Abstract
Background: Children with spastic hand impairments resulting from cerebral palsy or neuromuscular disorders often exhibit a restricted range of motion and diminished functional use. Rehabilitation devices that assist joint mobilization can enhance therapeutic outcomes, yet few solutions target pediatric populations. Methods: This study aimed to design, implement, and preliminarily evaluate a biomechanical device tailored to promote flexo-extension, radial–ulnar deviation, and supination movements in spastic hands of school-aged children. A prototype combining a motor-driven actuation system, adjustable wrist and finger supports, and a MATLAB-based graphical user interface was developed. Two participants (aged 8 and 10) with clinically diagnosed spastic hemiparesis underwent 25-minute sessions over 15 consecutive days. Joint angles were recorded before and after each session using an electro-goniometer. Data normality was assessed via the Shapiro–Wilk test, and pre–post differences were analyzed with the Wilcoxon signed-rank test (α = 0.05). Results: Both participants demonstrated consistent increases in their active range of motion across all measured planes. Median flexo-extension improved by 12.5° (p = 0.001), ulnar–radial deviation by 7.3° (p = 0.002), and supination by 9.1° (p = 0.001). No adverse events occurred, and device tolerance remained high throughout the intervention. Conclusions: The device facilitated statistically significant enhancements in joint mobility in a small pediatric cohort, supporting its feasibility and safety in spastic hand rehabilitation. These preliminary findings warrant larger controlled trials to confirm the device’s efficacy, optimize treatment protocols, and assess its long-term functional benefits.
1. Introduction
Pediatric spastic hand impairments, predominantly resulting from cerebral palsy and neuromuscular disorders, affect approximately 2–3 per 1000 children worldwide, with significant implications for motor development and quality of life. Unlike adult rehabilitation, pediatric interventions must account for ongoing neural plasticity, anatomical growth, and developmental milestones. The hand’s critical role in cognitive development and daily activities makes early and effective rehabilitation essential in optimizing functional outcomes in affected children [1].
Injuries to the corticospinal system can affect motor skills in people with cerebral palsy (CP), especially those with unilateral CP. Despite these challenges, there is considerable potential to improve and develop hand function. Appropriate interventions can mitigate mirror movements, which are involuntary movements of the opposite hand during voluntary activities [2,3]. The variability in the ability to develop hand function, which is influenced by the extent and location of brain injury, offers opportunities for individualized rehabilitation [4]. Spastic hand patterns, characterized by abnormal muscle tone and coordination, are common in cerebral palsy because of the disruption of neural pathways and the loss of inhibitory control of the central nervous system. However, new forms of treatment can address these challenges [5].
Although the recovery of motor control can be hampered by spasticity and random motor spasms, advanced interventions and methods can help people regain functional control [6]. In addition, there is a significant correlation between more complex motor tasks and cognitive demands, which underlines the importance of cognitive factors in the development and improvement of motor skills [7].
Understanding the neural mechanisms underlying spastic patterns in hand and motor skills is essential in developing specific therapies and interventions that can substantially improve motor function in people with cerebral palsy and other motor impairments [3].
A holistic approach is needed, combining a variety of interventions and therapeutic technologies to restore motor skills and control spastic hand patterns, especially in those who have suffered a stroke. Sensorimotor disturbances in the upper extremities are common after a stroke. It is estimated that between 50% and 70% of patients experience persistent deficits, such as paresis and spasticity, which significantly affect their performance in daily activities and quality of life [8].
Biomechanical devices have been developed to inhibit spastic patterns in the hands of patients with neurological diseases via a variety of innovative technologies and methods that can aid in both the assessment and rehabilitation of spasticity. These devices are designed to provide specific torsion and velocity stimuli to the affected muscles, allowing a detailed analysis of the response of the stretch reflex and joint stiffness [9,10]. For example, Jo et al. [10] developed a 3D-printed manual orthosis that can be combined with a drive system based on hand movement. The system uses a field-programmable gate array (FPGA) and detects biological signals, such as electroencephalograms (EEGs) and electromyograms (EMGs). Its objective is to help patients who have suffered a spinal cord injury or a stroke improve their motor function. The wrist-operated flexor hinge orthosis (WDFHO) developed by Rout and Prasanth [11] is another mechanical device that has shown remarkable improvements in pinch strength and hand dexterity in patients with lower cervical spine injuries, highlighting its effectiveness in restoring hand function. Similarly, a soft and portable robotic hand with an elastomer-based bidirectional soft actuator provides active control of finger flexion and extension, allowing better control of grip strength during activities of daily living (ADLs) [12]. Radial shock wave therapy (ReSW) has also been researched. Studies have shown that this intervention can significantly reduce muscle tone and stiffness and improve clinical evaluations after treatment. These findings suggest that ReS can be an effective option for treating peripheral components of spasticity, providing relief and improving the quality of life of patients [13]. Low-cost devices have also been developed to rehabilitate grip function; these devices use elastic bands and microcontrollers for data recording, making advanced rehabilitation technology more accessible [14]. Finally, advances in bioelectric prostheses, which incorporate robust control strategies and sensory feedback, have improved dexterity and grasping patterns, and sensory feedback plays a crucial role in the development of the device, as it enhances the user’s sense of ownership and control [15]. Together, these biomechanical devices represent a significant advance in the rehabilitation of spastic patterns of the hands, as they offer effective and personalized solutions that address the mechanical and neural aspects of spasticity.
Despite these advancements, existing pediatric spastic hand rehabilitation devices show many limitations which are addressed in this study. Current systems predominantly focus on adult demographics and seldom consider children’s anthropometric differences or developmental needs [11,12,13,14,15]. Numerous devices prioritize single-plane movements or require intricate setup protocols, limiting their application in resource-limited environments such as those in Ecuador, and they lack interfaces designed for clinicians treating pediatric patients.
Therefore, this research aimed to design, implement, and preliminarily evaluate a biomechanical device specifically tailored to pediatric spastic hand rehabilitation in the Ecuadorian context. The objectives were to (1) develop a multi-plane movement system accommodating pediatric anthropometry, (2) integrate user-friendly control interfaces suitable for clinical settings, and (3) assess feasibility and safety through pilot testing with affected children.
This research presents an innovative mechanism that simultaneously performs wrist flexion–extension, radial–ulnar deviation, and supination, using high-precision servomotors governed by a MATLAB-Arduino system, enabling real-time parameter modification and automated data logging. The device features anatomically adjustable support customized to the average pediatric arm dimensions, including both length and circumference, which are crucial in assessing growth and diagnosing conditions such as malnutrition and limb abnormalities. According to a study by Edmond et al., normal values for upper extremity length and circumference in children aged 0 to 17 years have been established, with growth rates being similar for both sexes and with no significant differences between the right and left extremities. These measurements are dependent on factors such as height, age, and sex for arm length and weight, age, and sex for forearm length [16]. The device employs a five-movement rehabilitation protocol specifically designed for children with spasticity. Its cost-effective, simple-to-assemble design establishes it as the original comprehensive pediatric spastic hand rehabilitation solution created and tested in Ecuador, offering a unique combination of functionality, adaptability, and accessibility.
In Ecuador, the National Council for the Equality of Disabilities reports that there are approximately 46,511 students with disabilities in basic education, with 41.47% presenting with physical disabilities. This substantial population underscores the urgent need for accessible, culturally appropriate rehabilitation technologies. The development of cost-effective, locally adapted devices could significantly impact therapeutic outcomes while building local capacity for pediatric rehabilitation. Figure 1 shows the rehabilitation mechanism.
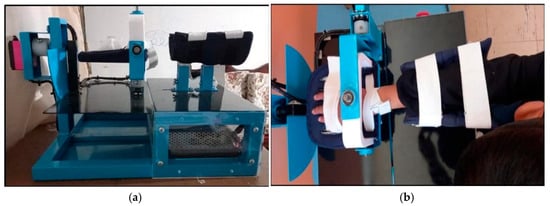
Figure 1.
Biomechanical device in clinical application. (a) Complete device assembly showing adjustable wrist and forearm supports with servomotor configuration. (b) Device in use, demonstrating wrist positioning and movement mechanism during therapy session.
2. Biomechanical Foundations
The human wrist joint complex comprises eight carpal bones forming radiocarpal and midcarpal articulations, enabling multi-planar movement that is essential in hand functions [17,18]. In healthy children, wrist mobility develops progressively, reaching adult ranges by the age of 12–14 years [19,20]. The five primary movements addressed by the device represent functionally critical motion planes: flexion/extension (sagittal plane), radial/ulnar deviation (frontal plane), and forearm supination (transverse plane) [20,21].
Spastic hand patterns in pediatric cerebral palsy result from upper motor neuron lesions affecting corticospinal tract development [17,18]. This leads to increased muscle tone, velocity-dependent resistance to passive movement, and the co-contraction of antagonist muscles [22]. The resulting biomechanical alterations include (1) a reduced passive range of motion due to soft-tissue contractures [23], (2) impaired selective motor control, limiting isolated joint movement [22], and (3) abnormal synergy patterns, restricting functional hand use [24].
The therapeutic rationale behind the five-movement protocol stems from neuroplasticity principles and biomechanical optimization [25,26]. Controlled passive mobilization at physiological velocities can (1) maintain the range of motion in the joints by preventing contracture formation, (2) provide proprioceptive input to facilitate motor learning, and (3) potentially modulate spasticity through reciprocal inhibition mechanisms [24,25]. The specific movement combinations were selected based on their contribution to functional activities of daily living, with flexion/extension enabling grasp–release patterns, deviation movements facilitating wrist stabilization, and supination supporting object manipulation [27,28].
Pediatric considerations include anatomical scaling (smaller joint dimensions and lower torque requirements), developmental factors (ongoing ossification and growth) [28], and neurological plasticity (enhanced capacity for motor learning compared to adults) [26]. The device parameters were calibrated to accommodate these differences, with maximum torques limited to 0.5 Nm and angular velocities restricted to 30°/s to ensure safety while providing an adequate therapeutic stimulus [29,30].
3. Materials and Methods
3.1. Mathematical Modeling and Control System
The biomechanical device operates through a multi-actuator system requiring precise kinematic and dynamic modeling for safe and effective operation [31,32]. The mathematical framework integrates forward kinematics, servomotor dynamics, and control system design to ensure accurate and reliable therapeutic movements suitable for pediatric populations [33].
The forward kinematics of each movement can be described using homogeneous transformation matrices, following established approaches for rehabilitation robotics [34]. For flexion/extension movement, the angular position is modeled as shown in Equation (1) [35]:
where represents the flexion/extension angle; represents the initial position; represents the angular velocity (limited to 30°/s for pediatric safety); represents the movement frequency.
For a typical therapeutic cycle with and :
At t = 5 s,
This kinematic approach ensures smooth, physiologically appropriate movement patterns while maintaining predictable trajectories essential for therapeutic efficacy [36].
The dynamic model for each servomotor considers the complete system dynamics, including motor inertia, damping, and patient-imposed resistance [31,32]. The torque equation for RDS5160 servomotors is expressed in Equation (2) [37]:
where J is the motor inertia (estimated at 0.0032 kg⋅m2 for RDS5160); B is the damping coefficient (0.0055 N⋅m⋅s/rad); K is the spring constant, and α is the angular acceleration; ω is the angular velocity; θ is the angular position; τload represents patient-imposed resistance.
For RDS5160 servomotors (flexion/extension and supination):
where , , providing a maximum torque of at 8.4 V with response speeds of 0.13 s/60°.
For FT6335M servomotors (radial/ulnar deviation):
where , , providing a maximum torque of at 8.4 V with response speeds of 0.2 s/60°.
For maximum therapeutic conditions with , , :
For RDS5160,
For FT5335M,
RDS5160 servos provide a maximum torque of 6.86 Nm (70 kg⋅cm) at 8.4 V with response speeds of 0.13 s/60° under optimal conditions. The calculated required torque (0.371 Nm) represents only 5.4% of maximum servo capacity, ensuring substantial safety margins. This comprehensive dynamic model accounts for both mechanical characteristics and human–robot interaction forces, ensuring accurate torque delivery while maintaining safety margins appropriate for pediatric applications [29].
The control system implements a PID controller optimized for human–robot interaction in rehabilitation settings [38,39]. The transfer function is defined in Equation (3):
With gains optimized for pediatric applications: , , ensuring stability while maintaining responsiveness to resistance changes [40]. The closed-loop system response can be calculated using the characteristic shown as Equation (4):
where represents the plant dynamics. Substituting values results in Equation (5):
The closed-loop transfer function becomes that shown in Equation (5):
For step response analysis, the settling time is calculated as , where (desired damping ratio) and (natural frequency). Therefore,
This ensures a rapid response while maintaining stability for safe pediatric interaction. These parameters were selected based on established pediatric rehabilitation robotics guidelines to provide appropriate stiffness and damping characteristics for safe interaction with children [29].
The multi-actuator system incorporates comprehensive safety protocols through the coordinated control of five independent RDS5160 servomotors [40]. Maximum torques are limited to 0.5 Nm across all movement planes, representing a significant safety margin below the servo’s 6.86 Nm maximum capability, with the real-time monitoring of patient-imposed resistance to prevent excessive forces [29]. The safety factor is calculated as shown in Equation (7) for RDS5160 and Equation (8) for FT6335M:
These safety margins (13.7× for RDS5160 and 7.0× for FT6335M) ensure that even under maximum therapeutic load, the system operates well within safe parameters for pediatric applications. The control architecture includes an emergency stop functionality with response times < 0.1 s and force limiting at 150% of therapeutic thresholds to ensure pediatric safety standards. The system employs adaptive impedance control to accommodate varying patient capabilities and spasticity levels, with force feedback loops continuously monitoring τload to adjust control parameters dynamically [41].
The power consumption analysis for the complete system is shown in Equation (9):
This low power consumption makes the system suitable for extended therapeutic sessions while maintaining energy efficiency standards for medical devices.
3.2. Study Design and Participants
This quasi-experimental pilot study involved two pediatric patients diagnosed with hand spasticity who were recruited from the Carlos Garbay Special Education Institute. Patients who had recently undergone surgery or experienced acute wrist injury were excluded.
The sample included N = 2 patients, suggestive of a preliminary pilot study focused on evaluating the practicality of the prototype and improving the protocol prior to a larger-scale phase. Because of the exploratory nature of this stage, a control group was omitted, with the goal of performing this comparison in subsequent trials with a greater number of individuals.
This study was designed as a proof-of-concept feasibility trial to assess device safety, tolerability, and preliminary efficacy signals prior to conducting a fully powered randomized controlled trial. The sample size (N = 2) was deemed appropriate for initial feasibility assessment while minimizing the exposure of vulnerable pediatric participants to experimental intervention.
3.3. Wrist Mobility Assessment
The biomechanical device was designed to address five primary wrist movements essential for functional hand use in pediatric spastic hand rehabilitation. These movements include all the therapeutic movements that the rehabilitation plan talks about:
Flexion: In this movement, the fingers bend or move toward the palm. This is important for holding and moving objects.
Extension: This move lets the fingers spread out from the palm, which helps with release patterns and opening the hand for everyday tasks.
Radial Deviation: This movement is performed by tilting the hand toward the thumb, providing wrist stabilization during lateral pinch and precision grip activities.
Ulnar Deviation: The ulnar deviation is opposite to the radial deviation, tilting the hand toward the little finger, supporting power grip and bimanual coordination tasks.
Supination: The movement of supination refers to the rotation when the palm is turned into an upward position, critical in object manipulation and tool use in activities of daily living.
Each of these movements has specific angles of mobility that have been measured from a neutral position, as described by the Association for the Study of Osteosynthesis (AO) and the American Academy of Orthopedic Surgeons (AAOS). The normative ranges for these movements in pediatric populations are shown in Table 1 and served as reference values for the therapeutic intervention.

Table 1.
Mobility ranges of the wrist.
3.4. Clinical Protocol
The intervention process took place at the Carlos Garbay Special Education Institute in Riobamba, Chimborazo Province. Both subjects followed a normal 15-day treatment plan that included daily 25-minute therapy sessions using the biomechanical rehabilitation gadget.
Each session involved controlled passive mobilization across all five movement planes (flexion/extension, radial/ulnar deviation, and supination) according to predetermined sequences programmed in the MATLAB R2022b version 9.13 interface. Movement velocities were limited to 30°/s with maximum torque restrictions of 0.5 Nm to ensure pediatric safety standards. Sessions were conducted at the same time each day to maintain consistency and minimize the effects of circadian rhythm on muscle tone.
Joint angles were recorded immediately before and after each session using a precision electro-goniometer (±0.1° resolution). Measurements were taken in the neutral position for each movement plane, with participants positioned in the standardized supine posture to ensure reproducibility. Each angle was tested three times, and the average was determined to achieve the most precise reading.
Professional physical therapists who work with children who have stiffness monitored all the lessons to make sure they were safe. To pause the intervention, participants had to be in pain (>3/10 on the pediatric pain scale) or show signs of stress or the device had to stop working. The emergency stop functionality was tested before each session to ensure a <0.1 s response time.
Clinical assessments included baseline demographic data, spasticity severity using the Modified Ashworth Scale, and functional hand use evaluation. Adverse events were documented using standardized forms and reviewed by the study physician within 24 h of occurrence.
3.5. Statistical Analysis
Shapiro–Wilk tests, which are acceptable for sample numbers under 50 per distribution, were used to determine whether the data were normal. Twenty-two measurements were taken of each movement angle variable in each condition, with sixty measurements taken before and after the intervention for each subject. The results were not distributed normally in all these cases (p < 0.001).
The Wilcoxon signed-rank test was used to compare the data before and after the intervention for each movement plane (flexion/extension, radial/ulnar deviation, and supination). This was necessary because the data was not parametric, and the design included repeated measures. The formula r = Z/√N was used to figure out the sizes of the effects. Z is the standardized test result, and N is the total number of observations. For all impact size values, 95% confidence intervals were included.
Version 26.0 of SPSS (IBM Corp., Armonk, NY, USA) was used for all result comparisons. For every comparison, the level of significance was set at 0.05. In this pilot study, which only had two subjects, the main goals were to estimate effect sizes and determine how useful the results would be in the real world. The power estimated for future larger randomized controlled studies will be based on these early results instead of testing a theory to its logical end. These early results will help in figuring out the power of future randomized controlled studies involving a larger group of participants.
3.6. Prototype Design
The integral system includes a power supply, which supplies power to the mechanism. In addition, programming has been developed via MATLAB R2022b version 9.13 software to control the mechanism and store data, which are saved in the PC. Figure 2 shows a general diagram of the system.
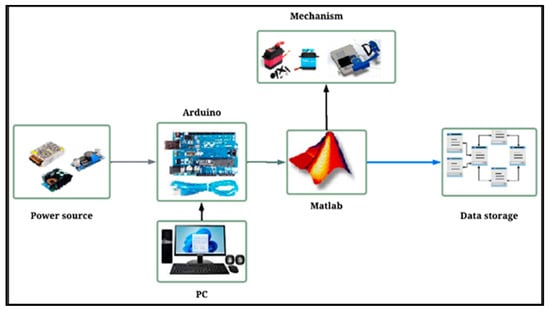
Figure 2.
General diagram of the system.
3.7. Electronic Elements
An Arduino board (Shenzhen Jin Da Peng Technology Co., Ltd., based in Shenzhen, Guangdong, China), which serves as the central control unit of the system, is one of the many electronic elements that have been selected. In addition, a switched source is used to convert alternating current into direct current and has a 12 V output. An XL4016 voltage step-down module is used, which powers the electronic components of the mechanism. This module includes an adjustable potentiometer to control the output voltage and current.
The various types of hand movements are executed by the RDS5160 and FT6335M servomotors. These servomotors were selected because they are durable, accurate, and compatible with the selected Arduino board.
The system includes a relay module. This module works as a switch and regulates the on and off pilot light, which turns on when the appliance is in operation. In addition, a buzzer is incorporated that emits a sound when the therapeutic exercises are finished, marking the end of the activity. The electrical scheme of the system is shown in Figure 3.
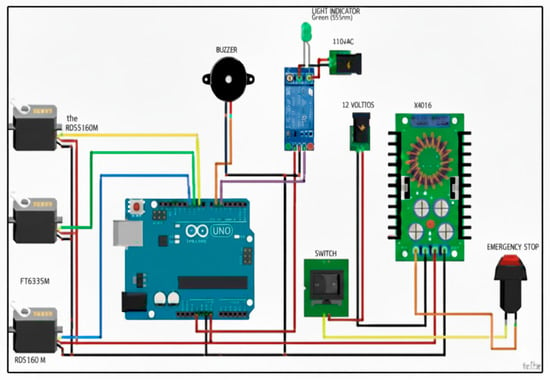
Figure 3.
Electrical diagram of the mechanism.
3.8. Manufacturing Materials
The device begins with a square base made of steel support, a high-strength material that provides solid support. To perform the desired movements, the servomotors are placed in strategic places. The FT5330M servomotors have specific positions. The first produces lateral movements in the lower part of the device at the base, where the wrist rests. The second is responsible for the movements of extension and flexion and is located next to the base of the wrist. At the front is RDS5160, the servomotor with the highest torque. It is fixed to a vertical support with screws, resulting in rotational movement. Nylon bushings are also inserted into the servomotor shafts to increase stability and reduce friction. Acrylic is used to cover the device, which protects it against external factors such as dust and allows the electronic components to be seen, facilitating inspection and maintenance. The design of the hand and forearm supports is based on the average arm measurement of each child. PLA is one of the most widely used filaments in 3D printing because it offers a solid foundation in terms of strength and torsion. It is used to create these supports. In addition, to provide greater comfort and stability during therapy, both supports are padded with Velcro. Figure 4 shows the manufacturing materials.
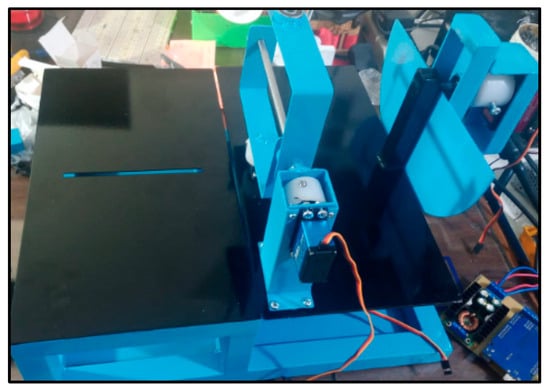
Figure 4.
Construction materials.
3.9. Adjustability Specifications
The device incorporates multiple adjustment mechanisms to accommodate pediatric anthropometric variations. Table 2 presents the adjustment ranges based on pediatric upper limb measurements for children aged 6–12 years.

Table 2.
Device adjustability specifications.
The adjustment mechanisms were validated through fitting tests on anatomical models representing the 5th to 95th percentile of pediatric arm dimensions. Each adjustment point includes visual markers to ensure reproducible positioning across therapy sessions.
3.10. Mechanism Interface
The mechanism interface, developed through the MATLAB GUI, offers an intuitive and easy-to-use interface that includes a variety of control types, such as buttons, sliders, and menu bars, among other properties. It consists of a start window, in which a username and password must be entered, and a control window, in which the various movements that the device can perform can be selected and configured, such as flexion, extension, radial deviation, ulnar deviation, and supination.
Each panel should include the desired number of sets and repetitions, as well as the angles of mobility and a maximum limit to ensure safety. In addition, the scrolling speed of the device can be adjusted. Finally, a help window is included that explains the various types of movements that the wrist performs, as well as their respective ranges of mobility. The control interface is presented in Figure 5.
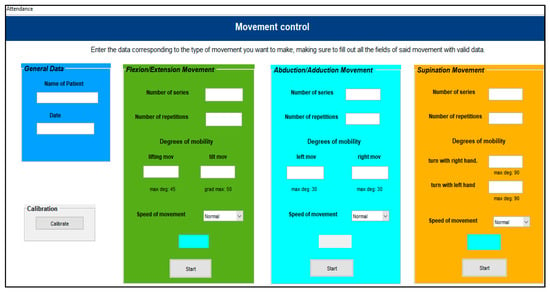
Figure 5.
System interface.
4. Results
Table 3 presents the Modified Ashworth Scale (MAS) scores for both participants before and after the intervention period, along with passive resistance torque measurements obtained during initial device calibration.

Table 3.
Spasticity and muscle tone assessment.
MAS Scores: 0 = no increase in muscle tone; 1 = slight increase in tone with catch and release; 1+ = slight increase in tone with catch followed by minimal resistance; 2 = more marked increase in tone through most of ROM; 3 = considerable increase in tone, passive movement difficult.
The reduction in MAS scores and resistance torque values indicates reduced spasticity following the intervention. All measured resistance values remained below the device’s safety threshold of 0.5 Nm, confirming appropriate safety margins throughout treatment.
Table 4 shows the measurements of the data obtained both before and after the mechanism of each of the variables was used.

Table 4.
Mean angles of mobility of the patients.
Table 5 shows the results of the normality tests, which indicated that the p value was less than 0.05, suggesting that the data did not follow a normal distribution. Consequently, a nonparametric hypothesis test was applied.

Table 5.
Normality test results of two patients.
To perform the nonparametric analysis, the Wilcoxon test was performed for related samples. This is because the data were analyzed before and after the use of the mechanism in each student.
The results obtained from Table 6 and Table 7 show that the p-value for both students’ wrist movements is less than 0.05. Therefore, it can be concluded that there is a significant difference in the data before and after the prototype is used.

Table 6.
Wilcoxon test for Patient 1.

Table 7.
Wilcoxon test for Patient 2.
Box diagrams corresponding to the different hand movements of the two participants before and after using the device can be observed in the following images, which allow for a clearer visualization of the changes observed after using the mechanism.
When the device was used, there was a small but significant improvement in all the movements of the first student, as demonstrated by an increase in the angles of mobility in all the movements analyzed.
Figure 6, Figure 7, Figure 8, Figure 9, Figure 10 and Figure 11 show box plots visualizing the wrist mobility angles (flexion, extension, ulnar deviation, radial deviation, and supination) of each patient before and after the sessions with the device. After using the prototype, Patient 1′s median flexion went from 37.60° to 40.68° and Patient 2′s median flexion went from 32.49° to 33.87°. The extension went from −33.80° to −30.36° and from 2.11° to 3.84°; the ulnar deviation went from 12.97° to 14.26° and from 8.02° to 9.53°; the radial deviation went from 2.47° to 3.77° and from 5.01° to 6.15°; and the supination went from 9.41° to 11.04° and from 7.07° to 8.44°. In addition, in all cases, the interquartile range after treatment was slightly lower, which means that the device had a more consistent effect and that the extreme outliers that had been present in the prior phase were no longer there. These changes in the graphs support the statistically significant gains observed using the Wilcoxon test (p < 0.001) and show that the prototype works in helping children with spasticity move their wrists more freely.
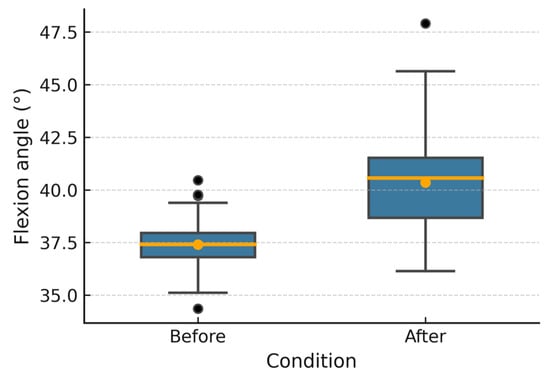
Figure 6.
Patient 1 flexion angle.
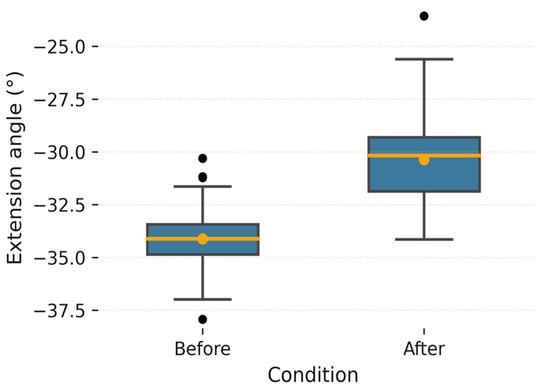
Figure 7.
Patient 1 extension angle. Patient 1 extension angle. The box plot shows the distribution of supination angles before and after the intervention. “The orange dot indicates the mean value, while the black dots represent individual measurements and potential outliers.
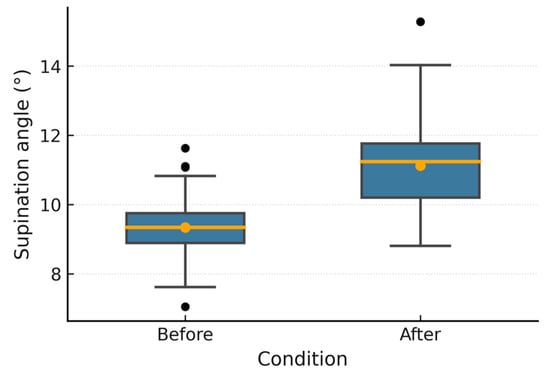
Figure 8.
Patient 1 supination angle.
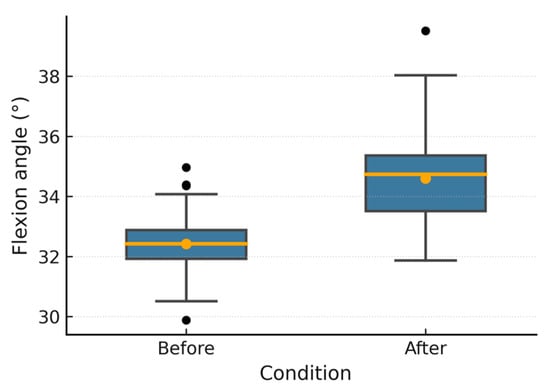
Figure 9.
Patient 2 flexion angle.
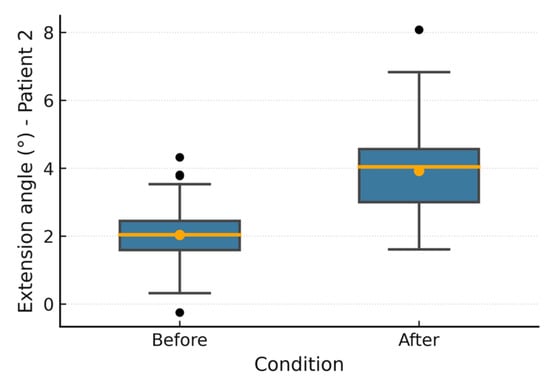
Figure 10.
Patient 2 extension angle.
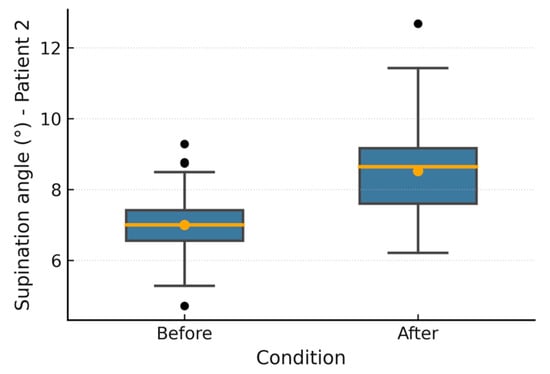
Figure 11.
Patient 2 supination angle.
5. Discussion
For children with spastic hemiparesis, this pilot feasibility study shows that a custom-designed biomechanical gadget can help them move their wrists more freely. There were statistically significant improvements in all movement directions for both subjects. The median improvements ranged from 1.14° to 3.44°, based on the movement and individual. Even though these improvements may not seem significant, they represent major steps forward for children who already had trouble moving around, especially considering that the intervention only lasted for 15 days.
The clinical importance of these changes in range of motion needs to be carefully interpreted in the context of managing stiffness in children. Previous studies have established that improvements of 2–5° in joint mobility can translate into meaningful functional gains in children with cerebral palsy, particularly when combined with occupational therapy interventions [17,23].
The observed improvements of 1.38–3.44° fall within this clinically relevant range, suggesting potential functional benefits.
Comparison with the existing literature reveals both similarities and differences in therapeutic outcomes. Heung et al. reported 3–7° improvements in wrist flexion using soft robotic actuators in adult populations. The pediatric cohort demonstrated improvements of 1.4–3.1° [16], a discrepancy that likely reflects both demographic factors (adult versus pediatric populations) and intervention parameters (daily sessions versus sessions three times weekly). The safety profile of the device also compares favorably with pharmacological treatments: mechanical intervention is reversible and adjustable with immediate cessation capability, whereas botulinum toxin injections carry risks of systemic weakness and require repeated administration.
The current biomechanical device demonstrates several technical constraints that limit its clinical applicability and may influence the interpretation of therapeutic outcomes. The servomotor resolution (0.29° per step) may be insufficient to detect subtle improvements in severely restricted joints, potentially underestimating therapeutic gains in the most severely impaired children [29]. Without real-time force input, it is not possible to adapt to changing muscle tone during treatment sessions, which would allow for better therapy dosing based on how each patient responded [31,41]. Also, the fixed-speed operation (30°/s) does not consider how different people can handle different speeds of movement, which could make it less useful for children who have severe stiffness or low pain limits44. Even though uniformity makes things safer and more consistent, it may not be the best healing stimulation for every person, as shown by the fact that the two cases had different reaction patterns.
The MATLAB–Arduino interface is easy for clinical operators to use, but there is a delay of about 50 to 100 microseconds between order and execution, which could make it harder to control movement precisely during important parts of treatment [43]. This delay may be particularly relevant when attempting to work within the narrow therapeutic window for spastic muscle relaxation [36]. The current system lacks the integration of electromyographic monitoring, preventing the real-time assessment of muscle activation patterns that could guide treatment modifications and optimize therapeutic protocols [39,41]. The mechanical design’s dependence on discrete servo positions limits the smoothness of movement transitions, potentially creating jerky motions that could trigger spastic responses rather than inhibit them [34]. This limitation may explain why some therapeutic gains were modest, as non-physiological movement patterns could activate protective reflexes in children with heightened muscle sensitivity [18,22].
The principal problem with this research is that we cannot definitively conclude whether the device intervention was the sole reason for the observed gains, as there was no control group receiving regular physical therapy. This design option was chosen for this pilot feasibility research to prioritize safety evaluation and protocol modification, ensuring that fragile pediatric participants were exposed to experimental intervention to the least possible extent. The very small number of participants (n = 2) further reduces the reliability and validity of the results. Since the size of the increases surpasses the usual test–retest variability for goniometric measures (±2–3°), the consistent improvements across all movement planes in both subjects indicate a real treatment effect. To determine the relative efficacy of mechanical-assisted vs. conventional manual treatment methods, future randomized controlled studies should include a control group that receives conventional physical therapy. Furthermore, although the findings were encouraging in the short term, the 15-day intervention period was not long enough to answer any questions about how effective the therapy would be in the long run, how long it should last, or whether maintenance would be necessary.
To overcome these technical problems in future versions of the device, higher-resolution servomotors, real-time force and EMG feedback systems, and adaptive velocity control algorithms that change based on each patient’s tolerance and spasticity severity should be added [38,44]. Improved system integration with low-latency interfaces and smooth, continuous motion profiles would make it easier to mimic the way that the body moves naturally. This could allow participants to feel more comfortable and enable the therapy to work better [33,34]. Implementing these technical improvements in the device’s clinical usefulness and treatment accuracy for juvenile spastic hand therapy would be hugely beneficial.
Regarding practical application, the development of the device within Ecuador’s healthcare system could also help other middle-income countries that are having trouble providing sufficient infant therapy equipment. The total cost of development, which includes design, materials, and testing, is only USD 3200, which is less than comparable foreign gadgets that cost USD 15–25,000. The possibility of manufacturing the equipment locally could further reduce costs while building its technical capacity. Obstacles to adoption include a lack of trained staff, repair infrastructure, and health insurance coverage for assistance technologies. To solve these issues, money should be spent on training programs and policies that support new tools for recovery.
These preliminary results show that biomechanical devices may be a good way to help children with weak hands in their recovery, but they also show that larger, more controlled studies are needed for greater certainty. The observed improvements in joint mobility, combined with excellent safety and tolerability profiles, justify advancement to appropriately powered randomized controlled trials, with comprehensive functional outcome assessments and extended follow-up periods, to assess the level of sustained therapeutic benefits and determine optimal implementation strategies.
6. Conclusions
This pilot research demonstrated the potential of a biomechanical device designed to improve wrist mobility in children with spastic hand conditions. Meaningful improvements were observed in both participating children over 15 days of daily therapy sessions. The first child’s movement range improved from 1.29° to 3.44°, and the second child’s movement range improved from 1.14° to 1.73°. These may not look like very high numbers, yet for children who have difficulty with typical hand movements, they represent a substantial improvement. Most significantly, both children finished all the sessions without any problems or pain, which shows that the gadget is safe and well tolerated.
Our device offers several advantages over existing rehabilitation equipment. The construction cost of approximately USD 3200 was found to be significantly lower than imported devices, which can reach USD 25,000, making it more accessible for healthcare facilities in Ecuador. The device’s adjustable design accommodates wrist circumferences from 10 to 16 cm and forearm lengths from 15 to 25 cm, covering the anthropometric range of children aged 6–12 years, as validated in this study, and the settings can be modified for each child’s needs by therapists through the computer interface. Therapy standardization is facilitated by the provision of consistent, controlled movements at safe speeds, while additional time is provided for therapists to focus on other treatment aspects.
However, it is recognized that the study’s conclusions are limited the testing, including only two children. To truly understand how effective this device is, future research needs to involve many more children; at least 30 would provide more reliable results. These studies should compare outcomes between children using the device and those receiving standard physical therapy. Wrist angle measurements are useful, but they do not reveal everything. Is it possible for children to handle a pencil better, eat with utensils, or play with their friends following treatment? Several months of observation would also show whether the improvements that these children are experiencing are long-lasting or whether they require ongoing therapy.
The device’s efficacy could be enhanced by implementing several upgrades. Real-time adaptation to each child’s condition could be achieved through the addition of sensors that detect muscle resistance levels. The detection of smaller improvements, which is particularly important in severely affected children, could be facilitated by enhanced motor precision. To make regular treatment more accessible, it could be possible to create portable versions suitable for use at home. Children in Ecuador and Latin America may benefit from this technology if local manufacturing capabilities are developed, therapist training programs are formed, and health institutions work together to provide insurance coverage.
The next step is to compare the gadget with standard physical therapy in a larger group (n ≥ 30 per group) in a randomized controlled experiment. Along with goniometric measures, this experiment should also include standardized functional evaluations such as the QUEST, AHA, and COPM (Canadian Occupational Performance Measure). The sustainability of benefits and appropriate maintenance treatment regimens may be determined through long-term follow-up at 3, 6, and 12 months.
Author Contributions
Conceptualization, P.S.V.-O., J.L.J.-T., L.G.S.-V., C.R.P.-O., D.V.I.B., and J.C.T.P.; methodology, P.S.V.-O., J.L.J.-T., L.G.S.-V., C.R.P.-O., and D.V.I.B.; software, P.S.V.-O., J.L.J.-T., L.G.S.-V., C.R.P.-O., D.V.I.B., and J.C.T.P.; validation, P.S.V.-O., J.L.J.-T., L.G.S.-V., C.R.P.-O., and D.V.I.B.; formal analysis, P.S.V.-O., J.L.J.-T., L.G.S.-V., C.R.P.-O., and D.V.I.B.; investigation, P.S.V.-O., J.L.J.-T., L.G.S.-V., C.R.P.-O., and D.V.I.B.; resources, P.S.V.-O., J.L.J.-T., L.G.S.-V., C.R.P.-O., and D.V.I.B.; data curation, P.S.V.-O., and L.G.S.-V.; writing—original draft preparation, L.G.S.-V.; writing—review and editing, P.S.V.-O., L.G.S.-V., and C.R.P.-O.; visualization, J.L.J.-T., and C.R.P.-O.; supervision, P.S.V.-O., J.L.J.-T., L.G.S.-V., C.R.P.-O., and D.V.I.B.; project administration, J.L.J.-T., and J.C.T.P.; funding acquisition, P.S.V.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the Ministry of Education of Ecuador through the Zonal Coordination of Education District 06D01-Chambo-Riobamba (Official Document No. MINEDUC-CZ3-06D01-2023-5486-O, dated 31 July 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. They are not publicly available because the participants are minors and the information is sensitive.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- McIntyre, S.; Goldsmith, S.; Webb, A.; Ehlinger, V.; Hollung, S.J.; McConnell, K.; Arnaud, C.; Smithers-Sheedy, H.; Oskoui, M.; Khandaker, G.; et al. Global Prevalence of Cerebral Palsy: A Systematic Analysis. Dev. Med. Child Neuro 2022, 64, 1494–1506. [Google Scholar] [CrossRef]
- Norton, J.; Sawicka, K. The Significance of Hand Movement Mirroring in Cerebral Palsy. Dev. Med. Child Neurol. 2016, 58, 655–656. [Google Scholar] [CrossRef]
- Pool, E.-M. Systems Biology Determinants of Motor Behavior in Humans. Ph.D. Thesis, Universität zu Köln, Cologne, Germany, 2015. [Google Scholar]
- Solodkin, A.; Hlustik, P.; Buccino, G. The Anatomy and Physiology of the Motor System in Humans. In Handbook of Psychophysiology; Cacioppo, J.T., Tassinary, L.G., Berntson, G., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 507–539. ISBN 978-0-511-54639-6. [Google Scholar]
- Bobath, K.; Bobath, B. The Importance of Memory Traces of Motor Efferent Discharges for Learning Skilled Movements. Dev. Med. Child. Neurol. 1974, 16, 620–628. [Google Scholar] [CrossRef]
- Chelette, T.L.; Repperger, D.W.; Phillips, C.A. Pattern Recognition of Spastic Motion. In Proceedings of the 1996 Fifteenth Southern Biomedical Engineering Conference, Dayton, OH, USA, 29–31 October 1996; pp. 423–426. [Google Scholar]
- Planinšec, J.; Pisot, R. Motor Coordination and Intelligence Level in Adolescents. Adolescence 2005, 41, 667–676. [Google Scholar]
- Piscitelli, D. Neurorehabilitation: Bridging Neurophysiology and Clinical Practice. Neurol. Sci. 2019, 40, 2209–2211. [Google Scholar] [CrossRef]
- Averta, G. A Novel Mechatronic System for Evaluating Elbow Muscular Spasticity Relying on Tonic Stretch Reflex Threshold Estimation. In Human-Aware Robotics: Modeling Human Motor Skills for the Design, Planning and Control of a New Generation of Robotic Devices; Averta, G., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 127–139. ISBN 978-3-030-92521-5. [Google Scholar]
- Joe, A.; Dickins, M.; Enticott, J.; Ogrin, R.; Lowthian, J. Community-Dwelling Older Women: The Association Between Living Alone and Use of a Home Nursing Service. J. Am. Med. Dir. Assoc. 2020, 21, 1273–1281.e2. [Google Scholar] [CrossRef]
- Shehata, A.W.; Rehani, M.; Jassat, Z.E.; Hebert, J.S. Mechanotactile Sensory Feedback Improves Embodiment of a Prosthetic Hand During Active Use. Front. Neurosci. 2020, 14, 263. [Google Scholar] [CrossRef] [PubMed]
- Heung, K.H.L.; Li, H.; Wong, T.W.L.; Ng, S.S.M. Assistive Robotic Hand with Bi-Directional Soft Actuator for Hand Impaired Patients. Front. Bioeng. Biotechnol. 2023, 11, 1188996. [Google Scholar] [CrossRef] [PubMed]
- Rout, B.K.; Prasanth, C.S. Modified Wrist Driven Flexor Hinge Splint for C6 Quadriplegic Patients. Int. J. Health. Sci. Res. 2020, 10, 260–263. [Google Scholar]
- Leng, Y.; Lo, W.L.A.; Hu, C.; Bian, R.; Xu, Z.; Shan, X.; Huang, D.; Li, L. The Effects of Extracorporeal Shock Wave Therapy on Spastic Muscle of the Wrist Joint in Stroke Survivors: Evidence From Neuromechanical Analysis. Front. Neurosci. 2021, 14, 580762. [Google Scholar] [CrossRef]
- Villalobos-Villegas, J.; Carrasquilla-Batista, A.; Vílchez-Monge, M.; Ortiz-León, G. Low Cost Mechatronic Device for Hand Grip Function Rehabilitation. In Proceeding of the 2022 8th International Engineering, Sciences and Technology Conference, Panama City, Panama, 19–21 October 2022; pp. 659–664. [Google Scholar]
- Akhbari, B.; Moore, D.C.; Laidlaw, D.H.; Weiss, A.C.; Akelman, E.; Wolfe, S.W.; Crisco, J.J. Predicting Carpal Bone Kinematics Using an Expanded Digital Database of Wrist Carpal Bone Anatomy and Kinematics. J. Orthop. Res. 2019, 37, 2661–2670. [Google Scholar] [CrossRef]
- Clewes, K.; Hammond, C.; Dong, Y.; Meyer, M.; Lowe, E.; Rose, J. Neuromuscular Impairments of Cerebral Palsy: Contributions to Gait Abnormalities and Implications for Treatment. Front. Hum. Neurosci. 2024, 18, 1445793. [Google Scholar] [CrossRef]
- Willaert, J.; Ting, L.H.; Van Campenhout, A.; Desloovere, K.; De Groote, F. Reduced Reciprocal Inhibition during Clinical Tests of Spasticity Is Associated with Impaired Reactive Standing Balance Control in Children with Cerebral Palsy. medRxiv 2023. [Google Scholar] [CrossRef]
- Edmonds, E.W.; Santago, A.C.; Saul, K.R. Functional Loss With Displacement of Medial Epicondyle Humerus Fractures: A Computer Simulation Study. J. Pediatr. Orthop. 2015, 35, 666–671. [Google Scholar] [CrossRef]
- Crisco, J.J.; Heard, W.M.; Rich, R.R.; Paller, D.J.; Wolfe, S.W. The Mechanical Axes of the Wrist Are Oriented Obliquely to the Anatomical Axes. J. Bone Jt. Surg. 2011, 93, 169–177. [Google Scholar] [CrossRef]
- Roda-Sales, A.; Jarque-Bou, N.J.; Bayarri-Porcar, V.; Gracia-Ibáñez, V.; Sancho-Bru, J.L.; Vergara, M. Electromyography and Kinematics Data of the Hand in Activities of Daily Living with Special Interest for Ergonomics. Sci. Data. 2023, 10, 814. [Google Scholar] [CrossRef]
- Graci, V.; O’Neill, M.; Bloss, M.; Akkem, R.; Paremski, A.C.; Sanders, O.; Prosser, L.A. A New Methodological Approach to Characterize Selective Motor Control in Children with Cerebral Palsy. Front. Hum. Neurosci. 2024, 18, 1330315. [Google Scholar] [CrossRef]
- Hedberg-Graff, J.; Granström, F.; Arner, M.; Krumlinde-Sundholm, L. Upper-limb Contracture Development in Children with Cerebral Palsy: A Population-based Study. Dev. Med. Child Neuro 2019, 61, 204–211. [Google Scholar] [CrossRef]
- Shuman, B.R.; Goudriaan, M.; Desloovere, K.; Schwartz, M.H.; Steele, K.M. Associations Between Muscle Synergies and Treatment Outcomes in Cerebral Palsy Are Robust Across Clinical Centers. Arch. Phys. Med. Rehabil. 2018, 99, 2175–2182. [Google Scholar] [CrossRef]
- Kachmar, O.O.; Kozyavkina, N.V.; Kushnir, A.D.; Kozyavkina, O.V. Neuroplasticity in Rehabilitation of Children with Cerebral Palsy. Int. Neurol. J. 2025, 21, 52–59. [Google Scholar] [CrossRef]
- Morgan, C.; Novak, I.; Dale, R.C.; Badawi, N. Optimising Motor Learning in Infants at High Risk of Cerebral Palsy: A Pilot Study. BMC Pediatr. 2015, 15, 30. [Google Scholar] [CrossRef]
- Reissner, L.; Fischer, G.; List, R.; Giovanoli, P.; Calcagni, M. Assessment of Hand Function during Activities of Daily Living Using Motion Tracking Cameras: A Systematic Review. Proc. Inst. Mech. Eng. H 2019, 233, 764–783. [Google Scholar] [CrossRef]
- Cavallo, F.; Mohn, A.; Chiarelli, F.; Giannini, C. Evaluation of Bone Age in Children: A Mini-Review. Front. Pediatr. 2021, 9, 580314. [Google Scholar] [CrossRef]
- Gonzalez, A.; Garcia, L.; Kilby, J.; McNair, P. Robotic Devices for Paediatric Rehabilitation: A Review of Design Features. Biomed. Eng. Online 2021, 20, 89. [Google Scholar] [CrossRef]
- Glinsky, J.A.; Harvey, L.A.; Ben, M. A New Clinical Device for Measuring Wrist Strength in People with Tetraplegia. Physiother. Theory Pract. 2010, 26, 342–346. [Google Scholar] [CrossRef]
- Brahmi, B.; Laraki, M.H.; Saad, M.; Rahman, M.H.; Ochoa-Luna, C.; Brahmi, A. Compliant Adaptive Control of Human Upper-Limb Exoskeleton Robot with Unknown Dynamics Based on a Modified Function Approximation Technique (MFAT). Robot. Auton. Syst. 2019, 117, 92–102. [Google Scholar] [CrossRef]
- Li, X.; Yang, Q.; Song, R. Performance-Based Hybrid Control of a Cable-Driven Upper-Limb Rehabilitation Robot. IEEE Trans. Biomed. Eng. 2021, 68, 1351–1359. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Z.; Yang, P.; Yu, S. ChMER: An Exoskeleton Robot with Active Body Weight Support Walker Based on Compliant Actuation for Children with Cerebral Palsy. Front. Bioeng. Biotechnol. 2025, 13, 1551039. [Google Scholar] [CrossRef]
- Firouzi, V.; Seyfarth, A.; Song, S.; Von Stryk, O.; Ahmad Sharbafi, M. Biomechanical Models in the Lower-Limb Exoskeletons Development: A Review. J. Neuroeng. Rehabil. 2025, 22, 12. [Google Scholar] [CrossRef]
- Goo, A.C.; Laubscher, C.A.; Wajda, D.A.; Sawicki, J.T. Preliminary Virtual Constraint-Based Control Evaluation on a Pediatric Lower-Limb Exoskeleton. Bioengineering 2024, 11, 590. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Wang, R.; Smith, C.; Gutierrez-Farewik, E.M. Modeling and Simulation of a Human Knee Exoskeleton’s Assistive Strategies and Interaction. Front. Neurorobot. 2021, 15, 620928. [Google Scholar] [CrossRef]
- Li, X.; Pan, Y.; Chen, G.; Yu, H. Multi-Modal Control Scheme for Rehabilitation Robotic Exoskeletons. Int. J. Robot. Res. 2017, 36, 759–777. [Google Scholar] [CrossRef]
- Yu, H.; Huang, S.; Chen, G.; Pan, Y.; Guo, Z. Human–Robot Interaction Control of Rehabilitation Robots With Series Elastic Actuators. IEEE Trans. Robot. 2015, 31, 1089–1100. [Google Scholar] [CrossRef]
- Jutinico, A.L.; Jaimes, J.C.; Escalante, F.M.; Perez-Ibarra, J.C.; Terra, M.H.; Siqueira, A.A.G. Impedance Control for Robotic Rehabilitation: A Robust Markovian Approach. Front. Neurorobot. 2017, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chen, Y. Development of an Intention-Based Adaptive Neural Cooperative Control Strategy for Upper-Limb Robotic Rehabilitation. IEEE Robot. Autom. Lett. 2021, 6, 335–342. [Google Scholar] [CrossRef]
- Wang, L.; Du, Z.; Dong, W.; Shen, Y.; Zhao, G. Hierarchical Human Machine Interaction Learning for a Lower Extremity Augmentation Device. Int. J. Soc. Robot. 2019, 11, 123–139. [Google Scholar] [CrossRef]
- Snyder, R.G.; Schneider, L.W.; Owings, C.L.; Reynolds, H.M.; Golomb, D.H.; Schork, M.A. Anthropometry of Infants, Children, and Youths to Age 18 for Product Safety Design; Final Report. Contract CPSC-C-75-0068; Highway Safety Research Institute, University of Michigan: Ann Arbor, MI, USA, 1977; p. 628. [Google Scholar]
- Wu, Q.; Wu, H. Development, Dynamic Modeling, and Multi-Modal Control of a Therapeutic Exoskeleton for Upper Limb Rehabilitation Training. Sensors 2018, 18, 3611. [Google Scholar] [CrossRef]
- Zimmermann, Y.; Sommerhalder, M.; Wolf, P.; Riener, R.; Hutter, M. ANYexo 2.0: A Fully Actuated Upper-Limb Exoskeleton for Manipulation and Joint-Oriented Training in All Stages of Rehabilitation. IEEE Trans. Robot. 2023, 39, 2131–2150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).