Abstract
Rice (Oryza sativa), a crucial global staple, grapples with environmental stress and resource constraints, necessitating sustainable farming. This review explores the transformative role of transcription factors (TFs) in revolutionizing rice agriculture and their potential impact on global food security. It underscores TFs’ pivotal role in gene expression, particularly in responding to environmental stimuli, presenting a promising avenue for enhancing rice resilience. Delving into key TF families in rice, it highlights their multifaceted roles in abiotic stress responses, defense mechanisms, yield improvement, nutrient uptake, seed development, photosynthesis, and flowering regulation. Specific TFs, including DREB (Dehydration-Responsive Element-Binding), WRKY, NAC, MYB (Myeloblastosis), AP2/ERF (APETALA2/Ethylene Responsive Factor), and bHLH (basic Helix–Loop–Helix), are examined for their contributions to stress resilience, defense mechanisms, and yield enhancement. Concrete examples from cutting-edge research illustrate the tangible benefits of harnessing these molecular regulators. However, manipulating TFs presents challenges, necessitating innovative approaches such as predictive models, collaborative field testing, and transparent communication to navigate intricate regulatory networks and regulatory hurdles. Ultimately, a promising future emerges where manipulating rice TFs leads to the development of resilient, high-yielding, and nutritious varieties. Embracing research advancements and addressing existing challenges is imperative to unlock the full potential of these concealed regulators, ensuring sustainable food security for a growing global population.
1. Introduction
Rice (Oryza sativa) plays a pivotal role at the forefront of global food security, as a staple for a substantial portion of the world’s population, with its global demand driven by its role as the primary dietary component for over half of the world’s population, particularly in Asia, which accounts for nearly 90% of rice production and consumption. The global rice market was valued at USD 376.54 billion in 2024 and is projected to reach USD 436.51 billion by 2029, growing at a CAGR of 3% during the forecast period []. Key contributions to this growth include the increasing preference for specialty rice varieties, advancements in rice mill machinery, and rising food and restaurant sectors. India and China, as leading producers, play a significant role in meeting both domestic needs and international trade demands, with India exporting substantial quantities of basmati rice and China maintaining high production levels to cater to its large domestic and global market []. However, the ever-increasing demand for rice, compounded by environmental stresses like climate change, compels a paradigm shift towards sustainable farming practices for this essential crop. The urgency of this shift is grounded in the realization that traditional cultivation methods may fall short in meeting the rising global demand amidst evolving environmental challenges []. With the global population continuing its upward trajectory, the call for sustainable agriculture becomes more pronounced, emphasizing the need for practices that ensure long-term environmental health, economic viability, and social equity [,]. In this context, the innovative approach of harnessing plant transcription factors emerges as a cutting-edge solution, holding immense potential to optimize rice yield while concurrently alleviating the impact of environmental stresses.
Beyond its fundamental role in nutrition, rice holds profound cultural, economic, and nutritional significance, particularly in Asia, where it enjoys the status of a dietary staple. The cultivation and consumption of rice become focal points with far-reaching impacts, making it a crucial subject for agricultural research and development endeavors []. The diverse array of rice varieties, spanning long-grain to short-grain and aromatic types, showcases its adaptability to various climates and preferences, further underlining its multifaceted importance []. Moreover, the economic significance of rice as a source of livelihood for numerous communities adds another layer to its complexity.
Transcription factors, as master regulators of gene expression in plants, emerge as central players influencing growth, development, and responses to environmental stresses. Their indispensable role in shaping a plant’s phenotype becomes apparent for adaptation to changing environmental conditions, highlighting their significance in the dynamic landscape of plant biology []. Unraveling how transcription factors orchestrate biological processes in rice becomes pivotal, given their role as molecular switches governing responses to external stimuli, including stresses like drought, pathogens, and temperature variations.
This pivotal role of transcription factors in plant biology sets the stage for an exploration of their function and regulation in rice. This endeavor holds immense potential, not only for elevating crop productivity and fortifying resilience against environmental challenges but also for enhancing the nutritional quality of rice []. Delving into the specific roles of transcription factors in rice, researchers aim to unravel the underlying molecular mechanisms influencing traits such as drought resistance, disease tolerance, and nutrient efficiency []. This knowledge not only contributes to a deeper understanding of fundamental plant biology but also provides tangible insights for developing new rice varieties tolerance to changing climate, ultimately meeting the demands of a growing global population (Figure 1).
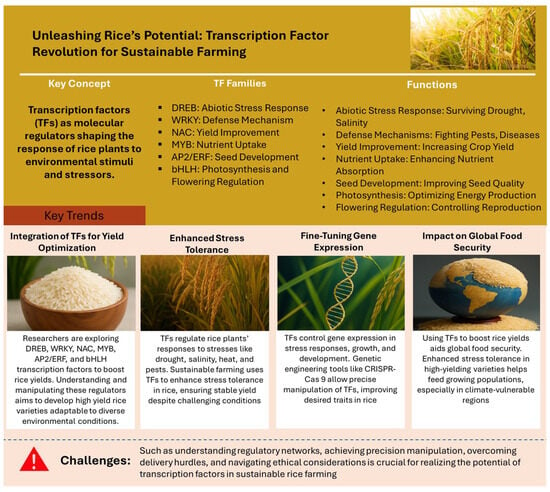
Figure 1.
Visual representation of transcription factors revolutionizing sustainable rice farming, optimizing yield amidst global demand and environmental challenges (Source: Authors’ creation).
1.1. Abiotic and Biotic Stress in Rice
Abiotic stresses such as drought, salinity, heat, cold, hypoxia, and nutrient deficiency severely affect rice growth, productivity, and grain quality. Among these, drought and salinity are the most prominent threats in rainfed and coastal rice-growing regions, respectively. Drought induces osmotic stress, leading to stomatal closure, reduced photosynthesis, and cellular damage. Salinity disrupts the ionic balance and causes oxidative stress. Temperature extremes impair reproductive development, particularly during panicle initiation and flowering. Hypoxia, commonly encountered under submergence or waterlogging, restricts the oxygen availability for root respiration, altering the cellular metabolism []. To survive these conditions, rice activates a cascade of molecular responses, many of which are orchestrated by transcription factors (TFs) that regulate stress-inducible genes.
Beyond abiotic factors, biotic stresses pose significant constraints on rice production. Pathogens such as Xanthomonas oryzae pv. oryzae (bacterial blight), Magnaporthe oryzae (rice blast), Rhizoctonia solani (sheath blight), and pests like brown planthopper and stem borers lead to major yield losses []. These organisms often secrete virulence factors or manipulate host immunity, triggering complex plant defense responses. Rice relies on a finely tuned transcriptional reprogramming to detect, signal, and counteract these threats. Tran-scription factors like WRKY, MYB, and NAC play essential roles in activating pathogene-sis-related (PR) genes, hypersensitive response (HR), and systemic acquired resistance (SAR). These TFs serve as critical hubs in integrating signaling molecules such as salicylic acid (SA), jasmonic acid (JA), and ethylene (ET), enabling precise spatiotemporal activa-tion of defense pathways [].
The TF families included in this review were selected based on their experimentally validated roles in regulating abiotic and/or biotic stress responses in rice (Table 1). Core families like DREB, MYB, WRKY, and NAC have been widely reported to modulate drought, salt, and pathogen responses. Emerging families like DST (a negative regulator of drought and salt), HD-ZIP (key to hypoxia adaptation), and bZIP (linked to ABA signal-ing) have been specifically included due to their mechanistic relevance. While some families like CO, PLT, and HSP are mentioned, the main focus is on TFs with direct stress-responsive gene targets and available functional data. This prioritization enables a more mechanistic and actionable review.

Table 1.
Major transcription factors involved in stress responses in rice.
In constructing this review, a conscious effort was made to balance extensively studied transcription factor families with those that are emerging as critical regulators in rice abiotic stress responses. In addition to canonical TFs like DREB, WRKY, NAC, MYB, and AP2/ERF, the inclusion of DST (Drought and Salt Tolerance), bZIP (Basic Leucine Zipper), and HD-ZIP (Homeodomain-Leucine Zipper) families offer a more comprehensive perspective on transcriptional regulation under complex environmental stresses []. While not as numerous in gene count as some other families, these TFs exert disproportionately large effects on rice stress physiology. For instance, DST, a zinc finger TF, functions as a negative regulator of drought and salt stress responses, and loss-of-function mutants exhibit enhanced water retention, ROS detoxification, and improved abiotic stress tolerance, a trait that has already reached the patent stage for agronomic application []. Similarly, bZIP TFs such as OsbZIP23 and OsbZIP46 are central to ABA signaling, modulating osmotic stress tolerance and influencing post-translational networks via phosphorylation and SUMOylation. The HD-ZIP family, notably OsHOX22 and OsHOX24, integrates ABA, ethylene, and hypoxia signaling, governing stress-inducible gene expression critical to survival under submergence and dehydration []. These TFs serve as molecular hubs that mediate cross-talk between developmental and stress-response pathways, representing high-value targets for precision breeding and CRISPR-mediated crop improvement. Their inclusion strengthens the review’s objective to present a multifaceted and translationally relevant synthesis of transcriptional regulation in rice agriculture.
1.1.1. Plant Transcription Factors in Rice
Transcription factors stand as the intricate architects of gene expression in rice, guiding the plant’s responses to internal and external cues with remarkable specificity. The rice genome hosts a diverse array of TFs, each belonging to distinct families marked by unique structural motifs and functional roles. Within this genomic landscape, TFs play a fundamental role in shaping various facets of rice biology, ranging from growth and development to adaptive responses against environmental stresses []. Among the key TF families in rice, the DREB family assumes a critical role in the plant’s resilience to water scarcity, activating stress-related genes to enhance drought tolerance []. The WRKY family, on the other hand, governs defense responses against pathogens, providing insights into strategies for developing disease-resistant rice varieties []. NAC TFs in rice exhibit multifaceted functions, influencing both developmental processes and responses to abiotic stresses, offering potential avenues for enhancing growth and stress tolerance []. The MYB family’s contributions to secondary metabolism and stress responses pave the way for manipulating rice traits to improve stress resilience and nutritional content [].
The AP2/ERF family in rice emerges as a key player in responding to abiotic stress and regulating yield-related traits, presenting opportunities for developing rice varieties with heightened stress resilience and improved productivity []. bHLH TFs in rice, in addition to their roles in normal plant growth, development, flower induction, and secondary metabolite biosynthesis, also play crucial roles in nutrient uptake and utilization, particularly in iron uptake and nutrient-related gene regulation, making them potential focal points for improving nutrient efficiency in alignment with sustainable agriculture practices []. Various other TF families in rice contribute to the intricate regulatory landscape, influencing traits like seed development, photosynthesis, and flowering time.
Understanding the nuanced roles of these TFs in rice not only enriches our comprehension of plant biology but also holds the promise of strategic genetic manipulation. This knowledge opens novel avenues for enhancing the adaptability, productivity, and nutritional quality of rice crops, crucial steps toward meeting the challenges posed by a changing climate and the demands of a growing global population.
1.1.2. DREB (Dehydration-Responsive Element-Binding)
The DREB family in rice stands out as a key player in orchestrating the plant’s response to the challenging conditions of drought stress. This family of transcription factors plays a pivotal role by directly binding to dehydration-responsive elements in the rice genome. In times of water scarcity, DREB transcription factors activate a cascade of stress-related genes, triggering molecular pathways that equip the plant to cope with and adapt to the arid environment []. The significance of DREB in rice becomes even more apparent when considering the intricate molecular mechanisms it employs to confer resilience to water deficit. As these transcription factors bind to dehydration-responsive elements, they act as molecular switches, initiating a series of biochemical and physiological changes within the plant. These changes include the upregulation of genes responsible for drought tolerance, water use efficiency, and osmotic regulation [].
The exploration of DREB family members unveils an understanding of rice’s adaptive strategies in arid environments. Different members of the DREB family may exhibit specificities in their activation of stress-related genes or responsiveness to varying degrees of water scarcity []. Investigating the expression patterns and regulatory interactions of DREB transcription factors contributes to a comprehensive grasp of how rice navigates the challenges posed by drought, shedding light on potential targets for genetic manipulation to enhance drought resilience. Within this framework, three major DREB subfamilies are identified in rice: OsDREB1, OsDREB2, and OsDREB3, each playing distinct roles in stress responses. Notably, OsDREB2A is crucial for conferring drought tolerance, while OsDREB1B shows promise for salinity tolerance. Other members like OsDREB1A and OsDREB3 also contribute to various stress responses. By focusing on rice-specific DREB subfamilies, the review offers insights into enhancing stress tolerance in rice crops [].
Overall, the DREB family’s involvement in the rice plant’s response to drought stress showcases the intricate and finely tuned regulatory networks at play. Unraveling the molecular intricacies of DREB-mediated drought tolerance not only deepens our understanding of plant stress responses but also provides valuable insights for the development of drought-resistant rice varieties, essential for ensuring food security in the face of changing climatic conditions.
The study conducted by Chengqi et al. underscores the significance of DREB genes in the context of molecular breeding for drought tolerance in rice []. While Chengqi et al. [] did not provide specific examples directly related to the utilization of DREB genes in molecular breeding techniques, they allude to potential strategies for their incorporation. Marker-assisted selection (MAS) is suggested as a means to identify and employ DNA markers linked to DREB genes associated with drought tolerance, facilitating targeted breeding efforts. Moreover, the study discusses the potential of quantitative trait loci (QTL) mapping to elucidate genomic regions harboring DREB genes or their regulatory elements, thereby aiding in the development of more efficient breeding strategies. Additionally, the paper speculates on the theoretical application of genome editing techniques such as CRISPR-Cas9 to precisely edit DREB genes or their regulatory elements, potentially directly enhancing drought tolerance in rice varieties. However, the ethical and regulatory challenges associated with genome editing are acknowledged. While specific examples using DREB genes in molecular breeding for drought tolerance are not provided in Chengqi et al.’s study, further exploration of the literature focused on this topic may yield more detailed insights into ongoing efforts and outcomes in this field [].
The study conducted by Al Azzawi et al. (2020) [] investigates the drought tolerance of five Iraqi rice cultivars through a comprehensive evaluation encompassing phenotypic, physiological, and gene expression analyses. Notably, Mashkab emerges as the most drought-tolerant cultivar, exhibiting superior performance in various parameters such as plant height, root length, chlorophyll content, and relative water content under stress conditions induced by polyethylene glycol (PEG) and water withholding []. Gene expression analysis, particularly focusing on OsDREB2A (Oryza sativa Dehydration-Responsive Element-Binding protein 2A), reveals higher expression levels in drought-tolerant cultivars compared to a drought-sensitive control, suggesting a potential association between OsDREB2A expression and drought tolerance []. These findings underscore the importance of identifying and characterizing drought-responsive genes like OsDREB2A in breeding programs aimed at developing rice varieties resilient to water scarcity, with Mashkab showing promising potential for further improvement and deployment in agricultural practices.
1.1.3. WRKY
The WRKY transcription factor family in rice occupies a crucial role in orchestrating the plant’s defense responses against a myriad of pathogens. Acting as regulatory switches, WRKY TFs play a pivotal role in fine-tuning the intricate molecular pathways that fortify the plant’s immune system []. Understanding the specific roles and functions of WRKY TFs in rice unveils mechanisms essential for bolstering the plant’s defense mechanisms, laying the foundation for the development of disease-resistant rice varieties.
In times of pathogenic invasion, WRKY TFs in rice act as sentinels, initiating a cascade of molecular events that activate defense-related genes. This includes the production of antimicrobial compounds, reinforcement of physical barriers, and the induction of programmed cell death to contain and neutralize the invading pathogens [,]. The WRKY family members display a level of specificity in their responses, with distinct TFs being activated in response to different pathogens or environmental cues.
The significance of WRKY TFs in rice extends beyond immediate defense reactions. By gaining insights into the regulatory roles of WRKY TFs, researchers can identify potential targets for genetic manipulation to enhance the plant’s innate ability to fend off pathogens. This in turn has implications for sustainable agriculture practices by reducing the dependence on chemical interventions and fostering environmentally friendly disease management strategies [].
This knowledge not only contributes to the scientific understanding of plant–pathogen interactions but also holds promise for practical applications in agriculture, addressing the ongoing challenges of global food security.
Liu et al. (2007) [] provide compelling evidence for the involvement of the rice transcription factor OsWRKY71 in the defense response against bacterial pathogens. Through meticulous experimentation, the researchers reveal that the expression of OsWRKY71 is upregulated in response to various defense signaling molecules and pathogen infection, indicating its role in the defense signaling cascade []. By employing transgenic approaches, they demonstrate that the overexpression of OsWRKY71 in rice leads to heightened resistance against the bacterial pathogen Xanthomonas oryzae pv. oryzae (Xoo). Intriguingly, this enhanced resistance is accompanied by the constitutive expression of two crucial marker genes in the defense signaling pathway, OsNPR1 and OsPR1b. These findings strongly suggest that OsWRKY71 functions as a transcriptional regulator, potentially operating upstream of OsNPR1 and OsPR1b, thereby activating pivotal genes for mounting an effective defense response in rice against bacterial pathogens. This study sheds light on the intricate molecular mechanisms governing rice immunity and offers promising insights for the development of novel strategies to enhance crop resistance to devastating bacterial diseases.
In the study conducted by Liu et al. (2016) [], they assessed the role of alternative splicing in regulating the activity of two rice WRKY transcription factor genes, WRKY62 and WRKY76, in response to pathogen attacks. They discovered that alternative splicing generates various versions of WRKY62 and WRKY76 transcripts, each showing distinct expression patterns when rice plants encounter pathogens. Through meticulous molecular investigations, the researchers revealed that specific variations of WRKY62 and WRKY76 contribute differently to the plant’s immune response []. Moreover, they shed light on the mechanisms controlling the alternative splicing of these WRKY genes, highlighting their crucial role in fine-tuning the defense mechanisms of rice. This study significantly advances our understanding of how rice defends itself against pathogens at the molecular level and provides valuable insights for improving disease resistance in crops.
1.1.4. NAC (NAM, ATAF1/2, CUC2)
The NAC transcription factor family in rice exhibits a diverse array of functions, contributing to the intricate balance between developmental processes and responses to abiotic stresses. NAC TFs are pivotal molecular players that influence the regulation of various genes, orchestrating the plant’s growth and adaptation to challenging environmental conditions []. Studying the roles of NAC TFs provides valuable in-sights into the complex interplay between growth modulation and stress tolerance strategies in rice crops.
In terms of developmental processes, NAC TFs in rice play regulatory roles in cell differentiation, lateral root formation, and shoot development. By modulating these fundamental processes, NAC TFs contribute to shaping the overall architecture and morphology of the rice plant. The multifaceted functions of NAC TFs extend beyond development, encompassing responses to abiotic stresses such as drought, salinity, and extreme temperatures [].
Understanding how NAC TFs respond to and mitigate the impact of abiotic stresses is crucial for developing rice varieties that can thrive in challenging environmental conditions. By deciphering the regulatory networks governed by NAC TFs, researchers can pinpoint specific genes and pathways associated with stress tolerance []. This knowledge, in turn, opens avenues for targeted genetic manipulation aimed at enhancing the resilience of rice crops to adverse environmental factors.
The significance of the NAC TF family in rice lies not only in its contribution to growth and development but also in its role as a key player in stress adaptation. Unraveling the specific functions of NAC TFs in these processes offers a holistic understanding of the molecular mechanisms that underpin the plant’s ability to navigate both developmental and stress-related challenges []. This integrated knowledge is pivotal for advancing strategies to breed rice varieties with improved growth characteristics and enhanced tolerance to diverse environmental stresses, ultimately contributing to the sustainability and productivity of rice agriculture.
In the study conducted by Bo et al. (2019) [], the researchers investigated the role of the NAC transcription factor gene, OsNAC041, in salt sensitivity in rice. Through targeted mutagenesis, the researchers disrupted OsNAC041’s function, resulting in a phenotype of increased salt sensitivity in rice plants []. This finding underscores the importance of OsNAC041 in regulating the plant’s response to salt stress. The study delved into the molecular mechanisms underlying this salt sensitivity phenotype, providing insights into the genetic basis of salt tolerance in rice. By elucidating OsNAC041’s role, the research contributes to our understanding of the complex regulatory networks involved in salt stress response in rice []. Furthermore, this discovery offers potential targets for genetic engineering approaches aimed at developing salt-tolerant rice varieties, thereby addressing a critical challenge in agricultural sustainability.
1.1.5. MYB (Myeloblastosis)
The MYB transcription factor family in rice plays a substantial role in the intricate regulation of secondary metabolism and responses to abiotic stresses. MYB TFs are pivotal molecular regulators that exert control over the expression of genes involved in diverse metabolic pathways, influencing both the plant’s adaptive responses to environmental challenges and its capacity to produce specialized metabolites []. Investigating the functions of the MYB family opens promising avenues for manipulating rice traits, offering opportunities to enhance stress resilience and nutritional content.
In terms of secondary metabolism, MYB TFs in rice govern the synthesis of various compounds, including flavonoids, phenolic acids, and lignin. These metabolites not only contribute to the plant’s defense mechanisms against environmental stresses but also play essential roles in overall plant development. MYB-mediated regulation of secondary metabolism is particularly crucial under abiotic stress conditions, such as drought, salinity, and extreme temperatures, where these metabolites act as antioxidants and contribute to the plant’s ability to withstand adversity [].
Moreover, MYB TFs in rice are involved in the regulation of genes associated with responses to abiotic stresses. Understanding how MYB TFs modulate these stress-responsive genes provides insights into the molecular mechanisms underpinning the plant’s ability to adapt and survive in challenging environmental conditions []. By unraveling these intricate regulatory networks, researchers can identify key nodes for genetic manipulation, potentially leading to the development of rice varieties with enhanced stress resilience.
The dual role of the MYB TF family in governing secondary metabolism and abiotic stress responses positions it as a central player in shaping the overall adaptability of rice plants. Manipulating the functions of MYB TFs offers a powerful approach for tailoring rice traits to meet the demands of a changing climate and evolving agricultural landscapes []. As researchers delve deeper into the specific functions of the MYB family in rice, they contribute not only to the fundamental understanding of plant biology but also to the development of innovative strategies for sustainable and resilient rice agriculture.
The transcriptome study conducted by Shehab et al. (2022) [] on two Egyptian rice varieties, Giza 177 and Giza 178, under salt stress conditions provides valuable insights into the genetic mechanisms underlying salt tolerance. The study revealed significant differences in gene expression patterns between the two varieties, particularly in response to salt stress. Interestingly, genes related to cell wall functions and oxidative stress response were found to be more abundant in the salt-tolerant variety, Giza 178, suggesting its enhanced ability to cope with salt-induced oxidative damage []. Moreover, Gene Ontology analysis highlighted the upregulation of transcripts involved in oxidoreductase, peroxidase, and antioxidant activities in Giza 178, further supporting its superior salt tolerance. Notably, Giza 178 also exhibited a higher number of expressed transcription factors, particularly members of the MYB family, suggesting their substantial role in orchestrating the response to salt stress. This finding aligns with previous research indicating the involvement of MYB transcription factors in regulating secondary metabolism and responses to abiotic stresses in rice []. Overall, the study enhances our understanding of salt stress adaptation in rice and provides valuable insights for breeding programs aimed at developing salt-tolerant rice varieties.
A study conducted to elucidate the involvement of R2R3-MYB found that R2R3-MYB plays a crucial role in mediating rice’s response to stressors, shedding light on the regulatory mechanisms underlying stress adaptation in rice []. The findings contribute to our understanding of stress biology in rice and offer insights into potential strategies for enhancing agricultural productivity and sustainability in the face of changing environmental conditions.
1.1.6. AP2/ERF (APETALA2/Ethylene Responsive Factor)
The AP2/ERF transcription factor family in rice assumes a crucial role in orchestrating responses to abiotic stress and finely regulating yield-related traits. The AP2/ERF family, known for its versatility, acts as a key molecular player in the plant’s ability to adapt to environmental challenges and optimize productivity []. Exploring the functions of AP2/ERF TFs presents exciting opportunities for the development of rice varieties that exhibit heightened stress resilience and improved productivity.
Under abiotic stress conditions such as drought, salinity, and extreme temperatures, the AP2/ERF family in rice acts as a central regulatory hub. These transcription factors modulate the expression of stress-responsive genes, facilitating the plant’s capacity to endure adverse conditions. By deciphering the specific roles of AP2/ERF TFs in stress response pathways, researchers can identify potential targets for genetic manipulation, aiming to enhance the plant’s ability to withstand and recover from environmental stresses [].
Beyond stress responses, the AP2/ERF family also influences yield-related traits in rice. This includes aspects such as flowering time, seed development, and overall biomass production. By fine-tuning these crucial traits, AP2/ERF TFs contribute to the plant’s reproductive success and overall productivity []. Understanding the regulatory mechanisms governing yield-related traits opens avenues for strategic interventions aimed at optimizing crop yield and ensuring food security.
The dual functionality of the AP2/ERF family in responding to abiotic stress and regulating yield-related traits positions it as a key player in the complex interplay between stress adaptation and crop productivity in rice. Harnessing the potential of AP2/ERF TFs through targeted genetic manipulation holds promise for the development of rice varieties that can thrive in challenging environments while maximizing agricultural output []. As researchers delve into the specific functions of the AP2/ERF family in rice, they contribute essential knowledge to the ongoing efforts towards sustainable and resilient rice agriculture, addressing the evolving needs of global food production.
Yongqi et al. (2020) [], investigated the molecular mechanisms underlying the initial imbibition stage of seed germination in rice. Through RNA-Seq analysis of rice seeds collected at this crucial developmental stage, the researchers aimed to identify differentially expressed genes and uncover AP2-domain-containing signaling regulators potentially involved in regulating seed germination []. The results of the study are expected to include the identification of genes that are differentially expressed during the initial imbibition stage compared to non-germinating seeds, along with the discovery of AP2-domain-containing regulators that may play key roles in initiating and coordinating the germination process. Functional annotation and pathway analysis of the identified genes and regulators are likely to provide insights into the biological processes and molecular pathways associated with seed germination in rice []. Overall, this research contributes to our understanding of the regulatory networks governing seed germination and may have implications for improving germination efficiency and agricultural practices in rice cultivation.
1.1.7. bHLH (basic Helix–Loop–Helix)
The bHLH transcription factor family in rice plays a central role in crucial processes of nutrient uptake and utilization, as well as in various stress responses. These bHLH TFs, with their distinctive structural motifs, serve as key regulators of nutrient-related genes, contributing to efficient nutrient management in rice crops. Additionally, certain bHLH genes have been implicated in drought tolerance, disease resistance, and other stress responses, further highlighting their significance in plant adaptation to environmental challenges. Specifically, their involvement in iron uptake and the regulation of genes associated with nutrient utilization positions them as prime targets for enhancing nutrient efficiency in rice, aligning with the principles of sustainable agriculture practices [].
One of the pivotal functions of bHLH TFs in rice is their involvement in the intricate mechanisms of iron uptake. These transcription factors play a regulatory role in the expression of genes associated with iron transport and homeostasis []. By finely tuning these processes, bHLH TFs contribute to the plant’s ability to acquire and utilize iron efficiently, which is essential for various physiological and biochemical processes.
Additionally, bHLH TFs in rice extend their influence on the broader realm of nutrient regulation. They participate in the orchestration of genes responsible for the uptake and utilization of various nutrients, ensuring a balanced and efficient nutrient utilization strategy within the plant. Understanding the intricacies of how bHLH TFs modulate nutrient-related genes provides valuable insights into the development of rice varieties with enhanced nutrient efficiency, a critical aspect for sustainable agricultural practices [].
As targets for improving nutrient efficiency, bHLH TFs offer a strategic avenue for genetic manipulation aimed at optimizing nutrient uptake and utilization in rice crops. This not only contributes to the plant’s overall health and productivity but also aligns with the broader goals of sustainable agriculture by minimizing resource inputs and maximizing crop yield. Delving into the specific functions of bHLH TFs in rice sheds light on the molecular underpinnings of nutrient regulation, paving the way for innovations in crop management practices and the development of nutrient-efficient rice varieties to meet the demands of a growing global population [].
Wei and Chen (2018) [] investigated the bHLH transcription factor family in three major cereal crops: rice, maize, and wheat. Through genome analysis, the authors identified and classified a total of 183, 231, and 571 bHLH genes in rice, maize, and wheat, respectively, categorizing them into 36 subfamilies based on sequence similarities and domain organization []. The evolutionary analysis suggests a common ancestry for all bHLH genes across these species, with evidence of diversifying selection over time. Moreover, the study examines the expression patterns of bHLH genes in various tissues and under different stress conditions, revealing diverse roles for these genes in response to environmental cues. Notably, the paper highlights specific bHLH genes implicated in drought tolerance, disease resistance, and other stress responses, supported by experimental evidence such as overexpression and RNA interference studies []. While nutrient management was not the primary focus, the paper provided indirect evidence suggesting the potential involvement of bHLH genes in nutrient uptake and utilization in rice. This is supported by the identification of subfamilies associated with nutrient-related processes, expression analysis indicating roles in relevant tissues and stresses, and examples of bHLH genes impacting nutrient-related processes, such as iron homeostasis and nutrient remobilization.
1.1.8. DST (Drought and Salt Tolerance) Gene
The Drought and Salt Tolerance (DST) gene encodes a C2H2-type zinc finger transcription factor that plays a pivotal role in modulating abiotic stress responses in rice. Uniquely, DST functions as a negative regulator, wherein its loss-of-function confers enhanced drought and salinity tolerance. This occurs through tight regulation of reactive oxygen species (ROS) levels and stomatal aperture control. DST transcriptionally activates genes such as Prx24, a peroxidase involved in ROS scavenging. Under normal conditions, this regulation helps maintain redox balance; however, under stress, overactivity can suppress critical stress adaptations. Studies have shown that dst mutant lines exhibit enhanced water retention, reduced H2O2 accumulation, and increased expression of heat-shock proteins, contributing to improved drought, salinity, and even heat tolerance. Furthermore, DST interacts with DCA1, a co-activator, to fine-tune ROS signaling. Given its well-defined repressor function and multiple downstream targets, DST represents a high-value candidate for CRISPR-based gene editing, particularly for developing rice varieties with robust abiotic stress tolerance without compromising growth [].
1.1.9. bZIP (Basic Leucine Zipper)
The bZIP transcription factor family comprises central regulators in ABA-mediated signaling networks, which are crucial for osmotic stress adaptation in rice. Members such as OsbZIP23, OsbZIP46, and OsbZIP72 have been functionally characterized by their roles in drought and salinity tolerance. Overexpression of OsbZIP23 leads to upregulation of late embryogenesis abundant (LEA) genes, such as LEA3-2 and Rab16A, contributing to osmoprotection and improved cell turgor under stress []. Functionally, OsbZIP23 is activated by SnRK2 kinases such as SAPK2 and is stabilized by SUMO protease OsOTS1, highlighting its integration within complex post-translational regulatory circuits []. Similarly, OsbZIP46, particularly in its constitutively active form (OsbZIP46CA1), enhances drought resilience by activating multiple ABA-responsive genes. These TFs also exhibit cross-talk with other pathways, including sugar signaling and ROS homeostasis, reinforcing their classification as master regulators of abiotic stress adaptation. Their functional versatility makes bZIP TFs attractive targets for transgenic overexpression and molecular breeding programs, especially in drought-prone environments [].
1.1.10. HD-ZIP (Homeodomain-Leucine Zipper)
HD-ZIP transcription factors, particularly OsHOX22 and OsHOX24, are integral components of the rice abiotic stress response network, with established roles in ABA signaling, osmotic stress, and hypoxia adaptation. These genes are rapidly upregulated upon exposure to salinity, dehydration, submergence, and ABA treatments []. HD-ZIP TFs function by binding to conserved homeobox motifs in promoter regions of stress-inducible genes, modulating responses such as cell wall remodeling, aquaporin regulation, and ethylene signaling. Functional studies indicate that suppression of OsHOX22 enhances drought and salt tolerance by decreasing ABA sensitivity and improving physiological resilience under stress []. In submerged conditions, HD-ZIPs act in coordination with ERF-VII family members to activate anaerobic metabolic pathways, promoting ethylene-mediated responses and ensuring energy production under hypoxia. These dual roles in developmental regulation and stress mitigation position HD-ZIPs as valuable assets for engineering climate-resilient rice cultivars. Their involvement in both growth and defense pathways underscores their utility in fine-tuning phenotypic plasticity in fluctuating environments [].
1.1.11. Other Important TF Families
The transcription factor (TF) families in rice, grouped under various categories, play diverse and crucial roles in various biological processes, showcasing their significance in shaping the adaptability and performance of rice plants. In seed development, ABI3/VP1 TFs, such as Abscisic Acid Insensitive 3 (ABI3) and Viviparous1 (VP1), orchestrate the expression of genes governing dormancy and maturation. Moving to photosynthesis, the GRF (Growth-Regulating Factor) TF family influences leaf growth, chloroplast development, and overall photosynthetic efficiency, optimizing the plant’s capacity to convert light into energy. CONSTANS (CO) TFs, essential for flowering time regulation, integrate environmental signals to precisely time floral initiation, influencing the reproductive success of rice. Pathogen resistance is fortified by TGA (TGACG-Binding Factor) TFs, which modulate defense-related gene expression, contributing to the plant’s ability to counteract pathogen attacks effectively. Additionally, PLT (Plethora) TFs are pivotal in root development, impacting root architecture through the regulation of meristem maintenance and lateral root formation. This multifaceted involvement across seed development, photosynthesis, flowering time regulation, pathogen resistance, and root development underscores the intricate interplay of TF families in shaping the resilience and productivity of rice plants []. These insights provide valuable avenues for targeted genetic manipulation, offering the potential to enhance specific traits and contribute to sustainable rice agriculture.
1.2. Abiotic Stress Responses in Rice
Abiotic stress in rice encompasses a range of adverse environmental conditions that significantly impact the growth and productivity of this vital crop. Drought stress, arising from inadequate water availability, poses a persistent threat to rice cultivation, affecting water-dependent physiological processes crucial for optimal growth. Heat stress, induced by elevated temperatures during critical growth stages, can disrupt metabolic functions and compromise overall yield. Cold stress, stemming from exposure to low temperatures during sensitive phases, can impede developmental processes. Salinity stress, a consequence of high soil salt content, hinders water uptake and nutrient absorption by rice plants []. Additionally, nutrient imbalances, whether deficiencies or toxicities, contribute to stress conditions. Recognizing these abiotic stress factors in rice cultivation is essential for devising strategies to enhance resilience []. The pivotal roles played by transcription factors, such as DREB, HSF (heat shock factor), and CBF/DREB, in mediating the plant’s response to these stressors open avenues for genetic manipulation and innovative interventions to develop stress-resistant rice varieties, ensuring sustainable agriculture in the face of environmental challenges [].
DREB TFs play a crucial role in rice’s response to drought stress, acting as guardians against water scarcity. These transcription factors activate genes vital for the plant’s survival in arid conditions. One of their key functions is triggering the production of osmoprotectants, specialized molecules that as-sist cells in retaining water and maintaining stability. Additionally, DREB induces the ex-pression of genes associated with antioxidant defense, helping the plant scavenge harmful free radicals generated under stress []. This multifaceted response orchestrated by DREB TFs enables rice plants to navigate and endure periods of drought.
In enhancing rice’s drought resistance, scientists are delving into genetic engineering approaches centered around DREB TFs. Overexpressing DREB genes within the rice genome represents a strategy to boost the plant’s natural stress response. This heightened response, triggered by an increased presence of DREB, leads to improved drought tolerance in rice varieties. Another avenue involves the introduction of DREB genes from other drought-resistant plants. By incorporating genetic material from naturally resilient species, researchers aim to broaden the rice plant’s repertoire of stress-fighting mechanisms []. These genetic engineering endeavors hold promises for developing rice varieties capable of withstanding and thriving in challenging water-scarce environments, contributing to global food security initiatives.
In response to elevated temperatures, HSFs play a pivotal role. Activated by heat stress, these transcription factors orchestrate the induction of heat shock proteins (HSPs). Functioning as cellular guardians, HSPs protect essential cellular structures and proteins from the detrimental effects of elevated temperatures []. Similarly, in cold stress situations, C-repeat binding factors (CBFs) and DREBs come to the forefront. These transcription factors, akin to their roles in drought tolerance, regulate the expression of genes associated with cryoprotectants. By inducing the production of cryoprotectants, CBFs and DREBs contribute to the preservation of critical cellular components, particularly in freezing temperatures [].
Hypoxia, a condition of low oxygen availability commonly encountered in waterlogged or submerged rice fields, presents a critical abiotic stress that disrupts aerobic respiration, impairs root metabolism, and reduces energy production. In response, rice activates a unique set of transcription factors that facilitate metabolic and physiological adaptation to oxygen-deficient environments. Central to this hypoxic response are AP2/ERF-VII transcription factors, particularly SUB1A, OsERF66, and OsERF67, which regulate genes involved in anaerobic metabolism such as alcohol dehydrogenase (ADH) and pyruvate decarboxylase (PDC), enabling the maintenance of ATP levels through fermentation pathways []. In parallel, members of the HD-ZIP family, notably OsHOX22 and OsHOX24, are rapidly upregulated under hypoxia and contribute to adaptive responses by modulating ethylene and abscisic acid (ABA) signaling. These transcription factors regulate genes involved in aquaporin transport, cell wall remodeling, and ROS detoxification, promoting morphological adjustments like aerenchyma formation, adventitious root development, and leaf elongation, which improve internal oxygen diffusion. SUB1A, in particular, suppresses elongation growth under complete submergence, conserving energy and enhancing survival. The coordinated action of these transcription factors allows rice to endure transient or prolonged flooding, underscoring their potential as molecular targets for engineering submergence-tolerant cultivars in flood-prone regions []. Continued research into the transcriptional regulation of hypoxia responses holds promise for improving rice resilience under climate change-induced hydrological extremes.
Crafting strategies for enhancing thermal resilience involves the concept of breeding for natural tolerance. This approach entails identifying and breeding rice varieties inherently equipped with heat or cold tolerance, leveraging the inherent genetic diversity within rice populations. Complementing this strategy is the advanced technique of modulating transcription factor expression through gene editing. This precision editing of HSFs, CBFs, and DREBs offers fine-tuned control, augmenting the plant’s intrinsic ability to navigate thermal stress []. Collectively, these strategies present innovative solutions to fortify rice crops against the unpredictable challenges posed by environmental fluctuations.
The study of transcription factors (TFs) in the context of rice’s response to abiotic stresses extends beyond well-established families like DREB, HSF, and CBF/DREB. Ongoing research delves into various other TF families, exploring their roles in salinity tolerance, nutrient uptake, and diverse physiological processes crucial for rice’s adaptability to challenging environmental conditions. In the realm of salinity tolerance, NAC TFs regulate genes involved in ion homeostasis, safeguarding cells from salt stress by maintaining a sodium–potassium balance []. AP2/ERF TFs induce stress-responsive genes, bolstering antioxidant defense and osmotic adjustment, while MYB TFs control the expression of genes related to proline biosynthesis, a key osmoprotectant []. The realm of nutrient uptake sees bHLH TFs modulating genes responsible for nutrient transporters, facilitating the efficient absorption of essential elements such as phosphorus and iron. Additionally, MYB TFs regulate genes involved in nitrogen metabolism, optimizing nitrogen uptake and utilization, and WRKY TFs contribute to iron uptake by influencing iron deficiency responses. Beyond these, TFs like GRF promote tillering and biomass accumulation, ABI3/VP1 regulate seed development and dormancy, and AP2/ERF along with NAC TFs contribute to photosynthesis and carbon fixation. CONSTANS (CO) TFs oversee flowering time, ensuring optimal adaptation to seasonal changes and maximizing grain production []. Understanding the intricate interplay between these TFs and their target genes holds immense potential for breeding stress-tolerant rice varieties, enhancing nutrient uptake efficiency, and optimizing overall plant growth, development, and yield in pursuit of resilient and productive rice plants.
Pradhan et al. (2019) [] pinpointed numerous stress-responsive transcription factors, including DREB2A, DREB2B, HSF1, HSF3, and CBF4, as potential key players in chilling stress tolerance. DREB2A and DREB2B, classified under the Dehydration-Responsive Element-Binding (DREB) family, were notably upregulated in the tolerant genotype under chilling stress, indicating their involvement in enhancing stress resilience []. Additionally, HSF1 and HSF3, recognized heat shock factors, showed increased expression in the tolerant genotype, hinting at their participation in the adaptation to chilling conditions. CBF4, belonging to the CBF family, also displayed elevated expression in the tolerant genotype, suggesting its contribution to chilling tolerance mechanisms. Furthermore, Pradhan et al. (2019) [] unveiled other potentially significant transcription factor families such as WRKY, bZIP, NAC, and MYB, each with possible roles in regulating chilling stress responses. Nonetheless, the research underscores the need for further investigation to elucidate the precise functions and interactions of these transcription factors within the intricate network of chilling stress response, emphasizing the importance of validation experiments and comprehensive analyses of their target genes for future breeding endeavors.
1.3. Enhancing Rice Defense Mechanisms
Pathogen resistance in crops, including rice, is a crucial aspect of agricultural re-search dedicated to developing strategies that protect plants from harmful microorganisms. This field of study aims to enhance the natural defense mechanisms of plants, enabling them to resist or tolerate attacks from various pathogens such as bacteria, viruses, and fungi []. By understanding the genetic and molecular mechanisms involved in pathogen resistance, researchers seek to develop resilient crop varieties that can withstand disease pressures, ultimately contributing to global food security and sustainable agriculture.
Rice, as a staple food crop, faces constant threats from various pathogens that can jeopardize yield and food security. A key avenue for bolstering its defense mechanisms involves the utilization of specific transcription factors, particularly WRKY and MYB. These transcription factors play pivotal roles in regulating the expression of genes associ-ted with pathogen resistance []. The WRKY family is known for its involvement in or-chestrating defense responses against a wide range of pathogens, while MYB transcrip-tion factors contribute to the regulation of genes related to disease resistance and stress tolerance []. Harnessing the potential of WRKY and MYB transcription factors presents a promising approach to fortify rice plants against pathogenic assaults, contributing to sustainable and resilient agricultural practices.
In the pursuit of enhancing rice’s resistance to pathogens, genetic modification emerges as a cutting-edge strategy. By incorporating targeted genetic modifications, scientists aim to equip rice plants with inherent capabilities to fend off specific diseases. This approach involves the introduction or manipulation of genes responsible for conferring resistance to prevalent pathogens. The genetic modification process allows for the development of disease-resistant rice varieties, reducing the reliance on chemical interventions and promoting environmentally sustainable agricultural practices []. While ethical considerations and regulatory frameworks are crucial in the implementation of genetic modification, the potential benefits in terms of increased crop yield and reduced susceptibility to diseases underscore the importance of exploring this avenue in fortifying the resilience of rice cultivation [].
Sun et al. (2013) [] investigates the functions of two NAC transcription factors, ONAC122 and ONAC131, in rice’s defense responses against the fungal pathogen Magnaporthe grisea, known for causing rice blast disease. It elucidates that these transcription factors are predominantly located in the nucleus and play pivotal roles in activating gene expression []. Notably, their expression levels surge following infection with Magnaporthe grisea, particularly in resistant rice plants. Furthermore, their upregulation is induced by defense hormones such as salicylic acid and jasmonic acid. Silencing of these genes results in the heightened susceptibility of rice to Magnaporthe grisea, highlighting their crucial role in defense mechanisms. Additionally, Sun et al. 2013 [] suggests that ONAC122 and ONAC131 regulate the expression of other defense-related genes, including LOX, PR1a, WRKY45, and NH1, thereby orchestrating a comprehensive defense response against the fungal pathogen. These findings deepen our understanding of the molecular mechanisms underlying rice’s defense against rice blast disease, offering insights into potential strategies for enhancing disease resistance in rice crops.
Nizolli et al. (2021) [] presented a comprehensive overview of methods and obstacles related to bolstering genetic resistance against rice blast disease in crop breeding and applied biotechnology in 2021. Rice blast, caused by the fungus Magnaporthe oryzae, poses a significant threat to rice crops globally, resulting in substantial yield reductions. The paper delved into various strategies aimed at combatting this disease, with a particular focus on genetic resistance as a sustainable and efficient approach for disease control []. It underscored the significance of unraveling the genetic foundations of resistance and comprehending the mechanisms underlying plant–pathogen interactions. Additionally, the article explored the utilization of diverse genetic reservoirs, including wild rice relatives and traditional landraces, to identify new resistance genes and alleles. Moreover, it addressed the challenges associated with developing durable resistance against rice blast, such as the rapid evolution of the pathogen and the intricacies of the rice genome. It advocated for interdisciplinary approaches that integrate genetics, genomics, and breeding methodologies to enhance resistance levels and broaden the genetic diversity of cultivated rice varieties. In essence, Nizolli et al. provide valuable insights into ongoing research efforts on rice blast resistance and outline future prospects for enhancing genetic resistance in rice cultivars, thereby contributing to sustainable rice production and global food security [].
1.4. Yield Improvement Strategies
Enhancing the yield of rice is a central objective in agricultural research, driven by the imperative to meet global food demands sustainably. Yield improvement strategies encompass a multifaceted approach, ranging from understanding key transcription factors like GRF for growth and biomass regulation to exploring the potential of AP2/ERF transcription factors for genetic manipulation aimed at increasing productivity []. These strategies integrate both molecular insights and practical applications, seeking to optimize growth conditions, nutrient management, and precision farming techniques. The overarching goal is to develop high-yielding rice varieties resilient to environmental challenges, contributing significantly to global food security.
In the pursuit of enhancing crop yield, a key focus lies in understanding and harnessing the potential of Growth-Regulating Factor (GRF) transcription factors. GRFs play a crucial role in the regulation of plant growth and biomass accumulation. Particularly in the context of rice cultivation, these transcription factors influence processes such as tillering and biomass development. By comprehending the intricate molecular pathways governed by GRFs, researchers aim to employ targeted strategies that optimize their activity, thereby promoting increased growth and biomass production in rice plants [].
Efforts to improve yield extend beyond understanding transcription factors to practical applications for rice growth enhancement. Implementing agronomic practices, nutrient management strategies, and precision farming techniques are integral components of this approach. By fine-tuning growth conditions, optimizing nutrient availability, and adopting innovative cultivation methods, farmers can enhance the overall growth and biomass accumulation in rice crops. This holistic approach ensures a comprehensive strategy for boosting yield through the effective utilization of available resources [].
Another avenue for yield improvement involves the exploration of AP2/ERF transcription factors and their potential impact on crop productivity. Through genetic manipulation techniques, researchers seek to modulate the expression of these transcription factors to enhance their positive influence on yield-related traits. AP2/ERF factors play a crucial role in stress responses and developmental processes, making them promising candidates for genetic interventions aimed at increasing yield in rice []. By manipulating the genetic makeup of rice plants to favorably influence these transcription factors, scientists aim to contribute to the development of high-yielding varieties capable of withstanding environmental challenges and ensuring food security.
Lu et al. (2022) [] delved into the intricate regulatory mechanisms underlying gibberellin (GA) signaling in rice and its impact on yield improvement strategies. Specifically, the study explored the role of the miR396-GRF-GIF-SWI/SNF module in modulating gene expression patterns crucial for enhancing rice yield. Through detailed molecular analyses, the researchers elucidated how miR396 negatively regulates the expression of GRFs, key transcription factors involved in rice growth and development []. Additionally, the paper highlighted the interaction between GRFs and GRF-Interacting Factors (GIFs), forming a complex that influences downstream gene expression. Furthermore, it underscored the involvement of the SWI/SNF chromatin remodeling complex in modulating the accessibility of target genes to transcription factors, thereby regulating rice growth and yield-related traits []. The experiments conducted in this study involved molecular techniques such as gene expression analysis, protein–protein interaction studies, and chromatin immunoprecipitation assays to elucidate the regulatory mechanisms of the miR396-GRF-GIF-SWI/SNF module in rice []. Overall, the research provides valuable insights into the molecular mechanisms governing yield improvement strategies in rice, offering potential avenues for crop enhancement and agricultural sustainability.
1.5. Nutrient Uptake and Utilization
Optimizing nutrient uptake and utilization is a critical aspect of rice cultivation, directly impacting crop yield and nutritional quality. This pursuit involves a detailed exploration of molecular mechanisms, with a specific focus on bHLH TFs in rice. These transcription factors govern the expression of genes responsible for nutrient transporters, influencing the absorption of key elements like phosphorus and iron. Concurrently, practical strategies for improving nutrient efficiency in rice crops encompass precision farming techniques and balanced fertilization practices []. By integrating both molecular insights and on-field applications, the aim is to enhance nutrient absorption and utilization, ensuring sustainable rice cultivation with increased productivity and nutritional benefits.
The efficient uptake and utilization of nutrients are essential for optimizing crop productivity, and in the context of rice, bHLH TFs play a pivotal role in this intricate process. These transcription factors modulate the expression of genes associated with nutrient transporters, influencing the absorption of vital elements such as phosphorus and iron. By understanding the regulatory mechanisms orchestrated by bHLH TFs, researchers aim to unlock strategies that enhance nutrient uptake efficiency in rice plants. This exploration of molecular pathways holds promise for developing nutrient-responsive varieties, contributing to sustainable agriculture and improved nutritional outcomes [].
Efforts to improve nutrient efficiency in rice crops extend beyond the molecular level to practical applications in agricultural management. Implementing precision farming techniques, adopting balanced fertilization practices, and employing innovative nutrient delivery systems are integral components of strategies aimed at maximizing nutrient utilization. By fine-tuning these approaches, farmers can enhance the overall nutrient efficiency of rice cultivation, ensuring optimal absorption and utilization of essential elements []. This comprehensive approach not only addresses the immediate needs of the crop but also contributes to environmental sustainability by minimizing excess nutrient runoff and potential ecological impacts.
The study conducted to delves into the pivotal role of the bHLH protein OsIRO3 in rice plants’ response to iron deficiency, shedding light on its significance for both plant survival and iron homeostasis maintenance []. Through a series of molecular and physiological analyses, the research elucidates the intricate regulatory mechanisms governed by OsIRO3 under conditions of limited iron availability in the soil. Notably, the findings reveal that OsIRO3 acts as a crucial transcription factor, orchestrating the expression of genes crucial for iron uptake and translocation within rice roots []. This regulatory role enables rice plants to adapt to iron-deficient environments effectively. Furthermore, the study demonstrates that the absence or impairment of OsIRO3 significantly compromises rice plants’ ability to acquire and utilize iron, ultimately impacting their survival and growth under iron-deficient conditions. These findings provide valuable insights into the molecular pathways involved in iron homeostasis regulation in rice and offer potential avenues for enhancing iron uptake efficiency and tolerance to iron deficiency in rice cultivation practices, thereby contributing to the advancement of global food security initiatives.
1.6. Seed Development and Quality Enhancement
Seed development and quality enhancement in rice are critical aspects of agricultural research geared towards ensuring robust crop yields and superior seed characteristics. At the heart of this endeavor are transcription factors, particularly the ABI3/VP1 factors, which play a central role in governing the genetic processes associated with seed formation, dormancy, and maturation. The intricate molecular pathways influenced by ABI3/VP1 impact crucial seed traits such as germination potential, storage compound accumulation, and dormancy regulation. Understanding and manipulating these transcription factors provide a promising avenue for researchers to enhance rice varieties, not only in terms of yield but also in the improvement of seed characteristics such as storability, viability, and nutritional content []. This holistic approach to seed development holds the potential to elevate both the productivity of rice cultivation and the quality of seeds harvested, addressing the dual goals of agricultural sustainability and meeting the demands of diverse stakeholders in the agricultural value chain.
Seed development and quality enhancement in rice are influenced significantly by the ABI3/VP1 transcription factors, which play a pivotal role. These transcription factors are instrumental in regulating the expression of genes associated with seed development, dormancy, and maturation. ABI3 (ABA INSENSITIVE3) and VP1 (VIVIPAROUS1) contribute to the coordination of various physiological processes critical for seed formation and subsequent quality. By understanding the intricate molecular pathways governed by ABI3/VP1, researchers aim to unlock strategies that positively influence seed traits, including germination potential, storability, and overall seed quality []. This exploration at the genetic level holds promise for developing rice varieties with improved seed characteristics, thereby enhancing both agricultural productivity and the quality of harvested seeds.
The influence of ABI3/VP1 transcription factors on rice seed characteristics is multifaceted. These factors play a central role in orchestrating the accumulation of storage compounds, such as starch and proteins, during seed development. Additionally, they contribute to the establishment of seed dormancy, influencing the timing of germination and ensuring optimal conditions for seedling establishment. The impact extends beyond the immediate growing season, affecting the storability and viability of harvested seeds. Understanding and manipulating the regulatory role of ABI3/VP1 transcription factors offer avenues for tailoring seed traits to meet diverse agricultural and market requirements. This comprehensive approach to seed development and quality enhancement in rice holds the potential to positively impact both farming practices and end-user satisfaction [].
Gong et al. extensively discuss the pivotal role of ABI3/VP1 transcription factors in orchestrating the hormonal regulation of rice seed germination []. These transcription factors are integral components of the abscisic acid (ABA) signaling pathway, which governs seed dormancy and germination. The authors elucidate how ABI3/VP1 factors regulate the expression of genes crucial for seed maturation and dormancy release, promoting the accumulation of storage reserves while inhibiting germination under unfavorable conditions through ABA synthesis []. Conversely, during seed imbibition and germination, ABI3/VP1 transcription factors are downregulated or inactivated, leading to a decrease in ABA levels and the activation of germination-promoting genes. Gong et al. further explored the modulation of ABI3/VP1 activity by various hormones, including ABA and gibberellins (GA), highlighting the intricate interplay between hormonal signals in governing rice seed germination []. Their comprehensive examination shed light on the molecular mechanisms underlying hormonal regulation during this critical stage of plant development, offering valuable insights for improving crop productivity and seedling establishment in rice cultivation.
1.7. Photosynthesis and Carbon Fixation in Rice
Photosynthesis and carbon fixation are fundamental processes that underpin the growth and productivity of rice, a staple crop supporting global food security. The regulation of these processes in rice plants is orchestrated by transcription factors, notably AP2/ERF and NAC. AP2/ERF transcription factors contribute to stress responses, including those affecting photosynthesis, while NAC transcription factors play a key role in governing genes associated with photosynthetic efficiency and carbon assimilation. As rice cultivation faces the challenges of diverse environmental conditions, understanding and harnessing the potential of these transcription factors become pivotal []. This exploration not only provides insights into the molecular intricacies of photosynthesis but also offers a pathway to develop resilient rice varieties capable of optimizing carbon fixation under varying climatic circumstances. In this context, strategies to improve photosynthetic efficiency, ranging from environmental optimization to genetic modifications, represent essential components in the quest for sustainable and enhanced rice crop yields.
Photosynthesis and carbon fixation, vital processes in rice cultivation, are intricately regulated by transcription factors, with AP2/ERF and NAC playing key roles. AP2/ERF transcription factors are involved in orchestrating stress responses, including those related to photosynthesis. Additionally, NAC transcription factors contribute to the regulation of genes associated with photosynthetic efficiency and carbon assimilation. Understanding the specific involvement of these transcription factors in the intricate dance of biochemical pathways during photosynthesis sheds light on potential avenues for optimizing rice plants for enhanced carbon fixation. The exploration of AP2/ERF and NAC transcription factors in the context of photosynthesis holds promise for developing resilient rice varieties capable of thriving under diverse environmental conditions, ultimately contributing to improved crop yield [].
Efforts to enhance photosynthetic efficiency in rice involve a multifaceted approach. Implementing strategies to optimize environmental conditions, such as light intensity and temperature, ensures the conducive operation of photosynthetic processes. Additionally, exploring genetic modifications to fine-tune the expression of genes associated with photosynthesis, guided by the insights from AP2/ERF and NAC transcription factors, offers a promising avenue. Advancements in crop management practices, including precision farming and nutrient optimization, further contribute to improving overall photosynthetic efficiency []. By integrating these strategies, researchers aim to unlock the full potential of rice plants, ensuring efficient carbon fixation and ultimately bolstering the productivity of this essential cereal crop.
Ambavaram et al. (2014) [] likely delve into the involvement of AP2/ERF and NAC transcription factor families in the coordinated regulation of photosynthesis and its impact on rice yield and stress tolerance. Both AP2/ERF and NAC transcription factor families are known for their pivotal roles in plant development, stress responses, and the regulatory networks governing photosynthesis []. In the context of rice, these transcription factors may modulate the expression of genes crucial to photosynthetic processes, including those associated with chlorophyll biosynthesis, light-harvesting complexes, and carbon assimilation pathways. Ambavaram et al. (2014) [] explored how AP2/ERF and NAC transcription factors respond to environmental cues like drought, heat, and salinity and elucidate their functions as key regulators of photosynthetic adaptation and stress tolerance in rice plants. By orchestrating the expression of genes involved in both photosynthesis and stress responses, AP2/ERF and NAC transcription factors may play significant roles in optimizing photosynthetic efficiency and enhancing rice yield under adverse environmental conditions. Moreover, the authors may discuss potential strategies for manipulating the activity of these transcription factors to improve rice resilience and productivity, offering promising avenues for crop enhancement and sustainable agriculture.
1.8. Regulation of Flowering Time in Rice
The regulation of flowering time in rice holds critical significance for the successful cultivation of this staple crop. Timing the transition from vegetative growth to the reproductive phase is intricately governed by molecular processes, with CONSTANS (CO) transcription factors playing a central role. These transcription factors act as key mediators in sensing and integrating environmental signals, particularly day length, to orchestrate the precise timing of flowering []. The regulation of flowering time not only impacts the synchronization of reproductive processes but also bears profound implications for crop yield. A nuanced understanding of the molecular mechanisms involved in this regulatory network provides insights that can be leveraged for crop management practices and breeding strategies, contributing to the development of rice varieties that thrive in diverse environmental conditions and maximize grain production []. As such, exploring the regulation of flowering time in rice is foundational to ensuring the adaptability and productivity of this essential cereal crop.
Understanding the intricate timing of rice flowering is paramount for optimizing crop development and ultimately influencing yield. CONSTANS transcription factors play a central role in this regulatory process. These transcription factors are key players in sensing and integrating environmental cues, such as day length, to finely orchestrate the transition from vegetative growth to reproductive development. The CO transcription factors act as molecular switches that trigger the flowering pathway, influencing the expression of downstream genes involved in this critical developmental stage. Investigating the specific functions and regulation of CONSTANS transcription factors provides valuable insights into the molecular mechanisms that govern flowering time in rice, offering potential avenues for crop management and breeding strategies [].
The influence of CONSTANS transcription factors on rice flowering has far-reaching implications for crop yield. The precise timing of flowering is crucial for synchronous pollination and optimal grain formation. By deciphering the role of CO transcription factors, researchers gain insights into how environmental factors impact flowering, allowing for the development of rice varieties that can adapt to varying conditions. Efficient regulation of flowering time contributes to uniform maturity and enhances the potential for maximizing grain production. Understanding the implications of CO transcription factors on rice flowering not only furthers our knowledge of plant development but also informs strategies for breeding resilient varieties with improved yield potential, ultimately addressing the dynamic challenges faced by rice cultivation [].
The research by Cai et al. (2021) [] offers detailed insights into the molecular mechanisms controlling flowering time in rice, focusing on the function of the DHD4 transcription factor. Their study reveals that DHD4, a member of the CONSTANS-like transcription factor family, is instrumental in delaying the heading date, which marks the onset of the reproductive phase in rice plants. They demonstrate that DHD4 achieves this delay by impacting the formation of the FAC (Fertilization-Independent Seed-Polycomb Repressive Complex) complex, a key regulator of flowering time. By influencing the assembly of the FAC complex, DHD4 effectively retards the transition from vegetative to reproductive growth stages in rice []. This research provides deeper insights into the molecular interactions governing the regulation of flowering time, highlighting the intricate interplay between transcription factors like DHD4 and other regulatory proteins in fine-tuning the timing of flowering in response to environmental signals such as day length and temperature. Overall, the study conducted by Cai et al. (2021) [] contributes significantly to our understanding of the genetic and molecular mechanisms controlling flowering time in rice, offering valuable insights into agricultural practices aimed at optimizing the yield and productivity in rice cultivation.
1.9. Challenges in Sustainable Rice Farming
While plant transcription factors offer promising avenues for optimizing yield in rice farming, several challenges impede their widespread application. Limited understanding of the complex gene regulatory networks and interactions involving transcription factors poses a significant hurdle. Precision in manipulating these factors to achieve desired outcomes, such as stress tolerance and increased yield, remains a considerable challenge. Additionally, the practical implementation of transcription factor-based strategies in diverse agroecosystems requires overcoming hurdles related to delivery methods, potential off-target effects, and scalability. Addressing these limitations is imperative for realizing the full potential of transcription factor applications in sustainable rice farming [].
As the application of transcription factors in agriculture advances, it is essential to consider the potential risks and ethical implications. Unintended environmental consequences, such as the impact on non-target organisms or ecosystem dynamics, warrant careful examination. Ethical considerations include concerns about genetic modifications and the potential long-term effects on biodiversity. Balancing the benefits of harnessing transcription factors for sustainable rice farming with the need to mitigate potential risks requires a comprehensive and transparent evaluation framework. Robust ethical guidelines and regulatory frameworks are crucial to guide the responsible and ethical use of transcription factor technologies in rice cultivation [].
2. Future Research Avenues
The future of sustainable rice farming hinges on addressing the current limitations and advancing research in key areas. Comprehensive exploration of gene regulatory networks, including the identification of novel transcription factors, will deepen our understanding of plant responses to environmental stresses. Research efforts should focus on developing precise and efficient transcription factor delivery systems, ensuring targeted and controlled applications. Furthermore, exploring CRISPR-based technologies for gene editing offers potential breakthroughs in tailoring transcription factors to specific agricultural needs []. Collaborative interdisciplinary research endeavors, incorporating insights from molecular biology, agronomy, and environmental science, are crucial for unlocking the full potential of transcription factors in sustainable rice farming. By fostering innovation, addressing challenges, and embracing ethical considerations, the field can pave the way for resilient and productive rice cultivation in response to global demand and environmental stresses.
3. Conclusions
Exploration into sustainable rice farming, particularly through the harnessing of plant transcription factors for yield optimization, has revealed promising avenues for addressing global food demand and mitigating environmental stresses. Key findings underscore the pivotal roles of TFs such as WRKY, MYB, AP2/ERF, NAC, and CONSTANS in orchestrating diverse aspects of rice growth, development, and stress responses. The intricate interplay among these molecular regulators offers a robust foundation for tailoring rice plants to thrive across varied agroecosystems. The implications for rice crop improvement are far-reaching, encompassing enhanced resistance to pathogens, improved nutrient uptake efficiency, increased biomass accumulation, optimized photosynthetic capacity, refined seed development, and precise regulation of flowering time. These advancements contribute not only to improved productivity but also to sustainability by reducing reliance on chemical inputs and lowering the environmental impact. Looking ahead, the future of transcription factor research in rice holds tremendous potential for innovation and collaboration. Overcoming current limitations, addressing biosafety concerns, and navigating ethical considerations will be crucial for the responsible application of these technologies. The integration of advanced tools such as CRISPR/Cas9 gene editing, along with the continued exploration of novel TFs, is poised to usher in a new era of resilient, high-yielding, and environmentally sustainable rice cultivation.
From a translational perspective, manipulation of key TFs through overexpression or genome editing has already shown promise. For instance, OsDREB2A overexpression enhances drought tolerance, while DST knockout improves both drought and salinity resistance. Similarly, OsNAC6 and OsbZIP23 have been employed to generate transgenic lines with improved osmotic stress tolerance. These and other TFs offer valuable targets for integration into marker-assisted selection and biotechnological strategies, contributing significantly to the development of climate-resilient rice varieties and the broader goal of global food security.
Author Contributions
H.G. and M.A.N.S. wrote the manuscript, and S.V.G.N.P. revised and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TFs | Transcription factors |
| DREB | Dehydration-responsive element-binding |
| WRKY | A group of transcription factors containing a conserved WRKY domain |
| NAC—NAM ATAF1/2, and CUC2 (NAC) | Transcription factors |
| MYB | Myeloblastosis transcription factors |
| AP2/ERF | APETALA2/ethylene responsive factor transcription factors |
| bHLH | Basic helix–loop–helix transcription factors |
References
- Mordor Intelligence Inc. Rice—Market Share Analysis, Industry Trends & Statistics, Growth Forecasts 2019–2029; Mordor Intelligence Inc.: Hyderabad, India, 2024. [Google Scholar]
- Rezvi, H.U.A.; Tahjib-Ul-Arif, M.; Azim, M.A.; Tumpa, T.A.; Tipu, M.M.H.; Najnine, F.; Dawood, M.F.A.; Skalicky, M.; Brestič, M. Rice and Food Security: Climate Change Implications and the Future Prospects for Nutritional Security. Food Energy Secur. 2023, 12, e430. [Google Scholar] [CrossRef]
- Polcyn, J.; Stratan, A.; Lopotenco, V. Sustainable Agriculture’s Contribution to Quality of Life. Sustainability 2023, 15, 16415. [Google Scholar] [CrossRef]
- Singh, P.P.; Priyam, A.; Singh, J.; Gupta, N. Biologically Synthesised Urea-Based Nanomaterial Shows Enhanced Agronomic Benefits in Maize and Rice Crops during Kharif Season. Sci. Hortic. 2023, 315, 111988. [Google Scholar] [CrossRef]
- Fukagawa, N.K.; Ziska, L.H. Rice: Importance for Global Nutrition. J. Nutr. Sci. Vitaminol. 2019, 65, S2–S3. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Srivastava, A.; Singh, S.; Mishra, S.; Mohan, S.; Singh, A.; Singh, A.K.; Jaiswal, H.K. Aromatic Rice of India: It’s Types and Breeding Strategies. In Integrative Advances in Rice Research; Huang, M., Ed.; IntechOpen: Rijeka, Croatia, 2021; ISBN 978-1-83969-600-8. [Google Scholar]
- Hoang, X.L.T.; Nhi, D.N.H.; Thu, N.B.A.; Thao, N.P.; Tran, L.-S.P. Transcription Factors and Their Roles in Signal Transduction in Plants under Abiotic Stresses. Curr. Genom. 2017, 18, 483–497. [Google Scholar] [CrossRef]
- Bo, W.; Zhaohui, Z.; Huanhuan, Z.; Xia, W.; Binglin, L.; Lijia, Y.; Xiangyan, H.; Deshui, Y.; Xuelian, Z.; Chunguo, W.; et al. Targeted Mutagenesis of NAC Transcription Factor Gene, OsNAC041, Leading to Salt Sensitivity in Rice. Rice Sci. 2019, 26, 98–108. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Y.; Wang, W.; Zhao, X.; Qin, Q.; Sun, F.; Hu, F.; Zhao, Y.; Li, Z.; Fu, B.; et al. Characterization of Transcription Factor Gene OSDRAP1 Conferring Drought Tolerance in Rice. Front. Plant Sci. 2018, 9, 94. [Google Scholar] [CrossRef]
- Sun, L.; Huang, L.; Hong, Y.; Zhang, H.; Song, F.; Li, D. Comprehensive Analysis Suggests Overlapping Expression of Rice Onac Transcription Factors in Abiotic and Biotic Stress Responses. Int. J. Mol. Sci. 2015, 16, 4306–4326. [Google Scholar] [CrossRef]
- Zhang, Q.; Teng, R.; Yuan, Z.; Sheng, S.; Xiao, Y.; Deng, H.; Tang, W.; Wang, F. Integrative Transcriptomic Analysis Deciphering the Role of Rice BHLH Transcription Factor Os04g0301500 in Mediating Responses to Biotic and Abiotic Stresses. Front. Plant Sci. 2023, 14, 1266242. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, P.; Suniti; Kumar, U.; Avni; Mann, A. Transcriptional Regulatory Network Involved in Drought and Salt Stress Response in Rice. In Salinity and Drought Tolerance in Plants: Physiological Perspectives; Springer: Singapore, 2023; pp. 237–274. ISBN 9789819946693. [Google Scholar]
- Das, A.; Pramanik, K.; Sharma, R.; Gantait, S.; Banerjee, J. In-Silico Study of Biotic and Abiotic Stress-Related Transcription Factor Binding Sites in the Promoter Regions of Rice Germin-like Protein Genes. PLoS ONE 2019, 14, e0211887. [Google Scholar] [CrossRef]
- Ramadoss, B.R.; Gangola, M.P.; Gurunathan, S. Chapter 4—NAC Transcription Factor Family in Rice: Recent Advancements in the Development of Stress-Tolerant Rice. In Transcription Factors for Abiotic Stress Tolerance in Plants; Wani, S.H., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 47–61. ISBN 978-0-12-819334-1. [Google Scholar]
- Oe, S.; Sasayama, D.; Luo, Q.; Fukayama, H.; Hatanaka, T.; Azuma, T. Growth Responses of Seedlings under Complete Submergence in Rice Cultivars Carrying Both the Submergence-Tolerance Gene SUB1A-1 and the Floating Genes SNORKELs. Plant Prod. Sci. 2022, 25, 70–77. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, M.; Wang, K.; Qu, A.; Hu, S.; Jiang, Q.; Yi, K.; Wang, F.; Cai, C.; Zhu, C.; et al. Rice DST Transcription Factor Negatively Regulates Heat Tolerance through ROS-Mediated Stomatal Movement and Heat-Responsive Gene Expression. Front. Plant Sci. 2023, 14, 1068296. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Zhang, Y.; Guo, J.; Liu, L.; Wang, C.; Wang, B.; Han, G. The Roles of HD-ZIP Proteins in Plant Abiotic Stress Tolerance. Front. Plant Sci. 2022, 13, 1027071. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, W.; Yamauchi, T.; Tsuda, K. Genetic Basis Controlling Rice Plant Architecture and Its Modification for Breeding. Breed. Sci. 2023, 73, 3–45. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Panda, S.K. Chapter 9—Drought Stress Responses and Its Management in Rice. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J.K., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 177–200. ISBN 978-0-12-814332-2. [Google Scholar]
- Singh, P.K.; Nag, A.; Arya, P.; Kapoor, R.; Singh, A.; Jaswal, R.; Sharma, T.R. Prospects of Understanding the Molecular Biology of Disease Resistance in Rice. Int. J. Mol. Sci. 2018, 19, 1141. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.A.; Abdullah-zawawi, M.R.; Zainal-abidin, R.A.; Sukiran, N.L.; Uddin, M.I.; Zainal, Z. A Review of Integrative Omic Approaches for Understanding Rice Salt Response Mechanisms. Plants 2022, 11, 1430. [Google Scholar] [CrossRef] [PubMed]
- Zargar, S.M.; Mir, R.A.; Ebinezer, L.B.; Masi, A.; Hami, A.; Manzoor, M.; Salgotra, R.K.; Sofi, N.R.; Mushtaq, R.; Rohila, J.S.; et al. Physiological and Multi-Omics Approaches for Explaining Drought Stress Tolerance and Supporting Sustainable Production of Rice. Front. Plant Sci. 2022, 12, 803603. [Google Scholar] [CrossRef]
- Ali, J.; Anumalla, M.; Murugaiyan, V.; Li, Z. Green Super Rice (GSR) Traits: Breeding and Genetics for Multiple Biotic and Abiotic Stress Tolerance in Rice BT—Rice Improvement: Physiological, Molecular Breeding and Genetic Perspectives; Ali, J., Wani, S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 59–97. ISBN 978-3-030-66530-2. [Google Scholar]
- Guo, J.; Sun, B.; He, H.; Zhang, Y.; Tian, H.; Wang, B. Current Understanding of BHLH Transcription Factors in Plant Abiotic Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 4921. [Google Scholar] [CrossRef]
- Sarkar, T.; Thankappan, R.; Mishra, G.P.; Nawade, B.D. Advances in the Development and Use of DREB for Improved Abiotic Stress Tolerance in Transgenic Crop Plants. Physiol. Mol. Biol. Plants 2019, 25, 1323–1334. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Gupta, K.; Lopato, S.; Agarwal, P. Dehydration Responsive Element Binding Transcription Factors and Their Applications for the Engineering of Stress Tolerance. J. Exp. Bot. 2017, 68, 2135–2148. [Google Scholar] [CrossRef]
- Chengqi, Z.; Yuxuan, Y.; Tian, Q.; Yafan, H.; Jifeng, Y.; Zhicheng, S. Drought-Tolerant Rice at Molecular Breeding Eras: An Emerging Reality. Rice Sci. 2024, 31, 179–189. [Google Scholar] [CrossRef]
- Al Azzawi, T.N.I.; Khan, M.; Hussain, A.; Shahid, M.; Imran, Q.M.; Mun, B.G.; Lee, S.U.; Yun, B.W. Evaluation of Iraqi Rice Cultivars for Their Tolerance to Drought Stress. Agronomy 2020, 10, 1782. [Google Scholar] [CrossRef]
- Song, Y.; Ai, C.; Jing, S.; Yu, D. Research Progress on Functional Analysis of Rice WRKY Genes. Rice Sci. 2010, 17, 60–72. [Google Scholar] [CrossRef]
- Pandey, S.P.; Somssich, I.E. The Role of WRKY Transcription Factors in Plant Immunity. Plant Physiol 2009, 150, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bai, X.; Wang, X.; Chu, C. OsWRKY71, a Rice Transcription Factor, Is Involved in Rice Defense Response. J. Plant Physiol. 2007, 164, 969–979. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Liang, X.; Zhou, X.; Yang, F.; Liu, J.; He, S.Y.; Guo, Z. Alternative Splicing of Rice WRKY62 and WRKY76 Transcription Factor Genes in Pathogen Defense. Plant Physiol. 2016, 171, 1427–1442. [Google Scholar] [CrossRef]
- Bian, Z.; Gao, H.; Wang, C. NAC Transcription Factors as Positive or Negative Regulators during Ongoing Battle between Pathogens and Our Food Crops. Int. J. Mol. Sci. 2020, 22, 81. [Google Scholar] [CrossRef]
- Shao, H.; Wang, H.; Tang, X. NAC Transcription Factors in Plant Multiple Abiotic Stress Responses: Progress and Prospects. Front. Plant Sci. 2015, 6, 902. [Google Scholar] [CrossRef]
- Anjali; Kumar, S.; Korra, T.; Thakur, R.; Arutselvan, R.; Kashyap, A.S.; Nehela, Y.; Chaplygin, V.; Minkina, T.; Keswani, C. Role of Plant Secondary Metabolites in Defence and Transcriptional Regulation in Response to Biotic Stress. Plant Stress 2023, 8, 100154. [Google Scholar] [CrossRef]
- Shehab, M.; Iovene, M.; Ciancio, A.; Colagiero, M.; Finetti-Sialer, M. Transcriptome Analysis Provides Novel Insights into Salt Stress Response in Two Egyptian Rice Varieties with Different Tolerance Levels. Rice Sci. 2022, 29, 499–502. [Google Scholar] [CrossRef]
- Cao, Y.; Li, K.; Li, Y.; Zhao, X.; Wang, L. MYB Transcription Factors as Regulators of Secondary Metabolism in Plants. Biology 2020, 9, 61. [Google Scholar] [CrossRef]
- Kang, L.; Teng, Y.; Cen, Q.; Fang, Y.; Tian, Q.; Zhang, X.; Wang, H.; Zhang, X.; Xue, D. Genome-Wide Identifi-cation of R2R3-MYB Transcription Factor and Expression Analysis under Abiotic Stress in Rice. Plants. 2022, 11, 1928. [Google Scholar] [CrossRef]
- Xie, Z.; Nolan, T.M.; Jiang, H.; Yin, Y. AP2/ERF Transcription Factor Regulatory Networks in Hormone and Abiotic Stress Responses in Arabidopsis. Front. Plant Sci. 2019, 10, 228. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, T.; Huang, K.; Jin, Y.M.; Li, Z.; Chen, M.; Yun, S.; Zhang, H.; Yang, X.; Chen, H.; et al. A Novel AP2/ERF Transcription Factor, OsRPH1, Negatively Regulates Plant Height in Rice. Front. Plant Sci. 2020, 11, 709. [Google Scholar] [CrossRef]
- Yongqi, H.; Jia, Z.; Defeng, F.; Zhibo, H.; Jiaming, L.; Yufei, Z.; Jinping, C.; Jifeng, Y.; Zhoufei, W. RNA-Seq Study Reveals AP2-Domain-Containing Signalling Regulators Involved in Initial Imbibition of Seed Germination in Rice. Rice Sci. 2020, 27, 302–314. [Google Scholar] [CrossRef]
- Gao, F.; Robe, K.; Gaymard, F.; Izquierdo, E.; Dubos, C. The Transcriptional Control of Iron Homeostasis in Plants: A Tale of BHLH Transcription Factors? Front. Plant Sci. 2019, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Lu, D.; Yu, Y.; Li, L.; Dong, W.; Xu, L.; Yang, Q.; Wan, X.; Liang, H. Genome-Wide Identification and Characterization of the BHLH Gene Family and Its Response to Abiotic Stresses in Carthamus Tinctorius. Plants 2023, 12, 3764. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Sharma, P.; Rani, M.; Laxmi, V.; Sahil; Sahi, C.; Satturu, V.; Katiyar-Agarwal, S.; Agarwal, M. Meta-QTL and Ortho Analysis Unravels the Genetic Architecture and Key Candidate Genes for Cold Tolerance at Seedling Stage in Rice. Physiol. Mol. Biol. Plants 2024, 30, 93–108. [Google Scholar] [CrossRef]
- Wei, K.; Chen, H. Comparative Functional Genomics Analysis of BHLH Gene Family in Rice, Maize and Wheat. BMC Plant Biol. 2018, 18, 309. [Google Scholar] [CrossRef]
- Zong, W.; Tang, N.; Yang, J.; Peng, L.; Ma, S.; Xu, Y.; Li, G.; Xiong, L. Feedback Regulation of ABA Signaling and Biosynthesis by a BZIP Transcription Factor Targets Drought-Resistance-Related Genes. Plant Physiol. 2016, 171, 2810–2825. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Zhang, C.; Caine, R.S.; Gray, J.; Sadanandom, A. Rice SUMO Protease Overly Tolerant to Salt 1 Targets the Transcription Factor, OsbZIP23 to Promote Drought Tolerance in Rice. Plant J. 2017, 92, 1031–1043. [Google Scholar] [CrossRef]
- Baoxiang, W.; Yan, L.; Yifeng, W.; Jingfang, L.; Zhiguang, S.; Ming, C.; Yungao, X.; Bo, X.; Bo, Y.; Jian, L.; et al. OsbZIP72 Is Involved in Transcriptional Gene-Regulation Pathway of Abscisic Acid Signal Transduction by Activating Rice High-Affinity Potassium Transporter OsHKT1;1. Rice Sci. 2021, 28, 257–267. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Khurana, J.P.; Jain, M. Characterization of Rice Homeobox Genes, OsHOX22 and OsHOX24, and Over-Expression of OsHOX24 in Transgenic Arabidopsis Suggest Their Role in Abiotic Stress Response. Front. Plant Sci. 2016, 7, 627. [Google Scholar] [CrossRef]
- Zhang, S.; Haider, I.; Kohlen, W.; Jiang, L.; Bouwmeester, H.; Meijer, A.H.; Schluepmann, H.; Liu, C.-M.; Ouwerkerk, P.B.F. Function of the HD-Zip I Gene Oshox22 in ABA-Mediated Drought and Salt Tolerances in Rice. Plant Mol. Biol. 2012, 80, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Trionfini, V.; Campi, M.; Welchen, E.; Chan, R.L.; Attallah, C.V. The Rice Transcription Factors OsHOX22 and OsHOX24 Oppositely Modulate the Lamina Joint Inclination. Environ. Exp. Bot. 2023, 213, 105433. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Kumari, S.; Nazir, F.; Khanna, R.R.; Gupta, R.; Chhillar, H. Defensive Role of Plant Hormones in Advancing Abiotic Stress-Resistant Rice Plants. Rice Sci. 2023, 30, 15–35. [Google Scholar] [CrossRef]
- Adhinarayanreddy, V.; Vargheese, A.; Dadi, S.; Uttarkar, A.; Niranjan, V.; Venkatraman, A.; Sheshshayee, M.; Sreeman, R.S.; Vemanna, P.V. A Simple and Rapid Oxidative Stress Screening Method of Small Molecules for Functional Studies of Transcription Factor. Rice Sci. 2022, 3, 402–406. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Pandit, E.; Nayak, D.K.; Behera, L.; Mohapatra, T. Genes, Pathways and Transcription Factors Involved in Seedling Stage Chilling Stress Tolerance in Indica Rice through RNA-Seq Analysis. BMC Plant Biol. 2019, 19, 352. [Google Scholar] [CrossRef]
- Devin, S.R.; Prudencio, Á.S.; Mahdavi, S.M.E.; Rubio, M.; Martínez-García, P.J.; Martínez-Gómez, P. Orchard Management and Incorporation of Biochemical and Molecular Strategies for Improving Drought Tolerance in Fruit Tree Crops. Plants 2023, 12, 773. [Google Scholar] [CrossRef]
- Fengfeng, F.; Meng, C.; Xiong, L.; Manman, L.; Huanran, Y.; Mingxing, C.; Ahmad, A.; Nengwu, L.; Shaoqing, L. Novel QTLs from Wild Rice Oryza Longistaminata Confer Strong Tolerance to High Temperature at Seedling Stage. Rice Sci. 2023, 30, 577–586. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Ye, M.; Lu, H.; Wang, D.; Chen, Q. Evolutionary History of the C-Repeat Binding Factor/Dehydration-Responsive Element-Binding 1 (CBF/DREB1) Protein Family in 43 Plant Species and Characterization of CBF/DREB1 Proteins in Solanum Tuberosum. BMC Evol. Biol. 2020, 20, 142. [Google Scholar] [CrossRef]
- Jung, K.H.; Seo, Y.S.; Walia, H.; Cao, P.; Fukao, T.; Canlas, P.E.; Amonpant, F.; Bailey-Serres, J.; Ronald, P.C. The Submergence Tolerance Regulator Sub1A Mediates Stress-Responsive Expression of AP2/ERF Transcription Factors. Plant Physiol. 2010, 152, 1674–1692. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.Y.; Ou, S.L.; Wang, M.C.; Yang, C.Y. Physiological Responses and Ethylene-Response AP2/ERF Factor Expression in Indica Rice Seedlings Subjected to Submergence and Osmotic Stress. BMC Plant Biol. 2023, 23, 372. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Jalmi, S.K.; Bhagat, P.K.; Verma, N.; Sinha, A.K. A BHLH Transcription Factor, MYC2, Imparts Salt Intolerance by Regulating Proline Biosynthesis in Arabidopsis. FEBS J. 2020, 287, 2560–2576. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; Zhang, A.; Ma, J.; Wang, T.; Yang, B.; Shuang, L.S.; Liu, M.; Li, J.; Xu, X.; Paterson, A.H.; et al. Joint QTL Mapping and Transcriptome Sequencing Analysis Reveal Candidate Flowering Time Genes in Brassica napus L. BMC Genom. 2019, 20, 21. [Google Scholar] [CrossRef]
- Ersong, Z.; Xuming, W.; Rumeng, X.; Feibo, Y.; Chao, Z.; Yong, Y.; Yang, C.; Jianping, C.; Chengqi, Y.; Jie, Z. Regulation of OsPR10a Promoter Activity by Phytohormone and Pathogen Stimulation in Rice. Rice Sci. 2021, 28, 442–456. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, X.; Yin, D.; Chen, D.; Luo, C.; Liu, H.; Huang, C. Advances in the Research on Plant WRKY Transcription Factors Responsive to External Stresses. Curr. Issues Mol. Biol. 2023, 45, 2861–2880. [Google Scholar] [CrossRef]
- Zafar, K.; Sedeek, K.E.M.; Rao, G.S.; Khan, M.Z.; Amin, I.; Kamel, R.; Mukhtar, Z.; Zafar, M.; Mansoor, S.; Mahfouz, M.M. Genome Editing Technologies for Rice Improvement: Progress, Prospects, and Safety Concerns. Front. Genome Ed. 2020, 2, 5. [Google Scholar] [CrossRef]
- Rothschild, J. Ethical Considerations of Gene Editing and Genetic Selection. J. Gen. Fam. Med. 2020, 21, 37–47. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, H.; Li, D.; Huang, L.; Hong, Y.; Ding, X.S.; Nelson, R.S.; Zhou, X.; Song, F. Functions of Rice NAC Transcriptional Factors, ONAC122 and ONAC131, in Defense Responses against Magnaporthe Grisea. Plant Mol. Biol. 2013, 81, 41–56. [Google Scholar] [CrossRef]
- Oliveira Nizolli, V.; Pegoraro, C.; Costa De Oliveira, A. Rice Blast: Strategies and Challenges for Improving Genetic Resistance Rice Blast: Strategies and Challenges for Improving Genetic Resistance ARTICLE. Crop Breed. Appl. Biotechnol. 2021, 21, 387721–387730. [Google Scholar] [CrossRef]
- Albahri, G.; Alyamani, A.A.; Badran, A.; Hijazi, A.; Nasser, M.; Maresca, M.; Baydoun, E. Enhancing Essential Grains Yield for Sustainable Food Security and Bio-Safe Agriculture through Latest Innovative Approaches. Agronomy 2023, 13, 1709. [Google Scholar] [CrossRef]
- Lu, Y.; Zeng, J.; Liu, Q. The Rice MiR396-GRF-GIF-SWI/SNF Module: A Player in GA Signaling. Front. Plant Sci. 2022, 12, 786641. [Google Scholar] [CrossRef]
- Kaur, P.; Saini, K.S.; Sharma, S.; Kaur, J.; Bhatt, R.; Alamri, S.; Alfagham, A.T.; Hussain, S. Increasing the Efficiency of the Rice–Wheat Cropping System through Integrated Nutrient Management. Sustainability 2023, 15, 12694. [Google Scholar] [CrossRef]
- Wang, F.; Itai, R.N.; Nozoye, T.; Kobayashi, T.; Nishizawa, N.K.; Nakanishi, H. The BHLH Protein OsIRO3 Is Critical for Plant Survival and Iron (Fe) Homeostasis in Rice (Oryza Sativa L.) under Fe-Deficient Conditions. Soil Sci. Plant Nutr. 2020, 66, 579–592. [Google Scholar] [CrossRef]
- Zhao, H.; Nie, K.; Zhou, H.; Yan, X.; Zhan, Q.; Zheng, Y.; Song, C.P. ABI5 Modulates Seed Germination via Feedback Regulation of the Expression of the PYR/PYL/RCAR ABA Receptor Genes. New Phytol. 2020, 228, 596–608. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Q.; Li, Y.; Yu, Y.; Liu, X.; Han, Y.; Luo, X.; Wu, X.; Ju, L.; Sun, J.; et al. Transcription Factors Rc and OsVP1 Coordinately Regulate Preharvest Sprouting Tolerance in Red Pericarp Rice. J. Agric. Food Chem. 2020, 68, 14748–14757. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic Acid Dynamics, Signaling, and Functions in Plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Gong, D.; He, F.; Liu, J.; Zhang, C.; Wang, Y.; Tian, S.; Sun, C.; Zhang, X. Understanding of Hormonal Regulation in Rice Seed Germination. Life 2022, 12, 1021. [Google Scholar] [CrossRef]
- Zaikina, E.A.; Rumyantsev, S.D.; Sarvarova, E.R.; Kuluev, B.R. Transcription Factor Genes Involved in Plant Response to Abiotic Stress Factors. Ecol. Genet. 2019, 17, 47–58. [Google Scholar] [CrossRef]
- Ambavaram, M.M.R.; Basu, S.; Krishnan, A.; Ramegowda, V.; Batlang, U.; Rahman, L.; Baisakh, N.; Pereira, A. Coordinated Regulation of Photosynthesis in Rice Increases Yield and Tolerance to Environmental Stress. Nat. Commun. 2014, 5, 5302. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Y.; Zhang, M.; Zhan, X.; Shen, X.; Yu, P.; Chen, D.; Liu, Q.; Sinumporn, S.; Hussain, K.; et al. The Rice CONSTANS-like Protein OsCOL15 Suppresses Flowering by Promoting Ghd7 and Repressing RID1. Biochem. Biophys. Res. Commun. 2018, 495, 1349–1355. [Google Scholar] [CrossRef]
- XIANG, C.; QU, L.; GAO, Y.; SHI, Y. Flower Development and Photoperiodic Control of Flowering in Rice. Rice Sci. 2013, 20, 79–87. [Google Scholar] [CrossRef]
- Cai, M.; Zhu, S.; Wu, M.; Zheng, X.; Wang, J.; Zhou, L.; Zheng, T.; Cui, S.; Zhou, S.; Li, C.; et al. DHD4, a CONSTANS-like Family Transcription Factor, Delays Heading Date by Affecting the Formation of the FAC Complex in Rice. Mol. Plant 2021, 14, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Hashim, N.; Mahadi, M.R.; Abdullah, A.F.; Wayayok, A.; Kassim, M.S.M.; Jamaluddin, A.M.M.A. Smart Farming for Sustainable Rice Production: An Insight into Application, Challenge, and Future Prospect. Rice Sci. 2024, 31, 47–61. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, J.W.; Li, J.; Han, B. Designing Future Crops: Challenges and Strategies for Sustainable Agriculture. Plant J. 2021, 105, 1165–1178. [Google Scholar] [CrossRef]
- Zegeye, W.A.; Tsegaw, M.; Zhang, Y.; Cao, L. CRISPR-Based Genome Editing: Advancements and Opportunities for Rice Improvement. Int. J. Mol. Sci. 2022, 23, 4454. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).