]. The authors summarize the mechanism in several important points, especially the antioxidant pathways, synthesis of osmotic regulatory metabolites and photosynthesis rates.
Based on this knowledge, possible applications of different biostimulants have been conducted; however, the nature of the molecular mechanisms remains elusive. Moreover, the effects on different stress conditions need to be elucidated by transcriptomic, metabolomic and proteomic studies.
Papaya, however, is a highly valuable and commercial tropical fruit, with a high demand and consumption. Recently, several TF, that control and confer heat tolerance, have been reported in papaya, and also, the implications of mRNA TF transcript movements have been revealed as a key factor related to its mechanisms [20,35,36,37].
Here, we demonstrate the impact of the foliar spray application of the CpRap2.4a, over-expressed in bacteria, in order to confer thermo-tolerance to Nicotiana tabaco, and how different physiological parameters suggest their improved tolerance. This novel application could shed some light on the generation of new and versatile technologies to couple heat and other abiotic stresses.
2. Materials and Methods
2.1. Cloning and Vectors Used for Recombinant Production of CpRap2.4a in E. coli
The C. papaya Rap2.4a gene, inserted into the pDEST17 vector, was transformed into cloning and expression E. coli strains, as previously described by Figueroa-Yañez et al., 2016 [20]. The cloned vector harbors an ampicillin resistance (AmpR) gene and introduces a six-histidine tag (6His-tag), fused to the recombinant protein product. The following bacterial strains were used: (a) for cloning and vector maintenance, DH5α was used and (b) for recombinant expression, BL21 DE3, Origami and Rosseta were used. Transformation was performed using the heat shock method, using calcium chloride (CaCl2) to increase the permeability of the bacterial cell membrane [20]. Transformed bacterial cells were separated into Petri dishes containing solid LB medium supplied with ampicillin, with 100 µg/mL as a selection marker. Three transformed bacterial colonies were selected from each strain, and grown in 5 mL of liquid LB medium supplied with 100 µg/mL ampicillin, for 12 h, at 200 rpm shaking, and at 37 °C. For clone maintenance 1 mL of this culture was taken and preserved in 30% glycerol at −80 °C ultra-congelation. Plasmid DNA was extracted using 1.5 mL of grown culture, using the alkaline lysis protocol to confirm transformation. Also, transformation was verified by end-point PCR, using the specific primers—forward 5′-ATG CCT CAA CCT ATT TCA AAC GC-3′ and reverse 5′-TCA TGA CAA TAT GGA GGC CCA AT-3′—to amplify the CpRap2.4a gene and using the plasmids extracted from transformed strains as a template. An expected fragment of 855 nt was confirmed by 1% agarose gel electrophoresis.
2.2. Transformation, Expression and Purification of CpRap2.4a
For expression cultures, a pre-inoculum was prepared in a glass tube with 3 mL of liquid LB medium containing 100 µg/mL ampicillin and 10 µL of 30% glycerol bacterial suspension. The tube was incubated for 12 h at 200 rpm and 37 °C. Then, the pre-inoculum was used to inoculate 100 mL of LB media, which was incubated under the conditions described for 5 h, or until the optical density was ~0.6 at 600 nm. The temperature was lowered to 25 °C, and an aliquot of the inducer Isopropyl β-D-1-thiogalactopyranoside (IPTG) was added at a final concentration of 1 mM. These conditions were maintained overnight.
Protein extraction was performed according to the protocol described by Spriestersbach et al., 2015 [38]. The media was removed by centrifugation at 4000 rpm at 4 °C for 8 min. The pellet was resuspended in Tris-HCl 50 mM pH 8 cell lysis buffer, containing the following enzyme mixture: pepstatin 10 μg/mL, leupeptin 10 μM, phenylmethanesulfonyl fluoride (PMSF) 1 mM and β-Mercaptoethanol (BME) 10 mM, to prevent protein degradation [39,40]. Cell disruption was then performed using high-frequency sonic waves. Soluble protein fractions were recovered by centrifugation at 12,000 rpm, 4 °C for 12 min, while the precipitate insoluble fraction was used for a second protein extraction. For this, last, the pellet was resuspended in a denaturing buffer containing 20 mM Tris HCl, pH 7.9, 8 M Urea, 0.1 M NaH2PO4, 0.5 M KCl and 20 mM imidazole, and sonicated for three time. A protease-degrading enzyme mixture was also used, and the supernatant was subsequently recovered by centrifugation at 10,000 rpm, 4 °C for 30 min. Both fraction samples were stored at −80 °C for further processing.
From both soluble and insoluble fractions, CpRap2.4a was purified with metal nickel (Qiagen® Hilden, Germany) ion-immobilized affinity chromatography (IMAC) [39]. Depending on the nature of the solubility of the protein, two protocols were followed. The protocol described in Guillen-Chable et al., 2020 [39] was used for soluble protein and the method from Loza-Muller et al., 2015 [40] was used for an insoluble fraction. Tagged proteins were eluted properly from the nickel resin, adjusting the imidazole concentration to 300 mM, and finally, the samples were stored at −80 °C for later analysis.
2.3. SDS-PAGE Electrophoresis for Protein Visualization
Recombinant CpRap2.4a purification was followed by SDS-PAGE containing 12% acrylamide under reductive and denaturant conditions. The gels were stained with 0.4% Coomassie R-250 Brilliant Blue in 10% acetic acid and 40% methanol for 30 min.
2.4. Inmunodetection of CpRap2.4a
Recombinantly produced CpRap2.4a was loaded in 12% acrylamide gel to perform SDS-PAGE, then transferred to a nitrocellulose membrane and blocked with 3% of BSA in PBS at room temperature for 1 h. The membrane was then incubated with a chicken polyclonal anti-CpRap2.4a antibody (1/3000) (H-140, Santa Cruz Biotechnology, Dallas, TX, USA), for 1 h at room temperature. Then, a secondary anti-chicken antibody was used to incubate for 1 h at room temperature, with three washes of 10 min each with PBS-T between incubations. Immunoblotting signals were analyzed by BioRad Imager System (BioRad). Also, for tag peptides, the primary antibody was anti-6xHis (1/5000) (Abcam, ab18184, mouse monoclonal) and anti-His IgG was the secondary antibody.
2.5. Plant Evaluation and Treatment of Sprayed rCaRap2.4a in Nicotiana tabacum
Wild type Nicotiana tabacum L. cv. SRI seedlings were planted in a potting mixture of Horti Pearl-Sunshine-Vermiculite-Peat moss (2:2:2:1, w/w/w/w). Plants were grown with a photoperiod of 16 h light/8 h dark (25 °C), with a photon flux density of 180 µmol m−2 s−1 for 8 weeks (Arroyo et al., 2016) [41]. For the temperature stress treatments, five N. tabacum plants were selected based on size and health similarity prior to the experiment, to maintain a uniform start point. The plants were then incubated near to 40 °C for the heat exposure condition. The stress phase and recovery phase are named regarding the period of stress itself, and measurements carried out after the stress period are labeled the recovery phase.
Physiological measurements were performed in vivo, using the portable photosynthesis system (LI-6400XT Portable Photosynthesis System, LI-COR Biosciences, Lincoln, USA). Five plants per experimental group were evaluated, selecting the third leaf, and counting from the apex to the base (excluding developing apical leaves). Five consecutive measurements were taken from each leaf to obtain a representative value per replicate. The parameters directly determined by the equipment were as follows: net photosynthesis rate, transpiration rate, stomatal conductance and internal CO2 concentration. From these values, the water use efficiency was indirectly calculated as the ratio of net photosynthesis to transpiration rate [19,36].
Immediately after each measurement, the leaves were harvested, placed in sterile centrifuge tubes and stored on ice for transport to the laboratory, where they were processed within a maximum of 1 h. Biochemical parameters were estimated by The rainbow protocol suggested in López-Hidalgo et al., 2020 [42].
2.6. Foliar Application of CpRap2.4a
For spraying, the supernatant obtained from protein extraction from the E. coli culture was used. The supernatant obtained was diluted 1:10 in non-sterile distilled water on day 0 of the experiment; plastic bottles with a standard sprayer were purchased at a local market. Each bottle had a capacity of 1 L. The bottles were stored at −20 °C and thawed only before spraying and were kept on ice for transport to the greenhouse.
Spraying was carried out on days 0, 7, 14 and 21, between 9 and 11 am, always after photographs and physiological sampling had been taken. On day 0, the spraying temperature was 28 °C, while on other days it was 38–41 °C, coinciding with the cubicle temperature. The volume sprayed per plant was approximately 5 mL, and care was taken to spray leaves on both sides and the stem.
2.7. Stomatal Behavior Visualization in Nicotiana tabacum Leaves by SEM
The analyzed plant samples were collected during the same sampling periods as the stress assay. The observation of the stomatal aperture was carried out in the central regions of the leaves, on the abaxial epidermal surface, using scanning electron microscopy (SEM). The assay was performed as follows: disks 3 cm in diameter were cut and bleached for 72 h in an 80% ethanol solution. Subsequently, they were dried under critical point conditions at 1072 psi/31 °C (Samdri®-795, Tousimis, Rockville, MD, USA). For visualization, the disks were mounted on metal stubs using conductive carbon adhesive tape (Electron Microscopy Sciences, Hatfield, PA, USA) and then sputter-coated with a fine 150 Å gold layer (Denton Vacuum Desk II, Moorestown, NJ, USA). To evaluate the stomatal aperture, magnifications of 400× were used to observe stomatal density, and 2000× were used to visualize individual stomata within the analyzed quadrant. Stomata were counted per quadrant, and their aperture was measured under the conditions of the stress assay. For each sample, five fields of view were observed, and within them, the aperture of five stomata were measured. Morphological analysis of the stomata was performed using a scanning electron microscope (Jeol, JSM-6360LV, Tokyo, Japan).
2.8. Statistical Analysis and Visualization
Several software programs were used for data analysis. The data obtained from the cultures were analyzed using STATISTITA version 7.0 to perform a mathematical adjustment of the Gompertz model to the cell culture. Electrophoresis gels were analyzed using GelAnalyser version 19.1, to identify the molecular weights of the signals of interest. The data obtained from the stress test were analyzed using the statistical software STATGRAPHICS Centurion XVI. For the physiological and biochemical measurements, each bar represents the average measurement from three independent samples for each condition. The data were analyzed by one-way analysis of variance (ANOVA), followed by the comparison of mean values using Tukey’s test at p < 0.01 in Statgraphics Centurion (StatPoint Technologies, Inc., Warrenton, VA, USA). Data were presented as mean ±SD error of three determinations per sample.
3. Results
3.1. Production and Confirmation of the Recombinant Protein CpRap2.4a
The recombinant protein CpRap2.4a was successfully produced in the bacterial expression system. Transformation of E. coli strains (DH5α, BL21, Origami and Rosetta) with the pDEST17 vector containing the gene of interest was confirmed by end-point PCR (Supplementary Figure S1 top left panel, lanes 3, 4, 5 and 6). A single band of approximately 855 bp, corresponding to the expected size for the gene obtained from C. papaya (lane 2), was observed in the transformed strains, while the negative control showed no amplification (Supplementary Figure S1, top right panel). Protein expression and purification were analyzed by SDS-PAGE electrophoresis. A band at approximately 34 kDa, corresponding to the theoretical molecular weight of the CpRap2.4a fusion protein, was visible in the supernatant fraction and in the elution from the Ni2+ affinity column, confirming soluble expression and successful purification of the recombinant protein (Supplementary Figure S1, bottom panel).
3.2. Biostimulant Activity of CpRap2.4a Applied by Spraying on N. tabacum
Under normal growth conditions, no visible phenotypic differences were observed between the control plant groups, those sprayed with the protein-free supernatant (Vector), or those sprayed with CpRap2.4a (Figure 1). However, after 14 days of exposure to high-temperature stress, a marked contrast was evident. Plants in the control group and those treated with the empty vector exhibited severe stress symptoms, including extensive chlorosis, loss of turgor, leaf curling and a marked reduction in growth. In contrast, plants treated with CpRap2.4a displayed a significantly more vigorous phenotype, with predominantly green leaves and greater leaf development (Figure 1, Stress panel). After 14 days of recovery under optimal conditions, the regrowth and recovery capacity were notably higher in the group treated with the recombinant protein, while the control and vector groups showed irreversible damage and minimal recovery (Figure 1, Recovery panel).
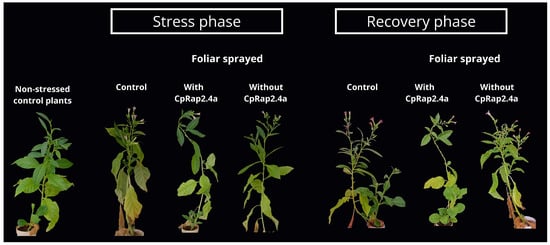
Figure 1.
Phenotypic effect of exogenous application of CpRap2.4a in tobacco plants under heat stress and recovery. Plants under optimal growth conditions (without stress) are at the left of the figure. Plants subjected to 14 days of high temperature stress were compared with those plants after 14 days of recovery. Control plants were sprayed with empty vector supernatant (protein extract without the induction of CpRap2.4a (without CrRap2.4a)), and plants were sprayed with supernatant containing the recombinant CpRap2.4a protein. Plants treated with CpRap2.4a displayed a noticeably more vigorous phenotype under stress and a superior recovery capacity compared to the control and empty vector groups.
3.3. Physiological and Biochemical Responses of N. tabacum to the Sprayed Application of CpRap2.4a
Analysis of physiological parameters revealed that treatment with CpRap2.4a significantly attenuated the negative effects of heat stress. Net photosynthesis and stomatal conductance decreased dramatically in both the control and vector groups during the stress phase, but remained at significantly higher levels in plants treated with the protein (Figure 2, top graphs). Consistently, water use efficiency (WUE) and relative water content (RWC) also showed a better recovery index in the CpRap2.4a sprayed group compared to the other two groups, indicating improved water homeostasis (Figure 2, middle graphs). Stomatal conductance showed a tendency to stabilize more quickly than treatments without the recombinant protein, indicating the facility of this transcription factor in the stomatal closing and opening processes to stabilize plant physiological processes. On the other hand, the internal CO2 concentration showed a complex pattern, consistent with stomatal and non-stomatal limitations of stress-induced photosynthesis (Figure 2, lower graphs).
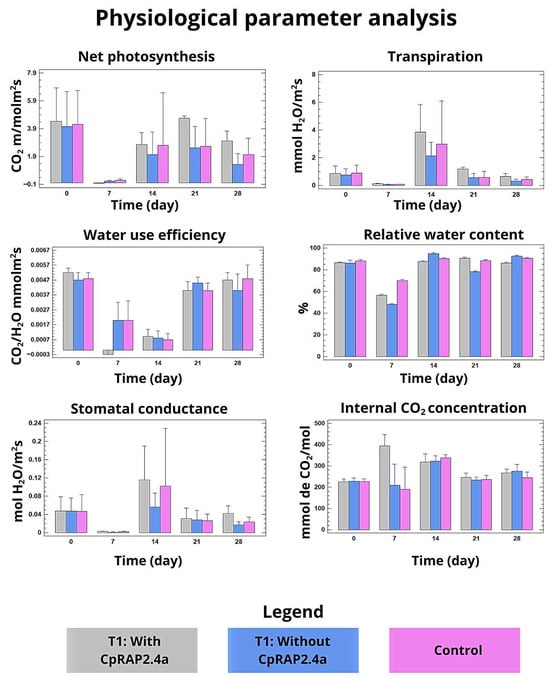
Figure 2.
Analysis of physiological parameters in tobacco plants treated with CpRap2.4a under heat stress. Statistical analysis of physiological parameters was conducted: net photosynthesis rate, stomatal conductance, water use efficiency, relative water content, stomatal conductance and internal CO2 concentration. The mean of three replicates per sample was added and the bars represent the mean ± standard deviation between treatments within each phase. The different sizes of the standard deviation bar indicate statistically significant differences (p < 0.05, Tukey’s test) between treatments within each phase.
At the biochemical level, treatment with CpRap2.4a helped maintain cellular integrity. Total chlorophyll and carotenoid content was less reduced in treated plants, reflecting less damage to the photosynthetic apparatus (Figure 3 upper graphs). In parallel, oxidative stress, quantified by malondialdehyde (MDA) levels, was significantly lower in the group sprayed with CpRap2.4a, suggesting less lipid peroxidation of membranes (Figure 2 middle graphs). Furthermore, treated plants showed an increase in the concentration of total phenolic compounds and free amino acids such as glycine, indicating a potential activation of antioxidant and osmoprotection mechanisms (Figure 2, middle and lower graphs).
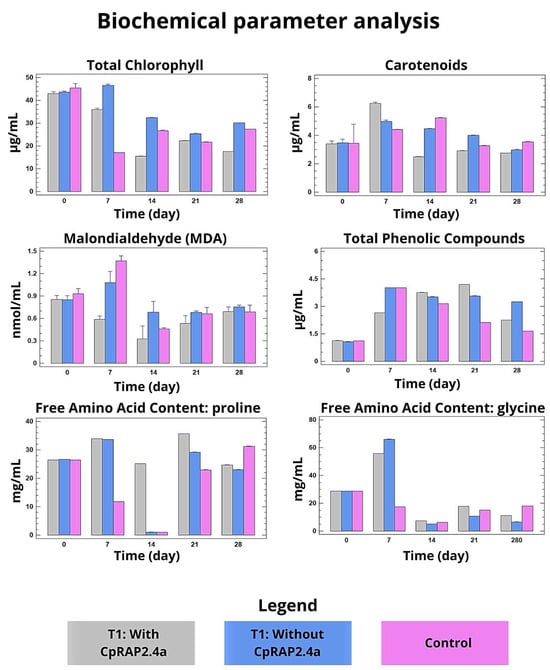
Figure 3.
Analysis of biochemical parameters in tobacco plants treated with CpRap2.4a under heat stress. The following biochemical parameters were measured: total chlorophyll content, carotenoid content, malondialdehyde concentration, total phenolic compounds concentration, free amino acids concentration, glycine and proline. The mean of three replicates per sample was compared; the bars represent the mean ± standard deviation between treatments within each phase. The different sizes of the standard deviation bar indicate statistically significant differences (p < 0.05, Tukey’s test) between treatments within each phase.
3.4. CpRap2.4a Regulates Stomatal Opening and Mitigates Stress at the Cellular Level
Scanning electron microscopy (SEM) allowed the evaluation of the impact of heat stress at the cellular level. Under stress conditions, stomata of control and vector plants were predominantly closed and showed signs of deformation and damage (Figure 4A,C, bottom graph). In contrast, plants treated with CpRAP2.4a presented a higher proportion of stomata with adequate opening and a more preserved morphology (Figure 4B, bottom graph). Quantitative analysis of the frequency of open stomata confirmed these observations: during the stress phase, the percentage of open stomata was significantly higher in the CpRAP2.4a group compared to the control and vector groups (Figure 3, bottom graph). After the recovery phase, stomata of treated plants showed a greater capacity to return to their normal open state (Figure 4A*–C*).
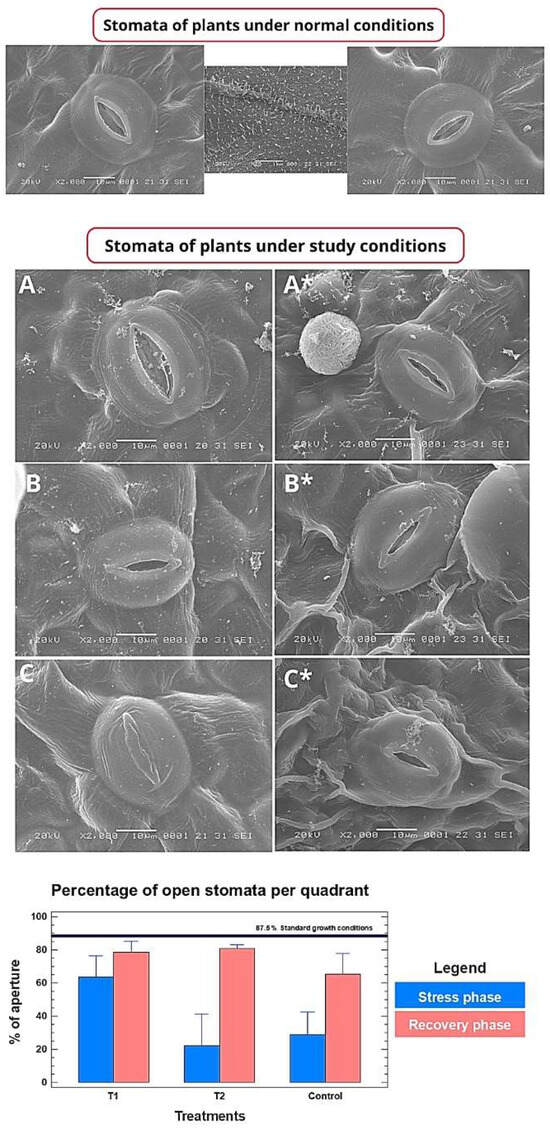
Figure 4.
Analysis of morphology and stomatal opening frequency. Top panel: Stomata from plants under normal (non-stress) conditions. Middle panels (A–C): Stomatal micrographs after 14 days of heat stress. Bottom panels (A,B,C*): Stomatal micrographs after 14 days of recovery: (A,A*): Control plants (untreated). (B,B*): Plants sprayed with CpRAP2.4a. (C,C*): Plants sprayed with empty vector supernatant. Bottom panel: Percentage of open stomata per quadrant during the stress and recovery phases. Bars represent mean ± standard deviation (n = 10). Different bar sizes indicate statistically significant differences (p < 0.05, Tukey’s test) between treatments within each phase.
4. Discussion
The present study demonstrates that exogenous application of the recombinant papaya protein, CpRap2.4a, confers significant heat stress tolerance to tobacco plants, as evidenced by substantial improvements in phenotype, physiological parameters, redox status and stomatal anatomy. Our findings suggest that this protein, belonging to the AP2/EREBP family of transcription factors, acts as an effective elicitor of plant defense responses when applied foliarly, representing a novel, non-transgenic biotechnological strategy.
The ability of CpRap2.4a to enhance heat stress tolerance is likely mediated by its function as a transcriptional regulator. Proteins that are homologous to Rap2.4 in Arabidopsis thaliana have been reported to bind to promoter elements of genes involved in the response to oxidative stress, such as those encoding antioxidant enzymes [19,43]. This is in direct agreement with our results, where we observed an increase in antioxidant enzyme activity and a significant reduction in MDA levels in plants treated with CpRap2.4a. The reduction in oxidative damage preserved the integrity of cell membranes, which in turn supported better water retention, as indicated by the higher relative water content and greater stability of the photosynthetic apparatus, reflected in higher chlorophyll levels.
At the physiological level, the remarkable maintenance of net photosynthetic rates and stomatal conductance in plants treated under stress is a crucial finding. The thermotolerance conferred by CpRap2.4a allowed stomata to maintain a more functional aperture, as confirmed by SEM analysis, facilitating better gas exchange and preventing leaves from overheating. A similar strategy of stomatal regulation to improve thermotolerance has been observed with the application of other biostimulants [44]. This improved stomatal function, combined with direct biochemical protection, and resulted in consistently greater water use efficiency: a highly desirable effect for crops under stress conditions.
The central novelty of our work lies in the successful exogenous application of the complete recombinant protein. While most previous studies have relied on transgenic overexpression of the genes encoding these proteins [45], we demonstrate that foliar application of the purified protein is sufficient to elicit a robust response. This suggests that CpRap2.4a could be perceived by cell surface receptors or internalized, subsequently activating the defense signaling cascade. This approach represents a practical, rapid and regulatory-friendly alternative to developing transgenic plants.
While our study establishes strong evidence for the protective effect of CpRap2.4a, the specific signaling pathways and target genes it regulates after exogenous application remain to be elucidated. Future transcriptomic studies (RNA-Seq) using the same experimental design would be ideal for identifying the gene network activated by this treatment. Furthermore, validating the efficacy of CpRap2.4a in crops of agricultural interest would be the next logical step toward its commercialization.
In conclusion, our results position the recombinant protein CpRap2.4a as a promising biostimulant to mitigate the impact of heat stress. Its mode of action, which integrates the enhancement of the antioxidant response, protection of photosynthesis and stomatal regulation, offers a multi-pronged solution to a complex problem. This work lays the foundation for the development of an innovative sustainable agriculture strategy, based on the application of protein elicitors.
5. Conclusions
The study demonstrates that it is possible to obtain a biologically active recombinant plant protein with agricultural biostimulant characteristics. Biochemical and physiological parameter tests after the foliar application of CpRap2.4a clearly demonstrate an effect and demonstrate heat stress tolerance. Probably, the mechanisms related to this effect occur due to the fact that CrRap2.4a can modulate the stomatal conductance and aperture during stress; however, more precisely, experiments related to the molecular process should be carried out to validate this. The use of the studied bacterial strain streamlines the scaling and optimization process, reducing costs compared to commercial protein-based biostimulants. Furthermore, it opens a new field of biotechnological development: the use of recombinant plant proteins as a crop improvement tool, without resorting to genetically modified crops.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/crops5060080/s1. Figure S1: Production and confirmation of recombinant CpRAP2.4a protein. Top left: Map of the bacterial expression vector pDEST17, used for cloning the CpRAP2.4a gene. Top right: Confirmation of transformation by end-point PCR. Lane 1: Negative control (strain without plasmid). Lanes 2-6: 855 bp PCR products corresponding to the CpRAP2.4a gene in E. coli strains DH5α, BL21(DE3), Origami and Rosetta, respectively. Bottom left: Expression analysis by SDS-PAGE (15%). Lane 1: BSA control (1 mg/mL). Lane 2: Elution from the Ni2+ affinity column. Lane 3: Cell lysis supernatant. Lane 4: Wash fraction. Lane 5: Elution from the Ni2+ affinity column after 10 days at −20 °C. The band corresponding to the recombinant protein CpRAP2.4a corresponds to a size of 34 kDa for the monomer and 67 for the dimer. Lower right: Immunodetection of the protein. The presence of the dimer is observed (faint solitary band), other bands are possible degradation (lower) or aggregation (upper) of the recombinant protein.
Author Contributions
Conceptualization, D.G.-S., F.G.-C., M.Á.H.-A., L.J.F.-Y., R.A.U.S., J.L.A., A.C.G.M., E.C. and L.C.R.-Z.; methodology, D.G.-S., F.G.-C., M.Á.H.-A. and L.J.F.-Y.; software, D.G.-S. and F.G.-C.; validation, R.A.U.S., J.L.A., A.C.G.M., E.C. and L.C.R.-Z.; formal analysis, D.G.-S., F.G.-C., M.Á.H.-A., L.J.F.-Y., R.A.U.S., J.L.A. and A.C.G.M.; investigation, D.G.-S., F.G.-C., M.Á.H.-A., L.J.F.-Y., R.A.U.S., J.L.A. and A.C.G.M.; resources, L.J.F.-Y. and L.C.R.-Z.; data curation, D.G.-S., F.G.-C., M.Á.H.-A.; writing—original draft preparation, D.G.-S., F.G.-C. and M.Á.H.-A.; writing—review and editing, D.G.-S., F.G.-C., L.J.F.-Y., E.C. and L.C.R.-Z.; visualization, D.G.-S. and F.G.-C.; supervision, L.J.F.-Y., E.C. and L.C.R.-Z.; project administration, L.J.F.-Y., E.C. and L.C.R.-Z.; funding acquisition, L.J.F.-Y., E.C. and L.C.R.-Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the associated research project, CF-2023-G-636: “CpRAP2.4a: A transcription factor (TF) involved in mitigating heat stress, to understand its long-distance transport mechanism in papaya sap”, approved under the Conahcyt 2023 Frontier Science Call (Convocatoria de Ciencia de Frontera 2023).
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We acknowledge Silvia B. Andrade Canto for her work and support in acquiring microscopic images using the SEM.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3544. [Google Scholar] [CrossRef] [PubMed]
- Leisner, C.P.; Potnis, N.; Sanz-Saez, A. Crosstalk and trade-offs: Plant responses to climate change-associated abiotic and biotic stresses. Plant Cell Environ. 2023, 46, 2946–2963. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Gómez, S. Abiotic and Biotic Stress Tolerance in Plants. In Molecular Stress Physiology of Plants; Rout, G.R., Das, A.B., Eds.; Springer: New Delhi, India, 2013; pp. 1–20. [Google Scholar] [CrossRef]
- Kan, Y.; Mu, X.R.; Gao, J.; Lin, H.X.; Lin, Y. The molecular basis of heat stress responses in plants. Mol. Plant 2023, 16, 1612–1634. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Complex plant responses to drought and heat stress under climate change. Plant J. 2024, 117, 1873–1892. [Google Scholar] [CrossRef]
- Smith, P.; Haberl, H.; Popp, A.; Erb Kheinz Lauk, C.; Harper, R.; Tubiello, F.N.; Pinto, A.d.S.; Jafari, M.; Sohi, S.; Masera, O.; et al. How much land-based greenhouse gas mitigation can be achieved without compromising food security and environmental goals? Glob. Change Biol. 2013, 19, 2285–2302. [Google Scholar] [CrossRef]
- Gregorio, J.; Hernández-Bernal, A.F.; Cordoba, E.; León, P. Characterization of evolutionarily conserved motifs involved in activity and regulation of the ABA-INSENSITIVE (ABI) 4 transcription factor. Mol. Plant 2014, 7, 422–436. [Google Scholar] [CrossRef]
- Brown, M.E.; Funk, C.C. Food Security Under Climate Change. Science 2008, 319, 580–581. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Yang, F.; Liu, C.Y.; Zhao, Y.-Q.; Li, M.-Y.; Lu, X.-J.; Ge, J.; Zhang, B.W.; Li, M.-Q.; Yang, Y.; et al. Transcriptome sequencing of garlic reveals key genes related to the heat stress response. Sci. Rep. 2024, 14, 15956. [Google Scholar] [CrossRef]
- Qian, R.; Hu, Q.; Ma, X.; Zhang, X.; Ye, Y.; Liu, H.; Gao, H.; Zheng, J. Comparative transcriptome analysis of heat stress responses of Clematis lanuginosa and Clematis crassifolia. BMC Plant Biol. 2022, 22, 138. [Google Scholar] [CrossRef]
- Mondal, S.; Karmakar, S.; Panda, D.; Pramanik, K.; Bose, B.; Singhal, R.K. Crucial plant processes under heat stress and tolerance through heat shock proteins. Plant Stress 2023, 10, 100227. [Google Scholar] [CrossRef]
- Gurley, W.B. HSP101: A Key Component for the Acquisition of Thermotolerance in Plants. Plant Cell 2000, 12, 457–460. [Google Scholar] [CrossRef]
- Schramm, F.; Larkindale, J.; Kiehlmann, E.; Ganguli, A.; Englich, G.; Vierling, E.; Von Koskull-Döring, P. A cascade of transcription factor DREB2A and heat stress transcription factor HsfA3 regulates the heat stress response of Arabidopsis. Plant J. 2008, 53, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Pietzenuk, B.; Markus, C.; Gaubert, H.; Bagwan, N.; Merotto, A.; Bucher, E.; Pecinka, A. Recurrent evolution of heat-responsiveness in Brassicaceae COPIA elements. Genome Biol. 2016, 17, 209. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, L.; Wang, L.; Wang, S.; Cheng, X. VrDREB2A, a DREB—Binding transcription factor from Vigna radiata, increased drought and high—salt tolerance in transgenic Arabidopsis thaliana. J. Plant Res. 2016, 129, 263–273. [Google Scholar] [CrossRef]
- Magar, M.M.; Liu, H.; Yan, G. Genome-Wide Analysis of AP2/ERF Superfamily Genes in Contrasting Wheat Genotypes Reveals Heat Stress-Related Candidate Genes. Front. Plant Sci. 2022, 13, 853086. [Google Scholar] [CrossRef]
- Xu, Z.S.; Chen, M.; Li, L.C.; Ma, Y.Z. Functions and Application of the AP2/ERF Transcription Factor Family in Crop Improvement. J. Integr. Plant Biol. 2011, 53, 570–585. [Google Scholar] [CrossRef]
- Xie, W.; Ding, C.; Hu, H.; Dong, G.; Zhang, G.; Qian, Q.; Ren, D. Molecular Events of Rice AP2/ERF Transcription Factors. Int. J. Mol. Sci. 2022, 23, 12013. [Google Scholar] [CrossRef]
- Najafi, S.; Sorkheh, K.; Nasernakhaei, F. Characterization of the APETALA2/Ethylene-responsive factor (AP2/ERF) transcription factor family in sunflower. Sci. Rep. 2018, 8, 11576. [Google Scholar] [CrossRef]
- Figueroa-Yañez, L.; Pereira-Santana, A.; Arroyo-Herrera, A.; Rodriguez-Corona, U.; Sanchez-Teyer, F.; Espadas-Alcocer, J.; Espadas-Gil, F.; Barredo-Pool, F.; Castaño, E.; Rodriguez-Zapata, L.C. RAP2.4a Is Transported through the Phloem to Regulate Cold and Heat Tolerance in Papaya Tree (Carica papaya cv. Maradol): Implications for Protection Against Abiotic Stress. PLoS ONE 2016, 11, e0165030. [Google Scholar] [CrossRef] [PubMed]
- Tolstyko, E.A.; Lezzhov, A.A.; Morozov, S.Y.; Solovyev, A.G. Phloem transport of structured RNAs: A widening repertoire of trafficking signals and protein factors. Plant Sci. 2020, 299, 110602. [Google Scholar] [CrossRef]
- Kehr, J.; Kragler, F. Long distance RNA movement. New Phytol. 2018, 218, 29–40. [Google Scholar] [CrossRef]
- Ostendorp, A.; Pahlow, S.; Krüßel, L.; Hanhart, P.; Garbe, M.Y.; Deke, J.; Giavalisco, P.; Kehr, J. Functional analysis of Brassica napus phloem protein and ribonucleoprotein complexes. New Phytol. 2017, 214, 1188–1197. [Google Scholar] [CrossRef]
- Kehr, J.; Buhtz, A. Long distance transport and movement of RNA through the phloem. J. Exp. Bot. 2008, 59, 85–92. [Google Scholar] [CrossRef]
- Murakami, T.; Qamar, S.; Lin, J.Q.; Schierle, G.S.K.K.; Rees, E.; Miyashita, A.; Costa, A.R.; Dodd, R.B.; Chan, F.T.S.; Michel, C.H.; et al. ALS/FTD Mutation-Induced Phase Transition of FUS Liquid Droplets and Reversible Hydrogels into Irreversible Hydrogels Impairs RNP Granule Function. Neuron 2015, 88, 678–690. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, X.; Shi, Y.; Xu, J.; Huang, B. Transgenic Tobacco Plants Overexpressing a Grass PpEXP1 Gene Exhibit Enhanced Tolerance to Heat Stress. PLoS ONE 2014, 9, e100792. [Google Scholar] [CrossRef]
- Hong, B.; Ma, C.; Yang, Y.; Wang, T.; Yamaguchi-Shinozaki, K.; Gao, J. Over-expression of AtDREB1A in chrysanthemum enhances tolerance to heat stress. Plant Mol. Biol. 2009, 70, 231–240. [Google Scholar] [CrossRef]
- Burnett, M.J.B.; Burnett, A.C. Therapeutic recombinant protein production in plants: Challenges and opportunities. Plants People Planet 2020, 2, 121–132. [Google Scholar] [CrossRef]
- Abu-Bakar, N.; Juri, N.M.; Abu-Bakar, R.A.H.; Sohaime, M.Z.; Badrun, R.; Sarip, J.; Hassan, M.Z.; Ahmad, K. Recombinant Protein Foliar Application Activates Systemic Acquired Resistance and Increases Tolerance Against Papaya Dieback Disease. Asian J. Agric. Rural. Dev. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Saudi, A.H. Effect of Foliar Spray with Seaweeds Extract on Growth, Yield and Seed Vigour of Bread Wheat Cultivars. Iraqi J. Agric. Sci. 2017, 48, 1313–1325. [Google Scholar] [CrossRef]
- Peña Calzada, K.; Olivera Viciedo, D.; Habermann, E.; Calero Hurtado, A.; Lupino Gratão, P.; De Mello Prado, R.; Lata-Tenesaca, L.F.; Martinez, C.A.; Celi, G.E.A.; Rodríguez, J.C. Exogenous application of amino acids mitigates the deleterious effects of salt stress on soybean plants. Agronomy 2022, 12, 2014. [Google Scholar] [CrossRef]
- Matysiak, K.; Kierzek, R.; Siatkowski, I.; Kowalska, J.; Krawczyk, R.; Miziniak, W. Effect of exogenous application of amino acids l-arginine and glycine on maize under temperature stress. Agronomy 2020, 10, 769. [Google Scholar] [CrossRef]
- Ismail, H.E.M.; Osman, A.; Sitohy, M.Z. Foliar spray of pea plants with modified egg albumin for enhancing growth and productivity. Sinai J. Appl. Sci. 2017, 6, 1–12. [Google Scholar] [CrossRef]
- Feng, D.; Jia, X.; Yan, Z.; Li, J.; Gao, J.; Xiao, W.; Shen, X.; Sun, X. Underlying mechanisms of exogenous substances involved in alleviating plant heat stress. Plant Stress 2023, 10, 100288. [Google Scholar] [CrossRef]
- David, G.-T.S.; Alejandro, P.-S.; Alejandro, Z.-B.J.; Enrique, C.; Francisco, E.-G.F.; Tonatiuh, A.-S.J.; Ángel, K.-L.M.; Felipe, S.-T.; Carlos, R.-Z.L. Transcriptomics and co-expression networks reveal tissue-specific responses and regulatory hubs under mild and severe drought in papaya (Carica papaya L.). Sci. Rep. 2018, 8, 14539. [Google Scholar]
- Hirano, S. Western Blot Analysis. In Nanotoxicity: Methods and Protocols; Reineke, J., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 87–97. [Google Scholar] [CrossRef]
- Harper, S.; Speicher, D.W. Purification of Proteins Fused to Glutathione S-Transferase. In Protein Chromatography: Methods and Protocols; Walls, D., Loughran, S.T., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 259–280. [Google Scholar] [CrossRef]
- Spriestersbach, A.; Kubicek, J.; Schäfer, F.; Block, H.; Maertens, B. Chapter One—Purification of His-Tagged Proteins. In Methods in Enzymology; Lorsch, J.R., Ed.; Academic Press: Oxford, UK, 2015; pp. 1–15. [Google Scholar] [CrossRef]
- Guillen-Chable, F.; Rodríguez Corona, U.; Pereira-Santana, A.; Bayona, A.; Rodríguez-Zapata, L.C.; Aquino, C.; Šebestová, L.; Vitale, N.; Hozak, P.; Castano, E. Fibrillarin ribonuclease activity is dependent on the gar domain and modulated by phospholipids. Cells 2020, 9, 1143. [Google Scholar] [CrossRef]
- Loza-Muller, L.; Rodríguez-Corona, U.; Sobol, M.; Rodríguez-Zapata, L.C.; Hozak, P.; Castano, E. Fibrillarin methylates H2A in RNA polymerase I trans-active promoters in Brassica oleracea. Front. Plant Sci. 2015, 6, 976. [Google Scholar] [CrossRef]
- Arroyo-Herrera, A.; Figueroa-Yáñez, L.; Castaño, E.; Santamaría, J.; Pereira-Santana, A.; Espadas-Alcocer, J.; Sánchez-Teyer, F.; Espadas-Gil, F.; Alcaraz, L.D.; López-Gómez, R.; et al. A novel Dreb2-type gene from Carica papaya confers tolerance under abiotic stress. Plant Cell Tiss. Organ Cult. 2016, 125, 119–133. [Google Scholar] [CrossRef]
- López-Hidalgo, C.; Meijón, M.; Lamelas, L.; Valledor, L. The rainbow protocol: A sequential method for quantifying pigments, sugars, free amino acids, phenolics, flavonoids and MDA from a small amount of sample. Plant Cell Environ. 2021, 44, 1977–1986. [Google Scholar] [CrossRef]
- Franco-Zorrilla, J.M.; López-Vidriero, I.; Carrasco, J.L.; Godoy, M.; Vera, P.; Solano, R. DNA-binding specificities of plant transcription factors and their potential to define target genes. Proc. Natl. Acad. Sci. USA 2014, 111, 2367–2372. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.D.; Ma, R.; Zheng, Y.X.; Xie, L.Q. Genome-wide identification of APX genes in flax (Linum usitatissimum) and functional characterization of LuAPX12 in osmotic and salinity stress responses. BMC Plant Biol. 2025, 25, 939. [Google Scholar] [CrossRef] [PubMed]
- Vaseva, I.I.; Simova-Stoilova, L.; Kostadinova, A.; Yuperlieva-Mateeva, B.; Karakicheva, T.; Vassileva, V. Heat-Stress-Mitigating Effects of a Protein-Hydrolysate-Based Biostimulant Are Linked to Changes in Protease, DHN, and HSP Gene Expression in Maize. Agronomy 2022, 12, 1127. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).