Abstract
TiO2 thin films are promising as photocatalysts to decompose organic compounds. In this study, TiO2 thin films were deposited by reactive radio-frequency (RF) magnetron sputtering under various flow rates of oxygen and argon gas. The results show that the photocatalytic activity decreases as the oxygen-gas ratio is increased to 30% or less, while the activity increases under oxygen-rich conditions. It was observed that the crystal structure changed from anatase to a composite of anatase and rutile, where the oxygen-gas ratio during RF sputtering is more than 40%. Interestingly, the oxygen vacancy concentration increased under oxygen-rich conditions, where the oxygen-gas ratio is more than 40%. The sample prepared under the most enriched oxygen condition, 70%, among our experiments exhibited the highest concentration of oxygen vacancy and the highest photocatalytic activity. Both the oxygen vacancies and the composite of anatase and rutile structure in the TiO2 films deposited under oxygen-rich conditions are considered responsible for the enhanced photocatalysis.
1. Introduction
Thin films of photocatalysts have been intensively studied as clean technology [,,]. Photocatalytic thin films can decompose various organic compounds in a solution using energy from sunlight, and it is relatively easy to separate the catalysts from the solution. Among many photocatalysts, TiO2 is promising due to its low cost, stable availability, high durability, and less risk to the human body and environment [,,,]. There are three major TiO2 crystalline forms: anatase, rutile, and brookite. The rutile phase is the most stable in bulk at room temperature and ambient pressure.
Several techniques can be used to deposit TiO2 thin films [], such as the sol-gel method [,], spray pyrolysis [], pulsed laser deposition [], e-beam evaporation [], chemical vapor deposition [], and sputtering []. Notably, reactive radio-frequency (RF) magnetron sputtering is a promising technique with desirable features such as excellent film adhesion and uniform distribution of thickness []. It is possible to form films with various electronic states and microstructures via changing sputtering conditions, such as RF power, deposition pressure, substrate temperature, and gas flow rate. Especially, it is the characteristic of reactive sputtering that can control oxygen contents. Then, the oxygen gas ratio (RO2: [O2 gas flow rate)]/[(Ar + O2) gas flow rate]) is one of the most critical parameters for photocatalytic activity [], although the effect of the RO2 is still controversial. Zhang et al. reported that TiO2 film deposited under a high argon flow rate absorbs more light irradiation, which results in more electron–hole pairs generated in the TiO2 film and thus enhanced photocatalytic activity []. Huang et al. and Chiou et al. demonstrated that the photocatalytic activity of TiO2 films is raised with increasing oxygen flow rate to some extent but finally dropped at the high values of RO2 [,]. Furthermore, the wide range of RO2 conditions was not tested without heating substrate (see Table S1 in Supporting Information).
In this study, we synthesized TiO2 thin films via reactive RF sputtering at the condition with the RO2 values ranging from 0 to 70%. In this study, we synthesized TiO2 thin films via reactive RF sputtering with changing RO2 and examined the parameters that influence photocatalytic activity. As the RO2 was increased, the photocatalytic activity decreased with the RO2 values up to 30% and increased with the RO2 values over 40%. We investigated the oxygen vacancy concentration, and it decreased with the RO2 values up to 30–40%, which is a general behavior under the oxygen-rich conditions [,]. On the other hand, interestingly, the vacancy concentration was increased with the RO2 values of more than 40%. In addition, other film properties also showed a change in their tendency at around RO2 = 40%. These are considered to involve enhanced photocatalytic activity to decompose an organic dye. A possible explanation of the phenomena will be provided to suggest suitable deposition conditions for highly active thin-film photocatalysts.
2. Experimental Details
2.1. Synthesis of TiO2 Thin Films
TiO2 thin films were deposited on a glass slide substrate using a reactive RF magnetron sputtering method with pure titanium (Ti) target. The deposition was performed without a substrate heater, while the temperature should be slightly raised by the sputtering energy. The distance between the substrate and the target was 70 mm. The glass slide substrates with the size of 24 × 48 × 1.2 mm were purchased from Toshinriko Co. Ltd (Tokyo, Japan). The sputtering equipment is RSC-MG2 produced by CryoVac Inc. (Osaka, Japan). The target is 99.9% pure titanium provided from Kojundo Chemical Laboratory Co., Ltd., (Sakado, Japan). Before deposition, pre-sputtering with a sputtering power of 300 W was conducted to clean the surface of the Ti target under Ar gas (100 sccm). The deposition time for a sample was 2 h with a sputtering power of 300 W. During the sputtering, the total flow rate of Ar and O2 gases was 100 sccm under a pressure of 4.0 × 10−3 Pa. Eight samples were prepared with different ratios of O2 gas (RO2: [O2 gas flow rate]/[(Ar + O2) gas flow rate)] = 5%, 10%, 20%, 30%, 40%, 50%, 60%, and 70%). The following characterization methods were conducted on these samples as deposited.
2.2. Characterization
The crystalline structures were characterized by X-ray diffractometry (XRD) (RINT2100CMJ, produced by Rigaku Co., Ltd., Tokyo, Japan) using Cu Kα radiation (λ = 1.54184 Å), operated at 40 kV and 30 mA.
The transmittance measurements of thin-film samples were performed using UV-vis spectroscopy (Lambda750S produced by PerkinElmer Co., Ltd., Waltham, MA, USA) in the wavelength range between 250 nm and 800 nm. The average transparency was determined in the visible light range from 380 nm to 740 nm. The band gaps of thin film samples were estimated using the Tauc plot method. The TiO2 was reported to have both a direct forbidden and an indirect allowed transition, where the latter transition dominates the optical absorption due to the weak strength of the former transition. Thus, we assumed only the indirect allowed transitions.
A field emission scanning electron microscope (FE-SEM, SU6600 produced by Hitachi High-Technologies Co., Ltd., Tokyo, Japan) was used to observe the surface morphology of the thin-film samples at an accelerating voltage of 20.0 kV. The average diameter was estimated via ImageJ.
The chemical composition and chemical bonding states of TiO2 thin films were investigated by X-ray photoelectron spectroscopy (XPS) analysis (JPS-9030 produced by JEOL Co., Ltd., Tokyo, Japan), operated at 10.0 kV and 20.0 mA. A standard MgKα X-ray source was employed, and the measurement area had a diameter of 10 mm. A high-etching-rate ion gun with Ar ions was employed to remove the contaminants and hydroxyl (OH) groups on the specimen surface. The approximate etched depth was 0.2 nm. The obtained data were analyzed using the software SPECSURF Analysis, which is built-in for JEOL XPS. The Ti3+ and Ti4+ peak areas were utilized to estimate oxygen vacancy concentrations (Ti3+ ratio: [Ti3+ peak area]/[(Ti3+ + Ti4+) peak area]) because one Ti3+ ion in TiO2 requires one oxygen vacancy to accommodate charge neutrality [].
2.3. Measurement of Photocatalytic Activity
The photocatalytic activity was evaluated via the decomposition rate of methyl orange (MO). The MO granular solid (purity: 99.9%; produced by Nacalai Tesque Inc., Kyoto, Japan) was used to prepare a MO solution with a concentration of 0.01 mmol/L, and 16 mL was utilized for photocatalytic measurements.
A xenon lamp (UXL-500D-O produced by Ushio Co., Ltd., Tokyo, Japan) located above the solution was used as the light source, which included both ultraviolet light and visible light (the spectra of the lamp were shown in Figure S1 in Supporting Information). The power of the lamp was 500 W. A tube with 15 cm (height) of water was placed between the Xenon lamp and the MO solution to filter the infrared light of the Xenon lamp irradiation and maintain the temperature. During the photoactivity measurement, the solution was stirred at 1200 rpm, and the concentration changes in MO were monitored every 20 min via spectrophotometry.
3. Results
3.1. XRD Measurements
Figure 1 shows XRD patterns of the TiO2 thin films deposited under various oxygen flow rates. The patterns indicate that the anatase structure is dominant except for RO2 = 70%. As the RO2 value increases (from 5%), the diffraction peak corresponding to the (110) plane of the rutile phase at around 2θ = 27° was not prominent until RO2 = 30–40%. However, at RO2 greater than 40%, the rutile structure was distinguished and became comparable with the anatase structure at RO2 = 70%. The crystallite sizes of the films estimated via Scherrer’s equation are shown in Table S1 in the Supporting Information. The crystallite sizes did not indicate the correlation with RO2.
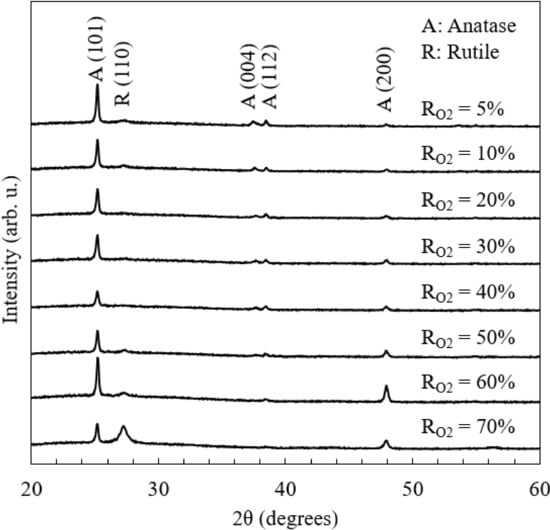
Figure 1.
XRD patterns of TiO2 thin films.
3.2. Optical Transmittance Spectra
Figure 2, Figure 3 and Figure 4 show the optical transmittance spectra of TiO2 thin films, an example of a Tauc plot based on the spectra, and the band gaps based on Tauc plots, respectively. As the RO2 value was increased, the band gap was also increased until RO2 = 20%, remained constant until 30–40%, and decreased over RO2 = 40%. The reported band gaps of the anatase and rutile structures are 3.2 eV and 3.0 eV, respectively, for bulk TiO2 []. The constant value, around 3.32 eV, at RO2 = 20–40% could be derived from the anatase structure, where the difference of 0.12 eV larger than 3.2 eV is explained by the tendency that the band gap of thin films is generally wider than that of bulk [,]. The relatively smaller values of band gap at RO2 = 5–10% should originate from the rutile structure, which was slightly detected in XRD results. The rutile structure has a narrower band gap than the anatase structure. The narrower band gaps in the other RO2 region should be due to the composite of the rutile and the anatase structure, which is consistent with XRD results. In fact, the rutile structure mostly disappeared in XRD patterns at RO2 = 20–40%. Therefore, the change in the band gap is mainly attributed to the crystal structure, though the film strain could modify the band gap value.
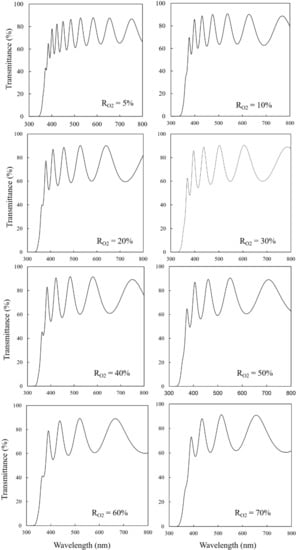
Figure 2.
Optical transmittance spectra of TiO2 thin films.
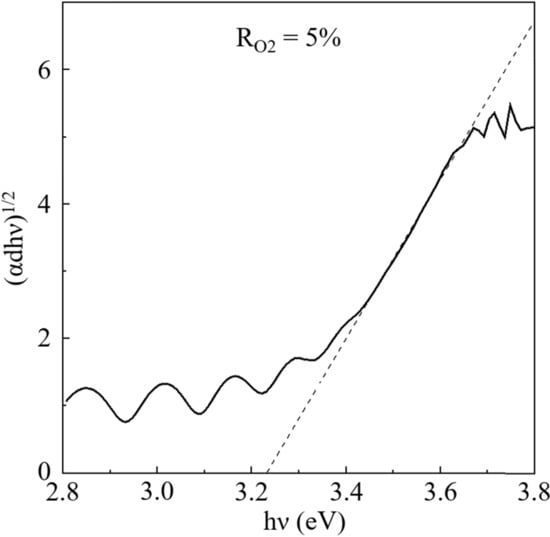
Figure 3.
An example of Tauc plot (the sample deposited under RO2 = 5%).
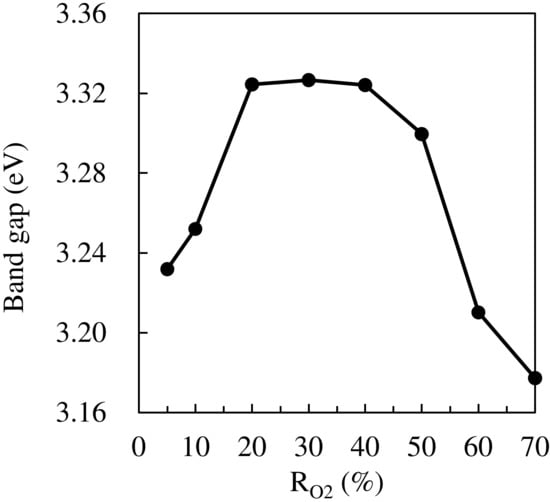
Figure 4.
Band gaps of TiO2 thin.
3.3. SEM Images
The surface morphologies were investigated by FE-SEM measurements (Figure 5). The average diameters were 23 and 25 nm for the film deposited under RO2 = 5% and 50%, respectively. Thus, it is suggested that the morphology does not change at the value of RO2 = 50%.
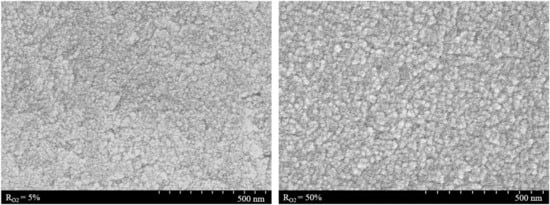
Figure 5.
Surface morphologies of TiO2 thin films prepared at RO2 = 5% (left) and 50% (right).
The thickness of TiO2 films was measured by the cross-sectional FE-SEM images and the interference of transmitted light. Figure 6 shows that the films gradually became thinner as the RO2 increased, indicating that the growth rate of the films became slower with increasing RO2. At high RO2 (low Ar gas ratio), the plasma generation generally becomes more difficult because Ar can more easily be dissociated than O2; the dissociation energy of the Ar was about 15.76 eV, and O2 was approximately 48.77 eV []. Therefore, a possible explanation of the thinner film at high RO2 is that the plasma sputtering rate becomes less intensive.
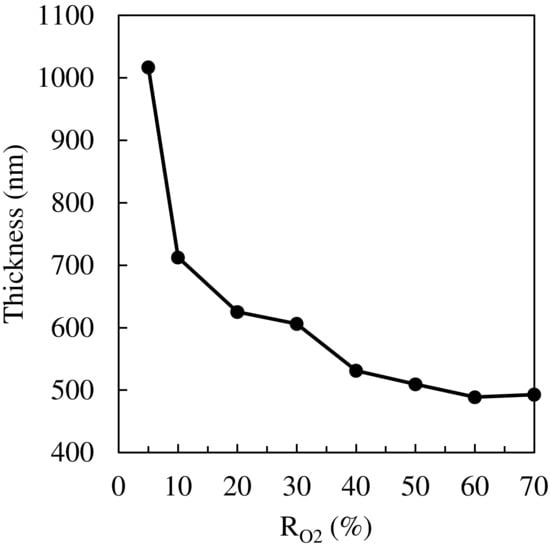
Figure 6.
Thickness of TiO2 thin films.
3.4. XPS Measurements
The XPS measurements were performed to examine the chemical states of Ti in proximity to surfaces of TiO2 thin films. Figure 7 shows the high-resolution narrow scan of XPS spectra around the Ti 2p3/2 spin orbital. The main peak (458.0 eV) is ascribed to the Ti4+ state, and the shoulder peak in the lower binding energy region (456.5 eV) is assigned to the Ti3+ state. The ratio of Ti3+ to Ti4+ corresponds to the oxygen vacancy ratio in TiO2 thin films because Ti3+ is generated when Ti4+ in TiO2 is reduced and releases oxygen []. Figure 8 summarizes the Ti3+ ratio changes as a function of RO2. It is noteworthy that the TiO2 film deposited under the most oxygen-rich condition (RO2 = 70%) has the highest amount of oxygen vacancies.
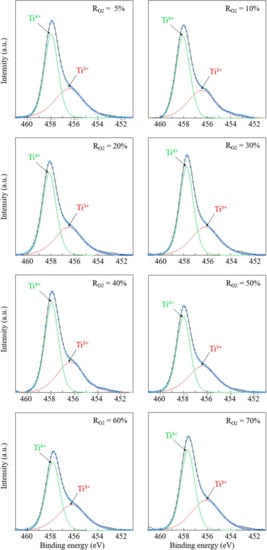
Figure 7.
XPS results for the binding energy of Ti3+ versus Ti4+ in TiO2 thin films.
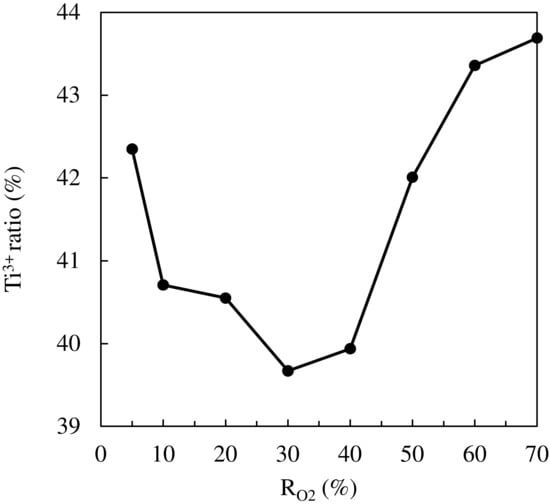
Figure 8.
The ratio of Ti3+ to Ti4+ in TiO2 thin films.
3.5. Photocatalytic Activity
Figure 9a shows the photocatalytic activity of the TiO2 thin films for MO degradation. As indicated by the control test (blank concentration) fluctuation, the error in the concentration measurement is at least 4.3%. To evaluate the photocatalytic activities, we employed the MO concentrations at 80 min (Figure 9b), where the decomposed MO amount is significant even considering the measurement error. We then found similar behavior in the degradation rate as well as other properties: that is, with the RO2 increase, the degradation rate is decreased until RO2 = 30%, while the rate is increased over RO2 = 40%. The TiO2 film synthesized under RO2 = 70% showed the highest photocatalytic activity. The correlation between catalytic activity and the other properties will be discussed in the next section.
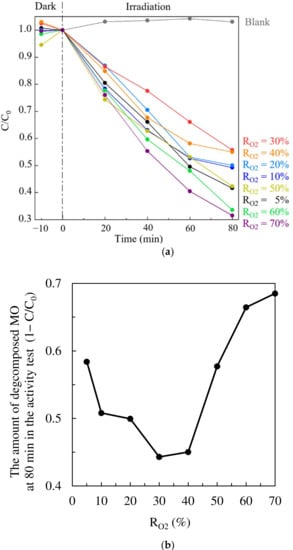
Figure 9.
The catalytic activity of TiO2 thin films for MO degradation. C0 and C are the concentrations of MO solution at the initial and at each minute, respectively. (a) The concentration changes over time and (b) the amount of decomposed MO at 80 min during the activity test (1−C/C0).
To check whether Ti3+ reacts with MO to become Ti4+, we estimated the amount of Ti3+ using the thickness of the films and compared them with the amount of decomposed MO after 80 min irradiation. The amount of decomposed MO is about six orders of magnitude higher than that of Ti3+ in TiO2 thin film. Therefore, the contribution of Ti3+ to the decomposition of MO, via sacrificing itself to become Ti4+, was negligible.
4. Discussion
4.1. Plausible Explanation of a Less Oxidized State Induced under the Oxygen-Rich Condition
In this section, we suggest a possible explanation for why the less oxidized state appeared in the TiO2 thin films prepared under the oxygen-rich condition, RO2 = 40–70%. According to a previous study [], oxygen-rich conditions facilitate the nucleation of the rutile structure. Similar behavior regarding crystal structure changes was also reported in another study; as RO2 was increased, the rutile structure disappeared first and appeared again when the synthesis condition became oxygen-rich, and they discussed these phenomena from reactive mechanics in the deposition [,]. Hence, the increase in the rutile structure in the oxygen-rich condition is reasonable. Furthermore, the ab initio calculation indicates that the rutile structure with (110) facets favors the oxygen vacancies on the surface, rather than the anatase structure with its most stable (101) facet, since their formation energy at the rutile (110) surface is smaller than that at the subsurface and much smaller than that in bulk rutile [,], which is consistent with our XPS results. Therefore, a higher concentration of oxygen vacancies is mainly observed due to an increased amount of the rutile structure, which is produced under oxygen-rich conditions.
4.2. Origin of High Photocatalytic Activity
The photocatalytic activity is generally related to the crystal structure [,,], band gap [], surface morphology [], and the amount of oxygen vacancy [,,]. However, the FE-SEM images show that the surface morphology did not change when comparing the samples prepared under RO2 = 5% and 50%. Hence, the surface morphology is unlikely related to the change in the degradation rate. The other three parameters (crystal structure, band gap, and the amount of oxygen vacancy) are then considered to further examine the origin of enhanced photocatalytic activity.
Figure 10 shows the relation between the photocatalytic activity and the band gap (a) or the Ti3+ ratio (b). Based on the XRD patterns, the TiO2 thin films synthesized under RO2 = 20–30% have only the anatase structure, whereas the samples prepared under RO2 = 5–10 and 40–70% were composites of the anatase and the rutile structure. Although the reference [] indicated that only one of the two structures was formed dominantly at RO2 = 70%, our sample showed both peaks are distinguished in XRD results, which seems derived from some other conditions. Generally, the anatase structure is more active than the rutile structure []. However, rutile/anatase composites could show higher activity than the anatase structure alone [,,], because the composite interface (heterojunction) may disturb the recombination of electrons and holes. In addition, the narrow band gap is preferable for absorbing more light, though a weak correlation between band gap and decomposition rate is seen, as shown in Figure 10a. Therefore, higher activity is likely influenced by the existence of composites.
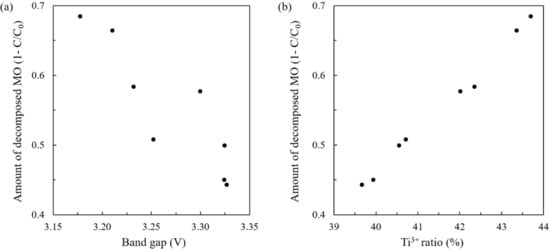
Figure 10.
The relationship between the amount of decomposed MO at 80 min during the activity test (1−C/C0) and (a) Ti3+ ratio and (b) band gap. C0 and C are the concentration of MO solution at the initial and at each minute, respectively.
Oxygen vacancies are also involved in promoting charge separation and disturbing the electron–hole recombination process, resulting in high photocatalytic activity [,,]. Then, a relatively large amount of oxygen vacancy in the TiO2 samples could be responsible for high photocatalytic activity. Although the pre-sputtering in XPS measurement removes the outermost surface layer (~0.2 nm) and detects the chemical composition in the subsurface, the oxygen vacancies in the subsurface layer could have a strong potential to enhance the photocatalytic activity []. Thus, the seemingly strong correlation shown in Figure 10b can be significant and explained in a consistent manner. It is noted that, though the discussed Ti3+ ratio may not be exactly the same with the as-sputtered surface due to some reduction of Ti4+ to Ti3+ via Ar ion etching, the difference of the reduction among samples could be negligible, and our XPS results on the tendency of Ti3+ ratio are inconsistent with the change in XRD patterns, band gap, and photocatalytic activity. It is then plausible that our XPS results are correlated to the oxygen vacancies in the original states. Therefore, the excellent correlation between Ti3+ ratio and degradation rate, as shown in Figure 10b, strongly suggests that the oxygen vacancies have a solid potential to enhance photocatalytic activity.
Hence, we can conclude that a large amount of oxygen vacancy, the narrower band gap, and the existence of rutile/anatase composites play an essential role in enhancing photocatalytic activity. It could be easy to prepare the thin films using reactive RF sputtering under the less oxygen flow condition to attain these three factors in the satisfactory range. However, this study shows that it is also possible or preferable in higher oxygen flow conditions.
5. Conclusions
The TiO2 thin films were prepared via reactive RF sputtering under various flow rates of oxygen and argon gas. We observed the highest photocatalytic activity for the films prepared under the most oxygen-enriched condition among the prepared samples, 70%. The origin of the enhanced activity is attributed to the composite of anatase/rutile structure and the increased oxygen vacancy despite the oxygen-rich sputtering condition. The reason why a less oxidized state appeared under the oxygen-enriched condition is as follows: the rutile structure prefers to grow under oxygen-rich conditions at around room temperature and tends to generate a higher concentration of oxygen vacancies. Therefore, this research presents the insight that the less oxidized state of TiO2 films can be prepared even in an oxygen-rich condition. The conditions could be useful for preparing highly photoactive TiO2 thin films.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/photochem2010011/s1, Figure S1: Spectral irradiance of the Xenon lamp; Table S1: The comparison of sputtering condition in several pieces of literature; Table S2: The crystallite size of TiO2 thin films estimated via Scherrer’s equation based on anatase (101) plane.
Author Contributions
Writing—original draft: T.O.; Formal analysis, Writing—review & editing, Validation: Y.Z.; K.N.I.; Data curation: T.O.; Y.Z.; H.O.; Investigation: T.O.; Y.Z.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Patel, N.; Fernandes, R.; Guella, G.; Kale, A.; Miotello, A.; Patton, B.; Zanchetta, C. Structured and nanoparticle assembled Co−B thin films prepared by pulsed laser deposition: A very efficient catalyst for hydrogen production. J. Phys. Chem. C 2008, 112, 6968–6976. [Google Scholar] [CrossRef]
- Anpo, M.; Takeuchi, M. The design and development of highly reactive titanium oxide photocatalysts operating under visible light irradiation. J. Catal. 2003, 216, 505–516. [Google Scholar] [CrossRef]
- Yu, J.G.; Zhao, X.J.; Zhao, Q.N. Effect of surface structure on photocatalytic activity of TiO2 thin films prepared by sol-gel method. Thin Solid Films 2000, 379, 7–14. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Hieu, V.Q.; Lam, T.C.; Khan, A.; Thi Vo, T.-T.; Nguyen, T.-Q.; Doan, V.D.; Tran, D.L.; Le, V.T.; Tran, V.A. TiO2/Ti3C2/g-C3N4 ternary heterojunction for photocatalytic hydrogen evolution. Chemosphere 2021, 285, 131429. [Google Scholar] [CrossRef] [PubMed]
- Hieu, V.Q.; Phung, T.K.; Nguyen, T.-Q.; Khan, A.; Doan, V.D.; Tran, V.A.; Le, V.T. Photocatalytic degradation of methyl orange dye by Ti3C2–TiO2 heterojunction under solar light. Chemosphere 2021, 276, 130154. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.K.; Liao, M.W.; Lai, C.W. Effects of oxygen flow ratios and annealing temperatures on raman and photoluminescence of titanium oxide thin films deposited by reactive magnetron sputtering. Thin Solid Films 2009, 518, 1415–1418. [Google Scholar] [CrossRef]
- Pomoni, K.; Vomvas, A.; Trapalis, C. Electrical conductivity and photoconductivity studies of TiO2 sol–gel thin films and the effect of N-doping. J. Non-Cryst. Solids 2008, 354, 4448–4457. [Google Scholar] [CrossRef]
- Natarajan, C.; Fukunaga, N.; Nogami, G. Titanium dioxide thin film deposited by spray pyrolysis of aqueous solution. Thin Solid Films 1998, 322, 6–8. [Google Scholar] [CrossRef]
- Terashima, M.; Inoue, N.; Kashiwabara, S.; Fujimoto, R. Photocatalytic TiO2 thin-films deposited by pulsed laser deposition technique. IEEJ Trans. Fundam. Mater. 2001, 121, 59–64. [Google Scholar] [CrossRef][Green Version]
- Sun, L.; Hou, P. Spectroscopic ellipsometry study on e-beam deposited titanium dioxide films. Thin Solid Films 2004, 455–456, 52–529. [Google Scholar] [CrossRef]
- Sun, H.; Wang, C.; Pang, S.; Li, X.; Tao, Y.; Tang, H.; Liu, M. Photocatalytic TiO2 films prepared by chemical vapor deposition at atmosphere pressure. J. Non-Cryst. Solids 2008, 354, 1440–1443. [Google Scholar] [CrossRef]
- Boukrouh, S.; Bensaha, R.; Bourgeois, S.; Finot, E.; Marco de Lucas, M.C. Reactive direct current magnetron sputtered TiO2 thin films with amorphous to crystalline structures. Thin Solid Films 2008, 516, 6353–6358. [Google Scholar] [CrossRef]
- Chiu, S.-M.; Chen, Z.-S.; Yang, K.-Y.; Hsu, Y.-L.; Gan, D. Photocatalytic activity of doped TiO2 coatings prepared by sputtering deposition. J. Mater. Process. Technol. 2007, 192–193, 60–67. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Rahman, K.H.; Wu, C.-C.; Chen, K.-C. A review on the pathways of the improved structural characteristics and photocatalytic performance of titanium dioxide (TiO2) thin films fabricated by the magnetron-sputtering technique. Catalysts 2020, 10, 598. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Zhu, S.; Wang, F. Influence of argon flow rate on TiO2 photocatalyst film deposited by dc reactive magnetron sputtering. Surf. Coat. Technol. 2004, 182, 192–198. [Google Scholar] [CrossRef]
- Huang, C.H.; Tsao, C.C.; Hsu, C.Y. Study on the photocatalytic activities of TiO2 films prepared by reactive RF sputtering. Ceram. Int. 2011, 37, 2781–2788. [Google Scholar] [CrossRef]
- Chiou, A.H.; Kuo, C.G.; Huang, C.H.; Wu, W.F.; Chou, C.P.; Hsu, C.Y. Influence of oxygen flow rate on photocatalytic TiO2 films deposited by RF magnetron sputtering. J. Mater. Sci. Mater. Electron. 2011, 23, 589–594. [Google Scholar] [CrossRef]
- Machda, F.; Ogawa, T.; Okumura, H.; Ishihara, K.N. Damp heat durability of Al-doped ZnO transparent electrodes with different crystal growth orientations. ECS J. Solid State Sci. Technol. 2019, 8, Q240–Q244. [Google Scholar] [CrossRef]
- Wang, L.-Q.; Baer, D.R.; Engelhard, M.H. Creation of variable concentrations of defects on TiO2(110) using low-density electron beams. Surf. Sci. 1994, 320, 295–306. [Google Scholar] [CrossRef]
- Yu, J.-G.; Yu, H.-G.; Cheng, B.; Zhao, X.-J.; Yu, J.C.; Ho, W.-K. The effect of calcination temperature on the surface microstructure and photocatalytic activity of TiO2 thin films prepared by liquid phase deposition. J. Phys. Chem. B 2003, 107, 13871–13879. [Google Scholar] [CrossRef]
- Paul, A.; John, J.C.; Augustine, S.; Sebastian, T.; Joy, A.; Joy, J.; Maria Michael, T. Comparison of photocatalytic efficiency of TiO2 and In2S3 thin films under UV and visible light irradiance. Adv. Mater. Lett. 2020, 11, 1–7. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.N.; Kumar, R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef]
- Farfan-Arribas, E.; Madix, R.J. Role of defects in the adsorption of aliphatic alcohols on the TiO2(110) surface. J. Phys. Chem. B 2002, 106, 10680–10692. [Google Scholar] [CrossRef]
- Löbl, P.; Huppertz, M.; Mergel, D. Nucleation and growth in TiO2 films prepared by sputtering and evaporation. Thin Solid Films 1994, 251, 72–79. [Google Scholar] [CrossRef]
- Zeman, P.; Takabayashi, S. Effect of total and oxygen partial pressures on structure of photocatalytic TiO2 films sputtered on unheated substrate. Surf. Coat. Technol. 2002, 153, 93–99. [Google Scholar] [CrossRef]
- Schiller, S.; Beister, G.; Sieber, W.; Schirmer, G.; Hacker, E. Influence of deposition parameters on the optical and structural properties of TiO2 films produced by reactive D.C. Plasmatron sputtering. Thin Solid Films 1981, 83, 239–245. [Google Scholar] [CrossRef]
- Cheng, H.; Selloni, A. Surface and subsurface oxygen vacancies in anatase TiO2 and differences with rutile. Phys. Rev. B 2009, 79, 092101 . [Google Scholar]
- Li, H.; Guo, Y.; Robertson, J. Calculation of TiO2 surface and subsurface oxygen vacancy by the screened exchange functional. J. Phys. Chem. C 2015, 119, 18160–18166. [Google Scholar] [CrossRef]
- Ohno, T.; Tokieda, K.; Higashida, S.; Matsumura, M. Synergism between rutile and anatase TiO2 particles in photocatalytic oxidation of naphthalene. Appl. Catal. A 2003, 244, 383–391. [Google Scholar] [CrossRef]
- Kho, Y.K.; Iwase, A.; Teoh, W.Y.; Mädler, L.; Kudo, A.; Amal, R. Photocatalytic H2 evolution over TiO2 nanoparticles. The synergistic effect of anatase and rutile. J. Phys. Chem. C 2010, 114, 2821–2829. [Google Scholar] [CrossRef]
- Su, R.; Bechstein, R.; Sø, L.; Vang, R.T.; Sillassen, M.; Esbjörnsson, B.; Palmqvist, A.; Besenbacher, F. How the anatase-to-rutile ratio influences the photoreactivity of TiO2. J. Phys. Chem. C 2011, 115, 24287–24292. [Google Scholar] [CrossRef]
- Nowotny, M.K.; Sheppard, L.R.; Bak, T.; Nowotny, J. Defect chemistry of titanium dioxide. Application of defect engineering in processing of TiO2-based photocatalysts. J. Phys. Chem. C 2008, 112, 5275–5300. [Google Scholar] [CrossRef]
- Wang, J.; Liu, P.; Fu, X.; Li, Z.; Han, W.; Wang, X. Relationship between oxygen defects and the photocatalytic property of ZnO nanocrystals in Nafion membranes. Langmuir 2009, 25, 1218–1223. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.Q.; Fu, X.; Zhang, N.; Xu, Y.J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Sclafani, A.; Herrmann, J.M. Comparison of the photoelectronic and photocatalytic activities of various anatase and rutile forms of titania in pure liquid organic phases and in aqueous solutions. J. Phys. Chem. 1996, 100, 13655–13661. [Google Scholar] [CrossRef]
- Lee, S.-H.; Yamasue, E.; Ishihara, K.N.; Okumura, H. Photocatalysis and surface doping states of N-doped TiOx films prepared by reactive sputtering with dry air. Appl. Catal. B 2010, 93, 217–226. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).