1. Introduction
Chemically modified electrodes have been investigated in order to increase the electrode performance, such as sensitivity, selectivity, and activity, over time. In the last decade, alumina-based electrodes have been investigated. It has been observed that the electrochemical characteristics of a glassy carbon electrode polished with alumina are influenced by the shape and structure of the alumina particles [
1,
2]. In HER and OER studies, graphene–alumina composite electrodes demonstrated efficiency comparable with platinum standards [
3]. The alumina type plays a crucial role in the electrochemical performance of an alumina-modified carbon paste electrode [
4]. The behavior of these electrodes at various pHs depended on the type and number of alumina surface groups. Although alumina is an insulating material, as a modifier of glassy carbon or carbon paste electrodes, it exhibits certain electroactivity. This feature of alumina has been exploited for the development of new sensors [
5,
6]. Alumina-supported metal–oxides have recently been investigated [
7,
8] as electrocatalysts. The role of alumina in these electrocatalysts is to serve as a support for metal–oxide, which is an electroactive component that provides chemical and hydrothermal stability. Alumina can also actively participate in electrochemical reactions by acting in synergy with metal–oxide nanoparticles. This aspect of alumina was the focus of this study.
The aim of this work was to investigate the behavior of alumina and alumina–Cu composite electrodes in alkaline media. Alumina is prone to corrosion in highly alkaline solutions [
9]. This reaction is often investigated in regard to possible corrosion inhibitors in alkaline aluminum–air batteries [
10]. However, this reaction might also be used for the development of sensors.
This paper presents a novel investigation of alumina–Cu samples prepared using a simple ion-exchange procedure as electrode materials for electroanalytical applications.
Two types of alumina, anhydrous (A) and trihydrate (T) alumina, were used due to their significantly different electrochemical behaviors [
4]. The observed variation was a consequence of the type and number of surface groups at the surface of the alumina particles. The electrochemical properties of the alumina–Cu composites were evaluated in both alkaline and neutral media. Their performance was assessed for the detection of glutamate and hydrogen peroxide by exploring their potential applications across different media and analytes. The novelty of this work lay in the strategic use of alumina as a supporting material to enhance the electrochemical properties of the electrode. By incorporating alumina, the copper-based electrode’s stability, conductivity, and overall performance were improved, which made it more effective for electroanalytical applications. This approach not only optimized the electrode’s functionality but also broadens its potential for detecting various analytes in different chemical environments.
The newly synthesized electrodes were applied for the detection of glutamic acid and hydrogen peroxide. Both of these analytes are important for human health. Glutamate, an ionized form of glutamic acid, is a neurotransmitter in the central nervous system [
11]. In the form of monosodium glutamate, it is often added to food as a flavor enhancer [
12]. Hydrogen peroxide is, among other things, used in the dairy industry [
13]. It can have an adverse effect on health in inappropriate concentrations [
14]. Therefore, accurate determinations of the concentrations of glutamic acid and H
2O
2 are of great importance. Electrochemical sensing techniques offer the advantages of accurate, fast, and low-cost measurements [
15,
16]. The development of non-enzymatic sensors is promising and the results presented in this study are a step in this direction. The application of nanomaterials and metal–oxide nanoparticles in the development of electrochemical sensors significantly improves their performances [
17,
18].
2. Materials and Methods
Industrial Al2O3 (designated as anhydrous alumina (A)) and Al2O3·3H2O (designated as trihydrate alumina (T)) were acquired from an alumina refinery (Alumina Ltd., Zvornik, Bosnia and Herzegovina). Copper-modified alumina samples were prepared by an ion-exchange procedure with a solution of 0.1 M CuSO4·5H2O. The alumina and solution in the ratio 1:20 were stirred with a magnetic stirrer at 500 rpm for 1 h at room temperature. The sample was filtered using a Büchner funnel and filter paper and rinsed with distilled water. The Cu–alumina samples were dried at 110 °C overnight and designated as A-Cu and T-Cu.
The diffuse reflectance UV–Vis (DR UV–Vis) spectra were recorded using a Shimadzu UV-2600 spectrophotometer (Shimadzu Corporation, Tokyo, Japan) equipped with an integrated sphere (ISR-2600 Plus (for UV-2600)). The DR spectra were measured in the range from 200 to 800 nm, with a scan speed of 60 nm/min. The DR spectra were converted to absorption spectra using the Kubelka–Munk function.
Electrochemical experiments were performed using the Autolab electrochemical workstation (Autolab PGSTAT302N, Metrohm-Autolab BV, Utrecht, The Netherlands) with a three-electrode cell composed of Ag/AgCl in 3 M KCl as the reference electrode, a platinum rod as the counter electrode, and a carbon paste electrode as the working electrode.
A carbon paste electrode (CPE) modified with alumina samples was prepared by hand-mixing 0.1 g carbon black (CB) (Vulcan-XC 72R, Cabot Corp., MA, USA) and 0.4 g of alumina sample with 1.2 g of paraffin oil. The composition was selected after the optimization experiment. The resulting paste was packed into a hollow (2 mm diameter; 0.0314 cm2) Teflon tube with the electrical contact provided by copper and used as a working electrode.
The electrochemical properties were tested by cyclic voltammetry (CV) and linear sweep voltammetry (LSV) in 0.1 M NaOH and 0.1 M Britton–Robinson buffer at pH 7. The response of the electrode in the presence of glutamic acid (GA) and H2O2 was tested by linear sweep voltammetry. The measurements were repeated three times for each concentration.
3. Results and Discussion
3.1. UV–Vis Diffuse Reflectance Spectra
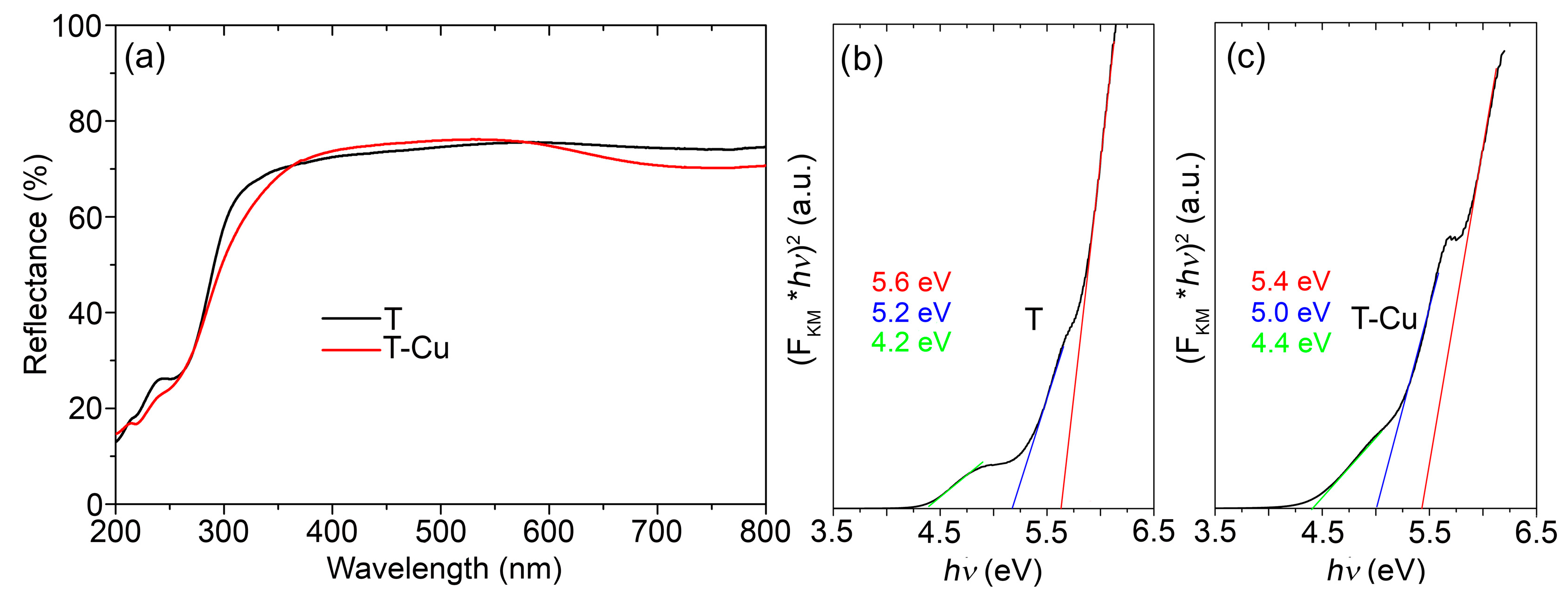
The DRS reflectance spectra of both pristine T alumina and T alumina doped with Cu ions (
Figure 1a) revealed that the samples mainly absorbed in the UV spectral region.
The only effect of doping by Cu ions was evidenced by the presence of the wide band placed between 600 and 800 nm, which, according to Fu et al. [
19], can be assigned to a Cu ion placed in an octahedral position in an alumina matrix coupled with oxygen vacancies, or to a transition between well-dispersed Cu species in the alumina matrix, which is a scenario expected in the case of low or medium levels of doping. The band gap energy of T alumina estimated from the Tauc plot (
Figure 1b) was 5.6 eV. Two additional electronic transitions could be detected, which were placed at 4.4 and 5.2 eV and originated from additional levels in the band gap of the material (oxygen vacancies or metal impurities). However, upon doping (
Figure 1c), no additional transitions were detected, except that the estimated band gap energy was slightly lower: 5.4 eV, which can be an indication of new electronic levels placed in the band gap but cannot be exclusively assigned to Cu dopant ions.
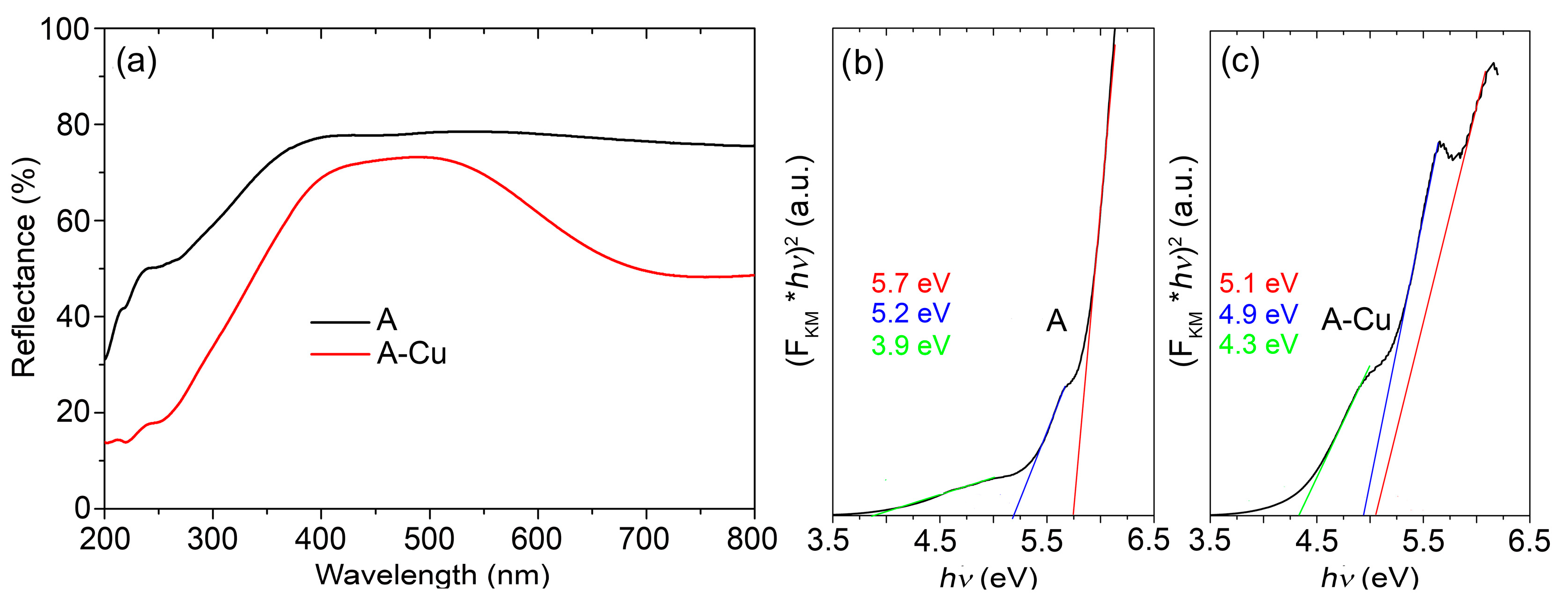
The DRS reflectance spectra (
Figure 2a) underlined a significant difference between pristine A and A alumina doped with Cu ions.
Although both samples absorbed mainly in the UV region, the A-Cu sample’s spectrum was characterized by the strong wide band found between 600 and 800 nm, which confirmed the high presence and good distribution of Cu ions in the alumina matrix. The band gap energy of A alumina estimated from the corresponding Tauc plot (
Figure 2b) was slightly higher than for T alumina, with a value of 5.7 eV, but both defect-related transitions were present and placed at energies of 3.9 eV and 5.2 eV. A significant lowering of the band gap energy of the doped A sample (
Figure 2c) to the value of 5.1 eV is an indication of a more uniform Cu ion distribution in A compared with the T alumina matrix.
3.2. Electrochemical Characterization
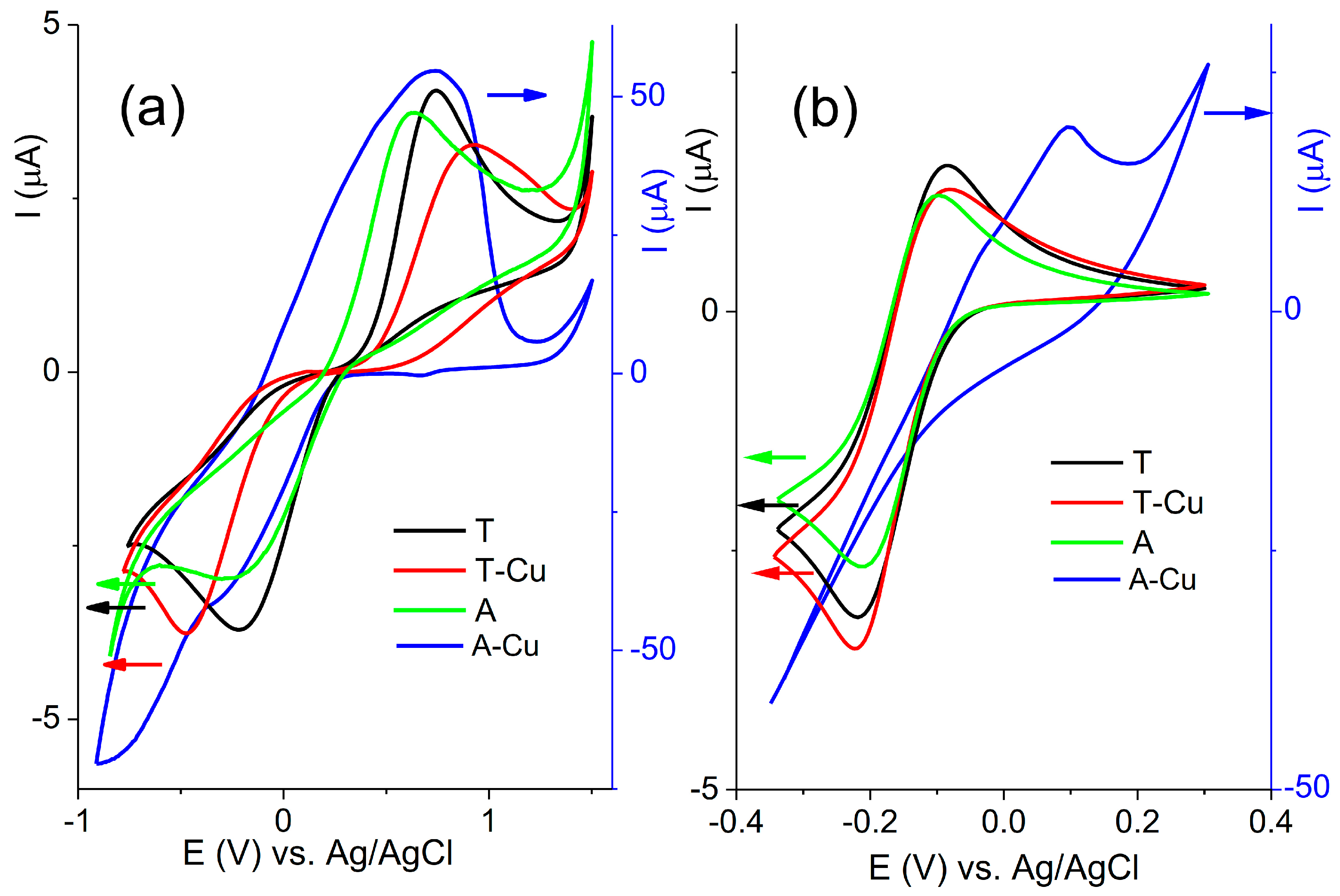
The electrochemical behavior of the carbon paste electrode modified with alumina samples was tested for two redox couples: ferri/ferrocyanide ([Fe(CN)
6]
3−/4−) and hexaamine ruthenium (III/II) ([Ru(NH
3)
6]
2+/3+) by cyclic voltammetry (
Figure 3).
The most noticeable feature of the presented cyclic voltammograms is that the currents recorded for the A-Cu-modified CPE were ten times greater than those recorded for the other three investigated electrodes (the currents of the A-Cu CPE are presented versus the right axis, while the currents of T, T-Cu, and A are presented versus the left axis; the currents are presented in this manner to accommodate the large differences in the current values recorded for the A-Cu electrode compared with the other three investigated electrodes). The reason is the different amounts of Cu2+ ions present at the surface of the A-Cu alumina compared with the T-Cu alumina. The copper redox process partially overlapped with the redox probe response, which complicated the peak identification.
The surface groups at the surface of the investigated alumina samples will influence the response of the electrodes to the two redox probes. The trihydrate (T) and anhydrous alumina (A) exhibited different responses due to different surface properties. There are several types of surface groups that can be found in alumina: various OH groups, but two major types are double-coordinated basal groups (≡Al
2OH) and singly coordinated edge sites (≡AlOH) [
20]; Lewis acid sites (Al
3+); and Al-O-Al bridges. The surface acidity of these samples is usually described by the “two pKa model”:
Depending on the type of alumina and the pH of the solution, the ratio and distribution of these surface groups will vary, which influences the response toward the redox probe. [Fe(CN)
6]
3−/4− is a negatively charged redox probe. A good electrochemical response can be expected for the surfaces that contain positively charged sites where a negatively charged redox probe would be electrostatically attracted [
21]. In the case of alumina, these positive sites can originate from Bronsted acid sites or Lewis acid sites. The electrode’s response toward the negative redox probe depends on the type of alumina: T or A. The presence of copper ions altered the distribution of these sites, which resulted in different responses. The peak-to-peak separations were 0.88 V and 0.73 V for the T and A electrodes. The peak-to-peak separation for T-Cu was increased to 1.30 V, indicating slower electron transfer kinetics. The estimation of the peak-to-peak separation for the A-Cu electrode was difficult due to the overlapping of the peaks that originated from the redox probe and copper redox processes. However, the cathodic current peak of A-Cu was significantly reduced in comparison with the A electrode, indicating a lower response toward the [Fe(CN)
6]
3−/4− redox probe. It was expected that during the ion-exchange procedure, copper ions were involved in the formation of Cu—O—Al bonds [
22], which effectively reduced the possible response toward a negative redox probe.
A similar trend was observed in the responses toward the positively charged redox probe [Ru(NH3)6]2+/3+. Negatively charged sites will mostly contribute to the response to a positively charged redox probe. The slightly lower currents were noticed for the A electrode in comparison with the T electrode. A significantly different response was noticed for the A-Cu electrode that was in accordance with a higher content of copper in the A-Cu sample.
3.3. Electrochemical Behavior and Analytical Properties in Alkaline Media
Cyclic voltammetry in 0.1 M NaOH was used to examine the electrochemical response of alumina with and without copper across a wide range of potentials (
Figure 4a).
The anodic behavior of the Al
2O
3 films was mainly investigated as a step in the corrosion behavior of aluminum. The anodic dissolution of aluminum was expected to proceed through the electrochemical formation of the oxide/hydroxide film. In the next step, this film was chemically dissolved through the formation of soluble [Al(OH)
4]
− [
23]. The alumina film formed in the electrochemical step could be composed of various species of oxides or oxy/hydroxides, Al
2O
3, Al(OH)
3, and/or AlOOH.
The anodic wave around 1 V was recorded for anhydrous alumina. The same wave was noticed in the cyclic voltammogram of trihydrate alumina, although the recorded current was significantly lower. The presence of copper on the surface of both the alumina samples resulted in higher currents in the CV of the copper-containing samples. The cathodic peak at around −1 V was also present for all the investigated samples, and an increase in the current was noticed for the samples with copper.
Within the potential range of −0.5 V to 0.5 V, distinct peaks that corresponded to the oxidation and reduction of copper species could be observed for the copper-containing electrodes. The currents for the A-Cu sample were significantly higher than those for the T-Cu sample, which can be attributed to the greater concentration of copper in the A-Cu sample.
In order to gain insight into possible origin of the anodic peak, the influence of the cathodic limit on the shape of the cyclic voltammogram was studied (
Figure 4b). The current of the anodic peak at around 1 V increased with the shift in the cathodic potential limit toward more negative values. Furthermore, this shift was followed by a change in the profile of copper species (inset figure in
Figure 4b). Two regions can be noticed in the cyclic voltammogram of the alumina–copper samples. Region I corresponds to the Cu(0) to Cu(I) oxidation, while region II corresponds to the Cu(I) to Cu(II) oxidation. This region usually contains two peaks, IIa and IIc, which corresponded to the formation of CuO and Cu(OH)
2. When the cathodic limit was placed at −1.4 V vs. Ag/AgCl, the middle peak IIb appeared. This peak most likely indicates the formation of the complex Cu(II) species (Cu(OH)
42−, HCuO
2−, or CuO
22−) [
24]. The further oxidation of copper, Cu(II)/Cu(III), has been reported to occur at potentials higher than 0.5 V. It was expected that the species formed at a high anodic potential include CuOOH and/or Cu(OH)
4−.
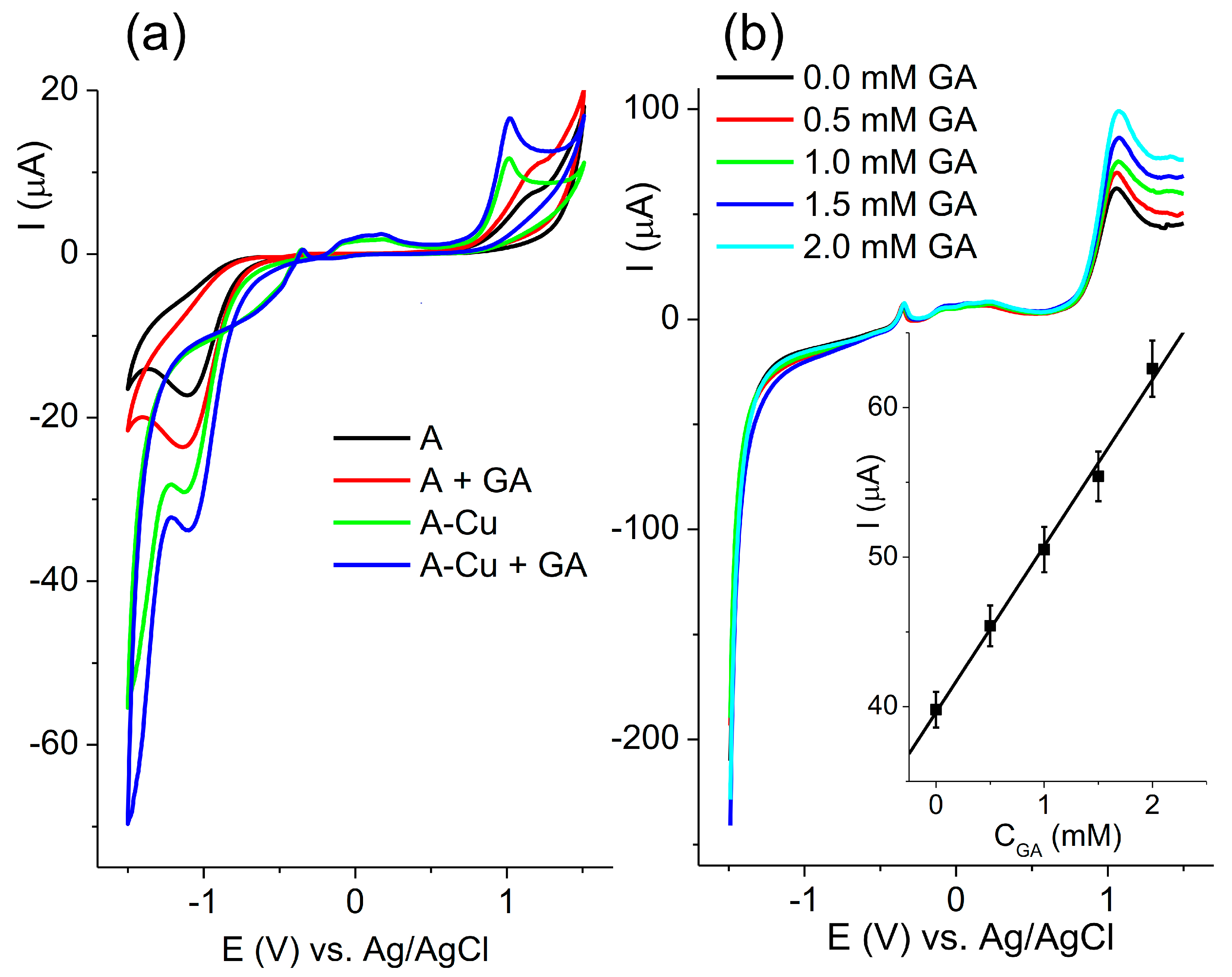
Since the greatest difference was noticed for the A and A-Cu electrodes, further investigation was focused on these two electrodes. The responses of the A and A-Cu electrodes toward 1 mM glutamic acid are presented in
Figure 5a.
The increase in the current response was observed in the potential area of the formation of oxy-hydroxide species. The electrooxidation of glutamic acid on nickel-based electrode was investigated, suggesting a mechanism where NiOOH reacts with glutamic acid to produce Ni(OH)
2 and 2-oxo glutaric acid [
25]. A similar mechanism can be proposed for the electrode investigated in this study since the formation of AlOOH and CuOOH is expected at the potential of glutamic acid oxidation. The A-Cu electrode was tested for its ability to sense glutamic acid. The LSV of the A-Cu electrode in 0.1 M NaOH contained various concentrations of glutamic acid and showed an increase in the currents with an increase in the concentration of glutamic acid (
Figure 5b) due to the oxidation of glutamic acid.
The cathodic limit was set to −1.5 V in order to obtain the highest response. The electrode composed of alumina without copper also showed an increase in the peak current with the addition of glutamic acid (Although the increase was less pronounced. The peak obtained at 1 V was used for plotting the calibration curve (inserted picture,
Figure 5b). The peak current vs. concentration plot for A-Cu was linear with a slope of 11.1 µA/mM.
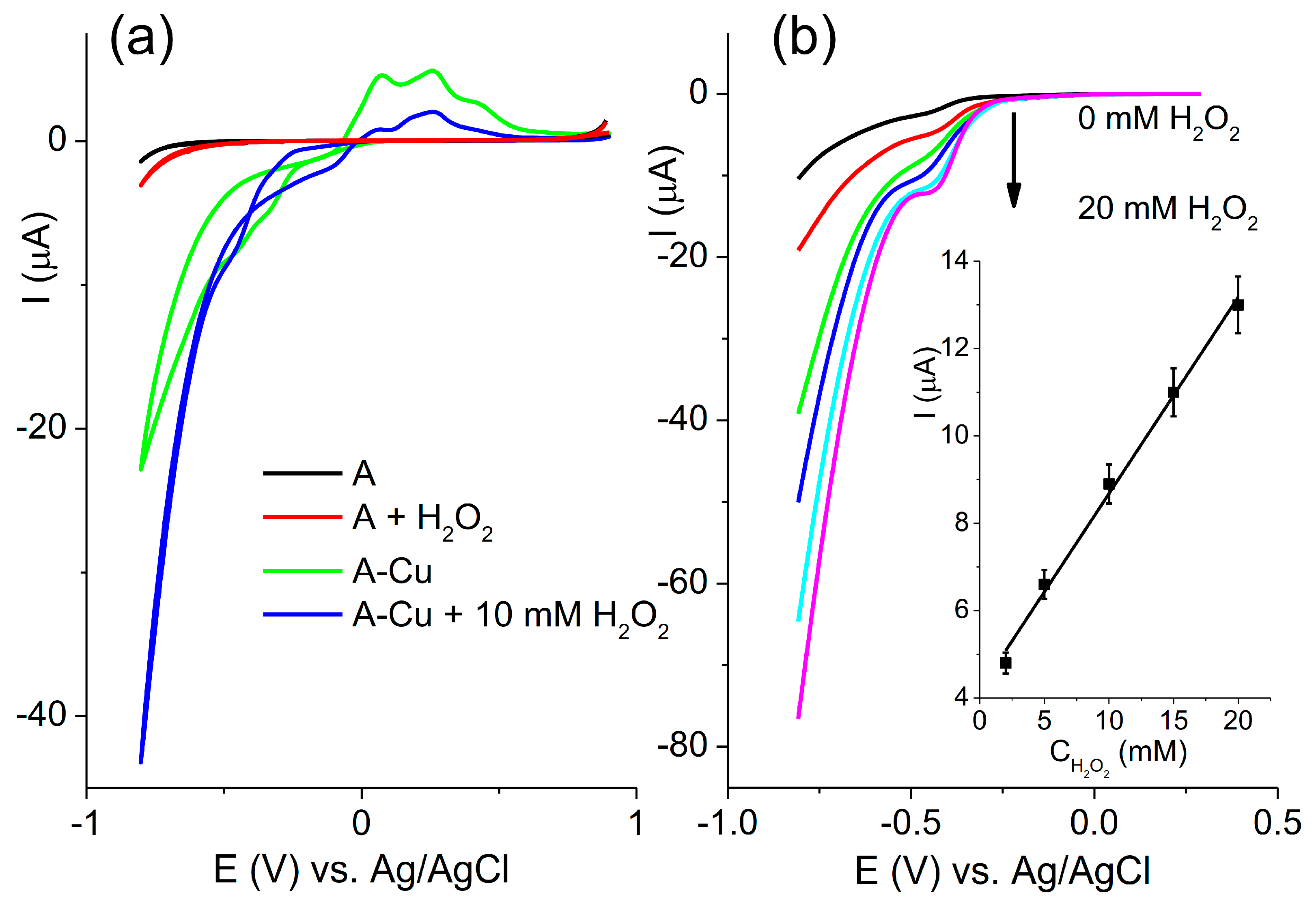
3.4. Electrochemical Behavior and Analytical Properties in Neutral Media
The electrochemical behavior of the A and A-Cu electrodes was investigated in a neutral solution of 0.1 M Britton–Robinson (BR) buffer at pH 7. The cyclic voltammograms recorded at a scan rate of 100 mVs
−1 in pure buffer and with the addition of 10 mM H
2O
2 are presented in
Figure 6a.
The response of the A electrode in neutral media with and without H
2O
2 was similar to each other. The only notable difference was observed at the edges of the applied potential window. In the neutral media, the regions of Cu oxidation at the A-Cu electrode were not distinctly separated, unlike in the alkaline solution. The cyclic voltammograms recorded without H
2O
2 revealed several anodic peaks. These peaks can be attributed to the formation of hydrous Cu
2O, CuO, and Cu(OH)
2 [
26]. In contrast, the cathodic peaks were not well resolved and appeared as a single current increase at potentials below −0.25 V. The CV recorded in the presence of H
2O
2 showed reduced currents of Cu oxidation due to the chemical oxidation of Cu by H
2O
2 [
27]. The direct oxidation of H
2O
2 can be seen at the potential around −0.1 V. This direct oxidation was further investigated at various concentrations of H
2O
2 using LSV, as shown in
Figure 6b, which includes a calibration curve as an inset. The plot of current versus concentration was linear, with a slope of 0.45 µA/mM.
4. Conclusions
Two types of alumina, anhydrous (A) and trihydrate (T) alumina, were used to obtain alumina–copper composites by an ion-exchange procedure.
The DRS spectra of the synthesized samples indicated that a more uniform distribution of Cu ions was achieved in the anhydrous alumina compared with the trihydrate alumina matrix. According to the Tauc plots used to estimate the band gap energies, the incorporation of Cu ions into the anhydrous alumina matrix resulted in a significant decrease in the optical band gap, which lowered it from an initial 5.7 eV to 5.1 eV.
The obtained samples were used as electrode materials. The electrochemical behavior of the alumina–Cu composites was investigated in both alkaline and neutral solutions using cyclic voltammetry. Characteristic peaks corresponding to the oxidation of Cu were recorded, along with the formation of oxy-hydroxide species at a high anodic potential in alkaline media. The electrooxidation of glutamic acid proceeds through a mechanism that involves these oxy-hydroxide species. The direct oxidation of H2O2 on the alumina–Cu samples in neutral media was used for the electroanalytical detection of H2O2.
Author Contributions
Investigation, T.N., D.P., N.A. and Z.M.; data curation and writing—review and editing, N.A. and Z.M.; conceptualization and writing—original draft preparation, Z.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Grant No. 451-03-136/2025-03/200026 and 451-03-136/2025-03/200017).
Data Availability Statement
The original contributions found in this study are included in this article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| A | Anhydrous alumina |
| T | Trihydrate alumina |
| CPE | Carbon paste electrode |
| CB | Carbon black |
| CV | Cyclic voltammetry |
| LSV | Linear sweep voltammetry |
| DRS | Diffuse reflectance spectra |
| GA | Glutamic acid |
References
- Lima, A.P.; dos Santos, W.T.P.; Nossol, E.; Richter, E.M.; Munoz, R.A.A. Critical evaluation of voltammetric techniques for antioxidant capacity and activity: Presence of alumina on glassy-carbon electrodes alters the results. Electrochim. Acta 2020, 358, 136925. [Google Scholar] [CrossRef]
- Lima, A.P.; Almeida, P.L.M.R.; Sousa, R.M.F.; Richter, E.M.; Nossol, E.; Munoz, R.A.A. Effect of alumina supported on glassy-carbon electrode on the electrochemical reduction of 2,4,6-trinitrotoluene: A simple strategy for its selective detection. J. Electroanal. Chem. 2019, 851, 113385. [Google Scholar] [CrossRef]
- Ma, X.X.; He, X.Q. Electronically tailoring 3D flower-like graphene via alumina doping and incorporating Co as an efficient oxygen electrode catalyst in both alkaline and acid media. J. Power Sources 2017, 353, 28. [Google Scholar] [CrossRef]
- Novaković, T.; Barudžija, T.; Čomor, M.; Banković, P.; Mojović, Z. Electrochemical behavior of different types of alumina. J. Electroanal. Chem. 2021, 895, 115542. [Google Scholar] [CrossRef]
- Ma, C.; Chang, Y.; Ye, W.; Shang, W.; Wang, C. Supercritical preparation of hexagonal γ-alumina nanosheets and its electrocatalytic properties. J. Colloid. Interf. Sci. 2008, 317, 148–154. [Google Scholar] [CrossRef]
- Yang, X.; Huang, W.; Li, Q.; Qi, B.; Zhang, W. Highly sensitive determination of paeonol using porous alumina microfibers modified electrode. J. Electroanal. Chem. 2017, 801, 98–103. [Google Scholar] [CrossRef]
- Khan, S.; Shah, S.S.; Janjua, N.K.; Yurtcan, A.B.; Nazir, M.T.; Katubi, K.M.; Alsaiari, N.S. Alumina supported copper oxide nanoparticles (CuO/Al2O3) as high-performance electrocatalysts for hydrazine oxidation reaction. Chemosphere 2023, 315, 137659. [Google Scholar] [CrossRef]
- Khan, S.; Shah, S.S.; Ahmad, A.; Yurtcan, A.B.; Jabeen, E.; Alshgari, R.A.; Janjua, N.K. Ruthenium and palladium oxide promoted zinc oxide nanoparticles: Efficient electrocatalysts for hydrazine oxidation reaction. J. Electroanal. Chem. 2022, 917, 116422. [Google Scholar] [CrossRef]
- Boukerche, I.; Djerad, S.; Benmansour, L.; Tifouti, L.; Saleh, K. Degradability of aluminum in acidic and alkaline solutions. Corros. Sci. 2014, 78, 343. [Google Scholar] [CrossRef]
- Daas, B.M.; Ghosh, P.K.; Mandal, B.; Ghosh, S.; Basumallick, I. Electrochemical analysis of aluminium corrosion in alkaline battery fluid under the influence of Alizarin Red S and Xylenol Orange. Results Chem. 2024, 7, 101405. [Google Scholar] [CrossRef]
- Meldrum, B.S. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J. Nutr. 2000, 130, 1007S–1015S. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.A.R.; Ribeiro, M.N.; Silva, G.A.; Rocha, L.C.R.; Pinheiro, A.C.M.; Nunes, C.A.; Carneiro, J.d.D.S. Temporal profile of flavor enhancers MAG, MSG, GMP, and IMP, and their ability to enhance salty taste, in different reductions of sodium chloride. J. Food Sci. 2020, 85, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- van Asselt, E.D.; Van der Fels-Klerx, H.J.; Marvin, H.J.P.; Van Bokhorst-van de Veen, H.; Groot, M.N. Overview of food safety hazards in the European dairy supply chain. Compr. Rev. Food Sci. Food Saf. 2017, 16, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Hemanth, S.; Sukumaran, M.K. Milk adulteration in Hyderabad, India-a comparative study on the levels of different adulterants present in milk. J. Chromatogr. Sep. Tech. 2014, 5, 1000212. [Google Scholar] [CrossRef]
- Schultz, J.; Uddin, Z.; Singh, G.; Howlader, M.M.R. Glutamate sensing in biofluids: Recent advances and research challenges of electrochemical sensors. Analyst 2020, 145, 321–347. [Google Scholar] [CrossRef]
- Alencar, L.M.; Silva, A.W.B.N.; Trindade, M.A.G.; Salvatierra, R.V.; Martins, C.A.; Souza, V.H.R. One-step synthesis of crumpled graphene fully decorated by copper-based nanoparticles: Application in H2O2 sensing. Sens. Actuators B Chem. 2022, 360, 131649. [Google Scholar] [CrossRef]
- Kumar, A.; Purohit, B.; Maurya, P.K.; Pandey, L.M.; Chandra, P. Engineered Nanomaterial Assisted Signal-amplification Strategies for Enhancing Analytical Performance of Electrochemical Biosensors. Electroanalysis 2019, 31, 1615–1629. [Google Scholar] [CrossRef]
- Fenzl, C.; Hirsch, T.; Baeumner, A.J. Nanomaterials as versatile tools for signal amplification in (bio)analytical applications. TrAC, Trends Anal. Chem. 2016, 79, 306–316. [Google Scholar] [CrossRef]
- Yang, L.F.H. Tailoring the Electronic Structure of Mesoporous Spinel γ-Al2O3 at Atomic Level: Cu-Doped Case. J. Phys. Chem. C 2014, 118, 14299–14315. [Google Scholar] [CrossRef]
- Tsyganenko, A.A.; Mardilovich, P.P. Structure of Alumina Surfaces. J. Chem. Soc. Faraday Trans. 1996, 92, 4843–4852. [Google Scholar] [CrossRef]
- Tonle, I.K.; Ngameni, E.; Walcarius, A. From clay- to organoclay-film modified electrodes: Tuning charge selectivity in ion exchange voltammetry. Electrochim. Acta 2004, 49, 3435–3443. [Google Scholar] [CrossRef]
- Leofanti, G.; Marsella, A.; Cremaschi, B.; Garilli, M.; Zecchina, A.; Spoto, G.; Bordiga, S.; Fisicaro, P.; Berlier, G.; Prestipino, C.; et al. Alumina-Supported Copper Chloride. J. Catal. 2001, 202, 279–295. [Google Scholar] [CrossRef]
- Pyun, S.-I.; Moon, S.-M. Corrosion mechanism of pure aluminium in aqueous alkaline solution. J. Solid State Electrochem. 2000, 4, 267–272. [Google Scholar] [CrossRef]
- Ambrose, J.; Barradas, R.G.; Shoesmith, D.W. Investigations of copper in aqueous alkaline solutions by cyclic voltammetry. J. Electroanal. Chem. Interf. Electrochem. 1973, 47, 47–64. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, T.; Niu, Y.; Ye, B.-C. Electrochemical detection of glutamate by metal–organic frameworks-derived Ni@NC electrocatalysts. Microchem. J. 2022, 175, 107229. [Google Scholar] [CrossRef]
- Metikoš-Huković, M.; Babić, R.; Paić, I. Copper corrosion at various pH values with and without the inhibitor. J. Appl. Electrochem. 2000, 30, 617–624. [Google Scholar] [CrossRef]
- Somasundrum, M.; Kirtikara, K.; Tanticharoen, M. Amperometric determination of hydrogen peroxide by direct and catalytic reduction at a copper electrode. Anal. Chmica Acta 1996, 319, 59–70. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).