Abstract
Under photoirradiation, Sc3N@Ih-C80 reacted readily with disilirane 1, silirane 4, and digermirane 7 to afford the corresponding 1:1 adducts, whereas Sc3N@D5h-C80 was recovered without producing those adducts. Based on these results, we described a novel method for the exclusive separation of Ih and D5h isomers of Sc3N@C80. The method includes three procedures: selective derivatization of Sc3N@Ih-C80 using 1, 4, and 7, facile HPLC separation of pristine Sc3N@D5h-C80 and Sc3N@Ih-C80 derivatives, and thermolysis of Sc3N@Ih-C80 derivatives to collect pristine Sc3N@Ih-C80. In addition, laser flash photolysis experiments were conducted to elucidate the reaction mechanism. Decay of the transient absorption of 3Sc3N@Ih-C80* was observed to be enhanced in the presence of 1, indicating the quenching process. When Sc3N@D5h-C80 was used, the transient absorption was much less intensive. Therefore, the quenching of 3Sc3N@D5h-C80* by 1 could not be confirmed. Furthermore, we applied time-dependent density functional theory (TD-DFT) calculations of the photoexcited states of Sc3N@C80 to obtain insights into the reaction mechanism.
1. Introduction
Endohedral metallofullerenes (EMFs) have been investigated extensively because of their fascinating structures based on electron transfer from encapsulated metal species to carbon cages [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. Among EMFs, trimetallic nitride template endohedral metallofullerenes (TNT-EMFs) constitute a major EMF family for which extensive studies have been conducted to ascertain and apply their remarkable properties [2,9,10]. In fact, Sc3N@Ih-C80 has been well studied among TNT-EMFs because of its high production yield [9]. A few years after the discovery of Sc3N@Ih-C80, the D5h isomer of Sc3N@C80 was isolated and characterized to demonstrate its higher reactivity than that of the Ih isomer [18,19]. For the synthesis of Sc3N@C80, however, separation of the Ih and D5h isomers by high-performance liquid chromatography (HPLC) is not efficient because the retention times of these isomers are mutually similar using commercial HPLC columns.
To date, many chemical procedures without HPLC separation have been reported for separation of mixtures of fullerenes based on their chemical reactivity differences [20]. For example, Diels–Alder reactions using a cyclopentadiene-functionalized resin followed by retro-addition was used to facilitate the separation of the Ih and D5h isomers of Sc3N@C80 and Lu3N@C80 [21]. Selective complexation procedures were developed using aminosilica and Lewis acids that precipitate with some EMFs [22,23]. These methods enabled separation of Sc3N@Ih-C80 in gram quantities. More recently, a selective oxidation procedure using a ferrocenium salt was applied to separate both isomers based on differences in their oxidation potentials. This procedure involves sequential column chromatographic separation of the unreactive Sc3N@Ih-C80 and the oxidized Sc3N@D5h-C80, which were subsequently recovered by reduction [24]. Although Sc3N@D5h-C80 and Sc3N@C68 were obtained as the same fraction in this method, it was subsequently reported that Sc3N@D5h-C80 was separated from Sc3N@C68 based on the predominant reactivity of the latter in methano-derivatization using a tosyl hydrazone reagent [25].
For our ongoing study of fullerene chemistry, disilirane 1 has been used as a versatile derivatizing reagent [15,16]. In general, 1 reacts with EMFs efficiently under visible light to afford the corresponding silylated EMFs. Furthermore, EMFs that exhibit less negative first reduction potentials are reactive toward 1 under both thermal and photochemical conditions [15,16]. These results led us to examine the reactivity of Sc3N@D5h-C80 for comparison with that of the corresponding Ih isomer. This report describes differences in the photochemical reactivities of the Ih and D5h isomers of Sc3N@C80 toward 1 as well as silirane 4 [26,27] and digermirane 7 [28,29]. Very interestingly, Sc3N@D5h-C80 was found to be photochemically inert toward 1–3, whereas Sc3N@Ih-C80 undergoes facile addition reactions under identical conditions. This result indicates a novel photochemical method for the exclusive separation of the Ih and D5h isomers of Sc3N@C80 as follows: (i) selective derivatization of Sc3N@Ih-C80 in mixtures of the Ih and D5h isomers, (ii) facile HPLC separation of Sc3N@D5h-C80 from the derivatized Sc3N@Ih-C80, and (iii) recovery of pristine Sc3N@Ih-C80 by thermolysis of its derivative.
In addition, the laser flash photolysis of Sc3N@C80 was conducted to elucidate differences in the reactivities of Ih and D5h isomers. To date, few examples of comparative studies of the photoreactions of fullerene isomers with different cage symmetries have been reported. We reported earlier that C2v-C78 undergoes photoreaction with 1 to afford the corresponding silylated C78, whereas D3-C78 was inert under the same conditions, indicating a procedure for the separation of C2v-C78 and D3-C78 [30]. More recently, the photodynamics of three isomers of Sc2C2@C82 have been reported as depending on the different fullerene cage symmetries although the intermolecular reactions of the photoexcited Sc2C2@C82 with organic molecules have not been examined yet [31]. For this study, we investigate the mechanistic origins for the difference in the reactivities of the Sc3N@C80 based on cage symmetries using spectroscopic and theoretical studies.
2. Results and Discussion
2.1. Separation of Sc3N@Ih-C80 and Sc3N@D5h-C80 Using Photochemical Functionalization
As described above, earlier reports have shown that Sc3N@Ih-C80 reacts with 1 under photolytic conditions to give the corresponding 1,2-adduct 2 and 1,4-adduct 3 [32]. To examine the reactivity of Sc3N@D5h-C80, a toluene solution of Sc3N@D5h-C80 and 1 was irradiated for 20 h using two 500 W halogen lamps (cut off < 400 nm) under an argon atmosphere (Scheme 1). However, Sc3N@D5h-C80 was found to be inert toward 1, as shown in the HPLC profiles of the photoreaction (Figure S1 in Supplementary Materials).
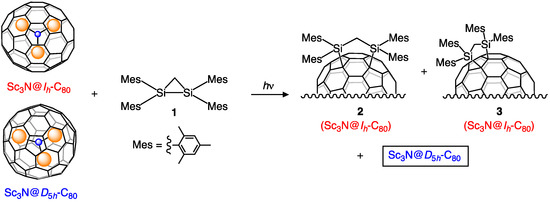
Scheme 1.
Selective silylation of Sc3N@Ih-C80 using 1.
This result led us to apply this photoreaction to the separation of the Ih and D5h isomers of Sc3N@C80. When a mixture of the Ih and D5h isomers and 1 in toluene was irradiated for 40 h, the Ih isomer was consumed with the concomitant formation of 2 and 3, whereas the D5h isomer remained intact (Figure 1). The pristine Sc3N@D5h-C80 and the mixture of 2 and 3 were separated easily from the reaction mixture by preparative HPLC without recycling procedures. Finally, thermal desilylation of the mixture of 2 and 3 was performed at 160–170 °C in o-dichlorobenzene (ODCB) for 20 h. Subsequent HPLC separation afforded pristine Sc3N@Ih-C80 (Figure S2). These procedures established a straightforward method to separate the Ih and D5h isomers of Sc3N@C80.
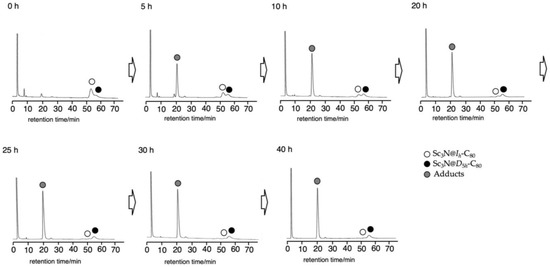
Figure 1.
HPLC profiles of the reaction mixture in the photoreaction of Sc3N@Ih-C80 and Sc3N@D5h-C80 with 1. HPLC conditions: 5PBB column (ϕ 4.6 × 250 mm), eluent: toluene, flow rate: 1.0 mL/min, detection wavelength: 330 nm.
Such a separation method employing silirane 4 as an alternative derivatizing reagent was also examined based on our earlier reported result obtained from the photochemical addition of 4 to Sc3N@Ih-C80 (Scheme 2) [26,27]. In fact, it was confirmed that 4 did not undergo an addition reaction with Sc3N@D5h-C80 under the photolytic condition used for Sc3N@Ih-C80 (Figure S3). As expected, 4 also worked well as a selective carbosilylating reagent for Sc3N@Ih-C80 without reaction with Sc3N@D5h-C80. Consequently, photoirradiation of a mixture of the Ih and D5h isomers and 4 in toluene for 60 h followed by HPLC separation gave pristine Sc3N@D5h-C80 and a mixture of 5a, 5b, and 6 [27], as shown in Figure 2. However, photochemical reactivity of 4 was somewhat lower than that of 1 considering the reaction time necessary to consume Sc3N@Ih-C80. In addition, thermal decomposition of the mixture of 5a, 5b, and 6 was performed at 160–170 °C in ODCB for 40 h to give Sc3N@Ih-C80 along with a recovered mixture of 5a, 5b, and 6 (Figure S4). This result indicates that thermal extrusion reactions of the addends in 5a, 5b, and 6 were less efficient than those of 2 and 3, which might reflect the relative stabilities of these adducts.
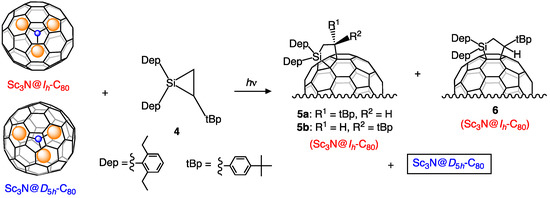
Scheme 2.
Selective carbosilylation of Sc3N@Ih-C80 using 4.
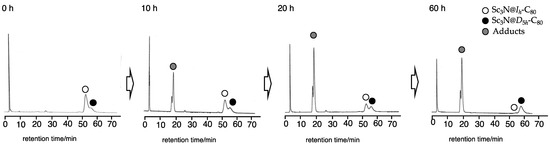
Figure 2.
HPLC profiles of the reaction mixture in the photoreaction of Sc3N@Ih-C80 and Sc3N@D5h-C80 with 4. HPLC conditions: 5PBB column (ϕ 4.6 × 250 mm), eluent: toluene, flow rate: 1.0 mL/min, detection wavelength: 330 nm.
An earlier report described that digermirane 7 is more reactive than its silicon analog 8 toward Lu3N@Ih-C80 under visible irradiation because of the excellent electron-donor property of 7 [29]. This remarkable result led us to evaluate 7 as a third candidate for use as a selective derivatizing reagent for Sc3N@Ih-C80. First, the photoreaction of 7 with Sc3N@Ih-C80 was performed in a manner similar to that used for 1 and 4. During photolysis, HPLC analysis indicated that a product peak developed intensively as the peak of Sc3N@Ih-C80 decreased (Figure S5). After consumption of Sc3N@Ih-C80, preparative HPLC separation of the reaction mixture afforded the 1,4-adduct 9 as the first example of germylated Sc3N@Ih-C80 derivative. The structure of 9 was established by X-ray crystallographic analysis as described below.
However, as expected, Sc3N@D5h-C80 did not react with 7 under identical conditions for prolonged photoirradiation (Figure S6). This result led us to apply this germylation reaction to the separation of the Ih and D5h isomers of Sc3N@C80 (Scheme 3). When a mixture of the Ih and D5h isomers and 7 in toluene was irradiated for 20 h, the Ih isomer was consumed with the formation of 9, whereas the D5h isomer remained intact (Figure 3). The adduct 9 and the pristine D5h isomer were separated easily using preparative HPLC. Finally, degermylation of 9 was accomplished by thermolysis at 130 °C in ODCB for 15 h (Figure S7). This thermolysis is apparently more efficient even at lower temperatures than in the cases of 2, 3, 5a, 5b, and 6, probably because of the lower bond energies of C–Ge bonds (242 kcal/mol) than those of C–C and C–Si bonds (348 kcal/mol and 301 kcal/mol, respectively) [33].
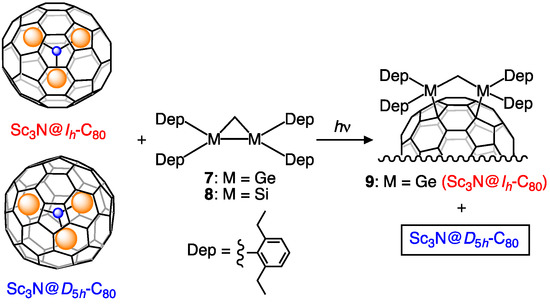
Scheme 3.
Selective germylation of Sc3N@Ih-C80 using 7.
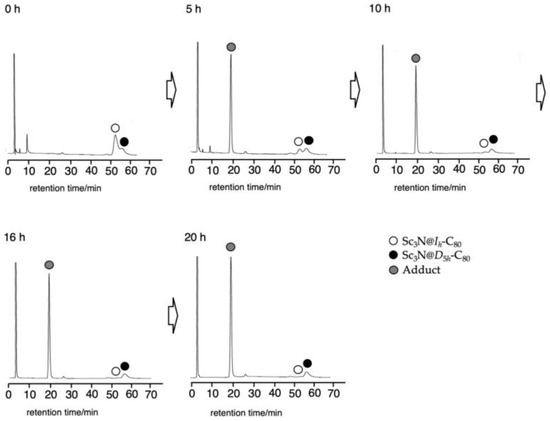
Figure 3.
HPLC profiles of the reaction mixture in the photolysis of Sc3N@Ih-C80 and Sc3N@D5h-C80 with 7. HPLC conditions: 5PBB column (ϕ 4.6 × 250 mm), eluent: toluene, flow rate: 1.0 mL/min, detection wavelength: 330 nm.
2.2. Characterization of Germylated Sc3N@Ih-C80 9
Structural analysis of 9 was conducted based on our earlier studies of the related derivatives 2, 10, 11, and 12 (Figure 4) [29,32]. The visible-near-IR (vis-NIR) spectrum of 9 closely resembles that of 2 (Figure 5). In addition, the NMR spectral features of 9 are similar to those of 11 and 12. The existence of two isomeric molecules is inferred because the 1H NMR spectrum shows two Ge-CH2-Ge methylene groups, respectively, as singlets at 2.33 and 2.57 ppm (Figure S8). In the 13C NMR spectrum, two methylene carbon signals that are attributable to Ge-CH2-Ge were observed at 23.04 and 26.81 ppm. The 13C NMR spectrum also shows two sets of four methyl groups, two sp3 carbon signals of the Ih-C80 cages, and a total of 102 sp2 carbon signals that are attributable to the Ih-C80 cages and the Dep ring carbons (Figure S9). These results indicate the existence of a mixture of conformational isomers of 1,4-adducts with C2 symmetries, as observed for 2, 10, 11, and 12. To examine the conformational exchange in 9, variable temperature (VT) 1H NMR experiments were performed between 303 and 363 K (Figure S10). As expected, the signals coalesced as the temperatures increased to show broad signals at 363 K. The spectrum at 303 K was reproduced when the NMR probe temperature was decreased to room temperature. The matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrum showed that no molecular ion peak expected for 9− was observed, although 1,1,4,4-tetraphenyl-1,3-butadiene (TPB), 9-nitroanthracene (9-NA), and 2,5-dihydroxybenzoic acid (DHB) were used as matrices. This result is attributable to the low stability of the molecular ion 9−, as reported for the MALDI-TOF spectrum of 12 [29].
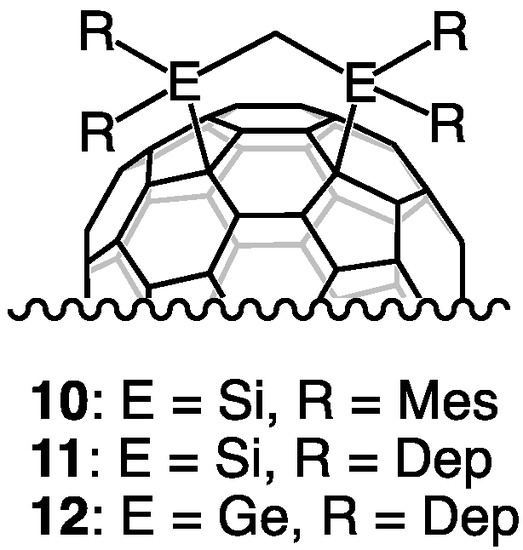
Figure 4.
Lu3N@Ih-C80-based 1,4-adducts.
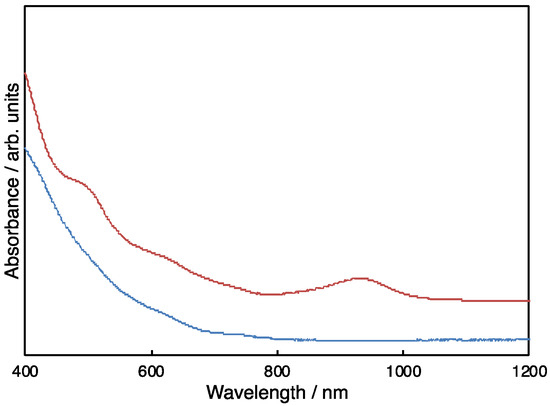
Figure 5.
Vis–NIR absorption spectra of 9 (red) and Sc3N@Ih-C80 (blue) in CS2.
Fortunately, the 1,4-addition structure of 9 was determined firmly using the following X-ray crystallographic analysis. Black block crystals of 9 suitable for X-ray diffraction were obtained using the liquid–liquid bilayer diffusion method with CS2 and hexane at 0 °C. The crystal structure of 9 shows two disordered positions in the Ih-C80 cage with occupancies of 0.72 and 0.28, whereas the digermirane addend is ordered (Figure S11). This result suggests that the crystal structure of 9 includes a pair of diasteromers, which is consistent with the NMR observations. Disorder also exists in the orientations of the Sc3N clusters. They involve six locations of Sc atoms with a common N atom position. These Sc atom sites fall into two Sc3N sets with occupancies of 0.68 and 0.32. Figure 6 presents orientation of the cage and the Sc3N cluster in 9 with major occupancies.
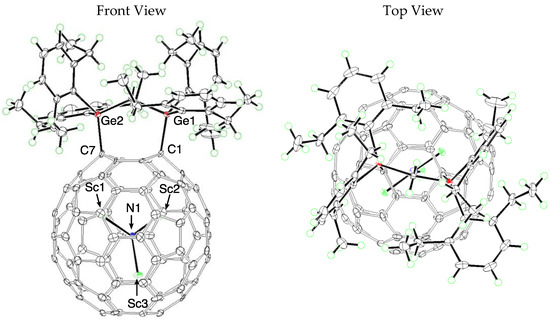
Figure 6.
ORTEP drawings of 9 showing thermal ellipsoids at the 50% probability level at 120 K. The CS2 molecule is omitted for clarity.
We have already reported that the redox properties of silylated and germylated EMFs are altered considerably compared to the corresponding pristine fullerenes because of electron-donating effects of the silyl and germyl groups [16]. The redox property of 9 was verified using cyclic voltammetry (CV) and differential pulse voltammetry (DPV), as shown in Figure 7. The first oxidation (Eox1) and first reduction (Ered1) potentials of 9 are shifted cathodically by 510 and 250 mV, respectively, relative to those of Sc3N@Ih-C80 as presented in Table 1. In addition, both Eox1 and Ered1 potentials of 9 are slightly more negative than those of the silylated derivative 2 [32]. Furthermore, the density functional theory calculations of 9 were conducted at the B3LYP/6-31G*~SDD level to obtain a basis for its electronic structure [34,35,36,37]. The optimized structure of 9 was calculated using an initial structure resembling that of the X-ray structure, as shown in Figure 6. The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) levels of 9 are higher than those of pristine Sc3N@Ih-C80 by 0.68 and 0.45 eV, respectively (Table 1). These changes of the HOMO and LUMO levels are qualitatively consistent with those of the redox potentials of 2 and Sc3N@Ih-C80.

Figure 7.
Cyclic voltammograms (CV) and differential pulse voltammograms (DPV) of 9 in ODCB containing 0.1 M (n-Bu)4NPF6. Conditions: working electrode, a glassy carbon electrode; counter-electrode, Pt wire; reference electrode, SCE; CV scan rates, 20 mV/s; DPV scan rate, 50 mV/s.

Table 1.
Redox potentials (V) a and calculated HOMO/LUMO levels (eV) of 2, 9, and Sc3N@Ih-C80.
2.3. Transient Absorption Spectroscopy of Photoreactions of Sc3N@C80
Laser flash photolysis experiments were conducted to shed light on the differences in the reactivities of the Ih and D5h isomers. Transient absorption spectra were observed in visible (420–760 nm) and NIR (810–1028 nm, 1055–1400 nm) regions using laser excitation at 387 nm. The gaps in the probed spectral range stem from the detection limits of the visible and NIR detectors, gap between 760 and 810 nm, and from the fundamental wavelength of the white light laser source, gap between 1028 and 1055 nm. In toluene, the absorption band of the triplet excited state of Sc3N@Ih-C80 (3Sc3N@Ih-C80*) was observed at λmax 520 nm, as shown in Figure 8 and Figure S12. A report of an earlier study described how the singlet excited state of Sc3N@Ih-C80 (1Sc3N@Ih-C80*) undergoes facile intersystem crossing (ISC) to give 3Sc3N@Ih-C80* with an absorption band around 500 nm [38]. Upon addition of 200 times equimolar amounts of 7, the decay of 3Sc3N@Ih-C80* was accelerated considerably, as shown in comparisons of decay plots both at 500 nm (Figure 9a,b) and at 1104 nm (Figure 9c,d).
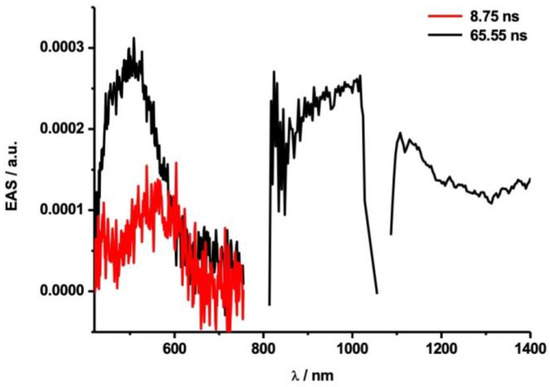
Figure 8.
Evolution-associated spectra (EAS) obtained by the photolysis of Sc3N@Ih-C80 (λex = 387 nm, E = 400 nJ; Figure S12) at their respective relative delay times. The transient absorption of the triplet excited state of Sc3N@Ih-C80 is shown in black and for toluene in red.
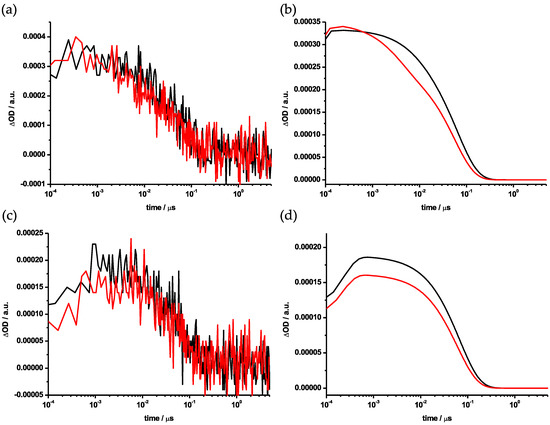
Figure 9.
Decay profiles of transient absorption (Figure 8 and Figure S12) using Sc3N@Ih-C80 in the absence (black) and the presence (red) of 200 times equimolar amounts of 7: (a) observed traces at 500 nm and (b) fitting plots, (c) observed traces at 1104 nm and (d) fitting plots. The residuals of the decay profiles are located in the supporting information (Figure S13).
However, when Sc3N@D5h-C80 was photolyzed under the same conditions, the observed transient absorption peak was much weaker than in the case of Sc3N@Ih-C80, as shown in Figure 10 and Figure S14. Singlet and triplet excited states of Sc3N@D5h-C80 (1Sc3N@D5h-C80* and 3Sc3N@D5h-C80*, respectively) have not been hitherto characterized by spectroscopic studies. Therefore, several mechanistic possibilities should be examined for the weak transient absorption in Figure 10. For example, the photoexcitation of Sc3N@D5h-C80 might not be as efficient as that of Sc3N@Ih-C80, although the former has a lower but comparable molar extinction coefficient at λ = 387 nm (excitation wavelength in laser flash photolysis) compared to that of the latter, as shown in Figure S16. Alternatively, if it is assumed that the molar extinction coefficient and the lifetime of 3Sc3N@D5h-C80* is not significantly different from those of 3Sc3N@Ih-C80*, then the weak absorption observed in Figure 10 suggests the low concentration of 3Sc3N@D5h-C80* under photolytic conditions. One possible explanation for this point is that the ISC process from 1Sc3N@D5h-C80* to 3Sc3N@D5h-C80* might be less effective than in the case of Sc3N@Ih-C80. In the presence of 200 times equimolar amounts of 7, no appreciable difference in intensity of the transient absorption at 500 nm was observed because intense absorption of the triplet excited state of toluene hindered the evaluation (Figure 11a,b) [39]. Although slight differences of intensity at 1215 nm were noted upon addition of 7 (Figure 11c,d), they are mostly attributable to the poor signal-to-noise ratio caused by the low intensity in the NIR region. As such, a quantitative analysis of the photoactivity of Sc3N@D5h-C80 and, in turn, a comparison with Sc3N@Ih-C80 is rendered impossible.
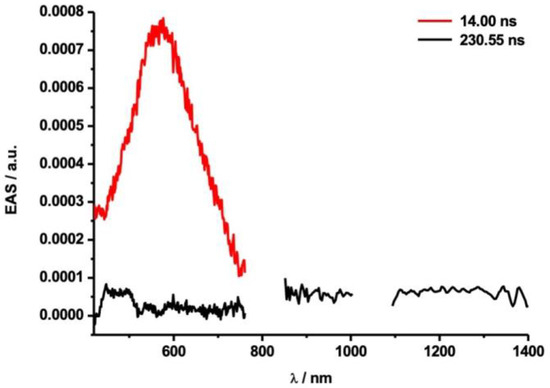
Figure 10.
Evolution-associated spectra (EAS) obtained by the photolysis of Sc3N@D5h-C80 (λex = 387 nm, E = 400 nJ; Figure S14) at their respective relative delay times. The transient absorption of the triplet excited state of Sc3N@D5h-C80 is shown in black and for toluene in red.

Figure 11.
Decay profiles of transient absorption (Figure 10 and Figure S14) using Sc3N@D5h-C80 in the absence (black) and the presence (red) of 200 times equimolar amounts of 7: (a) observed traces at 500 nm and (b) fitting plots, (c) observed traces at 1215 nm and (d) fitting plots. The residuals of the decay profiles are located in the supporting information (Figure S15).
2.4. Theoretical Calculations of Photoreactions of Sc3N@C80
To gain insight into the photochemical processes of Sc3N@C80, we applied time-dependent density functional theory (TD-DFT) [40] calculations on the ten lowest excited states including the singlet states (Sn; n = 1, 2, …) and triplet states (Tn; n = 1, 2, …), respectively, for the Ih and D5h isomers of Sc3N@C80. The corresponding electronic excitation energies are schematized in Figure 12. Major orbital transition configurations are presented in Tables S1 and S2.
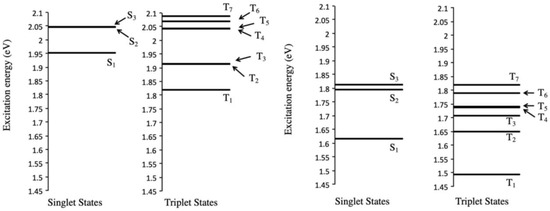
Figure 12.
Calculated energies of singlet and triplet excited states of (a) Sc3N@Ih-C80 and (b) Sc3N@D5h-C80.
According to Kasha’s rule [41], when the energy gaps between two singlet states (Sn and Sm) are small, internal conversion (IC) processes from energetically higher singlet states to lower singlet states are enhanced more effectively than the ISC processes. In the case of Sc3N@Ih-C80, there are the triplet excited states T2 and T3, with energies closely approximating those of the lowest singlet excited state S1. The energy differences between S1-T2 and S1-T3 are, respectively, 0.0387 and 0.0381 eV (Figure 12a). In the case of Sc3N@D5h-C80, T1 is energetically lower than S1, which is lower than other triplet states Tn (n > 1). S1 and T1 are different from each other in energy by 0.1239 eV (Figure 12b). However, these energy differences do not explain the efficiencies of the ISC processes in Sc3N@C80. Alternatively, the efficiency of the ISC processes might depend on the spin-orbit coupling (SOC) interaction between the singlet and triplet excited states. The behaviors of encapsulated metal clusters inside the carbon cages might therefore affect enhancement of the SOC interaction. Further understanding of the photoreactivities of Sc3N@C80 must await investigations of those photoexcited states of the corresponding Ih and D5h isomers.
It was proposed in an earlier report of the relevant literature that the photoreaction of C60 and 1 proceeds via the electron donor–acceptor interaction between 3C60* and 1 based on a quenching experiment of 3C60* by 1 [42]. The Rehm–Weller equation [43,44] for estimating the free energy change ΔG of electron transfer (ET) between 1 and 3C60* in non-polar solvents such as toluene exhibited a positive value. Therefore, results suggest that the ET between 1 and 3C60* is not efficient in non-polar solvents, but that process is regarded as possible because the ΔG value is not so large [42]. Additionally, it has been reported that photoirradiation of C60 and siliranes that possess benzylsilane structures, which are good electron donors, afforded the corresponding adducts [45]. In contrast, when siliranes without benzylsilane structures were used as substrates, the photoaddition reaction proceeded very slowly.
Based on these results, the ΔG values for the ET processes from 1 to the excited triplet states of Sc3N@Ih-C80 and Sc3N@D5h-C80 were calculated using the oxidation potential (Eox) of 1 (+0.27 V vs. Fc/Fc+) [42] and the first reduction potentials (Ered1) of Sc3N@Ih-C80 (−1.26 V vs. Fc/Fc+) [21] and Sc3N@D5h-C80 (−1.33 V vs. Fc/Fc+) [21], respectively. Assuming that the IC process from higher excited triplet states Tn (n >1) to the lowest state T1 occurs rapidly, the energies of the T1 states were evaluated using TD-DFT calculations as 1.82 eV for Sc3N@Ih-C80 and 1.49 eV for Sc3N@D5h-C80. As a result, the ΔG values were estimated as +10.36 kcal/mol for Sc3N@Ih-C80 and +19.50 kcal/mol for Sc3N@D5h-C80 [43,46,47]. These values are positive, as in the case of C60, but the value of the Ih isomer is small, whereas that of the D5h isomer is nearly twice as large as that of the Ih isomer. These results suggest that the photoinduced electron transfer process of 3Sc3N@D5h-C80* should be less efficient than that of 3Sc3N@Ih-C80* even if they take place. Based on these estimations, the poor electron acceptor property of 3Sc3N@D5h-C80* might decrease its photochemical reactivity toward 1, 4, and 7.
3. Experimental Section
Separation of Sc3N@C80 using 1: A mixture of the Ih and D5h isomers of Sc3N@C80 (2.7 mg) and 1 (58 mg) in toluene (20 mL) was degassed using freeze–pump–thaw cycles under reduced pressure in a Pyrex tube (ϕ20 mm). The solution was irradiated for 40 h with two 500 W halogen lamps using an aqueous sodium nitrite filter solution (cutoff < 400 nm) under an argon atmosphere. Preparative HPLC separation with a Buckyprep-M column of the reaction mixture afforded pristine Sc3N@D5h-C80 (0.7 mg) and a mixture of 2 and 3 (2.2 mg).
Thermal desilylation of 2 and 3: A solution of 2 and 3 (2.1 mg) in ODCB (5 mL) was heated at 160–170 °C under an argon atmosphere in a Schlenk tube in the dark for 20 h. After removal of ODCB in vacuo, Sc3N@Ih-C80 (0.8 mg) was obtained by preparative HPLC separation with a Buckyprep-M column.
Selective carbosilylation of Sc3N@C80 using 4: A mixture of Ih and D5h isomers of Sc3N@C80 (2.6 mg) and 4 (61 mg) in toluene (20 mL) was irradiated as described above for 60 h. Preparative HPLC separation with a Buckyprep-M column of the reaction mixture afforded pristine Sc3N@D5h-C80 (0.5 mg) and a mixture of 5a, 5b, and 6 (2.5 mg).
Thermal decarbosilylation of 5a, 5b, and 6: A solution of 5a, 5b, and 6 (2.1 mg) in ODCB (5 mL) was heated at 160–170 °C under an argon atmosphere in a Schlenk tube in the dark for 40 h. After removal of ODCB in vacuo, Sc3N@Ih-C80 (0.9 mg) was obtained along with a recovered mixture of 5a, 5b, and 6 (0.7 mg) by preparative HPLC separation with a Buckyprep-M column.
Photoreaction of Sc3N@Ih-C80 with 7: A solution of Sc3N@Ih-C80 (2.0 mg) and 7 (12.6 mg) in toluene (15 mL) was irradiated for 5 h as described above. Preparative HPLC separation with a Buckyprep-M column of the reaction mixture afforded 9 (2.1 mg). Spectral data for 9: The following NMR data are described based on the existence of two conformational isomers with C2 symmetries. 1H NMR (500 MHz, CS2/CDCl3 (1:1), 298 K) δ 7.33–7.28 (m, 6H), 7.22 (t, J = 7.5 Hz, 2H), 7.18 (d, J = 7.5 Hz, 2H), 7.13 (d, J = 7.5 Hz, 2H), 7.10 (t, J = 7.5 Hz, 2H), 7.02–6.96 (m, 8H), 6.91 (d, J = 7.5 Hz, 2H), 3.75 (dq, J = 7.5 Hz, 15 Hz, 2H), 3.68–3.43 (m, 8H), 3.31 (dq, J = 7.5 Hz, 15 Hz, 2H), 3.09 (dq, J = 7.5 Hz, 15 Hz, 2H), 2.98–2.74 (m, 8H), 2.63 (dq, J = 7.5 Hz, 15 Hz, 2H), 2.57 (s, 2H), 2.51–2.36 (m, 8H), 2.33 (s, 2H), 1.70 (t, J = 7.5 Hz, 6H), 1.43 (t, J = 7.5 Hz, 6H), 0.82 (t, J = 7.5 Hz, 6H), 0.76 (t, J = 7.5 Hz, 6H), 0.66–0.63 (m, 18H), 0.58 (t, J = 7.5 Hz, 6H); 13C NMR (125 MHz, CS2/CDCl3 (1:1), 298 K) δ 178.97, 176.15, 153.01, 152.92, 152.67, 152.58, 151.85, 151.68, 150.91, 150.87, 149.81, 149.67, 149.06, 148.45(2set), 147.72, 147.62, 147.42, 147.27, 147.22, 147.05, 147.00, 146.85, 146.67, 146.56, 146.52, 146.45, 146.38, 145.91, 146.88, 145.55, 145.52, 145.44, 145.36, 145.23, 144.97, 144.78, 144.58, 144.32, 143.16, 142.75, 142.37, 142.24, 141.95, 141.75, 141.15, 141.06, 140.88, 140.74, 140.64, 140.54, 140.45, 140.37, 139.21, 138.67, 138.53, 138.47, 137.51, 137.48, 136.04, 136.02, 135.91, 135.60, 135.43, 135.41, 135.29, 135.25, 134.95, 134.88, 134.82, 134.58, 134.44, 134.31, 134.22, 133.54, 133.17, 133.11, 132.51, 132.08, 132.00, 129.94, 129.59, 129.42, 129.32, 127.76, 127.67, 127.64, 126.88, 126.46, 124.03, 123.94, 116.40, 115.54, 59.09, 33.35, 32.87, 32.43, 32.11, 30.25, 29.92, 29.26, 29.04, 26.81, 23.04, 15.14, 15.06, 14.98, 14.78, 13.73; vis-NIR (CS2) λmax 926 nm; MALDI-TOF MS (TPB) m/z 1109 (Sc3N@C80−).
Selective germylation of Sc3N@C80 using 7: A mixture of Ih and D5h isomers of Sc3N@C80 (2.4 mg) and 7 (65 mg) in toluene (20 mL) was irradiated as described above for 20 h. Preparative HPLC separation with a Buckyprep-M column of the reaction mixture afforded pristine Sc3N@D5h-C80 (0.7 mg) and 9 (2.4 mg).
Thermal degermylation of 9: A solution of 9 (2.4 mg) in ODCB (5 mL) was heated at 130 °C under an argon atmosphere in a Schlenk tube in the dark for 15 h. After the removal of ODCB in vacuo, Sc3N@Ih-C80 (1.1 mg) was obtained by preparative HPLC separation using a Buckyprep-M column.
X-ray crystallography of 9: Black block crystals suitable for X-ray diffraction were obtained using the liquid–liquid bilayer diffusion method with solutions of 9 in CS2 using hexane as a poor solvent at 0 °C. Single-crystal X-ray diffraction data of 9 were collected on a Saturn70 CCD diffractometer (Rigaku Corp.) equipped with a nitrogen-gas flow low-temperature apparatus providing a constant temperature at 120 K. Crystal data for Sc3N@Ih-C80(Dep2Ge)2CH2(9)·1.5(CS2): C122.5H54Ge2Sc3NS3: Mr = 1915.90, black block, 0.25 × 0.13 × 0.07 mm, λ = 0.71069 Å, monoclinic, space group P21/n (no. 14), a = 19.5525(17), b = 20.9254(16), c = 19.6564(16) Å, β =111.0114(5)°, T = 120 K, V= 7506(11) Å3, Z = 4, 168,721 reflections measured, 16,520 unique (Rint = 0.0572), which were used for all calculations, 2θmax = 54.20; min/max transmission=0.782/0.941 (numerical absorption correction applied); the structure was solved using a direct method using SIR2014 [48] and was refined with SHELXL [49]. The final wR(F2) was 0.0927 (all data), conventional R1 = 0.0424 computed for 16,316 reflections with I >2σ (I) using 1937 parameters with 876 restraints. Crystallographic computations were performed with Yadokari-XG 2009 [50]. CCDC2127212 (9) contains the supplementary crystallographic data for this paper, and is obtainable free of charge from the Cambridge Crystallographic Data Centre.
Computational Methods: The computations were performed with the density functional theory (DFT) approach, namely using Becke’s three parameter functional [34] combined with the non-local Lee–Yang–Parr correlation functional [35] (B3LYP). The basis set applied to H, C, N, and Si atoms is the standard 6-31G* basis [36] whereas Sc and Ge atoms are treated in the SDD basis set [37] with the SDD effective core potential (the combined basis set is coded B3LYP/6-31G*~SDD). The geometry optimizations were performed with the analytically constructed energy gradients. In the optimized B3LYP/6-31G*~SDD geometries, the electronic excitation energies were evaluated with the time-dependent (TD) DFT response-theory method [40], again at the B3LYP/6-31G*~SDD level. The computations were performed using the Gaussian 09 program package [51] (See Supplementary Materials).
Transient Absorption Spectroscopy: The excitation was performed with an amplified CPA-2110 titanium:sapphire laser (1 kHz; 150 fs pulse width; 400 nJ laser energy) from Clark-MXR Inc. EOS SYSTEM (0–10 μs) from Ultrafast Systems, working with 1 kHz pump laser at 387 nm wavelength. Probing was performed with a 2 kHz continuous white light fiber laser. Data evaluation has been conducted by means of multiwavelength and global analysis using the GloTarAn package [52].
4. Conclusions
Photoreactions of Sc3N@Ih-C80 and 1, 4, and 7 afforded the corresponding 1:1 adducts, whereas Sc3N@D5h-C80 was found to be inert under identical photolytic conditions. The derivatives of Sc3N@Ih-C80 and pristine Sc3N@D5h-C80 were separated easily by HPLC without recycling processes. In addition, pristine Sc3N@Ih-C80 was recovered by thermolytic decomposition of the corresponding photoadducts. These procedures provide a novel method for the exclusive separation of Sc3N@Ih-C80 and Sc3N@D5h-C80. In laser flash photolysis experiments, the decay of transient absorption for 3Sc3N@Ih-C80* was accelerated in the presence of 7. In contrast, for Sc3N@D5h-C80, the transient absorption was too weak to offer a basis for interaction of the corresponding triplet excited states with 7. In turn, a meaningful conclusion regarding the reactivity differences between Sc3N@Ih-C80 and Sc3N@D5h-C80 was hampered. It is, however, expected that the electron-transfer processes between the 3Sc3N@D5h-C80* and 1 are much less likely to occur than those of Sc3N@Ih-C80, judging from the corresponding values of changes of free energies ΔG. Therefore, the photochemical inertness of Sc3N@D5h-C80 toward 1, 4, and 7 might be partly attributed to the lower electron-acceptor ability of 3Sc3N@D5h-C80*. Further investigations of the dependence of the photochemical reactivities of EMFs on their structures including the carbon cage symmetries and the encapsulated metals are in progress.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/photochem2010010/s1, Materials and General Methods and Complete list of authors for Ref 51, Figure S1: HPLC profiles of the photoreaction of Sc3N@D5h-C80 with 1, Figure S2: HPLC profiles of the thermolysis of the mixture of 2 and 3, Figure S3: HPLC profiles of the photoreaction of Sc3N@D5h-C80 with 4, Figure S4: HPLC profiles of the thermolysis of the mixture of 5a, 5b, and 6, Figure S5: HPLC profile of the reaction mixture of Sc3N@Ih-C80 and 7, Figure S6: HPLC profiles of the photolysis of Sc3N@D5h-C80 with 7, Figure S7: HPLC profiles of the thermolysis of 9, Figure S8: 500 MHz 1H NMR spectrum of 9 recorded at 293 K in CS2/CDCl3 (1:3), Figure S9: 125 MHz 13C NMR spectra of 9 recorded at 293 K in CS2/CDCl3 (1:3), Figure S10: 500 MHz VT 1H NMR spectra of 9 recorded in toluene-d8, Figure S11: Disorder of C80 cage and Sc3N cluster with occupancies in the crystal of 9, Figure S12: Raw data of all the transient absorption measurements of Sc3N@Ih-C80, Figure S13: Residual measurements of Sc3N@Ih-C80 in the absence and the presence of 7, Figure S14: Raw data of all the transient absorption measurements of Sc3N@D5h-C80, Figure S15: Residual measurements of Sc3N@D5h-C80 in the absence and the presence of 7, Figure S16: UV-Visible Spectra of Sc3N@Ih-C80 and Sc3N@D5h-C80 in toluene, Figure S17: Pure toluene reference measurements, Table S1: Ten lowest excited states of Sc3N@Ih-C80 calculated by TD-B3LYP/6-31G*~SDD, Figure S18: Selected molecular orbitals of Sc3N@Ih-C80, Table S2: Ten lowest excited states of Sc3N@D5h-C80 calculated by TD-B3LYP/6-31G*~SDD, Figure S19: Selected molecular orbitals of Sc3N@D5h-C80, Table S3: Cartesian coordinates of optimized structures.
Author Contributions
M.K. and T.A. conceived and designed the experiments; K.M., S.F. and S.K. performed the photoreactions and characterized the products; M.Y. (Masanori Yasui) and K.M. conducted the X-ray crystallography; M.Y. (Michio Yamada) and Y.M. contributed to the analysis of the products; I.P. and D.M.G. conducted the transient absorption spectroscopy; Z.S., F.U. and L.A. performed the calculations; M.K., M.F., S.N. and T.A. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (B) (No. 24350019) and (C) (No. 17K05797 and 20K05469) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; by the MetaCentrum (LM2010005) and CERIT-SC (CZ.1.05/3.2.00/08.0144) computing facilities, by the Charles University Centre of Advanced Materials/ CUCAM (CZ.02.1.01/0.0/0.0/15_003/0000417); by the NSFC (51602097, 51 602 112, 51 672 093, 51 772 195); and by the Deutsche Forschungsgemeinschaft (DFG) as part of SFB 953 “Synthetic Carbon Allotropes”.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Akasaka, T.; Nagase, S. (Eds.) Endofullerenes: A New Family of Carbon Clusters; Kluwer: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Dunsch, L.; Yang, S. Metal nitride cluster fullerenes: Their current state and future prospects endohedral fullerenes. Small 2007, 8, 1298–1320. [Google Scholar] [CrossRef]
- Chaur, M.N.; Melin, F.; Ortiz, A.L.; Echegoyen, L. Chemical, electrochemical, and structural properties of endohedral metallofullerenes. Angew. Chem. Int. Ed. 2009, 48, 7514–7538. [Google Scholar] [CrossRef]
- Yamada, M.; Akasaka, T.; Nagase, S. Endohedral metal atoms in pristine and functionalized fullerene cages. Acc. Chem. Res. 2010, 43, 92–102. [Google Scholar] [CrossRef]
- Akasaka, T.; Wudl, F.; Nagase, S. (Eds.) Chemistry of Nanocarbons; Wiley: Chichester, UK, 2010. [Google Scholar]
- Maeda, Y.; Tsuchiya, T.; Lu, X.; Takano, Y.; Akasaka, T.; Nagase, S. Current progress on the chemical functionalization and supramolecular chemistry of M@C82. Nanoscale 2011, 3, 2421–2429. [Google Scholar] [CrossRef]
- Lu, X.; Akasaka, T.; Nagase, S. Chemistry of endohedral metallofullerenes: The role of metals. Chem. Commun. 2011, 47, 5942–5957. [Google Scholar] [CrossRef]
- Lu, X.; Feng, L.; Akasaka, T.; Nagase, S. Current status and future developments of endohedral metallofullerenes. Chem. Soc. Rev. 2012, 41, 7723–7760. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Stevenson, S.; Dorn, H.C. Trimetallic nitride template endohedral metallofullerenes: Discovery, structural characterization, reactivity, and applications. Acc. Chem. Res. 2013, 46, 1458–1557. [Google Scholar] [CrossRef]
- Rivera-Nazario, D.M.; Pinzón, J.R.; Stevenson, S.; Echegoyen, L.A. Buckyball maracas: Exploring the inside and outside properties of endohedral fullerenes. J. Phys. Org. Chem. 2013, 26, 194–205. [Google Scholar] [CrossRef]
- Yamada, M.; Akasaka, T.; Nagase, S. Carbene additions to fullerenes. Chem. Rev. 2013, 113, 7209–7264. [Google Scholar] [CrossRef]
- Lu, X.; Akasaka, T.; Nagase, S. Carbide cluster metallofullerenes: Structure, properties, and possible origin. Acc. Chem. Res. 2013, 46, 1627–1635. [Google Scholar] [CrossRef]
- Popov, A.A.; Yang, S.; Dunsch, L. Endohedral fullerenes. Chem. Rev. 2013, 113, 5989–6113. [Google Scholar] [CrossRef]
- Nagase, S. Theory and calculations of molecules containing heavier main group elements and fullerenes encaging transition metals: Interplay with experiment. Bull. Chem. Soc. Jpn. 2014, 87, 167–195. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Akasaka, T. Emergence of highly elaborated π-space and extending its functionality based on nanocarbons: New vistas in the fullerene world. Bull. Chem. Soc. Jpn. 2014, 87, 1289–1314. [Google Scholar] [CrossRef] [Green Version]
- Kako, M.; Nagase, S.; Akasaka, T. Functionalization of Endohedral Metallofullerenes with Reactive Silicon and Germanium Compounds. Molecules 2017, 22, 1179. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Liu, M.T.H.; Nagase, S.; Akasaka, T. New Horizons in Chemical Functionalization of Endohedral Metallofullerenes. Molecules 2020, 25, 3626. [Google Scholar] [CrossRef]
- Duchamp, J.C.; Demortier, A.; Fletcher, K.R.; Dorn, D.; Iezzi, E.B.; Glass, T.; Dorn, H.C. An isomer of the endohedral metallofullerene Sc3N@C80 with D5h symmetry. Chem. Phys. Lett. 2003, 375, 655–659. [Google Scholar] [CrossRef]
- Krause, M.; Dunsch, L. Isolation and Characterization of Two Sc3N@C80 Isomers. ChemPhysChem 2004, 5, 1445–1449. [Google Scholar] [CrossRef]
- Wang, Z.; Omachi, H.; Shinohara, H. Non-Chromatographic Purification of Endohedral Metallofullerenes. Molecules 2017, 22, 718. [Google Scholar] [CrossRef] [Green Version]
- Cai, T.; Xu, L.; Anderson, M.R.; Ge, Z.; Zuo, T.; Wang, X.; Olmstead, M.M.; Balch, A.L.; Gibson, H.W.; Dorn, H.C. Structure and Enhanced Reactivity Rates of the D5h Sc3N@C80 and Lu3N@C80 Metallofullerene Isomers: The Importance of the Pyracylene Motif. J. Am. Chem. Soc. 2006, 128, 8581–8589. [Google Scholar] [CrossRef]
- Stevenson, S.; Mackey, M.A.; Coumbe, C.E.; Phillips, J.P.; Elliott, B.; Echegoyen, L. Rapid Removal of D5h Isomer Using the “Stir and Filter Approach” and Isolation of Large Quantities of Isomerically Pure Sc3N@C80 Metallic Nitride Fullerenes. J. Am. Chem. Soc. 2007, 129, 6072–6073. [Google Scholar] [CrossRef]
- Stevenson, S.; Mackey, M.A.; Pickens, J.E.; Stuart, M.A.; Confait, B.S.; Phillips, J.P. Selective Complexation and Reactivity of Metallic Nitride and Oxometallic Fullerenes with Lewis Acids and Use as an Effective Purification Method. Inorg. Chem. 2009, 48, 11685–11690. [Google Scholar] [CrossRef] [Green Version]
- Cerón, M.R.; Li, F.F.; Echegoyen, L. An efficient method to separate Sc3N@C80 Is and D5h isomers and Sc3N@C78 by selective oxidation with acetylferrocenium [Fe(COCH3C5H4)Cp]+. Chem. Eur. J. 2013, 19, 7410–7415. [Google Scholar] [CrossRef]
- Cerón, M.R.; Izquierdo, M.; Alegret, N.; Valdez, J.A.; Rodríguez-Fortea, A.; Olmstead, M.M.; Balch, A.L.; Poblet, J.M.; Echegoyen, L. Reactivity differences of Sc3N@C2n (2n = 68 and 80). Synthesis of the first methanofullerene derivatives of Sc3N@D5h-C80. Chem. Commun. 2016, 52, 64–67. [Google Scholar] [CrossRef] [Green Version]
- Kako, M.; Sugiura, T.; Akasaka, T. Photochemical addition of silirane to endohedral metallofullerene: Electronic properties of carbosilylated Sc3N@Ih-C80. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 201–206. [Google Scholar] [CrossRef]
- Kako, M.; Sugiura, T.; Miyabe, K.; Yasui, M.; Yamada, M.; Maeda, Y.; Guo, J.-D.; Nagase, S.; Akasaka, T. Preparation, Structural Determination, and Characterization of Electronic Properties of [5,6]- and [6,6]-Carbosilylated Sc3N@Ih-C80. Chem. Asian J. 2017, 12, 1391–1399. [Google Scholar] [CrossRef]
- Sato, K.; Kako, M.; Mizorogi, N.; Tsuchiya, T.; Akasaka, T.; Nagase, S. Bis-silylation of Lu3N@Ih-C80: Considerable variation in the electronic structures. Org. Lett. 2012, 14, 5908–5911. [Google Scholar] [CrossRef]
- Kako, M.; Miyabe, M.; Sato, K.; Suzuki, M.; Mizorogi, N.; Wang, W.-W.; Yamada, M.; Maeda, Y.; Olmstead, M.M.; Balch, A.L.; et al. Preparation, structural determination, and characterization of electronic properties of bis-silylated and bis-germylated Lu3N@Ih-C80. Chem. Eur. J. 2015, 21, 16411–16420. [Google Scholar] [CrossRef]
- Han, A.H.; Wakahara, T.; Maeda, Y.; Akasaka, T.; Fujitsuka, M.; Ito, O.; Yamamoto, K.; Kako, M.; Kobayashi, K.; Nagase, S. A new method for separating the D3 and C2v isomers of C78. New J. Chem. 2009, 33, 497–500. [Google Scholar] [CrossRef]
- Wu, B.; Hu, J.; Cui, P.; Jiang, L.; Chen, Z.; Zhang, Q.; Wang, C.; Luo, Y. Visible-Light Photoexcited Electron Dynamics of Scandium Endohedral Metallofullerenes: The Cage Symmetry and Substituent Effects. J. Am. Chem. Soc. 2015, 137, 8769–8774. [Google Scholar] [CrossRef]
- Wakahara, T.; Iiduka, Y.; Ikenaga, O.; Nakahodo, T.; Sakuraba, A.; Tsuchiya, T.; Maeda, Y.; Kako, M.; Akasaka, T.; Yoza, K.; et al. Characterization of the bis-silylated endofullerene Sc3N@C80. J. Am. Chem. Soc. 2006, 128, 9919–9925. [Google Scholar] [CrossRef]
- Shriver, D.F.; Atkins, P.W.; Langford, C.H. Inorganic Chemistry, 2nd ed.; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular-orbital studies of organic molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Cao, X.Y.; Dolg, M. Segmented contraction scheme for small-core lanthanide pseudopotential basis sets. J. Mol. Struct. THEOCHEM 2002, 581, 139–147. [Google Scholar] [CrossRef]
- Pinzón, J.R.; Gasca, D.C.; Sankaranarayanan, S.G.; Bottari, G.; Torres, T.; Guldi, D.M.; Echegoyen, L. Photoinduced Charge Transfer and Electrochemical Properties of Triphenylamine Ih-Sc3N@C80 Donor-Acceptor Conjugates. J. Am. Chem. Soc. 2009, 131, 7727–7734. [Google Scholar] [CrossRef] [Green Version]
- Additionally reference transient absorption measurements on pure toluene were performed (λex = 387 nm, E = 400 nJ), in order to independently prove the observed features in the respective Sc3N@Ih-C80 and Sc3N@D5h-C80 measurements (Figure 8 and Figure 10; red species). In these measurements the exact same signal with a maximum at 555 nm could be observed, due to the very high energy density of the laser excitation, thus confirming the triplet excited state signature of toluene (Figure S17).
- Casida, M.E.; Jamorski, C.; Casida, K.C.; Salahub, D.R. Molecular excitation energies to high-lying bound states from time-dependent density-functional response theory: Characterization and correction of the time-dependent local density approximation ionization threshold. J. Chem. Phys. 1998, 108, 4439–4449. [Google Scholar] [CrossRef]
- Kasha, M. Characterization of electronic transitions in complex molecules. Disc. Faraday Soc. 1950, 9, 14–19. [Google Scholar] [CrossRef]
- Akasaka, T.; Maeda, Y.; Wakahara, T.; Okamura, M.; Fujitsuka, M.; Ito, O.; Kobayashi, K.; Nagase, S.; Kako, M.; Nakadaira, Y.; et al. Novel Metal-free bis-silylation: C60-sensitized reaction of disilirane with benzonitrile. Org. Lett. 1999, 1, 1509–1512. [Google Scholar] [CrossRef]
- The ΔG values were calculated according to the Rehm-Weller equation [44] as follows: ΔG (kcal/mol) = 23.06[Eox(D/D+) − Ered(A/A−) − ΔE* + ε], where Eox(D/D+), Ered(A/A−), and ΔE* respectively represent the oxidation potential of electron-donor, the reduction potential of electron-acceptor, and the energies of excited states of electron-acceptors. Coulombic interaction energy ε in toluene (0.74) was calculated according to methods described in the literatures [46,47].
- Rehm, D.; Weller, A. Kinetics of Fluorescence Quenching by Electron and H-Atom Transfer. Isr. J. Chem. 1970, 8, 259–271. [Google Scholar] [CrossRef]
- Nagatsuka, J.; Sugitani, S.; Kako, M.; Nakahodo, T.; Mizorogi, N.; Ishitsuka, M.O.; Maeda, Y.; Tsuchiya, T.; Akasaka, T.; Gao, X.; et al. Photochemical addition of C60 with siliranes: Synthesis and characterization of carbosilylated and hydrosilylated C60 derivatives. J. Am. Chem. Soc. 2010, 132, 12106–12120. [Google Scholar] [CrossRef]
- Mattay, J.; Runsink, J.; Rumbach, T.; Ly, C.; Gersdorf, J. Selectivity and charge transfer in photoreactions of donor-acceptor systems. 5. Selectivity and Charge Transfer in Photoreactions of α, α, α-Trifluorotoluene with Olefins. J. Am. Chem. Soc. 1985, 107, 2557–2558. [Google Scholar] [CrossRef]
- Mattay, J.; Runsink, J.; Gersdorf, J.; Rumbach, T.; Ly, C. Selectivity and charge transfer in photoreactions of α,α,α-trifluorotoluene with olefins. Helv. Chem. Acta 1986, 69, 442–455. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G. Crystal structure determination and refinement via SIR2014. J. Appl. Cryst. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Kabuto, C.; Akine, S.; Nemoto, T.; Kwon, E. Release of Software (Yadokari-XG 2009) for Crystal Structure Analyses. J. Cryst. Soc. Jpn. 2009, 51, 218–224. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Rev. C.01; Gaussian Inc.: Wallingford, CT, USA, 2013; (See Supplementary Materials for the complete list of authors.). [Google Scholar]
- Snellenburg, J.J.; Laptenok, S.P.; Seger, R.; Mullen, K.M.; van Stokkum, I.H.M. Glotaran: A Java-Based Graphical User Interface for the R Package TIMP. J. Stat. Softw. 2012, 49, 1–22. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).