Reproductive Ecology of the Freshwater Snail, Pila globosa, Considering Environmental Factors in a Tropical Freshwater Swamp Forest
Abstract
1. Introduction
2. Materials and Methods
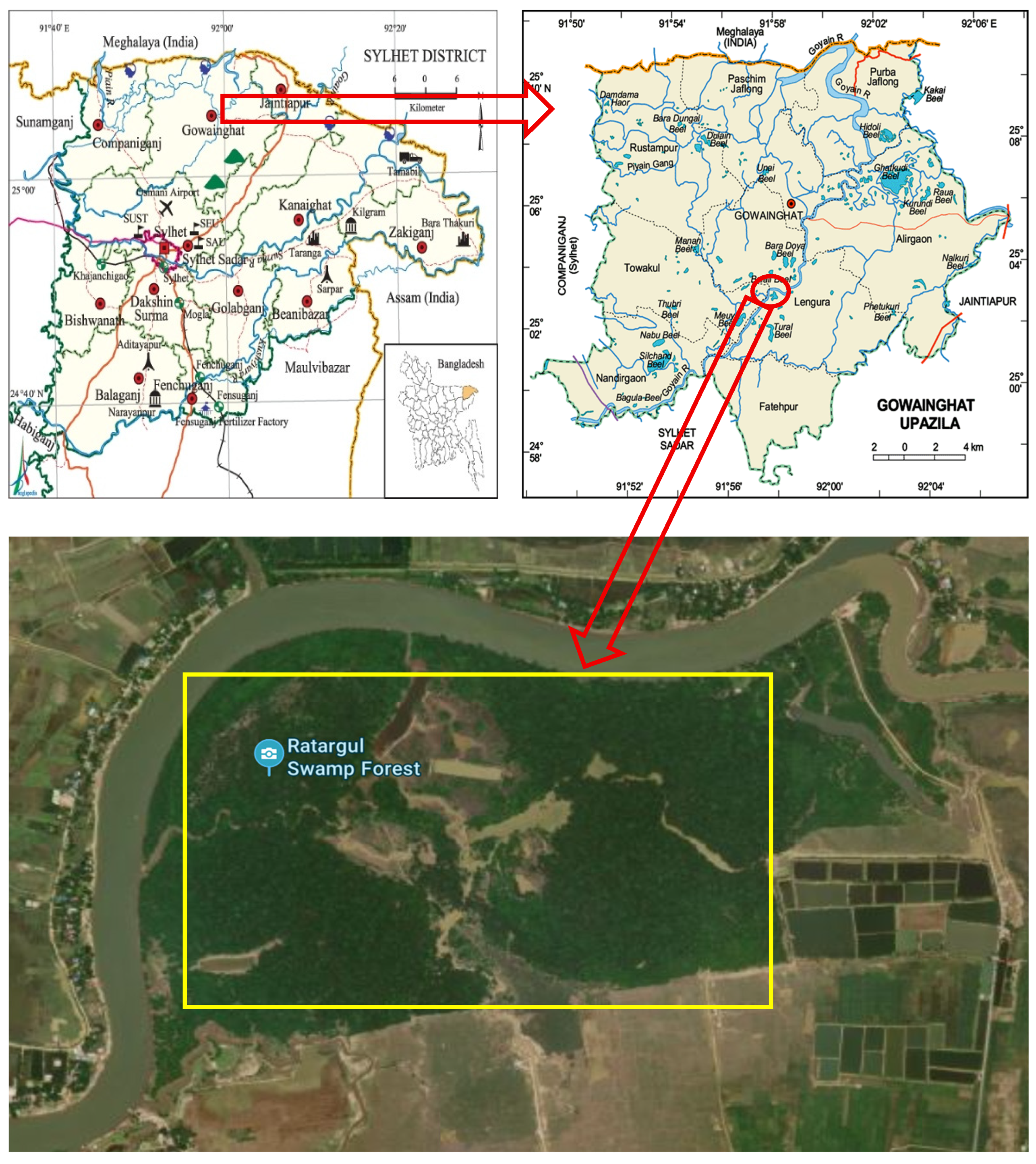
2.1. Study Area, Water Qualities, Sample Collection, and Morphometric Measurement
2.2. Tools for the Analysis of Morphometrics
2.3. Gonadal Maturation Phases and Histological Procedure
2.4. Statistical Analyses
3. Results
3.1. Physical and Chemical Properties of Water
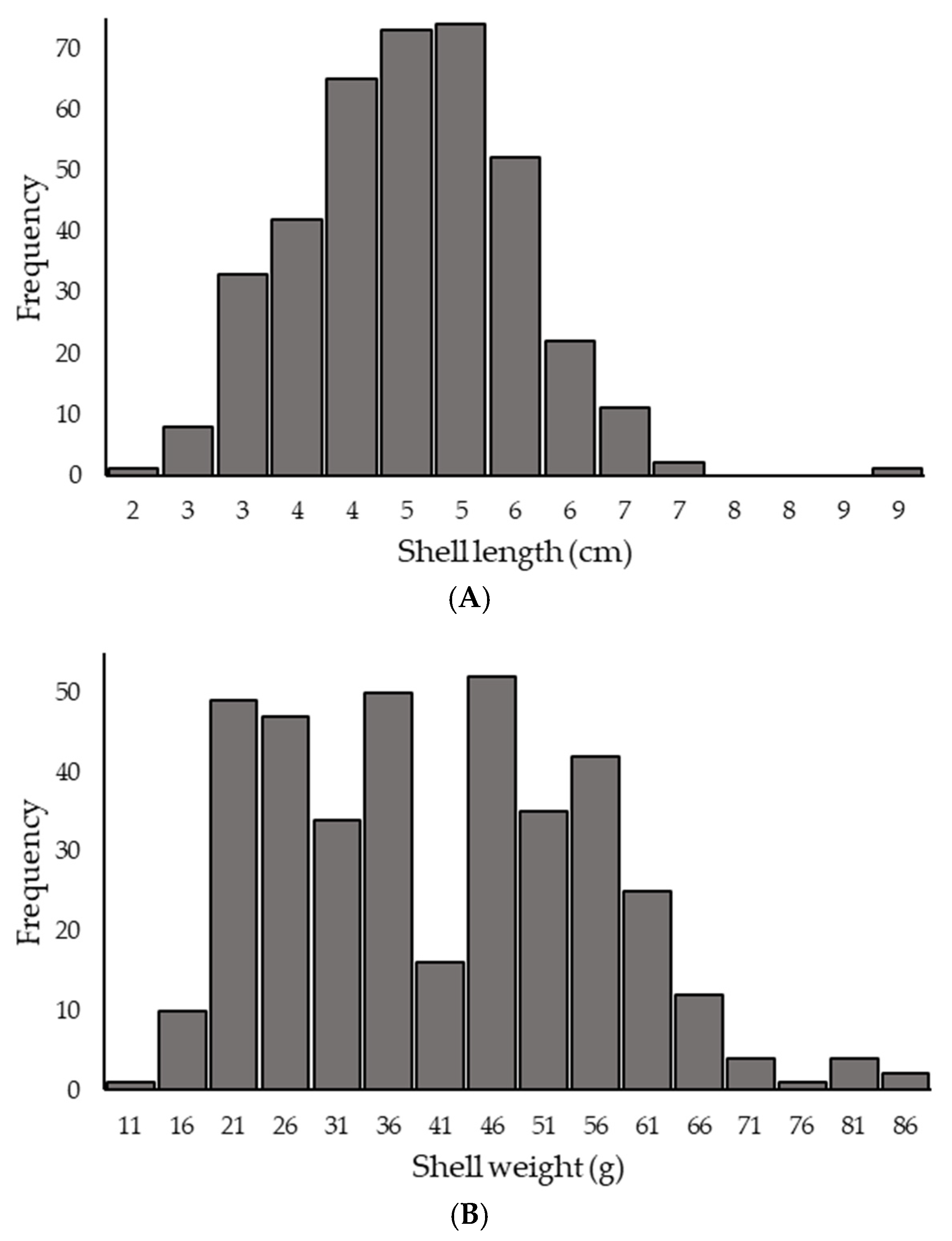
3.2. Morphometric Measurement of P. globosa
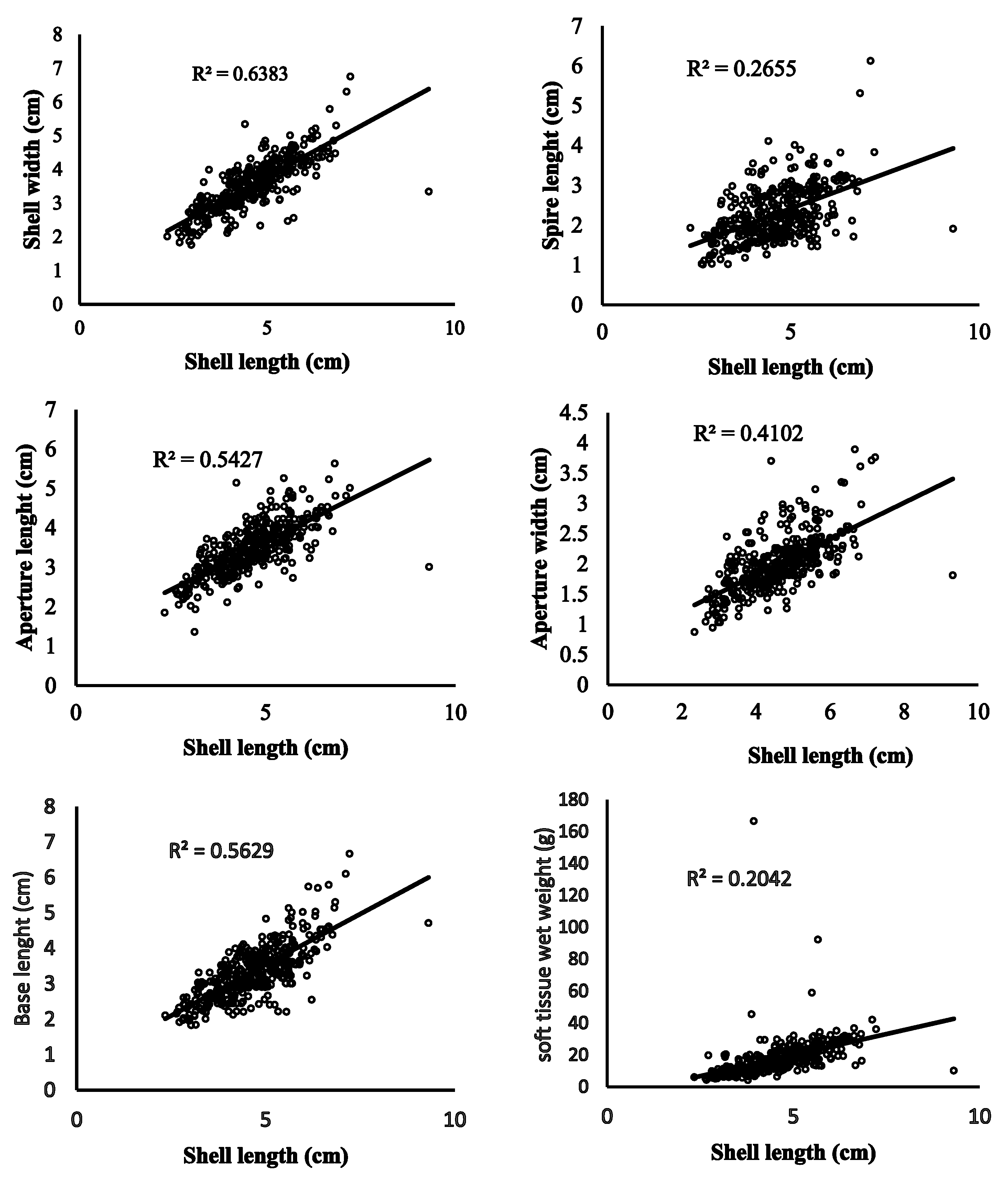
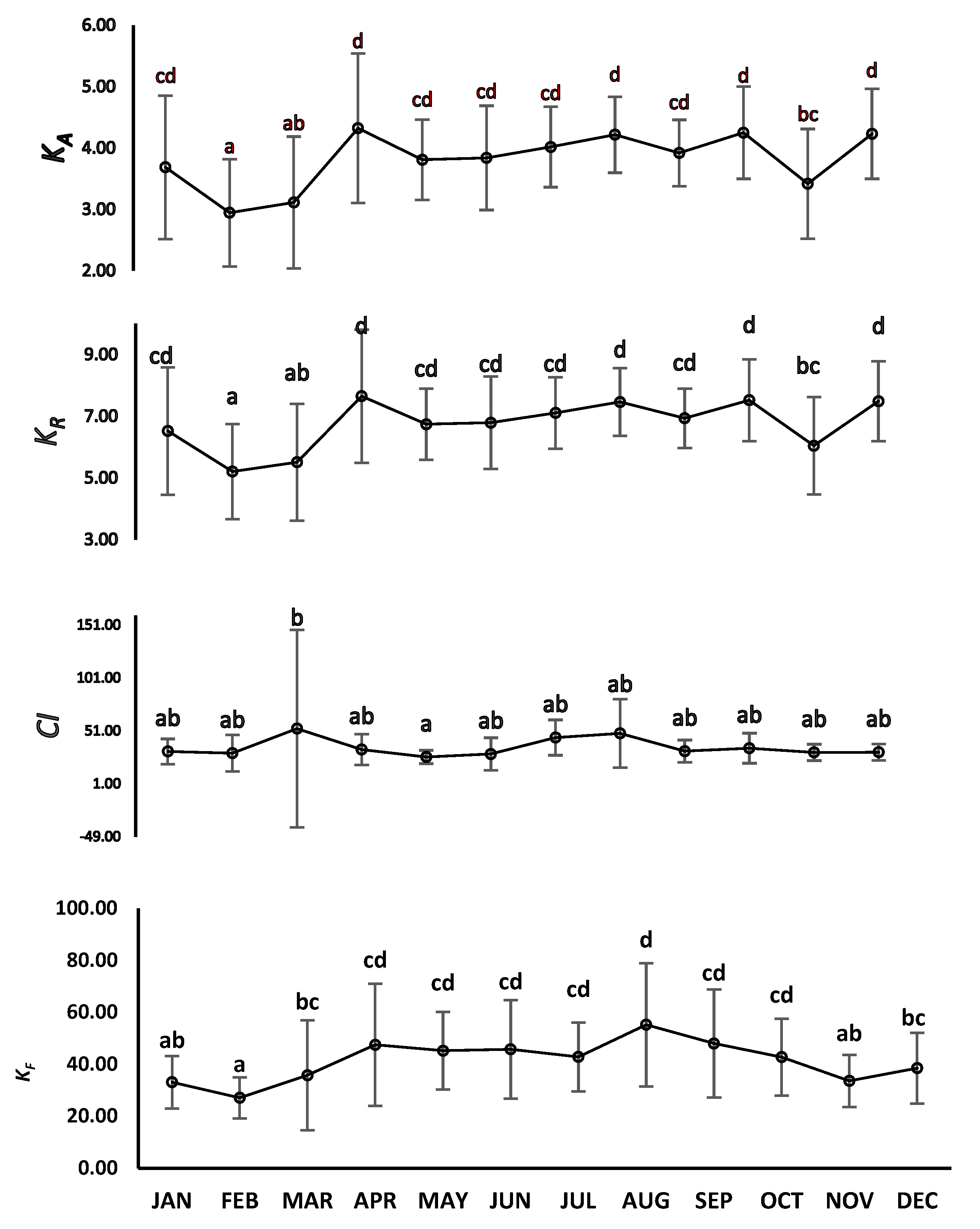
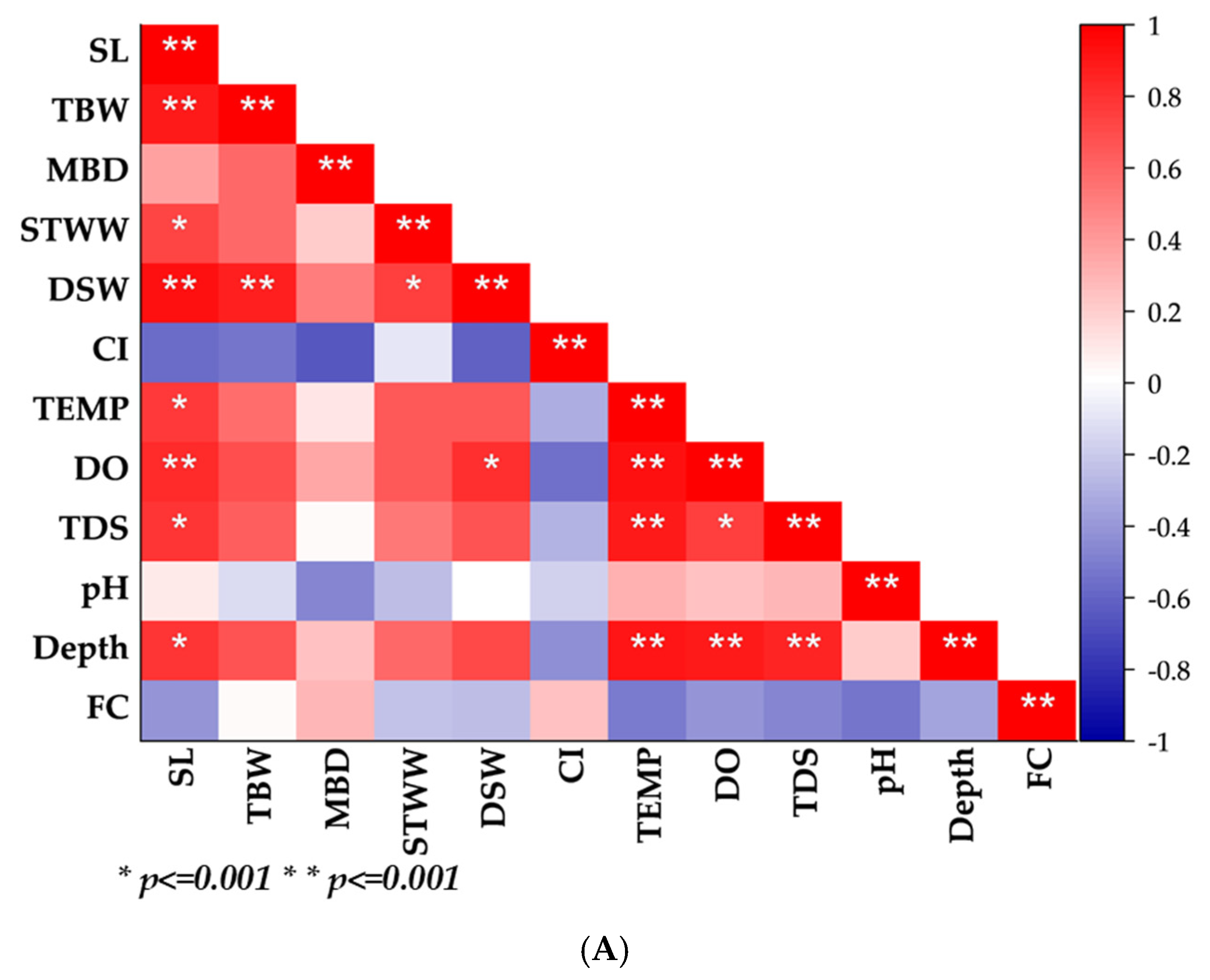
3.3. Length–Weight Parameters, Correlation, Condition Indices, and Sex Ratio
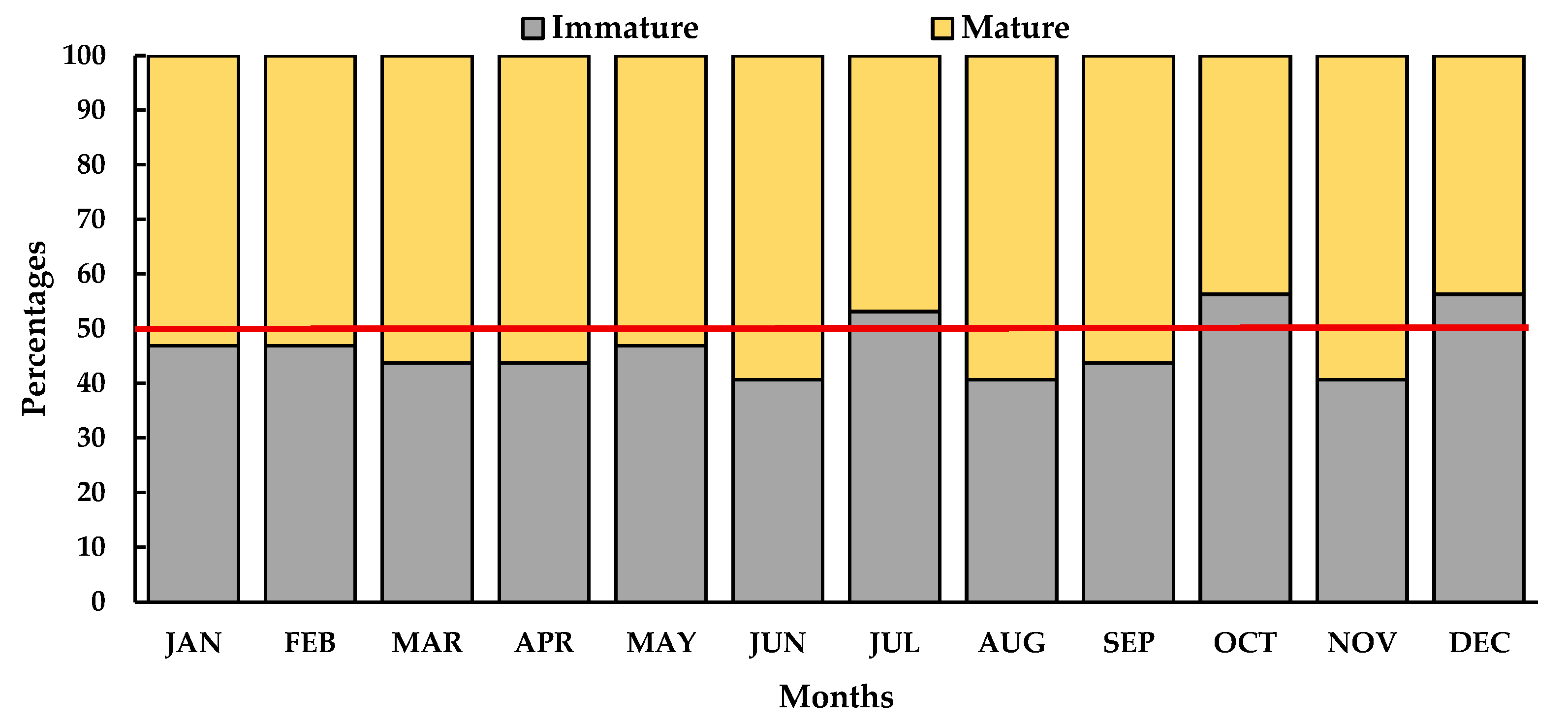
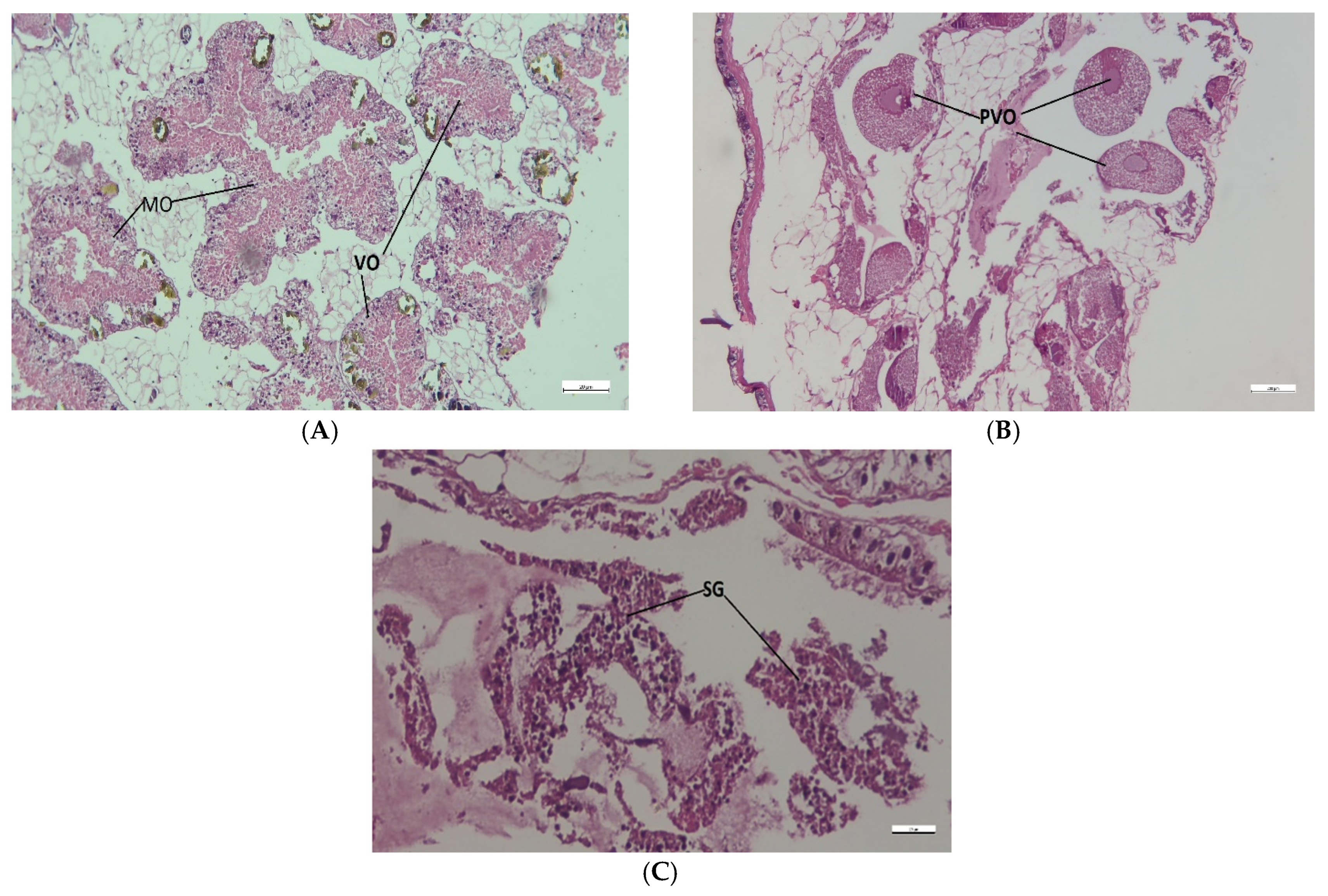
3.4. Dynamics of Gonadal Maturation Phases and Histological Gonads
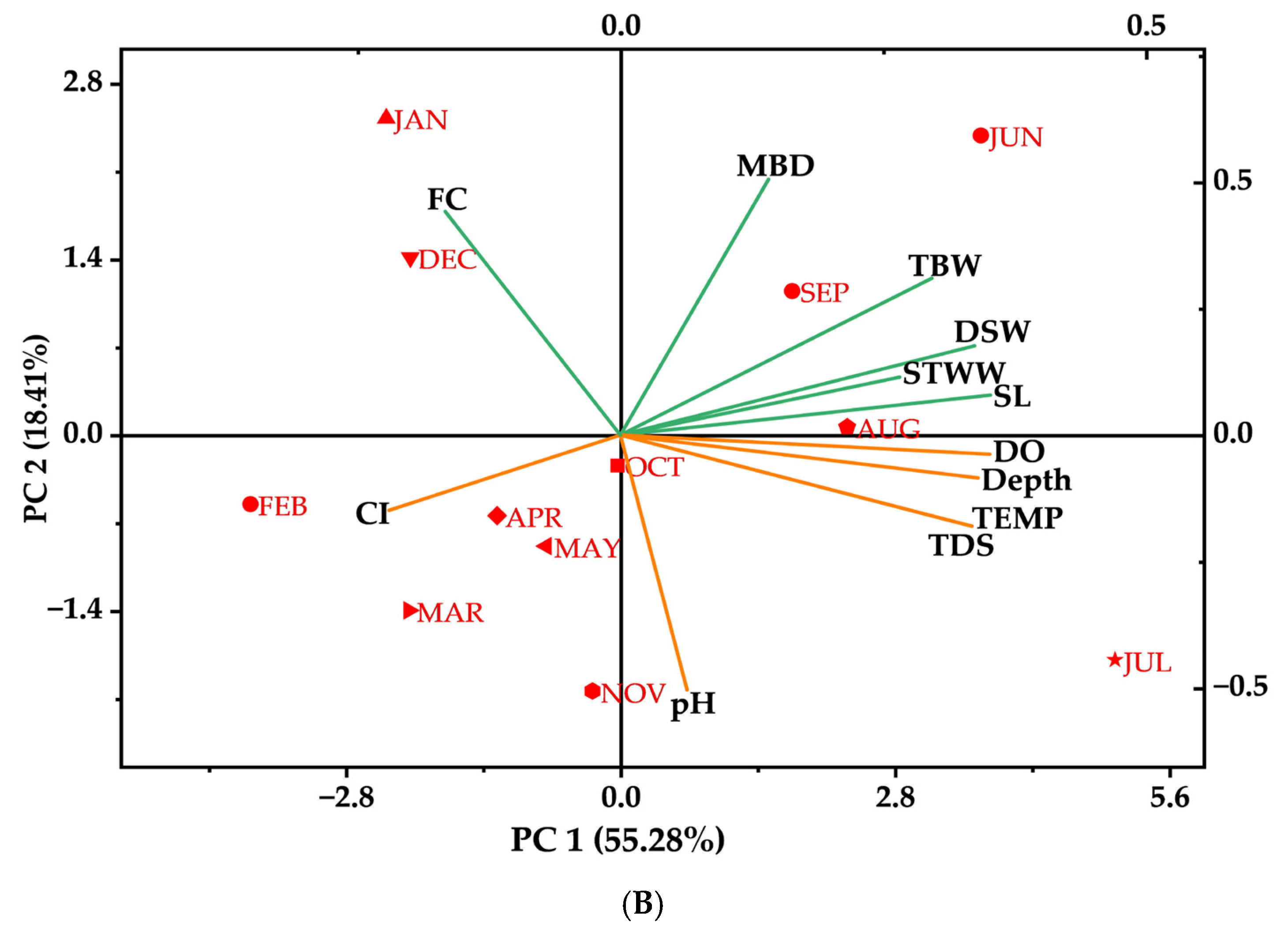
3.5. The Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hwang, S.; Lee, Y.; Kim, M.; Kim, B.-H. Filter Feeding and Carbon and Nitrogen Assimilation of a Freshwater Bivalve (Unio douglasiae) on a Toxic Cyanobacterium (Microcystis aeruginosa). Appl. Sci. 2021, 11, 9294. [Google Scholar] [CrossRef]
- Gheoca, V.; Benedek, A.M.; Schneider, E. Exploring Land Snails’ Response to Habitat Characteristics and Their Potential as Bioindicators of Riparian Forest Quality. Ecol. Indic. 2021, 132, 108289. [Google Scholar] [CrossRef]
- Jahan, M.S.; Akter, M.S.; Sar, M.M. Growth Ecology of Pila globosa (Swainson) (Gastropoda: Pilidae) in Simulated Habitat. Pakistan J. Biol. Sci. 2001, 4, 581–584. [Google Scholar] [CrossRef]
- Panda, F.; Pati, S.G.; Das, K.; Samanta, L.; Sahoo, D.K.; Paital, B. Biochemical and Molecular Responses of the Freshwater Snail Pila Sp. to Environmental Pollutants, Abiotic, and Biotic Stressors. Front. Environ. Sci. 2022, 10, 1033049. [Google Scholar] [CrossRef]
- Budha, P.B.; Madhyastha, A.; Dutta, J. Pila globosa. The IUCN Red List of Threatened Species 2010, e.T166694A6261849. Available online: https://www.iucnredlist.org/species/166694/6261849 (accessed on 1 January 2025).
- Shathi, U.H.; Rahman, M.R.; Rahman, R. Ecology and Bio-Economics of Freshwater Apple Snail Pila globosa in Natore District of Bangladesh. J. Sustain. Environ. Manag. 2022, 1, 332–338. [Google Scholar] [CrossRef]
- Jahan, M.S.; Islam, M.R.; Rahman, M.R.; Alam, M.M. Induced Breeding of Pila globosa (Swainson 1822) (Gastropoda: Prosobranchia) for Commercial Farming. Univ. J. Zool. Rajshahi Univ. 1970, 26, 35–39. [Google Scholar] [CrossRef]
- Nahid, S.; Henriksson, P.; Wahab, M. Value-Chain Analysis of Freshwater Apple Snail (Pila globosa)Used for on-Farm Feeds in the Freshwater Prawnfarming Sector in Bangladesh. Int. J. Agric. Res. Innov. Technol. 2013, 3, 22–30. [Google Scholar] [CrossRef]
- Nath, R.D.; Lifat Rahi, M.; Sipar Hossain, G.; Anisul Huq, K.; Anisul, K. Marketing Status of Fresh Water Snail in Khulna District. Bangladesh Res. Pub. J 2008, 1, 337–347. [Google Scholar]
- Panda, F.; Pati, S.G.; Bal, A.; Samanta, L.; Paital, B. Redox Regulatory System in Semi-Sessile Amphibious Indian Apple Snail Pila globosa for Future Ecotoxic Studies. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2022, 93, 443–450. [Google Scholar] [CrossRef]
- Pati, S.G.; Panda, F.; Samanta, L.; Paital, B. Spatio-Temporal Changes in Oxidative Stress Physiology Parameters in Apple Snail Pila globosa as a Function of Soil Mg, Ca, Organic Carbon and Aquatic Physico-Chemical Factors. Environ. Geochem. Health 2023, 45, 2591–2610. [Google Scholar] [CrossRef]
- Panda, F.; Pati, S.G.; Anwar, T.N.; Samanta, L.; Paital, B. Seasonal Variation of Water Quality Modulated Redox Regulatory System in the Apple Snail Pila globosa and Its Use as a Bioindicator Species in Freshwater Ecosystems across India. Water 2022, 14, 3275. [Google Scholar] [CrossRef]
- Nair, D.M. Developments in Molluscs Farming in Southeast Asia. In Responsible Aquaculture Development in Southeast Asia, Proceedings of the Seminar-Workshop on Aquaculture Development in Southeast Asia, Iloilo, Philippines, 12–14 October 1999; Southeast Asian Fisheries Development Center the Aquaculture Department: Iloilo, Philippines, 1999; pp. 103–114. [Google Scholar]
- Hossain, M.A.; Sarker, T.R.; Sutradhar, L.; Hussain, M.; Iqbal, M.M. Toxic Effects of Chlorpyrifos on the Growth, Hemocytes Counts, and Vital Organ’s Histopathology of Freshwater Mussel, Lamellidens marginalis. J. King Saud Univ.—Sci. 2023, 35, 102482. [Google Scholar] [CrossRef]
- Saha, B.; Jahan, M.; Hossain, M. Morphometrics, Length-Weight Relationship and Ecological Factors Affecting the Habitat of Pila globosa (Swainson, 1822) (Mesogastropoda: Pilidae) Located in Rajshahi University Campus. Bangladesh J. Sci. Ind. Res. 2016, 51, 121–128. [Google Scholar] [CrossRef]
- Ghosh, S.; Jung, C.; Meyer-Rochow, V.B. Snail as Mini-Livestock: Nutritional Potential of Farmed Pomacea canaliculata (Ampullariidae). Agric. Nat. Resour. 2017, 51, 504–511. [Google Scholar] [CrossRef]
- Rohaeni, E.S.; Subhan, A.; Hanifah, V.W.; Bakrie, B.; Sumantri, I. Effects of Feeding Alabio Ducks with Fresh Golden Snail on Egg Production and Quality. J. Hunan Univ. Nat. Sci. 2021, 48, 305–313. [Google Scholar]
- Akther, S.; Hossain, M.A.; Pandit, D.; Chowdhury, T.R.; Mian, S. Morphometrics and Reproductive Characteristics of the Freshwater Crab Sartoriana spinigera from the Habitat of Ratargul Swamp Forest, Bangladesh: An Approach to Conservation. Scientifica 2024, 2024, 4550875. [Google Scholar] [CrossRef]
- Chakraborty, A.; Begum, N.; Anam, U.; Gupta, T.; Iqbal, M.M.; Hossain, M.A. Growth Factors and Condition Indices of Some Air-Breathing and Catfishes from Hakaluki Haor, Bangladesh. J. Appl. Ichthyol. 2025, 2025, 7463844. [Google Scholar] [CrossRef]
- Kitai, H.; Kakuda, U.; Goto, S.G.; Shiga, S. Photoperiod Controls Egg Laying and Caudodorsal Cell Hormone Expression but Not Gonadal Development in the Pond Snail Lymnaea stagnalis. J. Comp. Physiol. A 2021, 207, 523–532. [Google Scholar] [CrossRef]
- Pewphong, R.; Kitana, J.; Kitana, N. Chronology of Gonadal Development in the Malayan Snail-Eating Turtle Malayemys Macrocephala. Zool. Stud. 2020, 59, e20. [Google Scholar] [CrossRef]
- Dominguez, E.; Abdala, V. Morphology and Evolution of the Wing Bullae in South American Leptophlebiidae (Ephemeroptera). J. Morphol. 2019, 280, 95–102. [Google Scholar] [CrossRef]
- Xu, X.; Wu, J.; Wang, K.; Yang, Y.; Yan, S. Locking of the Operculum in a Water Snail: Theoretical Modeling and Applications for Mechanical Sealing. J. Theor. Biol. 2019, 464, 104–111. [Google Scholar] [CrossRef]
- Mititelu, M.; Stanciu, G.; Drăgănescu, D.; Ioniță, A.C.; Neacșu, S.M.; Dinu, M.; Stefan-van Staden, R.-I.; Moroșan, E. Mussel Shells, a Valuable Calcium Resource for the Pharmaceutical Industry. Mar. Drugs 2021, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Riisgård, H.U.; Lüskow, F.; Pleissner, D.; Lundgreen, K.; López, M.Á.P. Effect of Salinity on Filtration Rates of Mussels Mytilus edulis with Special Emphasis on Dwarfed Mussels from the Low-Saline Central Baltic Sea. Helgol. Mar. Res. 2013, 67, 591–598. [Google Scholar] [CrossRef]
- Holt, W.V.; Brown, J.L.; Comizzoli, P. Reproductive Science as an Essential Component of Conservation Biology. Adv. Exp. Med. Biol. 2014, 753, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Kawsar, M.A.; Hossain, M.A.; Pandit, D.; Kabir, M.A.; Alam, M.T. Evaluation of Well-Being Status of Near-Threatened Gangetic Leaf Fish Nandus nandus (Hamilton, 1822) in the Kawadighi Haor: Implications to Haor Fishery Management in the Northeastern Bangladesh. Conservation 2023, 3, 175–190. [Google Scholar] [CrossRef]
- Khan, A.; Karim, M.R.; Mohammed; Kibria, M.G.; Sinha, K.; Sultana, F.; Mukul, S.A.; Arfin-Khan, M.A.S. Anthropogenic Disturbance Modifies Tree Functional Traits in the Only Remnant Swamp Forest of Bangladesh. Front. Ecol. Evol. 2023, 11, 1062764. [Google Scholar] [CrossRef]
- Jahan, K.M.; Zakaria, A. Environmental Movement and the Conservation of Forest: A Case Study on Ratargul Swamp Forest of Sylhet, Bangladesh. Asian Res. J. Arts Soc. Sci. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Refat, N.; Ador, M.A.H.; Sagor, P.S.; Raihan, F.; Joarder, M.A.M. Linkages among Biodiversity, Ecotourism and Livelihood of Wetland Communities: A Case Study of Ratargul Swamp Forest, Bangladesh. Environ. Dev. Sustain. 2024, 27, 16525–16548. [Google Scholar] [CrossRef]
- Kunda, M.; Ray, D.; Pandit, D.; Harun-Al-Rashid, A. Establishment of a Fish Sanctuary for Conserving Indigenous Fishes in the Largest Freshwater Swamp Forest of Bangladesh: A Community-Based Management Approach. Heliyon 2022, 8, e09498. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Khan, M.A.A.; Kashem, M.A.; Hoque, S. Plant Community Composition in Relation to Soil Physico-Chemical Properties of the Ratargul Swamp Forest, Bangladesh. Dhaka Univ. J. Biol. Sci. 2016, 25, 1–8. [Google Scholar] [CrossRef]
- Panda, F.; Pati, S.G.; Bal, A.; Mathur, S.; Nirmaladevi, R.; Paital, B. Temporal Morphometric Analyses of Pila globosa in India for Its Use in Aquaculture and Food Industry. J. Basic Appl. Zool. 2021, 82, 17. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hussain, M.; Sarker, T.R.; Saha, S.; Iqbal, M.M. Reproductive and Morphometric Traits of Freshwater Mussel Lamellidens marginalis and Associated Hydrology in the Ratargul Freshwater Swamp Forest, Bangladesh. Egypt. J. Aquat. Res. 2023, 49, 161–170. [Google Scholar] [CrossRef]
- Uddin, M.J.; Jeung, H.D.; Yang, H.S.; Kim, B.K.; Ju, S.J.; Choi, K.S. Quantitative Assessment of Reproductive Effort of the Manila Clam Ruditapes philippinarum in a Lagoon on Jeju Island (Korea) Using Enzyme-Linked Immunosorbent Assay. Invertebr. Reprod. Dev. 2013, 57, 316–324. [Google Scholar] [CrossRef]
- Htun-Han, M. The Reproductive Biology of the Dab Limanda limanda (L.) in the North Sea: Seasonal Changes in the Ovary. J. Fish Biol. 1978, 13, 351–359. [Google Scholar] [CrossRef]
- Froese, R.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.C.; Dimarchopoulou, D.; Scarcella, G.; Probst, W.N.; Dureuil, M.; Pauly, D. A New Approach for Estimating Stock Status from Length Frequency Data. ICES J. Mar. Sci. 2018, 75, 2004–2015. [Google Scholar] [CrossRef]
- Hattori, R.S.; Tashiro, S.; Zhang, Y.; Kakuta, N.; Yokota, M.; Strüssmann, C.A.; Yamamoto, Y. Demonstration of Viability and Fertility and Development of a Molecular Tool to Identify YY Supermales in a Fish with Both Genotypic and Environmental Sex Determination. Ecol. Evol. 2018, 8, 7522–7528. [Google Scholar] [CrossRef]
- Le Cren, E.D. The Length-Weight Relationship and Seasonal Cycle in Gonad Weight and Condition in the Perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201–219. [Google Scholar] [CrossRef]
- Caspers, H. Methods for Assessment of Fish Production in Fresh Waters; Ricker, W., Ed.; Blackwell Scientific Publications: Oxford, UK, 1969; Volume 54. [Google Scholar]
- Lee, J.H. Gonadal Development and Reproductive Cycle of the Top Shell, Omphalius rusticus (Gastropoda: Trochidae). Korean J. Biol. Sci. 2001, 5, 37–44. [Google Scholar] [CrossRef]
- Dennis, M.M.; Molnár, K.; Kriska, G.; Lőw, P. Mollusca. In Invertebrate Histology; Wiley: Oxford, UK, 2021; pp. 87–132. [Google Scholar]
- Rodrigues, C.C.; Caixeta, M.B.; Araújo, P.S.; Gonçalves, B.B.; Araújo, O.A.; Silva, L.D.; Rocha, T.L. Gonadal Histopathology and Inflammatory Response in the Freshwater Snail Exposed to Iron Oxide Nanoparticles and Ferric Chloride: Insights into Reproductive Nanotoxicity. Aquat. Toxicol. 2021, 237, 105910. [Google Scholar] [CrossRef]
- Gu, Q.H.; Husemann, M.; Wu, H.H.; Dong, J.; Zhou, C.J.; Wang, X.F.; Gao, Y.N.; Zhang, M.; Zhu, G.R.; Nie, G.X. Phylogeography of Bellamya (Mollusca: Gastropoda: Viviparidae) Snails on Different Continents: Contrasting Patterns of Diversification in China and East Africa. BMC Evol. Biol. 2019, 19, 82. [Google Scholar] [CrossRef]
- Prieto, G.I. Caution Ahead: Reassessing the Functional Morphology of the Respiratory Organs in Amphibious Snails. PeerJ 2021, 9, e12161. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Krupanidhi, S. Regression Analysis of Shell Morphology of a Few of Endemic Aquatic and Terrestrial Gastropods as a Prelude to Their Conservation Strategy. Acta Sci. Biotechnol. 2020, 1, 2–7. [Google Scholar]
- Mantale, A.; Patil, M.; Shaikh, M.; Lakwal, V. Length—Weight Relationship of Fresh Water Pulmonate Snail. J. Emerg. Technol. Innov. Res. 2018, 5, 168–173. [Google Scholar]
- Jaiswar, A.K.; Kulkarni, B.G. Length-Weight Relationship of Intertidal Molluscs from Mumbai, India. J. Indian Fish. Assoc. 2002, 29, 55–63. [Google Scholar]
- Ngor, P.B.; Sor, R.; Prak, L.H.; So, N.; Hogan, Z.S.; Lek, S. Mollusc Fisheries and Length-Weight Relationship in Tonle Sap Flood Pulse System, Cambodia. Ann. Limnol. 2018, 54, 34. [Google Scholar] [CrossRef]
- Siddique, M.A.; Khatun, M.A.; Rahman, M.M.; Ahmed, G.U.; Moniruzzaman, M.; Jasim Uddin, M. Annual Gametogenic Cycle of the Freshwater Pearl Mussel, Lamellidens marginalis (Lamarck, 1819) Collected from a Perennial Lentic Habitat of Bangladesh. Molluscan Res. 2020, 40, 36–43. [Google Scholar] [CrossRef]
- Hussain, M.; Hossain, M.A.; Begum, M.; Roy, N.C. Freshwater Mussel (Lamelliedens marginalis) to Reduce the Lead (Pb) Bioaccumulation in Aquaculture of Stinging Catfish, Heteropneustes fossilis. J. Appl. Aquac. 2023, 35, 605–621. [Google Scholar] [CrossRef]
- Thomas, D.; Rekha, M.U.; Angel, J.R.J.; Sreekanth, G.B.; Thiagarajan, G.; Subburaj, R.; Kailasam, M.; Vijayan, K.K. Effects of Salinity Amendments on the Embryonic and Larval Development of a Tropical Brackishwater Ornamental Silver Moony Fish, Monodactylus argenteus (Linnaeus, 1758). Aquaculture 2021, 544, 737073. [Google Scholar] [CrossRef]
- Gondal, M.A.; Waheed, Q.; Tariq, S.; Haider, W.; Khan, A.; Rasib, Q.; Ahmed, H. Morpho-Ecological Study of Freshwater Mollusks of Margalla Foothills, Pakistan. Pak. J. Zool. 2020, 52, 863–874. [Google Scholar] [CrossRef]
- Hossain, M.A.; Chowdhury, T.; Chowdhury, G.; Schneider, P.; Hussain, M.; Das, B.; Iqbal, M.M. Impact of Pb Toxicity on the Freshwater Pearl Mussel, Lamellidens marginalis: Growth Metrics, Hemocyto-Immunology, and Histological Alterations in Gill, Kidney, and Muscle Tissue. Toxics 2023, 11, 475. [Google Scholar] [CrossRef]
- Chatterjee, A.; Jain, M.; Roy, U.S.; Kumar Mukhopadhyay, S. Limnochemical Factors Influencing the Seasonal Population Density, Secondary Production, and Calcium-to-Tissue Ratio in the Freshwater Limpet Septaria lineata (Archaeogastropoda: Neritidae). Turkish J. Zool. 2008, 32, 245–252. [Google Scholar]
- Camara, I.A.; Bony, Y.K.; Diomandé, D.; Edia, O.E.; Konan, F.K.; Kouassi, C.N.; Gourne, G.; Pointier, J.P. Freshwater Snail Distribution Related to Environmental Factors in Banco National Park, an Urban Reserve in the Ivory Coast (West Africa). African Zool. 2012, 47, 160–168. [Google Scholar] [CrossRef]
- Pandit, D.; Haque, M.M.; Harun-Al-Rashid, A.; Sarker, B.; Hossain, M.A.; Schneider, P.; Kunda, M. Spatiotemporal Variations in Water Quality of the Transboundary Shari-Goyain River, Bangladesh. Sustainability 2023, 15, 5218. [Google Scholar] [CrossRef]
- Hoang, T.C.; Tong, X. Influence of Water Quality on Zinc Toxicity to the Florida Apple Snail (Pomacea paludosa) and Sensitivity of Freshwater Snails to Zinc. Environ. Toxicol. Chem. 2015, 34, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Islam, M.S.; Moniruzzaman, M.; Ahmed, K.K.U. Natural Breeding of Freshwater Apple Snail Pila globosa (Swainson) in Pond and Aquarium. Bangladesh J. Fish. 2020, 32, 45–54. [Google Scholar] [CrossRef]
- García-ulloa, M.; del Carmen Gallo-Garcia Maria, M.; Rodríguez-gonzález, H.; Góngora-gómez, A.; Ponce-palafox, J.T. Morphometric Relationship of Weight and Length of Cultured Freshwater Snail, Pomacea Patula (Baker, 1922), at Three Different Life Stages. J. World Aquac. Soc. 2008, 39, 842–846. [Google Scholar] [CrossRef]
- Wu, J.Y.; Wu, Y.T.; Li, M.C.; Chiu, Y.W.; Liu, M.Y.; Liu, L.L. Reproduction and Juvenile Growth of the Invasive Apple Snails Pomacea canaliculata and P. scalaris (Gastropoda: Ampullariidae) in Taiwan. Zool. Stud. 2011, 50, 61–68. [Google Scholar]
- Tamburi, N.E.; Martín, P.R. Feeding Rates and Food Conversion Efficiencies in the Apple Snail Pomacea Canaliculata (Caenogastropoda: Ampullariidae). Malacologia 2009, 51, 221–232. [Google Scholar] [CrossRef]
- Annate, S.; Ng, T.H.; Sutcharit, C.; Panha, S. Mating and Egg-Laying Behaviour of the Southeast Asian Apple Snail Pila Pesmei (Morlet, 1889) (Caenogastropoda, Ampullariidae). Zookeys 2025, 1231, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Humayun-Bin-Akram, M.; Masum, K.M. Forest Degradation Assessment of Ratargul Special Biodiversity Protection Area for Conservation Implications. Forestist 2020, 70, 77–84. [Google Scholar] [CrossRef]
- Kanan, A.H. Characterization and Conservation of Wetlands with Global Change Dynamics: A Case Study on Ratargul Swamp Forest. Master’s Thesis, Universidade de Lisboa, Lisbon, Portugal, 2016. [Google Scholar]
- Akter, S.; Mozahid, M.N.; Iqbal, M.H. Valuation of Ratargul Swamp Forest Conservation: Does Climate Knowledge Matter? Trees For. People 2024, 17, 100638. [Google Scholar] [CrossRef]
- Tirado, T.; Saura, M.; Rolán-Alvarez, E.; Quesada, H. Historical Biogeography of the Marine Snail Littorina saxatilis Inferred from Haplotype and Shell Morphology Evolution in NW Spain. PLoS ONE 2016, 11, e0161287. [Google Scholar] [CrossRef] [PubMed]
- Lantin-Olaguer, I.; Bagarinao, T.U. Gonadal Maturation, Fecundity, Spawning, and Timing of Reproduction in the Mud Snail, Cerithidea cingulata, a Pest in Milkfish Ponds in the Philippines. Invertebr. Reprod. Dev. 2001, 39, 195–207. [Google Scholar] [CrossRef]
| Months | Temperature (°C) | DO (mg/L) | EC (µS cm−1) | TDS (ppm) | pH | Transparency (cm) | Depth (m) |
|---|---|---|---|---|---|---|---|
| January | 17.22 ± 0.85 a | 5.06 ± 0.19 | 72.21 ± 0.87 d | 33.98 ± 0.70 d | 6.35 ± 0.23 bc | 19.83 ± 0.62 b | 1.14 ± 0.41 a |
| February | 25.35 ± 0.93 c | 5.35 ± 0.88 | 76.39 ± 1.01 d | 36.82 ± 0.23 d | 6.57 ± 0.19 c | 33.20 ± 1.29 c | 1.36 ± 0.48 a |
| March | 23.46 ± 0.42 c | 3.46 ± 0.21 | 62.84 ± 0.51 b | 31.83 ± 0.24 d | 6.11 ± 0.06 ab | 10.53 ± 1.44 ab | 0.69 ± 0.15 a |
| April | 28.07 ± 0.45 d | 3.42 ± 0.54 | 61.65 ± 0.63 b | 29.25 ± 0.25 d | 5.66 ± 0.28 ab | 8.57 ± 0.42 a | 1.07 ± 0.37 a |
| May | 27.74 ± 0.16 d | 4.82 ± 0.56 | 31.80 ± 0.83 a | 15.14 ± 0.33 a | 6.27 ± 0.26 bc | 12.83 ± 0.62 b | 1.99 ± 0.55 ab |
| June | 27.38 ± 1.00 d | 5.14 ± 0.55 | 66.60 ± 0.92 c | 29.66 ± 0.48 d | 7.48 ± 0.25 d | 17.20 ± 0.86 b | 3.05 ± 0.18 b |
| July | 29.09 ± 0.10 d | 4.79 ± 0.64 | 70.63 ± 0.46 d | 31.14 ± 0.07 d | 7.31 ± 0.21 d | 22.85 ± 0.24 b | 2.96 ± 0.32 b |
| August | 29.50 ± 0.44 d | 4.81 ± 0.75 | 71.93 ± 0.30 d | 27.49 ± 0.09 d | 6.40 ± 0.06 c | 100.27 ± 1.37 e | 2.77 ± 0.29 b |
| September | 29.35 ± 0.55 d | 5.29 ± 1.22 | 73.53 ± 0.15 d | 20.43 ± 0.21 d | 6.51 ± 0.11 c | 57.66 ± 0.59 d | 2.04 ± 0.42 ab |
| October | 27.23 ± 0.23 e | 5.57 ± 0.33 | 69.70 ± 0.49 d | 18.29 ± 0.05 b | 5.52 ± 0.30 a | 16.66 ± 0.81 b | 1.71 ± 0.60 ab |
| November | 24.41 ± 0.54 c | 5.50 ± 0.94 | 66.83 ± 0.20 c | 19.18 ± 0.23 c | 5.55 ± 0.24 a | 15.75 ± 0.41 b | 1.30 ± 0.43 a |
| December | 20.42 ± 0.64 b | 5.14 ± 0.16 | 70.60 ± 0.52 d | 22.66 ± 0.05 d | 5.69 ± 0.19 ab | 14.33 ± 0.34 b | 1.07 ± 0.29 a |
| Months | Shell Length (cm) | Shell Weight (g) | Shell Width (cm) | Spiral Length (cm) | Aperture Length (cm) | Aperture Width (cm) | Base Length (cm) | Soft Tissue Wet Weight (g) |
|---|---|---|---|---|---|---|---|---|
| January | 4.95 ± 0.63 bc | 41.47 ± 17.32 bc | 3.64 ± 0.50 bc | 2.08 ± 0.53 b | 3.32 ± 0.55 bc | 3.49 ± 0.44 ab | 1.95 ± 0.36 bc | 18.53 ± 7.38 ab |
| February | 4.94 ± 0.88 bc | 33.49 ± 16.88 ab | 4.04 ± 0.73 d | 2.82 ± 1.05 d | 3.68 ± 0.73 c | 3.54 ± 0.68 ab | 2.21 ± 0.50 bc | 20.50 ± 11.02 ab |
| March | 4.73 ± 1.42 bc | 31.61 ± 15.42 a | 3.44 ± 0.88 ab | 2.26 ± 0.65 bc | 3.42 ± 0.98 c | 3.38 ± 0.74 a | 2.04 ± 0.61 bc | 23.93 ± 30.34 b |
| April | 4.63 ± 0.93 bc | 42.18 ± 14.60 bc | 3.43 ± 0.83 ab | 2.02 ± 0.47 ab | 3.43 ± 0.73 c | 3.35 ± 0.51 a | 1.96 ± 0.39 bc | 16.53 ± 5.93 ab |
| May | 4.29 ± 0.87 ab | 34.38 ± 12.88 ab | 3.42 ± 0.71 ab | 2.06 ± 0.59 ab | 3.42 ± 0.75 c | 3.38 ± 0.64 a | 2.11 ± 0.46 bc | 13.56 ± 6.62 a |
| June | 4.36 ± 0.98 ab | 35.23 ± 14.29 ab | 3.48 ± 0.80 bc | 2.23 ± 0.71 bc | 3.48 ± 0.95 c | 3.46 ± 0.68 a | 2.17 ± 0.55 bc | 14.17 ± 7.13 a |
| July | 4.57 ± 0.82 bc | 39.63 ± 12.81 bc | 3.31 ± 0.65 ab | 2.73 ± 0.49 bc | 2.82 ± 0.57 ab | 3.33 ± 0.53 a | 1.91 ± 0.34 bc | 18.15 ± 6.42 ab |
| August | 4.12 ± 0.84 a | 34.85 ± 10.15 ab | 3.17 ± 0.59 a | 2.53 ± 0.49 cd | 2.78 ± 0.57 a | 3.27 ± 0.48 a | 1.88 ± 0.25 ab | 15.54 ± 5.24 ab |
| September | 4.37 ± 1.09 bc | 36.39 ± 15.42 ab | 3.47 ± 0.81 ab | 2.21 ± 0.66 ab | 3.21 ± 0.73 bc | 3.38 ± 0.80 a | 1.80 ± 0.43 a | 15.25 ± 6.99 ab |
| October | 4.80 ± 0.90 bc | 45.02 ± 14.39 bc | 3.77 ± 0.67 bc | 2.29 ± 0.43 bc | 3.36 ± 0.60 bc | 3.49 ± 0.64 a | 1.90 ± 0.38 bc | 19.97 ± 6.57 ab |
| November | 4.75 ± 0.80 bc | 36.07 ± 14.71 ab | 3.62 ± 0.50 bc | 1.97 ± 0.56 a | 3.39 ± 0.53 bc | 3.50 ± 0.51 ab | 1.90 ± 0.33 bc | 17.94 ± 6.62 ab |
| December | 5.13 ± 0.93 c | 49.20 ± 15.23 c | 3.98 ± 0.64 cd | 2.32 ± 0.51 cd | 3.65 ± 0.57 c | 4.00 ± 0.72 b | 2.25 ± 0.50 c | 22.59 ± 7.16 ab |
| Equation | N | a | b | 95% Confidence Interval | R2 | p Value | Mw | |
|---|---|---|---|---|---|---|---|---|
| a | b | |||||||
| BW = a × SLb | 384 | 0.55 | 1.50 | 0.49–0.52 | 1.60–1.64 | 0.58 | 0.000 | 0.97 |
| BW = a × BLb | 0.79 | 1.27 | 0.81–0.85 | 1.29–1.31 | 0.27 | 0.000 | ||
| BW = a × SPLb | 0.85 | 1.27 | 0.74–0.78 | 1.44–1.51 | 0.41 | 0.000 | ||
| BW = a × APLb | 0.95 | 1.15 | 0.80–0.84 | 1.43–1.50 | 0.35 | 0.000 | ||
| BW = a × APWDb | 0.75 | 1.50 | 0.75–0.78 | 1.48–1.53 | 0.45 | 0.000 | ||
| BW = a × STWWb | 1.24 | 1.05 | 1.24–1.25 | 1.13–1.17 | 0.30 | 0.000 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, S.; Hossain, M.A.; Chowdhury, G.; Hussain, M.; Pandit, D.; Kunda, M.; Schneider, P.; Iqbal, M.M. Reproductive Ecology of the Freshwater Snail, Pila globosa, Considering Environmental Factors in a Tropical Freshwater Swamp Forest. Conservation 2025, 5, 43. https://doi.org/10.3390/conservation5030043
Das S, Hossain MA, Chowdhury G, Hussain M, Pandit D, Kunda M, Schneider P, Iqbal MM. Reproductive Ecology of the Freshwater Snail, Pila globosa, Considering Environmental Factors in a Tropical Freshwater Swamp Forest. Conservation. 2025; 5(3):43. https://doi.org/10.3390/conservation5030043
Chicago/Turabian StyleDas, Suhel, Mohammad Amzad Hossain, Gourab Chowdhury, Monayem Hussain, Debasish Pandit, Mrityunjoy Kunda, Petra Schneider, and Mohammed Mahbub Iqbal. 2025. "Reproductive Ecology of the Freshwater Snail, Pila globosa, Considering Environmental Factors in a Tropical Freshwater Swamp Forest" Conservation 5, no. 3: 43. https://doi.org/10.3390/conservation5030043
APA StyleDas, S., Hossain, M. A., Chowdhury, G., Hussain, M., Pandit, D., Kunda, M., Schneider, P., & Iqbal, M. M. (2025). Reproductive Ecology of the Freshwater Snail, Pila globosa, Considering Environmental Factors in a Tropical Freshwater Swamp Forest. Conservation, 5(3), 43. https://doi.org/10.3390/conservation5030043









