Abstract
This work successfully synthesized green zinc oxide nanoparticles using extracts from strawberry guava leaves (Psidium cattleianum Sabine). Additionally, the reducing effect of the antioxidant extracts obtained through traditional techniques, such as infusion and maceration, was studied and compared against an emerging unconventional technology like ultrasound assisted extraction. Regarding the physical and chemical characteristics, it was found that all three systems were confined within a wavelength range of 357 to 370 nm (UV-vis) and sizes from 60 to 140 nm for the ultrasound-assisted nanoparticles (SEM), corroborated with DLS (134 ± 60 nm). Through X-ray diffraction, the hexagonal wurtzite structure was elucidated, and it was observed that ultrasound favored a higher percentage of crystallinity (98%) compared to the infusion (84%) and maceration (72%). This could be correlated with different functional groups via FTIR and with thermal events associated with thermogravimetric curves, where the total biomass weight loss was lower for nanoparticles using ultrasound extract (6.25%), followed by maceration (15.55%) and infusion (18.01%) extracts. Furthermore, these nanostructures were evaluated against clinically relevant pathogens, including Salmonella enteritidis, Staphylococcus aureus, Escherichia coli O157:H7, and Pseudomonas aeruginosa, assessing bacterial growth inhibition using the microdilution technique, and achieving inhibitions of 75%. Biofilm activity was evaluated through Congo red and crystal violet assays, where ultrasound-derived NPs proved to be good inhibitors for all pathogens. Finally, the toxicity of the nanoparticles was analyzed against peripheral blood leukocytes from goats as well as on the 3 T3-L1 cell line used in anti-obesity assays; the nanoparticles proved to be suitable in all concentrations reaching around 100% cell viability, positioning them as good candidates for diverse industrial applications that align with the principles of green chemistry towards a circular economy.
1. Introduction
Following the Sustainable Development Goals and the principles of circular economy, green technologies that impact waste minimization must be implemented []. Technologies such as the fabrication of green nanoparticles adopted as global environmental issues are increasing, with chemical and physical processes for nanoparticle production being among the causes of these issues [,].
The green synthesis of nanoparticles is a cost-effective and responsible option that combines efficiency, sustainability, and environmental compatibility, making it a key tool for advancing nanotechnology in the 21st century []. Numerous studies demonstrate that a vast arsenal of biological materials, such as plants, fungi, bacteria, algae, and yeasts, can be utilized [,]. In the case of plants as a biological source, they can reduce metal salts to produce stable metallic nanosystems due to their content of terpenoids, alkaloids, steroids, flavonoids, sugars, and their derivatives with amino, hydroxyl, carboxyl, allyl, alkoxy, and thiol groups. Thus, bio-fabricated nanoparticles exhibit biological activities comparable to those obtained by traditional methods [,,].
Zinc oxide nanoparticles have drawn attention due to their applications in electronics, optics, and biomedical systems. These particles, in comparison with other oxide nanoparticles, are inexpensive to produce and easily prepared []. As a semiconductor, ZnO nanoparticles exert great applications, such as hydrogen obtaining through photocatalysis when combined with ZnIn2 S4 [] and improving UV ray protection in cotton fabrics []. Zinc oxide nanoparticles synthesized through green methods have shown superior biocompatibility and improved biological attributes compared to traditional chemical methods []. Compared to other metal oxides with antibacterial capabilities, zinc oxide-based nanostructures have been more lethal to bacteria and less reactive toward human cells [,]. However, most used technology for zinc oxide nanoparticles production through green synthesis utilizes extracts from the infusion or decoction of plant leaves, while alternative, eco-friendlier extraction methods are currently being explored []. Unconventional techniques such as ultrasound-assisted extraction, microwave extraction, supercritical fluids, enzyme-assisted extraction, deep eutectic solvent extraction, shock wave extraction, and voltage extraction exist []. Ultrasound-assisted extraction has been extensively documented for obtaining secondary metabolites from plants due to its efficiency, low cost, and speed compared to traditional extraction methods such as infusion, decoction, maceration, or Soxhlet extraction, as ultrasound increases the permeability and diffusivity of the solvent in natural matrices, breaking down cell walls and membranes to facilitate solvent entry into the biological material [,].
Psidium cattleianum Sabine is native to Brazil and is popularly known worldwide as strawberry guava, little guava, ‘guayabita del Perú’, ‘araçá-amarelo’, ‘araçá-vermelho’, cherry guava, and Chinese guava [,,]. Traditional medicine uses its leaves for their anti-inflammatory, anti-diarrheal, anti-diabetic, antimicrobial, antihemorrhagic, antitumor, and ulcer-fighting activities []. Additionally, it possesses antioxidant properties due to the presence of phenolic compounds such as hydroxycinnamic acids, isoflavonoids, terpenes, sesquiterpenes like β-caryophyllene, and various aromatic compounds, thus becoming popular in fields such as cosmetics, food, and industry [,,]. Apart from the pharmacology effect that P. cattleianum exerts, its benefits in nutrition due to the presence of carbohydrates, protein, lipids, and minerals have led to an increase in its production and, thus, availability [].
Green synthesis of ZnO nanoparticles has been achieved using extracts of the Psidium genera, especially the guajava species through the decoction method, and their antibacterial and cytotoxic activities have been analyzed; however, P. cattleianum have not been used for these nanoparticles, and their antibiofilm activity of different pathogens has not been reported (Table 1). While traditional methods like decoction, Soxhlet, and maceration commonly require high levels of energy, longer time, or toxic solvents, the alternative extractions methods, such as ultrasound-assisted, use eco-friendly solvents, do not consume high energy, the extraction time is reduced, and integrity of bioactive compounds is kept.

Table 1.
Summary of ZnO nanoparticles obtained with Psidium extracts and biological applications.
This work aimed to employ three extraction techniques to obtain bioactive compounds (infusion, maceration, and ultrasound) from Psidium cattleianum leaves to use them in the green synthesis of zinc oxide nanoparticles, analyzing the content of phenolic compounds, flavonoids, and tannins, as well as their antioxidant response through the DPPH method, iron chelation capacity, and the TAOC technique of the particles obtained. These extracts were responsible for reducing zinc oxide nanoparticles for the first time, whose characterization was carried out by UV-vis, SEM, XRD, TGA, and FTIR studies. Furthermore, their biological capacity was evaluated by inhibiting pathogens such as Salmonella enteritidis, Staphylococcus aureus, Pseudomonas aeruginosa, and enterohemorrhagic Escherichia coli (O157:H7). Additionally, the nanosystems obtained were evaluated for toxicity in goats’ peripheral blood cells and in the 3 T3-L1 cell line used in anti-obesity challenges. With the findings presented here, a new option emerges for the diversification and functionality of novel nanoparticles, aligning with the true principles of green chemistry.
2. Materials and Methods
2.1. Extractions
2.1.1. Raw Material
The leaves of Psidium cattleianum were collected in Guadalajara, Mexico (20°39′25″ N 103°19′31″ W). They were washed and disinfected with a 20 mg/L sodium hypochlorite solution and then rinsed with distilled water. The fresh leaves were ground using a mortar for infusion and ultrasound extractions. Another portion of leaves was used for maceration extraction, where the leaves were dried in a forced convection oven (L-C Oven, Mechanically Convected, ON, Canada) at 40 °C for 24–48 h. After this period, the leaves were ground and sieved to achieve a particle size of less than 1 mm.
2.1.2. Infusion
For the extraction, 1 g of ground fresh leaves was placed in 100 mL of distilled water and brought to a boil for 5 min. The mixture was then filtered using Whatman No. 1 filter paper (El Crisol, Jalisco, Mexico). The crude extract underwent rotary evaporation until dryness was achieved to obtain the concentrated extract, which was then refrigerated and protected from light until use.
2.1.3. Ultrasound Extraction
The fresh leaves (1 g) were placed in 100 mL of distilled water. Subsequently, the mixture was subjected to ultrasonic energy using an ice bath for 30 min at 20 kHz and 60% amplitude (Ultrasonic PZ-300 LI, XMSJ, Shanghai, China). Afterward, the samples were filtered and processed under the same conditions as the infusion extraction.
2.1.4. Maceration
In order to compare it with a traditional extraction method, the dry leaf was placed in 70% ethyl alcohol in a 1:10 ratio. The samples were left to macerate in darkness for 7 days, after which they were filtered, and the solvent was subsequently removed by rotary evaporation. The extracts were stored in darkness at 4 °C.
2.2. Phytochemical Analysis
2.2.1. Total Polyphenol Content (TPC)
In a 2 mL tube, 125 µL of the sample (100–1000 µg/mL) was placed, and 750 µL of distilled water and 62.5 µL of Folin–Ciocalteu reagent (Sigma-Aldrich, Saint Louis, MO, USA) were added. Subsequently, 250 µL of distilled water and 187.5 µL of 20% Na2 CO3 were added, and the mixture was homogenized and incubated in darkness for 2 h. After this period, 200 µL of each reaction were placed in 96-well microplates, and their absorbance was measured at 750 nm in a microplate reader (BioRad, Hercules, CA, USA). A calibration curve of gallic acid (Sigma-Aldrich, Saint Louis, MO, USA) was constructed, and the results were expressed as mg equivalents of gallic acid per g of dry sample (mg GAE/g) [].
2.2.2. Total Flavonoid Content (TFC)
In a 2 mL tube, 125 µL of extract, 500 µL of distilled water, and 37.5 µL of 5% sodium nitrite (Sigma-Aldrich, Saint Louis, MO, USA) were added. The mixture was incubated at room temperature for 5 min, after which 37.5 µL of 10% aluminum chloride (Golden-Bell, Jalisco, Mexico) was added. The mixture was incubated for an additional 6 min, then 300 µL of distilled water and 250 µL of 1 M sodium hydroxide (NaOH) were added. The resulting mixture was homogenized, and 200 µL was transferred to a 96-well microplate and read at a wavelength of 510 nm (BioRad, Hercules, CA, USA). A calibration curve was constructed with quercetin (Sigma-Aldrich, Saint Louis, MO, USA), and the results were expressed as mg equivalents of quercetin per gram of dry extract (mg QE/g) [].
2.2.3. Total Condensed Tannins Content (CTC)
In a 2 mL tube, 1 mL of vanillin (Sigma-Aldrich, Saint Louis, MO, USA) (at 4% in methanol), 133 μL of the extract, and 500 μL of concentrated hydrochloric acid (Jalmek, Nuevo León, Mexico) were added. The mixture was vortexed and then incubated for 15 min. A total of 200 μL of the solution was transferred to a 96-well plate, and the absorbance was read at 500 nm (BioRad, Hercules, CA, USA). A calibration curve was established with catechin (Sigma-Aldrich, Saint Louis, MO, USA), and the results were expressed as mg equivalents of catechin per g of dry extract (mg CE/g) [,].
2.3. Antioxidant Capacities
2.3.1. DPPH Radical Assay
A stock solution of the DPPH• radical at 0.004% in methanol (Sigma-Aldrich, Saint Louis, MO, USA) was prepared, and 50 µL of this solution plus 50 µL of the extract to be evaluated were immediately added to a 96-well plate. The mixture was incubated for 30 min in dark conditions and then read at 515 nm on a plate reader (BioRad, Hercules, CA, USA). BHT (Sigma-Aldrich, Saint Louis, MO, USA) was used as a positive control, and the percentage of radical inhibition was expressed using Equation (1) [].
2.3.2. Metal Ion Chelating Activity Assay
In a 1.5 mL tube, 50 μL of FeCl2 (0.66 mM) (Sigma-Aldrich, Saint Louis, MO, USA) and 250 μL of the sample were added. The mixture was incubated for 15 min at 37 °C with agitation (every 5 min in the vortex). Subsequently, 200 μL of Ferrozine (1.66 mM) (Sigma-Aldrich, Saint Louis, MO, USA) was added, and the resulting mixture was vortexed and incubated for 10 min at 37 °C. After this time, the mixture was centrifuged at 14,000 × g for 5 min at 25 °C. The supernatants were placed in 100 µL aliquots in a 96-well microplate and then read at 565 nm (BioRad, Hercules, CA, USA). EDTA (Sigma-Aldrich, Saint Louis, MO, USA) was used as a positive control, and the chelating power was calculated using Equation (2) [].
2.3.3. Total Antioxidant Capacity (TAOC) Assay
The total antioxidant capacity (TAOC) was conducted using the ammonium molybdate method []. In a 2 mL tube, 900 μL of the stock solution (0.6 M sulfuric acid (Golden-Bell, Jalisoc, Mexico) + 28 mM sodium phosphate (Golden-Bell, Jalisco, Mexico) + 4 mM ammonium molybdate, 1:1:1 (Sigma-Aldrich, Saint Louis, MO, USA)) was added, followed by 100 μL of the sample. The mixture was heated to 95 °C in a dry bath for 1.5 h. Afterward, it was allowed to cool, and aliquots of 200 μL were transferred to a titration microplate, and the absorbances were recorded at 695 nm on a reader (BioRad, Hercules, CA, USA). Ascorbic acid (Sigma-Aldrich, Saint Louis, MO, USA) was used as a positive control, and the results were expressed as mM of ascorbic acid per gram of dry extract (mM AA/g).
2.4. Synthesis of Zinc Oxide Nanoparticles
A zinc sulfate solution (Golden Bell, Mexico) was prepared by dissolving 11.5 g in 50 mL of distilled water. The solution was stirred for 30 min, after which 10 mL of Psidium cattleianum leaf extract (infusion, ultrasound, or maceration) was added drop by drop. The mixture was stirred for an additional 30 min. During this process, 0.1 M sodium hydroxide (Golden Bell, Mexico) was added until the pH reached 12 (Hanna, HI98115, Shanghai, China). The reaction was left to stir continuously for 12 h at room temperature. The precipitate was centrifuged with two washes using distilled water at 10,000 rpm for 10 min at 25 °C (LaboGene LZ-1580 R, Bjarkesvej, Denmark). The precipitate was collected and dried in a domestic microwave oven (Daewoo, DMDP07 S2 CW, Seoul, Republic of Korea) at a power of 300 W. Subsequently, it was ground in an agate mortar until a fine powder was obtained. This procedure was repeated for each of the extracts obtained to produce nanoparticles.
2.5. Nanoparticles Characterization
2.5.1. UV-VIS-NIR Spectroscopic Analysis
The UV-vis-NIR spectra of the nanoparticle samples were acquired at room temperature using a Duetta Fluorescence and Absorbance Spectrometer (Horiba Scientific, VS70-MC, Anyang, Republic of Korea), which uses quartz cuvettes of different path lengths. Ethanol was used as a solvent to disperse nanoparticles with sonication.
2.5.2. Band Gap Analysis
The optical band gap (Eg) of ZnO NPs was estimated with the absorption spectra through Tauc’s formula (Equation (3)).
where B is a constant related to the material, hv is the photon energy (eV), h is the Plank’s constant, v is the frequency of the photon, Eg is the optical band (eV), and n is an exponent, it can be considered as 2 or ½ to direct transition or indirect transition, respectively. Finally, α is the absorption coefficient (cm−1) that can be calculated using α = 2.302 A. The Eg was estimated as the photon energy at which a linear fitting of the (αE)1/2 function around the absorption edge equals zero.
2.5.3. Scanning Electron Microscopy (SEM)
SEM equipment (JSM-IT300, JEOL, Tokyo, Japan) was used at 10 kV and 40,000× to visualize the morphology and size.
2.5.4. X-Ray Diffraction
The dry nanoparticles were grounded in an agate mortar and placed on a slide to collect the diffractograms on an X-ray diffractometer (Malvern Panalytical Empyrean, Grovewood Road, UK) with CuKα radiation, using a 2θ angle from 5° to 70° with a step of 0.02 and a collection time of 30 s per step. The degree of crystallinity was calculated by Equation (4) [].
where AC is the crystalline area and AA is the amorphous area.
2.5.5. Infrared Spectroscopy by Fourier Transform (FTIR)
The ZnO NPs samples were measured by FTIR in an absorbance mode with KBr pellets on a Nicolet iS20 FTIR spectrometer (Thermo Scientific, Waltham, MA, USA).
2.5.6. Thermogravimetric Analysis (TGA)
The thermal analysis was performed using a TGA Q500 analyzer (TA Instruments, New Castle, DE, USA) equipment with a heating ramp of 10 °C/min and nitrogen flow of 40 mL/min.
2.5.7. Zeta Potential and Particle Size
For zeta potential analysis and particle size, nanoparticles were dispersed in HPLC water (Fermont, Nuevo León, Mexico) and sonicated for 10 min in an ultrasonic bath at 40 kHz and 120 W. Dispersions were analyzed in a Zetasizer Nano (Malvern, UK), and measurements were taken at 25 °C.
2.6. Antimicrobial Capacity of Nanoparticles
Escherichia coli O157:H7, Salmonella enteritidis, Pseudomonas aeruginosa, and Staphylococcus aureus were inoculated in Mueller–Hinton (MH) broth for 18–24 h at 37 °C. Subsequently, the concentration was measured using optical density at 600 nm (OD600, Optizen POP, Daejeon, Republic of Korea). The strains were adjusted by serial dilution to achieve a final 1 × 106 cells/mL concentration. Then, aliquots of 20 µL were inoculated into 160 µL of MH broth with 20 µL of ZnO NPs at final concentrations of 5 mg/mL, 8 mg/mL, and 10 mg/mL in a 96-well microplate. In the control wells, 20 μL of the strains were added to 180 μL of MH broth. The microplate was then incubated for 18 to 24 h at 37 °C. The plates were read on a BioRad iMarkTM device at 600 nm. The bacterial growth inhibition rate (BGIR) was estimated using Equation (5) [,].
2.7. Antibiofilm Evaluation of Nanoparticles
2.7.1. Congo Red Assay
The bacteria were inoculated in Luria Bertani (LB) broth for 18–24 h at 37 °C. Subsequently, the concentration was measured using OD600, and the strains were adjusted using serial dilution to achieve a final concentration of 1 × 106 cells/mL. Then, 20 µL aliquots were inoculated into 160 µL of LB broth with 20 µL of ZnO NPs at 5, 8, and 10 mg/mL concentrations in a 96-well microplate. In the control wells, 20 µL of the strains were added to 180 µL of LB broth. The microplate was then incubated for 18 to 24 h at 37 °C. After incubation, 50 µL aliquots from each microplate well were inoculated onto Congo red agar prepared with Mueller–Hinton Agar supplemented with 5% glucose and Congo red dye (Golden-Bell, Mexico). These were aerobically incubated for 24 h at 37 °C. The color of the individual colonies was inspected after 24 h. According to the degree of biofilm formation, dark colonies with crystalline consistency indicate strong biofilm production (−, considered as no inhibition), and weak slime producers usually remain pink (+++, considered a strong inhibitor) [,,].
2.7.2. Violet Crystal Assay
The procedure followed the methodology reported by [], albeit with minor modifications. Biofilm acquisition commenced from microdilution assays (Section 2.6). Following the incubation period, 100 μL from each well were retrieved and added to a new 96-well microplate, after which the liquid was gently shaken. Subsequently, two washes were performed by immersion in distilled water. The resulting biofilm was stained with 125 µL of crystal violet (Golden-Bell, Mexico) in each microplate well and incubated at room temperature for 15 min. After incubation, the crystal violet solution was removed, and four washes with distilled water were conducted. Excess water was eliminated by vigorously shaking the plate over a paper towel. The plate was then left to dry for 3 h. To quantify biofilm formation, 125 µL of 30% acetic acid was added to each microplate well to solubilize the crystal violet. The plate was then incubated at room temperature for another 15 min. After this second incubation, 125 µL from each well were transferred to a new microplate. The absorbance was quantified using a microplate reader at 540 nm. Biofilm inhibition percentage was calculated using Equation (6) []. The following categories were considered: Good inhibitors (50%) and Weak inhibitors (<50%).
where Abssample is the absorbance of all the reagents and nanomaterials and Abscontrol is the absorbance of all the reagents without nanomaterials.
2.8. Nanoparticles Toxicity
2.8.1. Primary Cell Culture and Cell Viability in Goats
Peripheral blood was collected from healthy Saanen × Nuvian crossbred goats into Vacutainer® tubes (Franklin Lakes, Bergen Country, NJ, USA) with heparin, following approval from the bioethics committee for animal experimentation at the Northwest Biological Research Center (CIBNOR La Paz, Mexico). The blood was transferred to 50 mL conical tubes and mixed with RPMI-1640 medium with heparin. From this mixture, 1.25 mL was transferred, and 2 mL of Histopaque®-1077 was added, followed by centrifugation at 1500 rpm for 20 min at 25 °C. After centrifugation, the leukocyte layer was extracted and transferred to a new tube, which was washed with PBS (2000 rpm, 10 min, 25 °C). Subsequently, the mixture was decanted, and 2 mL of RPMI medium was added to the mix with 10% fetal bovine serum (FBS). Finally, in a 96-well plate, 90 μL of leukocytes (1 × 106 cells/mL) were combined with 10 μL of ZnO NPs and incubated at 37 °C with 5% CO2 for approximately 24 h. After this period, 10% resazurin (Sigma-Aldrich, Saint Louis, MO, USA) was added, and the mixture was incubated for 4 h at 37 °C with 5% CO2. The absorbance was then measured at 560 nm excitation and 590 nm emission using a Varioskan™ Flash Multimode Reader (ThermoScientific, Waltham, MA, USA) [].
2.8.2. Cell Viability on 3 T3 L-1
T3-L1 Cell Culture and Differentiation
3 T3-L1 preadipocytes (ATCC® CL-173™) were obtained from the Immunology Laboratory at the University Center for Health Sciences, University of Guadalajara. The cells were seeded in Petri dishes at a density of 1 × 105 cells per plate. Dulbecco’s Modified Eagle Medium (Sigma-Aldrich; Saint Louis, MO, USA) supplemented with 10% calf bovine serum (CBS; Cytiva; HyClone, Marlborough, MA, USA) and 1% antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin (Gibco; Thermo Fisher Scientific; Waltham, MA, USA)) was used for preadipocyte seeding and proliferation. The medium was replaced every 2–3 days until 100% confluence was reached. Then, the adipogenic differentiation process started using a differentiation kit (3 T3-L1 Differentiation Kit; Sigma-Aldrich; Saint Louis, MO, USA), following the manufacturer’s protocol. On day 0, the medium for proliferation was changed to a differentiation medium that contained DMEM/F-12 (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (Thermo Fisher Scientific, Waltham, MA, USA), 1% antibiotics, and an adipogenic cocktail including 500 µM 3-isobutyl-1-methylxanthine (IBMX), 1 µM dexamethasone, 1.5 µg/mL insulin, and 1 µM rosiglitazone (3 T3-L1 Differentiation Kit; Merck KGa, Boston, MA, USA) for 3 days. On day 3, the differentiation medium was replaced with a maintenance medium composed of DMEM/F12 with 10% FBS, 1% antibiotics, and 5 μg/mL insulin, which was replaced every 2–3 days until the 8-day differentiation period was completed. All procedures were conducted under constant conditions of 37 °C and 5% CO2 in a cell incubator (Sheldon Manufacturing, Inc., Cornelius, OR, USA).
T3-L1 Cells Treatment
Three study groups were formed: negative control (preadipocytes), adipocytes, and ZnO NPs (adipocytes stimulated with NPs). The vehicle group was stimulated with 0.5% DMSO. A dose–response curve was conducted with the nanoparticles using 1, 2, 4, 8, and 16 µg/mL of nanoparticles over 48 h.
MTT Assay on 3 T3-L1 Adipocytes
3 T3-L1 cells were seeded at 5 × 103 cells/well in 96-well plates. Four study groups were formed: negative control (preadipocytes), adipocytes, ZnO NPs (adipocytes stimulated with NPs), and the vehicle group, which was stimulated with 0.5% DMSO. A dose–response curve was conducted using 1, 2, 4, 8, and 16 µg/mL of nanoparticles for 48 h. Then, a 1 mg/mL MTT solution (Invitrogen; Thermo Fisher Scientific, Inc.; Waltham, MA, USA) was added to the adipocytes, and the cells were incubated for 1 h at 37 °C. The formazan crystals were dissolved using an extraction buffer (20% SDS and 50% dimethylformamide), followed by spectrophotometric measurement at 570 nm using a microplate reader (MultiScan GO; Thermo Fisher Scientific, Inc., Waltham, MA, USA) [].
Oil Red O Staining
3 T3-L1 cells were seeded at 5 × 103 cells/well in 96-well plates and subjected to the adipogenic differentiation process. After completing the 8-day differentiation period, the four study groups were formed, and a dose–response curve was conducted using 1, 2, 4, 8, and 16 µg/mL of nanoparticles for 48 h. Then, the cells were fixed with a 10% formaldehyde solution (v/v) for 60 min at room temperature and washed three times with distilled water. Lipid droplets in the adipocytes were stained with Oil Red O solution (Merck KGaA, Boston, MA, USA) for 15 min and rewashed three times with distilled water. The Oil Red O stain was dissolved after adding 100% isopropanol (IBI Scientific, Dubuque, IA, USA), and absorbance was measured at 515 nm using a microplate reader (Multiskan GO; Thermo Fisher Scientific, Inc., Waltham, MA, USA) [].
2.9. Statistical Analysis
All experiments were performed in triplicate and expressed as mean ± standard deviation (SD). They were subsequently subjected to an analysis of variance (ANOVA) followed by a Tukey post hoc test at a significance level of α = 0.05. The statistical package Statgraphics Centurion XIX (The Plains, VA, USA) was used for these analyses.
3. Results and Discussion
3.1. Phytochemical Content of Psidum Cattleianum Leaves
Table 2 shows the results of total polyphenols, flavonoids, and condensed tannins for the three extraction methods employed on Psidium cattleianum leaves. For polyphenols, the highest quantity was observed for the infusion technique (269.89 mg GAE/g at concentrations of 1 mg/mL of the sample), followed by ultrasound (239.28 mg GAE/g), and maceration (168.41 mg GAE/g) (p < 0.05). A similar trend was also observed for tannin content, with infusion and maceration presenting the highest values (13.67 and 10.59 mg CE/g, respectively) (p < 0.05). Previous studies have reported a polyphenol content in Psidium cattleianum leaves similar to this work using ultrasound techniques with optimized extraction conditions, yielding 275.75 mg GAE/g [], and extracts obtained through ultrasonic baths during winter and summer, reporting values of 144 and 101 mg GAE/g, respectively, which are lower than those found in this study [].

Table 2.
Total polyphenol, flavonoid, and condensed tannin content of Psidium cattleianum leaf extracts.
Regarding flavonoid content, higher amounts were observed for the ultrasound and maceration techniques (483.25 and 482.5 mg QE/g). Values for Psidium cattleianum fruits have been reported at 4.98 mg QE/g [], with the values reported in this study for leaf extracts being higher across all three techniques employed.
It has been documented that ancestral practices in tea preparation, such as infusion and decoction, surpass the content of compounds derived from hydroxycinnamic acid compared to techniques like ultrasound or maceration with ethyl alcohol. One possible explanation for using infusion or decoction techniques is using hot plates to achieve greater extraction of hydroxycinnamic acid derivatives []. It should be noted that the unconventional method of ultrasound does not present significant differences compared to infusion regarding polyphenols, making it a good alternative if the aim is to reduce extraction time without compromising other classes of bioactive compounds affected by heat, for example, the high flavonoid content found (483.25 mg QE/g) compared to infusion [,]. It is known that techniques such as ultrasound and maceration favor extraction in the flavonoid family by not compromising the temperature of the process. Furthermore, in the case of ultrasound, the mass transfer of plant material to the solvent is enhanced by cavitation [].
Extraction with ultrasound energy applies cavitation and mechanical mixing. This procedure allows the breakdown of cells and liberation of different components such as polyphenols, flavonoids, and tannins; also, since it is a non-thermal method, their degradation due to heat exposure is minimized []. Although maceration produces extracts rich in polyphenol components, their concentration is related to the relation of solvent used, and it requires extended time compared to infusion and ultrasound [].
3.2. Antioxidant Activities of Psidium Cattleianum Extracts
Figure 1 shows the antioxidant responses of P. cattleianum leaf extracts measured by the DPPH, chelating activity, and TAOC techniques. For infusion extraction, regarding the ability to sequester the DPPH radical (Figure 1A), concentrations starting from 100 µg/mL were able to inhibit the radical by over 50%, with no significant differences compared to the positive control (BHT) (p > 0.05). For chelating activity (Figure 1B), at concentrations of 400 µg/mL, inhibitions close to 80% were achieved, although with lower activity than the positive control EDTA (p < 0.05). Figure 1C represents the TAOC antioxidant activity, where at 600 µg/mL, it reaches an activity close to 400 mM equivalents of ascorbic acid, which is greater than the positive control (p < 0.05). Similar trends can be observed for the ultrasound methods (Figure 1D–F) and maceration, the latter only showing results for the DPPH assays (Figure 1G, achieving inhibitions close to 60%) and TAOC (Figure 1I, reaching values close to 400 mM equivalents of ascorbic acid). However, for chelating capacity (Figure 1H), maceration did not perform favorably, as it only reached values around 10% with marked significant differences compared to the control (p < 0.05).
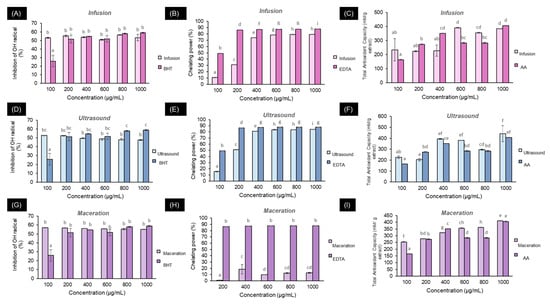
Figure 1.
Antioxidant capacity of Psidium cattleianum extracts for the different extraction methods: (A,D,G) DPPH assay; (B,E,H) Chelating activity assay; (C,F,I) Total antioxidant activity. Different lowercase letters indicate significant differences according to Tukey’s post hoc test at p < 0.05. For each figure, lowercase letters indicate significant differences (p > 0.05).
As observed for quantifying phytochemicals, infusion and ultrasound extraction methods were favored for their antioxidant capacity compared to maceration. In addition to the polarity of both methods using water as a solvent, in the specific case of infusion, the abundance of hydroxycinnamic acids likely contributes to the antioxidant response due to their ability to sequester free radicals and chelate metal ions []. Chelating capacity is favored at physiological pH; thus, the chemical structure is dependent on pH and, consequently, its binding with metals []. Evidence shows that anionic forms are more susceptible to binding with heavy metals such as Fe than neutral compounds, as reported for caffeic acid, which, in pH-dependent environments, demonstrated different chelating capacities against Fenton-induced oxidative damage [,,].
3.3. Characterization of Zinc Oxide Nanoparticles
The UV-vis absorption spectrum (Figure 2a) reflects the maximum absorbance around 365 nm for zinc oxide nanoparticles synthesized by the infusion method, 357 nm for the maceration method, and 371 nm for the ultrasound method. Additionally, the band gaps are 3.42 eV for infusion (Figure 2b), 3.38 eV for ultrasound (Figure 2c), and 3.4 eV for maceration (Figure 2d), with these values related to electron transitions from the valence band to the conduction band. The negative value from the band gap is related to lower compressive stress from compact ZnO to nanostructures, which is also related to photocatalytic and biological activities []. Similar results have been reported for zinc oxide nanoparticles reduced with extracts from Mussaenda frondosa L. and Cassia auriculata [,], as well as for the reduction in ZnO NPs using extracts from Psidium guajava that exhibit UV ray protection in cotton fabrics and antimicrobial activity against E. coli, S. aureus, and C. albicans [,,]. To our knowledge, this is the first report on synthesizing ZnO NPs using extracts from Psidium cattleianum.
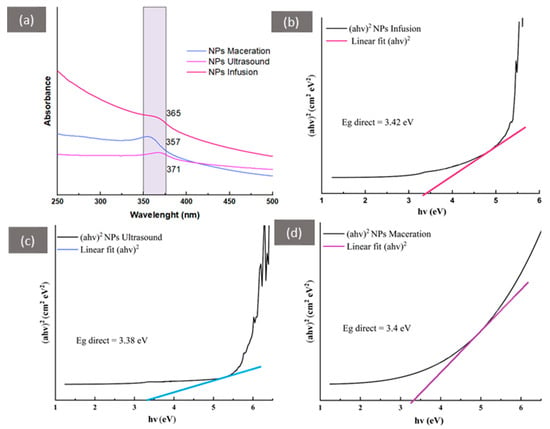
Figure 2.
UV-vis spectroscopy of zinc oxide nanoparticles synthesized with different extracts of Psidium cattleianum: (a) Characteristic region confirming nanoparticle confinement; (b–d) Band gap calculations (Tauc’s plot) for ZnO NPs formed with the different extraction methods.
Figure 3 shows the diffractograms for the synthesized nanoparticles, which were compared with international databases, with 2θ angles of 31.83°, 34.5°, 36.28°, 47.53°, 56.58°, 62.88°, 66.17°, 67.96°, and 69.06°, corresponding to Miller indices (100), (002), (101), (102), (110), (103), (200), (112), and (201), matching the ICDD card #361451, and indicating the hexagonal crystalline structure corresponding to wurtzite []. This result is corroborated by other nanosystems synthesized via green methods for forming ZnO NPs. To visualize a possible difference among the three nanosystems, a higher percentage of crystallinity was calculated using the ultrasound extract (98%), followed by infusion (84%) and maceration (72%) extracts. These variations in crystallinity are mainly attributed to different components obtained through different extraction methods, which help to stabilize and form the nanoparticles []. These results are comparable to a study where zinc oxide nanoparticles were synthesized with extracts from Eucalyptus globulus leaves, achieving a crystallinity percentage of 99.49% [].
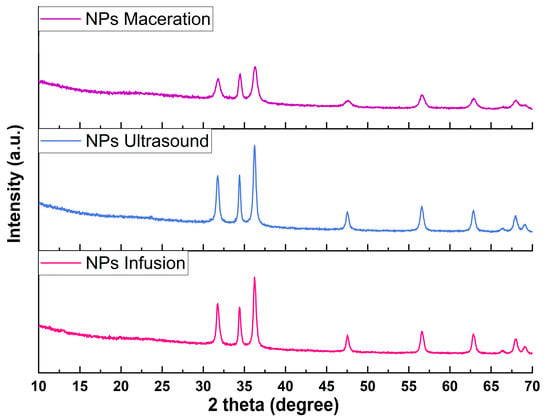
Figure 3.
X-ray diffractograms of zinc oxide nanoparticles.
Figure 4 shows the morphologies corresponding to the ZnO NPs. Nanoparticle size estimated with SEM for infusion was of 214.80 ± 122 nm, for maceration of 389.70 ± 20 nm, and ultrasound assisted nanoparticles exhibited sizes of 102.19 ± 40 nm. This distribution can be observed in Figure 4a–c. The structure observed in the three micrographs corresponds to irregular hexagonal shapes typical of wurtzite, and agglomerations become more evident in Figure 4c. It has been reported that the presence of functional groups such as alcohol, alkane and carboxylic acid, contribute to the formation of spherical and hexagonal structures in nanoparticle synthesis []. These functional groups can be corroborated in the FTIR analysis shown below.
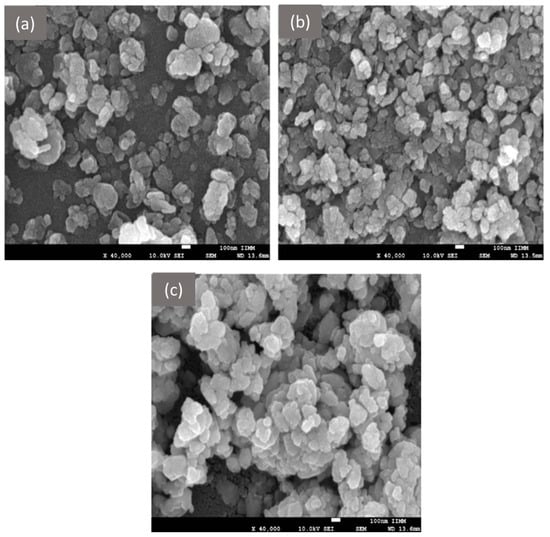
Figure 4.
Morphology obtained by SEM of ZnO NPs: (a) Infusion NPs, (b) Ultrasound NPs, and (c) Maceration NPs.
Values of different sizes considered not only single particles but also agglomerations that could be seen in the micrographs. To elucidate better the particle size, distribution, and Z-potential, ZnO NPs were analyzed in a zeta sizer; results are shown in Table 3.

Table 3.
Particle size, polydispersity index, and zeta-potential of NPs infusion, NPs maceration, and NPs ultrasound obtained through Dynamic Light Scattering.
The nanoparticles produced with the ultrasound extract had the smallest size, lower PDI, and most negative Zeta potential. This results in a more stable nanoparticle in solution since they will likely not aggregate; also, the size distribution is more homogeneous than the ones obtained with infusion and maceration, and with smaller size, biological activities such as antimicrobial performance can be enhanced [,]. The ultrasound extract had higher TPC than maceration and TFC than infusion (Table 2); this combination of compounds may lead to these characteristics of the nanoparticles obtained [,]. It has been reported that smaller particle sizes exhibit more negative z-potentials. The composition of the extract used for green synthesis of nanoparticles impacts the size and z-potential since different components may increase the ion-reduction rate; also, the concentration of these reducing agents changes depending on the extraction method applied []. The results obtained for ultrasound-assisted zinc oxide nanoparticles in this study are similar to those reported by Yedurkar et al. [] with Ixora coccinea leaf extract, resembling both size and z-potential of nanoparticles (145 nm, −49.19 mV, respectively).
The infrared spectra (Figure 5) show the contributions of functional groups for the ZnO NPs synthesized with different extracts. It can be observed that the three spectra share similarities in the regions approximately at 3400, 1390, and 418 cm⁻¹, corresponding to the OH groups, C-H bending, and Zn-O, respectively. The band’s intensity at 3400 cm⁻¹ can be attributed to the forming of intra- and intermolecular hydrogen bonds due to interaction with phenolic compounds. The region around 1390 cm⁻¹ can be attributed to C-H vibrational bending due to the presence of proteins in the extract []. The metal bond appears near 418 cm⁻¹ and confirms the 400 to 500 cm⁻¹ range, which verifies the formation of zinc oxide nanoparticles []. As can be seen, there are differences between the spectra with some contributions (Table 4), which may be due to the nature of the extract and its quantity of secondary metabolites. For example, a difference is observed with maceration, where a signal at 2931 cm⁻¹ appears, corresponding to the stretching vibration of C-H present in certain phytochemical groups, and this vibration generates another signal at 1608 cm⁻¹ corresponding to C=C vibration. These signals have been reported for ethanolic extracts of Cucurbita pepo L. for the bio reduction in zinc nanoparticles and align with our findings []. Additionally, it has been reported that the C-O bond may appear in the range of 1049 to 1131 cm⁻¹ and is attributed to flavonoids and terpenes characteristic of plants. For infusion, bands at 1131 and 1059 cm⁻¹ can be observed, while ultrasound only presents a contribution at 1049 cm⁻¹. Maceration also shows a contribution at 1122 cm⁻¹, possibly related to phenols []. Other differences found between the spectra indicate that NPs using infusion or ultrasound exhibit more vibrations compared to maceration; for instance, the contribution of Zn-OH or interactions of the OH group with zinc oxide nanoparticles (895, 833 and 601 cm⁻¹) does not appear for maceration and could be associated with the protonation of acidic groups on the surface of the ZnO NPs [,], which could have been present in the extract as part of hydroxycinnamic acid derivatives [].
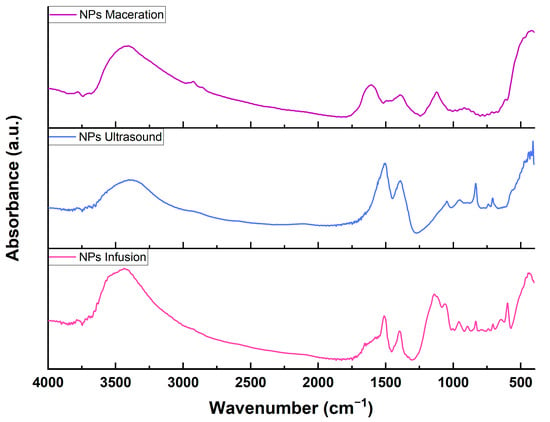
Figure 5.
Fourier Transform Infrared Spectroscopy of zinc oxide nanoparticles reduced with extracts of Psidium cattleianum.

Table 4.
Contributions of functional groups via FTIR spectroscopy of ZnO NPs formed with different extracts.
Just as the IR spectra show differences in their fingerprints, similar observations can be made in Figure 6 for thermogravimetric analysis. Between 100 and 200 °C, the evaporation of water is observed, while above 200 °C, losses of phytochemicals associated with the extracts that facilitate the reduction in the nanoparticles are noted. The lowest total weight loss is observed for the NPs synthesized by ultrasound (6.25%, Figure 6c), followed by maceration (15.55%, Figure 6d), and finally infusion (18.01%, Figure 6b). This suggests that the NPs obtained from the ultrasonic extract were more selective for phenolic compounds than other aromatic structures or primary plant metabolites, as indicated by fewer peaks in their derivatives or decomposition stages []. No losses are observed above 600 °C, which ultimately confers thermal stability to all three systems []. Other studies have correlated the amount of phenolic compounds and small proteins through TGA analysis, suggesting two decomposition stages: the first around 200–300 °C corresponding to phenolic compounds added to the surface of the nanoparticles, and above 400 °C attributed to the loss of resistant aromatic compounds present in the extracts, as seen in the case of gold nanoparticles synthesized with Verbascum thapsus and Ricinus communis [], and in similar cases such as the synthesis of ZnO nanoparticles using pineapple peels []. Thermal events similar to those in this study have also been reported for ZnO nanoparticles synthesized with extracts from Passiflora foetida peels, where the thermogram shows a decomposition associated with phytochemicals of around 3.9% and a total weight loss of approximately 6%, with this study reporting a peak related to phytochemicals of 2.459% and a total loss of 6.25% []. These findings are consistent with those reported here for XRD analysis, as the NPs using ultrasonic extracts exhibited greater crystallinity and, through TGA, showed lower weight loss, resembling traditional chemical synthesis, making it a good candidate for being environmentally friendly.
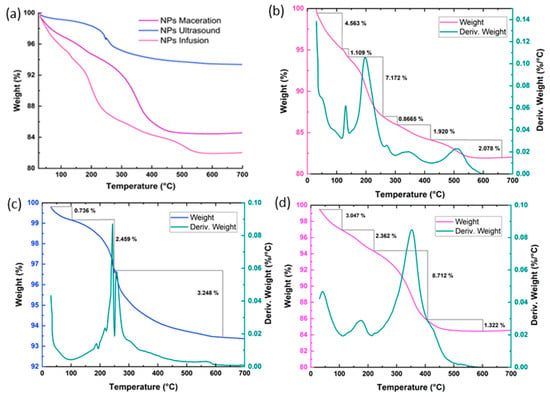
Figure 6.
Thermogravimetric analysis of nanoparticles reduced with extracts of Psidium cattleianum: (a) Thermograms of the NPs; (b) Thermogram and the first derivative of the NPs using infusion extract; (c) Thermogram and the first derivative of the NPs using ultrasound extract; and (d) Thermogram and the first derivative of the NPs using maceration extract.
3.4. Antibacterial and Antibiofilm Activities
Figure 7 shows the antimicrobial activity of the ZnO NPs, where a dose-dependent response can be observed for all treatments and bacteria. The ZnO NPs obtained by ultrasound favored inhibitions exceeding 75% for all bacteria except for P. aeruginosa (p < 0.05), which reached values close to 60% reduction. For the infusion-derived ZnO NPs, the highest inhibition value recorded was for Salmonella enteritidis, with a percentage above 75%, while for the other bacteria, values above 60% were noted. In contrast, the ZnO NPs from maceration showed slight differences, exceeding 60% inhibition for all bacteria except for P. aeruginosa, which recorded inhibition values around 40% at the highest concentrations. It is known that ZnO nanoparticles inhibit the viability of a wide range of bacteria by inducing damage to microbial cells through the generation of hydrogen peroxide from the surface of ZnO NPs and can also enter bacteria by interacting with phosphorus- and sulfur-containing compounds, such as bacterial DNA []. ZnO nanoparticles have a negative charge, as previously mentioned in Table 3, so they can interact with the cell walls of gram-negative and positive bacteria. The antibacterial mechanism has not been completely elucidated; still, it has been proposed that nanoparticles can attach to different parts of the cell wall, either with porins and lipopolysaccharides in gram-negative bacteria or only to peptidoglycan of the gram-positive bacteria, which conducts to disruption and, thus, cell lysis []. Similar studies using ZnO NPs with Saraca asoca extract against Bacillus subtilis show a dose-dependent response and optimal inhibition at 5 mg/mL, likely due to maximum absorption capacity in the medium []. In this study, the dose was slightly higher (10 mg/mL). Still, we selected a panel of clinical pathogens, such as enterohemorrhagic E. coli, providing new data on the use of zinc NPs. Another similar study highlights the importance of evaluating challenging pathogens such as vancomycin-resistant S. aureus, where the authors found that at a dose of 10 mg/mL, the growth of the resistant bacteria was inhibited entirely []. It has been demonstrated that the antibacterial capabilities of ZnO nanoparticles correlate directly with their dose and the surface area of the particles, meaning that size and morphology are also subject to the synthesis technique or the origin of the phytochemicals used, as in the case of green synthesis [,].
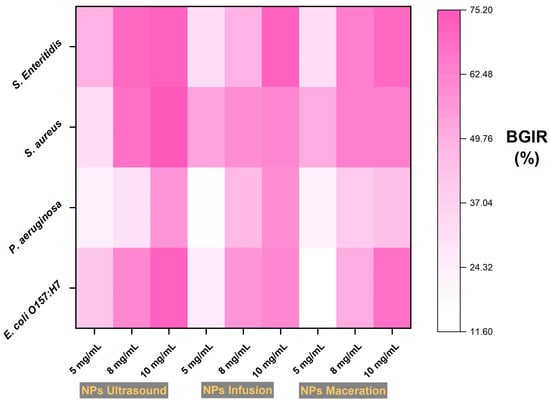
Figure 7.
Percentages of bacterial inhibition using ZnO NPs with extracts of Psidium cattleianum. BGIR: Bacterial Growth Inhibition Rate.
For comparison between aqueous extracts (infusion and ultrasound) and given that the NPs from maceration exhibited lower activity against P. aeruginosa, the antibiofilm activity of the NPs was evaluated, excluding the ZnO NPs from maceration. As shown in Table 5, results of biofilm inhibition using Congo red and crystal violet methods are presented. At the highest concentrations, weak inhibitions are observed for infusion regarding P. aeruginosa and S. aureus, achieving reduction percentages of 40.22% and 34.30%, respectively, in the crystal violet assay, with qualitative values of intermediate inhibitors marked as (++) in Congo red assay. For the ultrasound-derived NPs at high concentrations in the crystal violet assay, all bacteria are categorized as good inhibitors. In the Congo red assay, they reach strong inhibitor categories (+++), as the typical black crystalline colonies were not observed.

Table 5.
Congo red and crystal violet assays for biofilm performances at different treatments on pathogenic bacteria.
Recently, zinc oxide (ZnO) nanoparticles have gained popularity due to their remarkable toxicity toward drug-resistant microorganisms. These nanoparticles have a distinct antimicrobial mechanism of action. First, they destroy the bacterial cell wall, then enter the cell, and finally accumulate in the cell membrane, leading to bacterial death. Additionally, ZnO NPs can alter the microenvironment surrounding the bacteria by releasing reactive oxygen species (ROS) and Zn²⁺ ions, causing damage to lipids, proteins, carbohydrates, and DNA through oxidative stress, lipid peroxidation, and disruption of critical cellular functions. Furthermore, these nanoparticles are preferred in the cosmetic and food industries, as the U.S. Food and Drug Administration considers them safe, categorizing them as an effective agent against biofilms in various processes []. ZnO NPs interaction with DNA can lead to biofilm formation irruption, as has been demonstrated by Hassani et al. [], in which research the pslA gene was found in bacterial biofilm treated with ZnO nanoparticles after formation, but the presence of this gene was significantly lower when nanoparticles were in contact before biofilm formation.
3.5. Toxicity
Figure 8 shows the viability of the ZnO NPs from infusion and ultrasound on peripheral blood leukocytes from goats, where both types of nanoparticles are demonstrated to be safe at all studied concentrations, showing no decrease in viability (p < 0.05). Other studies have shown that in mouse splenocytes, concentrations above 5 µg/mL reduce viability by more than 60% []. The authors suggested that toxicity depends on the size and surface charge of zinc nanoparticles, with negatively charged NPs having a more significant influence on toxicity. Other authors have investigated the interaction of ZnO nanoparticles with different cell lines, resulting in Zn ion uptake or ROS formation effective against cancer cells []. Leukocytes may increase myeloperoxidase and catalase activities, which can control ROS negative effects []. In this study, we analyzed the zeta potential of ZnO NPs using a Zetasizer Nano (Malvern, UK), finding that all three systems were negatively charged, with values of −43.2 mV for the ultrasound-derived NPs, followed by −33.6 mV for the infusion-derived NPs.
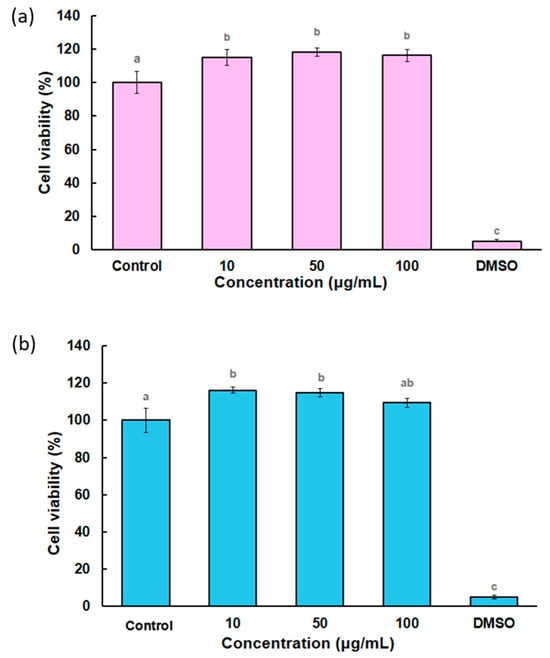
Figure 8.
Percentages of cell viability in peripheral blood from goats: (a) ZnO NPs infusion (pink bars) and (b) ZnO NPs ultrasound (blue bars). Different letters indicate significant differences (p < 0.05).
This makes the nanoparticles studied in this research good candidates for various applications, as a 100% viability was observed even at a concentration of 100 µg/mL, similar to the values reported for viabilities using a mouse-derived spermatogonia cell line (GC-1), where similar values were achieved in that study using a synthetic chalcone with antioxidant capacity to mitigate the toxic effect of ZnO NPs []. This could pave the way for future nanotoxicity studies, as green synthesis using different extracts or biological sources may confer different toxicity-related activities to each formed nanosystem, thereby reducing the negative impact of purely chemical nanoparticles, such as ZnO.
Finally, the ZnO infusion-derived NPs were chosen to evaluate other viabilities with antiadipogenic applications, as the infusion is the most documented technique in green synthesis. They exhibited the same viabilities as the ultrasound-derived NPs in the goat leukocyte assay. In this regard, Figure 9 presents the MTT assay in 3 T3-L1 cells, where around 100% of the viabilities are observed for all studied concentrations. The ZnO NP doses were not toxic to cell viability; however, no effect was observed on reducing lipid droplet content (oil red staining assay). Although the 16 µg/mL dose slightly decreased, these results were not statistically significant. Muthuraman et al. [] conducted a study with 3 T3-L1 cells and evaluated cytotoxicity through the SRB assay for doses ranging from 0.001 to 20 mg/L. The higher doses, 15 and 20 mg/L, were toxic to the cells and were therefore discarded. They worked with doses of 1 mg/L, 2 mg/L, and 4 mg/L. Regarding lipid droplet accumulation, they performed Oil Red O staining, as was performed in this study, and reported an increase in lipid accumulation with all three doses of ZnO nanoparticles. This demonstrates that ZnO nanoparticles do not reduce lipid droplet accumulation in 3 T3-L1 cells; conversely, they showed more significant accumulation. This finding could promote future investigations into using nanoparticles as adjuvants in cellular differentiation in anti-obesogenic models.
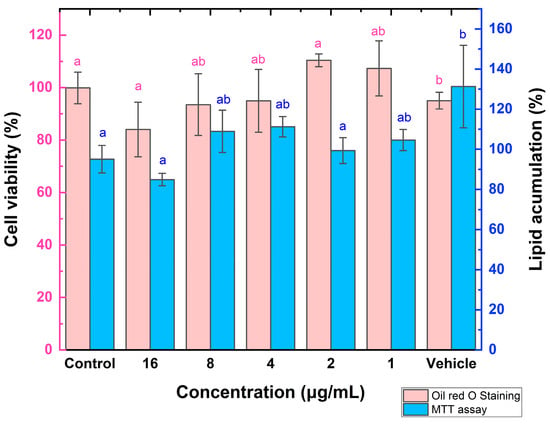
Figure 9.
Percentages of cell viability and lipid accumulation in 3 T3-L1 cells. Different letters indicate significant differences (p < 0.05).
4. Conclusions
The effect of the extraction method on the reduction in zinc oxide nanoparticles was evaluated, where hexagonal wurtzite-like morphologies were obtained, confirmed by UV-vis, XRD, and SEM analyses, in addition to thermogravimetric and FTIR analyses. The zinc oxide nanoparticles synthesized using the ultrasound extract were favored in terms of morphology, crystallinity, and functionalization. Furthermore, they demonstrated the ability to reduce pathogenic gram-positive and gram-negative bacteria and a strong antibiofilm capacity designated as a potent inhibitor of biofilm formation for all studied strains. Additionally, this nanosystem showed no cytotoxicity in the evaluated cells and provides insights into global trends in green synthesis by assessing different extracts from plants, algae, or microorganisms for the functionalization of zinc oxide nanoparticles with biological activities, corresponding to the extraction method of bioactive or phytochemical compounds. Overall, green synthesis using ultrasonic energy provided a more efficient zinc oxide nanoparticle with smaller particle size, higher crystallinity, and antimicrobial activity against foodborne pathogens compared to traditional extracts, highlighting that ultrasound is a green technology that does not require high levels of energy and uses eco-friendly solvents.
Author Contributions
Conceptualization, J.M.S.-J., M.R.-B. and J.J.R.-V.; methodology, C.I.P.-H., P.R.O.-S. and M.L.-O.; software, J.M.S.-J.; validation, C.I.P.-H., A.F.-G., C.A.V.-C. and M.E.M.-R.; formal analysis, J.M.S.-J., A.F.-G., M.R.-B. and J.J.R.-V.; investigation, C.I.P.-H., J.M.S.-J. and C.A.V.-C.; resources, M.R.-B., A.F.-G., J.J.R.-V., M.E.M.-R. and J.M.S.-J.; data curation, C.I.P.-H., C.A.V.-C. and J.M.S.-J.; writing—original draft preparation, J.M.S.-J.; writing—review and editing, C.A.V.-C. and L.M.A.-E.; visualization, J.M.S.-J.; supervision, C.A.V.-C. and L.M.A.-E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Animal procedures followed the Guide for Care and Use of Laboratory Animals of the National Institute of Health (USA) and the Institutional Animal Care and Use Committee (IACUC) of Centro de Investigaciones Biológicas del Noroeste, S.C., La Paz, Baja California Sur, México. Permit Number: IACUC-20190179.
Data Availability Statement
Data are contained within the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Authors thanks to CONHACyT México.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Becker, J.; Manske, C.; Randl, S. Green Chemistry and Sustainability Metrics in the Pharmaceutical Manufacturing Sector. Curr. Opin. Green Sustain. Chem. 2022, 33, 100562. [Google Scholar] [CrossRef]
- Naiel, B.; Fawzy, M.; Halmy, M.W.A.; Mahmoud, A.E.D. Green Synthesis of Zinc Oxide Nanoparticles Using Sea Lavender (Limonium pruinosum L. Chaz.) Extract: Characterization, Evaluation of Anti-Skin Cancer, Antimicrobial and Antioxidant Potentials. Sci. Rep. 2022, 12, 20370. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, P.; Maity, D.; Awasthi, R. Nano-Green: Harnessing the Potential of Plant Extracts for Sustainable Antimicrobial Metallic Nanoparticles. J. Drug Deliv. Sci. Technol. 2024, 94, 105488. [Google Scholar] [CrossRef]
- Hernández-Díaz, M.N.; Torres-Valencia, N.; Miranda-Arámbula, M.; Ríos-Cortés, A.M.; Fernández-Luqueño, F.; López-Gayou, V.; López-Valdez, F. El rol de las plantas silvestres o cultivables de México en la síntesis de nanopartículas. Mundo Nano. Rev. Interdiscip. En Nanocienc. Y Nanotecnol. 2024, 17, 1e–17e. [Google Scholar] [CrossRef]
- Villagrán, Z.; Anaya-Esparza, L.M.; Velázquez-Carriles, C.A.; Silva-Jara, J.M.; Ruvalcaba-Gómez, J.M.; Aurora-Vigo, E.F.; Rodríguez-Lafitte, E.; Rodríguez-Barajas, N.; Balderas-León, I.; Martínez-Esquivias, F. Plant-Based Extracts as Reducing, Capping, and Stabilizing Agents for the Green Synthesis of Inorganic Nanoparticles. Resources 2024, 13, 70. [Google Scholar] [CrossRef]
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles–An eco-friendly approach. Resour. Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Zhou, L.; Fang, Q.; Liu, M.; Farhan, S.; Yang, S.; Wu, Y. Strong built-in electric field-assisted ZnO/ZnIn2S4 S-scheme heterostructure to promote photocatalytic hydrogen production. Inorg. Chem. 2024, 63, 21202–21211. [Google Scholar] [CrossRef]
- Zayed, M.; Othman, H.; Ghazal, H.; Hassabo, A.G. Psidium guajava leave extract as reducing agent for synthesis of zinc oxide nanoparticles and its application to impart multifunctional properties for cellulosic fabrics. Biointerface Res. Appl. Chem. 2021, 11, 13535–13556. [Google Scholar] [CrossRef]
- Al-darwesh, M.Y.; Ibrahim, S.S.; Mohammed, M.A. A Review on Plant Extract Mediated Green Synthesis of Zinc Oxide Nanoparticles and Their Biomedical Applications. Results Chem. 2024, 7, 101368. [Google Scholar] [CrossRef]
- Ahmad Mir, S.; Shrotriya, V.; Al-Muhimeed, T.I.; Amzad Hossain, M.; Zaman, M.B. Metal and Metal Oxide Nanostructures Applied as Alternatives of Antibiotics. Inorg. Chem. Commun. 2023, 150, 110503. [Google Scholar] [CrossRef]
- Riahi, S.; Ben Moussa, N.; Lajnef, M.; Jebari, N.; Dabek, A.; Chtourou, R.; Guisbiers, G.; Vimont, S.; Herth, E. Bactericidal Activity of ZnO Nanoparticles against Multidrug-Resistant Bacteria. J. Mol. Liq. 2023, 387, 122596. [Google Scholar] [CrossRef]
- Torres-Ortiz, D.; García-Alcocer, G.; Berumen-Segura, L.C.; Estévez, M. Green Extraction of Secondary Metabolites from Plants: Obstacles, Current Status, and Trends. Sustain. Chem. Environ. 2024, 8, 100157. [Google Scholar] [CrossRef]
- Aguilar-Villalva, R.; Molina, G.A.; España-Sánchez, B.L.; Díaz-Peña, L.F.; Elizalde-Mata, A.; Valerio, E.; Azanza-Ricardo, C.; Estevez, M. Antioxidant Capacity and Antibacterial Activity from Annona Cherimola Phytochemicals by Ultrasound-Assisted Extraction and Its Comparison to Conventional Methods. Arab. J. Chem. 2021, 14, 103239. [Google Scholar] [CrossRef]
- Arya, P.; Kumar, P. Comparison of Ultrasound and Microwave Assisted Extraction of Diosgenin from Trigonella Foenum Graceum Seed. Ultrason. Sonochem. 2021, 74, 105572. [Google Scholar] [CrossRef]
- Savoldi, T.L.; Glamoclija, J.; Sokovic, M.; Goncalves, J.E.; Ruiz, S.P.; Linde, G.A.; Gazim, Z.C.; Colauto, N.B. Actividad antimicrobiana del aceite esencial de hojas de Psidium cattleianum Afzel. ex Sabine. Boletín Latinoam. Y Del Caribe De Plantas Med. Y Aromáticas 2020, 19, 614–627. [Google Scholar] [CrossRef]
- Gomes, J.L.; da Araujo, J.R.S.; de Araújo, S.S.; de Oliveira, P.L.; de Veras, B.O.; Feitoza, G.S.; de Oliveira, A.P.; de Lira Júnior, J.S.; de Pereira, R.C.A.; do Vale Martins, L.; et al. Psidium cattleianum Sabine and P. myrtoides, O. Berg Fruits: A Comparative Composition, Antioxidant, and Safety Use. S. Afr. J. Bot. 2024, 172, 109–115. [Google Scholar] [CrossRef]
- dos Santos Pereira, E.; Vinholes, J.; Franzon, R.C.; Dalmazo, G.; Vizzotto, M.; Nora, L. Psidium cattleianum Fruits: A Review on Its Composition and Bioactivity. Food Chem. 2018, 258, 95–103. [Google Scholar] [CrossRef]
- Zandoná, G.P.; Bagatini, L.; Woloszyn, N.; de Souza Cardoso, J.; Hoffmann, J.F.; Moroni, L.S.; Stefanello, F.M.; Junges, A.; Rombaldi, C.V. Extraction and Characterization of Phytochemical Compounds from Araçazeiro (Psidium cattleianum) Leaf: Putative Antioxidant and Antimicrobial Properties. Food Res. Int. 2020, 137, 109573. [Google Scholar] [CrossRef]
- Patel, S. Exotic Tropical Plant Psidium Cattleianum: A Review on Prospects and Threats. Rev. Environ. Sci. Biotechnol. 2012, 11, 243–248. [Google Scholar] [CrossRef]
- González-Silva, N.; Nolasco-González, Y.; Aguilar-Hernández, G.; Sáyago-Ayerdi, S.G.; Villagrán, Z.; Acosta, J.L.; Montalvo-González, E.; Anaya-Esparza, L.M. Ultrasound-Assisted Extraction of Phenolic Compounds from Psidium cattleianum Leaves: Optimization Using the Response Surface Methodology. Molecules 2022, 27, 3557. [Google Scholar] [CrossRef]
- Ferreira Macedo, J.G.; Linhares Rangel, J.M.; de Oliveira Santos, M.; Camilo, C.J.; Martins da Costa, J.G.; Maria de Almeida Souza, M. Therapeutic Indications, Chemical Composition and Biological Activity of Native Brazilian Species from Psidium genus (Myrtaceae): A Review. J. Ethnopharmacol. 2021, 278, 114248. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Subramani, K.; Raju, S.A.K.P.M.; Rangaraj, S.; Venkatachalam, R. Psidium guajava leaf extract-mediated synthesis of ZnO nanoparticles under different processing parameters for hydrophobic and antibacterial finishing over cotton fabrics. Prog. Org. Coat. 2018, 124, 80–91. [Google Scholar] [CrossRef]
- Boopathi, T.S.; Suksom, S.; Suriyaprakash, J.; Hirad, A.H.; Alarfaj, A.A.; Thangavelu, I. Psidium guajava-mediated green synthesis of Fe-doped ZnO and Co-doped ZnO nanoparticles: A comprehensive study on characterization and biological applications. Bioprocess Biosyst. Eng. 2024, 47, 1271–1291. [Google Scholar] [CrossRef]
- Ramya, V.; Kalaiselvi, V.; Kannan, S.K.; Shkir, M.; Ghramh, H.A.; Ahmad, Z.; Nithiya, P.; Vidhya, N. Facile Synthesis and Characterization of Zinc Oxide Nanoparticles Using Psidium guajava Leaf Extract and Their Antibacterial Applications. Arab. J. Sci. Eng. 2022, 47, 909–918. [Google Scholar] [CrossRef]
- Sheta, M.H.; Abd El-Wahed, A.H.; Elshaer, M.A.; Bayomy, H.M.; Ozaybi, N.A.; Abd-Elraheem, M.A.M.; El-Sheshtaway, A.N.A.; El-Serafy, R.S.; Moustafa, M.M.I. Green synthesis of zinc and iron nanoparticles using Psidium guajava leaf extract stimulates cowpea growth, yield, and tolerance to saline water irrigation. Horticulturae 2024, 10, 915. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Hassan, R.G. New Biogenic Nanoparticles as Natural Products in Medicine Production and Water Pathogen Elimination, Characterization, Cytotoxic Evaluation and Antimicrobial Resistivity of Zinc Oxide Nanoparticles from Psidium guajava Leaves. Egypt J. Chem. 2023, 66, 1839–1850. [Google Scholar] [CrossRef]
- Fouda, A.; Abdel-Rahman, M.A.; Eid, A.M.; Selim, S.; Ejaz, H.; Alruwaili, M.; Manni, E.; Almuhayawi, M.S.; Al Jaouni, S.K.; Hassan, S.E.D. Investigating the Potential of Green-Fabricated Zinc Oxide Nanoparticles to Inhibit the Foodborne Pathogenic bacteria Isolated from Spoiled Fruits. Catalysts 2024, 14, 427. [Google Scholar] [CrossRef]
- Rani, N.; Kumar, S.; Kumar, K. Synergistic influence of the hybridization between green carbon dots adorned Psidium guajava extracted zinc oxide for boosted photocatalytic efficiency. Biomass Conv. Bioref. 2024, 1–16. [Google Scholar] [CrossRef]
- Thejashwini, P.P.; Chandrika, R.; Madhusudhan, M.C.; Joshi, S.M.; Ali, D.; Alarifi, S.; Jogaiah, S.; Geetha, N. Psidium guajav-mediated zinc oxide nanoparticles as a multifunctional, microbicidal, antioxidant and antiproliferative agent against destructive pathogens. Bioprocess Biosyst. Eng. 2024, 47, 1571–1584. [Google Scholar] [CrossRef]
- Son, N.N.; Thanh, V.M.; Huong, N.T. Anticancer Activities of Zinc Oxide Nanoparticles Synthesized Using Guava Leaf extract. ChemistrySelect 2023, 8, e202303214. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Angulo, C.; Silva-Jara, J. Antibacterial and Immunomodulatory Activity of Moringa (Moringa oleifera) Seed Extract in Longfin Yellowtail (Seriola rivoliana) Peripheral Blood Leukocytes. Aquac. Res. 2021, 52, 4076–4085. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of Condensed Tannins Using Acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Canabady-Rochelle, L.L.S.; Harscoat-Schiavo, C.; Kessler, V.; Aymes, A.; Fournier, F.; Girardet, J.-M. Determination of Reducing Power and Metal Chelating Ability of Antioxidant Peptides: Revisited Methods. Food Chem. 2015, 183, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Barzinjy, A.A.; Azeez, H.H. Green Synthesis and Characterization of Zinc Oxide Nanoparticles Using Eucalyptus Globulus Labill. Leaf Extract and Zinc Nitrate Hexahydrate Salt. SN Appl. Sci. 2020, 2, 991. [Google Scholar] [CrossRef]
- Aguilar-Ávila, D.S.; Reyes-Becerril, M.; Velázquez-Carriles, C.A.; Hinojosa-Ventura, G.; Macías-Rodríguez, M.E.; Angulo, C.; Silva-Jara, J.M. Biogenic Ag2O Nanoparticles with “Hoja Santa” (Piper auritum) Extract: Characterization and Biological Capabilities. Biometals 2024, 37, 971–982. [Google Scholar] [CrossRef]
- Romero-García, D.M.; Velázquez-Carriles, C.A.; Gomez, C.; Velázquez-Juárez, G.; Silva-Jara, J.M. Tannic Acid-Layered Hydroxide Salt Hybrid: Assessment of Antibiofilm Formation and Foodborne Pathogen Growth Inhibition. J. Food Sci. Technol. 2023, 60, 2659–2669. [Google Scholar] [CrossRef]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Cameotra, S.S.; Pal, R. Antibiofilm Efficacy of Silver Nanoparticles against Biofilm of Extended Spectrum β-Lactamase Isolates of Escherichia Coli and Klebsiella Pneumoniae. Appl. Nanosci. 2014, 4, 859–868. [Google Scholar] [CrossRef]
- Neihaya, H.Z.; Zaman, H.H. Investigating the Effect of Biosynthesized Silver Nanoparticles as Antibiofilm on Bacterial Clinical Isolates. Microb. Pathog. 2018, 116, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.J.; Falkiner, F.R.; Keane, C.T. New Method for Detecting Slime Production by Coagulase Negative Staphylococci. J. Clin. Pathol. 1989, 42, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Microtiter Dish Biofilm Formation Assay. Available online: https://app.jove.com (accessed on 18 December 2024).
- Córdova, N.M.; Becerril, M.R.; Angulo, M.; Angulo, C. Immunobiological Effects of Marine Debaryomyces Hansenii-Derived Lysates on Goat Peripheral Blood Leukocytes. Trop. Subtrop. Agroecosyst. 2021, 25, 1–14. [Google Scholar] [CrossRef]
- Preciado-Ortiz, M.E.; Martínez-López, E.; Pedraza-Chaverri, J.; Medina-Campos, O.N.; Rodríguez-Echevarría, R.; Reyes-Pérez, S.D.; Rivera-Valdés, J.J. 10-Gingerol Increases Antioxidant Enzymes and Attenuates Lipopolysaccharide-Induced Inflammation by Modulating Adipokines in 3T3-L1 Adipocytes. Antioxidants 2024, 13, 1093. [Google Scholar] [CrossRef]
- Dacoreggio, M.V.; Moroni, L.S.; Kempka, A.P. Antioxidant, Antimicrobial and Allelopathic Activities and Surface Disinfection of the Extract of Psidium cattleianum Sabine Leaves. Biocatal. Agric. Biotechnol. 2019, 21, 101295. [Google Scholar] [CrossRef]
- Moraes, L.d.L.S.; Rodrigues, N.R.; Dal Forno, A.H.; Tambara, A.L.; Boldori, J.R.; Vizzotto, M.; Quatrin, A.; Emanuelli, T.; Denardin, C.C. Araçá (Psidium cattleianum Sabine) Ethanol Extracts Increase Lifespan and Alleviate Oxidative Stress in Caenorhabditis Elegans. J. Agric. Food Res. 2023, 11, 100505. [Google Scholar] [CrossRef]
- İnce, A.; Şahin, S.; Şümnü, S. Extraction of Phenolic Compounds from Melissa Using Microwave and Ultrasound. Turk. J. Agric. For. 2013, 37, 69–75. [Google Scholar] [CrossRef]
- Papoti, V.T.; Totomis, N.; Atmatzidou, A.; Zinoviadou, K.; Androulaki, A.; Petridis, D.; Ritzoulis, C. Phytochemical Content of Melissa officinalis L. Herbal Preparations Appropriate for Consumption. Processes 2019, 7, 88. [Google Scholar] [CrossRef]
- Brahmi, F.; Blando, F.; Sellami, R.; Mehdi, S.; De Bellis, L.; Negro, C.; Haddadi-Guemghar, H.; Madani, K.; Makhlouf-Boulekbache, L. Optimization of the Conditions for Ultrasound-Assisted Extraction of Phenolic Compounds from Opuntia ficus-indica [L.] Mill. Flowers and Comparison with Conventional Procedures. Ind. Crops Prod. 2022, 184, 114977. [Google Scholar] [CrossRef]
- Selka, A.; Moutombi, F.J.N.; Jean-François, J.; Touaibia, M. Hydroxycinnamic Acids and Their Related Synthetic Analogs: An Update of Pharmacological Activities. Mini Rev. Med. Chem. 2022, 22, 1516–1544. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, G. On the Inhibition of Hydroxyl Radical Formation by Hydroxycinnamic Acids: The Case of Caffeic Acid as a Promising Chelating Ligand of a Ferrous Ion. J. Phys. Chem. A 2019, 123, 9560–9566. [Google Scholar] [CrossRef] [PubMed]
- Psotová, J.; Lasovský, J.; Vicar, J. Metal-Chelating Properties, Electrochemical Behavior, Scavenging and Cytoprotective Activities of Six Natural Phenolics. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub. 2003, 147, 147–153. [Google Scholar] [CrossRef]
- Samavati, A.; Awang, A.; Samavati, Z.; Ismail, A.F.; Othman, M.H.D.; Velashjerdi, M.; Rostami, A. Influence of ZnO nanostructure configuration on tailoring the optical bandgap: Theory and experiment. Mater. Sci. Eng. B 2021, 263, 114811. [Google Scholar] [CrossRef]
- Jayappa, M.D.; Ramaiah, C.K.; Kumar, M.A.P.; Suresh, D.; Prabhu, A.; Devasya, R.P.; Sheikh, S. Green Synthesis of Zinc Oxide Nanoparticles from the Leaf, Stem and in Vitro Grown Callus of Mussaenda frondosa L.: Characterization and Their Applications. Appl. Nanosci. 2020, 10, 3057–3074. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P.; Saravanan, K.; Manogar, P.; Johnson, J.; Vinoth, E.; Mayakannan, M. Green Synthesis and Characterization of Biocompatible Zinc Oxide Nanoparticles and Evaluation of Its Antibacterial Potential. Sens. Biol. Sens. Res. 2021, 31, 100399. [Google Scholar] [CrossRef]
- Gupta, R.; Malik, P.; Das, N.; Singh, M. Antioxidant and Physicochemical Study of Psidium guajava Prepared Zinc Oxide Nanoparticles. J. Mol. Liq. 2019, 275, 749–767. [Google Scholar] [CrossRef]
- Elia, P.; Zach, R.; Hazan, S.; Kolusheva, S.; Porat, Z.E.; Zeiri, Y. Green synthesis of gold nanoparticles using plant extracts as reducing agents. Int. J. Nanomed. 2014, 9, 4007–4021. [Google Scholar] [CrossRef]
- Yedurkar, S.; Maurya, C.; Mahanwar, P. Biosynthesis of zinc oxide nanoparticles using Ixora coccinea leaf extract—A green approach. Open J. Synth. Theory Appl. 2016, 5, 1–14. [Google Scholar] [CrossRef]
- Khalafi, T.; Buazar, F.; Ghanemi, K. Phycosynthesis and Enhanced Photocatalytic Activity of Zinc Oxide Nanoparticles Toward Organosulfur Pollutants. Sci. Rep. 2019, 9, 6866. [Google Scholar] [CrossRef]
- El-Belely, E.F.; Farag, M.M.S.; Said, H.A.; Amin, A.S.; Azab, E.; Gobouri, A.A.; Fouda, A. Green Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) Using Arthrospira Platensis (Class: Cyanophyceae) and Evaluation of Their Biomedical Activities. Nanomaterials 2021, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Azmi, S.N.H.; Alam, M. Exploring the Anti-Corrosion, Photocatalytic, and Adsorptive Functionalities of Biogenically Synthesized Zinc Oxide Nanoparticles. Inorganics 2024, 12, 199. [Google Scholar] [CrossRef]
- Mankad, M.; Patil, G.; Patel, S.; Patel, D.; Patel, A. Green Synthesis of Zinc Oxide Nanoparticles Using Azadirachta Indica A. Juss. Leaves Extract and Its Antibacterial Activity against Xanthomonas orzyae pv. oryzae. Ann. Phytomed. Int. J. 2016, 5, 76–86. [Google Scholar] [CrossRef]
- Balogun, S.W.; James, O.O.; Sanusi, Y.K.; Olayinka, O.H. Green Synthesis and Characterization of Zinc Oxide Nanoparticles Using Bashful (Mimosa pudica), Leaf Extract: A Precursor for Organic Electronics Applications. SN Appl. Sci. 2020, 2, 504. [Google Scholar] [CrossRef]
- Faheem, M.; Siddiqi, H.M.; Habib, A.; Shahid, M.; Afzal, A. ZnO/Zn(OH)2 Nanoparticles and Self-Cleaning Coatings for the Photocatalytic Degradation of Organic Pollutants. Front. Environ. Sci. 2022, 10, 965925. [Google Scholar] [CrossRef]
- Klinbumrung, A.; Panya, R.; Pung-Ngama, A.; Nasomjai, P.; Saowalakmeka, J.; Sirirak, R. Green Synthesis of ZnO Nanoparticles by Pineapple Peel Extract from Various Alkali Sources. J. Asian Ceram. Soc. 2022, 10, 755–765. [Google Scholar] [CrossRef]
- Soto, K.M.; Luzardo-Ocampo, I.; López-Romero, J.M.; Mendoza, S.; Loarca-Piña, G.; Rivera-Muñoz, E.M.; Manzano-Ramírez, A. Gold Nanoparticles Synthesized with Common Mullein (Verbascum thapsus) and Castor Bean (Ricinus communis) Ethanolic Extracts Displayed Antiproliferative Effects and Induced Caspase 3 Activity in Human HT29 and SW480 Cancer Cells. Pharmaceutics 2022, 14, 2069. [Google Scholar] [CrossRef]
- Khan, M.; Ware, P.; Shimpi, N. Synthesis of ZnO Nanoparticles Using Peels of Passiflora Foetida and Study of Its Activity as an Efficient Catalyst for the Degradation of Hazardous Organic Dye. SN Appl. Sci. 2021, 3, 528. [Google Scholar] [CrossRef]
- Bai, D.-P.; Lin, X.-Y.; Huang, Y.-F.; Zhang, X.-F. Theranostics Aspects of Various Nanoparticles in Veterinary Medicine. Int. J. Mol. Sci. 2018, 19, 3299. [Google Scholar] [CrossRef]
- Hoseinzadeh, E.; Alikhani, M.Y.; Samarghandi, M.R.; Shirzad-Siboni, M. Antimicrobial potential of synthesized zinc oxide nanoparticles against gram positive and gram negative bacteria. Desalination Water Treat. 2014, 52, 4969–4976. [Google Scholar] [CrossRef]
- Agrawal, A.; Sharma, R.; Sharma, A.; Gurjar, K.C.; Kumar, S.; Chatterjee, S.; Pandey, H.; Awasthi, K.; Awasthi, A. Antibacterial and Antibiofilm Efficacy of Green Synthesized ZnO Nanoparticles Using Saraca asoca Leaves. Environ. Sci. Pollut. Res. 2023, 30, 86328–86337. [Google Scholar] [CrossRef] [PubMed]
- Jasim, N.A.; Al-Gasha’a, F.A.; Al-Marjani, M.F.; Al-Rahal, A.H.; Abid, H.A.; Al-Kadhmi, N.A.; Jakaria, M.; Rheima, A.M. ZnO Nanoparticles Inhibit Growth and Biofilm Formation of Vancomycin-resistant S. aureus (VRSA). Biocatal. Agric. Biotechnol. 2020, 29, 101745. [Google Scholar] [CrossRef]
- Haiouani, K.; Hegazy, S.; Alsaeedi, H.; Bechelany, M.; Barhoum, A. Green Synthesis of Hexagonal-like ZnO Nanoparticles Modified with Phytochemicals of Clove (Syzygium aromaticum) and Thymus capitatus Extracts: Enhanced Antibacterial, Antifungal, and Antioxidant Activities. Materials 2024, 17, 4340. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Partoazar, A. Targeting Bacterial Biofilm-Related Genes with Nanoparticle-Based Strategies. Front. Microbiol. 2024, 15, 1387114. [Google Scholar] [CrossRef]
- Hassani, S.M.; Nakhaei, M.M.; Forghanifard, M.M. Inhibitory effect of zinc oxide nanoparticles on pseudomonas aeruginosa biofilm formation. Nanomed. J. 2015, 2, 121–128. [Google Scholar]
- Kim, C.-S.; Nguyen, H.-D.; Ignacio, R.M.; Kim, J.-H.; Cho, H.-C.; Maeng, E.H.; Kim, Y.-R.; Kim, M.-K.; Park, B.-K.; Kim, S.-K. Immunotoxicity of Zinc Oxide Nanoparticles with Different Size and Electrostatic Charge. Int. J. Nanomed. 2014, 9, 195–205. [Google Scholar] [CrossRef]
- Anders, C.B.; Chess, J.J.; Wingett, D.G.; Punnoose, A. Serum proteins enhance dispersion stability and influence the cytotoxicity and dosimetry of ZnO nanoparticles in suspension and adherent cancer cell models. Nanoscale Res. Lett. 2015, 10, 1–22. [Google Scholar] [CrossRef]
- Babin, K.; Antoine, F.; Goncalves, D.M.; Girard, D. TiO2, CeO2 and ZnO nanoparticles and modulation of the degranulation process in human neutrophils. Toxicol. Lett. 2013, 221, 57–63. [Google Scholar] [CrossRef]
- Vassal, M.; Pereira, C.D.; Martins, F.; Silva, V.L.M.; Silva, A.M.S.; Senos, A.M.R.; Costa, M.E.V.; de Pereira, M.L.; Rebelo, S. Different Strategies to Attenuate the Toxic Effects of Zinc Oxide Nanoparticles on Spermatogonia Cells. Nanomaterials 2022, 12, 3561. [Google Scholar] [CrossRef]
- Pandurangan, M.; Jin, B.Y.; Kim, D.H. ZnO Nanoparticles Upregulates Adipocyte Differentiation in 3T3-L1 Cells. Biol. Trace. Elem. Res. 2016, 170, 201–207. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).