Abstract
Organic luminogens (OLs) with piezochromic (PC) properties have attracted significant attention for their varied applications in chemical sensors, organic optoelectronic devices, biological imaging, etc. In this work, we designed and synthesized three donor–acceptor–donor- or donor–acceptor-structured OLs with different donor or acceptor moieties. Their photophysical properties in both dilute solution and aggregated states were studied through various spectroscopic analytical methods, and their PC properties were investigated under mechanical grinding (MG) conditions. The OLs containing cyanostilbene moiety exhibited a photoemission shift up to ~45 nm after simple grinding, while that was only ~10 nm for cyanostyrene-containing OL. Combined with the powder X-ray diffraction analysis, the incorporation of the cyanostilbene moiety is inferred to play an important role in inducing the apparent PC properties. Our study not only reports novel OLs with good PC properties, but also discusses the structure–property relationships in order to provide guidance for future rational design and the development of novel PC materials.
1. Introduction
Organic luminogens (OLs) have attracted significant interdisciplinary attention for their excellent fluorescent properties, large structural diversity, easy synthesis, and functionalization, and have been widely applied in chemical sensors, organic optoelectronic devices, biological imaging, etc. [1,2,3,4,5,6]. One particularly intriguing aspect of OLs is their ability to exhibit piezochromic (PC) properties, wherein they undergo color or fluorescence changes in response to external force stimulation [7,8,9]. This phenomenon may stem from alterations in either molecular chemical structure, molecular conformation, or solid-state structure in the aggregates [10,11,12]. As a result of these PC properties, this type of OLs holds promising application potentials in information encryption and wearable technologies, showcasing significant scientific merit and practical utility [13,14]. Thus, there is high demand for the development of novel OLs with PC properties.
To date, various PC materials have been developed, including tetraphenylethylene functionalized materials [15,16], triphenylamine (TPA)-based donor–acceptor fluorophores [17,18], coumarin derivatives [19,20,21], etc. In parallel, cyanostilbene (CS) is a widely applied building block to construct donor–acceptor- (D–A) or donor–acceptor–donor (D–A–D)-structured OLs, many of which are sensitive to external force stimuli and exhibit PC properties [22,23,24]. In particular, the functionalized CS derivatives may have special photophysical properties, e.g., dual state emission (DSE) [25,26,27]. Therefore, developing CS-based OLs has attracted lots of research interest in terms of developing PC materials. However, even though some molecular designing principles for developing OLs with distinct PC properties have been proposed, i.e., introducing non-planar building blocks, realizing the rational design of PC OLs is still challenging.
Recently, a series of donor–acceptor–donor (D–A–D)-structured OLs with CS as the acceptor, and TPA and pyrene (Py) as the donors, respectively, were reported by our group (Scheme 1) [28,29]. These materials possess different molecular conformations, i.e., linear-shaped for PYPHTPA and TCpP and curved-shaped for TmCP and TCmP. Accordingly, the former exhibit apparent PC properties upon grinding; in contrast, the latter essentially do not show obvious color or PL changes under similar experimental conditions. Regarding the linear-shaped materials, since they are both composed of three moieties, an intriguing question as to which part plays the essential roles to induce their PC properties is raised. To answer this question, in this study, three novel OLs, namely NCSPy, NCPy, and TCS are designed and synthesized. The newly designed compounds have similar molecular conformations to the reported linear-shaped PC materials, but the building moieties are varied accordingly. Specifically, the TPA, CS, and Py moieties are substituted with other building units or removed in a stepwise manner. The photophysical and PC properties of these compounds were studied, and their structure–property relationships were discussed. Our study not only reports novel PC materials with different building moieties, but also offers valuable insights into the future design of novel PC materials.
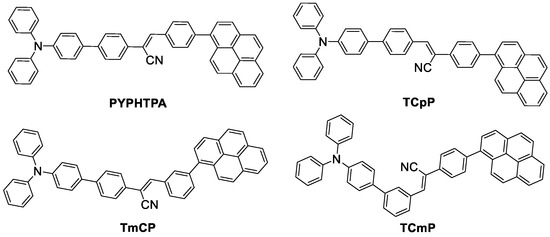
Scheme 1.
Molecular structures of the reported D–A–D-structured OLs with distinct PC properties (PYPHTPA reported in Ref. [28], TCpP, TmCP and TCmP reported in Ref. [29], respectively).
2. Materials and Methods
All the chemicals and solvents were purchased and used from commercial suppliers without further purification. The solvents used for UV-Vis absorption and fluorescence measurements were of analytical grade. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Bruker Avance 400 MHz spectrometer (Bruker Corporation, Germany), with CDCl3 as the solvent and tetramethylsilane (TMS) as the internal standard. The triple quadrupole mass spectra were measured using atmospheric chemical ionization (APCI) equipment (Thermo Fisher Scientific, Rochester, MN, USA). UV-Vis absorption spectra were recorded using a TU-1901 spectrometer from Beijing Purkinje General Instrument Co., Ltd. (Beijing, China), with samples in a quartz cuvette (path length 1 cm). Fluorescence spectra were obtained using a Hitachi FL-7100 (Hitachi High Technologies Corporation, Tokyo, Japan). The theoretical calculation was carried out using the Gaussian 09 program package at B3LYP/6-31G** level, with empirical dispersion correction considered. The powder X-ray diffraction measurements were performed on a Rigaku SmartLab X-ray diffractometer (Rigaku Corporation, Tokyo, Japan).
3. Results
3.1. Synthetic Route to the Target Compounds
Scheme 2 shows the synthetic route to the target compounds. Firstly, 4-bromophenylacetonitrile (1) was reacted with 4-(dimethylamino)phenylboronic acid pinacol ester (2) and 4-(diphenylamino)phenylboronic acid (4) to give the acetonitrile-based intermediates 3 and 5, respectively, through Suzuki coupling reactions [30]. Similarly, 1-pyrenylboronic acid (6) and 4-bromobenzaldehyde (7) were reacted to produce the aldehyde-based intermediate 8 [31]. Subsequently, 3 was reacted with 8 and 1-pyrenealdehyde (9) separately via Knoevenagel condensation to yield the target compounds NCSPy and NCPy. Another target compound TCS was prepared from 5 and benzaldehyde (10) through a similar reaction. Detailed synthetic procedures and characterization of the target compounds can be found in the supplementary information.
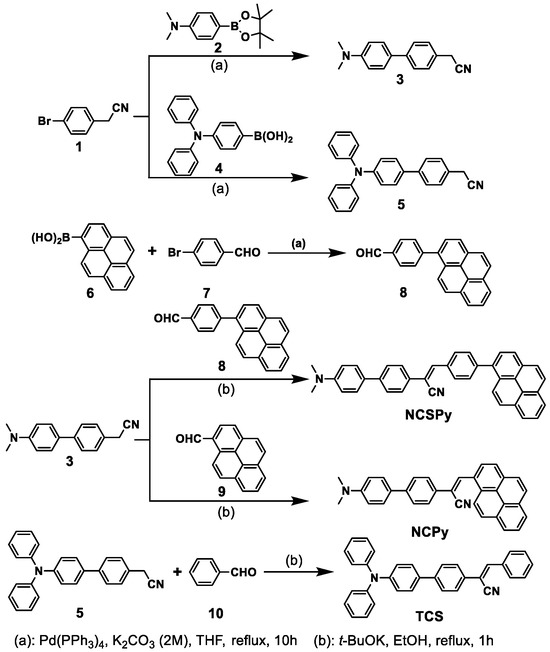
Scheme 2.
Synthetic route to the target compounds.
3.2. Photophysical Properties in Diluted Solution and the Aggregated State
The photophysical properties of the target compounds in diluted solution and the aggregated state were studied via UV-Vis absorption and photoluminescence (PL) spectroscopies, and Figure 1 shows the corresponding normalized spectra. In diluted solution (toluene (Tol), 10−5 M), the compounds all exhibit one main absorption band in the range of ~350–500 nm (Figure 1a, dashed lines). Featuring identical electron-donating groups ((4-dimethylamino)phenyl unit), NCSPy and NCPy exhibit similar absorption spectra and maximums of absorption (λmax) of 397 nm and 406 nm, respectively. On the other hand, with the TPA unit as the electron-donating group, the absorption spectrum of TCS is slightly blue-shifted, with λmax of 380 nm.
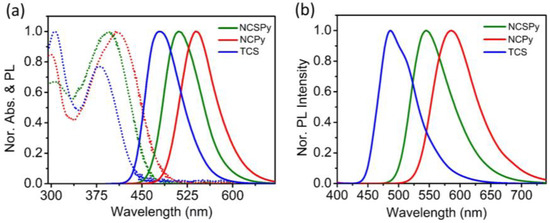
Figure 1.
(a) The normalized UV-Vis absorption spectra (dashed line) and PL spectra (solid line) of the three compounds in toluene and the (b) normalized PL spectra of the three compounds in the aggregated state.
In the PL spectra, the compounds in toluene exhibit one main emission band, with the maximum of emission (λPL) at 511 nm, 539 nm, and 479 nm for NCSPy, NCPy, and TCS, respectively. The intense absorption and strong PL emission of these compounds in diluted solution suggest the potential occurrence of intramolecular charge transfer (ICT) during the photon excitation and relaxation process [32,33]. The relative fluorescence quantum yields (Φ) of NCSPy and TCS are 0.34 and 0.48 in Tol, respectively, while that of NCPy is only 0.10. When the solvent is changed to THF, the Φ of all these compounds decrease.
Upon aggregation, the λPL of NCSPy and NCPy are both largely red-shifted compared to in toluene, (33 nm for NCSPy with λPL of 544 nm, 75 nm for NCPy with λPL of 614 nm). In contrast, the λPL of TCS (482 nm) exhibits only a slight red-shift by 3 nm. These results indicate that aggregation has a more significant influence on the photophysical properties of the D–A–D-structured compounds with a (4-dimethylamino)phenyl donor unit. Table 1 summarizes the UV-Vis absorption and PL peaks of the three compounds in the solution and the aggregated state.

Table 1.
Summarized UV-Vis absorption and PL peaks of the three compounds in the solution and the aggregated state, as well as their Φ values in Tol and THF.
3.3. Solvatochromic and Solvatokinetic Effects
The solvatochromic and solvatokinetic effects of the compounds were studied in various organic solvents with different polarities. As shown in Figure 2, as the solvent polarity increases (from Tol to ACN), the λPL of all the compounds progressively red-shift (510→615 nm for NCSPy, 539→630 nm for NCPy, and 479→578 nm for TCS), indicating a positive solvatochromic effect. On the other hand, the PL intensities of all the compounds progressively decrease, suggesting a positive solvatokinetic effect.
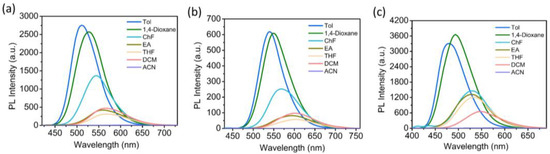
Figure 2.
PL spectra of NCSPy (a), NCPy (b), TCS (c) in various organic solvents (Tol = toluene, ChF = trichloromethane, EA = ethyl acetate, THF = tetrahydrofuran, DCM = dichloromethane, ACN = acetonitrile).
Based on the analysis of UV-Vis absorption spectra (Figure S1) and PL spectra of the three compounds in different solvents, the corresponding Stokes shifts (Δν) could be calculated. The solvent orientation polarizabilities were fitted and plotted according to the Lippert–Mataga Equations (1) and (2) (Figure S2), in which Δν is Stokes shift, h is Planck’s constant, c is the speed of light, a is the Onsager cavity radius, and μe and μg are the dipole moments in the excited and ground states, respectively. Δf in Equation (1) is the solvent polarity function, which can be obtained from Equation (2), where ε is the dielectric constant and n is the refractive index.
The slopes of the plots (LM slope) are 14,947 for NCSPy, 8895 for NCPy, and 13,116 for TCS. This indicates strong correlation effects between solute and solvent molecules, further supporting that molecular excitation and relaxation in dilute solutions are based on ICT processes.
3.4. Aggregation-Induced Emission Properties
The aggregation-induced emission properties of the compounds were studied in the THF/H2O mixed solvents. Figure 3 shows the corresponding PL spectra (Figure 3a–c), and the changes of λPL and intensities of PL emission peaks (Figure 3d–f). As the fraction of H2O increases from 0 to ~40%, in which case clear solutions can be continuously and obviously observed, the three compounds have not yet aggregated under these conditions. However, the solvent polarity gradually increases, causing red shifts of λPL and a decrease in PL intensities. These phenomena are consistent with their positive solvatochromic and solvatokinetic effects, in which the non-radiative decay may be enhanced through the stabilization of ICT in higher-polarity environments. With the further increase in water fraction, the λPL of the three compounds exhibit obvious blue-shift, accompanied by a significant increase in PL intensity, suggesting the occurrence of molecular aggregation. In the high water fraction level (99%), the PL intensities of TCS aggregates are only slightly lower than the initial state in pure THF solution; thus, it can be classified as a dual-state emission (DSE) material. On the other hand, the PL intensities of NCSPy and NCPy both exhibit apparent decrease.
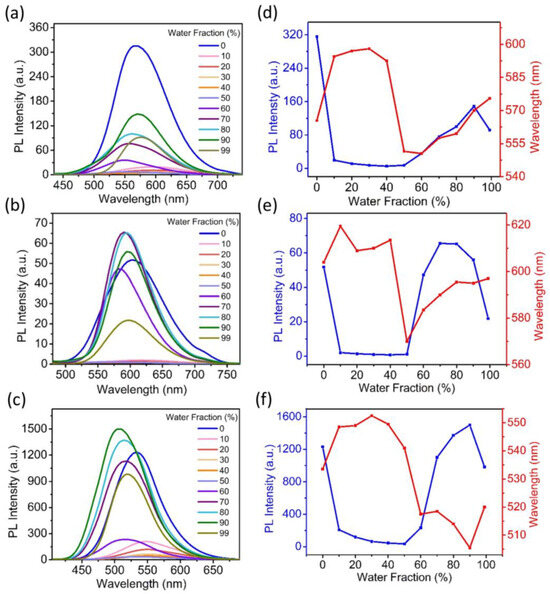
Figure 3.
The PL spectra of NCSPy (a), NCPy (b), and TCS (c) in THF/H2O mixed solvents with different H2O fractions; (d–f) the corresponding PL shifts and intensity changes as the increase of H2O fractions in THF/H2O mixed solvents.
3.5. Theoretical Calculations
The molecular conformation, frontier molecular orbitals, and energy levels of the target compounds in the gas phase were studied using theoretical calculations based on density functional theory (DFT) at the B3LYP/6-31G** level. As shown in Figure 4, NCSPy and TCS, both possessing a cyanostilbene moiety, exhibit relatively linear-shaped molecular conformations. In contrast, the molecular conformation of NCPy, which is connected via a cyanostyrene unit, is relatively twisted.
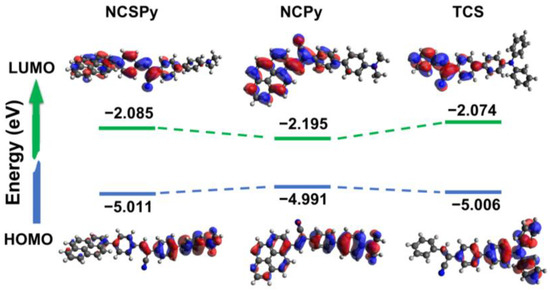
Figure 4.
Frontier molecular orbitals and energy levels of the three compounds.
The highest occupied molecular orbital (HOMO) coefficients of the compounds are primarily located on the (4-dimethylamino)phenyl or TPA units. On the other hand, the lowest unoccupied molecular orbital (LUMO) coefficients of the compounds are predominantly distributed over the cyanostilbene or cyanostyrene moieties, and those only have partial distribution on the pyrene units in NCSPy and NCPy. The distinct distribution of the HOMO and LUMO coefficients of these molecules strongly indicate the existence of an ICT process.
The HOMO and LUMO energy levels of NCSPy (−5.011 eV for HOMO and −2.085 eV for LUMO) and TCS (−5.006 eV for HOMO and −2.074 eV for LUMO) are quite similar, while those of NCPy are quite different (−4.991 eV for HOMO and −2.195 eV for LUMO). As a result, the latter has narrower bandgap (2.796 eV) than the formers (2.926 eV for NCSPy and 2.932 eV for TCS). These results suggest that changing the acceptor unit from cyanostilbene to cyanostyrene has a more significant influence on the frontier molecular energy levels and bandgaps in these materials. The narrowest bandgap of NCPy is consistent with its longest λabs and λPL in the solution and the aggregated state. Table 2 summarizes the energy levels and bandgaps of the three molecules.

Table 2.
Summarized frontier molecular energy levels and bandgaps of the three compounds.
3.6. Piezochromic Properties upon Mechanical Grinding
The piezochromic properties of these materials upon mechanical grinding were studied by grinding the crystalline aggregates using an agate mortar and pestle. As illustrated in Figure 5, after grinding, the λPL of NCPy exhibits a slight red-shift (10 nm) from 615 nm to 625 nm. In sharp contrast, the PL spectra of NCSPy and TCS red-shift significantly by up to 44 nm (λPL shifts from 545 nm to 589 nm) and 45 nm (λPL shifts from 482 nm to 527 nm), which demonstrates apparent PC properties. Both the differences in the molecular structures and changes in the aggregation behaviors can be the reasons behind the different PC properties, and the latter will be discussed vide infra.
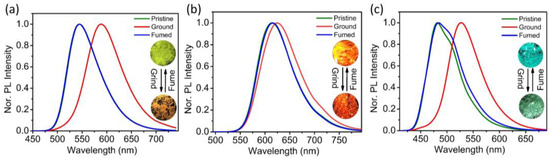
Figure 5.
Piezochromic properties of NCSPy (a), NCPy (b), and TCS (c) upon mechanical grinding and after vapor fuming.
After DCM vapor treatment, the PL spectra of the ground powders of the three compounds could essentially be restored to their original states. This favorable recovery indicates that the PC behaviors were not caused by permanent chemical structural changes in the molecules during grinding. The inset photographs in Figure 5 display the PL changes in the powdered samples before and after grinding (or after steam treatment) upon excitation at 365 nm, where distinct fluorescence shifts are directly observable: NCSPy changes from light green to orange and TCS shifts from blue to yellow-green. Table 3 summarizes the PL peak changes for the three compounds before and after grinding, as well as after vapor treatment. Moreover, these materials show good reversibility in their PC properties, and the emission changes are quite robust during five repeated grinding/vapor fuming cycles (Figure S3).

Table 3.
Summarized shifts in PL peaks before and after grinding and after vapor fuming.
3.7. Mechanical Grinding-Induced Crystallinity Change
To further understand the mechanisms of the different PC behaviors of the materials upon MG, powder X-ray diffraction (PXRD) analysis was performed for the compounds under different states (Figure 6). The PXRD patterns of the three compounds in their pristine state exhibit sharp and intense diffraction peaks, indicating the good crystallinity of the aggregates. After grinding, the intensities of the diffraction peaks significantly decrease, suggesting that the crystalline aggregates change into an amorphous state. Following recovery from organic solvent fuming, the diminished diffraction peaks can basically recover back to their original sharp states. Even though the changes between the crystalline and amorphous states of OLs can explain the PC mechanisms of many materials, in this work this explanation is not solid enough. This is because the crystallinity changing trends of these materials are similar, while their PC properties are different. Instead, the differences in the molecular structures of the three compounds should play a more important role in causing the different PC properties. More specifically, the cyanostilbene moiety may play an important role in inducing large PL shifts upon MG, which is the case for NCSPy and TCS.
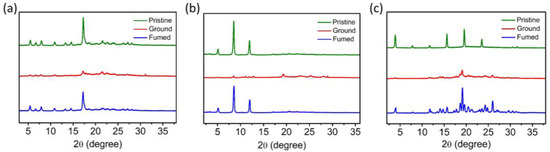
Figure 6.
Powder XRD patterns of the aggregates in a pristine state (blue line), upon grinding (red line), and after vapor exposure: (a) NCSPy, (b) NCPy, and (c) TCS.
4. Conclusions
In conclusion, a series of organic luminogenes (NCSPy, NCPy, and TCS) with different electron donor and acceptor moieties were designed and synthesized, and their photophysical and PC properties were investigated. Changing the donor units and the acceptor moieties can change the photophysical properties accordingly. This is particularly obvious for the cyanostyrene moieties, which lower both the HOMO and LUMO energy levels and narrow the bandgap most significantly. More interestingly, the PC properties of these materials are also tuned, which is obvious for NCSPy and TCS, with PL shifts up to ~45 nm, while only slight for NCPy, with a PL shift of 10 nm. The existence of the cyanostilbene moiety in NCSPy and TCS is anticipated to play important roles in their large PC responses. Our study not only reports novel OLs with different PC properties, but also provides certain guidance for the future development of sensitive force-stimuli-responsive materials.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/photochem5040036/s1, Detailed synthetic procedures for the target compounds; Figure S1: UV-Vis absorption spectra of NCSPy (a), NCPy (b) and TCS (c) in various organic solvents, Figure S2: Linearly fitted Stokes shifts and orientation polarizability of NCSPy (a), NCPy (b) and TCS (c) in various solvents based on the Lippert-Mataga equation, Figure S3: Reversibility of the PC properties of NCSPy (a), NCPy (b) and TCS (c) upon repeated grindings and vapor fuming cycles; Figure S4: 1H NMR spectrum of NCSPy in CDCl3, Figure S5: 1H NMR spectrum of NCPy in CDCl3, Figure S6: 1H NMR spectrum of TCS in CDCl3, Figure S7: 13C NMR spectrum of TCS in CDCl3, Figure S8: HR-MS spectra of NCSPy, Figure S9: HR-MS spectra of NCPy, Figure S10: HR-MS spectra of TCS; Table S1: summarized Stokes shifts.
Author Contributions
Conceptualization, H.S. and C.W.; formal analysis, M.Y., Y.C. and X.Y.; data curation, Z.W., B.X. and X.D.; writing—original draft preparation, M.Y.; writing—review and editing, C.W.; supervision, C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 22205003.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy.
Acknowledgments
C.W. acknowledges the financial support from the National Natural Science Foundation of China (22205003) and the Start-up Foundation for Talents of Anhui University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mako, T.L.; Racicot, J.M.; Levine, M. Supramolecular Luminescent Sensors. Chem. Rev. 2019, 119, 322–477. [Google Scholar] [CrossRef]
- Wang, H.; Ji, X.; Page, Z.A.; Sessler, J.L. Fluorescent materials-based information storage. Mater. Chem. Front. 2020, 4, 1024–1039. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Q.; Feng, W.; Li, F. Luminescent Chemodosimeters for Bioimaging. Chem. Rev. 2013, 113, 192–270. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Hu, W.; Stoddart, J.F. Color-Tunable Supramolecular Luminescent Materials. Adv. Mater. 2022, 34, 2105405. [Google Scholar] [CrossRef] [PubMed]
- Kenry; Chong, K.C.; Liu, B. Reactivity-Based Organic Theranostic Bioprobes. Acc. Chem. Res. 2019, 52, 3051–3063. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Chen, J.; Wang, D.; Liu, Z.; Han, G.; Liu, B.; Han, M.; Zhang, R.; Liu, G.; Zhang, Z. Real-time quantification of nuclear RNA export using an intracellular relocation probe. Chin. Chem. Lett. 2022, 33, 3865–3868. [Google Scholar] [CrossRef]
- Sagara, Y.; Kato, T. Mechanically induced luminescence changes in molecular assemblies. Nat. Chem. 2009, 1, 605–610. [Google Scholar] [CrossRef]
- Chi, Z.; Zhang, X.; Xu, B.; Zhou, X.; Ma, C.; Zhang, Y.; Liu, S.; Xu, J. Recent advances in organic mechanofluorochromic materials. Chem. Soc. Rev. 2012, 41, 3878–3896. [Google Scholar] [CrossRef]
- Chung, K.; Kwon, M.S.; Leung, B.M.; Wong-Foy, A.G.; Kim, M.S.; Kim, J.; Takayama, S.; Gierschner, J.; Matzger, A.J.; Kim, J. Shear-Triggered Crystallization and Light Emission of a Thermally Stable Organic Supercooled Liquid. ACS Cent. Sci. 2015, 1, 94–102. [Google Scholar] [CrossRef]
- Li, A.; Xu, S.; Bi, C.; Geng, Y.; Cui, H.; Xu, W. Piezochromic mechanism of organic crystals under hydrostatic pressure. Mater. Chem. Front. 2021, 5, 2588–2606. [Google Scholar] [CrossRef]
- Nagura, K.; Saito, S.; Yusa, H.; Yamawaki, H.; Fujihisa, H.; Sato, H.; Shimoikeda, Y.; Yamaguchi, S. Distinct Responses to Mechanical Grinding and Hydrostatic Pressure in Luminescent Chromism of Tetrathiazolylthiophene. J. Am. Chem. Soc. 2013, 135, 10322–10325. [Google Scholar] [CrossRef]
- Gong, Y.-B.; Zhang, P.; Gu, Y.-R.; Wang, J.-Q.; Han, M.-M.; Chen, C.; Zhan, X.-J.; Xie, Z.-L.; Zou, B.; Peng, Q.; et al. The Influence of Molecular Packing on the Emissive Behavior of Pyrene Derivatives: Mechanoluminescence and Mechanochromism. Adv. Opt. Mater. 2018, 6, 1800198. [Google Scholar] [CrossRef]
- Yang, J.; Fang, M.; Li, Z. Organic luminescent materials: The concentration on aggregates from aggregation-induced emission. Aggregate 2020, 1, 6–18. [Google Scholar] [CrossRef]
- Meng, X.; Qi, G.; Li, X.; Wang, Z.; Wang, K.; Zou, B.; Ma, Y. Spiropyran-based multi-colored switching tuned by pressure and mechanical grinding. J. Mater. Chem. C 2016, 4, 7584–7588. [Google Scholar] [CrossRef]
- Yang, Z.; Chi, Z.; Mao, Z.; Zhang, Y.; Liu, S.; Zhao, J.; Aldred, M.P.; Chi, Z. Recent advances in mechano-responsive luminescence of tetraphenylethylene derivatives with aggregation-induced emission properties. Mater. Chem. Front. 2018, 2, 861–890. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef]
- Gayathri, P.; Pannipara, M.; Al-Sehemi, A.G.; Anthony, S.P. Triphenylamine-based stimuli-responsive solid state fluorescent materials. New J. Chem. 2020, 44, 8680–8696. [Google Scholar] [CrossRef]
- Lee, J.; Yuk, S.; Namgoong, J.; Kim, J. Mechanofluorochromism of Triphenylamine-BODIPY: Effect of twisted intramolecular charge transfer and restriction in rotation on fluorescence. Dyes Pigments 2021, 185, 108864. [Google Scholar] [CrossRef]
- Fu, S.; Jia, H.; Meng, X.; Wang, C.; Li, Q.; Li, L.; Yang, J.; Niu, H. Fine-tuning the molecular conformation and packing structures of coumarin-based luminogens to achieve distinct piezochromic properties upon mechanical grinding and under hydrostatic pressures. Mater. Horiz. 2025, 12, 293–302. [Google Scholar] [CrossRef]
- Traven, V.F.; Cheptsov, D.A.; Svetlova, J.I.; Ivanov, I.V.; Cuerva, C.; Lodeiro, C.; Duarte, F.; Dunaev, S.F.; Chernyshev, V.V. The role of the intermolecular π···π interactions in the luminescence behavior of novel coumarin-based pyrazoline materials. Dyes Pigments 2021, 186, 108942. [Google Scholar] [CrossRef]
- Yang, M.; Guo, J.; Fu, S.; Zhang, Y.; Yang, L.; Li, Q.; Bao, Y.; Wang, C. Packing structures and piezochromic properties engineering through F functionalization in 4-position substituted coumarin based organic luminogens. Dyes Pigments 2025, 243, 113055. [Google Scholar] [CrossRef]
- An, B.-K.; Kwon, S.-K.; Jung, S.-D.; Park, S.Y. Enhanced Emission and Its Switching in Fluorescent Organic Nanoparticles. J. Am. Chem. Soc. 2002, 124, 14410–14415. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, Y. Cyanostilbene-based intelligent organic optoelectronic materials. J. Mater. Chem. C 2013, 1, 1059–1065. [Google Scholar] [CrossRef]
- Martínez-Abadía, M.; Giménez, R.; Ros, M.B. Self-Assembled α-Cyanostilbenes for Advanced Functional Materials. Adv. Mater. 2018, 30, 1704161. [Google Scholar] [CrossRef] [PubMed]
- Belmonte-Vázquez, J.L.; Amador-Sánchez, Y.A.; Rodríguez-Cortés, L.A.; Rodríguez-Molina, B. Dual-State Emission (DSE) in Organic Fluorophores: Design and Applications. Chem. Mater. 2021, 33, 7160–7184. [Google Scholar] [CrossRef]
- Qiu, Q.; Xu, P.; Zhu, Y.; Yu, J.; Wei, M.; Xi, W.; Feng, H.; Chen, J.; Qian, Z. Rational Design of Dual-State Emission Luminogens with Solvatochromism by Combining a Partially Shared Donor–Acceptor Pattern and Twisted Structures. Chem. Eur. J. 2019, 25, 15983–15987. [Google Scholar] [CrossRef] [PubMed]
- König, N.F.; Mutruc, D.; Hecht, S. Accelerated Discovery of α-Cyanodiarylethene Photoswitches. J. Am. Chem. Soc. 2021, 143, 9162–9168. [Google Scholar] [CrossRef]
- Huang, Z.; Tang, F.; He, F.; Kong, L.; Huang, J.; Yang, J.; Ding, A. Pyrene and triphenylamine substituted cyanostyrene and cyanostilbene derivatives with dual-state emission for high-contrast mechanofluorochromism and cell imaging. Org. Chem. Front. 2022, 9, 5118–5124. [Google Scholar] [CrossRef]
- Shen, H.; Fu, X.; Wang, Y.; Shi, Q.; Xu, X.; Li, L.; Li, Q.; Yang, J.; Wang, C. Molecular conformation and cyano regio-isomerization effects to the piezochromic properties of cyanostilbene-bridged donor-acceptor-donor structured organic luminogens. J. Mol. Struct. 2025, 1327, 141198. [Google Scholar] [CrossRef]
- Ouyang, M.; Zhuo, C.; Cao, F.; Pan, G.; Lv, C.; Yang, S.; Li, C.; Zhang, C.; Sun, J.; Zhang, Y. Organogelator based on long alkyl chain attached excimer precursor: Two channels of TICT, highly efficient and switchable luminescence. Dyes Pigments 2020, 180, 108433. [Google Scholar] [CrossRef]
- Mayurachayakul, P.; Thianchai, J.; Attakul, N.; Pratumyot, K.; Sukwattanasinitt, M.; Srikittiwanna, K.; Niamnont, N. Novel pyrenylbenzylidene-malononitrile derivative mixed with cellulose acetate electrospun nanofibrous sheets for hydrazine detection. New J. Chem. 2024, 48, 16095–16106. [Google Scholar] [CrossRef]
- Grabowski, Z.R.; Rotkiewicz, K.; Rettig, W. Structural Changes Accompanying Intramolecular Electron Transfer: Focus on Twisted Intramolecular Charge-Transfer States and Structures. Chem. Rev. 2003, 103, 3899–4032. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chi, W.; Qiao, Q.; Tan, D.; Xu, Z.; Liu, X. Twisted intramolecular charge transfer (TICT) and twists beyond TICT: From mechanisms to rational designs of bright and sensitive fluorophores. Chem. Soc. Rev. 2021, 50, 12656–12678. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).