Efficient Room-Temperature Luminescence of Indole-5-Carboxamide in Poly(vinyl alcohol) Films
Abstract
1. Introduction
2. Materials and Methods
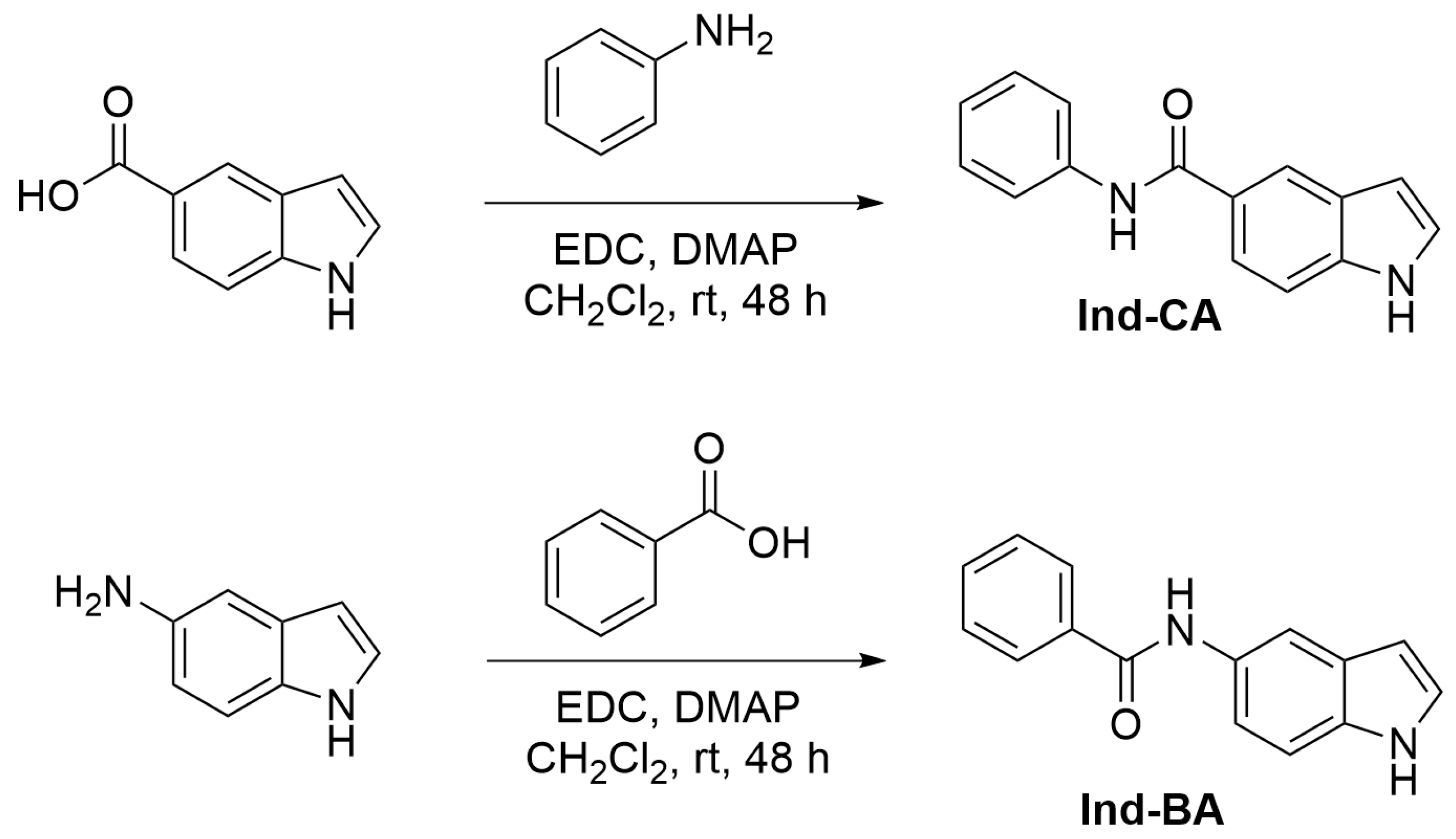
2.1. Synthesis
2.2. PVA Film Preparation
2.3. Absorption Measurements
2.4. Steady-State Fluorescence Measurements
2.5. Fluorescence Anisotropy Measurements
2.6. Fluorescence Quantum Yield Measurements
2.7. Fluorescence Lifetime Measurements
2.8. Phosphorescence Spectra Measurements
2.9. Phosphorescence Lifetime Measurements
3. Results and Discussion
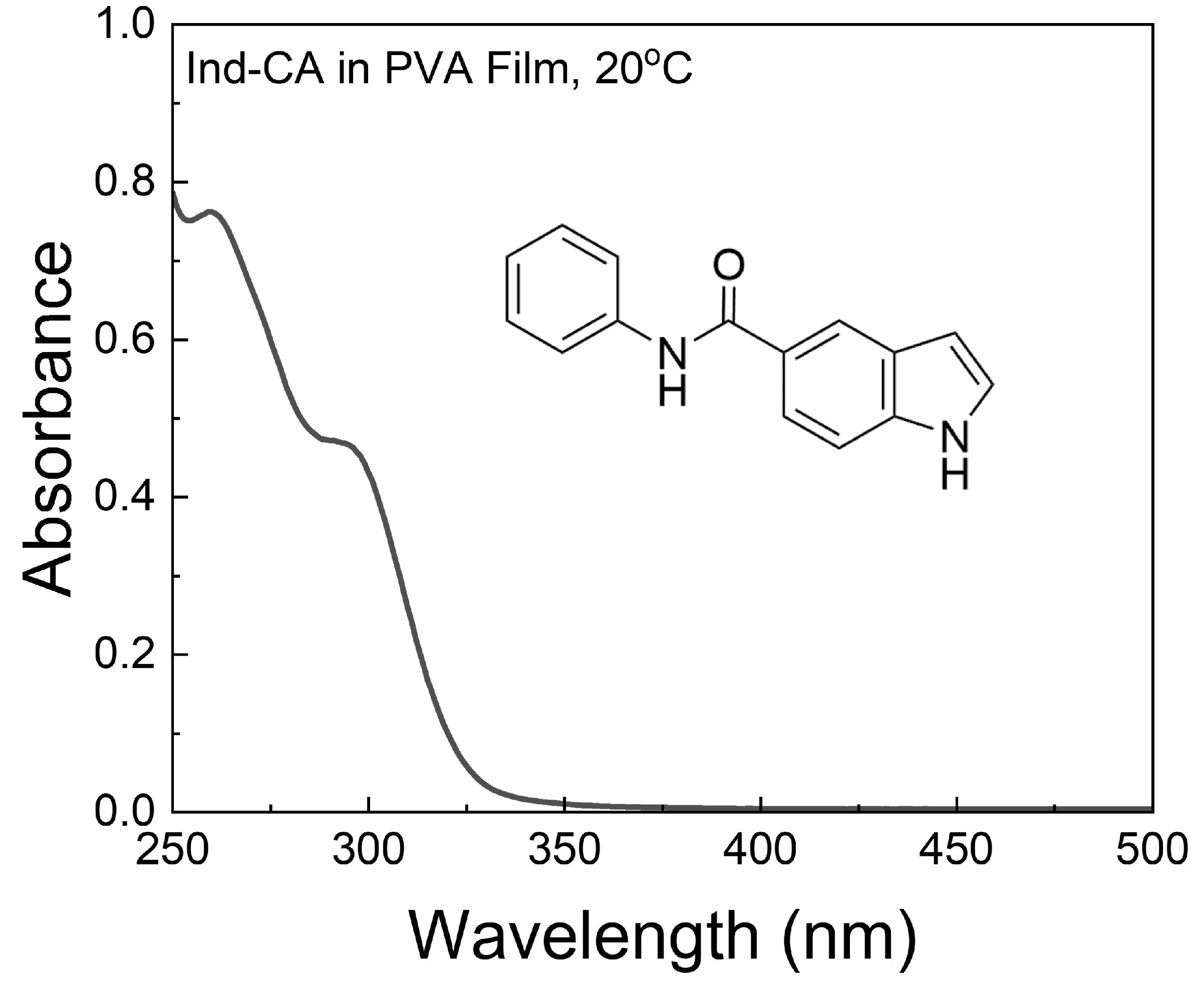
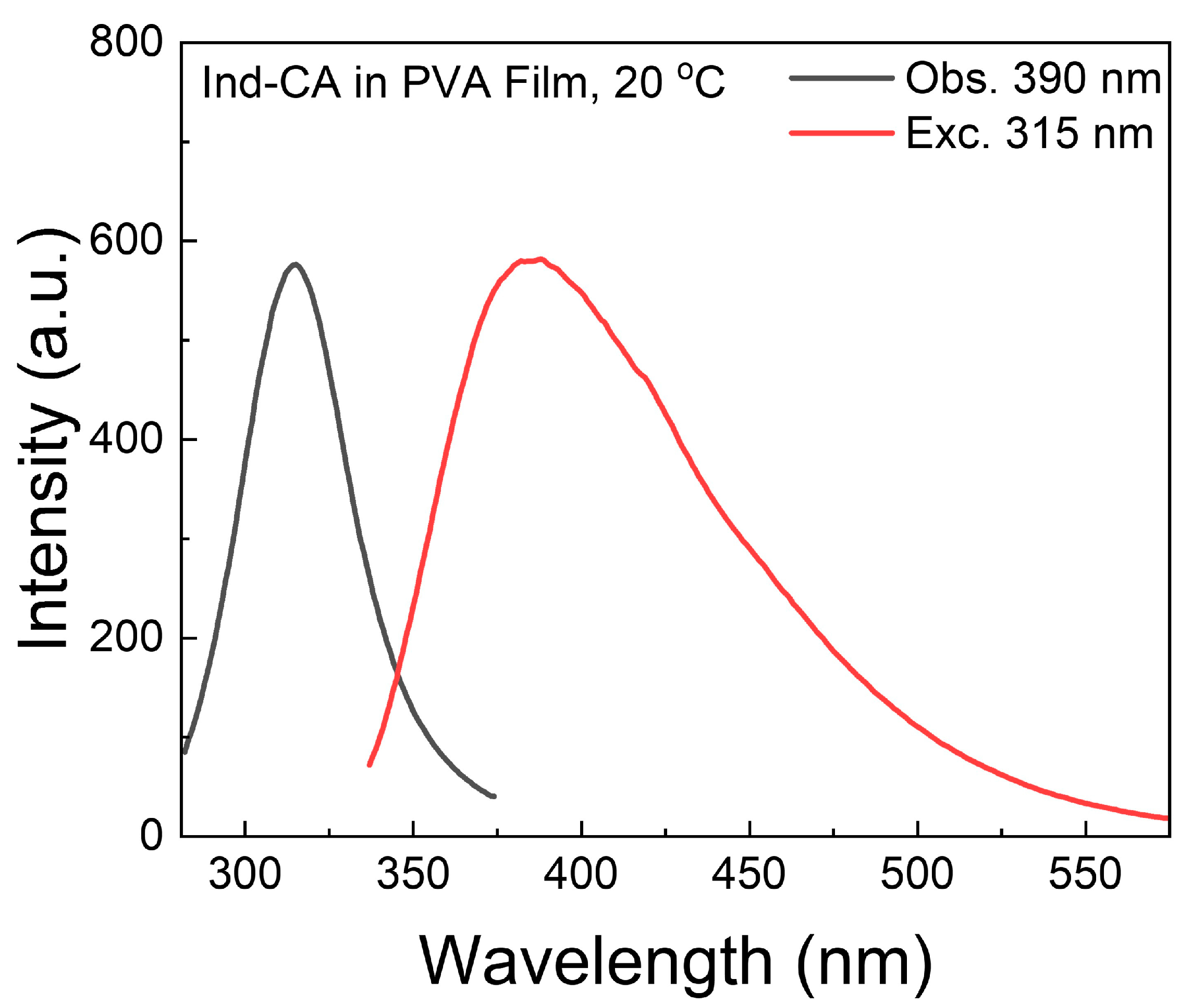
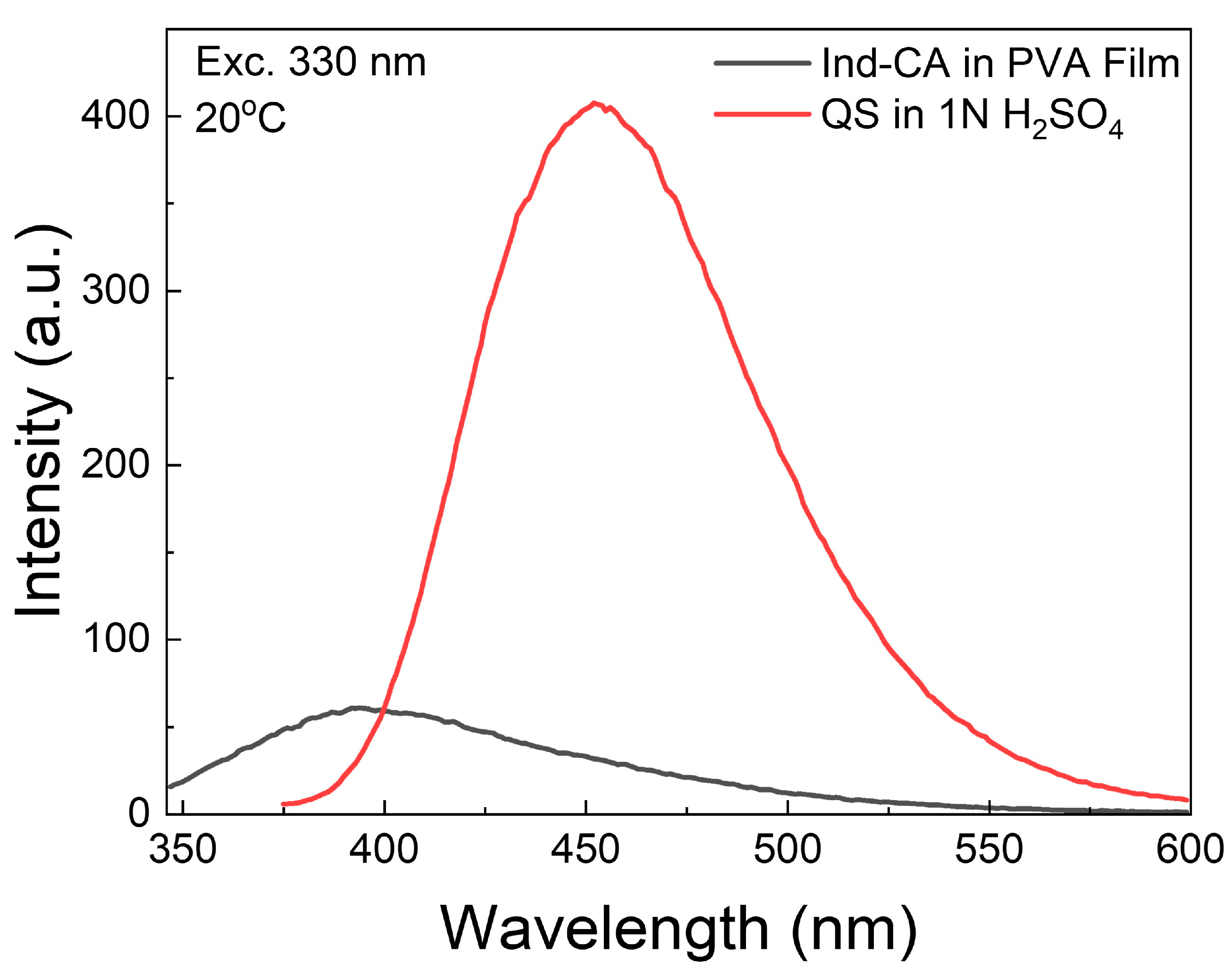
3.1. Absorption and Fluorescence Spectra of Ind-CA
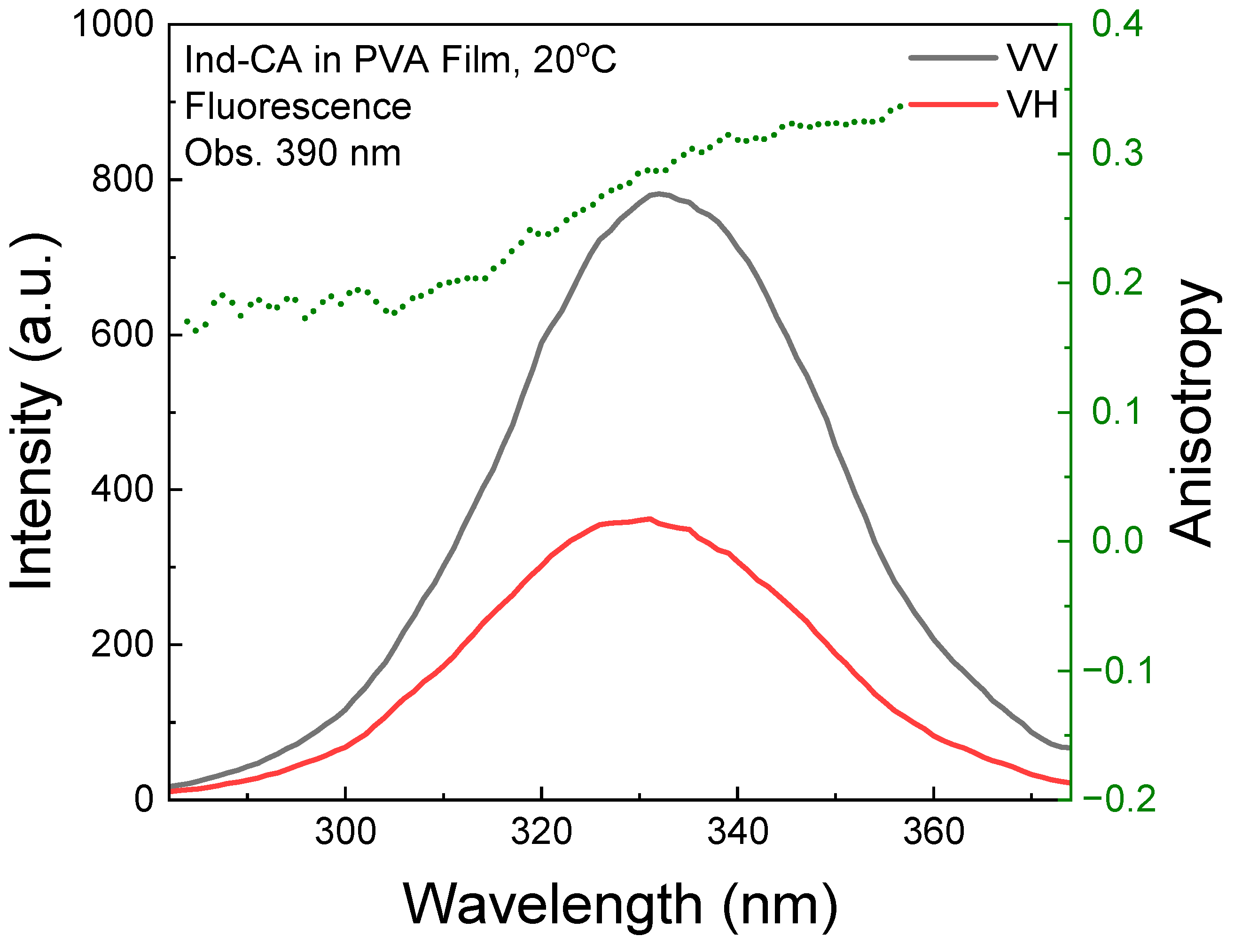
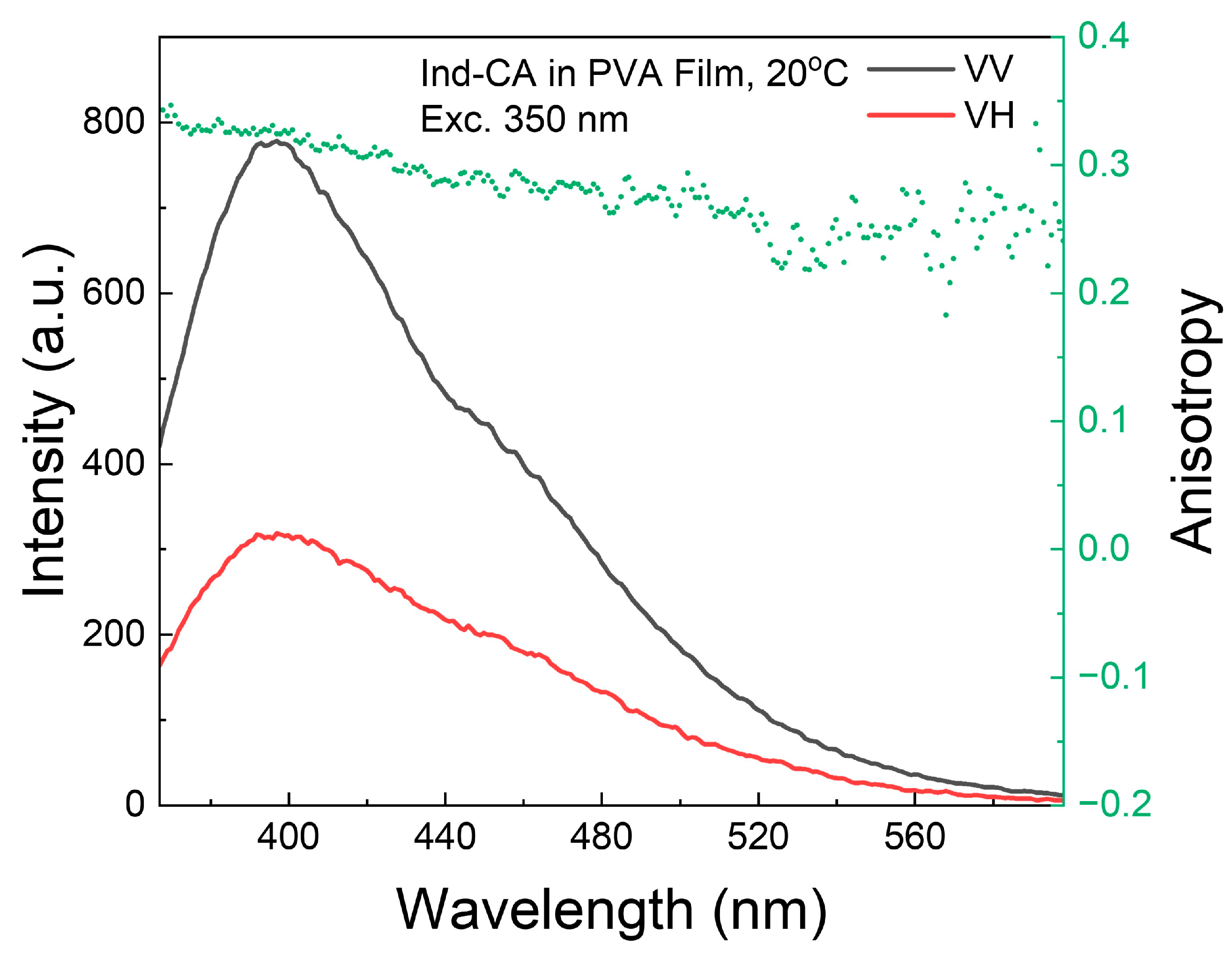
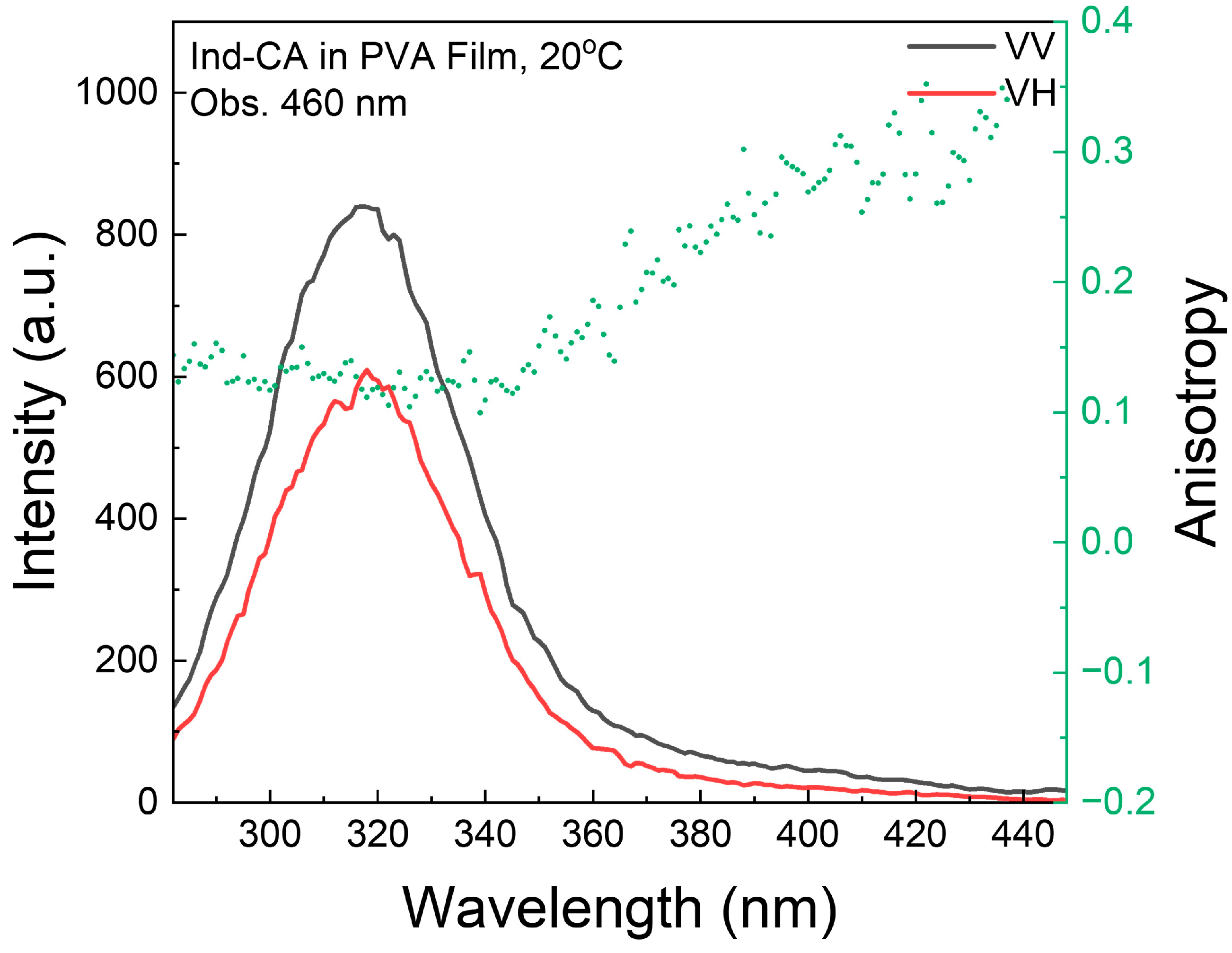
3.2. Fluorescence Anisotropy
3.3. Fluorescence Quantum Yield
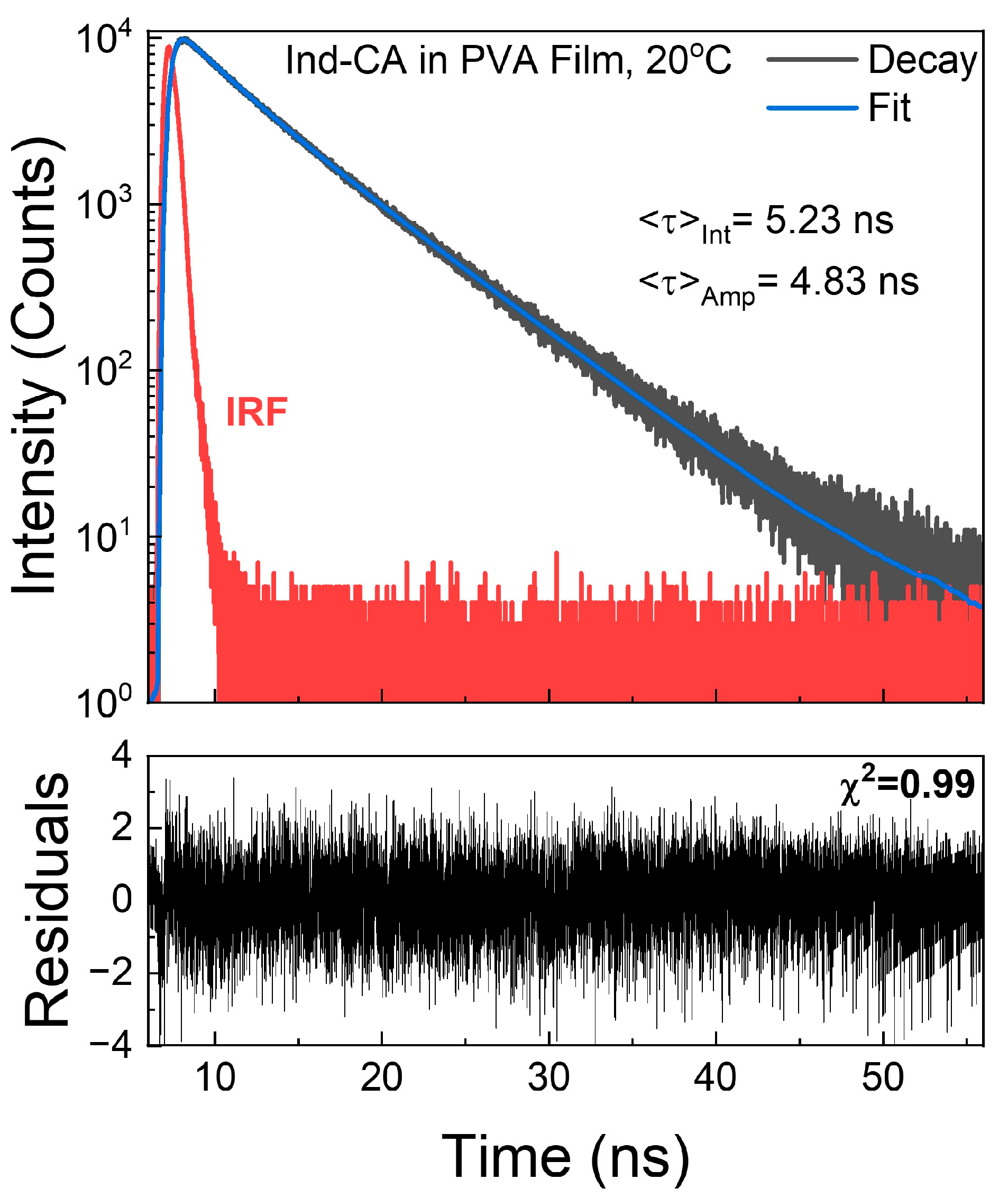
3.4. Fluorescence Lifetime
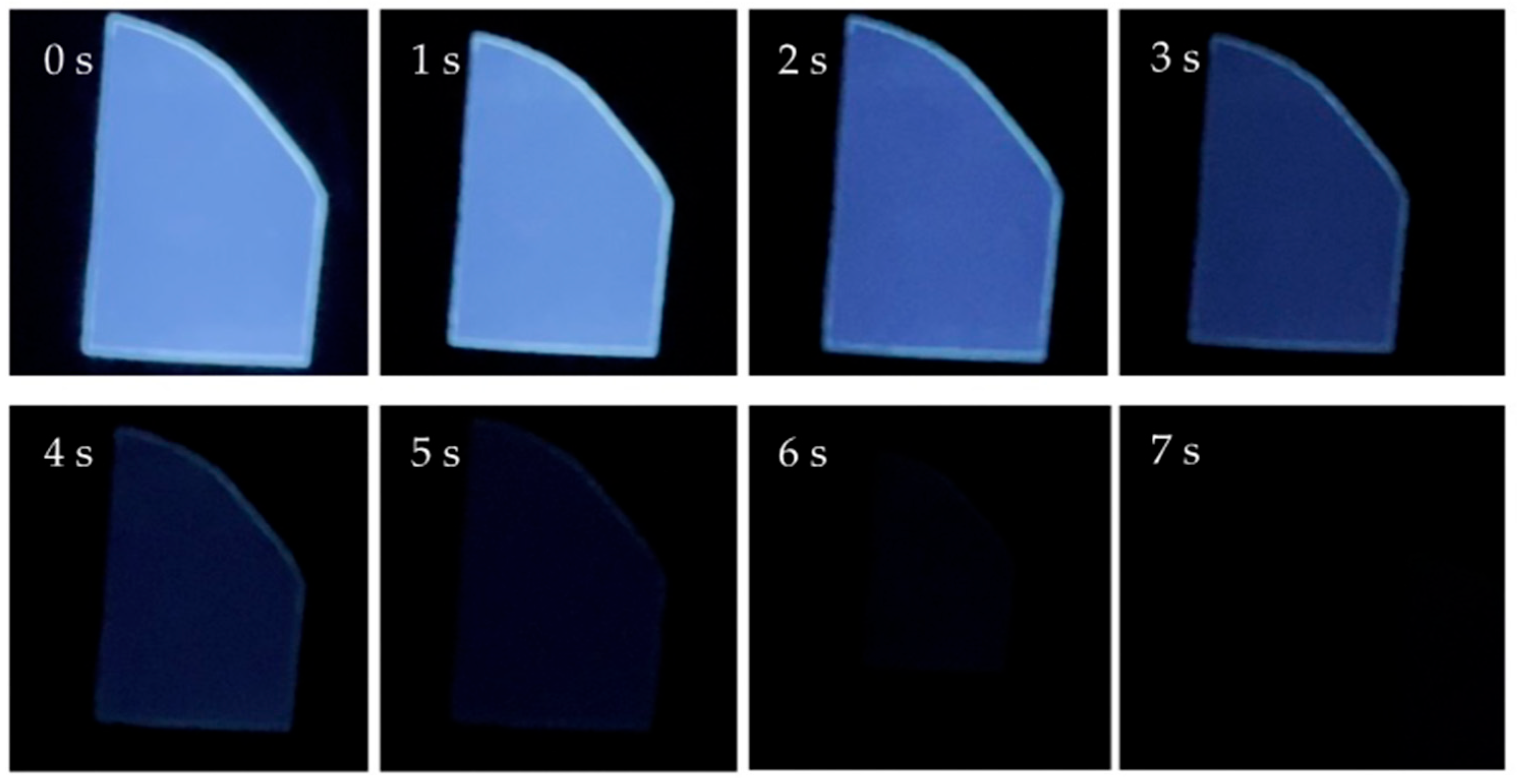
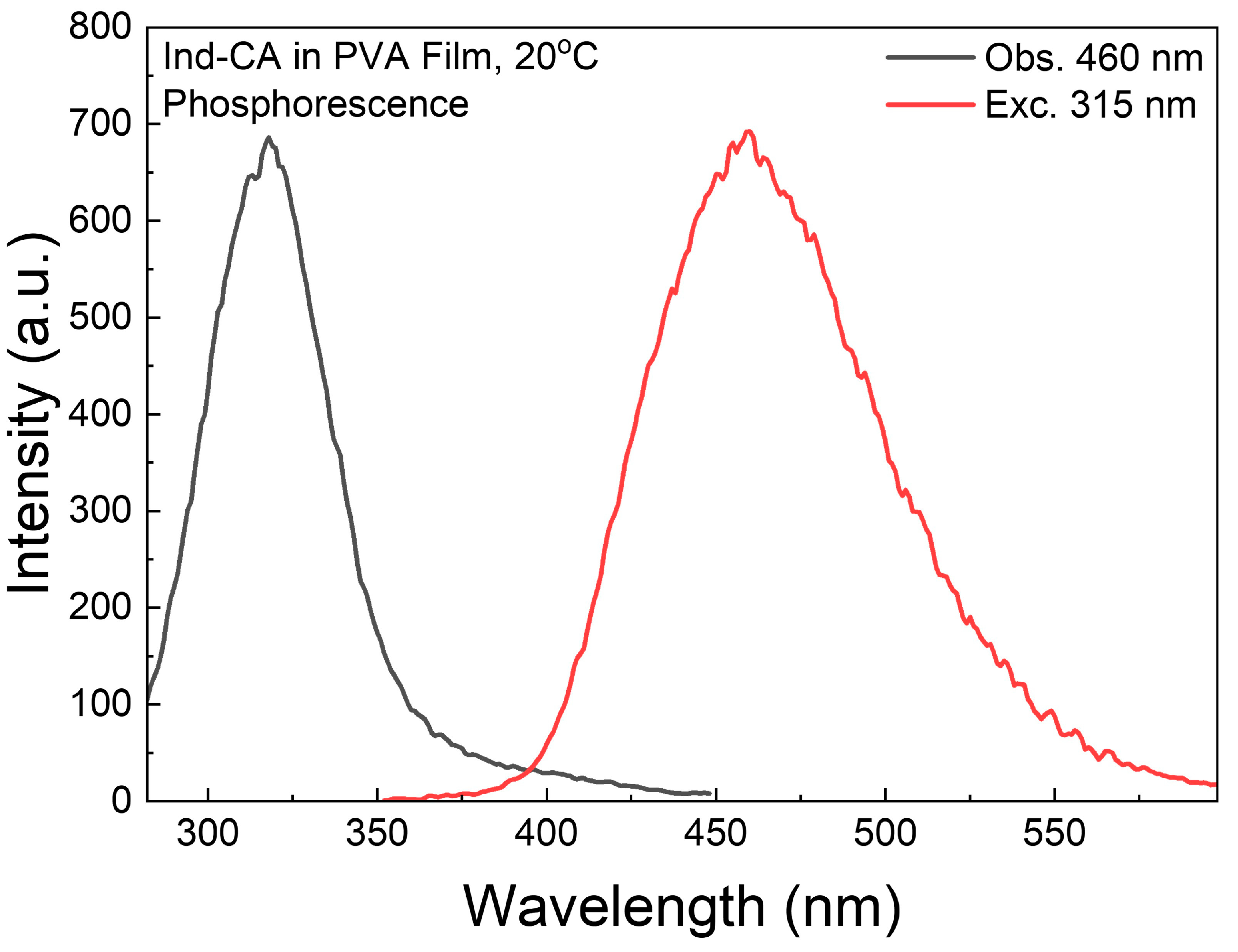
3.5. Room-Temperature Phosphorescence
3.6. Phosphorescence Spectra
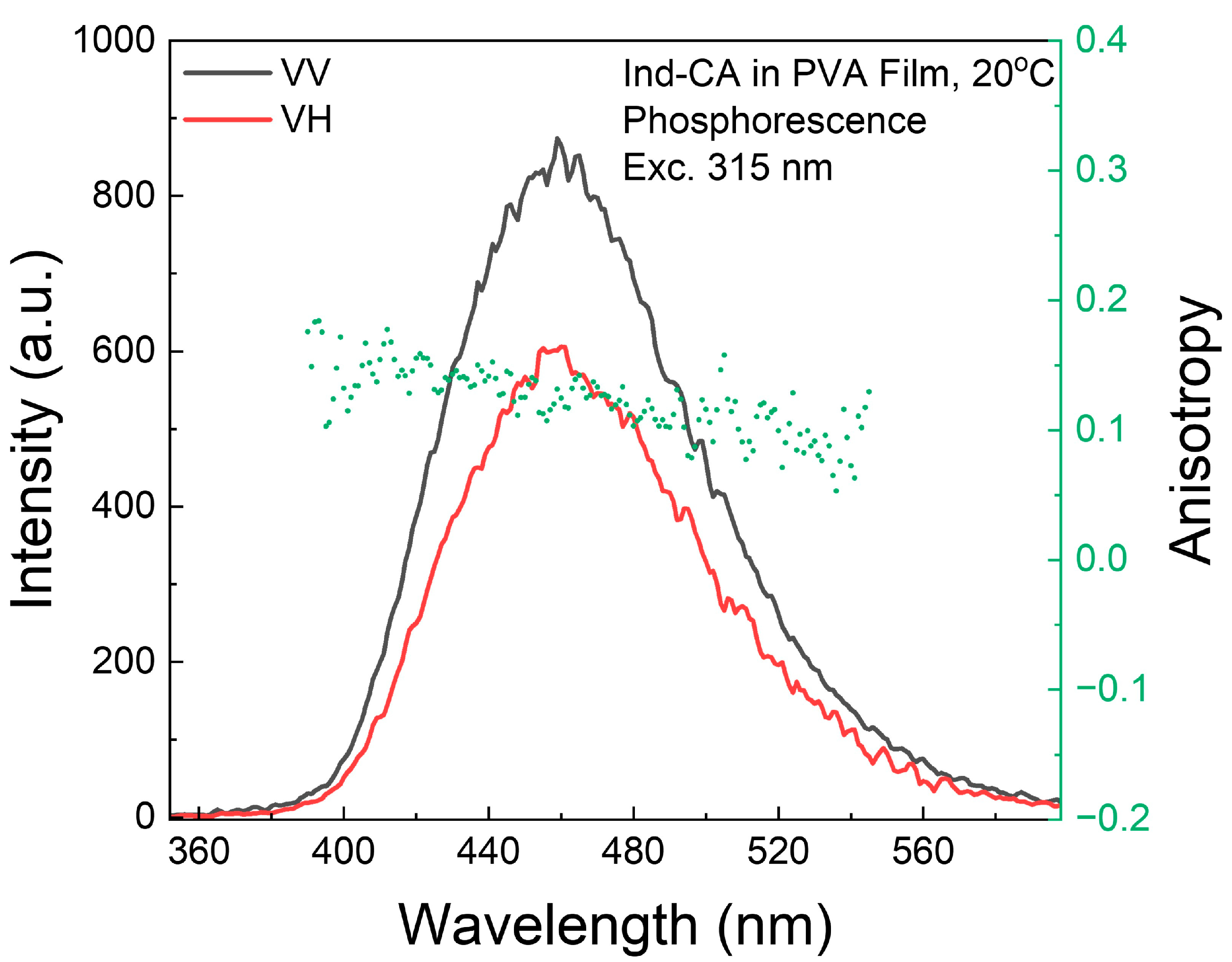
3.7. Phosphorescence Anisotropy
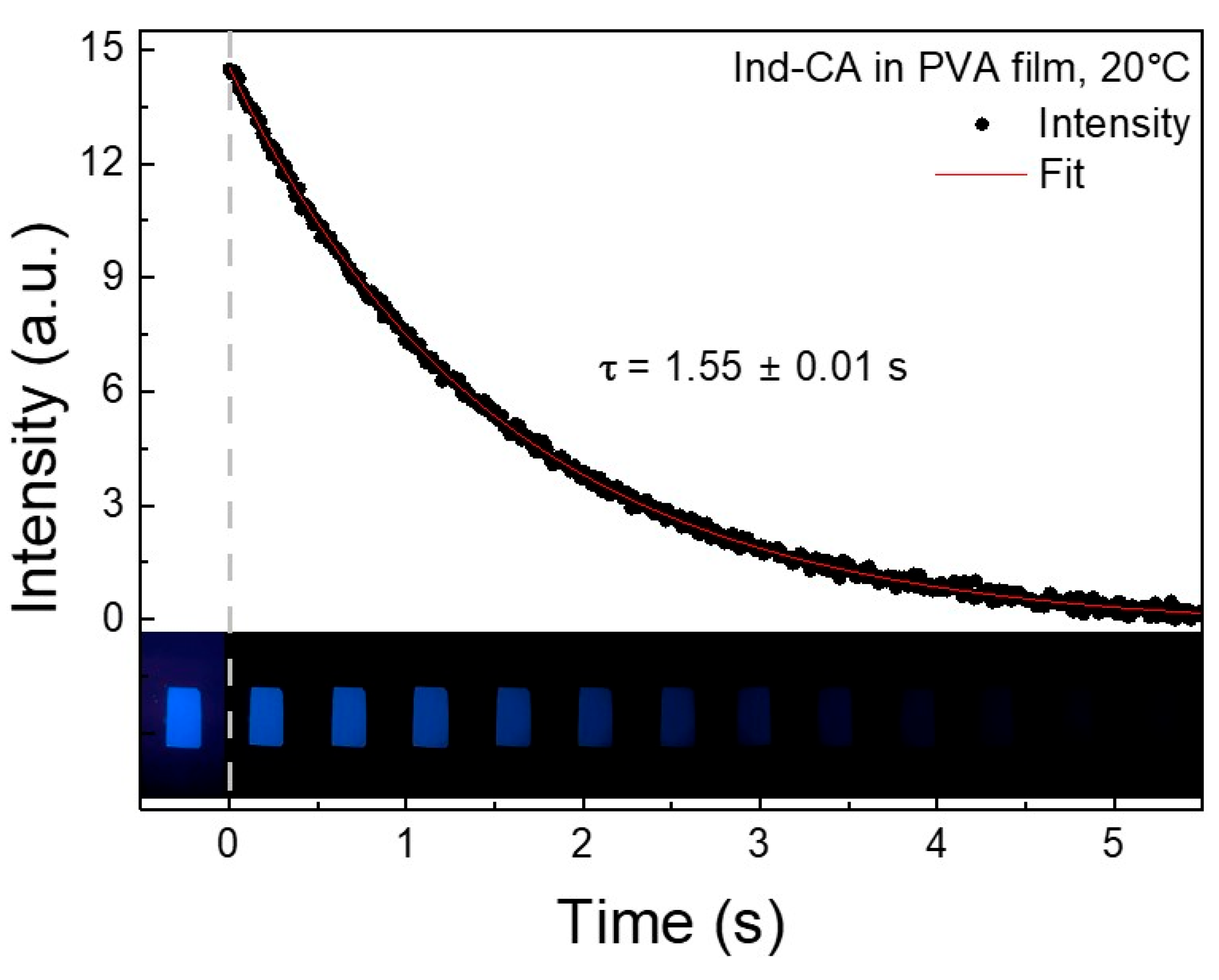
3.8. Phosphorescence Lifetime
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ind-CA | N-phenyl-1H-Indole-5-carboxamide |
| Ind-BA | N-1H-indole-5-ylbenzamide |
| PVA | Polyvinyl Alcohol |
| RTP | Room-Temperature Phosphorescence |
References
- Qiu, J.; Li, Y.; Jia, Y. Persistent Phosphors: From Fundamentals to Applications; Woodhead Publishing: Cambridge, UK, 2020. [Google Scholar]
- Gao, R.; Yan, D.; Evans, D.G.; Duan, X. Layer-by-layer assembly of long-afterglow self-supporting thin films with dual-stimuli-responsive phosphorescence and antiforgery applications. Nano Res. 2017, 10, 3606–3617. [Google Scholar] [CrossRef]
- Su, Y.; Phua, S.Z.F.; Li, Y.; Zhou, X.; Jana, D.; Liu, G.; Zhao, Y. Ultralong room temperature phosphorescence from amorphous organic materials toward confidential information encryption and decryption. Sci. Adv. 2018, 4, eaas9732. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, S.; Lin, W.; Zhang, K.Y.; Lv, W.; Huang, X.; Huo, F.; Yang, H.; Jenkins, G.; Zhao, Q.; et al. Smart responsive phosphorescent materials for data recording and security protection. Nat. Commun. 2014, 5, 3601. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fang, M.; Li, Z. Stimulus-responsive room temperature phosphorescence materials: Internal mechanism, design strategy, and potential application. Acc. Mater. Res. 2021, 2, 644–654. [Google Scholar] [CrossRef]
- Fateminia, S.M.A.; Mao, Z.; Xu, S.; Yang, Z.; Chi, Z.; Liu, B. Organic nanocrystals with bright red persistent room-temperature phosphorescence for biological applications. Angew. Chem. Int. Ed. 2017, 56, 12160–12164. [Google Scholar] [CrossRef]
- Vanderkooi, J.; Calhoun, D.; Englander, S. On the prevalence of room-temperature protein phosphorescence. Science 1987, 236, 568–569. [Google Scholar] [CrossRef]
- Gacintov, N.; Brenner, H. The triplet state as a probe of dynamics and structure in biological macromolecules. Photochem. Photobiol. 1989, 50, 841–858. [Google Scholar] [CrossRef]
- Ceresa, L.; Chavez, J.; Kitchner, E.; Kimball, J.; Gryczynski, I.; Gryczynski, Z. Imaging and detection of long-lived fluorescence probes in presence of highly emissive and scattering background. Exp. Biol. Med. 2022, 247, 1840–1851. [Google Scholar] [CrossRef]
- Zhao, W.; He, Z.; Tang, B.Z. Room-temperature phosphorescence from organic aggregates. Nat. Rev. Mater. 2020, 5, 869–885. [Google Scholar] [CrossRef]
- Wu, Z.; Nitsch, J.; Marder, T.B. Persistent room-temperature phosphorescence from purely organic molecules and multi-component systems. Adv. Opt. Mater. 2021, 9, 2100411. [Google Scholar] [CrossRef]
- Nidhankar, A.D.; Goudappagouda; Wakchaure, W.C.; Babu, S.S. Efficient metal-free organic phoshpors. Chem. Sci. 2021, 12, 4216–4236. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dong, M.; Li, Z.; Wang, P.; Liang, F.-S. Recent advances of room-temperature phosphorescence and long persistent luminescence by doping system of purely organic molecules. Dyes Pigm. 2022, 204, 110400. [Google Scholar] [CrossRef]
- Shen, X.; Wu, W.; Yang, C. Recent progress in solid-state room temperature afterglow based on pure organic small molecules. Molecules 2024, 29, 3236. [Google Scholar] [CrossRef]
- Papp, S.; Vanderkooi, J. Tryptophan phosphorescence at room temperature as a tool to study protein structure and dynamics. Photochem. Photobiol. 1989, 49, 775–784. [Google Scholar] [CrossRef]
- Fischer, C.; Gafni, A.; Steel, D.; Schauerte, J. The triplet-state lifetime of indole in aqueous and viscous environments: Significance to the interpretation of room temperature phosphorescence in proteins. J. Am. Chem. Soc. 2002, 124, 10359–10366. [Google Scholar] [CrossRef] [PubMed]
- Schlyer, B.; Schauerte, J.; Steel, D.; Gafni, A. Time-resolved room temperature protein phosphorescence: Nonexponential decay from single emitting tryptophans. Biophys. J. 1994, 67, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Strambini, G.; Gonelli, M.; Galley, W. Room temperature phosphorescence of Trp 314 as monitor of subunit communication in alcohol dehydrogenase from horse liver. Biochemistry 1990, 29, 203–208. [Google Scholar] [CrossRef]
- Kawski, A.; Gryczynski, I.; Gryczynski, Z. Fluorescence and phosphorescence anisotropy spectra of indole in poly (vinyl alcohol) film at room temperature. Z. Naturforsch. A 1994, 49, 1091–1092. [Google Scholar] [CrossRef]
- Kowalska-Baron, A.; Chan, M.; Gałęcki, K.; Wysocki, S. Photophysics of indole, tryptophan and N-acetyl-L-tryptophanamide (NATA): Heavy atom effect. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 98, 282–289. [Google Scholar] [CrossRef]
- Kowalska-Baron, A.; Gałęcki, K.; Wysocki, S. Room temperature phosphorescence study on the structural flexibility of single tryptophan containing proteins. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 134, 380–387. [Google Scholar] [CrossRef]
- Chavez, J.; Ceresa, L.; Kitchner, E.; Kimball, J.; Shtoyko, T.; Fudala, R.; Borejdo, J.; Gryczynski, Z.; Gryczynski, I. On the possibility of direct triplet state excitation of indole. J. Photochem. Photobiol. B Biol. 2020, 208, 111897. [Google Scholar] [CrossRef] [PubMed]
- Chavez, J.; Ceresa, L.; Kimball, J.; Kitchner, E.; Gryczynski, Z.; Gryczynski, I. Room temperature luminescence of 5-chloroindole. J. Mol. Liq. 2022, 360, 119482. [Google Scholar] [CrossRef]
- Kowalska-Baron, A.; Gałęcki, K.; Wysocki, K. Photophysics of indole-2-carboxylic acid (I2C) and indole 5-carboxylic acid (I5C): Heavy atom effect. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 116, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wen, G.; Li, K.; Xiong, H.; Zhang, J.; Lu, S.; Chen, X. Room temperature phosphorescence of heavy-atom-free indole carboxylic acid/polyacrylamide: Low cost, long lifetime and good luminescence efficiency. Dyes Pigm. 2022, 205, 110481. [Google Scholar] [CrossRef]
- Ma, H.; Peng, Q.; An, Z.; Huang, W.; Shuai, Z. Efficient and long-lived room-temperature organic phosphorescence: Theoretical descriptors for molecular designs. J. Am. Chem. Soc. 2018, 141, 1010–1015. [Google Scholar] [CrossRef]
- Gu, L.; Shi, H.; Miao, C.; Wu, Q.; Cheng, Z.; Cai, S.; Gu, M.; Ma, C.; Yao, W.; Gao, Y. Prolonging the lifetime of ultralong organic phosphorescence through dihydrogen bonding. J. Mater. Chem. C. 2018, 6, 226–233. [Google Scholar] [CrossRef]
- Yao, X.; Ma, H.; Wang, X.; Wang, H.; Wang, Q.; Zou, X.; Song, Z.; Jia, W.; Li, Y.; Mao, Y. Ultralong organic phosphorescence from isolated molecules with repulsive interactions for multifunctional applications. Nat. Commun. 2022, 13, 4890. [Google Scholar] [CrossRef]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence: Principles and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- McGlynn, P.; Azumi, T.; Kinoshita, M. Molecular Spectroscopy of the Triplet State; Prentice-Hall: Englewood Cliffs, NJ, USA, 1969. [Google Scholar]
- El-Sayed, M.A.; Brewer, R.G. Polarization of the π * π and π *-n Phosphorescence Spectra of N-Heterocyclics. J. Phys. Chem. 1963, 39, 1623–1628. [Google Scholar] [CrossRef]
- Lim, E.C.; Yu, J.M.H. Vibronic Spin-Orbit Interactions in Heteroaromatic Molecules. I. Polycyclic Monazines. J. Chem. Phys. 1967, 47, 3270–3275. [Google Scholar] [CrossRef]
- Lee, D.; Bolton, O.; Kim, B.C.; Youk, J.H.; Takayama, S.; Kim, J. Room Temperature Phosphorescence of Metal-Free Organic Materials in Amorphous Polymer Matrices. J. Am. Chem. Soc. 2013, 135, 6325–6329. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, G.; Liu, Z.-W.; Bian, Z.-Q.; Huang, C.-H. Room-temperature phosphorescence from purely organic materials. Chin. Chem. Lett. 2016, 27, 1231–1240. [Google Scholar] [CrossRef]
- Cai, S.; Ma, H.; Shi, H.; Wang, H.; Wang, X.; Xiao, L.; Ye, W.; Huang, K.; Cao, X.; Gan, N.; et al. Enabling long-lived organic room temperature phosphorescence in polymers by subunit interlocking. Nat. Commun. 2019, 10, 4247. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Gao, H.; Zhang, S.; Zheng, S.; Wang, Y.; Zhao, Z.; Ding, D.; Yang, B.; Zhang, Y.; Yuan, W.Z. Achieving persistent, efficient, and robust room-temperature phosphorescence from pure organics for versatile applications. Adv. Mater. 2019, 31, 1807222. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Y.; Huo, M.; Liu, Y. Phosphorescence resonance energy transfer from purely organic supramolecular assembly. Nat. Rev. Chem. 2023, 7, 854–874. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, J.-F.; Dodd, R.H. Regioselective ortho-directed metalation and electrophilic substitution of indole–And indoline-5-(N-phenyl) carboxamides. Heterocycles 2001, 55, 2289–2304. [Google Scholar] [CrossRef]
- Macor, J.E.; Blank, D.H.; Fox, C.B.; Lebel, L.A.; Newman, M.E.; Post, R.J.; Ryan, K.; Schmidt, A.W.; Schulz, D.W.; Koe, B.K. 5-[(3-Nitropyrid-2-yl) amino] indoles: Novel serotonin agonists with selectivity for the 5-HT1D receptor. Variation of the C3 substituent on the indole template leads to increased 5-HT1D receptor selectivity. J. Med. Chem. 1994, 37, 2509–2512. [Google Scholar] [CrossRef]
- Gryczynski, Z.; Gryczynski, I. Practical Fluorescence Spectroscopy; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Eaton, D.F. Reference materials for fluorescence measurement. Pure Appl. Chem. 1988, 60, 1107–1114. [Google Scholar] [CrossRef]
- Sillen, A.; Engelborghs, Y. The correct use of ‘average’ fluorescence parameters. Photochem. Photobiol. 1998, 67, 475–486. [Google Scholar] [CrossRef]
- Jameson, D.M. Introduction to Fluorescence; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Lee, B.; Gryczynski, I.; Gryczynski, Z. Demonstration of intermolecular triplet−singlet FRET in dye doped PVA films at room temperature. J. Phys. Chem. A 2025, 129, 2734–2737. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.; Jablonska, A.; Sagoo, R.; Pham, D.; Pham, T.T.; Dzyuba, S.V.; Gryczynski, Z.; Gryczynski, I. Efficient Room-Temperature Luminescence of Indole-5-Carboxamide in Poly(vinyl alcohol) Films. Photochem 2025, 5, 14. https://doi.org/10.3390/photochem5020014
Lee B, Jablonska A, Sagoo R, Pham D, Pham TT, Dzyuba SV, Gryczynski Z, Gryczynski I. Efficient Room-Temperature Luminescence of Indole-5-Carboxamide in Poly(vinyl alcohol) Films. Photochem. 2025; 5(2):14. https://doi.org/10.3390/photochem5020014
Chicago/Turabian StyleLee, Bong, Agnieszka Jablonska, Rajveer Sagoo, Danh Pham, Trang Thien Pham, Sergei V. Dzyuba, Zygmunt Gryczynski, and Ignacy Gryczynski. 2025. "Efficient Room-Temperature Luminescence of Indole-5-Carboxamide in Poly(vinyl alcohol) Films" Photochem 5, no. 2: 14. https://doi.org/10.3390/photochem5020014
APA StyleLee, B., Jablonska, A., Sagoo, R., Pham, D., Pham, T. T., Dzyuba, S. V., Gryczynski, Z., & Gryczynski, I. (2025). Efficient Room-Temperature Luminescence of Indole-5-Carboxamide in Poly(vinyl alcohol) Films. Photochem, 5(2), 14. https://doi.org/10.3390/photochem5020014






