Simple Summary
This study implemented a 12-week exercise program for 13 female breast cancer survivors, aged 35–53, focusing on quality of life, self-esteem, and depression. Participants engaged in twice-weekly sessions of aerobic and resistance training exercises. Results showed significant improvements in quality of life, particularly in future health prospects and arm symptoms, and in self-esteem. Although depressive symptoms decreased, the change was not statistically significant. The study underscores the importance of physical exercise as a supportive method for managing breast cancer therapy and its side effects. It highlights the need to demystify exercise post-cancer diagnosis, especially among less active groups, promoting healthier lifestyles. Community-based programs and educational initiatives are recommended to enhance the adoption of physical activity, optimizing recovery and improving overall well-being for breast cancer survivors.
Abstract
Controlled study designs usually report that physical exercise improves the health of women living with breast cancer. However, many of these women are not sufficiently active to experience the benefits of exercise. The main objective was to analyze the effect of a physical exercise program on quality of life, self-esteem, and depression in breast cancer survivors. Thirteen participants (46.54 ± 6.31 years old) completed the exercise intervention. Three patient-reported questionnaires were used: Supplementary Questionnaire Breast Cancer Module (QLQ-BR23), Beck Depression Inventory (BDI), and Rosenberg Self-Esteem Scale (RSES). All participants had significantly improved self-esteem (p = 0.004). Although there were no statistically significant changes in depression, there was a notable decrease in scores (6.39 ± 4.75 vs. 5.00 ± 4.75; p = 0.080). Regarding quality of life, significant improvements were observed in “future perspectives” (p = 0.047) and “arm symptoms” (p = 0.015). No significant changes were noted in the other variables. Our results suggest that physical exercise is an effective strategy that positively affects breast cancer survivors’ quality of life and self-esteem. The results reinforce the need for community-based exercise programs for breast cancer survivors. Healthcare professionals should promote physical exercise to improve health outcomes before, during, and after treatment.
1. Introduction
Physical exercise is widely recognized as an effective and safe non-pharmacological intervention to mitigate the side effects of cancer and its treatments, primarily by enhancing patients’ quality of life [1]. It significantly contributes to physical and psychological well-being in a non-invasive, non-toxic, and economically accessible manner for patients [2].
The American Cancer Society recommends that cancer survivors engage in regular physical activities, maintaining 150–300 min of moderate physical activity per week, and avoid sedentary behavior [3]. Physical exercise has been shown to reduce the risk of mortality (by 28–44%) and recurrence (by 21–35%) [4]. According to the American Cancer Society [3], both moderate and vigorous physical activities serve as protective factors against breast cancer. Cormie et al. [4] further state that exercise alleviates side effects associated with treatments, such as fatigue, anxiety, depression, stress, sleep disturbances, self-image issues, and physical health problems. Gilchrist et al. [5] emphasize that sedentary behavior can increase cancer mortality risk, which can be mitigated by substituting sedentary behavior with physical activity.
Several studies have shown that physical exercise interventions in women with breast cancer lead to significant improvements in their quality of life [6,7,8,9], notably in aerobic fitness [9,10], flexibility [7], fatigue [6,7], depression [10,11], and anxiety [12].
This investigation is important because physical exercise promotes a healthy lifestyle, thereby providing physiological and psychological well-being [13,14,15,16]. Additionally, it can help reduce the side effects of treatments and may also reduce the risk of recurrence and mortality from this disease [14,15,17].
Despite the recognized benefits of exercise for cancer treatment, many women with breast cancer are insufficiently active to reap these advantages, often due to a lack of knowledge, motivation, access to specialists, and referrals from physicians. Community-based studies offer several significant scientific benefits, enhancing both research and community welfare. This research ensures that findings are relevant and applicable to local contexts, leading to more effective interventions. Active community participation increases engagement, acceptance, and adherence to health recommendations, resulting in more successful implementations of interventions. Additionally, it enhances ecological validity, make research outcomes more credible and useful for everyday situations. Scientific literature supports these advantages, highlighting the importance of community involvement in research [18].
The main objective of this study was to analyze the effect of a physical exercise program on quality of life (body image, sexuality, future perspectives, side effects of chemotherapy, and breast and arm symptoms), self-esteem, and depression in breast cancer survivors.
2. Materials and Methods
2.1. Study Design
The present study utilized a longitudinal design, initially applying a baseline assessment to participants who joined the exercise program. This initial evaluation included body mass index (BMI), the International Physical Activity Questionnaire (IPAQ), Cancer History Questionnaire, Beck Depression Inventory, Rosenberg Self-Esteem Scale, and the Supplementary Questionnaire Breast Cancer Module. The pre-test was conducted before starting the physical exercise program, and the post-test was conducted at the end of the program, after 12 weeks. At the end of the program, the breast cancer survivors completed the Beck Depression Inventory, Rosenberg Self-Esteem Scale, and the Supplementary Questionnaire Breast Cancer Module again. The Borg Rating of Perceived Exertion Scale was used to control the intensity of exercise during the sessions.
These questionnaires were completed individually, without interaction or exchange of information between the participants. Prior explanations were given, and during the completion of the questionnaires, any doubts or questions that arose were addressed to ensure understanding.
2.2. Exercise Intervention
Based on the evidence and international clinical guidelines for exercise practice in breast cancer patients [19,20,21,22], a physical exercise program was structured specifically for this population over 12 weeks. The program consisted of two sessions per week, each lasting 60 min. The intensities of the exercises were measured using the Borg Rating of Perceived Exertion Scale at the end of the sessions, ranging between 12 and 14, which indicates a subjective perception of effort within the “somewhat hard” and “hard” intensity intervals. Most sessions took place in a classroom at a school in the city of Vila Real, while some sessions, on good weather days, were held in the city park. This aimed to provide a different training environment where participants could engage with various stimuli, enjoy social interaction, and connect with nature.
The exercise sessions were divided into four parts, each with specific objectives: (i) warm-up (approximately 10 min) aimed to gradually increase the heart rate and body temperature and activate proper breathing while mobilizing large muscle groups. This part included aerobic choreography or walking, primarily focusing on cardiovascular/aerobic training. (ii) Mobilization (approximately 10 min) consisted of breathing exercises, stretching, and the main joint mobilizations (shoulder, elbow, wrist, hip, knee, and ankle). The goal was to improve the range of motion in the upper limbs and prevent osteoarticular injuries. (iii) Main part (approximately 30 min) combined training with a focus on muscular resistance (strength) using body weight, fitballs, resistance bands, and dumbbells of various weights. Exercises were selected to be easily transferable to daily tasks, such as squatting/sitting, standing, and pulling, along with core-strengthening exercises. During this part, the exercises were performed in a circuit format, each lasting one minute. Each exercise sequence was repeated three times. In this part, each participant was advised to choose the alternative that best suited their physical condition, resulting in two different intensities: beginner (easier) and advanced (harder). (iv) Relaxation (duration approximately 10 min) aimed to return each participant to a calm state through slow walking and stretching, reducing respiratory and heart rates, increasing joint range of motion, and improving flexibility
During the 12-week intervention, there were no dropouts from the intervention group.
2.3. Participants
All study participants were recruited through the Breast Unit of the Centro Hospitalar de Trás-os-Montes e Alto Douro. They were informed that their participation in the study was voluntary and that all data provided and collected were confidential. Participants were free to withdraw from the study at any time. After being informed about the study’s objectives, all participants signed the informed consent statement in accordance with the Declaration of Helsinki.
The sample consisted of 13 women who had experienced oncological disease, specifically breast cancer, aged between 35–53 (46.54 ± 6.31) years. The average time from diagnosis to the start of the program was 20.46 (±30.66) months. All women underwent breast surgery, with 9 having conservative surgery and 4 undergoing mastectomies. Regarding treatment types, 10 women underwent combined chemotherapy and radiotherapy, 3 received radiotherapy, and 12 had hormone therapy.
The average body mass index (BMI) was 24.52 (±4.18) kg/m2, with 6 women being above the recommended weight. According to the IPAQ responses, 8 women had low physical activity levels, while 5 had moderate levels.
Participants were selected based on the following criteria: (i) women diagnosed with breast cancer; (ii) authorization from the oncologist to engage in physical exercise; (iii) agreement and signing of the informed consent form; and (iv) attending a minimum of six monthly exercise sessions.
2.4. Measurements
Initially, to better understand the sample and plan the intervention program in the most accurate and individualized manner, we assessed BMI (through weight and height measurements), the International Physical Activity Questionnaire-Short Version (to evaluate physical activity levels), and the Oncological Disease History Questionnaire (covering the date of oncological diagnosis, knowledge of the diagnosis, prognosis, physical symptoms, and current treatment). Subsequently, both at baseline and post-test, we applied three self-report questionnaires to evaluate the dependent variables under study.
- -
- Beck Depression Inventory:
The Beck Depression Inventory (BDI) is a 21-item, self-report rating inventory that measures characteristic attitudes and symptoms of depression [23]. This inventory was translated and validated into Portuguese by Vaz Serra and Abreu [24]. Internal consistency for the BDI ranges from 0.73 to 0.92, with a mean of 0.86 [25].
- -
- Rosenberg Self-Esteem Scale (RSE):
The purpose of the 10-item RSE scale is to measure global self-esteem. Originally intended as a measure of self-esteem for adolescents, Rosenberg’s Self-Esteem Scale is probably the most widely used measure of self-esteem for adult populations. This scale was translated and validated into Portuguese by Santos [26]. The RSE demonstrates a Guttman scale coefficient of reproducibility of 0.92, indicating excellent internal consistency [27].
- -
- Supplementary Questionnaire Breast Cancer Module (EORTC QLQ-BR23):
The Breast Cancer Module incorporates five multi-item scales to assess body image, sexual functioning, systemic therapy side effects, breast symptoms, and arm symptoms. In addition, single items assess sexual enjoyment, future perspective, and being upset by hair loss. This questionnaire was developed by Fayers et al. [28] and translated by EORTC. The reliability of this instrument was assessed with Cronbach’s alpha values ranging from 0.564 (body image) to 0.812 (sexual functioning), indicating internal consistencies ranging from acceptable to high [29].
2.5. Statistical Analysis
Statistical analysis was performed with the SPSS 26.0 (Statistical Package for the Social Sciences). Quantitative variables were described as mean and standard deviation (SD), and categorical variables were described in absolute frequency. Gaussian normal distribution was tested by the Kolmogorov–Smirnov test. To assess the impact of exercise intervention on the investigated parameters, a paired Student’s t-test with a 95% confidence interval was employed.
3. Results
Figure 1 presents descriptive statistics for the psychological variables of self-esteem and depression, based on pre-test and post-test assessments.
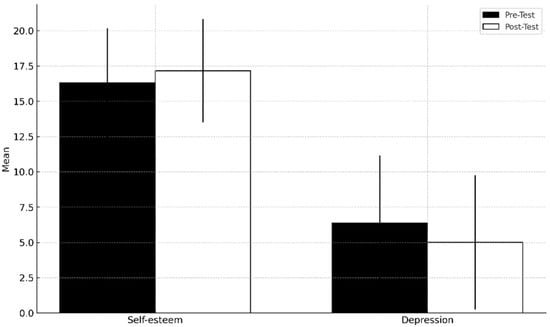
Figure 1.
Mean and standard deviation in self-esteem and depression.
Table 1 compares pre-test and post-test assessments, highlighting the effects of physical exercise on the psychological variables, self-esteem and depression.

Table 1.
Comparison of pre-test and post-test scores of self-esteem and depression.
The analysis of the results in Table 1 reveals a significant improvement in self-esteem when comparing pre-test and post-test scores (p = 0.004). Regarding depression, no significant improvements were observed (p = 0.080); however, a slight improvement was noted in the scores obtained in the post-test (6.39 vs. 5.00).
Figure 2 presents descriptive statistics for quality of life variables, based on pre-test and post-test assessments.
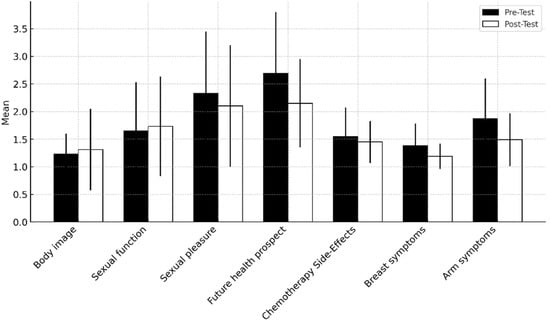
Figure 2.
Mean and standard deviation of quality of life variables.
Table 2 compares pre-test and post-test scores, illustrating changes in quality of life induced by physical exercise in the sample.

Table 2.
Comparison of pre-test and post-test scores for quality of life.
Regarding the functional scale, the results in Table 2 demonstrate an improvement in future health prospects (p = 0.047). In the symptom scale, significant improvements were observed in arm symptoms (p = 0.015). However, an analysis of the remaining subscales indicates that while there were improvements in body image, chemotherapy side-effects, breast symptoms, and sexual function, these results did not reach statistical significance.
4. Discussion
This study implemented a physical exercise program specifically designed for 13 female breast cancer survivors aged between 35 and 53, aiming to analyze its effect on quality of life, self-esteem, and depression. The program design adhered to the latest literature recommendations for this population, suggesting at least 150 min per week of moderate-intensity exercise or 75 min of vigorous-intensity exercise, including strength exercises targeting large muscle groups at least twice a week. We aimed to individualize the exercises and incorporate progressions and sequences to maximize benefits and minimize risks, given the specific nature of the sample. Additionally, we emphasized constant motivational reinforcement and individual feedback throughout the sessions, which helped retain all participants, despite the program’s demands (e.g., travel, availability, and schedule).
Before the intervention program, only four (31%) of the women in the sample reported engaging in physical exercise, averaging 2 to 3 times per week. The reduction in physical activity following a cancer diagnosis was noted and confirmed by authors such as Courneya et al. [29]. Several studies reported lower levels of physical activity and increased sedentary behavior in individuals diagnosed with cancer [30,31].
In terms of the intensity reported throughout the intervention, it ranged from 12 to 14 on the Borg scale, indicating that participants trained with a subjective perception of effort within the intervals considered “somewhat hard” and “hard”. The literature identified light to moderate intensity as effective in reducing depressive symptoms and improving quality of life and psychological well-being [32,33] in women who have survived breast cancer. This demonstrates that lower exercise intensities also promote significant benefits.
The results of this study showed significant improvements in self-esteem. However, regarding depression, although there was a slight decrease in scores, the improvement was not statistically significant. There were also significant improvements in some quality of life subscales, namely “future prospects” and “arm symptoms”. However, slight, non-significant improvements were observed in other subscales, such as body image, physical effects of chemotherapy, breast symptoms, and sexual function. Steindorf et al. [34] also reported no significant improvement in depression following a twice-weekly exercise intervention over 12 weeks in 80 women with breast cancer. However, they did note significant improvements in quality of life, body image, future prospects, and sexual function.
Sprod et al. [33] compared the physiological and psychological outcomes after 3 and 6 months of exercise in 97 breast cancer survivors. They reported additional benefits in physiological and psychological components, particularly in depression, after 24 weeks of intervention. These findings align with those of Burgess et al. [35], who concluded that women in remission showed similar levels of depressive symptoms and anxiety, compared to the general female population. Moreover, the meta-analysis by Krebber et al. [36] shows that the prevalence of depression is higher during cancer treatments, decreasing after therapies end, affecting 9% of cancer survivors.
Regarding self-esteem, our results indicate significant improvements in this variable. Similar results have been reported in other studies. Courneya et al. [30] evaluated the effects of physical exercise during breast cancer treatment and reported improvements in some quality of life components, particularly self-esteem. They also demonstrated that these effects were maintained in the long term, after 6 months. These results are relevant, as breast cancer survivors undergo challenging treatments that significantly affect self-esteem. The improvements in this quality of life aspect may be due to the group nature of the exercise sessions, fostering relationships among participants. Another reason could be the various motor tasks performed during the sessions, diverting focus from the disease.
The quality of life instrument used provided a specific dimension of quality of life using two scales: the functional scale and the symptoms scale. Statistically significant improvements in arm symptoms were recorded on the symptoms scale. However, improvements in chemotherapy side effects and breast symptoms were slight and not significant. Similar results were observed in the meta-analysis by Mishra et al. [37], verifying the effectiveness of physical exercise on general quality of life and symptom scales in cancer survivors. Travier et al. [38] evaluated the effects of an 18-week exercise program on 102 women with breast cancer and found slight, non-significant improvements in quality of life, depression, and anxiety in the intervention group.
On the functional scale, the improvements in sexual function, although not significant, were consistent with findings from other studies. Ramos and Patrão [39] studied the impact of exercise on marital relationship quality and sexual satisfaction in about 30 women who underwent surgery (mastectomy or conservative surgery) and subsequent treatments. Our results can be attributed to the significant increase in self-esteem: issues with personal appreciation (appearance and self-esteem) can lead to greater inhibition, embarrassment, and body non-acceptance, resulting in sexual dissatisfaction. Thus, improvements in self-esteem may have indirectly affected the sample’s sexuality in our study.
Regarding future health prospects, significant improvements were observed in this dimension. Shobeiri et al. [40] conducted a randomized clinical trial to assess the contribution of aerobic exercise to the quality of life of women with breast cancer, reporting that aerobic training improved overall health status and increased functional and symptom scale values in the intervention group, compared to the control group.
As for body image, dissatisfaction with appearance is a frequently reported concern among patients who have undergone surgery and treatments, negatively impacting identity, confidence, self-esteem, and body satisfaction. Our results showed a slight improvement, consistent with findings by Shobeiri et al. [40] and Remondes-Costa et al. [41]. Paulo et al. [8] evaluated the impact of a physical exercise program on quality of life in breast cancer survivors, concluding that the intervention helped improve body image and issues related to depression. The pilot study by Pinto et al. [42] also revealed that physical exercise improved the body image of women with this condition.
We know that self-esteem is directly related to body image, and physical exercise can provide psychological benefits, particularly in the mentioned variables. This may explain the results obtained in our study, despite the non-significant improvements in body image.
Studies such as the one by Schmitz et al. [43] report that physical exercise enhances self-esteem among female breast cancer survivors, demonstrating an improvement in body image resulting from physical activity. Similarly, research by Ligibel [44], Hayes et al. [45], and Vallance et al. [46] indicates that women who engage in physical exercise report feeling better and more satisfied with their appearance, with increased self-esteem and reduced symptoms of depression and anxiety. The fact that the exercise program in this study was conducted with women who had the same illness, treatments, and physical and emotional challenges may have contributed to improving future perspectives, self-esteem, body image satisfaction, self-perception, relationships with others, motivation to exercise, and the sharing of experiences, fears, and challenges throughout the program.
It is noteworthy, however, that despite both national and international recommendations from various health institutions, there is still a need to focus on motivational strategies to encourage individuals to take proactive steps for their own health. Even with studies scientifically proving the benefits of adopting active lifestyle habits, the general trend among the Portuguese population towards increasingly sedentary habits and occupations due to long work hours or family routines affects positive decision-making centered on quality of life and well-being. This often leads to neglecting basic needs in favor of other life aspects.
Motivating women with breast cancer to maintain a physically active lifestyle during and after diagnosis is crucial for promoting good emotional, social, and mental health. Group exercise can facilitate this, making the practice more enjoyable through conversation, experience exchange, interaction, and mutual progression, reducing the challenges posed by the disease. The new friendships, expanded social circles, and concern for each other contribute to feelings of usefulness, increasing confidence, good humor, self-esteem, and commitment to physical exercise. Additionally, the small physical changes observed during the program, such as improved arm mobility, increased muscle mass, and reduced fatigue, can be transferred to daily life, particularly in performing household tasks. These aspects help participants feel their lives are returning “to normal” and view the future more positively.
To ensure this practice, a collaborative effort is needed among professionals involved in this process, including oncologists, physiatrists, physiotherapists, exercise technicians, and exercise physiologists—a multidisciplinary team working for the common good of cancer patients. The main strength of our study is the implementation of a physical exercise program specifically aimed at the recovery of women with breast cancer, tailored to each individual’s health status.
The study’s limitations include the small number of participants, limiting the statistical treatment of the results. Another limitation could be the total duration of the program. The program duration was based on scientific literature, showing positive results in physical exercise programs after 12 weeks. Finally, the twice-weekly frequency may have been insufficient to provide more consistent results for this study.
5. Conclusions
This study highlights the practical application of structured exercise programs in improving the well-being of breast cancer survivors. By demonstrating significant improvements in self-esteem and certain aspects of quality of life, the research underscores the value of incorporating physical exercise into rehabilitation protocols. The community-based approach enhances engagement and adherence, making it a viable option for broader implementation. These findings provide a foundation for healthcare professionals to advocate for regular physical activity as part of comprehensive cancer care.
In addition, future research should focus on long-term follow-up to assess the sustained benefits of physical exercise on breast cancer survivors, using bigger samples. Investigating different types of exercise, varying intensities, and the inclusion of other supportive therapies could provide a more comprehensive understanding of how to optimize exercise interventions for this population.
Author Contributions
Conceptualization, E.M.R.T.d.C.C. and C.A.V.; methodology E.M.R.T.d.C.C., C.A.V. and S.C.F.F.; formal analysis, E.M.R.T.d.C.C., C.A.V. and H.I.A.M.; investigation, H.I.A.M.; writing—original draft preparation, E.M.R.T.d.C.C., C.A.V. and H.I.A.M.; writing—review and editing, E.M.R.T.d.C.C., C.A.V., H.I.A.M. and S.C.F.F.; visualization and supervision E.M.R.T.d.C.C., C.A.V. and S.C.F.F.; project administration, E.M.R.T.d.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
E.M.R.T.C.C.: C.A.V. and S.C.F.F. were funded by National Funds by FCT—Foundation for Science and Technology under the following project: UIDB/04045/2020 (https://doi.org/10.54499/UIDB/04045/2020, accessed on 19 August 2024).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the e UTAD Ethics Committee (CE-UTAD) (Ref. Doc 34-CE-UTAD-2022, approval date: 12 May 2022).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Oei, S.L.; Thronicke, A.; Matthes, H.; Schad, F. Assessment of integrative non-pharmacological interventions and quality of life in breast cancer patients using real-world data. Breast Cancer 2021, 28, 608–617. [Google Scholar] [CrossRef]
- Bruce, J.; Mazuquin, B.; Mistry, P.; Rees, S.; Canaway, A.; Hossain, A.; Williamson, E.; Padfield, E.J.; Lall, R.; Richmond, H.; et al. Exercise to prevent shoulder problems after breast cancer surgery: The PROSPER RCT. Health Technol. Assess. 2022, 26, 1–124. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society Guideline for Diet and Physical Activity for Cancer Prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Cormie, P.; Zopf, E.M.; Zhang, X.; Schmitz, K.H. The Impact of Exercise on Cancer Mortality, Recurrence, and Treatment-Related Adverse Effects. Epidemiol. Rev. 2017, 39, 71–92. [Google Scholar] [CrossRef]
- Gilchrist, S.C.; Howard, V.J.; Akinyemiju, T.; Judd, S.E.; Cushman, M.; Hooker, S.P.; Diaz, K.M. Association of Sedentary Behavior With Cancer Mortality in Middle-aged and Older US Adults. JAMA Oncol. 2020, 6, 1210–1217. [Google Scholar] [CrossRef]
- Baumann, F.T.; Bieck, O.; Oberste, M.; Kuhn, R.; Schmitt, J.; Wentrock, S.; Zopf, E.; Bloch, W.; Schüle, K.; Reuss-Borst, M. Sustainable impact of an individualized exercise program on physical activity level and fatigue syndrome on breast cancer patients in two German rehabilitation centers. Support. Care Cancer 2016, 25, 1047–1054. [Google Scholar] [CrossRef]
- Leclerc, A.F.; Foidart-Dessalle, M.; Tomasella, M.; Coucke, P.; Devos, M.; Bruyère, O.; Bury, T.; Deflandre, D.; Jerusalem, G.; Lifrange, E.; et al. Multidisciplinary Rehabilitation Program after Breast Cancer: Benefits on Physical Function, Anthropometry and Quality of Life. Eur. J. Phys. Rehabil. Med. 2017, 53, 633–642. [Google Scholar] [CrossRef]
- Paulo, T.R.; Rossi, F.E.; Viezel, J.; Tosello, G.T.; Seidinger, S.C.; Simões, R.R.; De Freitas, R.; Freitas, I.F. The impact of an exercise program on quality of life in older breast cancer survivors undergoing aromatase inhibitor therapy: A randomized controlled trial. Health Qual. Life Outcomes 2019, 17, 17. [Google Scholar] [CrossRef]
- Wirtz, P.; Baumann, F.T. Physical Activity, Exercise and Breast Cancer—What Is the Evidence for Rehabilitation, Aftercare, and Survival? A Review. BRC 2018, 13, 92–100. [Google Scholar] [CrossRef]
- Dolan, L.B.; Barry, D.; Petrella, T.; Davey, L.; Minnes, A.; Yantzi, A.; Marzolini, S.; Oh, P. The Cardiac Rehabilitation Model Improves Fitness, Quality of Life, and Depression in Breast Cancer Survivors. J. Cardiopulm. Rehabil. Prev. 2018, 38, 246–252. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Sweeney, F.C.; Stewart, C.; Buchanan, T.A.; Spicer, D.; Tripathy, D.; et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: A randomized controlled trial. Breast Cancer Res. 2018, 20, 124. [Google Scholar] [CrossRef]
- Blount, D.S.; McDonough, D.J.; Gao, Z. Effect of Wearable Technology-Based Physical Activity Interventions on Breast Cancer Survivors’ Physiological, Cognitive, and Emotional Outcomes: A Systematic Review. J. Clin. Med. 2021, 10, 2015. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Pekarek, L.; Guijarro, L.G.; Castellanos, A.J.; Sanchez-Trujillo, L.; García-Honduvilla, N.; Álvarez-Mon, M.; Buján, J.; et al. Physical Activity as an Imperative Support in Breast Cancer Management. Cancers 2021, 13, 55. [Google Scholar] [CrossRef]
- Liska, T.M.; Kolen, A.M. The role of physical activity in cancer survivors’ quality of life. Health Qual. Life Outcomes 2020, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- del-Rosal-Jurado, A.; Romero-Galisteo, R.; Trinidad-Fernández, M.; González-Sánchez, M.; Cuesta-Vargas, A.; Ruiz-Muñoz, M. Therapeutic Physical Exercise Post-Treatment in Breast Cancer: A Systematic Review of Clinical Practice Guidelines. J. Clin. Med. 2020, 9, 1239. [Google Scholar] [CrossRef] [PubMed]
- García-Chico, C.; López-Ortiz, S.; Peñín-Grandes, S.; Pinto-Fraga, J.; Valenzuela, P.L.; Emanuele, E.; Ceci, C.; Graziani, G.; Fiuza-Luces, C.; Lista, S.; et al. Physical Exercise and the Hallmarks of Breast Cancer: A Narrative Review. Cancers 2023, 15, 324. [Google Scholar] [CrossRef]
- Zagalaz-Anula, N.; Mora-Rubio, M.J.; Obrero-Gaitán, E.; Del-Pino-Casado, R. Recreational physical activity reduces breast cancer recurrence in female survivors of breast cancer: A meta-analysis. Eur. J. Oncol. Nurs. 2022, 59, 102162. [Google Scholar] [CrossRef]
- Israel, B.A.; Schulz, A.J.; Parker, E.A.; Becker, A.B. Review of Community-Based Research: Assessing Partnership Approaches to Improve Public Health. Annu. Rev. Public Health 1998, 19, 173–202. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 8th ed.; Lippincott, Williams & Wilkins: Baltimore, MD, USA, 2010; pp. 228–232. ISBN 9780781769020. [Google Scholar]
- Blanchard, C.M.; Courneya, K.S.; Stein, K. American cancer society’s SCS II. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: Results from the American Cancer Society’s SCS-II. J. Clin. Oncol. 2008, 26, 2198–2204. [Google Scholar] [CrossRef]
- Ballard-Barbash, R.; George, S.; Alfano, C.; Schmitz, K. Physical activity across the cancer continuum. Oncology 2013, 27, 589–592. [Google Scholar]
- Courneya, K.S.; Mackey, J.R.; McKenzie, D.C. Exercise for breast cancer survivors: Research evidence and clinical guidelines. Physician Sportsmed. 2002, 30, 33–42. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Vaz Serra, A.S.; Abreu, J.L. Aferição dos quadros clínicos depressivos: Ensaio de aplicação do inventário depressivo de Beck a uma amostra portuguesa de doentes deprimidos. Sep. Coimbra Méd. 1973, 20, 623–644. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Garbin, M.G. Psychometric properties of the beck depression inventory: Twenty-five years of evaluation. Clin. Psychol. Rev. 1988, 8, 77–100. [Google Scholar] [CrossRef]
- Santos, P.J. Validação da Rosenberg Self-Esteem Scale Numa Amostra de Estudantes do Ensino Superior; III Conferência Internacional Avaliação Psicológica; Formas e Contextos: Braga, Portugal, 2008. [Google Scholar]
- Rosenberg, M. Conceiving the Self; Basic Books: New York, NY, USA, 1979. [Google Scholar]
- Fayers, P.M.; Aaraonson, N.; Bjordal, K.; Curran, D.; Groevold, M. EORTC QLQ-C30. In Scoring Manual, 2nd ed.; European Organization for Research and Treatment of Cancer Quality of Life Study Group: Brussels, Portugal, 1999. [Google Scholar]
- Pestana, M.; Gageiro, L. Análise de Dados para Ciências Sociais. In A Complementaridade Do SPSS, 5th ed.; Edições Silabo: Lisboa, Portugal, 2005. [Google Scholar]
- Courneya, K.S.; Segal, R.J.; Mackey, J.R.; Gelmon, K.; Reid, R.D.; Friedenreich, C.M.; McKenzie, D.C. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J. Clin. Oncol. 2007, 25, 4396–4404. [Google Scholar] [CrossRef] [PubMed]
- Valenti, M.; Porzio, G.; Aielli, F.; Verna, L.; Cannita, K.; Manno, R.; Masedu, F.; Marchetti, P.; Ficorella, C. Physical exercise and quality of life in breast cancer survivors. Int. J. Sci. 2008, 5, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.M.; Frierson, G.M.; Rabin, C.; Trunzo, J.J.; Marcus, B.H. Home-based physical activity intervention for breast cancer patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 3577–3587. [Google Scholar] [CrossRef]
- Sprod, L.K.; Hsieh, C.C.; Hayward, R.; Schneider, C.M. Three versus Six Months of Exercise Training in Breast Cancer Survivors. Breast Cancer Res. Treat. 2010, 121, 413–419. [Google Scholar] [CrossRef]
- Steindorf, K.; Schmidt, M.E.; Klassen, O.; Ulrich, C.M.; Oelmann, J.; Habermann, N.; Potthoff, K. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: Results on cancer-related fatigue and quality of life. Ann. Oncol. 2014, 25, 2237–2243. [Google Scholar] [CrossRef]
- Burgess, C.; Cornelius, V.; Love, S.; Graham j Richards, M.; Ramirez, A. Depression and anxiety in women with early breast cancer: Five-year observational cohort study. BMJ 2005, 330, 702. [Google Scholar] [CrossRef]
- Krebber, A.M.; Buffart, L.M.; Kleijn, G.; Riepma, I.C.; De Bree, R.; Leemans, C.R.; Becker, A.; Brug, J.; Van Straten, A.; Cuijpers, P.; et al. Prevalence of depression in cancer patients: A meta- analysis of diagnostic interviews and self-report instruments. Psycho-Oncol. 2014, 23, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.I.; Scherer, R.W.; Geigle, P.M.; Berlanstein, T.O.; Gotay, C.C.; Snyder, C. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst. Rev. 2012, 15, CD007566. [Google Scholar]
- Travier, N.; Velthuis, M.J.; Steins Bisschop, C.N.; van den Buijs, B.; Monninkhof, E.M.; Backx, F.; Los, M.; Erdkamp, F.; Bloemendal, H.J.; Rodenhuis, C.; et al. Effects of an 18-week exercise programme started early during breast cancer treatment: A randomised controlled trial. BMC Med. 2015, 13, 121. [Google Scholar] [CrossRef]
- Ramos, A.S.; Patrão, I. Imagem corporal da mulher com cancro de mama: Impacto na qualidade do relacionamento conjugal e na satisfação sexual. Anál. Psicol. 2005, 3, 295–304. [Google Scholar] [CrossRef]
- Shobeiri, F.; Masoumi, S.Z.; Nikravesh, A.; Moghadam, R.H.; Karami, M. The impact of aerobic exercise on quality of life in women with breast cancer: A randomized controlled trial. J. Res. Health Sci. 2016, 16, 127–132. [Google Scholar]
- Remondes-Costa, S.; Jimenéz, F.; Pais-Ribeiro, J. Imagem corporal, sexualidade e qualidade de vida no cancro da mama. Psicol. Saúde Doenças 2012, 13, 327–339. [Google Scholar]
- Pinto, B.M.; Clark, M.M.; Maruyama, N.C.; Feder, S.I. Psychological and fitness changes associated with exercise participation among women with breast cancer. Psycho-Oncol. 2003, 12, 118–126. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvão, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine Roundtable on Exercise Guidelines for Cancer Survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef]
- Ligibel, J. Lifestyle factors in cancer survivorship. JCO 2012, 30, 3697–3704. [Google Scholar] [CrossRef]
- Hayes, S.; Spence, R.R.; Galvão, D.; Newton, R. Australian association for exercise and sport science position stand: Optimizing cancer outcomes through exercise. J. Sci. Med. Sport 2009, 12, 428–434. [Google Scholar] [CrossRef]
- Vallance, J.; Courneya, K.; Plotnikoff, R.; Mackey, J. Analyzing theoretical mechanisms of physical activity behavior change in breast cancer surviviors: Results from the activity promotion (action) trail. Ann. Behav. Med. 2008, 35, 150–158. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).