Support Surface Chemistry Evolution During the Preparation of Metal Oxide–Activated Carbon Catalysts by Wet Impregnation: A FT-IR Spectroscopy Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of the MO/AC Samples
2.3. Characterisation of the MO/AC Samples
3. Results and Discussion
3.1. Preparation of the MO/AC Samples
3.1.1. Yield
3.1.2. Elemental Analysis
3.2. Characterisation of the Surface Chemistry of the MO/AC Samples
3.2.1. FT-IR Spectroscopy
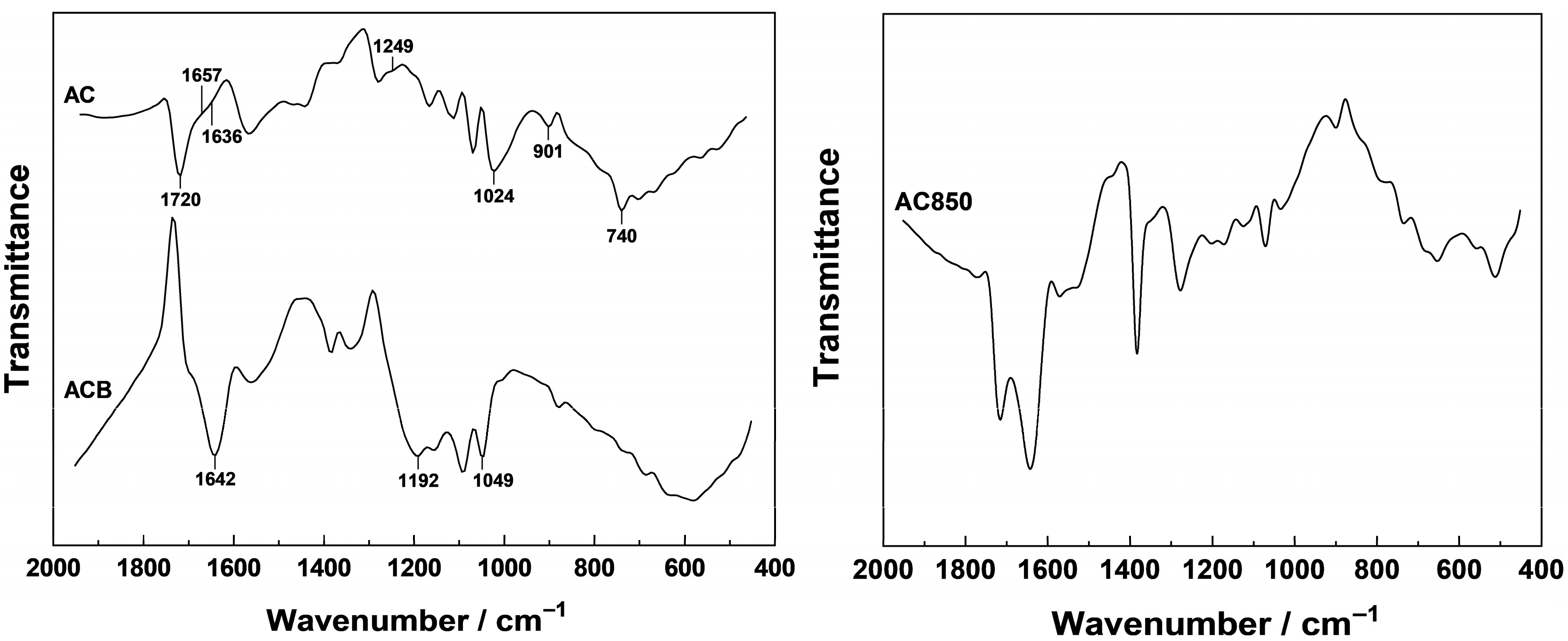
AC and “Blank” Samples
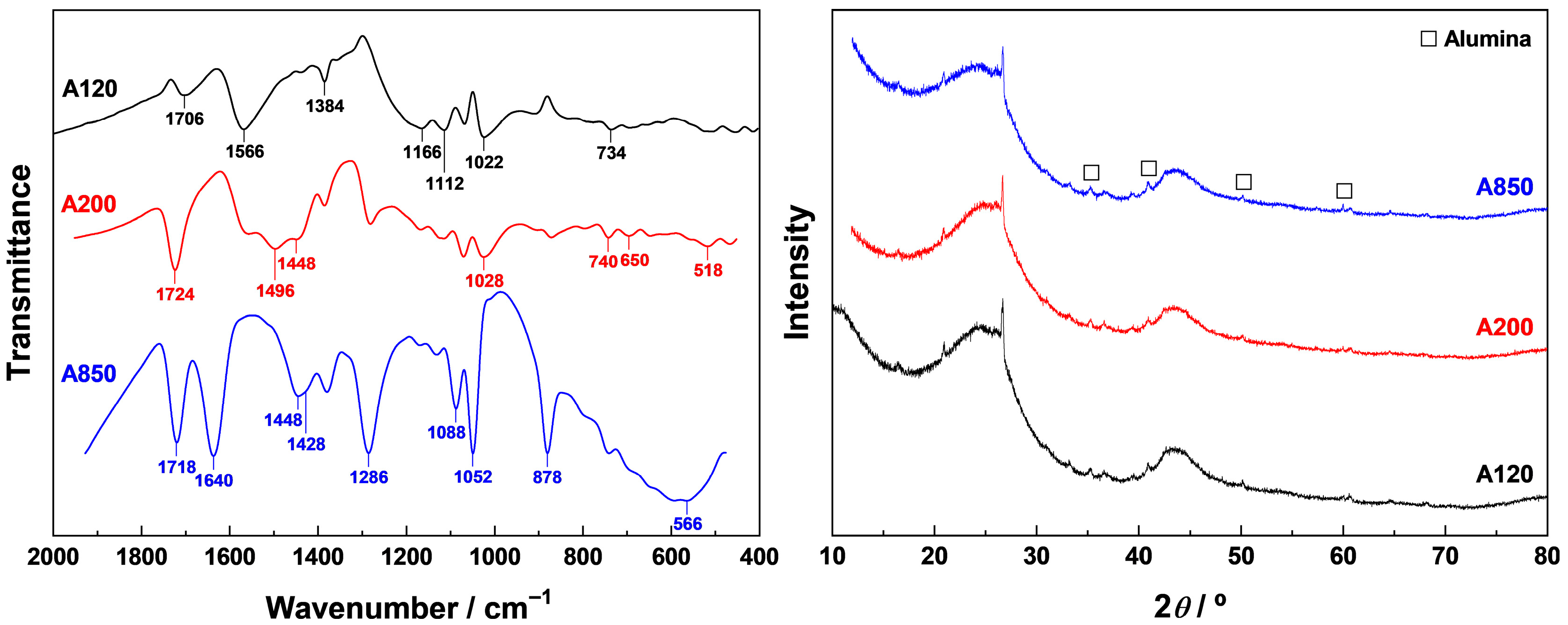
Samples AT
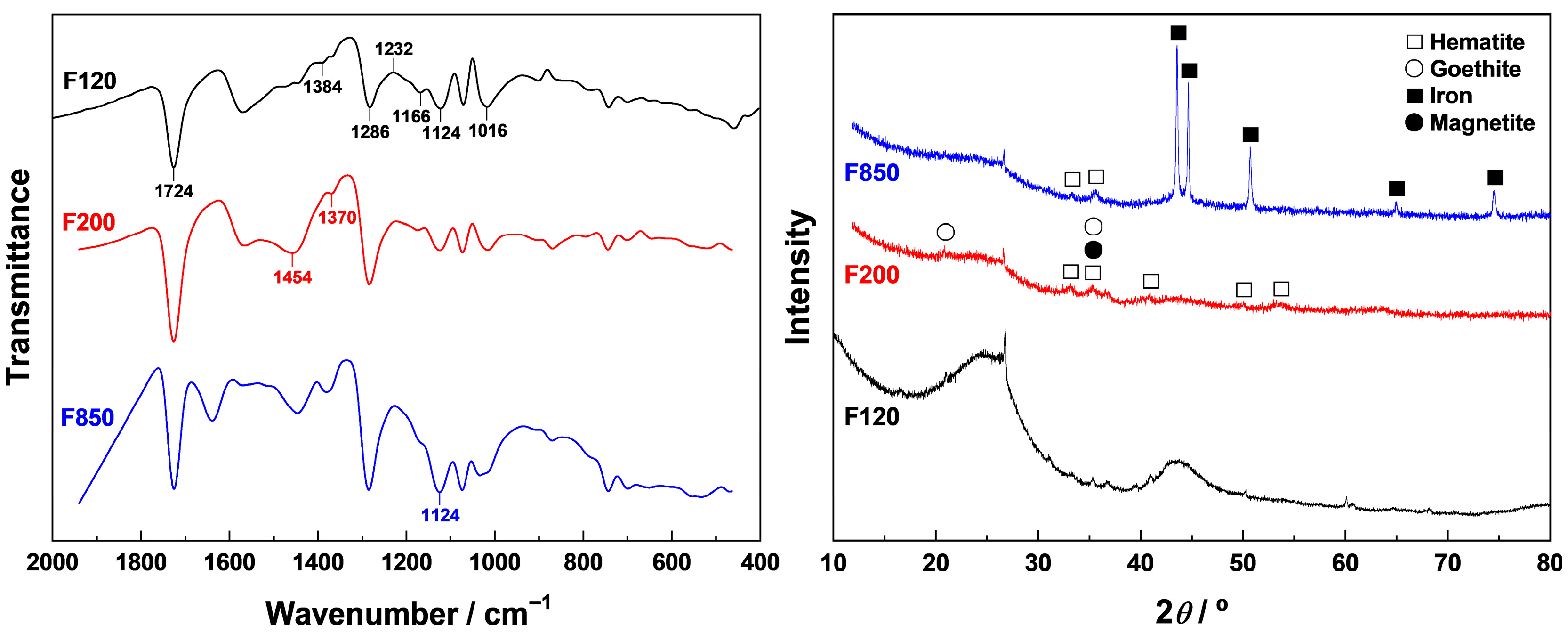
Samples FT
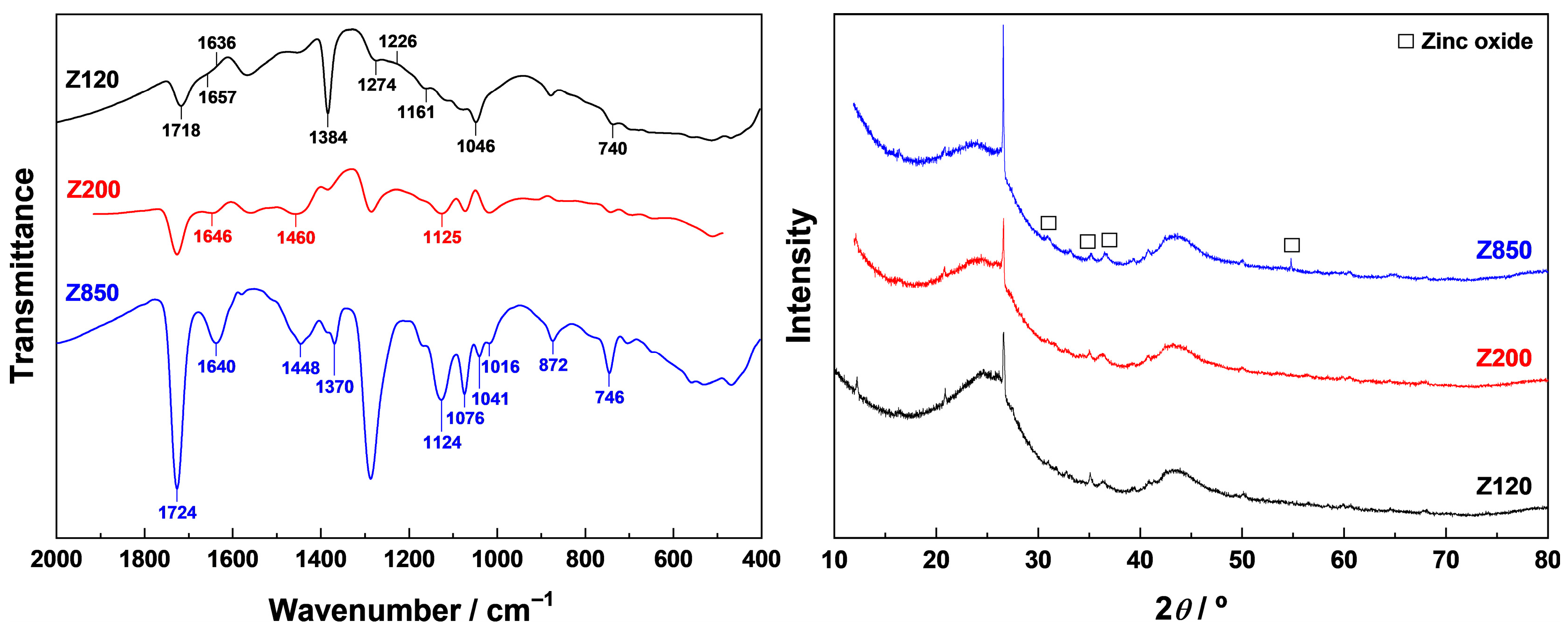
Samples ZT
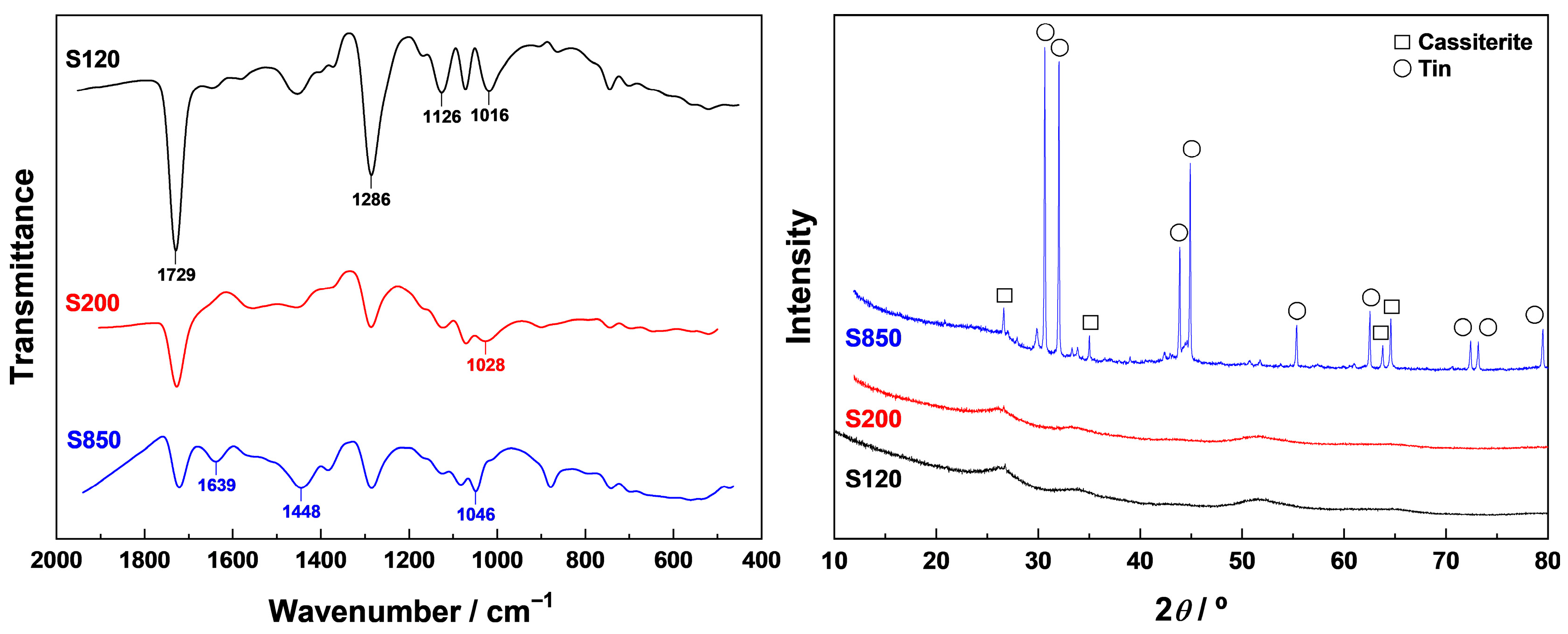
Samples ST
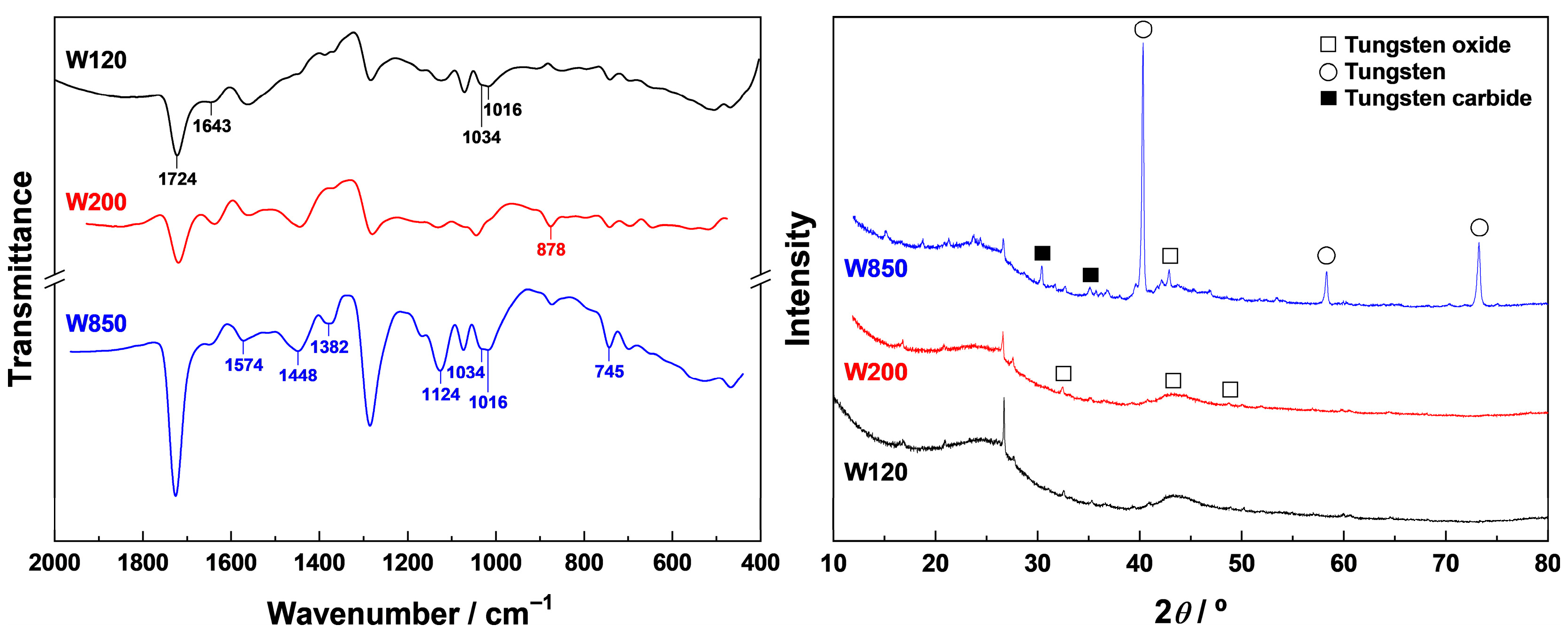
Samples WT
3.2.2. pH of the Point of Zero Charge
4. Conclusions
- The impregnation of AC and subsequent oven-drying in the preparation of the S1 samples usually result in the formation of phenolic hydroxyl and carboxylic acid groups by oxidation of chromene, pyrone, and ether-type structures initially present in the surface of the raw support. The extent of these oxidations fairly well correlated with the oxidising power of the precursor aqueous solutions, particularly those prepared from metal nitrate and SnCl2 salts.
- The chemical changes undergone by the AC surface as a result of the heat treatment of the S1 samples at 200 °C or 850 °C under an inert atmosphere are markedly stronger for the higher temperature. In the case of the S3 samples, a drastic restructuring of the carbon surface was observed, leading to the formation of carboxylic acid groups, 4-pyrone groups, coordinated metal carboxylates, and C-O-M atomic groupings. In addition, carbothermal reduction reactions also occurred, further influencing both the surface chemistry and the evolution of the supported metal phases.
- The chemical transformations observed in the AC support surface because of the preparation of the S1 samples have been essentially attributed to the oxidising action of the different chemical species present in the impregnation solution or generated during the soaking step, as well as to the behaviour of the supported MOs as oxidation catalysts. Upon subsequent heat treatment at 200 °C or 850 °C, the chemical modifications have been associated with the presence of chemisorbed oxygen in the oven-dried samples, together with the carbothermal reduction of MOs and other thermal effects taking place at high temperatures.
- Although all the employed metal precursors significantly modified the AC surface chemistry, clear differences were observed. Fe(NO3)3 and SnCl2 precursors caused the strongest oxidative effects, Zn(NO3)2 induced comparatively milder modifications, Al(NO3)3 likely favoured the formation of 4-pyrone structures at high temperature, and Na2WO4 promoted both oxidation and carburisation. Overall, these differences highlight the pivotal role of the metal precursor in determining the evolution of the surface chemistry of the carbon support during the preparation of MO/AC catalysts.
- The pHpzc values as a rule decreased markedly upon impregnation and oven-drying at 120 °C, with the extent depending on the precursor salt, while they underwent a notable increase when heating at 200 °C and especially 850 °C. These trends reflect progressive chemical changes in oxygen-containing surface functionalities, particularly in the chemical state of carboxylic acid groups.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Védrine, J.C. Heterogeneous Catalysis on Metal Oxides. Catalysts 2017, 7, 341. [Google Scholar] [CrossRef]
- Védrine, J.C. (Ed.) Metal Oxides in Heterogeneous Catalysis; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-811631-9. [Google Scholar]
- Védrine, J.C. Importance, Features and Uses of Metal Oxide Catalysts in Heterogeneous Catalysis. Chin. J. Catal. 2019, 40, 1627–1636. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. Activated Carbon; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 9780080444635. [Google Scholar]
- Rodríguez-Reinoso, F.; Sepúlveda-Escribano, A. Carbon as Catalyst Support. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 131–155. ISBN 9780470178850. [Google Scholar]
- Barroso-Bogeat, A.; Fernández-González, C.; Alexandre-Franco, M.; Gómez-Serrano, V. Activated Carbon as a Metal Oxide Support: A Review. In Activated Carbon: Classifications, Properties and Applications; Kwiatkowski, J.F., Ed.; Nova Science Publishers: New York, NY, USA, 2012; pp. 297–318. ISBN 9781612096841. [Google Scholar]
- Matos, I.; Bernardo, M.; Fonseca, I. Porous Carbon: A Versatile Material for Catalysis. Catal. Today 2017, 285, 194–203. [Google Scholar] [CrossRef]
- Bahuguna, A.; Kumar, A.; Krishnan, V. Carbon-Support-Based Heterogeneous Nanocatalysts: Synthesis and Applications in Organic Reactions. Asian J. Org. Chem. 2019, 8, 1263–1305. [Google Scholar] [CrossRef]
- Iwanow, M.; Gärtner, T.; Sieber, V.; König, B. Activated Carbon as Catalyst Support: Precursors, Preparation, Modification and Characterization. Beilstein J. Org. Chem. 2020, 16, 1188–1202. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, S.; Kumar, A.; Lai, C.W.; Naushad, M.; Shehnaz; Iqbal, J.; Stadler, F.J. Activated Carbon as Superadsorbent and Sustainable Material for Diverse Applications. Adsorpt. Sci. Technol. 2022, 2022, 4184809. [Google Scholar] [CrossRef]
- Auer, E.; Freund, A.; Pietsch, J.; Tacke, T. Carbons as Supports for Industrial Precious Metal Catalysts. Appl. Catal. A Gen. 1998, 173, 259–271. [Google Scholar] [CrossRef]
- Yang, Y.; Chiang, K.; Burke, N. Porous Carbon-Supported Catalysts for Energy and Environmental Applications: A Short Review. Catal. Today 2011, 178, 197–205. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F. The Role of Carbon Materials in Heterogeneous Catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Perego, C.; Villa, P. Catalyst Preparation Methods. Catal. Today 1997, 34, 281–305. [Google Scholar] [CrossRef]
- Barroso-Bogeat, A.; Alexandre-Franco, M.; Fernández-González, C.; Gómez-Serrano, V. Preparation of Activated Carbon-Metal Oxide Hybrid Catalysts: Textural Characterization. Fuel Process. Technol. 2014, 126, 95–103. [Google Scholar] [CrossRef]
- Barroso-Bogeat, A.; Alexandre-Franco, M.; Fernández-González, C.; Gómez-Serrano, V. Preparation and Microstructural Characterization of Activated Carbon-Metal Oxide Hybrid Catalysts: New Insights into Reaction Paths. J. Mater. Sci. Technol. 2015, 31, 806–814. [Google Scholar] [CrossRef]
- Barroso-Bogeat, A.; Alexandre-Franco, M.; Fernández-González, C.; Gómez-Serrano, V. FT-IR Analysis of Pyrone and Chromene Structures in Activated Carbon. Energy Fuels 2014, 28, 4096–4103. [Google Scholar] [CrossRef]
- Newcombe, G.; Hayes, R.; Drikas, M. Granular Activated Carbon: Importance of Surface Properties in the Adsorption of Naturally Occurring Organics. Colloids Surf. A Physicochem. Eng. Asp. 1993, 78, 65–71. [Google Scholar] [CrossRef]
- Lopez-Ramon, M.V.; Stoeckli, F.; Moreno-Castilla, C.; Carrasco-Marin, F. On the Characterization of Acidic and Basic Surface Sites on Carbons by Various Techniques. Carbon 1999, 37, 1215–1221. [Google Scholar] [CrossRef]
- Barroso-Bogeat, A.; Alexandre-Franco, M.; Fernández-González, C.; Gómez-Serrano, V. Preparation of Activated Carbon-Metal (Hydr)Oxide Photocatalysts under Different Heating Conditions. Chemical Aspects. Bol. Grupo Esp. Carbón 2016, 42, 22–26. [Google Scholar]
- Barroso-Bogeat, A.; Alexandre-Franco, M.; Fernández-González, C.; Gómez-Serrano, V. Activated Carbon Surface Chemistry: Changes upon Impregnation with Al(III), Fe(III) and Zn(II)-Metal Oxide Catalyst Precursors from NO3− Aqueous Solutions. Arab. J. Chem. 2019, 12, 3963–3976. [Google Scholar] [CrossRef]
- Gurrath, M.; Kuretzky, T.; Boehm, H.P.; Okhlopkova, L.B.; Lisitsyn, A.S.; Likholobov, V.A. Palladium Catalysts on Activated Carbon Supports. Influence of Reduction Temperature, Origin of the Support and Pretreatments of the Carbon Surface. Carbon 2000, 38, 1241–1255. [Google Scholar] [CrossRef]
- Baes, C.F.; Mesmer, R.S. The Hydrolysis of Cations; John Wiley & Sons: New York, NY, USA, 1976. [Google Scholar]
- Brown, P.L.; Ekberg, C. Hydrolysis of Metal Ions; Wiley: Hoboken, NJ, USA, 2016; ISBN 9783527330102. [Google Scholar]
- Barroso-Bogeat, A.; Alexandre-Franco, M.; Fernández-González, C.; Gómez-Serrano, V. Preparation of Activated Carbon-Metal (Hydr)Oxide Materials by Thermal Methods. Thermogravimetric-Mass Spectrometric (TG-MS) Analysis. J. Anal. Appl. Pyrolysis 2015, 116, 243–252. [Google Scholar] [CrossRef]
- Barroso-Bogeat, A.; Alexandre-Franco, M.; Fernández-González, C.; Macías-García, A.; Gómez-Serrano, V. Preparation of Activated Carbon-SnO2, TiO2, and WO3 Catalysts. Study by FT-IR Spectroscopy. Ind. Eng. Chem. Res. 2016, 55, 5200–5206. [Google Scholar] [CrossRef]
- Nakanishi, K.; Solomon, P.H. Infrared Absorption Spectroscopy, 2nd ed.; Solomon, P.H., Ed.; Holden-Day: San Francisco, CA, USA, 1977. [Google Scholar]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- González-Domínguez, J.M.; Fernández-González, C.; Alexandre-Franco, M.; Gómez-Serrano, V. Surface Chemistry of Cherry Stone-Derived Activated Carbon Prepared by H3PO4 Activation. Processes 2024, 12, 149. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds. Tables of Spectral Data; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Buckó, Á.; Kutus, B.; Peintler, G.; Kele, Z.; Pálinkó, I.; Sipos, P. Stability and Structural Aspects of Complexes Forming Between Aluminum(III) and D-Heptagluconate in Acidic to Strongly Alkaline Media: An Unexpected Diversity. J. Mol. Liq. 2020, 314, 113645. [Google Scholar] [CrossRef]
- Gam, S.; Maghrebi, K.; Alkhaldi, S.; Khemiri, N.; Abderrabba, M.; Messaoudi, S. Exploration of the Mechanism of the Condensation Reaction of Al(OH)4− with a D-Gluconate Using Density Functional Theory. Struct. Chem. 2023, 34, 1759–1773. [Google Scholar] [CrossRef]
- Martin, D.S.; Cole, R.J.; Haq, S. Creating a Functionalized Surface: The Adsorption of Terephthalic Acid onto Cu(110). Phys. Rev. B Condens. Matter Mater. Phys. 2002, 66, 155427. [Google Scholar] [CrossRef]
- Martin, D.S.; Cole, R.J.; Haq, S. Investigating the Adsorption of Oxalic Acid onto Cu(110) to Create a Chemically Functionalised Surface. Surf. Sci. 2003, 539, 171–181. [Google Scholar] [CrossRef]
- van den Brand, J.; Blajiev, O.; Beentjes, P.C.J.; Terryn, H.; de Wit, J.H.W. Interaction of Anhydride and Carboxylic Acid Compounds with Aluminum Oxide Surfaces Studied Using Infrared Reflection Absorption Spectroscopy. Langmuir 2004, 20, 6308–6317. [Google Scholar] [CrossRef] [PubMed]
- Curtis, N.F.; Curtis, Y.M. Some Nitrato-Amine Nickel(II) Compounds with Monodentate and Bidentate Nitrate Ions. Inorg. Chem. 1965, 4, 804–809. [Google Scholar] [CrossRef]
- Grube, M.; Lin, J.G.; Lee, P.H.; Kokorevicha, S. Evaluation of Sewage Sludge-Based Compost by FT-IR Spectroscopy. Geoderma 2006, 130, 324–333. [Google Scholar] [CrossRef]
- Mihaylov, M.Y.; Zdravkova, V.R.; Ivanova, E.Z.; Aleksandrov, H.A.; St. Petkov, P.; Vayssilov, G.N.; Hadjiivanov, K.I. Infrared Spectra of Surface Nitrates: Revision of the Current Opinions Based on the Case Study of Ceria. J. Catal. 2021, 394, 245–258. [Google Scholar] [CrossRef]
- Kiss, A.B.; Keresztury, G.; Farkas, L. Raman and i.r. Spectra and Structure of Boehmite (y-AlOOH). Evidence for the Recently Discarded D172h Space Group. Spectrochim. Acta 1980, 36, 653–658. [Google Scholar] [CrossRef]
- Music, S.; Dragcevic, O.; Popovic, S. Hydrothermal Crystallization of Boehmite from Freshly Precipitated Aluminium Hydroxide. Mater. Lett. 1999, 40, 269–274. [Google Scholar] [CrossRef]
- Ram, S. Infrared Spectral Study of Molecular Vibrations in Amorphous, Nanocrystalline and AlO(OH)·aH2O Bulk Crystals. Infrared Phys. Technol. 2001, 42, 547–560. [Google Scholar] [CrossRef]
- Ruan, H.D.; Frost, R.L.; Kloprogge, J.T.; Duong, L. Far-Infrared Spectroscopy of Alumina Phases. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2002, 58, 265–272. [Google Scholar] [CrossRef]
- Dickie, S.A.; McQuillan, A.J. In-Situ Infrared Spectroscopic Studies of Adsorption Processes on Boehmite Particle Films: Exchange of Surface Hydroxyl Groups Observed upon Chelation by Acetylacetone. Langmuir 2004, 20, 11630–11636. [Google Scholar] [CrossRef]
- Feng, Y.; Lu, W.; Zhang, L.; Bao, X.; Yue, B.; Iv, Y.; Shang, X. One-Step Synthesis of Hierarchical Cantaloupe-like AlOOH Superstructures via a Hydrothermal Route. Cryst. Growth Des. 2008, 8, 1426–1429. [Google Scholar] [CrossRef]
- Boumaza, A.; Favaro, L.; Lédion, J.; Sattonnay, G.; Brubach, J.B.; Berthet, P.; Huntz, A.M.; Roy, P.; Tétot, R. Transition Alumina Phases Induced by Heat Treatment of Boehmite: An X-Ray Diffraction and Infrared Spectroscopy Study. J. Solid. State Chem. 2009, 182, 1171–1176. [Google Scholar] [CrossRef]
- Rahimi-Tabar, F.; Hosseini-Monfared, H. Low Temperature Synthesis of Nanosized Boehmite Powder from Sodium Aluminate by Template-Free Hydrothermal Method. Next Mater. 2025, 7, 100456. [Google Scholar] [CrossRef]
- Davydov, A.A. Infrared Spectroscopy of Adsorbed Species on the Surface of Transition Metal Oxides; John Wiley & Sons: Chichester, UK, 1990. [Google Scholar]
- Mukha, S.A.; Antipova, I.A.; Medvedeva, S.A.; Saraev, V.V.; Larina, L.I.; Tsyrenzhapov, A.V.; Sukhov, B.G. Synthesis and Properties of Metal Chelates Based on Natural γ-Pyrone Maltol. Chem. Sustain. Dev. 2007, 15, 448–458. [Google Scholar]
- Shevchenko, L.L. Infrared Spectra of Salts and Complexes of Carboxylic Acids and Some of Their Derivatives. Russ. Chem. Rev. 1963, 32, 201–207. [Google Scholar] [CrossRef]
- Obydennov, D.L.; Röschenthaler, G.-V.; Sosnovskikh, V.Y. An Improved Synthesis and Some Reactions of Diethyl 4-Oxo-4H-Pyran-2,5-Dicarboxylate. Tetrahedron Lett. 2013, 54, 6545–6548. [Google Scholar] [CrossRef]
- Bankauskaite, A.; Baltakys, K. The Hydrothermal Synthesis of Hydrotalcite by Using Different Partially Soluble and Insoluble in Water Mangesium and Aluminium Components. Sci. Sinter. 2011, 43, 261–275. [Google Scholar] [CrossRef]
- Nimkar, A.; Ramana, M.M.V.; Betkar, R.; Ranade, P.; Mundhe, B. CsOH/γ-Al2O3: A Heterogeneous Reusable Basic Catalyst for One-Pot Synthesis of 2-Amino-4,6-Diaryl Pyrimidines. New J. Chem. 2016, 40, 2541–2546. [Google Scholar] [CrossRef]
- Jafar Tafreshi, M.; Masoomi Khanghah, Z. Infrared Spectroscopy Studies on Sol-Gel Prepared Alumina Powders. Medziagotyra 2015, 21, 28–31. [Google Scholar] [CrossRef][Green Version]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Palacios, E.G.; Juárez-López, G.; Monhemius, A.J. Infrared Spectroscopy of Metal Carboxylates: II. Analysis of Fe(III), Ni and Zn Carboxylate Solutions. Hydrometallurgy 2004, 72, 139–148. [Google Scholar] [CrossRef]
- Otero, V.; Sanches, D.; Montagner, C.; Vilarigues, M.; Carlyle, L.; Lopes, J.A.; Melo, M.J. Characterisation of Metal Carboxylates by Raman and Infrared Spectroscopy in Works of Art. J. Raman Spectrosc. 2014, 45, 1197–1206. [Google Scholar] [CrossRef]
- Justi, M.; de Freitas, M.P.; Silla, J.M.; Nunes, C.A.; Silva, C.A. Molecular Structure Features and Fast Identification of Chemical Properties of Metal Carboxylate Complexes by FTIR and Partial Least Square Regression. J. Mol. Struct. 2021, 1237, 130405. [Google Scholar] [CrossRef]
- Sousa, E.T.; da Silva, M.M.; de Andrade, S.J.; Cardoso, M.P.; Silva, L.A.; de Andrade, J.B. Evaluation of Thermal Stability of Quinones by Thermal Analysis Techniques. Thermochim. Acta 2012, 529, 1–5. [Google Scholar] [CrossRef]
- Barroso-Bogeat, A.; Alexandre-Franco, M.; Fernández-González, C.; Gómez-Serrano, V. Surface Morphological Characterization of Activated Carbon-Metal (Hydr)Oxide Composites: Some Insights into the Role of the Precursor Chemistry in Aqueous Solution. J. Dispers. Sci. Technol. 2020, 41, 1743–1753. [Google Scholar] [CrossRef]
- Gadgil, M.M.; Sasikala, R.; Kulshreshtha, S.K. CO Oxidation over Pd/SnO2 Catalyst. J. Mol. Catal. 1994, 87, 297–310. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Y.-C. Total Oxidation of CH4 on Sn-Cr Composite Oxide Catalysts. Appl. Catal. B 2001, 35, 85–94. [Google Scholar] [CrossRef]
- Iyuke, S.E.; Ahmadun, F.-R. Adsorption and Solid Catalysed Reaction between Activated Carbon Impregnated with SnO2 and CO at Ordinary Temperature. Appl. Surf. Sci. 2002, 187, 37–44. [Google Scholar] [CrossRef]
- Ragupathy, S.; Sathya, T. Synthesis and Characterization of SnO2 Loaded on Groundnut Shell Activated Carbon and Photocatalytic Activity on MB Dye under Sunlight Radiation. J. Mater. Sci. Mater. Electron. 2016, 27, 5770–5778. [Google Scholar] [CrossRef]
- Can, F.; Courtois, X.; Duprez, D. Tungsten-based Catalysts for Environmental Applications. Catalysts 2021, 11, 703. [Google Scholar] [CrossRef]
- Deepa, M.; Sharma, N.; Varshney, P.; Varma, S.P.; Agnihotry, S.A. FTIR Investigations of Solid Precursor Materials for Sol-Gel Deposition of WO3 Based Electrochromic Films. J. Mater. Sci. 2000, 35, 5313–5318. [Google Scholar] [CrossRef]
- Sharma, N.; Deepa, M.; Varshney, P.; Agnihotry, S.A. FTIR Investigations of Tungsten Oxide Electrochromic Films Derived from Organically Modified Peroxotungstic Acid Precursors. Thin Solid Films 2001, 401, 45–51. [Google Scholar] [CrossRef]
- Hoffmann, P.; Galindo, H.; Zambrano, G.; Rincón, C.; Prieto, P. FTIR Studies of Tungsten Carbide in Bulk Material and Thin Film Samples. Mater. Charact. 2003, 50, 255–259. [Google Scholar] [CrossRef]
- Abbas, R.K.; Musa, K.M. Using Raman Shift and FT-IR Spectra as Quality Indices of Oil Bit PDC Cutters. Petroleum 2019, 5, 329–334. [Google Scholar] [CrossRef]
- Daniel, M.F.; Desbat, B.; Lassegues, J.C.; Gerand, B.; Figlarz, M. Infrared and Raman Study of WO3 Tungsten Trioxides and WO3 XH2O Tungsten Trioxide Hydrates. J. Solid. State Chem. 1987, 67, 235–247. [Google Scholar] [CrossRef]
- Menéndez, J.Á.; Phillips, J.; Xia, B.; Radovic, L.R. On the Modification and Characterization of Chemical Surface Properties of Activated Carbon: In the Search of Carbons with Stable Basic Properties. Langmuir 1996, 12, 4404–4410. [Google Scholar] [CrossRef]
- Fuente, E.; Menéndez, J.A.; Díez, M.A.; Suárez, D.; Montes-Morán, M.A. Infrared Spectroscopy of Carbon Materials: A Quantum Chemical Study of Model Compounds. J. Phys. Chem. B 2003, 107, 6350–6359. [Google Scholar] [CrossRef]
| Precursor | Precursor/AC Mass Ratio | pH | Oven-Drying or Heat Treatment Temperature | |||||
|---|---|---|---|---|---|---|---|---|
| 120 °C | 200 °C | 850 °C | ||||||
| Yield/wt.% | Code | Yield/wt.% | Code | Yield/wt.% | Code | |||
| - | - | 5.1 | 98 | ACB | 97 | AC200 | 95 | AC850 |
| Al(NO3)3∙9H2O | 1/1 | 2.9 | 102 | A120 | 93 | A200 | 90 | A850 |
| Fe(NO3)3∙9H2O | 1/1 | 1.5 | 114 | F120 | 94 | F200 | 81 | F850 |
| Zn(NO3)2∙6H2O | 1/1 | 5.2 | 103 | Z120 | 91 | Z200 | 84 | Z850 |
| SnCl2∙2H2O | 1/1 | 1.4 | 149 | S120 | 96 | S200 | 68 | S850 |
| Na2WO4∙2H2O | 1/1 | 9.5 | 106 | W120 | 96 | W200 | 95 | W850 |
| Series | Sample | C/wt.% | H/wt.% | N/wt.% | S/wt.% | Odiff./wt.% | M/wt.% 1 |
|---|---|---|---|---|---|---|---|
| - | AC | 86.50 | 0.51 | 0.26 | 0.64 | 12.09 | - |
| S1 | ACB | 85.63 | 1.69 | 0.64 | 0.48 | 11.56 | - |
| A120 | 72.83 | 1.16 | 0.49 | 0.56 | 24.96 | 3.94 | |
| F120 | 72.48 | 0.85 | 0.44 | 0.59 | 25.64 | 32.95 | |
| Z120 | 77.98 | 0.86 | 0.61 | 0.60 | 19.95 | 7.25 | |
| S120 | 52.32 | 1.25 | 0.06 | 0.37 | 46.00 | 46.48 | |
| W120 | 78.32 | 0.98 | 0.20 | 0.61 | 19.89 | 5.17 | |
| S2 | AC200 | 84.68 | 0.69 | 0.25 | 0.66 | 13.72 | - |
| A200 | 76.44 | 1.19 | 0.57 | 0.55 | 21.25 | 5.05 | |
| F200 | 71.08 | 1.24 | 0.46 | 0.55 | 26.67 | 18.70 | |
| Z200 | 79.62 | 1.09 | 0.54 | 0.56 | 18.19 | 7.73 | |
| S200 | 54.44 | 1.04 | 0.04 | 0.42 | 44.06 | 52.13 | |
| W200 | 77.66 | 1.03 | 0.28 | 0.58 | 20.45 | 4.09 | |
| S3 | AC850 | 84.21 | 0.76 | 0.26 | 0.67 | 14.10 | - |
| A850 | 79.29 | 1.09 | 0.41 | 0.51 | 18.70 | 4.57 | |
| F850 | 80.45 | 0.54 | 0.42 | 0.57 | 18.02 | 21.23 | |
| Z850 | 82.92 | 0.86 | 0.33 | 0.63 | 15.26 | 0.24 | |
| S850 | 72.51 | 0.57 | 0.25 | 0.52 | 26.15 | 29.74 | |
| W850 | 76.80 | 0.94 | 0.14 | 0.60 | 21.52 | 4.54 |
| Sample | pHpzc | Sample | pHpzc | Sample | pHpzc |
|---|---|---|---|---|---|
| AC | 10.50 | ||||
| A120 | 5.20 | A200 | 5.45 | A850 | 9.20 |
| F120 | 4.00 | F200 | 4.10 | F850 | 8.80 |
| Z120 | 6.30 | Z200 | 6.50 | Z850 | 9.80 |
| S120 | <1.60 | S200 | <1.60 | S850 | 9.10 |
| W120 | 7.90 | W200 | 7.35 | W850 | 9.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogeat-Barroso, A.; Alexandre-Franco, M.F.; Fernández-González, C.; Serrano, V.G. Support Surface Chemistry Evolution During the Preparation of Metal Oxide–Activated Carbon Catalysts by Wet Impregnation: A FT-IR Spectroscopy Analysis. Compounds 2025, 5, 36. https://doi.org/10.3390/compounds5030036
Bogeat-Barroso A, Alexandre-Franco MF, Fernández-González C, Serrano VG. Support Surface Chemistry Evolution During the Preparation of Metal Oxide–Activated Carbon Catalysts by Wet Impregnation: A FT-IR Spectroscopy Analysis. Compounds. 2025; 5(3):36. https://doi.org/10.3390/compounds5030036
Chicago/Turabian StyleBogeat-Barroso, Adrián, María Francisca Alexandre-Franco, Carmen Fernández-González, and Vicente Gómez Serrano. 2025. "Support Surface Chemistry Evolution During the Preparation of Metal Oxide–Activated Carbon Catalysts by Wet Impregnation: A FT-IR Spectroscopy Analysis" Compounds 5, no. 3: 36. https://doi.org/10.3390/compounds5030036
APA StyleBogeat-Barroso, A., Alexandre-Franco, M. F., Fernández-González, C., & Serrano, V. G. (2025). Support Surface Chemistry Evolution During the Preparation of Metal Oxide–Activated Carbon Catalysts by Wet Impregnation: A FT-IR Spectroscopy Analysis. Compounds, 5(3), 36. https://doi.org/10.3390/compounds5030036








