Synthesis, Structure, and Anticancer Activity of a Dinuclear Organoplatinum(IV) Complex Stabilized by Adenine
Abstract
:1. Introduction
2. Materials and Methods
3. Results
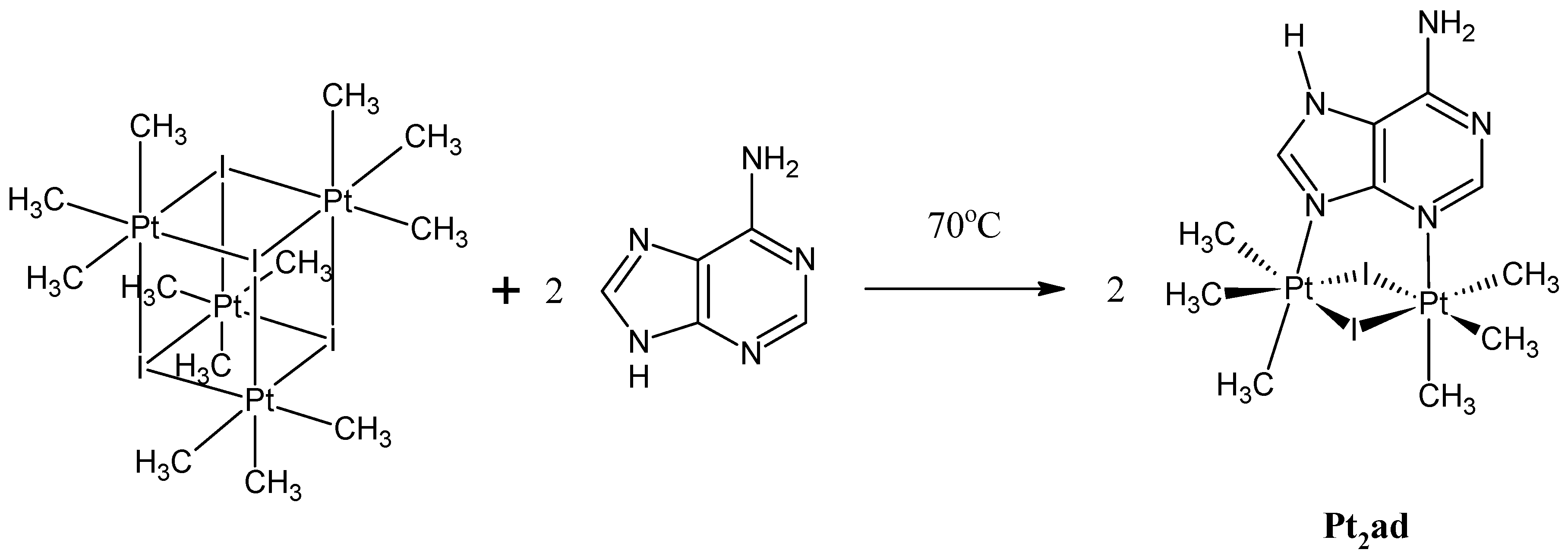
3.1. Synthesis and Spectroscopic Data
3.2. Single Crystal X-Ray Structure
3.3. In Vitro Anticancer Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richardson, D.L.; Eskander, R.N.; O’Malley, D.M. Advances in Ovarian Cancer Care and Unmet Treatment Needs for Patients with Platinum Resistance: A Narrative Review. J. Am. Med. Assoc. Oncol. 2023, 9, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Baxevanos, P.; Mountzios, G. Novel Chemotherapy Regimens for Advanced Lung Cancer: Have We Reached a Plateau? Ann. Transl. Med. 2018, 6, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Di Maio, M. Platinum-Based Chemotherapy in Advanced Non-Small Cell Lung Cancer: Optimal Number of Treatment Cycles. Expert Rev. Anticancer Ther. 2016, 16, 653–660. [Google Scholar] [CrossRef]
- Rottenberg, S.; Disler, C.; Perego, P. The Re-Discovery of Platinum-Based Cancer Therapy. Nat. Rev. Cancer 2021, 21, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Krishnamurthy, S. Cellular Responses to Cisplatin-Induced DNA Damage. J. Nucleic Acids 2010, 2010, 201367. [Google Scholar] [CrossRef]
- Eastman, A. Activation of Programmed Cell Death by Anticancer Agents: Cisplatin as a Model System. Cancer Cells 1990, 2, 275–280. [Google Scholar]
- Casini, A.; Reedijk, J. Interactions of Anticancer Pt Compounds with Proteins: An Overlooked Topic in Medicinal Inorganic Chemistry? Chem. Sci. 2012, 3, 3135–3144. [Google Scholar] [CrossRef]
- Zamora, A.; Wachter, E.; Vera, M.; Heidary, D.K.; Rodríguez, V.; Ortega, E.; Fernández-Espín, V.; Janiak, C.; Glazer, E.C.; Barone, G.; et al. Organoplatinum(II) Complexes Self-Assemble and Recognize AT-Rich Duplex DNA Sequences. Inorg. Chem. 2021, 60, 2178–2187. [Google Scholar] [CrossRef]
- Veclani, D.; Tolazzi, M.; Cerón-Carrasco, J.P.; Melchior, A. Intercalation Ability of Novel Monofunctional Platinum Anticancer Drugs: A Key Step in Their Biological Action. J. Chem. Inf. Model. 2021, 61, 4391–4399. [Google Scholar] [CrossRef]
- Wang, F.-Y.; Liu, R.; Huang, K.-B.; Feng, H.-W.; Liu, Y.-N.; Liang, H. New Platinum(II)-Based DNA Intercalator: Synthesis, Characterization, and Anticancer Activity. Inorg. Chem. Commun. 2019, 105, 182–187. [Google Scholar] [CrossRef]
- Pages, B.J.; Garbutcheon-Singh, K.B.; Aldrich-Wright, J.R. Platinum Intercalators of DNA as Anticancer Agents. Eur. J. Inorg. Chem. 2017, 2017, 1613–1624. [Google Scholar] [CrossRef]
- Tan, Y.; Xiang, D.; Pei, J.; Tan, X. Novel Anti-Tumor Compound and Application Thereof in Preparing Anti-Tumor Drugs. U.S. Patent 2020/0377545 A1, 3 December 2020. [Google Scholar]
- Leitão, M.I.P.S.; Orsini, G.; Murtinheira, F.; Gomes, C.S.B.; Herrera, F.; Petronilho, A. Synthesis, Base Pairing Properties and Biological Activity Studies of Platinum(II) Complexes Based on Uracil Nucleosides. Inorg. Chem. 2023, 62, 16412–16425. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, S.; Falanga, A.P.; Greco, F.; Piccialli, G.; Oliviero, G.; Borbone, N. State of Art in the Chemistry of Nucleoside-Based Pt(II) Complexes. Bioorg. Chem. 2023, 131, 106325. [Google Scholar] [CrossRef] [PubMed]
- De Castro, F.; De Luca, E.; Girelli, C.R.; Barca, A.; Romano, A.; Migoni, D.; Verri, T.; Benedetti, M.; Fanizzi, F.P. First Evidence for N7-Platinated Guanosine Derivatives Cell Uptake Mediated by Plasma Membrance Transport Processes. J. Inorg. Biochem. 2022, 226, 111660. [Google Scholar] [CrossRef] [PubMed]
- Pullen, S.; Hiller, W.G.; Lippert, B. Regarding the Diamagnetic Components in Rosenberg’s “Platinum Pyrimidine Blues”: Species in the Cis-Pt(NH3)2-1-Methyluracil System. Inorg. Chim. Acta 2019, 494, 168–180. [Google Scholar] [CrossRef]
- Montagner, D.; Gandin, V.; Marzano, C.; Longato, B. Synthesis, Characterization and Cytotoxic Properties of Platinum(II) Complexes Containing Nucleosides Adenosine and Cytidine. J. Inorg. Biochem. 2011, 105, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Cleare, M.J.; Hoeschele, J.D. Studies on the Antitumor Activity of Group VIII Transition Metal Complexes. Part I. Platinum(II) Complexes. Bioinorg. Chem. 1973, 2, 187–210. [Google Scholar] [CrossRef]
- Mueller, K.; Schütz, C.; Rüffer, T.; Bette, M.; Kaluderović, G.N.; Steinborn, D.; Schmidt, H. Synthesis, Characterization, Structures and in vitro Antitumor Activity of Platinum(II) Complexes Bearing Adeninato or Methylated Adeninato Ligands. Inorg. Chim. Acta 2020, 507, 119539. [Google Scholar] [CrossRef]
- Aseman, M.D.; Aryamanesh, S.; Shojaeifard, Z.; Hemmateenejad, B.; Nabavizadeh, S.M. Cycloplatinated(II) Derivatives of Mercaptopurine Capable of Binding Interactions with HSA/DNA. Inorg. Chem. 2019, 58, 16154–16170. [Google Scholar] [CrossRef]
- Liskova, B.; Zerzankova, L.; Novakova, O.; Kostrhunova, H.; Travnicek, Z.; Brabec, V. Cellular Response to Antitumor Cis-Dichlorido Platinum(II) Complexes of CDK Inhibitor Bohemine and Its Analogues. Chem. Res. Toxicol. 2012, 25, 500–509. [Google Scholar] [CrossRef]
- Dvořák, Z.; Štarha, P.; Trávníček, Z. Evaluation of In Vitro Cytotoxicity of 6-Benzylaminopurine Carboplatin Derivatives against Human Cancer Cell Lines and Primary Human Hepatocytes. Toxicol. Vitr. 2011, 25, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Trávníček, Z.; Štarha, P.; Popa, I.; Vrzal, R.; Dvořák, Z. Roscovitine-Based CDK Inhibitors Acting as N-Donor Ligands in the Platinum(II) Oxalato Complexes: Preparation, Characterization, and In Vitro Cytotoxicity. Eur. J. Med. Chem. 2010, 45, 4609–4614. [Google Scholar] [CrossRef] [PubMed]
- Dvořák, L.; Popa, I.; Štarha, P.; Trávníček, Z. In Vitro Cytotoxic-Active Platinum(II) Complexes Derived from Carboplatin and Involving Purine Derivatives. Eur. J. Inorg. Chem. 2010, 2010, 3441–3448. [Google Scholar] [CrossRef]
- Vrzal, R.; Štarha, P.; Dvořák, Z.; Trávníček, Z. Evaluation of In Vitro Cytotoxicity and Hepatotoxicity of Platinum(II) and Palladium(II) Oxalato Complexes with Adenine Derivatives and Carrier Ligands. J. Inorg. Biochem. 2010, 104, 1130–1132. [Google Scholar] [CrossRef]
- Štarha, P.; Trávníček, Z.; Popa, I. Platinum(II) Oxalato Complexes with Adenine-Based Carrier Ligands Showing Significant In Vitro Antitumor Activity. J. Inorg. Biochem. 2010, 104, 639–647. [Google Scholar] [CrossRef]
- Scűčová, L.; Trávníček, Z.; Zatloukal, M.; Popa, I. Novel Platinum(II) and Palladium(II) Complexes with Cyclin-Dependent Kinase Inhibitors: Synthesis, Characterization and Antitumour Activity. Bioorg. Med. Chem. 2006, 14, 479–491. [Google Scholar] [CrossRef]
- Maloň, M.; Trávníček, Z.; Marek, R.; Strnad, M. Synthesis, Spectral Study and Cytotoxicity of Platinum(II) Complexes with 2,9-Disubstituted-6-Benzylaminopurines. J. Inorg. Biochem. 2005, 99, 2127–2138. [Google Scholar] [CrossRef]
- Trávníček, Z.; Maloň, M.; Zatloukal, M.; Doležal, K.; Strnad, M.; Marek, J. Mixed Ligand Complexes of Platinum(II) and Palladium(II) with Cytokinin-Derived Compounds Bohemine and Olomoucine: X-ray Structure of [Pt(BohH+-N7)Cl3 · 9/5 H2O {Boh = 6-(Benzylamino)-2-[(3-Hydroxypropyl)-Amino]-9-Isopropylpurine, Bohemine}. J. Inorg. Biochem. 2003, 94, 307–316. [Google Scholar] [CrossRef]
- Baranowska-Kortylewicz, J.; Pavlik, E.J.; Smith, W.T., Jr.; Flanigan, R.C.; Van Nagell, J.R., Jr.; Ross, D.; Kenady, D.E. Dichloro(6-aminoethylaminopurine)platinum(II) and its Hydroxy Analogues: Synthesis and Preliminary Evaluation. Inorg. Chim. Acta 1985, 108, 91–98. [Google Scholar] [CrossRef]
- Arabi, A.; Cogley, M.O.; Fabrizio, D.; Stitz, S.; Howard, W.A.; Wheeler, K.A. Anticancer Activity of Nonpolar Pt(CH3)2I2{bipy} Is Found to be Superior among Four Similar Organoplatinum(IV) Complexes. J. Mol. Struct. 2023, 1274, 134551. [Google Scholar] [CrossRef]
- Clark, H.C.; Manzer, L.E. Reactions of (π-1,5-Cyclooctadiene)organoplatinum(II) Compounds and the Synthesis of Perfluoroalkylplatinum Complexes. J. Organomet. Chem. 1973, 59, 411–428. [Google Scholar] [CrossRef]
- Štarha, P.; Vančo, J.; Trávníček, Z. Platinum Complexes Containing Adenine-Based Ligands: An Overview of Selected Structural Features. Coord. Chem. Rev. 2017, 332, 1–29. [Google Scholar] [CrossRef]
- Appleton, T.G. NMR Spectroscopy, Heteronuclei, La-Hg. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Lindon, J.C., Tranter, G.E., Koppenaal, D.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 342–345. [Google Scholar]
- Sheldrick, G.M. SADABS and TWINABS—Program for Area Detector Absorption Corrections; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Barbour, L.J. X-Seed 4: Updates to a Program for Small-Molecule Supramolecular Crystallography. J. Appl. Cryst. 2020, 53, 1141–1146. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.; Kunkel, M.W.; White, S.L.; Wishka, D.G.; Lopez, O.D.; Bowles, L.; Brady, P.S.; Ramsey, P.; Grams, J.; Rohrer, T.; et al. Targeted Investigational Oncology Agents in the NCI60: A Phenotypic Systems-Based Resource. Mol. Cancer. Ther. 2023, 22, 1270–1279. [Google Scholar] [CrossRef]
- Shoemaker, R.H. The NCI60 Human Tumor Cell Line Anticancer Drug Screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a High-Flux Anticancer Drug Screen Using a Diverse Panel of Cultured Human Tumor Cell Lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef]
- Khan, B.T.; Kumari, S.V.; Goud, G.N. Complexes of Platinum (II & IV) with Adenine & Guanine. Indian J. Chem. A 1982, 21, 264–267. [Google Scholar]
- Ebert, K.H.; Massa, W.; Donath, H.; Lorberth, J.; Seo, B.-S.; Herdtweck, E. Organoplatinum Compounds: VI. Trimethylplatinum Thiomethylate and Trimethylplatinum Iodide. The Crystal Structures of [Pt(CH3)3S(CH3)]4 and [Pt(CH3)3I]4 ∙ 0.5 CH3I. J. Organomet. Chem. 1998, 559, 203–207. [Google Scholar] [CrossRef]
- Zhu, X.; Rusanov, E.; Kluge, R.; Schmidt, H.; Steinborn, D. Synthesis, Characterization, and Structure of [{PtMe3(9-MeA)}3] (9-MeA = 9-Methyladenine): A Cyclic Trimeric Platinum(IV) Complex with a Nucleobase. Inorg. Chem. 2002, 41, 2667–2671. [Google Scholar] [CrossRef]
- Batsanov, S.S. Van der Waals Radii of Elements. Inorg. Mater. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Gazdar, A.F.; Minna, J.D. NCI Series of Cell Lines: An Historical Perspective. J. Cell. Biochem. Suppl. 1996, 24, 1–11. [Google Scholar] [CrossRef]
- Yamori, T.; Matsunaga, A.; Sato, S.; Yamazaki, K.; Komi, A.; Ishizu, K.; Mita, I.; Edatsugi, H.; Matsuba, Y.; Takezawa, K.; et al. Potent Antitumor Activity of MS-247, a Novel DNA Minor Groove Binder, Evaluated by an In Vitro and In Vivo Human Cancer Cell Line Panel. Cancer Res. 1999, 59, 4042–4049. [Google Scholar] [PubMed]
- Rajput, A.; San Martin, I.D.; Rose, R.; Beko, A.; LeVea, C.; Sharratt, E.; Mazurchuk, R.; Hoffman, R.M.; Brattain, M.G.; Wang, J. Characterization of HCT116 Human Colon Cancer Cells in an Orthotopic Model. J. Surg. Res. 2008, 147, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Fogh, J.; Trempe, G. Human Tumor Cells In Vitro; Springer: Boston, MA, USA, 1975; pp. 115–159. [Google Scholar]
- Siekmann, W.; Tina, E.; Von Sydow, A.K.; Gupta, A. Effect of Lidocaine and Ropivacaine on Primary (SW480) and Metastatic (SW620) Colon Cancer Cell Lines. Oncol. Lett. 2019, 18, 395–401. [Google Scholar] [CrossRef]
- Rutka, J.T.; Giblin, J.R.; Høifødt, H.K.; Dougherty, D.V.; Bell, C.W.; McCulloch, J.R.; Davis, R.L.; Wilson, C.B.; Rosenblum, M.L. Establishment and Characterization of a Cell Line from a Human Gliosarcoma. Cancer Res. 1986, 46, 5893–5902. [Google Scholar]
- Fodstad, O.; Aamdal, S.; McMenamin, M.; Nesland, J.M.; Pihl, A. A New Experimental Metastasis Model in Athymic Nude Mice, the Human Malignant Melanoma LOX. Int. J. Cancer 1988, 41, 442–449. [Google Scholar] [CrossRef]
- Fogh, J.; Fogh, J.M.; Orfeo, T. One Hundred and Twenty-Seven Cultured Human Tumor Cell Lines Producing Tumors in Nude Mice. J. Natl. Cancer Inst. 1977, 59, 221–226. [Google Scholar] [CrossRef]
- Chambers, A.F. MDA-MB-435 and M14 Cell Lines: Identical but not M14 Melanoma. Cancer Res. 2009, 69, 5292–5293. [Google Scholar] [CrossRef]
- Rae, J.M.; Creighton, C.J.; Meck, J.M.; Haddad, B.R.; Johnson, M.D. MDA-MB-435 cells are derived from M14 Melanoma cells––a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res. Treat. 2007, 104, 13–19. [Google Scholar] [CrossRef]
- Carey, T.E.; Takahashi, T.; Resnick, L.A.; Oettgen, H.F.; Old, L.J. Cell surface antigens of human malignant melanoma. I. Mixed hemadsorption assays for humoral immunity to cultured autologous melanoma cells. Proc. Natl. Acad. Sci. USA 1976, 73, 3278–3282. [Google Scholar] [CrossRef] [PubMed]
- Sustarsic, E.G.; Junnila, R.K.; Kopchick, J.J. Human metastatic melanoma cell lines express high levels of growth hormone receptor and respond to GH treatment. Biochem. Biophys. Res. Commun. 2013, 441, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.W.; Laub, P.B.; Beesley, J.S.; Ozols, R.F.; Hamilton, T.C. Increased platinum-DNA damage tolerance is associated with cisplatin resistance and cross-resistance to various chemotherapeutic agents in unrelated human ovarian cancer cell lines. Cancer Res. 1997, 57, 850–856. [Google Scholar] [PubMed]
- Blayney, J.K.; Davison, T.; McCabe, N.; Walker, S.; Keating, K.; Delaney, T.; Greenan, C.; Williams, A.R.; McCluggage, W.G.; Capes-Davis, A.; et al. Prior knowledge transfer across transcriptional data sets and technologies using compositional statistics yields new mislabelled ovarian cell line. Nucleic Acids Res. 2016, 44, e137. [Google Scholar] [CrossRef]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P.J. In vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef]
- Cailleau, R.; Young, R.; Olivé, M.; Reeves, W.J.J., Jr. Breast tumor cell lines from pleural effusions. Natl. Cancer Inst. 1974, 53, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Neve, R.M.; Chin, K.; Fridlyand, J.; Yeh, J.; Baehner, F.L.; Fevr, T.; Clark, L.; Bayani, N.; Coppe, J.-P.; Tong, F.; et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006, 10, 515–527. [Google Scholar] [CrossRef]
- Cailleau, R.; Olivé, M.; Cruciger, Q.V.J. Long-term human breast carcinoma cell lines of metastatic origin: Preliminary characterization. In Vitro 1978, 14, 911–915. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy Plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Pouryasin, Z.; Yousefi, R.; Nabavizadeh, S.M.; Rashidi, M.; Hamidizadeh, P.; Alavianmehr, M.-M.; Moosavi-Movahedi, A.A. Anticancer and DNA Binding Activities of Platinum(IV) Complexes: Importance of Leaving Group Departure Rate. Appl. Biochem. Biotechnol. 2014, 172, 2604–2617. [Google Scholar] [CrossRef] [PubMed]
- Escolà, A.; Crespo, M.; López, C.; Quirante, J.; Jayaraman, A.; Polat, I.H.; Badía, J.; Baldomà, L.; Cascante, M. On the Stability and Biological Behavior of Cyclometallated Pt(IV) Complexes with Halido and Aryl Ligands in the Axial Positions. Bioorg. Med. Chem. 2016, 24, 5804–5815. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M. Cyclometallated Platinum(IV) Compounds as Promising Antitumour Agents. J. Organomet. Chem. 2019, 879, 15–26. [Google Scholar] [CrossRef]
- Tan, M.-X.; Wang, Z.-F.; Qin, Q.-P.; Huang, X.-L.; Zou, B.-Q.; Liang, H. Complexes of Platinum(II/IV) with 2-Phenylpyridine Derivatives as a New Class of Promising Anticancer Agents. Inorg. Chem. Commun. 2019, 108, 107510. [Google Scholar] [CrossRef]
- Annunziata, A.; Amoresano, A.; Cucciolito, M.E.; Esposito, R.; Ferraro, G.; Iacobucci, I.; Imbimbo, P.; Lucignano, R.; Melchiorre, M.; Monti, M.; et al. Pt(II) Versus Pt(IV) in Carbene Glycoconjugate Antitumor Agents: Minimal Structural Variations and Great Performance Changes. Inorg. Chem. 2020, 59, 4002–4014. [Google Scholar] [CrossRef]
- Bauer, E.; Domingo, X.; Balcells, C.; Polat, I.H.; Crespo, M.; Quirante, J.; Badía, J.; Baldomà, L.; Font-Bardia, M.; Cascante, M. Synthesis, Characterization and Biological Activity of New Cyclometallated Platinum(IV) Iodido Complexes. Dalton Trans. 2017, 46, 14973–14987. [Google Scholar] [CrossRef]
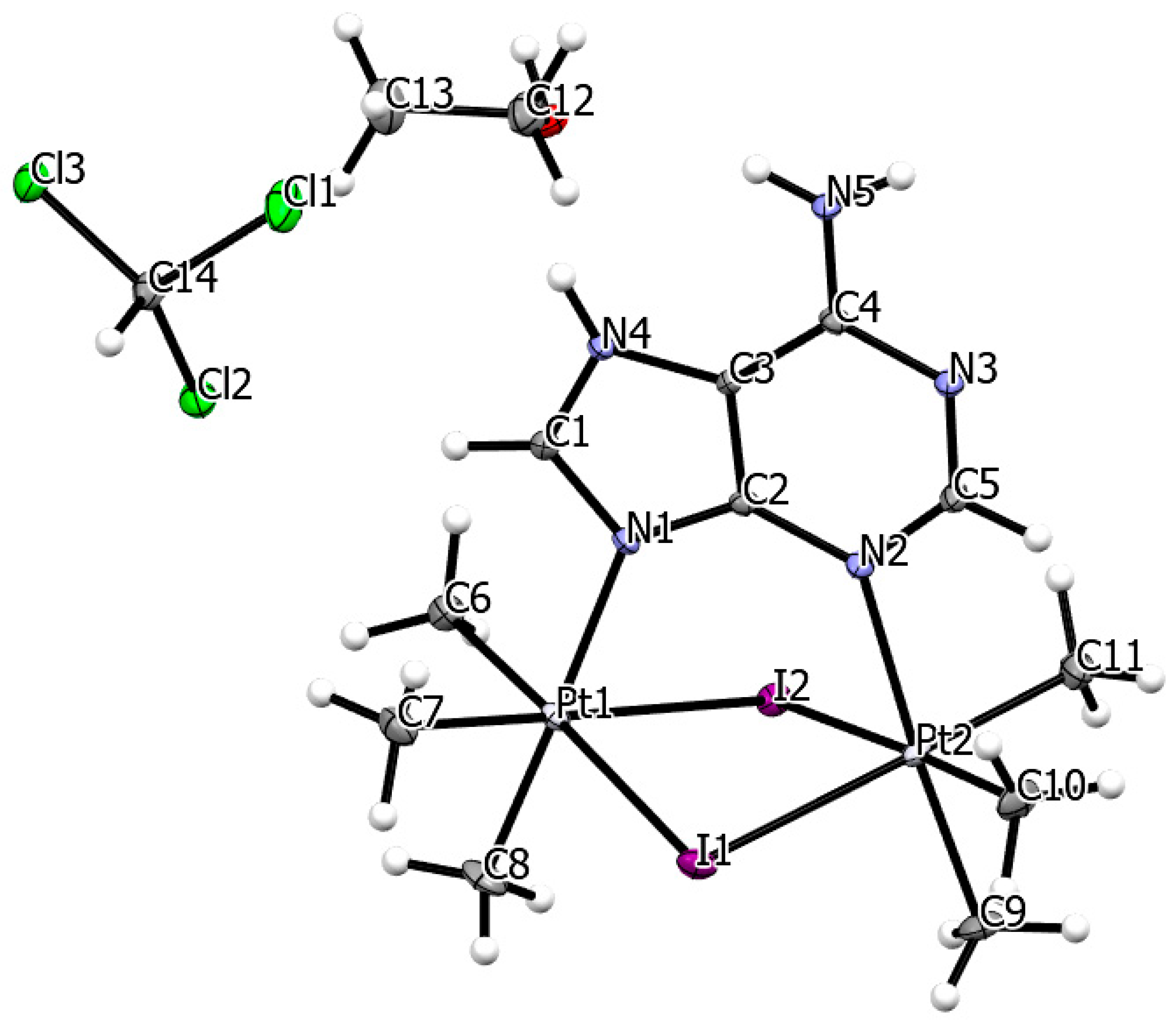
| formula | C14H30Cl3I2N5OPt2 | |
| molecular weight, g mol−1 | 1034.76 | |
| temperature, K | 100.00 | |
| wavelength, Å | 0.71073 | |
| lattice | triclinic | |
| space group | P−1 | |
| cell constants | ||
| a, Å | 10.1669(9) | |
| b, Å | 10.5929(9) | |
| c, Å | 13.0266(11) | |
| α, deg. | 92.574(3) | |
| β, deg. | 109.885(3) | |
| γ, deg. | 103.182(4) | |
| volume, Å3 | 1272.97(19) | |
| Z | 2 | |
| ρ (calc.) g cm−3 | 2.700 | |
| absorption coefficient, mm−1 | 13.732 | |
| F(000) | 940 | |
| crystal size, mm3 | 0.337 × 0.189 × 0.066 | |
| θ range | 1.992 to 33.778° | |
| index ranges | −15 ≤ h ≤ 15 −16 ≤ k ≤ 16 −20 ≤ l ≤ 20 | |
| reflections collected | 83,153 | |
| independent reflections | 10,210 [Rint = 0.0328] | |
| coverage, independent reflections | 100% | |
| absorption correction | Multi scan | |
| max. and min. transmission | 0.7467 and 0.3892 | |
| refinement method | Full-matrix least-squares on F2 | |
| data/restraints/parameters | 10,210/1/263 | |
| goodness-of-fit on F2 | 1.085 | |
| final R indices [I > 2σ(I)] | R1 = 0.0223 | wR2 = 0.0460 |
| R indices (all data) | R1 = 0.0282 | wR2 = 0.0475 |
| largest difference peak and hole | 1.413 &–1.998 e. Å−3 | |
| Bond | Bond Length (Å) | Bond Angle | Degrees |
| Pt(1)–N(1) | 2.203(2) | Pt(1)–I(1)–Pt(2) | 87.111(9) |
| Pt(1)–I(1) | 2.7548(3) | N(1)–Pt(1)–I(1) | 89.22(6) |
| Pt(1)–I(2) | 2.8004(3) | N(1)–Pt(1)–I(2) | 87.14(6) |
| Pt(1)–C(6) | 2.064(3) | N(1)–Pt(1)–C(6) | 93.04(10) |
| Pt(1)–C(7) | 2.055(3) | N(1)–Pt(1)–C(7) | 91.37(10) |
| Pt(1)–C(8) | 2.051(3) | N(1)–Pt(1)–C(8) | 178.54(11) |
| Pt(1) ------ Pt(2) | 3.805 | C(6)–Pt(1)–I(1) | 177.73(9) |
| Pt(2)–N(2) | 2.207(2) | C(6)–Pt(1)–I(2) | 91.30(8) |
| Pt(2)–I(1) | 2.7672(3) | C(6)–Pt(1)–C(7) | 89.70(12) |
| Pt(2)–I(2) | 2.7887(3) | I(1)–Pt(1)–I(2) | 88.976(8) |
| Pt(2)–C(10) | 2.054(3) | Pt(2)–I(2)–Pt(1) | 88.966(9) |
| Pt(2)–C(11) | 2.053(3) | N(2)–Pt(2)–I(1) | 89.78(6) |
| Pt(2)–C(9) | 2.050(3) | N(2)–Pt(2)–I(2) | 90.31(6) |
| N(2)–Pt(2)–C(10) | 91.23(10) | ||
| N(2)–Pt(2)–C(11) | 90.09(10) | ||
| N(2)–Pt(2)–C(9) | 176.21(10) | ||
| C(10)–Pt(2)–I(1) | 89.51(9) | ||
| C(10)–Pt(2)–I(2) | 177.83(9) | ||
| C(10)–Pt(2)–C(11) | 91.58(13) | ||
| I(1)–Pt(2)–I(2) | 88.966(9) |
| Type of Cancer | Cell Line, Reference | GI50, μM | TGI, μM | LC50, μM |
| Non-Small Cell Lung | NCI-H522, [45] | 1.51 | 3.70 | 18.6 |
| Colon Cancer | HCC-2998, [46] | 13.1 (10.7) | 31.7 (25.5) | 76.8 (60.8) |
| HCT-116, [47] | 3.43 (9.24) | 15.5 (>100) | 52.5 (>100) | |
| HT29, [48] | 5.99 (7.81) | 21.2 (>100) | 63.6 (>100) | |
| SW-620, [49] | 3.37 (2.72) | 11.4 (>100) | 39.2 (>100) | |
| Central Nervous System | SF-539, [50] | 1.47 (0.600) | 2.76 (7.67) | 5.20 (>100) |
| Melanoma | LOX IMVI, [51] | 3.85 (0.961) | 18.4 (7.93) | 58.2 (>100) |
| Malme-3M, [52] | 1.95 (1.85) | 8.19 (4.58) | 39.2 (>100) | |
| M14, [53] | 2.08 (1.71) | 14.8 (7.68) | 63.4 (35.1) | |
| MDA-MB-435, [54] | 6.19 (2.77) | 18.4 (14.4) | 46.2 (42.7) | |
| SK-MEL-28, [55] | 5.10 (2.99) | 17.4 (12.8) | 42.4 (99.5) | |
| UACC-62, [56] | 3.18 (1.34) | 15.7 (9.39) | 42.0 (37.2) | |
| Ovarian Cancer | OVCAR-5, [57,58] | 2.35 (4.38) | 11.9 (46.4) | 35.2 (>100) |
| Renal Cancer | A498, [59] | 19.7 (12.7) | 37.8 (26.4) | 72.6 (54.9) |
| RXF-393, [40] | ---------- | 2.63 (4.58) | 5.99 (24.2) | |
| Breast Cancer | MDA-MB-231, [60] | 1.62 (28.1) | 8.79 (>100) | 60.2 (>100) |
| BT-549, [61] | 3.56 (3.36) | 16.5 (44.9) | 61.2 (>100) | |
| MDA-MB-468, [62] | 1.49 (0.233) | 3.85 (0.744) | 19.2 (4.50) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Brien, A.M.; Wheeler, K.A.; Howard, W.A. Synthesis, Structure, and Anticancer Activity of a Dinuclear Organoplatinum(IV) Complex Stabilized by Adenine. Compounds 2025, 5, 16. https://doi.org/10.3390/compounds5020016
O’Brien AM, Wheeler KA, Howard WA. Synthesis, Structure, and Anticancer Activity of a Dinuclear Organoplatinum(IV) Complex Stabilized by Adenine. Compounds. 2025; 5(2):16. https://doi.org/10.3390/compounds5020016
Chicago/Turabian StyleO’Brien, Alisha M., Kraig A. Wheeler, and William A. Howard. 2025. "Synthesis, Structure, and Anticancer Activity of a Dinuclear Organoplatinum(IV) Complex Stabilized by Adenine" Compounds 5, no. 2: 16. https://doi.org/10.3390/compounds5020016
APA StyleO’Brien, A. M., Wheeler, K. A., & Howard, W. A. (2025). Synthesis, Structure, and Anticancer Activity of a Dinuclear Organoplatinum(IV) Complex Stabilized by Adenine. Compounds, 5(2), 16. https://doi.org/10.3390/compounds5020016








