Abstract
Neurodegenerative diseases, including Alzheimer’s disease (AD), represent one of the main global health challenges. Cannabis sativa synthesizes spermidine-type alkaloids, whose potential biological activities have been little studied. This study aimed to isolate bioactive alkaloids from an alkaloid-enriched extract (AEE) of C. sativa roots throughout a bioguided approach using conventional chromatographic techniques based on AChE and BuChE inhibitory activities. A qualitative and semiquantitative analysis by UPLC-ESI-MS/MS as well as molecular modeling simulations were performed. In addition, predictive in silico analyses were conducted to assess toxicity properties. The alkaloids cannabisativine (CS) and anhydrocannabisativine (ACS) were isolated, and showed highly selective BuChE inhibitory activity. The molecular modeling study revealed a conserved interaction profile across both alkaloids, indicating the amino acids TRP82, GLU197, TYR440, and HIS438 as the major contributors involved in the complex formation. Finally, CS and ACS exhibited low in silico predictive toxicity values. In conclusion, CS and ACS alkaloids emerge as new selective BuChE inhibitors with therapeutic potential that deserves the attention from the field of pharmacology in neurodegenerative disease research. Additionally, this approach promotes innovation and environmental sustainability through the use of C. sativa roots.
1. Introduction
Cannabis sativa L., a plant known since ancient times, has been recognized for its diverse uses as a source of fiber, food, oil, and for both recreational and religious purposes. Their therapeutic potential was emphasized, particularly in the management of chronic pain, depression, and inflammation [1]. Other studies reported antioxidant, antibacterial, anti-inflammatory, anti-acetylcholinesterase [2], anticoagulant, insecticide, anticancer, anti-aflatoxigenic, antifungal, cytotoxic, anti-elastase, anti-collagenase, neuroprotective (anti-Alzheimer’s, anti-epilepsy, and anti-Parkinson’s, to treat substance- and alcohol-use disorders), and dermo cosmetic (anti-tyrosinase, anti-collagenase, and anti-elastase) properties [1,3,4,5]. Additionally, the synergistic relationship between terpenoids and cannabinoids was highlighted, showcasing their complementary roles in enhancing therapeutic efficacy for treating pain, psychiatric disorders, cancer, and various other conditions [6].
The secondary metabolite profile of Cannabis sativa has been extensively investigated, with approximately 750 compounds including cannabinoids and non-cannabinoids, and a wide range of biological activities have been documented, particularly those linked to central nervous system modulation [7,8]. A diverse spectrum of lesser-known phytocannabinoids, alongside terpenes, stilbenoids, lignanamides, carotenoids, flavonoids, and alkaloids types, exhibit a wide range of pharmacological activities by modulating the endocannabinoid system and engaging additional biochemical pathways [9,10,11]. The multifunctional spanning antioxidative, anti-inflammatory, and neural regulation actions of the mentioned compounds highlight their therapeutic relevance in neurodegeneration research [4].
Although the C. sativa exploitations are focused on the unfertilized female inflorescences, as the principal source of pharmacologically active constituents, other plant tissues also harbor unique unexplored biochemical profiles. For instance, the cannabis roots are rich in other compounds, including triterpenoids, friedelin, epifriedelane, alkaloids, cannabisativine (CS), and anhydrocannabisativine (ACS). Likewise, the ethnopharmacological data of decoctions, juices, and teas of the Cannabis roots indicate that these preparations could be used for the treatment of inflammation, fever, infection, and pain, as well as relief of symptoms related to labor and postpartum hemorrhages [1,2,5,12]. Furthermore, Ferrini et al. [13] proposed utilizing cannabis roots as a natural source of epi-friedelanol and friedelin, which are antioxidants and anti-inflammatory bioactive compounds with highly appealing properties. The leaves contain terpenes, polyphenols, cannabinoids, and alkaloids [1], and Fasakin et al. [14] explored this in cannabis leaves CS, cannabimine C, and ACS. The first identification of a non-quaternary alkaloid from the roots of C. sativa L. was reported as CS [15], while ACS was reported for first time by Elsohly et al. [16]
Considering the diverse pharmacological applications of the C. sativa, it becomes evident that plant components commonly discarded during processing, such as stems, leaves, and roots, may contain bioactive alkaloids with complementary properties. These underexplored tissues represent a promising reservoir of therapeutic compounds, particularly relevant to neurodegenerative diseases like Alzheimer’s disease (AD), which pose one of the most pressing global health challenges today.
AD is a multifactorial neurodegenerative condition characterized by severe degeneration of cholinergic neurons and a marked reduction in acetylcholine levels, which contribute to synaptic dysfunction. Its onset involves the accumulation of amyloid-β (Aβ) peptides forming senile plaques and hyperphosphorylated Tau protein, leading to neurofibrillary tangles (NFTs). The pathological processes include neuroinflammation, mitochondrial and bioenergetic impairments, oxidative stress, and vascular abnormalities. Additional contributing factors include metal ion dysregulation, glutamate-induced excitotoxicity, microbiota–gut–brain axis disruptions, and defective autophagic pathways [17]. Alterations in the cholinesterases levels led to a cholinergic deficit in the brain, marked by reduced levels of acetylcholine. Understanding the interplay between AChE and BuChE in both normal and pathological conditions offers promising compelling therapeutic opportunities [18]. BuChE inhibition may also mitigate abnormal Aβ aggregation, further justifying the targeting of BuChE in AD treatment. Pharmacologically, BuChE and its modulators are linked to therapies for AD, multiple sclerosis, cocaine addiction, and organophosphate poisoning [19]. On the other hand, in vitro studies demonstrated that BuChE inhibitors reduce pro-inflammatory cytokines levels such as IL-1β and TNF-α in peripheral blood mononuclear cells, highlighting their immunomodulatory role [20]. Complementary findings reinforce this anti-inflammatory potential, showing that altered BuChE activity correlates with neuroinflammation markers in cerebrospinal fluid [19,21]. Selective inhibition of BuChE has emerged as a promising strategy for neurodegenerative and metabolic disorders, owing to its dual role in preserving acetylcholine levels and attenuating neuroinflammation [21,22]. Novel BuChE-selective compounds further demonstrated dual effect in cholinergic restoration and cytokine suppression [23,24], underscoring BuChE’s evolving role from a peripheral biomarker to a prime therapeutic target in AD intervention strategies. To date, no studies have been reported regarding the biological activity of CS and ACS alkaloids isolated from Cannabis leaves or roots, leaving their potential therapeutic effects unexplored.
This work aimed to isolate bioactive alkaloids from C. sativa roots using a bioguided fractionation strategy based on AChE and BuChE inhibitory activity. To further investigate the molecular characteristics of the isolated compounds, in silico analyses were conducted to assess their binding interactions with the target cholinesterase enzymes.
The alkaloids CS and ACS were isolated through a bioactivity-guided approach from the alkaloid extract by conventional chromatographic techniques, and the AChE and BuChE-inhibitory activities are reported. The inhibitory effect of CS and ACS against BuChE were highly selective, and for further insight into the experimental results, an in silico study was performed employing docking simulations and molecular dynamics, revealing a conserved interaction profile across both alkaloids, especially on ACS.
2. Materials and Methods
2.1. Chemicals
Methanol (MeOH), sulfuric acid (H2SO4), ethyl ether (Et2O), ammonium hydroxide (NH4OH), ethyl acetate (EtOAc), and anhydrous sodium sulfate (Na2SO4) were purchased from Laboratorios Cicarelli (Buenos Aires, Argentina). Silica gel 60 0.063–0.2 mm from Macherey-Nagel GmbH & Co. KG (Düren, Germany) and TLC Silica gel 60 F254 plates (20 × 20 cm) Merck KGaA were used. AChE from Electrophorus electricus (electric eel), BuChE from equine serum, dipotassium hydrogen phosphate (K2HPO4), sodium dihydrogen phosphate (NaH2PO4), sodium chloride (NaCl), disodium hydrogen phosphate (Na2HPO4), 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB), acetylthiocholine iodide (ATC), butyrylthiocholine iodide (BTC), Galanthamine (Gal), and Donepezil (Don) were obtained from Sigma-Aldrich, St. Louis, MO, USA.
2.2. Plant Material and Extraction
Roots of Cannabis sativa var. ultraviolet were kindly provided by CANME, a public institution in San Juan province, Argentina (Programa de Investigación Plataformas Estratégicas (PIPE) N°1400-000091-2022). Fresh roots of C. sativa var. ultraviolet (512 g) were collected in March 2024, after flowering period, and were dried under air current at 40 °C until constant weight. The dried material was milled, resulting in 250 g of dried roots. It was subjected to maceration with MeOH (2 × 2500 mL, for 72 h each time), kept in darkness at room temperature, filtered, combined, and concentrated under reduced pressure, to give the methanolic extract (ME) 6.25 g, yielding 2.5% w/w referred to dry starting material. The ME was dissolved in a 2% (v/v) H2SO4 to pH 3.5–4. Three extractions with Et2O (3 × 100 mL) were performed to remove fats and nonpolar compounds. The remaining aqueous phase was alkalinized to pH 9−10 with 20% (w/v) NH4OH and subjected to extractions with AcOEt (3 × 100 mL) to recover the alkaloids. The AcOEt fraction was separated, and anhydrous sodium sulphate was added to remove any remaining water. Finally, the solvent was evaporated to dryness using a rotary evaporator (Waltham, MA, USA) in order to obtain the alkaloid-enriched extract (AEE; 45.9 mg) yielding 0.0198%.
2.3. Bioguided Isolation
The alkaloids were isolated through a bioactivity-guided approach from the AEE by conventional chromatographic techniques, evaluating in each separation instance, their inhibitory effect against AChE and BuChE by a modified Ellman’s method, and selecting the active fractions for further isolation. Consequently, the AEE was chromatographed on a silica gel flash column (6 cm length, 2.5 cm i.d.), eluted with a gradient from EtOAc 100% to EtOAc: MeOH (8:2) giving three fractions; F1 (29 mg), F2 (3.7 mg), and F3 (14 mg). After being assayed, F3 showed selective BuChE inhibitory activity. Then, F3 was subjected to semi-preparative TLC (mobile phase EtOAc/MeOH − 9:1) to afford Cannabisativine (CS) (3.2 mg) and anhydrocannabisativine (ACS) (2.5 mg), which were evaluated against BuChE.
2.4. Ultra Performance Liquid Chromatography (UPLC) Analysis
The LC-MS analysis was performed in an ACQUITY H–Class UPLC instrument equipped with a XEVO TQ-S micro triple quadrupole mass spectrometer (Waters Corp, Milford, MA, USA) with electrospray ionization (ESI). An UPLC ACQUITY BEH C18 (1.7 μm, 2.1 mm × 50 mm) column was used for separation at 35 °C.
The mobile phase for semiquantitation analysis consisted of A (0.1% formic acid), B (acetonitrile), and C (methanol) with a flow rate of 0.2 mL/min. The gradient conditions were as follows: initially, 90%A—5%B—5%C; 3 min, 70%A—15%B—15%C; 3.10 min, 90%A—5%B—5%C and hold for 2 min; completing a total of 5 min. The AEE was prepared at 65 ppm. Caffeine was used as standard for calibration curve at the concentrations 10, 15, 20, 25, and 30 ppm. The samples were dissolved in a mixture of methanol/water (50:50) and filtered through a membrane filter (0.22 µm). The injection volume was 10 µL. The capillary, cone, and collision energies were 3 kV, 34 V, and 20 eV, respectively. The data were acquired in ESI positive mode using MS2 scan (60−1000 Da) and daughters (m/z 195, 364, 382) function. The data were processed using MassLynx Software V4.2 (Waters, Milford, MA, USA).
2.5. Microplate Assay for AChE and BuChE Inhibitory Activities
Cholinesterases inhibitory activities were performed according to Ellman et al. [25] with modifications [26]. In each well of a 96-well microplate, 50 µL of AChE or BuChE enzyme solution (0.25 U/mL, in phosphate-buffer saline (PBS): 8 mM K2HPO4, 2.3 mM NaH2PO4, 0.15 M NaCl, pH 7.5) and 50 µL of each sample dissolved in the same buffer were added. The plates were incubated at room temperature for 30 min. Subsequently, 100 µL of the substrate solution, containing DTNB and ATC or BTC (0.6 mM), prepared in a saline solution with Na2HPO4 (pH 7.5), was added. The absorbance at 405 nm was recorded using a Thermo Scientific Multiskan FC spectrophotometer (Waltham, MA, USA), 5 min after the reaction began. To calculate the IC50 values, the concentrations of the AEE, fractions, and the alkaloids assayed ranged from 1 to 200 (µg/mL or µM). Gal and Don were used as positive controls and PBS was used as a negative control. The enzyme-inhibitory activity was calculated as a percentage using PBS without inhibitor.
2.6. Molecular Docking Study
2.6.1. Automated Docking Setup
The X-ray crystal structure corresponding to HsBuChE (PDB code: 1P0I) [27] was obtained from the Protein Data Bank (http://www.rcsb.org (accessed on 22 July 2025)) and used as the receptor. Before the calculations, any non-amino acid residues were removed. Both ligands, CS and ACS (compounds CID: 442846 and 14106160, respectively), were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/ (accessed on 22 July 2025)). Molecular docking was performed with AutoDock4 [28], treating the receptor structure as rigid. Grid box dimensions were defined as 50 × 50 × 50 in the X, Y, and Z directions, with a spacing resolution of 0.375 Å, centered at the enzyme’s active site. Gasteiger charges were assigned to the ligands, and all non-polar hydrogen atoms were merged. All possible torsions of the ligand were allowed to rotate, and 200 docking poses were generated. The maximum number of energy evaluations was set to 2.5 × 106, and the maximum number of generations to 2.7 × 104, with all remaining parameters left at their default values. The resulting poses were grouped into clusters based on the root-mean-square deviation (RMSD) of their backbones and then ranked according to their predicted binding free energies. The conformation from the most populated cluster displaying the lowest relative free energy was selected for MD simulations.
2.6.2. Molecular Dynamics Simulations
MD simulations were carried out using the AMBER22 [29] software package with the ff14SB [30] and GAFF [31] force fields. Complex assembly was performed with the Leap module, and ligand parameters were generated using the antechamber module. Each selected docked conformation was solvated in an octahedral periodic box filled with TIP3P water molecules [32], ensuring a 10 Å buffer from the solute in all directions. To neutralize the systems, Na+ or Cl− ions were added as required. The Sander module was then used to minimize the potential energy of each system for 5000 steps with the steepest-descent algorithm. Next, each system was equilibrated under constant volume conditions for 2500 ps, during which the temperature was gradually raised from 0 to 300 K using a Langevin thermostat [33] at a collision frequency of 1.0 ps−1. The SHAKE algorithm [34] was applied to constrain all bonds involving hydrogen, allowing an integration time step of 2 fs. Production MD simulations were executed in triplicate at 298 K for a total of 50 ns each, resulting in 150 ns of simulated time per complex. The Particle Mesh Ewald (PME) method [35] was used with a grid spacing of 1.2 Å, a spline interpolation order of 4, and a real-space cutoff of 10 Å. Analyses of the simulation trajectories were conducted using CPPTRAJ [36].
2.6.3. MM-GBSA Free Energy Decomposition
The relative binding free energies for each complex were evaluated using the MM_PBSA module in Amber version 22, followed by a decomposition of the total free energy to identify the critical BuChE active-site residues interacting with the ligands. Snapshots corresponding to the last 30 ns of each trajectory were collected, and explicit water molecules along with counter ions were removed before analysis [29].
2.7. Preliminary Toxicological Assessment
The ProTox-3.0 web tool (https://comptox.charite.de/protox3/ (accessed on 22 July 2025)) [37] was employed to predict the toxicological profiles of cannabisativine and anhydrocannabisativine, including acute toxicity, organ-specific effects, genotoxicity, and potential cholinesterase inhibition. In parallel, the SwissADME web tool (http://www.swissadme.ch (accessed on 22 July 2025)) [38] was used to predict ADME-related properties (Absorption, Distribution, Metabolism, and Excretion), including gastrointestinal absorption, topological polar surface area (TPSA), lipophilicity (cLogP), hydrogen-bonding capacity, drug-likeness, blood–brain barrier permeability, and cytochrome P450 inhibition.
2.8. Statistical Data Analysis
All measurements from the AChE and BuChE inhibition assays were performed in triplicate, and IC50 values were expressed as the mean ± standard deviation (SD) of three independent experiments. Data were analyzed using Prism 10.4.1 (GraphPad Software Inc., San Diego, CA, USA).
3. Results
3.1. UPLC-MS/MS Analysis
For the qualitative and semiquantitative analysis, several UPLC-ESI-MS/MS and DI-ESI-MS/MS measurements were carried out for the C. sativa alkaloids-enriched extract (AEE), fractions, and the isolated compounds. The alkaloids were identified based on the m/z species and their fragmentation patterns, comparing the results obtained with those in the literature. The AEE DI-MS analysis revealed the m/z ions; 382, 364 as well as the m/2z ions; 182.6 and 191.6, which were tentatively assigned to cannabisativine (CS) and anhydrocannabisativine (ACS) spermidine alkaloids and would correspond for the species [M+H+]+ and [M+2H+]2+, respectively. In order to confirm the alkaloids identity, UPLC-ESI-MS/MS and DI-MS/MS analysis were performed for the AEE, F3 fraction, ACS, and CS (daughters analysis of m/z 364 and 382) (Figures S1–S3). Thus, in all samples, the results for ACS [M+H+]+ = 364(7) displayed the following fragments: 208(100), 171(20), 250(12), 129(9), 112(5), 198(4), and 181(1) which were in agreement with those of ACS found in the literature [16,39,40,41]. Regarding CS [M+H+]+ = 382(44) the fragments observed were m/z 96(100), 226(62), 198(27), 112(9), 126(8), 157(7), 364(5), 129(5), 143(5), 264(4), 171(3), and 208(3), confirming the identity for CS [15,39,40,41,42]. The proposed ESI(+)-MS/MS main fragments structures for ACS and CS, are available in Supplementary Materials (Table S1 and S2).
In regard to the alkaloid content analysis, CS and ACS were semiquantified by UHPLC-ESI-MS/MS related to dry weight of C. sativa roots, obtaining concentration values of 0.002% (20.45 mg/kg dw) and 0.0042% (42.88 mg/kg dw) (w/w), respectively. The linear correlation curve was r2 = 0.988735 and the linear equation was y = 199931 x − 62742.5 (Figure S4). The structures of the compounds are shown in Figure 1. Although the structural differences among caffeine, CS, and ACS are considerable, these results provide an approximation that allows us to estimate the potential levels of these natural products in C. sativa roots. A quantitative analysis using a closely related derivative will be performed in the future.

Figure 1.
Chemical structures of the identified alkaloids from C. sativa roots.
3.2. Cholinesterases Inhibitory Bioassay
The AEE, along with its fractions and isolated compounds (CS and ACS), were evaluated for in vitro inhibitory activity against acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE). The results are summarized in Table 1, expressed as half-maximal inhibitory concentration (IC50) values, and selectivity indices (SI) as IC50 AChE/IC50 BuChE. Under the experimental conditions, the standard compounds galanthamine (Gal) and donepezil (Don) exhibited IC50 values of 0.97 ± 1.33 µM and 0.06 ± 0.01 µM against AChE, and 15.60 ± 1.25 µM and 4.71 ± 0.11 µM against BuChE, respectively. The AEE showed inhibition against BuChE (IC50 = 51.52 ± 2.90 µg/mL), while for AChE it was inactive (IC50 = 311.24 ± 13.02 µg/mL). After flash chromatography, three fractions (F1–F3) were obtained and assessed for cholinesterase inhibition. Notably, F3 exhibited strong inhibitory activity only for BuChE (IC50 = 13.40 ± 1.04 µg/mL), whereas F1 and F2 were inactive against both enzymes. The bioguided isolation yielded the alkaloids CS and ACS (Figure 1), showing selective activity against BuChE IC50 26.38 ± 0.95 µM; SI ≥ 7.58, and IC50 24.97 ± 1.08; SI ≥ 8.00, respectively.

Table 1.
Acetylcholinesterase and butyrylcholinesterase inhibitory activities of the alkaloid-enriched extract, fractions, cannabisativine, and anhydrocannabisativine from Cannabis sativa roots.
3.3. In Silico Toxicological and ADME Profiling
Both alkaloids, CS and ACS, were subjected to in silico toxicological evaluation using the ProTox-3.0 platform [37]. These compounds were classified as toxicity class 4, with a predicted LD50 of 418 mg/kg−1. While the neuro- and respiratory toxicity were estimated to have moderate probability, the hepatotoxicity, nephrotoxicity, cardiotoxicity, cytotoxicity, mutagenicity, and carcinogenicity were predicted inactive (Table 2). A subtle difference emerged in immunotoxicity classifying CS as marginal, while ACS remained inactive.

Table 2.
In silico toxicity and pharmacokinetic parameters predicted for CS and ACS.
Regarding oral bioavailability, both alkaloids satisfied Lipinski’s rule-of-five and were predicted to have high gastrointestinal absorption, according to SwissADME [38]. Their physicochemical properties, including topological polar surface area (TPSA), calculated partition coefficient (cLogP), and hydrogen-bonding capacity, were in accordance with this prediction. The blood–brain barrier (BBB) properties for both compounds were predicted to be permeable by ProTox-3.0; however, SwissADME predicted permeability only for ACS (Table 2).
3.4. Molecular Modeling
Molecular docking studies were applied to obtain accurate predictions of protein–ligand interaction for CS and ACS. To evaluate the dynamic behavior of the compounds over time, molecular dynamics simulations were conducted. These analyses provided insight into the stability and adaptability of the ligand–enzyme complexes. The final stage involved calculating the binding free energies based on the trajectories obtained from MD simulations. A per-residue decomposition analysis was also performed to identify key amino acids in the active site of BuChE that contribute to the intermolecular interactions within each complex. Both ligands, CS and ACS, bind to the catalytic gorge of human BuChE, interacting with key amino acids commonly associated with ligand binding in this enzyme. A comparative breakdown of key observations, overall binding position, and orientation are shown in Figure 2 and Figure 3 (panels A and B).
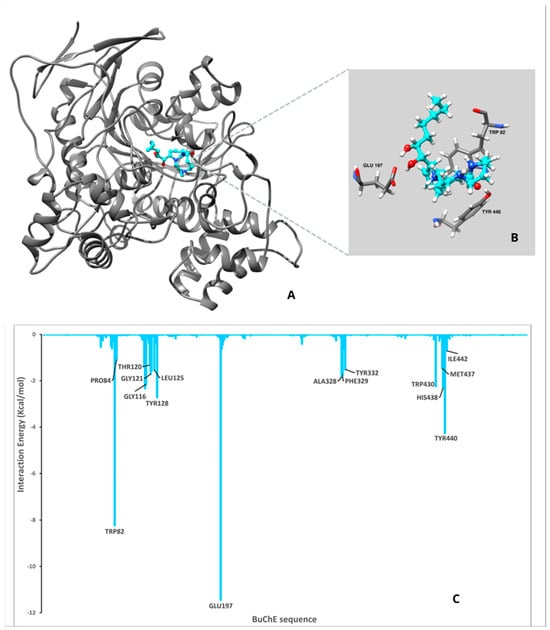
Figure 2.
Results for CS Molecular Dynamics (MD) analysis. (A) The complex between ligand and enzyme obtained after the MD: BuChE is shown in gray and the ligand in cyan. (B) Active site zoom showing the strongest interaction between the ligand and the amino acids. (C) per residues analysis for the complex, the bars indicate the interaction energy between an amino acid and the ligand. “-“ represents negative values in Y-axis numbers.
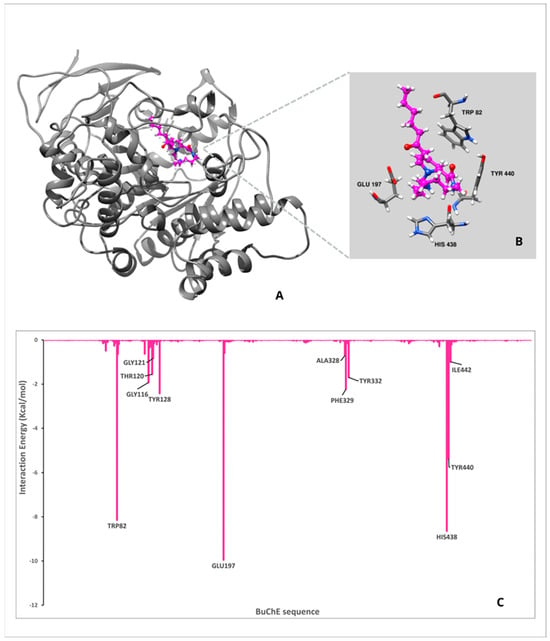
Figure 3.
Results for ACS Molecular Dynamics (MD) analysis. (A) The complex between ligand and enzyme obtained after the MD: BuChE is shown in gray and the ligand in magenta. (B) Active site zoom showing the strongest interaction between the ligand and the amino acids. (C) Per-residue analysis for the complex, the bars indicate the interaction energy between an amino acid and the ligand. “-“ represents negative values in Y-axis numbers.
In both complexes, the ligands occupy the same general region in the BuChE catalytic site, suggesting they interact with the same set of critical residues: TRP82, GLU197, TYR440, and HIS438. The close-up views (panels B) confirm that each ligand establishes contact via aromatic stacking (notably with TRP82 and TYR440) and hydrogen bonds (with GLU197 and HIS438). The bar charts display how each residue in the BuChE sequence contributes to the total interaction energy with the ligand. Negative values indicate favorable (stabilizing) contributions; larger negative peaks suggest more significant binding interactions. Regarding CS, the amino acids TRP82 and GLU197 emerge as major contributors, reinforcing their role as key anchoring residues. However, the magnitude of these peaks might differ compared to ACS. Likewise, TYR440, HIS438, and PHE329 show notable negative energy contributions, indicating aromatic and/or hydrogen-bond interactions that stabilize the ligand. Some additional residues, such as LEU125 and TYR128, would also contribute to binding, although these interactions slightly differ from those observed with ACS.
For ACS, the residues TRP82 and GLU197 show particularly large negative energy contributions, indicating strong stabilizing interactions. To a lesser extent, TYR440 and HIS438 also contribute to the stabilization of the complex. Additional residues such as TYR128, THR120, and GLY116 provide moderate yet favorable contributions, suggesting ancillary interactions that help position or stabilize the ligand in the binding pocket.
Both ligands rely on the same fundamental set of anchoring residues, but subtle variations in the contribution of individual residues may explain any differences in binding affinity or specificity. Comparison of the key peaks, TRP82 vs. GLU197, in each system may provide insights into how each ligand orients within the active site and whether it engages in stronger hydrogen bonding, π–π stacking, or electrostatic interactions.
In summary, both CS and ACS interact with key BuChE residues, especially TRP82 and GLU197. Variations in peak magnitudes from the per-residue energy decomposition suggest that, while both ligands bind in a similar region, their interaction patterns vary, which may influence overall binding affinity and inhibitory activity.
4. Discussion
The Cannabis roots have been comparatively overlooked, receiving far less scientific attention than the aerial parts, despite their traditional use in treating fever, inflammation, infections, and arthritis [7]. This work investigates the neuroprotective potential of cannabisativine (CS) and anhydrocannabisativine (ACS) isolated from C. sativa roots through a bioguided fractionation strategy centered on BuChE inhibition. To further characterize the molecular features of both alkaloids, in silico docking analyses were performed, revealing favorable binding interactions with the BuChE active site.
The alkaloids in C. sativa are mainly in the roots and leaves [42,43]. Nowadays, the cannabis roots are discarded in the production of medicinal cannabis, and represent between 30% and 50% of the plant biomass. To the best of our knowledge, the spermidine-type alkaloids, CS and ACS, have not been investigated for their potential biological activities [5]. CS and ACS are polyamines subclassified as dihydroperiphylline type alkaloids [43].
The semiquantitative analysis of the isolated alkaloids revealed that CS accounted for 0.002% and ACS for 0.004% related to dry weight of C. sativa roots. These values were notably higher than those previously reported, approximately two-fold higher, suggesting that this extraction method may be more efficient. Considering that under acidic thermal conditions CS may undergo dehydration, it is plausible that during the extraction process, partial conversion of CS to ACS occurs [15,16].
The bioguided isolation process revealed that the inhibitory activities were BuChE-selective for AEE, F3, and the isolated alkaloids. Likewise, the inhibitory potency progressively increased during the bioguided isolation, with F3 showing four-fold and CS and ACS five-fold higher activity than AEE, indicating that these alkaloids are responsible for the inhibitory effect in the AEE. In this regard, Gagné et al. [2] reported antimicrobial, anti-inflammatory, anticholinesterase, and antioxidant activities for different cannabis roots extracts (aqueous, alcohol, and acid-base), indicating the presence of several phenolic compounds, cannabinoids, terpenoids, amino acids, and nitrogen-containing compounds as responsible for the studied biological activities. Likewise, aqueous extracts from cannabis roots with antitussive, expectorant, anti-inflammatory, antiasthmatic, and slight toxic effects were reported [39,44]. Additionally, de Lima Araújo et al. [41] described its antidysmenorrheic and nociceptive effects, recognizing CS, ACS, p-coumaroyltyramine, and feruloyltyramine as the active compounds. However, all the reported bioativities were only evaluated for the C. sativa roots extracts, while the isolated compounds were not tested.
In order to gain deeper insight into the observed experimental outcomes, a molecular modeling investigation was carried out over BuChE. Molecular docking and molecular dynamics simulations were performed to evaluate the interactions of CS and ACS with BuChE to identify the most favorable conformations.
The dynamic simulations indicated that CS and ACS are capable of binding within the BuChE catalytic site in a manner analogous to Gal and Don interaction on CAS and PAS, respectively [45]. These findings suggest a conserved interaction pattern across all these alkaloids, supporting their inhibitory activity. In summary, a comparative study of two similar alkaloids, which differ primarily in their interaction with GLU197, revealed that ACS interacts with nearly three times the intensity of CS. Perhaps the absence of hydroxyl groups in ACS allows it to penetrate more deeply into the BuChE active-site gorge, thereby promoting stronger interactions with CAS and influencing overall binding strength and potential inhibitory activity.
Regarding in silico toxicity and pharmacokinetic parameters, the predictions for CS and ACS indicate that these compounds are not active against AChE (Table 2). These results are in agreement with those of the AChE in vitro assay. In terms of metabolic interactions, neither CS nor ACS were predicted to significantly inhibit any major cytochrome P450 (CYP450) isoforms. However, ProTox-3.0 predictions identified a low-confidence potential interaction between CS and CYP2D6.
ProTox-3.0 prediction indicated favorable BBB permeability for both alkaloids, while SwissADME predicted permeability only for ACS; this difference may be attributed to its lower polarity and fewer hydrogen bond donors relative to CS [46]. In addition, the CS and ACS alkaloids were also identified as P-glycoprotein (P-gp) substrates, which may reduce their effective accumulation in the central nervous system despite predicted permeability. Regarding to metabolism, no relevant inhibition of major CYP450 isoforms was predicted. However, a low-confidence interaction with CYP2D6 was indicated for ACS by ProTox-3.0. These results suggest that both alkaloids display acceptable toxicological profiles, promising pharmacokinetic characteristics, and potential for central nervous system accessibility, particularly ACS. The moderate neuro- and respiratory toxicity predictions, along with the potential CYP2D6 interaction, highlight aspects requiring further experimental validation.
Considering that BuChE has recently gained relevance as a target in neurodegenerative diseases research [47], CS and ACS alkaloids would emerge as promising compounds in the research for future potential treatments. In the early stages of AD, AChE inhibitors are employed to enhance synaptic acetylcholine (ACh) levels. AChE activity declines by approximately 50% as the disease advances, while BuChE activity increases up to 900% in late-stage of the AD [19,21]. Under these conditions, BuChE becomes the predominant enzyme responsible for ACh hydrolysis, thereby exacerbating cholinergic deficits and highlighting its compensatory substitution for AChE activity [47]. This abnormal upregulation reinforces its potential as an emerging therapeutic target in AD treatment strategies [19,21,47]. Inhibition of BuChE is generally accompanied by fewer adverse effects [47]. In this regard, the ProTox 3.0 results predicted that CS and ACS exhibited moderate toxicity with equal values of LD50 of 418 [mg/kg, classified as Class4, being less toxic at higher doses compared to Gal (LD50 = 85 mg/kg, Class 3), and similar to Don (LD50 = 505 mg/kg, Class 4), making it an attractive target for therapeutic intervention as BuChE inhibitors.
5. Conclusions
The bioguided phytochemical study from C. sativa roots led to the identification of cannabisativine (CS) and anhydrocannabisativine (ACS) spermidine-type alkaloids showing low in silico predictive toxicity values and highly selective inhibitory activity, as well as favorable binding affinities and interaction patterns against BuChE.
Cannabis roots, typically discarded as waste during medicinal cannabis production, can be transformed into a valuable source of bioactive compounds with potential applications in neurodegenerative disorders. In conclusion, CS and ACS alkaloids emerge as new selective BuChE inhibitors with therapeutic potential that deserves attention from the field of pharmacology and medicinal chemistry for its potential to drive global therapeutic innovation while reinforcing environmental sustainability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/compounds5030035/s1, Figure S1: Cannabisativine (CS) MS/MS spectrum (daughters of 382); Figure S2: Anhydrocannabisativine (ACS) MS/MS spectrum (daughters of 364); Figure S3: MS spectrum of the alkaloid-enriched extract from C. sativa roots (DI-ESI-MS). Red arrows indicate [M+H]+ species of CS and ACS, while blue arrows their [M+2H]2+ species.; Figure S4: Caffeine calibration curve for CS and ACS quantification.; Table S1: Proposed ESI(+)-MS/MS main fragments structures for cannabisativine.; Table S2: Proposed ESI(+)-MS/MS main fragments structures for anhydrocannabisativine.
Author Contributions
Conceptualization, J.E.O. and G.E.F.; methodology, D.C., J.E.O., C.W.A.-F., and O.L.-C.; software, C.W.A.-F., A.G., and O.L.-C.; formal analysis, J.E.O., C.W.A.-F., and G.E.F.; investigation J.E.O., C.W.A.-F., and G.E.F.; resources, J.E.O. and G.E.F.; writing—original draft preparation, J.E.O., C.W.A.-F., and G.E.F.; writing—review and editing J.E.O. and G.E.F.; supervision G.E.F.; project administration, J.E.O. and G.E.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by Programa de Investigación Plataformas Estratégicas (PIPE) N°1400-000091. 2022 SECITI Gobierno de la Provincia de San Juan, CICITCA- UNSJ.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to express our gratitude to Julian Moreno and Mariano Distefano for their technical assistance. C.W.A-F. and O.L-C. hold a fellowship from CONICET. G.E.F., which belongs to CONICET. Generative AI tools were not utilized. The authors independently conceptualized, integrated, and finalized the graphical abstract using Canva (www.canva.com, accessed on 22 July 2025).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hourfane, S.; Mechqoq, H.; Bekkali, A.Y.; Rocha, J.M.; El Aouad, N. A Comprehensive Review on Cannabis sativa Ethnobotany, Phytochemistry, Molecular Docking and Biological Activities. Plants 2023, 12, 1245. [Google Scholar] [CrossRef]
- Gagné, V.; Merindol, N.; Boucher, R.; Boucher, N.; Desgagné-Penix, I. Rooted in therapeutics: Comprehensive analyses of Cannabis sativa root extracts reveal potent antioxidant, anti-inflammatory, and bactericidal properties. Front. Pharmacol. 2024, 15, 1465136. [Google Scholar] [CrossRef]
- Karimi, I.; Yousofvand, N.; Hussein, B.A. In vitro cholinesterase inhibitory action of Cannabis sativa L. Cannabaceae and in silico study of its selected phytocompounds. Silico Pharm. 2021, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, B.S.; Subbanna, S. Unveiling the potential of Phytocannabinoids: Exploring Marijuana’s lesser-known constituents for neurological disorders. Biomolecules 2024, 14, 1296. [Google Scholar] [CrossRef] [PubMed]
- Boucher, R.; Germain, H.; Desgagné-Penix, I. Exploring the Lesser-Known Bioactive Natural Products of Plant Species of the Genus Cannabis L.: Alkaloids, Phenolic Compounds, and Their Therapeutic Potential. Plants 2025, 14, 1372. [Google Scholar] [CrossRef] [PubMed]
- Ferber, S.G.; Namdar, D.; Hen-Shoval, D.; Eger, G.; Koltai, H.; Shoval, G.; Shbiro, L.; Weller, A. The “Entourage Effect”: Terpenes Coupled with Cannabinoids for the Treatment of Mood Disorders and Anxiety Disorders. Curr. Neuropharmacol. 2020, 18, 87–96. [Google Scholar] [CrossRef]
- Siracusa, L.; Ruberto, G.; Cristino, L. Recent Research on Cannabis sativa L.: Phytochemistry, New Matrices, Cultivation Techniques, and Recent Updates on Its Brain-Related Effects (2018–2023). Molecules 2023, 28, 3387. [Google Scholar] [CrossRef]
- Fordjour, E.; Manful, C.F.; Sey, A.A.; Javed, R.; Pham, T.H.; Thomas, R.; Cheema, M. Cannabis: A multifaceted plant with endless potentials. Front. Pharmacol. 2023, 15, 1200269. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary metabolites profiled in cannabis inflorescences, leaves, stem barks, and roots for medicinal purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef]
- Lowe, H.; Steele, B.; Bryant, J.; Toyang, N.; Ngwa, W. Non-cannabinoid metabolites of Cannabis sativa L. with therapeutic potential. Plants 2021, 10, 400. [Google Scholar] [CrossRef]
- Ryz, N.R.; Remillard, D.J.; Russo, E.B. Cannabis roots: A traditional therapy with future potential for treating inflammation and pain. Cannabis Cannabinoid Res. 2017, 2, 210–216. [Google Scholar] [CrossRef]
- Ferrini, F.; Donati Zeppa, S.; Fraternale, D.; Carrabs, V.; Annibalini, G.; Verardo, G.; Gorassini, A.; Albertini, M.C.; Ismail, T.; Fimognari, C.; et al. Characterization of the Biological Activity of the Ethanolic Extract from the Roots of Cannabis sativa L. Grown in Aeroponics. Antioxidants 2022, 11, 860. [Google Scholar] [CrossRef]
- Fasakin, O.W.; Oboh, G.; Ademosun, A.O.; Lawal, A.O. The modulatory effects of alkaloid extract of Cannabis sativa, Datura stramonium, Nicotiana tabacum and male Carica papaya on neurotransmitter, neurotrophic and neuroinflammatory systems linked to anxiety and depression. Inflammopharmacology 2022, 30, 2447–2476. [Google Scholar] [CrossRef]
- Lotter, H.L.; Abraham, D.J.; Turner, C.E.; Knapp, J.E.; Schiff, P.L., Jr.; Slatkin, D.J. Cannabisativine, a new alkaloid from Cannabis sativa L. root. Tet. Lett. 1975, 16, 2815–2818. [Google Scholar] [CrossRef]
- Elsohly, M.A.; Turner, C.E.; Phoebe, C.H., Jr.; Knapp, J.E.; Schiff, P.L., Jr.; Slatkin, D.J. Anhydrocannabisativine, a new alkaloid from Cannabis sativa L. J. Pharm. Sci. 1978, 67, 124. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Yue, S.; Qin, R.; Du, X.; Wu, Y.; Zhangsun, D.; Luo, S. Recent Advances in Drug Development for Alzheimer’s Disease: A Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 3905. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, G.; Greig, N.H.; Khan, J.A.; Kamal, M.A. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol. Disord. Drug Targets 2014, 13, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Sang, Z.; Huang, S.; Tan, W.; Ban, Y.; Wang, K.; Fan, Y.; Chen, H.; Zhang, Q.; Liang, C.H.; MI, J.; et al. Discovery of novel butyrylcholinesterase inhibitors for treating Alzheimer’s disease. Acta Pharm. Sinica B 2025, 15, 2134–2155. [Google Scholar] [CrossRef]
- Reale, M.; Nicola, M.D.; Velluto, L.; D’Angelo, C.; Costantini, E.; Lahiri, D.K.; Kamal, M.A.; Yu, Q.-Y.; Greig, N.H. Selective acetyl-and butyrylcholinesterase inhibitors reduce amyloid-β ex vivo activation of peripheral chemo-cytokines from Alzheimer’s disease subjects: Exploring the cholinergic anti-inflammatory pathway. Curr. Alzheimer Res. 2014, 11, 608–622. [Google Scholar] [CrossRef]
- Sun, T.; Zhen, T.; Harakandi, C.H.; Wang, L.; Guo, H.; Chen, Y.; Sun, H. New insights into butyrylcholinesterase: Pharmaceutical applications, selective inhibitors and multitarget-directed ligands. Europ. J. Med. Chem. 2024, 275, 116569. [Google Scholar] [CrossRef]
- Mallick, R.; Basak, S.; Chowdhury, P.; Bhowmik, P.; Das, R.K.; Banerjee, A.; Paul, S.; Pathak, S.; Duttaroy, A.K. Targeting Cytokine-Mediated Inflammation in Brain Disorders: Developing New Treatment Strategies. Pharmaceuticals 2025, 18, 104. [Google Scholar] [CrossRef] [PubMed]
- Raspudić, A.; Odak, I.; Mlakić, M.; Jelčić, A.; Bulava, K.; Karadža, K.; Milašinović, V.; Šagud, I.; Pongrac, P.; Štefok, D.; et al. Heterostilbene Carbamates with Selective and Remarkable Butyrylcholinesterase Inhibition: Computational Study and Physico-Chemical Properties. Biomolecules 2025, 15, 825. [Google Scholar] [CrossRef]
- Hasni, F.; Daoud, I.; Melkemi, N. Study of Acetylcholinesterase and Butyrylcholinesterase (AChE/BuChE) Inhibition Using Molecular Modelling Methods. Chem. Proc. 2023, 14, 77. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.J.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Ortiz, J.E.; Pigni, N.B.; Andujar, S.A.; Roitman, G.; Suvire, F.D.; Enriz, R.D.; Tapia, A.; Bastida, J.; Feresin, G.E. Alkaloids from Hippeastrum argentinum and their cholinesterase-inhibitory activities: An in vitro and in silico study. J. Nat. Prod. 2016, 79, 1241–1248. [Google Scholar] [CrossRef]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar] [CrossRef]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Software news and updates AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Case, D.A.; Aktulga, M.H.; Belfon, K.; Ben-Shalom, I.Y.; Berryman, J.T.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.; Cisneros, G.A.; Cruzeiro, V.W.D.; et al. Amber 2023 Reference Manual; University of California: San Francisco, CA, USA, 2023; Available online: https://ambermd.org/doc12/Amber23.pdf (accessed on 22 July 2025).
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general Amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Izaguirre, J.A.; Catarello, D.P.; Wozniak, J.M.; Skeel, R.D. Langevin stabilization of molecular dynamics. J. Chem. Phys. 2001, 114, 2090. [Google Scholar] [CrossRef]
- Ryckaert, J.P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2024, 52, 513–520. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Menezes, P.M.N.; Pereira, E.C.V.; Lima, K.S.B.; da Silva, B.A.O.; Brito, M.C.; de Lima Araújo, T.C.; Neto, J.A.; de Araujo Ribeiro, L.A.; Silva, F.S.; Rolim, L.A. Chemical analysis by LC-MS of Cannabis sativa root samples from Northeast Brazil and evaluation of antitussive and expectorant activities. Planta Med. 2022, 88, 1223–1232. [Google Scholar] [CrossRef]
- Menezes, P.M.N.; Araújo, T.C.L.; Pereira, E.C.V.; Neto, J.A.; Silva, D.S.; Brito, M.C.; Lima, K.S.B.; Monte, A.P.O.; Matos, M.H.T.; Lima, R.S.; et al. Investigation of antinociceptive, antipyretic, antiasthmatic, and spasmolytic activities of Brazilian Cannabis sativa L. roots in rodents. J. Ethnopharm. 2021, 278, 114259. [Google Scholar] [CrossRef]
- de Lima Araújo, T.C.; Menezes, P.M.N.; Ribeiro, T.F.; Macêdo, C.A.F.; de Souza, N.A.C.; Lima, K.S.B.; Figueredo, H.F.; Silva, F.S.; Rolim, L.A. Cannabis sativa L. roots from Northeast Brazil reduce abdominal contortions in a mouse model of primary dysmenorrhea. J. Ethnopharm. 2024, 318, 116891. [Google Scholar] [CrossRef]
- Turner, C.E.; Hsu, M.H.; Knapp, J.E. Isolation of Cannabisativine, an alkaloid from Cannabis sativa root. J. Pharm. Sc. 1976, 65, 1084–1085. [Google Scholar] [CrossRef] [PubMed]
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary metabolism in cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Lima, K.S.B.; Silva, M.E.G.C.; Araújo, T.C.L.; Silva, C.P.F.; Santos, B.L.; Ribeiro, L.A.A.; Menezes, P.M.N.; Silva, M.G.; Lavor, E.M.; Silva, F.S.; et al. Cannabis roots: Pharmacological and toxicological studies in mice. J. Ethnopharmacol. 2021, 271, 113868. [Google Scholar] [CrossRef]
- Adarvez-Feresin, C.W.; Ortiz, J.E.; Piñeiro, M.D.; Parravicini, O.; Enriz, R.D.; Garro, A.D.; Feresin, G.E. Inhibitory effect of galantamine and donepezil combination against cholinesterase: An in silico and in vitro study. Arch. Der Pharm. 2024, 357, 2300581. [Google Scholar] [CrossRef]
- Sánchez-Martínez, J.D.; Valdés, A.; Gallego, R.; Suárez-Montenegro, Z.J.; Alarcón, M.; Ibañez, E.; Alvarez-Rivera, G.; Cifuentes, A. Blood–Brain Barrier Permeability Study of Potential Neuroprotective Compounds Recovered from Plants and Agri-Food by-Products. Front. Nutr. 2022, 9, 924596. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Li, Q.; Xiong, B.; Chen, Y.; Feng, F.; Liu, W.; Sun, H. Structure and therapeutic uses of butyrylcholinesterase: Application in detoxification, Alzheimer’s disease, and fat metabolism. Med. Res. Rev. 2021, 41, 858–901. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).