Targeting the Hippo- Yes-Associated Protein/Transcriptional Coactivator with PDZ-Binding Motif Signaling Pathway in Primary Liver Cancer Therapy
Abstract
Simple Summary
Abstract
1. Introduction
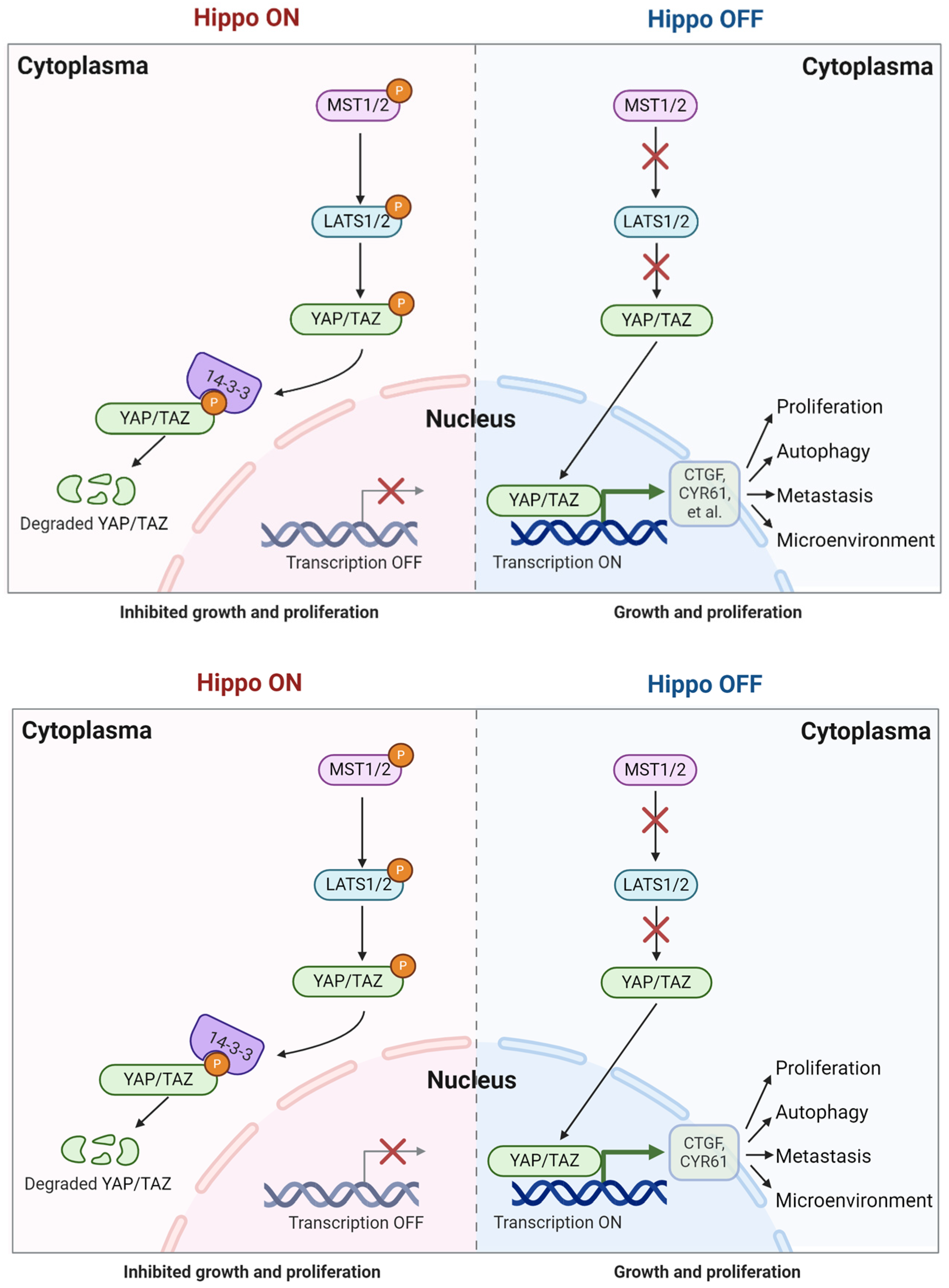
2. Hippo/YAP Signaling Pathway
3. Role of the Hippo-YAP/TAZ Signaling Pathway in Primary Liver Cancer
3.1. Cell Proliferation
3.2. Autophagy
3.3. Tumor Invasion and Metastasis
3.4. The Tumor Microenvironment
4. The Hippo/YAP Pathway in Liver Cancer Drug Resistance
5. Targeting the Hippo/YAP Signaling Pathway in Primary Liver Cancer
5.1. Targeting Upstream Kinases MST/LATS
5.2. Direct Regulation of YAP/TAZ
6. Drugs Targeting Hippo/YAP Signaling in Primary Cancer Therapy
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.J.; von Felden, J.; Garcia-Lezana, T.; Sarcognato, S.; Villanueva, A. Tumour evolution in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Piao, Y.; Guo, F.; Wei, J.; Chen, Y.; Dai, X.; Zhang, X. Current progress of pig models for liver cancer research. Biomed. Pharmacother. 2023, 165, 115256. [Google Scholar] [CrossRef]
- Patel, S.H.; Camargo, F.D.; Yimlamai, D. Hippo signaling in the liver regulates organ size, cell fate, and carcinogenesis. Gastroenterology 2017, 152, 533–545. [Google Scholar] [CrossRef]
- Kriz, V.; Korinek, V. Wnt, RSPO and Hippo Signaling in the Intestine and Intestinal Stem Cells. Genes 2018, 9, 20. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Yang, Y. Hepatic Hippo signaling inhibits development of hepatocellular carcinoma. Clin. Mol. Hepatol. 2020, 26, 742–750. [Google Scholar] [CrossRef]
- Cunningham, R.; Hansen, C.G. The Hippo pathway in cancer: YAP/TAZ and TEAD as therapeutic targets in cancer. Clin. Sci. 2022, 136, 197–222. [Google Scholar] [CrossRef]
- Ortega, á.; Vera, I.; Diaz, M.P.; Navarro, C.; Rojas, M.; Torres, W.; Parra, H.; Salazar, J.; Sanctis, J.D.; Bermúdez, V. The YAP/TAZ signaling pathway in the tumor microenvironment and carcinogenesis: Current knowledge and therapeutic promises. Int. J. Mol. Sci. 2021, 23, 430. [Google Scholar] [CrossRef]
- Huang, J.; Wu, S.; Barrera, J.; Matthews, K.; Pan, D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 2005, 122, 421–434. [Google Scholar] [CrossRef]
- Chan, S.W.; Lim, C.J.; Chen, L.; Chong, Y.F.; Huang, C.; Song, H.; Hong, W. The Hippo pathway in biological control and cancer development. J. Cell. Physiol. 2011, 226, 928–939. [Google Scholar] [CrossRef]
- Mranda, G.M.; Xiang, Z.P.; Liu, J.J.; Wei, T.; Ding, Y. Advances in prognostic and therapeutic targets for hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The Hippo signaling pathway. Front. Oncol. 2022, 12, 937957. [Google Scholar] [CrossRef] [PubMed]
- Justice, R.W.; Zilian, O.; Woods, D.F.; Noll, M.; Bryant, P.J. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995, 9, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Y.; Jiang, J.; An, Y.; Ye, P.F.; Zhang, C.C.; Sun, N.N.; Miao, S.N.; Chai, M.Q.; Liu, W.M.; Yang, M.; et al. Angiotensin II type-2 receptor signaling facilitates liver injury repair and regeneration via inactivation of Hippo pathway. Acta Pharmacol. Sin. 2024, 45, 1201–1213. [Google Scholar] [CrossRef]
- Lee, N.Y.; Choi, M.G.; Lee, E.J.; Koo, J.H. Interplay between YAP/TAZ and metabolic dysfunction-associated steatotic liver disease progression. Arch. Pharm. Res. 2024, 47, 558–570. [Google Scholar] [CrossRef]
- Kumar, R.; Hong, W. Hippo Signaling at the Hallmarks of Cancer and Drug Resistance. Cells 2024, 13, 564. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, Y.; Jiang, Y.; Wang, K.; Wang, X.; Zhou, D.; Wang, Y.; Yu, R.; Zhou, X. YAP promotes autophagy and progression of gliomas via upregulating HMGB1. J. Exp. Clin. Cancer Res. 2021, 40, 99. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Mai, J.; Peng, H.; Wan, J.; Sun, T. YAP in pancreatic cancer: Oncogenic role and therapeutic strategy. Theranostics 2021, 11, 1753–1762. [Google Scholar] [CrossRef]
- Hayashi, H.; Higashi, T.; Yokoyama, N.; Kaida, T.; Sakamoto, K.; Fukushima, Y.; Ishimoto, T.; Kuroki, H.; Nitta, H.; Hashimoto, D.; et al. An imbalance in TAZ and YAP expression in hepatocellular carcinoma confers cancer stem cell-like behaviors contributing to disease progression. Cancer Res. 2015, 75, 4985–4997. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Jiang, X.W.; Li, W.F.; Guo, G.; Gong, J.P.; Ding, X. Clinicopathological and prognostic significance of Yes- associated protein expression in hepatocellular carcinoma and hepatic cholangiocarcinoma. Tumour Biol. 2016, 37, 13499–13508. [Google Scholar] [CrossRef]
- Nishio, M.; Hamada, K.; Kawahara, K.; Sasaki, M.; Noguchi, F.; Chiba, S.; Mizuno, K.; Suzuki, S.O.; Dong, Y.; Tokuda, M.; et al. Cancer susceptibility and embryonic lethality in Mobla/lb double-mutant mice. J. Clin. Investig. 2012, 122, 4505–4518. [Google Scholar] [CrossRef]
- Leask, A.; Nguyen, J.; Naik, A.; Chitturi, P.; Riser, B.L. The role of yes activated protein (YAP) in melanoma metastasis. iScience 2024, 27, 109864. [Google Scholar] [CrossRef]
- Felley-Bosco, E.; Stahel, R. Hippo/YAP pathway for targeted therapy. Transl. Lung Cancer Res. 2014, 3, 75–83. [Google Scholar] [PubMed]
- Qi, S.; Zhu, Y.; Liu, X.; Li, P.; Wang, Y.; Zeng, Y.; Yu, A.; Wang, Y.; Sha, Z.; Zhong, Z.; et al. WWC proteins mediate LATS1/2 activation by Hippo kinases and imply a tumor suppression strategy. Mol. Cell 2022, 82, 1850–1864. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.H.; Nousiainen, M.; Chalamalasetty, R.B.; Schäfer, A.; Nigg, E.A.; Silljé, H.H. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 2005, 24, 2076–2086. [Google Scholar] [CrossRef]
- Dhanaraman, T.; Singh, S.; Killoran, R.C.; Singh, A.; Xu, X.; Shifman, J.M.; Smith, M.J. RASSF effectors couple diverse RAS subfamily GTPases to the Hippo pathway. Sci. Signal. 2020, 13, eabb4778. [Google Scholar] [CrossRef]
- Delgado, I.L.S.; Carmona, B.; Nolasco, S.; Santos, D.; Leitão, A.; Soares, H. MOB: Pivotal conserved proteins in cytokinesis, cell architecture and tissue homeostasis. Biology 2020, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Moroishi, T.; Mottier-Pavie, V.; Plouffe, S.W.; Hansen, C.G.; Hong, A.W.; Park, H.W.; Mo, J.S.; Lu, W.; Lu, S.; et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat. Commun. 2015, 6, 8357. [Google Scholar] [CrossRef]
- Li, F.L.; Fu, V.; Liu, G.; Tang, T.; Konradi, A.W.; Peng, X.; Kemper, E.; Cravatt, B.F.; Franklin, J.M.; Wu, Z.; et al. Hippo pathway regulation by phosphatidylinositol transfer protein and phosphoinositides. Nat. Chem. Biol. 2022, 18, 1076–1086. [Google Scholar] [CrossRef]
- Hao, X.; Zhang, Y.; Shi, X.; Liu, H.; Zheng, Z.; Han, G.; Rong, D.; Zhang, C.; Tang, W.; Wang, X. CircPAK1 promotes the progression of hepatocellular carcinoma via modulation of YAP nucleus localization by interacting with 14-3-3ζ. J. Exp. Clin. Cancer Res. 2022, 41, 281. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, L.; Tumaneng, K.; Wang, C.Y.; Guan, K.L. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF (β-TRCP). Genes Dev. 2010, 24, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Zha, Z.Y.; Zhou, X.; Zhang, H.; Huang, W.; Zhao, D.; Li, T.; Chan, S.W.; Lim, C.J.; Hong, W.; et al. The Hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF β-TrCP E3 ligase. J. Biol. Chem. 2010, 285, 37159–37169. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, N.; Figel, S.A.; Wilson, K.E.; Morrison, C.D.; Gelman, I.H.; Zhang, J. PTPN14 interacts with and negatively regulates the oncogenic function of YAP. Oncogene 2013, 32, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Remue, E.; Meerschaert, K.; Vanloo, B.; Boucherie, C.; Gfeller, D.; Bader, G.D.; Sidhu, S.S.; Vandekerckhove, J.; Gettemans, J.; et al. Functional complexes between YAP2 and ZO-2 are PDZ domain-dependent, and regulate YAP2 nuclear localization and signalling. Biochem. J. 2010, 432, 461–472. [Google Scholar] [CrossRef]
- Feng, X.; Degese, M.S.; Iglesias-Bartolome, R.; Vaque, J.P.; Molinolo, A.A.; Rodrigues, M.; Zaidi, M.R.; Ksander, B.R.; Merlino, G.; Sodhi, A.; et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell 2014, 25, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, H.; Wang, Z.; Jia, M.; Guo, J.; Jin, J.; Li, X.; Meng, D.; Lin, L.; He, A.R.; et al. Loss of SPTBN1 suppresses autophagy via SETD7-mediated YAP methylation in hepatocellular carcinoma initiation and development. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 949–973.e7. [Google Scholar] [CrossRef]
- Zhai, L.; Yang, X.; Dong, J.; Qian, L.; Gao, Y.; Lv, Y.; Chen, L.; Chen, B.; Zhou, F. O-GlcNAcylation mediates endometrial cancer progression by regulating the Hippo-YAP pathway. Int. J. Oncol. 2023, 63, 90. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kang, J.G.; Kang, M.J.; Park, J.H.; Kim, Y.J.; Kweon, T.H.; Lee, H.W.; Jho, E.H.; Lee, Y.H.; Kim, S.I.; et al. O-GlcNAcylation on LATS2 disrupts the Hippo pathway by inhibiting its activity. Proc. Natl. Acad. Sci. USA 2020, 117, 14259–14269. [Google Scholar] [CrossRef]
- Yang, S.; Xu, W.; Liu, C.; Jin, J.; Li, X.; Jiang, Y.; Zhang, L.; Meng, X.; Zhan, J.; Zhang, H. LATS1 K751 acetylation blocksactivation of Hippo signalling and switches LATS1 from a tumor suppressor to an oncoprotein. Sci. China Life Sci. 2022, 65, 129–141. [Google Scholar] [CrossRef]
- Zhao, B.; Tumaneng, K.; Guan, K.L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 2011, 13, 877–883. [Google Scholar] [CrossRef]
- de Haan, L.R.; van Golen, R.F.; Heger, M. Molecular Pathways Governing the Termination of Liver Regeneration. Pharmacol. Rev. 2024, 76, 500–558. [Google Scholar] [CrossRef]
- Yu, F.X.; Meng, Z.; Plouffe, S.W.; Guan, K.L. Hippo pathway regulation of gastrointestinal tissues. Ann. Rev. Physiol. 2015, 77, 201–227. [Google Scholar] [CrossRef]
- Kim, W.; Khan, S.K.; Liu, Y.; Xu, R.; Park, O.; He, Y.; Cha, B.; Yang, Y. Hepatic Hippo signaling inhibits protumoural microenvironment to suppress hepatocellular carcinoma. Gut 2018, 67, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, S.; Zhang, Y.; Jia, J.; Wang, J.; Liu, X.; Zhang, J.; Song, X.; Ribback, S.; Cigliano, A.; et al. TAZ is indispensable for c-MYC-induced hepatocarcinogenesis. J. Hepatol. 2022, 76, 123–134. [Google Scholar] [CrossRef]
- Grijalva, J.L.; Huizenga, M.; Mueller, K.; Rodriguez, S.; Brazzo, J.; Camargo, F.; Sadri-Vakili, G.; Vakili, K. Dynamic alterations in Hippo signaling pathway and YAP activation during liver regeneration. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G196–G204. [Google Scholar] [CrossRef] [PubMed]
- Ba, M.C.; Long, H.; Cui, S.Z.; Tang, Y.Q.; Wu, Y.B.; Zhang, X.L.; Tang, H.S.; Bai, S.X. Multivariate comparison of B-ultrasound guided and laparoscopic continuous circulatory hyperthermic intraperitoneal perfusion chemotherapyfor malignant ascites. Surg. Endosc. 2013, 27, 2735–2743. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Noon, L.A.; Akat, K.M.; Ybanez, M.D.; Lee, T.F.; Berres, M.L.; Fujiwara, N.; Goossens, N.; Chou, H.I.; Parvin-Nejad, F.P.; et al. Autophagy is a gatekeeper of hepatic differentiation and carcinogenesis by controlling the degradation of Yap. Nat. Commun. 2018, 9, 4962. [Google Scholar] [CrossRef]
- Park, H.; Lee, Y.; Lee, K.; Lee, H.; Yoo, J.E.; Ahn, S.; Park, Y.N.; Kim, H. The clinicopathological significance of YAP/TAZ expression in hepatocellular carcinoma with relation to hypoxia and stemness. Pathol. Oncol. Res. 2021, 27, 604600. [Google Scholar] [CrossRef]
- Xiao, H.; Jiang, N.; Zhou, B.; Liu, Q.; Du, C. TAZ regulates cell proliferation and epithelial-mesenchymal transition of human hepatocellular carcinoma. Cancer Sci. 2015, 106, 151–159. [Google Scholar] [CrossRef]
- Yan, Y.C.; Meng, G.X.; Yang, C.C.; Yang, Y.F.; Tan, S.Y.; Yan, L.J.; Ding, Z.N.; Ma, Y.L.; Dong, Z.R.; Li, T. Diacylglycerol lipase alpha promotes hepatocellular carcinoma progression and induces lenvatinib resistance by enhancing YAP activity. Cell Death Dis. 2023, 14, 404. [Google Scholar] [CrossRef]
- Guan, L.; Li, T.; Ai, N.; Wang, W.; He, B.; Bai, Y.; Yu, Z.; Li, M.; Dong, S.; Zhu, Q.; et al. MEIS2C and MEIS2D promote tumor progression via Wnt/beta-catenin and hippo/YAP signaling in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 417. [Google Scholar] [CrossRef]
- Lu, C.; Rong, D.; Zhang, B.; Zheng, W.; Wang, X.; Chen, Z.; Tang, W. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: Challenges and opportunities. Mol. Cancer 2019, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Li, Y.; Ren, Y.; Cao, M.; Yang, G.; Luo, J.; Hu, Z.; Deng, H.; Deng, M.; Liu, B.; et al. A new strategy for overcoming drug resistance in liver cancer: Epigenetic regulation. Biomed. Pharmacother. 2024, 176, 116902. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.S.; Wang, L.Y.; Wang, Y.W.; Tsai, M.M.; Lin, T.K.; Liao, C.J.; Yeh, C.T.; Lin, K.H. Evaluation and Application of Drug Resistance by Biomarkers in the Clinical Treatment of Liver Cancer. Cells 2023, 12, 869. [Google Scholar] [CrossRef] [PubMed]
- Nussinov, R.; Tsai, C.J.; Jang, H. Anticancer drug resistance: An update and perspective. Drug Resist. Updat. 2021, 59, 100796. [Google Scholar] [CrossRef]
- Thompson, B.J. YAP/TAZ: Drivers of Tumor Growth, Metastasis, and Resistance to Therapy. Bioessays 2020, 5, e1900162. [Google Scholar] [CrossRef]
- Mohajan, S.; Jaiswal, P.K.; Vatanmakarian, M.; Yousefi, H.; Sankaralingam, S.; Alahari, S.K.; Koul, S.; Koul, H.K. Hippo pathway: Regulation, deregulation and potential therapeutic targets in cancer. Cancer Lett. 2021, 507, 112–123. [Google Scholar] [CrossRef]
- Huo, X.; Zhang, Q.; Liu, A.M.; Tang, C.; Gong, Y.; Bian, J.; Luk, J.M.; Xu, Z.; Chen, J. Overexpression of Yes-associated protein confers doxorubicin resistance in hepatocellular carcinoma. Oncol. Rep. 2013, 29, 840–846. [Google Scholar] [CrossRef]
- Tao, Y.; Shan, L.; Xu, X.; Jiang, H.; Chen, R.; Qian, Z.; Yang, Z.; Liang, B.; Zheng, H.; Cai, F.; et al. Huaier augmented the chemotherapeutic sensitivity of oxaliplatin via downregulation of YAP in hepatocellular carcinoma. J. Cancer 2018, 93, 962–3970. [Google Scholar] [CrossRef]
- Dai, X.Y.; Zhuang, L.H.; Wang, D.D.; Zhou, T.Y.; Chang, L.L.; Gai, R.H.; Zhu, D.F.; Yang, B.; Zhu, H.; He, Q.J. Nuclear translocation and activation of YAP by hypoxia contributes to the chemoresistance of SN38 in hepatocellular carcinoma cells. Oncotarget 2016, 7, 6933–6947. [Google Scholar] [CrossRef]
- Sun, T.; Mao, W.; Peng, H.; Wang, Q.; Jiao, L. YAP promotes sorafenib resistance in hepatocellular carcinoma by upregulating surviving. Cell. Oncol. 2021, 44, 689–699. [Google Scholar] [CrossRef]
- Hyun, J.; Al, A.M.; Dutta, R.K.; Oh, S.H.; Xiang, K.; Zhou, X.; Maeso-Díaz, R.; Caffrey, R.; Sanyal, A.J.; Freedman, J.A.; et al. Dysregulation of the ESRP2-NF2-YAP/TAZ axis promotes hepatobiliary carcinogenesis in non-alcoholic fatty liver disease. J. Hepatol. 2021, 75, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Xu, Q.; Xu, N. Long non-coding RNA LOC107985656 represses the proliferation of hepatocellular carcinoma cells through activation of the tumor-suppressive Hippo pathway. Bioengineered 2021, 12, 7964–7974. [Google Scholar] [CrossRef] [PubMed]
- Höffken, V.; Hermann, A.; Pavenstädt, H.; Kremerskothen, J. WWC Proteins: Important Regulators of Hippo Signaling in Cancer. Cancers 2021, 13, 306. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhou, Y.; Qian, M.; Xu, M.; Wang, J.; Zhang, Y.; Song, X.; Wang, H.; Lin, S.; Ren, C.; et al. TBX3 functions as a tumor suppressor downstream of activated CTNNB1 mutants during hepatocarcinogenesis. J. Hepatol. 2021, 75, 120–131. [Google Scholar] [CrossRef]
- Yang, X.M.; Cao, X.Y.; He, P.; Li, J.; Feng, M.X.; Zhang, Y.L.; Zhang, X.L.; Wang, Y.H.; Yang, Q.; Zhu, L.; et al. Overexpression of Rac GTPase Activating Protein 1 Contributes to Proliferation of Cancer Cells by Reducing Hippo Signaling to Promote Cytokinesis. Gastroenterology 2018, 155, 1233–1249.e22. [Google Scholar] [CrossRef]
- Fan, Y.; Du, Z.; Ding, Q.; Zhang, J.; Op, d.w.m.; Gerbes, A.L.; Liu, M.; Steib, C.J. SEPT6 drives hepatocellular carcinoma cell proliferation, migration and invasion via the Hippo/YAP signaling pathway. Int. J. Oncol. 2021, 58, 25. [Google Scholar] [CrossRef]
- Chen, R.; Zhu, S.; Fan, X.G.; Wang, H.; Lotze, M.T.; Zeh, H.J.; Billiar, T.R.; Kang, R.; Tang, D. High mobility group protein B1 controls liver cancer initiation through yes-associated protein-dependent aerobic glycolysis. Hepatology 2018, 67, 1823–1841. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Jiang, W.; Yang, W.; Yang, C.; Yang, X.; Chen, K.; Hu, Y.; Shen, G.; Lu, L.; Cheng, F.; et al. Epigenetically modulated miR-1224 suppresses the proliferation of HCC through CREB-mediated activation of YAP signaling pathway. Mol. Ther. Nucleic Acids 2021, 23, 944–958. [Google Scholar] [CrossRef]
- Jin, Y.; Yang, R.; Ding, J.; Zhu, F.; Zhu, C.; Xu, Q.; Cai, J. KAT6A is associated with sorafenib resistance and contributes to progression of hepatocellular carcinoma by targeting YAP. Biochem. Biophys. Res. Commun. 2021, 585, 185–190. [Google Scholar] [CrossRef]
- Chen, M.; Wu, L.; Tu, J.; Zhao, Z.; Fan, X.; Mao, J.; Weng, Q.; Wu, X.; Huang, L.; Xu, M.; et al. miR-590-5p suppresses hepatocellular carcinoma chemoresistance by targeting YAP1 expression. EBioMedicine 2018, 35, 142–154. [Google Scholar] [CrossRef]
- Driskill, J.H.; Pan, D. The Hippo pathway in liver homeostasis and pathophysiology. Annu. Rev. Pathol. Mech. 2021, 16, 299–322. [Google Scholar] [CrossRef]
- Higashi, T.; Hayashi, H.; Ishimoto, T.; Takeyama, H.; Kaida, T.; Arima, K.; Taki, K.; Sakamoto, K.; Kuroki, H.; Okabe, H. miR-9-3p plays a tumour-suppressor role by targeting TAZ (WWTR1) in hepatocellular carcinoma cells. Br. J. Cancer 2015, 113, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fang, L.; Yu, W.; Wang, Y. MicroRNA-125b suppresses the migration and invasion of hepatocellular carcinoma cells by targeting transcriptional coactivator with PDZ-binding motif. Oncol. Lett. 2015, 9, 1971–1975. [Google Scholar] [CrossRef]
- Yang, N.; Chen, T.; Wang, L.; Liu, R.; Niu, Y.; Sun, L.; Yao, B.; Wang, Y.; Yang, W.; Liu, Q.; et al. CXCR4 mediates matrix stiffness-induced downregulation of UBTD1 driving hepatocellular carcinoma progression via YAP signaling pathway. Theranostics 2020, 10, 5790–5801. [Google Scholar] [CrossRef]
- Zhu, H.; Yan, F.; Yuan, T.; Qian, M.; Zhou, T.; Dai, X.; Cao, J.; Ying, M.; Dong, X.; He, Q.; et al. USP10 promotes proliferation of hepatocellular carcinoma by deubiquitinating and stabilizing YAP/TAZ. Cancer Res. 2020, 80, 2204–2216. [Google Scholar] [CrossRef]
- Qian, M.; Yan, F.; Wang, W.; Du, J.; Yuan, T.; Wu, R.; Zhao, C.; Wang, J.; Lu, J.; Zhang, B.; et al. Deubiquitinase JOSD2 stabilizes YAP/TAZ to promote cholangiocarcinoma progression. Acta Pharm. Sin. B 2021, 11, 4008–4019. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, D.D.; Yuan, T.; Yan, F.J.; Zeng, C.M.; Dai, X.Y.; Chen, Z.B.; Chen, Y.; Zhou, T.; Fan, G.H.; et al. Multikinase inhibitor CT-707 targets liver cancer by interrupting the hypoxia-activated IGF-1R-YAP axis. Cancer Res. 2018, 78, 3995–4006. [Google Scholar] [CrossRef]
- Wu, J.; Chai, H.; Li, F.; Ren, Q.; Gu, Y. SETD1A augments sorafenib primary resistance via activating YAP in hepatocellular carcinoma. Life Sci. 2020, 260, 118406. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhong, F.J.; Xu, C.; Li, Y.M.; Zhao, Y.R.; Cao, M.M.; Yang, L.Y. Programmed cell death 10 promotes metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma via PP2Ac-mediated YAP activation. Cell Death Dis. 2021, 12, 849. [Google Scholar] [CrossRef]
- Liu, S.; Xu, X.; Fang, Z.; Ning, Y.; Deng, B.; Pan, X.; He, Y.; Yang, Z.; Huang, K.; Li, J. Piezo1 impairs hepatocellular tumor growth via deregulation of the MAPK-mediated YAP signaling pathway. Cell Calcium 2021, 95, 102367. [Google Scholar] [CrossRef]
- Yu, H.; He, J.; Su, G.; Wangl, Y.; Fang, F.; Yang, W.; Gu, K.; Fu, N.; Wang, Y.; Shen, Y.; et al. Fluid shear stress activates YAP to promote epithelial-mesenchymal transition in hepatocellular carcinoma. Mol. Oncol. 2021, 15, 3164–3183. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhao, Q.; An, L.; Jiao, S.; Li, R.; Sang, Y.; Liao, J.; Nie, P.; Wen, F.; Ju, J.; et al. A TNFR2-hnRNPK axis promotes primary liver cancer development via activation of YAP signaling in hepatic progenitor cells. Cancer Res. 2021, 81, 3036–3050. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Jiang, Y.; Miao, X.; Wu, Z.; Liu, H.; Gong, W. Tadalafil enhances the therapeutic efficacy of BET inhibitors in hepatocellular carcinoma through activating Hippo pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166267. [Google Scholar] [CrossRef]
- Chen, T.; Sun, D.; Wang, Q.; Zhou, T.; Tan, J.; Xu, C.; Cheng, H.; Shen, W. α-Hederin inhibits the proliferation of hepatocellular carcinoma cells via Hippo-Yes-associated protein signaling pathway. Front. Oncol. 2022, 12, 839603. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, K.; Jiang, R.; Li, D.Y.; Guo, X.X.; Zhou, P.; Tang, J.F.; Li, L.S.; Zeng, D.; Hu, L.; et al. Evodiamine inhibits proliferation and promotes apoptosis of hepatocellular carcinoma cells via the Lippo-Yes-Associated protein signaling pathway. Front. Oncol. 2022, 12, 839603. [Google Scholar]
- Wang, H.; Wang, R.; Huang, D.; Li, S.; Gao, B.; Kang, Z.; Tang, B.; Xie, J.; Yan, F.; Liang, R.; et al. Homoharringtonine exerts anti-tumor effects in hepatocellular carcinoma through activation of the Hippo pathway. Front. Pharmacol. 2021, 12, 592071. [Google Scholar] [CrossRef]
- Zhao, D.; Xia, L.; Geng, W.; Xu, D.; Zhong, C.; Zhang, J.; Xia, Q. Metformin suppresses interleukin-22 induced hepatocellular carcinoma by upregulating Hippo signaling pathway. J. Gastroenterol. Hepatol. 2021, 36, 3469–3476. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Yuan, W.; Hao, S.; Wang, M.; Wang, F.; Xuan, H. Bioactive components and mechanisms of poplar propolis in inhibiting proliferation of human hepatocellular carcinoma HepG2 cells. Biomed. Pharmacother. 2021, 144, 112364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Niu, Y.; Wang, Z.; Xu, X.; Li, Y.; Ma, L.; Wang, J.; Yu, Y. Corosolic acid inhibits cancer progression by decreasing the level of CDK19-mediated O-GlcNAcylation in liver cancer cells. Cell Death Dis. 2021, 12, 889. [Google Scholar] [CrossRef]
- Shan, L.; Li, Y.; Jiang, H.; Tao, Y.; Qian, Z.; Li, L.; Cai, F.; Ma, L.; Yu, Y. Huaier restrains proliferative and migratory potential of hepatocellular carcinoma cells partially through decreased Yes-associated protein 1. J. Cancer 2017, 8, 4087–4097. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Hayashi, H.; Kitano, Y.; Yamamura, K.; Kaida, T.; Arima, K.; Taki, K.; Nakagawa, S.; Okabe, H.; Nitta, H.; et al. Statin attenuates cell proliferative ability via TAZ (WWTR1) in hepatocellular carcinoma. Med. Oncol. 2016, 33, 123. [Google Scholar] [CrossRef] [PubMed]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the roots of cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef] [PubMed]
- Fitamant, J.; Kottakis, F.; Benhamouche, S.; Tian, H.S.; Chuvin, N.; Parachoniak, C.A.; Nagle, J.M.; Perera, R.M.; Lapouge, M.; Deshpande, V.; et al. YAP inhibition restores hepatocyte differentiation in advanced HCC, leading to tumor regression. Cell Rep. 2015, 10, 1692–1707. [Google Scholar] [CrossRef]
- Yimlamai, D.; Christodoulou, C.; Galli, G.G.; Yanger, K.; Pepe-Mooney, B.; Gurung, B.; Shrestha, K.; Cahan, P.; Stanger, B.Z.; Camargo, F.D. Hippo pathway activity influences liver cell fate. Cell 2014, 157, 1324–1338. [Google Scholar] [CrossRef]
- Lin, K.C.; Park, H.W.; Guan, K.L. Regulation of the Hippo Pathway Transcription Factor TEAD. Trends Biochem. Sci. 2017, 42, 862–872. [Google Scholar] [CrossRef]
- Ma, D.; Luo, Q.; Song, G. Matrix stiffening facilitates stemness of liver cancer stem cells by YAP activation and BMF inhibition. Biomater. Adv. 2024, 163, 213936. [Google Scholar] [CrossRef]
- Luo, W. Nasopharyngeal carcinoma ecology theory: Cancer as multidimensional spatiotemporal “unity of ecology and evolution” pathological ecosystem. Theranostics 2023, 13, 1607–1631. [Google Scholar] [CrossRef]
| Status | Molecules | Targets | Function |
|---|---|---|---|
| Hippo on | ESRP2/NF2 | MST1/2 | Loss of ESRP2/NF2 function permits sustained YAP/TAZ activity that drives hepatocyte proliferation, advantaging the growth of cells with mutations that enable them to survive chronic oncogenic stress [62]. |
| lncRNA LOC107985656/miR-106b-5p | LATS1 | LOC107985656 regulated the expression of LATS1 by acting as a sponge for absorbing miR-106b-5p in HCC cells [63]. | |
| WWC | LATS1/2 | WWC proteins positively regulate the Hippo pathway via the activation of LATS1/2 kinases and the subsequent cytoplasmic accumulation of phosphorylated YAP [64]. | |
| TBX3/PLD1 | LATS2 | TBX3 inhibited HCC cell growth, as well as YAP/TAZ activation, by promoting the overexpression of LATS2 via suppressing transcriptional target PLD1 [65]. | |
| Hippo off | RACGAP1 | LATS1/2 | RACGAP1 promotes the proliferation of HCC cells by reducing the activation of LATS1/2 [66]. |
| SEPT6 | LATS1 | SEPT6 facilitates F-actin formation, which induces LATS1 dephosphorylation, inhibits Hippo signaling, and upregulates YAP expression and nuclear translocation [67]. |
| Regulation Level | Molecules | Function |
|---|---|---|
| Transcription | HMGB1/GABPα | The binding of HMGB1 to GABPα promotes the expression YAP at the transcriptional level [68]. |
| miR-1224/CREB | By binding with CREB, miR-1224 could repress the transcription and the activation of YAP [69]. | |
| KAT6A | KAT6A was associated with sorafenib resistance and contributes to the progression of HCC by targeting YAP expression [70]. | |
| miR-590-5p | YAP is regulated by microRNA-590-5p and is critical for HCC chemoresistance by regulating the expression of stemness markers and ATP-binding cassette transporters [70]. | |
| ARID1A (AT-rich interaction domain 1A) | ARID1A was discovered to bind to YAP, inhibiting its transcriptional output [72]. | |
| Translation | MicroRNA-9-3p | MicroRNA-9-3p acts as a tumor suppressor miR by targeting TAZ expression in HCC cells [73]. |
| MicroRNA-125b | miR-125b may be involved in the tumorigenesis of HCC at least in part by the suppression of TAZ [74]. | |
| Ubiquitination | CXCR4/UBTD1 | CXCR4 decreases the levels of UBTD1, which is involved in the proteasome-dependent degradation of YAP [75]. |
| USP10 | USP10 promotes the proliferation of hepatocellular carcinoma by deubiquitinating and stabilizing YAP/TAZ [76]. | |
| JOSD2 | Deubiquitinase JOSD2 stabilizes YAP/TAZ to promote cholangiocarcinoma progression [77]. | |
| Methylation | SPTBN1/SETD7 | SPTBN1 positively regulated the expression of the suppressor of SETD7 to promote YAP methylation, which leads to YAP degradation and inactivation [36]. |
| Phosphorylation | IGF1R | The depletion of IGF1R increased the p-YAP, which denoted the loss of YAP function [78]. |
| SETD1A | SETD1A deficiency impairs YAP phosphorylation and activation. In contrast, SETD1A enhances YAP activation to induce sorafenib primary resistance in HCC [79]. | |
| PDCD10 | PDCD10 directly binds to the catalytic subunit of protein phosphatase 2A (PP2Ac) and increases its enzymatic activity, leading to dephosphorylation of the YAP, which contributes to YAP nuclear translocation and transcriptional activation [80]. | |
| Nucleus translocation | Piezo1/MAPK | Piezo1 activates the mitogen-activated protein kinase (MAPK) pathway and then integrates with YAP signaling to control the nuclear translocation of YAP and the regulation of its target genes [81]. |
| FSS | FSS induces the translocation of YAP from the cytomembrane to the nucleus, contributes to epithelial–mesenchymal transition (EMT), and enhances metastasis in hepatocellular carcinoma [82]. | |
| Stabilization | TNFR2–hnRNPK | TNFR2–hnRNPK acted downstream of TNFα–TNFR2 signaling to directly interact with and stabilize YAP on target gene promoters genome-wide, therefore coregulating the expression of YAP target genes [83]. |
| Target | Drug | Function |
|---|---|---|
| MST1/LATS1 | Tadalafil | Tadalafil blocks YAP/TAZ protein expression by activating the Hippo pathway and enhances the therapeutic efficacy of BET inhibitors in hepatocellular carcinoma treatment [84]. |
| α-Hederin | α-Hederin treatment effectively enhanced MST1 and LATS1 gene expression while downregulated YAP gene expression in HepG2 and SMMC-7721 cells [85]. | |
| MST1/2 and LATS1 | Evodiamine | Evodiamine activates MST1/2 and upregulates LATS1 phosphorylation, leading to phosphorylation and decreased nuclear translocation of YAP [86]. |
| Homoharringtonine | Homoharringtonine treatment increased the phosphorylation levels of MST1/2 and LATS1, significantly inhibiting HCC cell growth by suppressing cell proliferation and colony formation [87]. | |
| MST1/2 and LATS1/2 | Metformin | Metformin directly inhibits LATS1/2 and activates MST1/2, phosphorylating YAP1, as a result, suppressing IL-22-mediated HCC progression [88]. |
| LATS2 | Poplar propolis | Poplar propolis obviously upregulated the levels of LATS2 and decreased the expression of YAP, TAZ, and their target protein in the nucleus [89]. |
| YAP | CT-707 | CT-707 has remarkable inhibitory activity against YAP function and exhibits prominent cytotoxicity under hypoxia on HCC cells [78]. |
| Corosolic acid | Corosolic acid can reduce YAP expression and O-GlcNAcylation by inhibiting the activity of CDK19 [90]. | |
| Trametes robiniophila Murr | Trametes robiniophila Murr treatment translocated YAP from the nucleus to the cytoplasm and further promoted the phosphorylation of YAP to be degraded by ubiquitination [91]. | |
| TAZ | Statin (fluvastatin and simvastatin) | TAZ expression was suppressed in HCC cells by fluvastatin and simvastatin treatment, which have anti-proliferative effects, induced apoptosis in HCC cells, and improved the prognosis of HCC patients [92]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Rui, L. Targeting the Hippo- Yes-Associated Protein/Transcriptional Coactivator with PDZ-Binding Motif Signaling Pathway in Primary Liver Cancer Therapy. Onco 2024, 4, 217-231. https://doi.org/10.3390/onco4030016
Wang Y, Rui L. Targeting the Hippo- Yes-Associated Protein/Transcriptional Coactivator with PDZ-Binding Motif Signaling Pathway in Primary Liver Cancer Therapy. Onco. 2024; 4(3):217-231. https://doi.org/10.3390/onco4030016
Chicago/Turabian StyleWang, Yina, and Liangyou Rui. 2024. "Targeting the Hippo- Yes-Associated Protein/Transcriptional Coactivator with PDZ-Binding Motif Signaling Pathway in Primary Liver Cancer Therapy" Onco 4, no. 3: 217-231. https://doi.org/10.3390/onco4030016
APA StyleWang, Y., & Rui, L. (2024). Targeting the Hippo- Yes-Associated Protein/Transcriptional Coactivator with PDZ-Binding Motif Signaling Pathway in Primary Liver Cancer Therapy. Onco, 4(3), 217-231. https://doi.org/10.3390/onco4030016






