Abstract
Herein, we present hybrid crown ether ligands with siloxane and ethylene oxide units and their coordination with the cations Li+, Na+, Mg2+ and Ca2+. The compounds prepared are (SiMe2O)2(C2H4O)3 (1, TrEGDS = Triethylenglycoldisiloxane) and (SiMe2O)2(C2H4O)4 (2, TeEGDS = Tetraethylenglycoldisiloxane)), as well as the metal complexes [Li(TrEGDS][GaI4] (3), [Na(TeEGDS)][GaI4] (4), [Mg(TrEGDS)][GaI4]2 (5) and [Ca(TeEGDS)][GaI4]2 (6). Single-crystal X-ray diffraction was used to study the prepared complexes and coordination modes in the solid state.
1. Introduction
The coordination chemistry of siloxanes was rarely represented until the year 2018 [1,2,3,4]. This is due to the weak donor abilities of these ligands compared to (crown) ethers. Taking into account ionic and covalent contributions, the Si–O bond is considered a polar covalent bond today. Negative hyperconjugation, p(O) → e*(Si–C/O), strengthens the Si–O bond but decreases Lewis basicity, which leads to weaker interactions between the siloxanes and the metal ions [5,6,7,8,9]. Scheme 1c shows the ligand (Me2SiO)5 as an example. In contrast, crown ethers (Scheme 1a) are very good ligands for metal cations, which are used very frequently and in various areas [10,11].
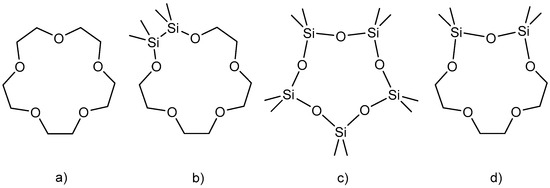
Scheme 1.
Examples of (a) crown ethers, (b) hybrid crown ethers bearing Si2Me4 units, (c) cyclosiloxanes and (d) this work.
Hybrid crown ethers are ligands containing carbon- and silicon-based fragments between the donor atoms. Such ligands with Si2Me4 units have already been described in the past (Scheme 1b). The ligands were synthesized in a ring closing reaction, and their coordination on s-block metal cations was studied. The prepared complexes bear disilane units for the purpose of imitating the C2H4 bridges of crown ethers and to obtain better coordination abilities [12,13].
Some of these ligands can be obtained by a template effect and show very high content of disilane units [13]. Despite the mentioned difficulties in the coordination of siloxanes, disiloxane fragments have also been successfully coordinated in the past. For example, Beckmann and co-workers were able to incorporate a disiloxane fragment into a five-membered ring and thus increase its basicity, enabling the formation of hydrogen bonds [8].
The Harder group were even able to coordinate the free hexamethyldisiloxane using a very Lewis-acidic Mg complex [14,15]. Only recently, Haynes et al. observed the coordination of disiloxane fragments by coordinating the dianionic di(amino)siloxane ligand (O(SiMe2NDipp)2)2− to calcium, strontium and barium ions [16].
We have incorporated disiloxane fragments into crown ethers (Scheme 1d) and report here on preliminary investigations into their coordination. With this study, we want to explore the coordination on hybrid crown ethers bearing disiloxane units. To investigate the coordination properties of such ligands, we reacted them with various s-block metal salts of the GaI4− anion. This easily accessible, weakly coordinating anion has been used in the past for coordination studies on alkali and alkaline earth metal cations [14].
2. Materials and Methods
All operations were performed under an argon atmosphere using the standard Schlenk technique. Silicon grease was not used for the reactions. PTFE paste was used instead. All solvents were dried with standard techniques, distilled and stored under argon [17]. GaI3 and the gallats were synthesized according to procedures from the literature [18,19,20]. Metal iodides were used as received.
3. Results
In recent works, we obtained a variety of coordination compounds of all alkali and earth alkali metal cations except beryllium with cyclosiloxanes with ring sizes up to (SiMe2O)9 (short D9) [19,20,21,22]. Crown ether complexes of these metals have been known for a long time and have been well examined [10,11].
The synthesis of the hybrid cyclic ligands (SiMe2O)2(C2H4O)3 (1, TrEGDS = Triethylenglycoldisiloxane) and (SiMe2O)2(C2H4O)4 (2, TeEGDS = Tetraethylenglycoldisiloxane) was carried out as shown in Scheme 2 by reaction of the corresponding glycol, (ClSiMe2)2O andNEt3 as a supporting base. For details of the synthesis and characterization see Supplementary Material (1: Figures S1–S3, S18, S23 and S24; 2: Figures S4–S6, S19, S25 and S26).
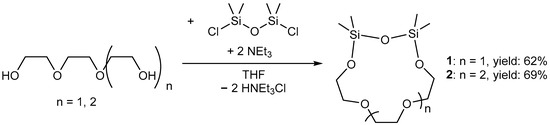
Scheme 2.
Preparation of ligands 1 and 2.
For the preparation of the desired coordination compounds, we suspended M(GaI4)z (M = Li, Na: z = 1; M = Mg, Ca: z = 2) in DCM (PhF for Ca(GaI4)2) and added the hybrid crown ethers 1 or 2, whereupon a clearing of the suspension was observed. After processing, we selectively obtained the target complexes [Li(TrEGDS)][GaI4] (3), [Na(TeEGDS)][GaI4] (4) and [Ca(TeEGDS)][GaI4]2 (6) in yields of 66–90% (Scheme 3). In contrast, the reaction starting from Mg(GaI4)2 proceeded non-selectively, with the formation of a large number of compounds, but the compound [Mg(TrEGDS)][GaI4]2 (5) could still be isolated from the product mixture in low yields.
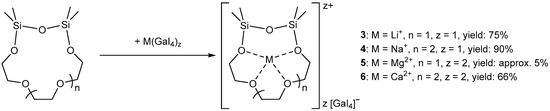
Scheme 3.
Preparation of complexes 3–6.
In the case of the Li+ cation, we used 1 as the ligand, which led to the formation of the complex [Li(TrEGDS)][GaI4] (3). For details of the synthesis and characterization see Supplementary Material (Figures S7–S10, S20, S27 and S28). Crystals of 3 suitable for single-crystal X-ray diffraction were obtained from a DCM solution (Figure 1).
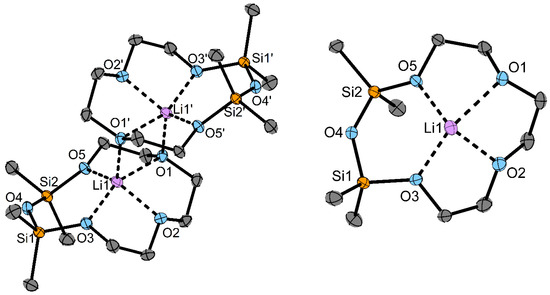
Figure 1.
(left) Dimeric structure of the cation of 3 in the solid state. (right) Top view of the cation of 3 in the solid state. Ellipsoids are shown at a 50% probability level. C-bonded H atoms, the DCM molecule and the anions are omitted for clarity. Selected bond lengths/pm: O1−Li1: 223.0(4), O2−Li1: 198.8(4), O3−Li1: 197.1(4), O5−Li1: 198.3(5). Selected bond angles/°: Si1–O4–Si2: 138.3(1).
Compound 3 crystallizes in the space group P21/n with one DCM molecule in the asymmetric unit (see Table S1 and Figure S31 for details). In the solid state, the formation of a dimer is observed by coordinating the Li atoms from four O atoms of one ligand and one O atom from another ligand to obtain a total coordination number (c. n.) of 5. The siloxane fragment is not coordinating, which underlines the weaker coordination ability of siloxane fragments compared to crown ethers. The distances of coordinating O atoms of the ligand to the Li atom are mostly in the range of 197.1(5)–198.7(5) pm; only O1 has a longer distance to the cation (223.0(5) pm), which is due to the coordination of two Li atoms. The complexes in the literature [Li(1,2-disila [12]crown-4)]SO3CF3 and [Li([12]crown-4)]SCN show slightly longer Li–O bonds in the ranges of 199.4(4)–212.6(4) pm and 205(1)–214(1) pm [23]. The siloxane angle in 3 of 138.3(1)° is slightly smaller than that of the non-coordinated siloxane fragments in the complex [Li(D6)][GaI4] (D6 = (SiMe2O)6) of 145.1(1)° [19].
The behavior in solution was studied via 1H, 13C, 29Si and 7Li NMR spectroscopy. Compound 3 shows a low-field shift in the respective NMR spectra for the SiMe2 units (1H: 0.29, 29Si: −0.5 ppm) compared to the free ligand 1 (1H: 0.09, 29Si: −12.9 ppm). This confirms that at least the outer oxygen atoms of the siloxane fragment (O3 and O5) coordinate to the metal cation even in the solution. Whether O4 also coordinates in solution cannot be determined from these data.
For Na, we chose ligand 2 due to its bigger cation size. The reaction led to the formation of the complex [Na(TeEGDS)][GaI4] (4). For details of the synthesis and characterization see Supplementary Material (Figures S11–S13, S21, S29 and S30). The molecular structure of 4 is shown in Figure 2.
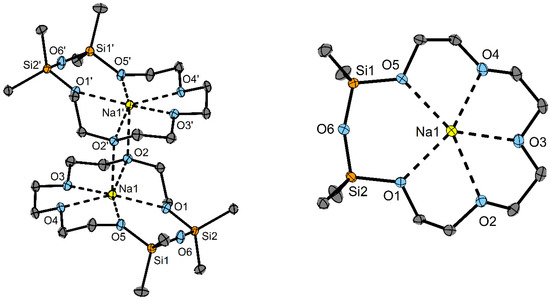
Figure 2.
(left) Dimeric structure of the cation in 4 in the solid state (right). Top view of the cation of 4 in the solid state. Ellipsoids are shown at a 50% probability level. C-bonded H atoms and anions are omitted for clarity. Selected bond lengths/pm: O1−Na1: 241.9(2), O2−Na1: 262.4(2), O3−Na1: 239.4(2), O4−Na1: 240.5(2), O5−Na1: 241.8(2). Selected bond angles/°: Si1–O6–Si2: 151.4(1).
Compound 4 crystallizes in the space group P21/n with one molecule in the asymmetric unit (Table S2 and Figure S32). As for compound 3, the formation of a dimer is observed. The Na atoms are coordinated from five O atoms of one ligand and one O atom from another ligand to obtain a total c. n. of six. Additionally, a weak interaction from one I atom of the anion is present (see SI). The siloxane fragment is, again, not coordinating.
The O–Na distances vary between 239.3(2) and 241.9(2) pm; only O2 has a longer distance to the cation (262.4(2) pm), which is again due to the bridging position between two sodium cations. The O–Na distances of the complexes from the literature [Na(1,2-disila[15]crown-5)]ClO4 (240.2(2)–250.8(2) pm) and [Na[15]crown-5]NCS (235.6(4)-246.1(3) are in the same range as in 4 [24]. The siloxane angle in 4 amounts to 151.4(1), which is slightly smaller than that of the non-coordinating siloxane fragments in the complex [Na(D6][GaI4], with 162.6(4)° [19].
Compound 4 shows, like compound 3, a low-field shift in the respective NMR spectra of the SiMe2 groups (1H: 0.22, 29Si: −3.6 ppm) in comparison to the free ligand 2 (1H: 0.10, 29Si: −11.9 ppm).
Subsequently, we explored the coordination behavior of ligands 1 and 2 with the earth alkali metal cations Mg2+ and Ca2+.
Unfortunately, the synthesis of the Mg compound led only to unselective reactions (for details Figure S14). We suppose this is due to the very hard Mg2+ ion, which undergoes strong interactions with siloxane fragments and leads to ring-opening reactions. The 1H NMR spectrum shows up to six different compounds. The chemical shifts suggest four different compounds in which a ligand containing SiMe2O groups coordinates to Mg2+ ions, and two silicon-containing compounds that do not coordinate to a metal cation. Nevertheless, we were able to obtain the crystal structure of [Mg(TrEGDS)][GaI4]2 (5, Table S3 and Figure S33), which is shown in Figure 3. As one of the by-products, we were able to characterize the glycol complex [Mg{(CH2OC2H4OH)2}2][GaI4]2 (5b, Table S4 and Figure S34).
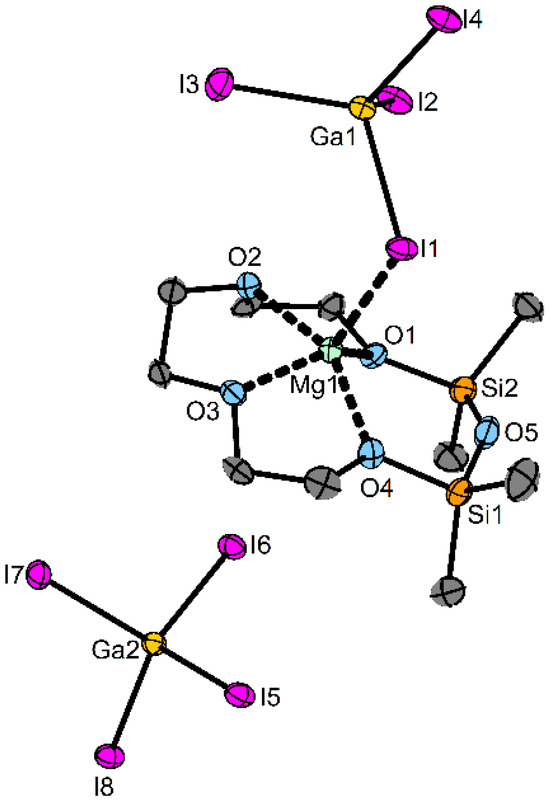
Figure 3.
Molecular structure of 5 in the solid state. Ellipsoids are shown at a 50% probability level. C-bonded H atoms are omitted for clarity. Selected bond lengths/pm: O1−Mg1: 200.1(5), O2−Mg1: 203.5(5), O3−Mg1: 202.5(5), O4−Mg1: 201.3(5), I1−Mg1: 276.9(2). Selected bond angles/°: Si1–O5–Si2: 146.0(3).
Compound 5 crystallizes in the space group P21/n with one molecule in the asymmetric unit. In contrast to the alkali metal ions, no dimerization of the cations appears in the solid state. Instead, one [GaI4]− ion coordinates the Mg atom in addition to the four carbon-bonded O atoms (c. n. 5). Even the very Lewis-acidic Mg2+ does not lead to a coordination of the siloxane fragment in the solid state. The distances of the four coordinating O atoms are in the range of 200.1(5)–203.5(5) pm, which is shorter than in [Mg(1,2-disila[15]crown-5)Br2] (222.3(2)–235.1(2)) pm due to the larger ring size in the literature. Compared to [(Ar[tBu]N)3Ti(μ-O)([12]crown-4)Mg(μ-CO3)Ti(N[tBu]Ar)3] (Ar = 3,5-Me2C6H3, 217.9(4)-226.3(4) pm), 5 has shorter O–Mg bonds due to the fact that the Mg2+ ion is located outside the crown ether. The Si–O–Si angle of 146.0(3)° is smaller than in the non-coordinating siloxane fragment in [Mg(D6)I2] with 161.2(1) [21,25].
For the bigger Ca2+ ion, we used 2 as a fitting ligand to obtain [Ca(TeEGDS)][GaI4]2 (6). For details of the synthesis and characterization see Supplementary Material (Figures S15–S17 and S22). The reaction was unselective in DCM but selective in fluorobenzene. The molecular structure of 6 is shown in Figure 4.
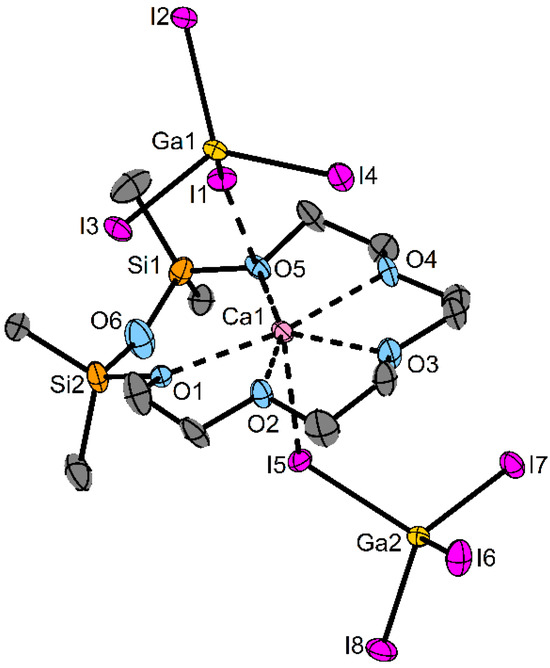
Figure 4.
Molecular structure of 6 in the solid state. Ellipsoids are shown at a 50% probability level. C-bonded H atoms, distortion and the second molecule of the asymmetric unit are omitted for clarity. Selected bond lengths/pm: O1−Ca1: 240.5(5), O2−Ca1: 239.4(5), O3−Ca1: 237.4(6), O4−Ca1: 240.9(5), O5−Ca1: 242.9(5), O7−Ca2: 240.1(5), O8−Ca2: 239.7(5), O9−Ca2: 239.9(5), O10−Ca2: 239.6(5), O11−Ca2: 241.7(5), I1−Ca1: 317.35(15), I5−Ca1: 319.68(14), I9−Ca2: 319.17(14), I13−Ca2: 317.19(14). Selected bond angles/°: Si1–O6–Si2: 153.8(5), Si3–O12–Si4: 152.2(4).
Compound 6 crystallizes in the space group P with two molecules in the asymmetric unit (Table S5 and Figure S35). As with compounds 3–5, the siloxane O atom is not coordinating. Overall, the Ca atom is coordinated by five oxygen atoms and one I atom from each anion to obtain a total c. n. of 7. The distances between O and Ca atoms vary between 237.4(6) and 242.9(5) pm and are similar to the values in [Ca(1,2-disila[18]crown-6)]OTf2 (243.6(1)–247.9(1) pm) but shorter than in [Ca(PhCOCHCOCF3)2(15-crown-5)] (254.8(2)–276.1(3) pm) due to the fact that the Ca2+ ion is located out of the crown ether plane. The siloxane angles are 152.2(4)–153.8(5)°, which is also quite fitting for the non-coordinating siloxane units in [Ca(D7)I2] (149.4(2)–152.8(2)°) [21,26]. The chemical shifts (1H: 0.41, 29Si: −2.4 ppm) are again low-field shifted compared to the free ligand.
4. Discussion
In this study, we report the preparation of the hybrid crown ethers TrEGDS (1, TrEGDS = Triethylenglycoldisiloxane, (SiMe2O)2(O(C2H4)3) and TeEGDS (2, TeEGDS = Tetraethylenglycoldisiloxane, (SiMe2O)2(O(C2H4)4) as well as their coordination compounds [Li(TrEGDS][GaI4] (3), [Na(TeEGDS)][GaI4] (4), [Mg(TrEGDS)][GaI4]2 (5) and [Ca(TeEGDS)][GaI4]2 (6). The investigations showed, based on X-ray diffraction studies, that disiloxane units (SiMe2–O–SiMe2) are, as expected, weaker coordinating in hybrid crown ethers than disilane units (Si2Me4–O–Si2Me4). In none of the coordination compounds obtained does the siloxane oxygen atom participate in the coordination. It can be seen that in such ligands, the disiloxane fragment merely acts as a bridge between the donor atoms. The reaction with the hard Mg2+ ion was unfortunately unselective following ring-opening reactions. For the alkali metal ions, the formation of dimers was observed in the solid state. In the future, we want to expand these investigations with higher homologues, mismatching conditions and template-assisted syntheses of these complexes and build hybrid crown ether complexes with higher numbers of siloxane units.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/compounds5020011/s1, Figure S1: 1H NMR spectrum of 1 (CD2Cl2, 500 MHz); Figure S2: 13C{1H} NMR spectrum of 1 (CD2Cl2, 126 MHz); Figure S3: 29Si{1H} NMR spectrum of 1 (CD2Cl2, 99 MHz); Figure S4: 1H NMR spectrum of 2 (CD2Cl2, 500 MHz; Figure S5: 13C{1H} NMR spectrum of 2 (CD2Cl2, 126 MHz); Figure S6: 29Si{1H} NMR spectrum of 2 (CD2Cl2, 99 MHz); Figure S7: 1H NMR spectrum of 3 (CD2Cl2, 500 MHz); Figure S8: 13C{1H} NMR spectrum of 3 (CD2Cl2, 126 MHz); Figure S9: 29Si{1H} NMR spectrum of 3 (CD2Cl2, 99 MHz); Figure S10: 7Li{1H} NMR spectrum of 3 (CD2Cl2, 194 MHz); Figure S11: 1H NMR spectrum of 4 (CD2Cl2, 500 MHz); Figure S12: 13C{1H} NMR spectrum of 4 (CD2Cl2, 126 MHz); Figure S13: 29Si{1H} NMR spectrum of 4 (CD2Cl2, 99 MHz); Figure S14: 1H NMR spectrum from the reaction of Mg(GaI4)2 with TrEGDS (CD2Cl2, 500 MHz); Figure S15: 1H NMR spectrum of 6 (CD2Cl2, 500 MHz); Figure S16: 13C{1H} NMR spectrum of 6 (CD2Cl2, 126 MHz); Figure S17: 29Si{1H} NMR spectrum of 6 (CD2Cl2, 99 MHz); Figure S18: IR spectrum of 1; Figure S19: IR spectrum of 2; Figure S20: IR spectrum of 3; Figure S21: IR spectrum of 4; Figure S22: IR spectrum of 6; Figure S23: ESI spectrum of 1; Figure S24: ESI+ HR spectrum (top: observed, bottom: calculated) of [Na(TrEGDS)]+; Figure S25: ESI+ spectrum of 2; Figure S26: ESI+ HR spectrum (top: observed, bottom: calculated) of [Na(TeEGDS)]+; Figure S27: CI+ spectrum of 3; Figure S28: CI+ HR spectrum (top: observed, bottom: calculated) of [H(TrEGDS)]+; Figure S29: ESI+ spectrum of 4; Figure S30: ESI+ HR spectrum (top: observed, bottom: calculated) of [Na(TeEGDS)]+; Figure S31: Dimeric form of 3×DCM in the solid state. Ellipsoids are shown at a 50% probability level. H atoms are omitted; Figure S32: Dimeric form of 4 in the solid state. Ellipsoids are shown at a 50% probability level. H atoms are omitted; Figure S33: Molecular structure of 5 in the solid state. Ellipsoids are shown at a 50% probability level. H atoms are omitted; Figure S34: Molecular structure of 5b in the solid state. Ellipsoids are shown at a 50% probability level. H atoms are omitted; Figure S35: Molecular structure of 6 in the solid state. Ellipsoids are shown at a 50% probability level. H atoms and the distortion are omitted; Table S1: Selected crystal structure data for the structure determination of compound 3×DCM; Table S2: Selected crystal structure data for the structure determination of compound 4; Table S3: Selected crystal structure data for the structure determination of compound 5; Table S4: Selected crystal structure data for the structure determination of compound 5b; Table S5: Selected crystal structure data for the structure determination of compound 6.
Author Contributions
Conceptualization, R.-M.R. and C.v.H.; synthesis, R.-M.R., D.J.S., A.L.R., L.R., K.B. and A.D.; data curation, R.-M.R., D.J.S., A.L.R., L.R., K.B. and A.D.; writing—original draft preparation, R.-M.R.; writing—review and editing, R.-M.R. and C.v.H.; visualization, R.-M.R.; supervision, C.v.H.; project administration, C.v.H.; funding acquisition, C.v.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deutsche Forschungsgemeinschaft (DFG), grant number HA 3466/8-3.
Data Availability Statement
Deposition Numbers CCDC 2412948 (3), 2412949 (4), 2412950 (5), 2412947 (5b) and 2412946 (6) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, accessed on 9 March 2025. For details on the crystal structures, see also ESI. Experimental details including all spectroscopic investigations can be found in the Supplementary Information (ESI).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| DCM | Dichloromethane |
| Et | Ethyl |
| NMR | Nuclear Magnetic Resonance |
| PhF | Fluorobenzene |
| PTFE | Polytetrafluoroethylene |
| TrEGDS | Triethylenglycoldisiloxane |
| TeEGDS | Tetraethylenglycoldisiloxane |
References
- Decken, A.; Passmore, J.; Wang, X. Cyclic Dimethylsiloxanes as Pseudo Crown Ethers: Syntheses and Characterization of Li (Me2SiO)5[Al{OC(CF3Li(Me2SiO)6[Al{OC(CF3Li(Me2SiO)6[Al{OC(CF3)3}4)3}4)2Ph}4]. Angew. Chem. Int. Ed. 2006, 45, 2773–2777. [Google Scholar] [CrossRef] [PubMed]
- Ernst, R.D.; Glöckner, A.; Arif, A.M. Crystal structure of hexakis(dimethyl-m-oxosilane)dibromozirconium(IV) bis(nonabromodizirconate(IV)), [Zr{(CH3)2SiO}6Br2][Zr2Br9]2. New Cryst. Struct. 2007, 222, 333–334. [Google Scholar] [CrossRef]
- Cameron, T.S.; Decken, A.; Krossing, I.; Passmore, J.; Rautiainen, J.M.; Wang, X.; Zeng, X. Reactions of a Cyclodimethylsiloxane (Me2SiO)6 with Silver Salts of Weakly Coordinating Anions; Crystal Structures of [Ag(Me2SiO)6][Al] ([Al] = [FAl{OC(CF3)3}3], [Al{OC(CF3)3}4]) and Their Comparison with [Ag(18-Crown-6)]2[SbF6]2. Inorg. Chem. 2013, 52, 3113–3126. [Google Scholar] [CrossRef] [PubMed]
- Sänger, I.; Gärtner, M.; Bolte, M.; Wagner, M.; Lerner, H.-W. New Aspects with Regard to Silanide Chemistry in Particular Formation and Structure of the First Disilyl Sodate [K(Me2SiO)7][(tBu3Si)2MeSi-Na-SiMe(SitBu3)2]. Z. Anorg. Allg. Chem. 2018, 644, 925–929. [Google Scholar] [CrossRef]
- Weinhold, F.; West, R. The Nature of the Silicon–Oxygen Bond. Organometallics 2011, 30, 5815–5824. [Google Scholar] [CrossRef]
- Moraru, I.-T.; Petrar, P.M.; Nemeş, G. Bridging a Knowledge Gap from Siloxanes to Germoxanes and Stannoxanes. A Theoretical Natural Bond Orbital Study. J. Phys. Chem. A 2017, 121, 2515–2522. [Google Scholar] [CrossRef]
- Fugel, M.; Hesse, M.F.; Pal, R.; Beckmann, J.; Jayatilaka, D.; Turner, M.J.; Karton, A.; Bultinck, P.; Chandler, G.S.; Grabowsky, S. Covalency and Ionicity Do Not Oppose Each Other-Relationship Between Si-O Bond Character and Basicity of Siloxanes. Chemistry 2018, 24, 15275–15286. [Google Scholar] [CrossRef]
- Grabowsky, S.; Hesse, M.F.; Paulmann, C.; Luger, P.; Beckmann, J. How to make the ionic Si-O bond more covalent and the Si-O-Si linkage a better acceptor for hydrogen bonding. Inorg. Chem. 2009, 48, 4384–4393. [Google Scholar] [CrossRef]
- Grabowsky, S.; Beckmann, J.; Luger, P. The Nature of Hydrogen Bonding Involving the Siloxane Group. Aust. J. Chem. 2012, 65, 785. [Google Scholar] [CrossRef]
- Pedersen, C.J. The Discovery of Crown Ethers (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 1021–1027. [Google Scholar] [CrossRef]
- Ritch, J.S.; Chivers, T. Silicon Analogues of Crown Ethers and Cryptands: A New Chapter in Host–Guest Chemistry? Angew. Chem. Int. Ed. 2007, 46, 4610–4613. [Google Scholar] [CrossRef] [PubMed]
- Dankert, F.; von Hänisch, C. Siloxane Coordination Revisited: Si−O Bond Character, Reactivity and Magnificent Molecular Shapes. Eur. J. Inorg. Chem. 2021, 2021, 2907–2927. [Google Scholar] [CrossRef]
- Dankert, F.; Donsbach, C.; Rienmüller, J.; Richter, R.M.; von Hänisch, C. Alkaline Earth Metal Template (Cross-)Coupling Reactions with Hybrid Disila-Crown Ether Analogues. Chem. Eur. J. 2019, 25, 15934–15943. [Google Scholar] [CrossRef] [PubMed]
- Pahl, J.; Elsen, H.; Friedrich, A.; Harder, S. Unsupported metal silyl ether coordination. Chem. Commun. 2018, 54, 7846–7849. [Google Scholar] [CrossRef]
- Thum, K.; Friedrich, A.; Pahl, J.; Elsen, H.; Langer, J.; Harder, S. Unsupported Mg-Alkene Bonding. Chem.—Eur. J. 2021, 27, 2513–2522. [Google Scholar] [CrossRef]
- Haynes, M.D.; O’Reilly, A.; Poole, A.J.M.; Roper, A.F.; Thum, S.; Morris, L.J.; Coles, M.P.; Fulton, J.R.; Harder, S.; Turner, Z.R.; et al. Heavier alkaline earth and heterobimetallic s-block “ate” complexes of a di(amido)siloxane ligand: Solid-state structure and dynamic solution-phase behavior. Dalton Trans. 2025, 54, 4542–4555. [Google Scholar] [CrossRef]
- Chai, C.L.L.; Armarego, W.L.F. Purification of Laboratory Chemicals, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Troyanov, S.I.; Krahl, T.; Kemnitz, E. Crystal structures of GaX3 (X = Cl, Br, I) and AlI3. Z. Kristallogr. 2004, 219, 88–92. [Google Scholar] [CrossRef]
- Richter, R.-M.; von Hänisch, C. Complexes of Alkali Metal and the Ammonium Ion with the Cyclodimethylsiloxane Ligands D7 and D8 (D = Me2SiO). Z. Anorg. Allg. Chem. 2024, 650, e202400036. [Google Scholar] [CrossRef]
- Richter, R.-M.; Heinrichs, J.; Weiss, P.; Subrati, Z.; von Hänisch, C. Complexes of Alkaline Earth Metal Ions with the Cyclodimethylsiloxane ligands D7, D8 and D9 (D = Me2SiO). Z. Anorg. Allg. Chem. 2024, 650, e202400178. [Google Scholar] [CrossRef]
- Dankert, F.; Weigend, F.; von Hänisch, C. Not Non-Coordinating at All: Coordination Compounds of the Cyclodimethylsiloxanes Dn (D = Me2SiO; n = 6, 7) and Group 2 Metal Cations. Inorg. Chem. 2019, 58, 15417–15422. [Google Scholar] [CrossRef]
- Dankert, F.; Erlemeier, L.; Ritter, C.; von Hänisch, C. On the molecular architectures of siloxane coordination compounds: (re-)investigating the coordination of the cyclodimethylsiloxanes Dn (n =5–8) towards alkali metal ions. Inorg. Chem. Front. 2020, 7, 2138–2153. [Google Scholar] [CrossRef]
- Groth, P.; Møllendal, H.; Seip, R.; Weidlein, J.; Spahiu, K. On the Crystal Structure of the (1:1) Complex between Lithium Thiocyanate and 1,4,7,10-Tetraoxacyclododecane at Room Temperature. Acta Chem. Scand. 1981, 35a, 463–465. [Google Scholar] [CrossRef]
- Nöth, H.; Warchhold, M. Sodium Hydro(isothiocyanato)borates: Synthesis and Structures. Eur. J. Inorg. Chem. 2004, 2004, 1115–1124. [Google Scholar] [CrossRef]
- Paparo, A.; Silvia, J.S.; Spaniol, T.P.; Okuda, J.; Cummins, C.C. Countercation Effect on CO2 Binding to Oxo Titanate with Bulky Anilide Ligands. Chem. Eur. J. 2018, 24, 17072–17079. [Google Scholar] [CrossRef]
- Pochekutova, T.S.; Khamylov, V.K.; Fukin, G.K.; Kurskii, Y.A.; Petrov, B.I.; Shavyrin, A.S.; Arapova, A.V. New calcium β-diketonate complexes: The monomeric complexes [Ca(PhCOCHCOCF3)2(15-crown-5)], [Ca(AdCOCHCOCF3)2(15-crown-5)] and the binuclear hydrated complex [{Ca(adtfa)(18-crown-6)(H2O)}{Ca(adtfa)3(H2O)}(EtOH)]. Synthesis, characterization and crystal structure. Polyhedron 2011, 30, 1945–1952. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).