Hybrid Biocatalysis with Photoelectrocatalysis for Renewable Furan Derivatives’ Valorization: A Review
Abstract
1. Introduction
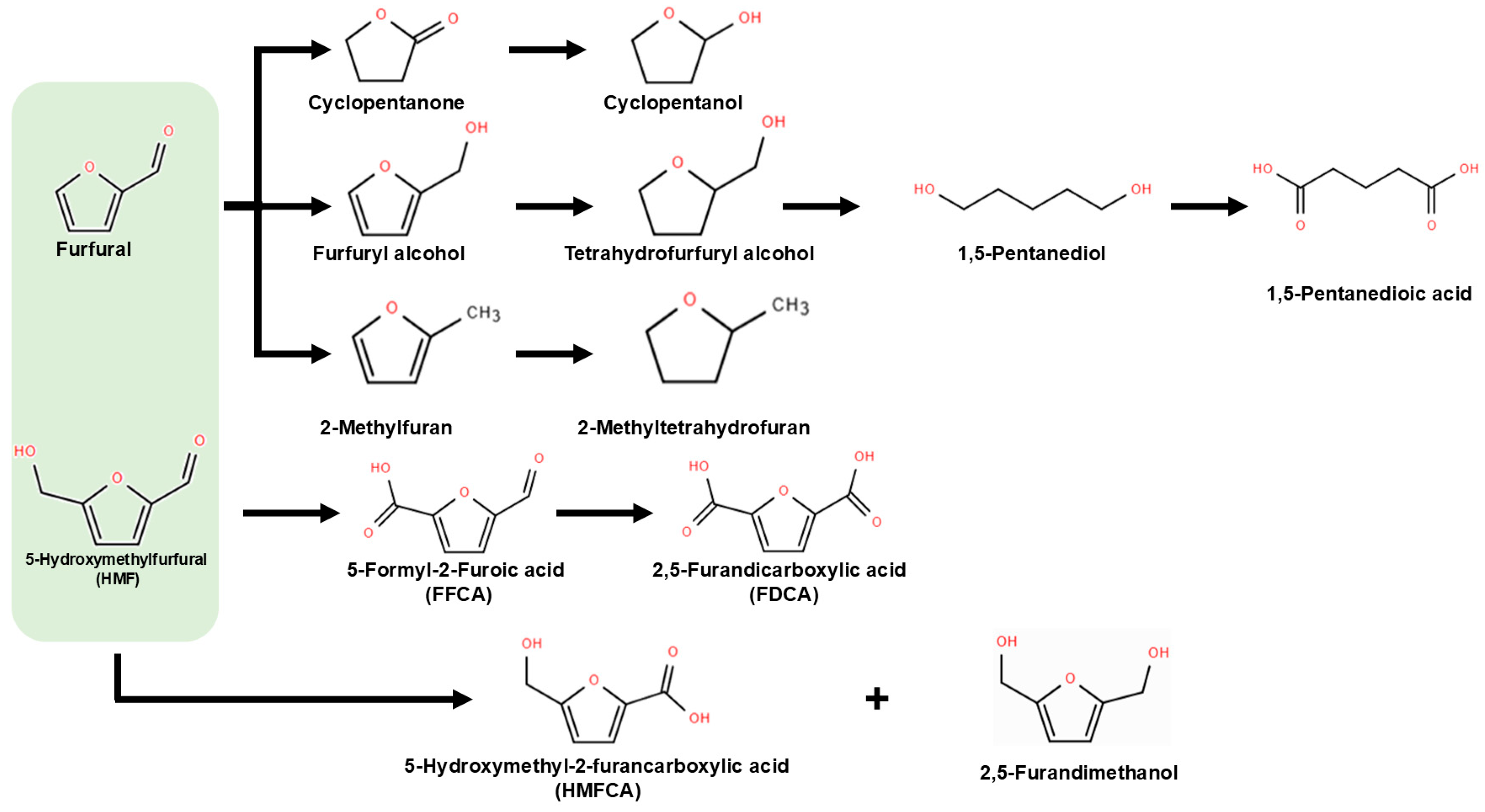
2. Renewable Furan Derivatives and Valorization
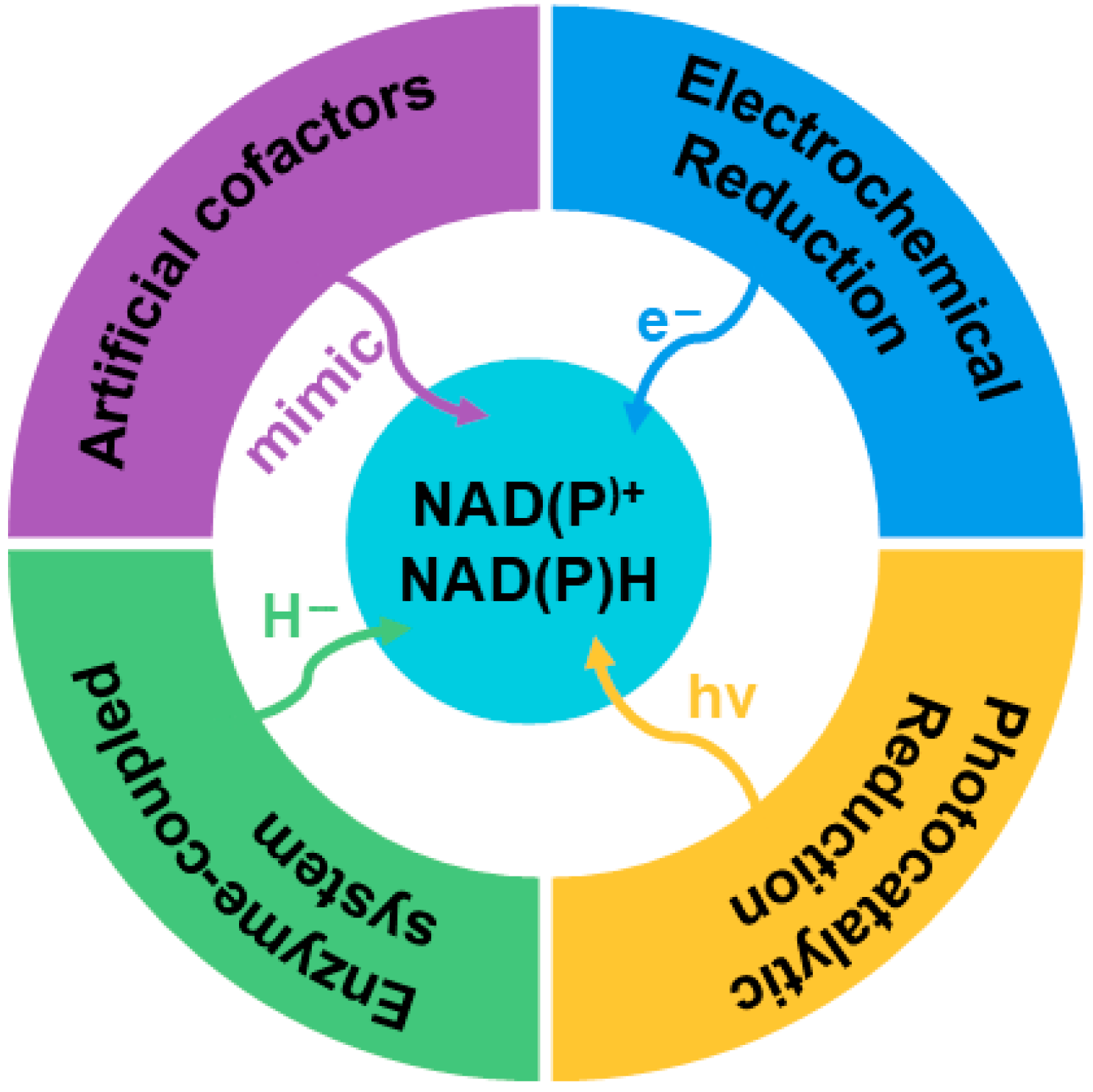
3. Principles of Hybrid Photoelectro-Biocatalysis
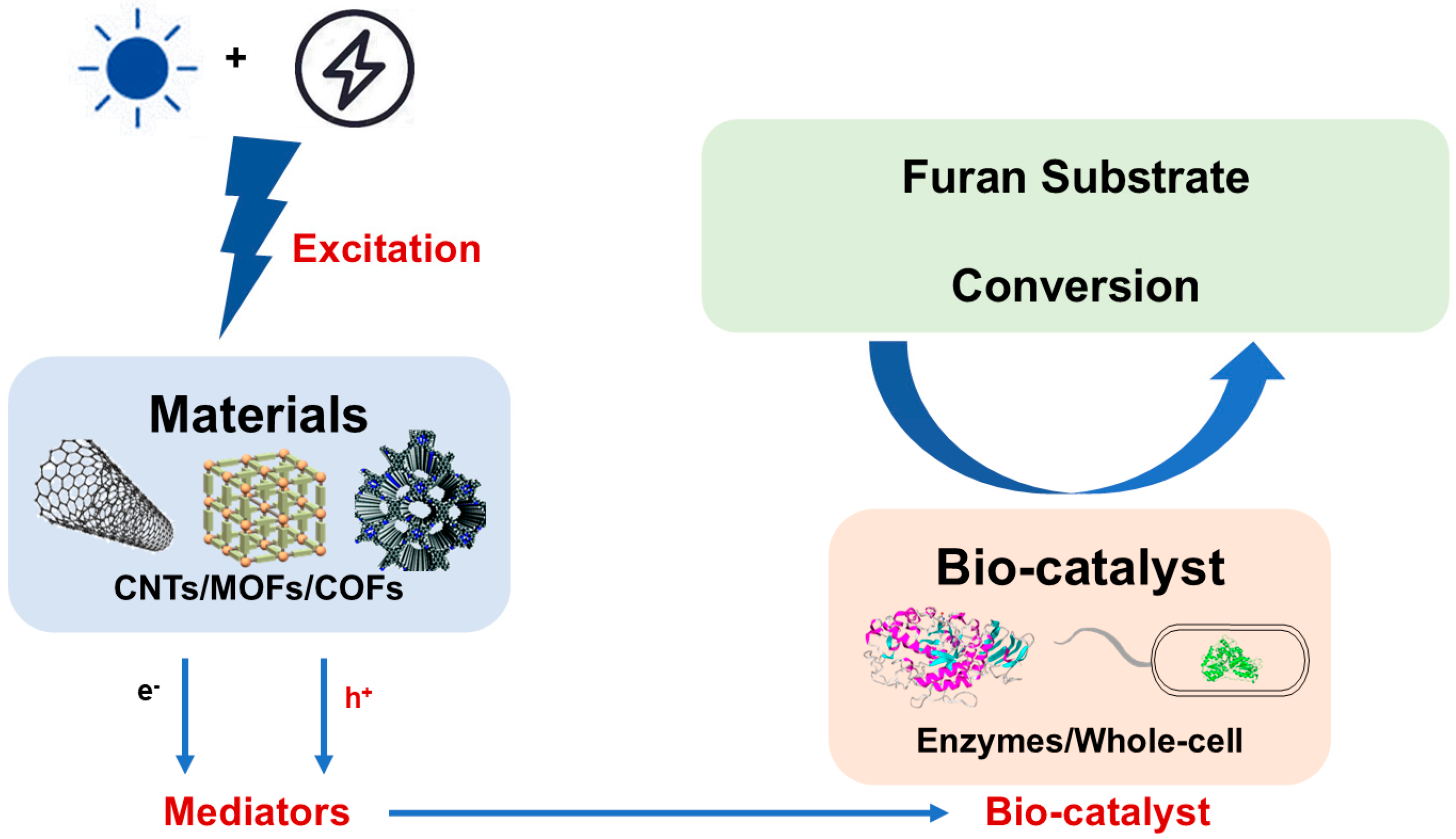
4. Representative Strategies and Systems
5. Applications in Furan Derivatives Transformation
6. Challenges and Outlook
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bielski, R.; Grynkiewicz, G. Furan platform chemicals beyond fuels and plastics. Green Chem. 2021, 23, 7458–7487. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, D.; Qin, Y.; Liu, D.; Zhao, X. High value-added monomer chemicals and functional bio-based materials derived from polymeric components of lignocellulose by organosolv fractionation. Biofuels Bioprod. Biorefining 2020, 14, 371–401. [Google Scholar] [CrossRef]
- Becerra, M.L.; Prieto, G.A.; Rendueles, M.; Diaz, M. Biological transformations of furanic platform molecules to obtain biomass-derived furans: A review. Biomass Convers. Biorefinery 2024, 14, 26611–26629. [Google Scholar] [CrossRef]
- Jaswal, A.; Singh, P.P.; Mondal, T. Furfural–a versatile, biomass-derived platform chemical for the production of renewable chemicals. Green Chem. 2022, 24, 510–551. [Google Scholar] [CrossRef]
- Jiang, Y.; Yun, J.; Pan, X. Renewable furan-based epoxy resins derived from 5-hydroxymethylfurfural and furfural. ACS Sustain. Chem. Eng. 2022, 10, 16555–16562. [Google Scholar] [CrossRef]
- Frank, N.; Leutzsch, M.; List, B. Bronsted Acid-Catalyzed Reduction of Furans. J. Am. Chem. Soc. 2025, 147, 7932–7938. [Google Scholar] [CrossRef]
- Batool, Z.; Singla, R.K.; Kamal, M.A.; Shen, B. Demystifying furan formation in foods: Implications for human health, detection, and control measures: A review. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70087. [Google Scholar] [CrossRef]
- Eid, N.; Ameduri, B.; Boutevin, B. Synthesis and properties of furan derivatives for epoxy resins. ACS Sustain. Chem. Eng. 2021, 9, 8018–8031. [Google Scholar] [CrossRef]
- Khurana, H.; Srivastava, M.; Chaudhary, D.; Gosain, T.P.; Kumari, R.; Bean, A.C.; Chugh, S.; Maiti, T.K.; Stephens, C.E.; Asthana, S. Identification of diphenyl furan derivatives via high throughput and computational studies as ArgA inhibitors of Mycobacterium tuberculosis. Int. J. Biol. Macromol. 2021, 193, 1845–1858. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sun, Y.; Liu, Q.; Li, A.; Wang, W.; Gu, W. Design, synthesis, and antifungal activity of novel thiophene/furan-1, 3, 4-oxadiazole carboxamides as potent succinate dehydrogenase inhibitors. J. Agric. Food Chem. 2021, 69, 13373–13385. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, L.; Li, B.-G. Poly (1,5-pentylene-co-2,2,4,4-tetramethyl cyclobutylene terephthalate) copolyesters with high Tg and improved ductility and thermal stability. Polymer 2021, 232, 124152. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Kandola, B.; Song, L.; Kan, Y.; Chen, J.; Hu, Y. A bio-based intrinsically flame-retardant epoxy vitrimer from furan derivatives and its application in recyclable carbon fiber composites. Polym. Degrad. Stab. 2023, 207, 110206. [Google Scholar] [CrossRef]
- Mukhtar, A.; Saqib, S.; Lin, H.; Shah, M.U.H.; Ullah, S.; Younas, M.; Rezakazemi, M.; Ibrahim, M.; Mahmood, A.; Asif, S. Current status and challenges in the heterogeneous catalysis for biodiesel production. Renew. Sustain. Energy Rev. 2022, 157, 112012. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.; Ashokkumar, M. Synergistic Catalysis for Algae Control: Integrating Sonocavitation and Chemical Catalysis. Catalysts 2025, 15, 784. [Google Scholar] [CrossRef]
- Bell, E.L.; Finnigan, W.; France, S.P.; Green, A.P.; Hayes, M.A.; Hepworth, L.J.; Lovelock, S.L.; Niikura, H.; Osuna, S.; Romero, E. Biocatalysis. Nat. Rev. Methods Primers 2021, 1, 46. [Google Scholar] [CrossRef]
- Trisolini, L.; Gambacorta, N.; Gorgoglione, R.; Montaruli, M.; Laera, L.; Colella, F.; Volpicella, M.; De Grassi, A.; Pierri, C.L. FAD/NADH dependent oxidoreductases: From different amino acid sequences to similar protein shapes for playing an ancient function. J. Clin. Med. 2019, 8, 2117. [Google Scholar] [CrossRef]
- Marreiros, B.C.; Sena, F.V.; Sousa, F.M.; Batista, A.P.; Pereira, M.M. Type II NADH: Quinone oxidoreductase family: Phylogenetic distribution, structural diversity and evolutionary divergences. Environ. Microbiol. 2016, 18, 4697–4709. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; McMillan, A.H.; Giansante, C.; Bergamini, G. NADH photoregeneration in a fully automated microfluidic setup. Energy Fuels 2024, 38, 12078–12086. [Google Scholar] [CrossRef]
- Li, N.; You, J.; Huang, L.; Zhang, H.; Wang, X.; He, L.; Gong, C.; Lin, S.; Zhang, B. Photoelectrochemical NADH regeneration on a polymer semiconductor-based photocathode. Green Chem. 2023, 25, 5247–5256. [Google Scholar] [CrossRef]
- Ma, B.; Sun, S.; He, H.; Lv, R.; Deng, J.; Huo, T.; Zhao, Y.; Yu, H.; Zhou, L. An efficient metal-free photocatalytic system with enhanced activity for NADH regeneration. Ind. Eng. Chem. Res. 2019, 58, 23567–23573. [Google Scholar] [CrossRef]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H. Catalytic conversion of lignocellulosic biomass into chemicals and fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, W.; Qiu, X.; Xu, C. Hydrothermal treatment of lignocellulosic biomass towards low-carbon development: Production of high-value-added bioproducts. EnergyChem 2024, 6, 100133. [Google Scholar] [CrossRef]
- De Jong, E.; Visser, H.A.; Dias, A.S.; Harvey, C.; Gruter, G.-J.M. The Road to Bring FDCA and PEF to the Market. Polymers 2022, 14, 943. [Google Scholar] [CrossRef]
- Davidson, M.G.; Elgie, S.; Parsons, S.; Young, T.J. Production of HMF, FDCA and their derived products: A review of life cycle assessment (LCA) and techno-economic analysis (TEA) studies. Green Chem. 2021, 23, 3154–3171. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Y.; Gao, S.; Lv, B.; Wu, Z. Noble Metal-Based Catalysts for Selective Oxidation of HMF to FDCA: Progress in Reaction Mechanism and Active Sites. Chemistry 2025, 7, 17. [Google Scholar] [CrossRef]
- Wolny, A.; Więcławik, D.; Zdarta, J.; Jurczyk, S.; Jesionowski, T.; Chrobok, A. Robust biocatalyst for the green continuous flow synthesis of esters from biomass-derived furfuryl alcohol and C8–C18 carboxylic acids. Green Chem. 2024, 26, 10829–10841. [Google Scholar] [CrossRef]
- Ye, J.; Ma, S.; Wang, B.; Chen, Q.; Huang, K.; Xu, X.; Li, Q.; Wang, S.; Lu, N.; Zhu, J. High-performance bio-based epoxies from ferulic acid and furfuryl alcohol: Synthesis and properties. Green Chem. 2021, 23, 1772–1781. [Google Scholar] [CrossRef]
- Huerta-Ochoa, S. Advantages and new potential applications of whole-cell biocatalysis. In Whole Cell Biocatalysis; Elsevier: Amsterdam, The Netherlands, 2025; pp. 1–19. [Google Scholar]
- Kuo, C.-H.; Huang, C.-Y.; Shieh, C.-J.; Dong, C.-D. Enzymes and biocatalysis. Catalysts 2022, 12, 993. [Google Scholar] [CrossRef]
- Santi, M.; Sancineto, L.; Nascimento, V.; Braun Azeredo, J.; Orozco, E.V.; Andrade, L.H.; Gröger, H.; Santi, C. Flow biocatalysis: A challenging alternative for the synthesis of APIs and natural compounds. Int. J. Mol. Sci. 2021, 22, 990. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Brady, D. Streamlining design, engineering, and applications of enzymes for sustainable biocatalysis. ACS Sustain. Chem. Eng. 2021, 9, 8032–8052. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Y.; Li, Y.; Zhang, H.; Li, H.; Yang, S. Advances in Diels–Alder/aromatization of biomass furan derivatives towards renewable aromatic hydrocarbons. Catal. Sci. Technol. 2022, 12, 1902–1921. [Google Scholar] [CrossRef]
- Annatelli, M.; Sánchez-Velandia, J.E.; Mazzi, G.; Pandeirada, S.V.; Giannakoudakis, D.; Rautiainen, S.; Esposito, A.; Thiyagarajan, S.; Richel, A.; Triantafyllidis, K.S. Beyond 2, 5-furandicarboxylic acid: Status quo, environmental assessment, and blind spots of furanic monomers for bio-based polymers. Green Chem. 2024, 26, 8894–8941. [Google Scholar] [CrossRef]
- Karmakar, G.; Tyagi, A.; Shah, A.Y. A comprehensive review on single source molecular precursors for nanometric group IV metal chalcogenides: Technologically important class of compound semiconductors. Coord. Chem. Rev. 2024, 504, 215665. [Google Scholar] [CrossRef]
- Yoo, H.; Lee, I.S.; Jung, S.; Rho, S.M.; Kang, B.H.; Kim, H.J. A review of phototransistors using metal oxide semiconductors: Research progress and future directions. Adv. Mater. 2021, 33, 2006091. [Google Scholar] [CrossRef]
- Zheng, X.; Li, C.; Mao, H.; Liu, X.; Guo, Y.; Wang, Y. Boosting 2, 5-Furandicarboxylic acid production via coating carbon over CeO2 in a Pt catalyst. Ind. Crops Prod. 2022, 186, 115168. [Google Scholar] [CrossRef]
- Keum, C.; Hirschbiegel, C.-M.; Chakraborty, S.; Jin, S.; Jeong, Y.; Rotello, V.M. Biomimetic and bioorthogonal nanozymes for biomedical applications. Nano Converg. 2023, 10, 42. [Google Scholar] [CrossRef]
- Mondal, D.; Snodgrass, H.M.; Gomez, C.A.; Lewis, J.C. Non-native site-selective enzyme catalysis. Chem. Rev. 2023, 123, 10381–10431. [Google Scholar] [CrossRef]
- Wang, K.-Y.; Zhang, J.; Hsu, Y.-C.; Lin, H.; Han, Z.; Pang, J.; Yang, Z.; Liang, R.-R.; Shi, W.; Zhou, H.-C. Bioinspired framework catalysts: From enzyme immobilization to biomimetic catalysis. Chem. Rev. 2023, 123, 5347–5420. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Q.-L.; Zou, R.; Xu, Q. Metal-organic frameworks for energy applications. Chem 2017, 2, 52–80. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Jiang, H.-L. Metal–organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, X.; Li, C.; Wang, M.; Li, H.; Yang, Q. Assembly of COFs layer and electron mediator on silica for visible light driven photocatalytic NADH regeneration. Appl. Catal. B Environ. 2022, 310, 121314. [Google Scholar] [CrossRef]
- Yu, B.; Wang, W.; Sun, W.; Jiang, C.; Lu, L. Defect engineering enables synergistic action of enzyme-mimicking active centers for high-efficiency tumor therapy. J. Am. Chem. Soc. 2021, 143, 8855–8865. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, J.; Xu, W.; Huang, J.; Jiao, L.; Tang, Y.; Chen, Y.; Yan, H.; Cao, S.; Zheng, L. Defect-engineered nanozyme-linked receptors. Small 2021, 17, 2101907. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, S.; Sengupta, S.; Mongal, B.N.; Naskar, S. Earth abundant transition metal complexes as molecular water oxidation catalysts. Coord. Chem. Rev. 2024, 504, 215679. [Google Scholar] [CrossRef]
- Zou, M.; Waldie, K.M. Redox-active ligand promoted multielectron reactivity at earth-abundant transition metal complexes. Inorg. Chem. Front. 2024, 11, 5795–5809. [Google Scholar] [CrossRef]
- Kamel, S.; Khattab, T.A. Recent advances in cellulose supported metal nanoparticles as green and sustainable catalysis for organic synthesis. Cellulose 2021, 28, 4545–4574. [Google Scholar] [CrossRef]
- Madani, M.; Hosny, S.; Alshangiti, D.M.; Nady, N.; Alkhursani, S.A.; Alkhaldi, H.; Al-Gahtany, S.A.; Ghobashy, M.M.; Gaber, G.A. Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes. Nanotechnol. Rev. 2022, 11, 731–759. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, C.; Chen, W.; Xie, Q.; Guo, Q.; Huang, B. Electrochemical-driven nanoparticulate catalysis for highly efficient dechlorination of chlorinated environmental pollutant. J. Catal. 2021, 395, 362–374. [Google Scholar] [CrossRef]
- Qamar, S.A.; Qamar, M.; Bilal, M.; Bharagava, R.N.; Ferreira, L.F.R.; Sher, F.; Iqbal, H.M. Cellulose-deconstruction potential of nano-biocatalytic systems: A strategic drive from designing to sustainable applications of immobilized cellulases. Int. J. Biol. Macromol. 2021, 185, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Mittal, R.; Bishnoi, N.R. Nanomaterial-Based Immobilization in Industrial Biocatalysis: Prospects, Gaps, and Challenges. In Enzyme Immobilization with Nanomaterials: Applications and Challenges; ACS Publications: Washington, DC, USA, 2025; pp. 81–103. [Google Scholar]
- Van Schie, M.M.; Spöring, J.-D.; Bocola, M.; de María, P.D.; Rother, D. Applied biocatalysis beyond just buffers–from aqueous to unconventional media. Options and guidelines. Green Chem. 2021, 23, 3191–3206. [Google Scholar] [CrossRef]
- Harrison, W.; Huang, X.; Zhao, H. Photobiocatalysis for abiological transformations. Acc. Chem. Res. 2022, 55, 1087–1096. [Google Scholar] [CrossRef]
- Lee, J.; Song, W.J. Photocatalytic C–O coupling enzymes that operate via intramolecular electron transfer. J. Am. Chem. Soc. 2023, 145, 5211–5221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, F.; Liu, D.; Liu, Q.; Song, H. Engineering extracellular electron transfer pathways of electroactive microorganisms by synthetic biology for energy and chemicals production. Chem. Soc. Rev. 2024, 53, 1375–1446. [Google Scholar] [CrossRef] [PubMed]
- King, A.J.; Weber, A.Z.; Bell, A.T. Understanding Photovoltage Enhancement in Metal–Insulator Semiconductor Photoelectrodes with Metal Nanoparticles. ACS Appl. Mater. Interfaces 2024, 16, 36380–36391. [Google Scholar] [CrossRef]
- Hasnan, N.S.N.; Nordin, N.A.; Mohamed, M.A. Synergistic interaction and hybrid association of conducting polymer photocatalysts/photoelectrodes for emerging visible light active photocatalytic applications. J. Mater. Chem. A 2024, 12, 27892–27931. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, C.; Zhan, P.; Liu, X.; Shan, H.; Wang, Y.; Wang, B.; Qin, P.; Cai, D.; Tan, T. A mediator-free enzyme carbonaceous cathode for bioelectrocatalytic reduction of furfural to furfuryl alcohol. Green Chem. 2025, 27, 3733–3742. [Google Scholar] [CrossRef]
- Zhao, P.; Kong, F.; Jiang, Y.; Qin, X.; Tian, X.; Cong, Z. Enabling peroxygenase activity in cytochrome P450 monooxygenases by engineering hydrogen peroxide tunnels. J. Am. Chem. Soc. 2023, 145, 5506–5511. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Liu, X.; Zhang, S.; Zhu, Q.; Zhao, H.; Ren, C.; Zhang, J.; Lu, L.; Cai, D.; Qin, P. Electroenzymatic reduction of furfural to furfuryl alcohol by an electron mediator and enzyme orderly assembled biocathode. ACS Appl. Mater. Interfaces 2023, 15, 12855–12863. [Google Scholar] [CrossRef]
- Zhou, Y.; He, Y.; Gao, M.; Ding, N.; Lei, J.; Zhou, Y. Efficient photocatalytic NADH regeneration with Rh-loaded Z-scheme mediator-free system. Chin. Chem. Lett. 2024, 35, 108690. [Google Scholar] [CrossRef]
- Hong, Y.H.; Nilajakar, M.; Lee, Y.-M.; Nam, W.; Fukuzumi, S. Artificial photosynthesis for regioselective reduction of NAD (P)+ to NAD (P) H using water as an electron and proton source. J. Am. Chem. Soc. 2024, 146, 5152–5161. [Google Scholar] [CrossRef]
- Liang, L.; Wang, Y.H.; Cui, C.X.; Deng, X.S.; Wang, S.L.; Guo, H.M.; Li, Y.; Niu, H.Y.; Mao, R. NADH Analogues Enable Metal-and Light-Free Decarboxylative Functionalization. Angew. Chem. Int. Ed. 2025, 64, e202415131. [Google Scholar] [CrossRef]
- Zhang, C.; Zhan, P.; Shan, H.; Ren, W.; Liu, Y.; Liu, X.; Zheng, S.; Liao, Z.; Cai, D.; Qin, P. Research on highly efficient photocatalytic selective oxidation of HMF to DFF using CdS@MXene Composites: Construction of Schottky junctions and mechanistic investigation. Chem. Eng. J. 2025, 506, 159946. [Google Scholar] [CrossRef]
- Kowalewska, B.; Jakubow, K. The impact of immobilization process on the electrochemical performance, bioactivity and conformation of glucose oxidase enzyme. Sens. Actuators B Chem. 2017, 238, 852–861. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.; Ribeiro, M.H. Enzyme immobilization and co-immobilization: Main framework, advances and some applications. Processes 2022, 10, 494. [Google Scholar] [CrossRef]
- Hou, M.; Yuan, J.; Dong, X.; Wang, Y.; Yang, S.; Gao, J. Engineering Oxygen-Independent NADH Oxidase Integrated with Electrocatalytic FAD Cofactor Regeneration. JACS Au 2024, 4, 3581–3592. [Google Scholar] [CrossRef]
- Bahukhandi, S.B.; Klein, A.S.; Mustafa, G.; Weyh, M.; Walter, A.; Thyrhaug, E.; Hauer, J.; Storch, G.; Zeymer, C. The Natural Redox Cofactor Pyrroloquinoline Quinone (PQQ) Enables Photocatalytic Radical Cyclizations. Angew. Chem. Int. Ed. 2025, 64, e202505431. [Google Scholar] [CrossRef]
- Chang, L.; Cui, H.; Liu, W.; Job Zhang, Y.H.P.; Zhang, L. Electrodriven ATP synthesis via a reconstructed thylakoid membrane. Angew. Chem. Int. Ed. 2025, 64, e202421120. [Google Scholar] [CrossRef] [PubMed]
- Burai, T.N.; Panay, A.J.; Zhu, H.; Lian, T.; Lutz, S. Light-driven, quantum dot-mediated regeneration of FMN to drive reduction of ketoisophorone by old yellow enzyme. ACS Catal. 2012, 2, 667–670. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X.; Willner, I. Integration of photoswitchable proteins, photosynthetic reaction centers and semiconductor/biomolecule hybrids with electrode supports for optobioelectronic applications. Adv. Mater. 2013, 25, 349–377. [Google Scholar] [CrossRef]
- Liu, W.; Wang, P. Cofactor regeneration for sustainable enzymatic biosynthesis. Biotechnol. Adv. 2007, 25, 369–384. [Google Scholar] [CrossRef]
- Nambiar, K.P.; Stauffer, D.M.; Kolodziej, P.A.; Benner, S.A. A mechanistic basis for the stereoselectivity of enzymic transfer of hydrogen from nicotinamide cofactors. J. Am. Chem. Soc. 1983, 105, 5886–5890. [Google Scholar] [CrossRef]
- Lauterbach, L.; Lenz, O.; Vincent, K.A. H2-driven cofactor regeneration with NAD (P)+-reducing hydrogenases. FEBS J. 2013, 280, 3058–3068. [Google Scholar] [CrossRef] [PubMed]
- Baumer, B.; Classen, T.; Pohl, M.; Pietruszka, J. Efficient nicotinamide adenine dinucleotide phosphate [NADP (H)] recycling in closed-loop continuous flow biocatalysis. Adv. Synth. Catal. 2020, 362, 2894–2901. [Google Scholar] [CrossRef]
- Roth, J.P.; Lovell, S.; Mayer, J.M. Intrinsic barriers for electron and hydrogen atom transfer reactions of biomimetic iron complexes. J. Am. Chem. Soc. 2000, 122, 5486–5498. [Google Scholar] [CrossRef]
- Soldevila-Barreda, J.J.; Fawibe, K.B.; Azmanova, M.; Rafols, L.; Pitto-Barry, A.; Eke, U.B.; Barry, N.P. Synthesis, characterisation and in vitro anticancer activity of catalytically active indole-based half-sandwich complexes. Molecules 2020, 25, 4540. [Google Scholar] [CrossRef]
- Jayathilake, B.S.; Bhattacharya, S.; Vaidehi, N.; Narayanan, S. Efficient and selective electrochemically driven enzyme-catalyzed reduction of carbon dioxide to formate using formate dehydrogenase and an artificial cofactor. Acc. Chem. Res. 2019, 52, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Kuk, S.K.; Singh, R.K.; Nam, D.H.; Singh, R.; Lee, J.K.; Park, C.B. Photoelectrochemical reduction of carbon dioxide to methanol through a highly efficient enzyme cascade. Angew. Chem. Int. Ed. 2017, 56, 3827–3832. [Google Scholar] [CrossRef]
- Brown, K.A.; Wilker, M.B.; Boehm, M.; Hamby, H.; Dukovic, G.; King, P.W. Photocatalytic regeneration of nicotinamide cofactors by quantum dot–enzyme biohybrid complexes. ACS Catal. 2016, 6, 2201–2204. [Google Scholar] [CrossRef]
- Ortiz de Montellano, P.R. Hydrocarbon hydroxylation by cytochrome P450 enzymes. Chem. Rev. 2010, 110, 932–948. [Google Scholar] [CrossRef]
- Campbell, A.S.; Jeong, Y.J.; Geier, S.M.; Koepsel, R.R.; Russell, A.J.; Islam, M.F. Membrane/mediator-free rechargeable enzymatic biofuel cell utilizing graphene/single-wall carbon nanotube cogel electrodes. ACS Appl. Mater. Interfaces 2015, 7, 4056–4065. [Google Scholar] [CrossRef]
- Dinh, T.H.; Lee, S.C.; Hou, C.Y.; Won, K. Diaphorase-viologen conjugates as bioelectrocatalysts for NADH regeneration. J. Electrochem. Soc. 2016, 163, H440. [Google Scholar] [CrossRef]
- Chen, X.; Cui, Y.; Feng, J.; Wang, Y.; Liu, X.; Wu, Q.; Zhu, D.; Ma, Y. Flavin oxidoreductase-mediated regeneration of nicotinamide adenine dinucleotide with dioxygen and catalytic amount of flavin mononucleotide for one-pot multi-enzymatic preparation of ursodeoxycholic acid. Adv. Synth. Catal. 2019, 361, 2497–2504. [Google Scholar] [CrossRef]
- Ganesan, V.; Sivanesan, D.; Yoon, S. Correlation between the structure and catalytic activity of [Cp* Rh (substituted bipyridine)] complexes for NADH regeneration. Inorg. Chem. 2017, 56, 1366–1374. [Google Scholar] [CrossRef]
- Sun, F.; Chong, R.; Yang, J.; Gao, S.; Meng, Z.; Liu, W. Activated-carbon supported two dimensional covalent organic frameworks for high-efficient coenzyme photo-regeneration. J. Environ. Chem. Eng. 2025, 13, 115142. [Google Scholar] [CrossRef]
- Choi, W.S.; Chisholm, M.F.; Singh, D.J.; Choi, T.; Jellison Jr, G.E.; Lee, H.N. Wide bandgap tunability in complex transition metal oxides by site-specific substitution. Nat. Commun. 2012, 3, 689. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.S.; Martin, A.; Thomas, B.; Li, A.; Dorn, R.W.; Gong, J.; Rossini, A.J.; Thuo, M.M. Synthesis of interface-driven tunable bandgap metal oxides. ACS Mater. Lett. 2020, 2, 1211–1217. [Google Scholar] [CrossRef]
- Li, X.-B.; Tung, C.-H.; Wu, L.-Z. Semiconducting quantum dots for artificial photosynthesis. Nat. Rev. Chem. 2018, 2, 160–173. [Google Scholar] [CrossRef]
- Skoog, S.; Elam, J.; Narayan, R. Atomic layer deposition: Medical and biological applications. Int. Mater. Rev. 2013, 58, 113–129. [Google Scholar] [CrossRef]
- Jenkins, P.A.; Boland, S.; Kavanagh, P.; Leech, D. Evaluation of performance and stability of biocatalytic redox films constructed with different copper oxygenases and osmium-based redox polymers. Bioelectrochemistry 2009, 76, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Garcia, L.; Casadevall, C. Bioinspired photocatalytic systems towards compartmentalized artificial photosynthesis. Commun. Chem. 2023, 6, 263. [Google Scholar] [CrossRef]
- Zhang, N.; Trépout, S.; Chen, H.; Li, M.-H. AIE polymer micelle/vesicle photocatalysts combined with native enzymes for aerobic photobiocatalysis. J. Am. Chem. Soc. 2022, 145, 288–299. [Google Scholar] [CrossRef]
- Kim, J.; Um, Y.; Han, S.; Hilberath, T.; Kim, Y.H.; Hollmann, F.; Park, C.B. Unbiased photoelectrode interfaces for solar coupling of lignin oxidation with biocatalytic C= C bond hydrogenation. ACS Appl. Mater. Interfaces 2022, 14, 11465–11473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shi, J.; Sun, Y.; Wu, Y.; Zhang, Y.; Cai, Z.; Chen, Y.; You, C.; Han, P.; Jiang, Z. Artificial thylakoid for the coordinated photoenzymatic reduction of carbon dioxide. ACS Catal. 2019, 9, 3913–3925. [Google Scholar] [CrossRef]
- Singh, R.; Musameh, M.; Gao, Y.; Ozcelik, B.; Mulet, X.; Doherty, C.M. Stable MOF@ enzyme composites for electrochemical biosensing devices. J. Mater. Chem. C 2021, 9, 7677–7688. [Google Scholar] [CrossRef]
- Zou, X.; Zhu, C.; Wang, Q.; Yang, G. Catalytic dehydration of hexose sugars to 5-hydroxymethylfural. Biofuels Bioprod. Biorefining 2019, 13, 153–173. [Google Scholar] [CrossRef]
- Mittal, A.; Black, S.K.; Vinzant, T.B.; O’Brien, M.; Tucker, M.P.; Johnson, D.K. Production of furfural from process-relevant biomass-derived pentoses in a biphasic reaction system. ACS Sustain. Chem. Eng. 2017, 5, 5694–5701. [Google Scholar] [CrossRef]
- Dargó, G.; Kis, D.; Ráduly, A.; Farkas, V.; Kupai, J. Furandicarboxylic Acid (FDCA): Electrosynthesis and Its Facile Recovery From Polyethylene Furanoate (PEF) via Depolymerization. ChemSusChem 2025, 18, e202401190. [Google Scholar] [CrossRef]
- Qin, Y.-Z.; Li, Y.-M.; Zong, M.-H.; Wu, H.; Li, N. Enzyme-catalyzed selective oxidation of 5-hydroxymethylfurfural (HMF) and separation of HMF and 2, 5-diformylfuran using deep eutectic solvents. Green Chem. 2015, 17, 3718–3722. [Google Scholar] [CrossRef]
- McKenna, S.; Mines, P.; Law, P.; Kovacs-Schreiner, K.; Birmingham, W.; Turner, N.; Leimkühler, S.; Carnell, A. The continuous oxidation of HMF to FDCA and the immobilisation and stabilisation of periplasmic aldehyde oxidase (PaoABC). Green Chem. 2017, 19, 4660–4665. [Google Scholar] [CrossRef]
- Seel, C.J.; Gulder, T. Biocatalysis fueled by light: On the versatile combination of photocatalysis and enzymes. ChemBioChem 2019, 20, 1871–1897. [Google Scholar] [CrossRef]
- Thiel, D.; Doknić, D.; Deska, J. Enzymatic aerobic ring rearrangement of optically active furylcarbinols. Nat. Commun. 2014, 5, 5278. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.R.; Cox, J.H.; Chiappini, N.D.; Roos, C.B.; McLoughlin, E.A.; Hejna, B.G.; Nguyen, S.T.; Ripberger, H.H.; Ganley, J.M.; Tsui, E. Photochemical and electrochemical applications of proton-coupled electron transfer in organic synthesis. Chem. Rev. 2021, 122, 2017–2291. [Google Scholar] [CrossRef] [PubMed]
- Lessa, M.D.; Stoyanov, S.R.; de Carneiro, J.W.M.; da Costa, L.M. Density functional theory investigation of the contributions of π-π stacking and hydrogen bonding with water to the supramolecular aggregation interactions of model asphaltene heterocyclic compounds. J. Mol. Model. 2024, 30, 145. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.R.; Lin, S.; Jacobsen, E.N. The Cation–π Interaction in Small-Molecule Catalysis. Angew. Chem. Int. Ed. 2016, 55, 12596–12624. [Google Scholar] [CrossRef]
- Troiano, D.; Orsat, V.; Dumont, M.-J. Status of biocatalysis in the production of 2,5-furandicarboxylic acid. ACS Catal. 2020, 10, 9145–9169. [Google Scholar] [CrossRef]
- Cadoux, C.; Milton, R.D. Recent enzymatic electrochemistry for reductive reactions. ChemElectroChem 2020, 7, 1974–1986. [Google Scholar] [CrossRef]
- Trapasso, G.; Mazzi, G.; Chícharo, B.; Annatelli, M.; Dalla Torre, D.; Aricò, F. Multigram synthesis of pure HMF and BHMF. Org. Process Res. Dev. 2022, 26, 2830–2838. [Google Scholar] [CrossRef]
- Wang, H.; Li, Q.; Kuang, X.; Xiao, D.; Han, X.; Hu, X.; Li, X.; Ma, M. Functions of aldehyde reductases from Saccharomyces cerevisiae in detoxification of aldehyde inhibitors and their biotechnological applications. Appl. Microbiol. Biotechnol. 2018, 102, 10439–10456. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zong, M.-H.; Li, N. Identifying a robust alcohol dehydrogenase for developing one-pot chemobiocatalytic route toward 2, 5-bis (hydroxymethyl) furan from glucose. Mol. Catal. 2025, 573, 114813. [Google Scholar] [CrossRef]
- Pan, X.; Wang, X.; Wu, S.; Xu, L.; Zhang, L.; Zhang, Z.; Li, B.; He, X.; Chang, S. Insights into the molecular mechanism of a new efficient whole-cell biocatalyst Enterobacter ludwigii YYP3 in 5-hydroxymethylfurfural reduction. Green Chem. 2022, 24, 8691–8704. [Google Scholar] [CrossRef]
- Aranha, D.J.; Gogate, P.R. A review on green and efficient synthesis of 5-hydroxymethylfurfural (HMF) and 2, 5-furandicarboxylic acid (FDCA) from sustainable biomass. Ind. Eng. Chem. Res. 2023, 62, 3053–3078. [Google Scholar] [CrossRef]
- Monti, D.; Ottolina, G.; Carrea, G.; Riva, S. Redox reactions catalyzed by isolated enzymes. Chem. Rev. 2011, 111, 4111–4140. [Google Scholar] [CrossRef]
- Osuna, S.; Jimenez-Oses, G.; Noey, E.L.; Houk, K. Molecular dynamics explorations of active site structure in designed and evolved enzymes. Acc. Chem. Res. 2015, 48, 1080–1089. [Google Scholar] [CrossRef]
- Selvaraj, C.; Rudhra, O.; Alothaim, A.S.; Alkhanani, M.; Singh, S.K. Structure and chemistry of enzymatic active sites that play a role in the switch and conformation mechanism. Adv. Protein Chem. Struct. Biol. 2022, 130, 59–83. [Google Scholar] [PubMed]
- Giannakoudakis, D.A.; Colmenares, J.C.; Tsiplakides, D.; Triantafyllidis, K.S. Nanoengineered electrodes for biomass-derived 5-hydroxymethylfurfural electrocatalytic oxidation to 2, 5-furandicarboxylic acid. ACS Sustain. Chem. Eng. 2021, 9, 1970–1993. [Google Scholar] [CrossRef]
- Li, Q.; Metthew Lam, L.; Xun, L. Biochemical characterization of ethanol-dependent reduction of furfural by alcohol dehydrogenases. Biodegradation 2011, 22, 1227–1237. [Google Scholar] [CrossRef]
- Aamer, E.; Thöming, J.; Baune, M.; Reimer, N.; Dringen, R.; Romero, M.; Bösing, I. Influence of electrode potential, pH and NAD+ concentration on the electrochemical NADH regeneration. Sci. Rep. 2022, 12, 16380. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, B.; Liang, H. Rhodium-based MOF-on-MOF difunctional core–shell nanoreactor for NAD (P) H regeneration and enzyme directed immobilization. ACS Appl. Mater. Interfaces 2023, 15, 3442–3454. [Google Scholar] [CrossRef]
- Zhao, G.; Yang, C.; Meng, W.; Huang, X. Recent advances in porous materials for photocatalytic NADH regeneration. J. Mater. Chem. A 2024, 12, 3209–3229. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ishizaka, T.; Kawanami, H. Reductive amination of furfural to furfurylamine using aqueous ammonia solution and molecular hydrogen: An environmentally friendly approach. Green Chem. 2016, 18, 487–496. [Google Scholar] [CrossRef]
- Ni, J.; Li, Q.; Gong, L.; Liao, X.-L.; Zhang, Z.-J.; Ma, C.; He, Y. Highly efficient chemoenzymatic cascade catalysis of biomass into furfurylamine by a heterogeneous shrimp shell-based chemocatalyst and an ω-transaminase biocatalyst in deep eutectic solvent–water. ACS Sustain. Chem. Eng. 2021, 9, 13084–13095. [Google Scholar] [CrossRef]
- Zhu, L.; Di, J.; Li, Q.; He, Y.-C.; Ma, C. Enhanced conversion of corncob into furfurylamine via chemoenzymatic cascade catalysis in a toluene–water medium. J. Mol. Liq. 2023, 380, 121741. [Google Scholar] [CrossRef]
- Xue, Z.; Wu, S.; Fu, Y.; Luo, L.; Li, M.; Li, Z.; Shao, M.; Zheng, L.; Xu, M.; Duan, H. Efficient light-driven reductive amination of furfural to furfurylamine over ruthenium-cluster catalyst. J. Energy Chem. 2023, 76, 239–248. [Google Scholar] [CrossRef]
- Secundo, F. Conformational changes of enzymes upon immobilisation. Chem. Soc. Rev. 2013, 42, 6250–6261. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Chen, C.; Lv, M.; Hu, H.; Huai, L.; Zhu, B.; Fan, S.; Wang, Q.; Zhang, J. 5-Hydroxymethylfurfural and its downstream chemicals: A review of catalytic routes. Adv. Mater. 2024, 36, 2311464. [Google Scholar] [CrossRef]
- Lecona-Vargas, C.S.a.; Dumont, M.-J. Advances in continuous flow production of 5-(Hydroxymethyl) furfural, 2, 5-Furandicarboxylic acid, 2, 5-Diformylfuran, and 2, 5-Dimethylfuran. Ind. Eng. Chem. Res. 2024, 63, 16222–16246. [Google Scholar] [CrossRef]
| Cofactor | Biocatalyst | Electrode Material | Regeneration Method | Performances | References |
|---|---|---|---|---|---|
| FAD | Oxygen-Independent NADH Oxidase | CNTs | DET | High efficiency, enabling efficient catalytic cycles | [67] |
| PQQMe3 | Aldose Sugar Dehydrogenases | Solution Phase reaction | Blue-light Irradiation | 76% yield of cyclized product | [68] |
| ATP | Spinach thylakoid membrane (TMs)-enriched ATPases | Nafion membrane | Electrodriven ATP synthesis via proton gradient | Max ATP production of 8.39 μM in 120 min | [69] |
| FMN | Old Yellow Enzyme | CdSe QDs | Light-driven FMN reduction via QDs | Full reduction in FMN a few seconds | [70] |
| Strategy | Mechanism | Material | Advantages | Limitations | References |
|---|---|---|---|---|---|
| DET | Electrode injects e− directly | Carbon nanotubes, graphene | Atomically efficient, no mediator loss | Enzyme orientation sensitive, instability | [76,82] |
| MET | e− mediators shuttle e− to NAD(P)+ | Methyl viologen, Rh-complex, COFs | Broad enzyme applicability | Mediator loss, side reactions | [83,84,85,86] |
| Artificial Cofactors | Synthetic analogs mimic NADH | BNAH, mNADH | Bypass natural NADH instability | Cost, not fully biocompatible | [62,63] |
| Enzymatic Cofactor Recycling | Secondary enzyme regenerates NADH | FDH | High turnover frequency | Requires sacrificial donors | [78] |
| Substrate | Target Product | Enzyme | Photo-/Electrocatalyst | Regeneration Strategy | Performance | Evaluation | Reference |
|---|---|---|---|---|---|---|---|
| HMF | FDCA | ADH, lipase | CdS@MXene | ROS-mediated oxidation | Yield ~90% | High sel., TRL4 | [64,100,101] |
| HMF | BHMF | ADH | Carbon electrode | Electro-NADH regeneration | Yield > 99% | >99% yield, low energy, TRL4 | [110,111,112] |
| HMF | DFF | Oxidase | Photoelectrode mediator | O2/ROS | Selectivity > 80% | 80% sel., O2 sensitive, TRL3 | [100,103,117] |
| Furfural | Furfuryl alcohol | ADH | Carbonaceous cathode | Mediator-free DET | High selectivity | High sel., mediator-free, TRL4 | [58,118] |
| Furfural | Furoic acid | Oxidase | Hybrid photocatalyst | ROS-mediated oxidation | Faradaic efficiency 70–85% | By-products, TRL3 | [117,122] |
| Furfural | Furfurylamine | Transaminase cascade | Ru-cluster photocatalyst | Light-driven tandem | High yield | High yield, costly Ru, TRL3 | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Liu, X.; Guo, B.; Qi, Y.; Lv, X.; Wang, B.; Cai, D. Hybrid Biocatalysis with Photoelectrocatalysis for Renewable Furan Derivatives’ Valorization: A Review. Photochem 2025, 5, 35. https://doi.org/10.3390/photochem5040035
Zheng S, Liu X, Guo B, Qi Y, Lv X, Wang B, Cai D. Hybrid Biocatalysis with Photoelectrocatalysis for Renewable Furan Derivatives’ Valorization: A Review. Photochem. 2025; 5(4):35. https://doi.org/10.3390/photochem5040035
Chicago/Turabian StyleZheng, Shize, Xiangshi Liu, Bingqian Guo, Yanou Qi, Xifeng Lv, Bin Wang, and Di Cai. 2025. "Hybrid Biocatalysis with Photoelectrocatalysis for Renewable Furan Derivatives’ Valorization: A Review" Photochem 5, no. 4: 35. https://doi.org/10.3390/photochem5040035
APA StyleZheng, S., Liu, X., Guo, B., Qi, Y., Lv, X., Wang, B., & Cai, D. (2025). Hybrid Biocatalysis with Photoelectrocatalysis for Renewable Furan Derivatives’ Valorization: A Review. Photochem, 5(4), 35. https://doi.org/10.3390/photochem5040035








